Abstract
Objectives
The aim of this study was to determine the risk of congenital malformations in offspring born to women with systemic lupus erythematosus (SLE).
Methods
This nationwide population-based study included Korean women who had a singleton pregnancy. The risk of congenital malformations in women with SLE was compared with those without SLE. Multivariable analyses were performed to estimate the OR of congenital malformations. In a sensitivity analysis, the risk of malformation was compared between the offspring of women with SLE and those of propensity-matched women without SLE.
Results
Of a total of 3 279 204 pregnant women, 0.1% had SLE and their offspring had a higher frequency of congenital malformations (17.13% vs 11.99%, p<0.0001). After adjustment for age, parity, hypertension, diabetes, and fetal sex, the SLE group was found to be associated with an increased risk of congenital malformations in the nervous system (adjusted OR (aOR, 1.90; 95% CI, 1.20 to 3.03), eye, ear, face, and neck (aOR, 1.37; 95% CI, 1.09 to 1.71), circulatory system (aOR, 1.91; 95% CI, 1.67 to 2.20), and musculoskeletal system (aOR, 1.26; 95% CI, 1.05 to 1.52). Even after propensity matching, some of the tendencies were maintained.
Conclusions
This nationwide population-based study in South Korea indicates that compared with the general population, neonates born to SLE mothers have a slightly increased risk of congenital malformations affecting the nervous system, head and neck, cardiovascular system, and musculoskeletal system. When a woman with lupus becomes pregnant, careful fetal ultrasound and newborn screening can be helpful in identifying the risk of potential malformations.
Keywords: Systemic Lupus Erythematosus; Epidemiology; Antibodies, Anticardiolipin
WHAT IS ALREADY KNOWN ON THIS TOPIC
Systemic lupus erythematosus (SLE) is associated with increased maternal and fetal pregnancy complications.
Children born to women with SLE are at an increased risk of adverse health outcomes such as neurodevelopmental disorders, congenital heart defects, haematologic malignancies and autoimmune disease.
WHAT THIS STUDY ADDS
Neonates born to mothers with SLE mother have a slightly increased risk of congenital malformations affecting the nervous system, eye/ear/face and neck, circulatory system and musculoskeletal system.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
When a woman with lupus becomes pregnant, careful fetal ultrasound and newborn screening can be helpful in identifying the risk of potential malformations.
Introduction
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease that often affects women of reproductive age. Compared with healthy women, women with SLE were at an increased risk of having unfavourable maternal and fetal pregnancy outcomes.1–8 Maternal complications include pre-eclampsia, lupus flare and increased mortality, while fetal complications involve abortion, preterm birth, intrauterine growth restriction, fetal death and congenital heart block. However, little is known about the risk of congenital malformations in the offspring of women with SLE.9
Pregnancy outcomes have improved dramatically in recent decades.10 Since SLE is no longer a contraindication to pregnancy, it is important to attend prepregnancy counselling and peripartum and postpartum risk management to mitigate the possibility of immediate and long-term adverse maternal and fetal outcomes.11 12 While transient growth restriction, heart block or neonatal lupus in offspring resolves with time without long-term consequences, severe congenital malformations have a life-long impact on offspring and their family members.13 14 Therefore, risk stratification of not only perinatal or postnatal complications but also malformations in the offspring of SLE mothers is critical. A large population-based study is needed, since determining the incidence of congenital malformations is hampered by the low prevalence of SLE.
The aim of this study was to determine the risk of overall and specific congenital malformations in neonates born to women with SLE as compared with mothers without SLE in a nationwide population-based cohort study.
Methods
Study data
This retrospective cohort study used claims data for Health Insurance Review and Assessment of the National Health Insurance Service (NHIS), National Health Screening Examination (NHSE) and National Health Screening Program for Infants and Children. NHIS Korea covers healthcare costs for over 95% of the entire Korean population. The database includes information on demographics, medical history, diagnosis and reimbursement for medical services such as diagnostic studies, medication prescriptions and treatment. The NHSE database comprises biannual health interviews and health examinations. NHSE is conducted once every 2 years for all citizens over the age of 20. The health interview questions contain information on demographics, socioeconomic status and lifestyle.
Study population
As patients with rare diseases are covered under the Individual Copayment Beneficiaries Program, which subsidises medical expenses for patients with a definitive diagnosis of SLE according to 1997 American College of Rheumatology criteria or 2012 Systemic Lupus International Collaborating Clinics criteria,15 16 International Classification of Diseases, 10th edition (ICD-10) is reliable to identify SLE. This population-based cohort study included pregnant Korean women who met the following criteria: (1) singleton pregnancy with live born delivery; (2) delivery between 2007 and 2015; and (3) participation in the NHSE within 4 years before pregnancy. Women with incomplete information on pregnancy and the neonatal outcome and those with multifetal gestation were excluded. Medical information before pregnancy was collected from the NHSE database. Women without SLE were chosen for propensity matching after matching for age, parity, baseline (ie, before pregnancy) hypertension, diabetes mellitus and year of delivery at a ratio of 1:5 and nearest neighbour matching.
Outcome measures
The diagnosis of congenital malformations was ascertained by the ICD-10. Women with SLE were identified by a diagnostic code (ICD-10 diagnostic code of M32)17 recorded within 4 years prior to pregnancy. The primary outcome was defined as the presence of a congenital malformation in the offspring. Congenital malformations were defined using ICD-10 codes during the first 12 months of life, indicating an organ-specific class of malformations, such as nervous system; eye, ear, face and neck; circulatory system; respiratory system; cleft lip and cleft palate; digestive system; genital organs; urinary system; musculoskeletal system; and other malformations. The secondary outcome included each of the organ-specific malformations.
Covariables
We selected four groups of variables that might be associated with congenital malformations in the cohort: maternal demographics such as age and parity; maternal comorbidities before pregnancy (such as pre-existing diabetes, pre-existing hypertension) and during pregnancy (gestational diabetes, and pre-eclampsia); and neonatal characteristics (sex and birth weight).18–20 Comorbidities before and during pregnancy were identified by ICD-10 codes.
Patient and public involvement
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Statistical analysis
Continuous variables are described as mean and SD and compared using Student’s t-test. Categorical variables are given as numbers and percentages and compared using the χ2 test. Multivariable logistic regression analysis was used to estimate the adjusted ORs (aOR) and 95% CIs for the calculation of the risk of malformation in neonates born to women with SLE in comparison to women without SLE. A generalised estimating equation was used to account for the familial correlation between offspring from a single mother.21 Additionally, propensity score matching analysis was conducted to address selection bias. Multivariable logistic regression was performed to derive propensity score with covariates such as maternal age, parity, hypertension, diabetes before pregnancy and year of delivery. A 1:5 matching algorithm was applied to minimise the potential confounding effects of variables on the incidence of congenital malformations. Analyses were performed using IBM SPSS Statistics for Windows, V.23. The level of statistical significance was set at p<0.05.
Results
Characteristics of the study population
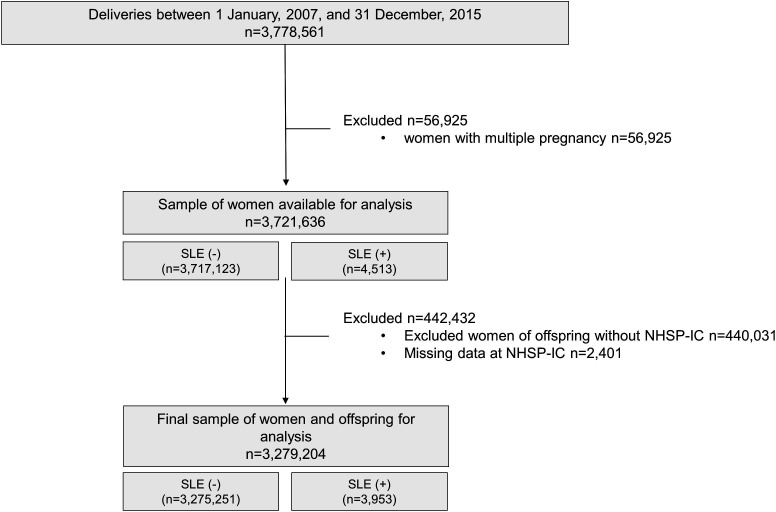
A total of 3 778 561 women who delivered between 2007 and 2015 underwent NHSE within 4 years before pregnancy. After excluding women with multifetal pregnancies and women with incomplete clinical data, a total of 3 279 204 women were included in the final analysis. Among them, 3953 (0.1%) had SLE (figure 1). Baseline characteristics are summarised in table 1. Women with SLE were older (31.8 vs 30.9 years, p<0.001) and more often primiparous (56.4% vs 52.7%, p<0.001) than those without SLE. More women with SLE had hypertension and diabetes mellitus than those without SLE.
Figure 1.
Study population. NHSP-IC, National Health Screening Program for Infants and Children; SLE, systemic lupus erythematosus.
Table 1.
Characteristics and pregnancy outcomes of the study population
| Characteristics | Non-maternal SLE (N=3 275 251) | Maternal SLE (N=3953) | P value |
| Age (years) | 30.87±3.87 | 31.82±3.70 | <0.0001 |
| Nulliparity | 1 727 073 (52.73) | 2229 (56.39) | <0.0001 |
| Comorbidity before pregnancy | |||
| Hypertension | 106 313 (3.25) | 745 (18.85) | <0.0001 |
| Diabetes | 157 014 (4.79) | 577 (14.60) | <0.0001 |
| Pregnancy outcomes | |||
| Gestational diabetes | 136 314 (4.16) | 193 (4.88) | 0.0234 |
| Pre-eclampsia | 60 855 (1.86) | 139 (3.52) | <0.0001 |
| Preterm birth | 85 095 (2.60) | 268 (6.78) | <0.0001 |
| Caesarean section | 1 182 025 (36.09) | 1691 (42.78) | <0.0001 |
| Placental abruption | 11 572 (0.35) | 28 (0.71) | 0.0002 |
| Placenta previa | 35 646 (1.09) | 80 (2.02) | <0.0001 |
| Neonatal outcomes | |||
| Neonatal sex, male | 1 687 580 (51.53) | 2076 (52.52) | 0.2124 |
| Mean birth weight (kg) | 3.21±0.46 | 3.06±0.55 | <0.0001 |
| Low birth weight | 115 666 (3.53) | 396 (10.02) | <0.0001 |
| Large for gestational age | 124 614 (3.80) | 89 (2.25) | <0.0001 |
Data are presented as number (%) or mean±SD.
SLE, systemic lupus erythematosus.
Pregnancy complications
More women with SLE developed obstetric complications that included gestational diabetes, pre-eclampsia, preterm birth, caesarean delivery, placental abruption and placenta previa. Newborns of SLE mothers had lower birth weights than those of mothers without SLE (table 1).
Congenital malformations
Congenital malformations were more frequent in the neonates born to women with SLE than in those born to women without SLE (17.13% vs 11.99%, p<0.0001). The circulatory system was the most common malformation in neonates born to SLE mothers, followed by the digestive system, musculoskeletal system and genital organs (table 2). Women with malformed offspring were more likely to be primiparous than women with healthy offspring. Further, they were more likely to have hypertension, diabetes before and during pregnancy and pre-eclampsia (online supplemental table 1).
Table 2.
Analysis of specific organ malformations
| Non-maternal SLE (n=3 275 251) |
Maternal SLE (n=3953) |
Unadjusted OR (95% CI) |
Adjusted OR* (95% CI) |
|
| Any congenital malformation | 392 803 (11.99) | 677 (17.13) | 1.51 (1.39 to 1.64) | 1.40 (1.28 to 1.52) |
| Q00–07 (congenital malformations of the nervous system) | 7148 (0.22) | 18 (0.46) | 2.09 (1.31 to 3.33) | 1.90 (1.20 to 3.03) |
| Q10–Q18 (congenital malformations of the eye, ear, face and neck) | 44 623 (1.30) | 78 (1.97) | 1.45 (1.16 to 1.82) | 1.37 (1.09 to 1.71) |
| Q20–Q28 (congenital malformations of the circulatory system) | 84 968 (2.59) | 216 (5.46) | 2.17 (1.89 to 2.49) | 1.91 (1.67 to 2.20) |
| Q30–Q34 (congenital malformations of the respiratory system) | 8785 (0.27) | 11 (0.28) | 1.04 (0.57 to 1.88) | 1.02 (0.57 to 1.85) |
| Q35–Q37 (cleft lip and cleft palate) | 3840 (0.12) | 6 (0.15) | 1.29 (0.58 to 2.88) | 1.19 (0.53 to 2.67) |
| Q38–Q45 (other congenital malformations of the digestive system) | 116 272 (3.55) | 155 (3.92) | 1.12 (0.96 to 1.32) | 1.08 (0.92 to 1.26) |
| Q50–Q56 (congenital malformations of the genital organs) | 24 572 (0.75) | 30 (0.76) | 1.01 (0.71 to 1.45) | 0.95 (0.66 to 1.36) |
| Q60–Q64 (congenital malformations of the urinary system) | 18 315 (0.56) | 28 (0.71) | 1.27 (0.87 to 1.84) | 1.20 (0.82 to 1.73) |
| Q65–Q79 (congenital malformations and deformations of the musculoskeletal system) | 72 337 (2.21) | 117 (2.96) | 1.35 (1.12 to 1.62) | 1.26 (1.05 to 1.52) |
| Q80–Q89 (other congenital malformations) | 11 943 (0.36) | 18 (0.46) | 1.25 (0.79 to 1.98) | 1.18 (0.74 to 1.87) |
*Adjusted for age, parity, hypertension, diabetes and neonatal sex.
CI, confidence interval; OR, odds ratio; SLE, systemic lupus erythematosus.
rmdopen-2022-002916supp001.pdf (141.7KB, pdf)
More offspring of women with SLE had congenital malformations of the nervous system, eye, ear, face and neck, circulatory system, and musculoskeletal system than those of women without SLE (table 2). In a logistic regression analysis adjusting for age, parity, hypertension, diabetes and fetal sex, SLE was significantly associated with an increased risk for congenital malformations affecting the nervous system (aOR), 1.90; 95% CI, 1.20 to 3.03), the circulatory system (aOR, 1.91; 95% CI, 1.67 to 2.20), eye, ear, face and neck (aOR, 1.37; 95% CI, 1.09 to 1.71) and musculoskeletal system (aOR, 1.26; 95% CI, 1.05 to 1.52). Maternal SLE, on the other hand, was not associated with an increased risk of malformation of the respiratory system, cleft lip and palate, digestive system, genital organs or urinary system.
Organ system-specific malformations
Table 2 and online supplemental table 2 show the association between SLE and types of congenital malformations within the organ system. In the nervous system, the frequency of congenital hydrocephalus was particularly increased (aOR, 3.18; 95% CI, 1.01 to 10.05) and the frequency of other congenital malformations of the brain also increased (aOR, 2.53; 95% CI, 1.13 to 5.66). Among head and neck abnormalities, malformations of eyelids, lacrimal apparatus and orbit (aOR, 1.35; 95% CI, 1.04 to 1.76) and hearing impairment (aOR, 3.01; 95% CI, 1.13 to 8.09) were more common in the newborns of patients with SLE. With respect to the circulatory system, congenital malformations of the cardiac septa (aOR, 2.05; 95% CI, 1.74 to 2.41) and great arteries (aOR, 1.84; 95% CI, 1.37 to 2.48) were particularly increased. In the musculoskeletal system, the risk of other congenital musculoskeletal deformities (aOR, 1.48; 95% CI, 1.01 to 2.19) and polydactyly (aOR, 1.97; 95% CI, 1.06 to 3.67) were particularly increased.
Sensitivity analysis
A sensitivity analysis was performed using propensity score matching to adjust for unbalanced baseline characteristics. After matching, there were no significant differences in major comorbidities before pregnancy between 3953 women with SLE and 19 765 matched controls (online supplemental table 3). Overall, congenital malformations were more frequent in neonates born to women with SLE than in those without SLE (17.3% vs 13.2%, p<0.0001). SLE remained significantly associated with an increased risk of congenital malformations of the cardiovascular system (aOR, 1.72; 95% CI, 1.47 to 2.02), eye/ear/face and neck (aOR, 1.38; 95% CI, 1.07 to 1.78) (table 3).
Table 3.
Risk of organ system-specific congenital malformations in the propensity-score matched cohorts
| Unadjusted OR (95% CI) |
Adjusted OR* (95% CI) |
|
| Any congenital malformation (Q00–Q89) | 1.25 (1.14 to 1.37) | 1.25 (1.14 to 1.37) |
| Q00–07 (congenital malformations of the nervous system) | 1.70 (0.99 to 2.91) | 1.71 (0.99 to 2.91) |
| Q10–Q18 (congenital malformations of the eye, ear, face and neck) | 1.38 (1.07 to 1.78) | 1.38 (1.07 to 1.78) |
| Q20–Q28 (congenital malformations of the circulatory system) | 1.72 (1.47 to 2.01) | 1.72 (1.47 to 2.02) |
| Q30–Q34 (congenital malformations of the respiratory system) | 1.10 (0.57 to 2.12) | 1.10 (0.57 to 2.11) |
| Q35–Q37 (cleft lip and cleft palate) | 0.97 (0.40 to 2.32) | 0.97 (0.40 to 2.32) |
| Q38–Q45 (other congenital malformations of the digestive system) | 0.90 (0.76 to 1.07) | 0.90 (0.76 to 1.07) |
| Q50–Q56 (congenital malformations of the genital organs) | 0.93 (0.63 to 1.39) | 0.93 (0.63 to 1.38) |
| Q60–Q64 (congenital malformations of the urinary system) | 1.08 (0.72 to 1.62) | 1.07 (0.71 to 1.62) |
| Q65–Q79 (congenital malformations and deformations of the musculoskeletal system) | 1.17 (0.95 to 1.43) | 1.17 (0.95 to 1.43) |
| Q80–Q89 (other congenital malformations) | 1.04 (0.62 to 1.72) | 1.04 (0.62 to 1.72) |
ORs and 95% CIs are shown. The control group was selected after matching for age, parity, hypertension, diabetes before pregnancy and year of delivery.
*Adjusted for age, parity, hypertension, diabetes and neonatal sex.
CI, confidence interval; OR, odds ratio.
Discussion
Main findings
The major findings of this study are as follows: (1) the risk of congenital malformations in neonates born to mothers with SLE was significantly increased compared with those born to mothers without SLE and (2) these congenital malformations preferentially involved the nervous system, eye/ear/face and neck, circulatory system and musculoskeletal system.
Comparison with findings from previous studies
The current study is the largest to demonstrate the increased risk of congenital malformations in offspring born to mothers with SLE. This is consistent with previous studies showing that children born to women with SLE are at an increased risk of adverse health outcomes such as neurodevelopmental disorders, congenital heart defects, haematologic malignancies and autoimmune disease.22 Another study using the national birth registry showed that congenital malformations were more prevalent among children born to SLE mothers than among those born to women without SLE (7.4% vs 2.8%).23 In this study, the frequency of congenital malformation in the offspring of SLE mothers and those of mothers without SLE was estimated at 17.13% and 11.99%, respectively, confirming that maternal SLE was associated with an increased risk of congenital malformation. The higher frequency of congenital malformations in the general population in this study could be explained by better diagnosing24 and/or reporting less severe congenital malformations using the ICD. In fact, the prevalence of anomalies have been increasing compared with that in the past, which is consistent with the major role of improved postnatal detection of less severe malformations.25
In this study, the frequency of congenital malformation in the cardiovascular system was higher (5.5% vs 2.6%, p<0.0001), which was comparable to the findings by Vinet et al9 who reported increased risk of the circulatory system in 719 SLE offspring compared with 8493 controls (5.1% vs 1.9%, p<0.0001). In addition, we also showed that pregnancies with SLE were also at increased risk for other organ system abnormalities such as nervous system, eye, ear, face and neck abnormalities as well as musculoskeletal system abnormalities (table 4).
Table 4.
Comparison with other studies
| Pregnancy, n | Target malformation | Conclusion | |
| Wallenius et al23 | 95 women with SLE 257 327 control |
Major congenital malformations | Major congenital malformations were more frequent in children with SLE (OR, 2.71; 95% CI, 1.25 to 5.86). |
| Vinet et al9 | 719 SLE offspring 8493 control children |
Congenital heart defects | Children born to women with SLE had a substantially increased risk of CHD in comparison with controls (OR, 2.62; 95% CI, 1.77 to 3.88). |
| Current study (2023) | 3953 women with SLE 3 275 251 control |
All kinds of congenital malformations | After adjustment for age, parity, hypertension, diabetes, and fetal sex, the SLE group was found to be associated with an increased risk of congenital malformations in the nervous system (adjusted OR (aOR, 1.90; 95% CI, 1.20 to 3.03), eye, ear, face and neck (aOR, 1.37; 95% CI, 1.09 to 1.71), circulatory system (aOR, 1.91; 95% CI, 1.67 to 2.20) and musculoskeletal system (aOR, 1.26; 95% CI, 1.05 to 1.52). |
CHD, congenital heart defect; SLE, systemic lupus erythematosus.
We also evaluated each type of malformation within organ systems. Some congenital malformations were particularly increased. In the cardiovascular system, congenital malformations of the cardiac septa and great arteries were particularly increased, consistent with results from prior studies that showed children born to women with SLE had an increased risk of congenital heart defects, including a specifically increased risk of atrial septal defect (ASD), ventricular septal defect (VSD) and valve anomalies.9 The increased risk of ASD and VSD were similar to that observed in our study. Here, we show that the risk of congenital malformation of the great arteries (coarctation of the aorta, atresia of the aorta, etc) was increased. While several studies have compared the risk of congenital malformations between SLE and non-SLE groups in a few organ systems, this study is one of the first to systematically evaluate all the organ systems. Interestingly, SLE in the mother did not affect the risk of malformation in all organ systems equally.
Clinical and research implications
SLE itself might cause some congenital malformations through the mechanism of autoantibodies, cytokines or treatment-associated side effects. Maternal anti-Ro/SS-A and anti-La/SS-B were associated with congenital heart block in newborns26–28 and neonatal lupus.29 Little is known about anti-Ro/SS-A and anti-La/SS-B and the development of other congenital malformations. Cardiac septation occurs early in embryogenesis and is usually completed around four to 7 weeks of gestation.30 It may be difficult to explain that the septal defect is caused by the antibody because the passage of maternal autoantibodies through the placenta usually occurs after 20 weeks. However, since most VSDs are thought to arise from foci of apoptosis within an already formed ventricular septum, the maternal anti-Ro/SS-A and anti-La/SS-B may prevent spontaneous closure of defects.9 Increased cytokines in patients with SLE might also contribute to cardiac malformation.31 32 Cytokines, such as Transforming growth factor-β (TGF-β), play an important role in the normal development of the heart.33 Multiple signalling pathways, including notch, Fibroblast growth factor (FGF) and TGF-β, have been implicated in outflow tract (aorta or pulmonary artery) development.34 Edwards et al found that transplacental acquisition of anti-Ro/SS-A antibodies has been associated with external hydrocephalus.35 It is possible that autoantibody-mediated inflammation is implicated in its pathogenesis.36 37
It may be quite possible that the observed risk of congenital malformation in pregnant women with SLE may be attributable to the medications prescribed to these women. Unfortunately, information on medication exposures was not available in the current study. Patients with SLE usually continue some medications such as corticosteroids, antimalarials and pregnancy-compatible immunosuppressants during pregnancy to control disease activity and major organ involvement. Corticosteroids are commonly used to treat SLE during pregnancy. Several studies have shown an association between in utero exposure and an increased risk of oral cleft.38 In a nationwide cohort study, no association was found between maternal use of oral corticosteroids and congenital heart defects.39 Another study found no evidence of an association between the use of corticosteroids and a risk of congenital malformations in offspring.40 Antimalarial drugs are commonly used in pregnant women with SLE to reduce disease activity and the risk of congenital heart block and neonatal lupus syndrome.41 Some investigators concluded that hydroxychloroquine is a safe drug in the treatment of SLE during pregnancy. There was no increased risk of congenital malformations in newborns.42 43 Recently, some studies suggest a small increased risk of malformations associated with the use of hydroxychloroquine in early pregnancy.44 45 However, the observed birth defects among hydroxychloroquine-exposed women did not present a clear pattern. Rather, discontinuing a drug during pregnancy increased the risk of flare and worsened pregnancy outcomes.46 Immunosuppressive drugs such as azathioprine are sometimes used, too. Several studies have found that azathioprine is relatively safe in pregnancy. The drug has not been associated with congenital defects in these studies.47–49 Mycophenolate mofetil is another widely used drug for the treatment of SLE. It is known to be teratogenic based on observational studies of pregnancies exposed to mycophenolate mofetil. Unintended pregnancies while on teratogenic drugs such as mycophenolate mofetil contribute to facial malformation observed in this study.50 Taken together, it is difficult to say that the effects of drugs on congenital malformations have been fully studied.
SLE is actually a disease with heterogeneous phenotypes. Therefore, it is hard to assume that specific disease phenotypes can be all responsible for the induction of certain malformations. However, the heterogeneous phenotypes of SLE can still share common biological mechanisms, such as interferon production, immune complex-mediated inflammation and vasculopathy. While the patients might be clinically in remission before conception, the serological activity might contribute to the proinflammatory milieu that drives the congenital malformations in various forms.
While increased relative risk of malformations in offspring born to mothers with SLE was observed, the absolute numbers of children with malformation are very small. Both relative risk and absolute numbers of malformation should be considered when counselling the patient. Calculated number needed to harm for any malformation is 19.45.
Strengths and limitations
This study was the first to determine the interaction of maternal SLE and neonatal congenital malformation in a large population-based study. The incidence of major congenital malformations in the general population is about 2.4%–6.9%.51 52 Therefore, it is difficult to analyse the relationship between maternal SLE and the risk of congenital malformation using a small cohort. However, because it is a government paid, biannual health screening examination, we were able to acquire data on lupus before pregnancy and assess its correlation with congenital malformations systematically in a large cohort.
This study has several limitations. First, we identified congenital malformations using ICD-10 diagnostic codes, which were recorded for reimbursement purposes. It is possible that the diagnosis of malformations was not correctly recorded during the postpartum workup of all newborns. A closer monitoring of children born to SLE mothers that can lead to the increased identification of asymptomatic or non-clinically significant malformations. Clinically, it is thought that major structural anomalies are the conditions that account for most of the deaths, morbidity and disability related to congenital anomalies. In studying malformation, it is also important to consider the severity of malformation. However, there is no fully defined definition of major malformation. Second, in our database, data on clinical and laboratory parameters such as antinuclear antibody, anti-Ro and anti-La autoantibodies, and antiphospholipid antibodies were not available. The effects of disease activity and autoantibody presence on congenital malformations would not be estimated. Anti-Ro and La antibodies, found in 40% of lupus women, cross the placenta and are associated with the development of neonatal lupus, with congenital heart block being the most characteristic cardiac manifestation.53 Third, the analysis accounting for medication exposures could not be performed; hence, the association with the occurrence of drug-induced congenital malformations could not be established. Therefore, the current findings need to be confirmed in a larger prospective cohort study. Fourth, there is a slight increase in the adjusted OR for malformations in SLE. Therefore, further research is needed to determine whether warnings should be issued in terms of public health and precautions to be implemented.
In conclusion, this nation-wide population study in South Korea indicates that neonates born to mothers with SLE have a slightly increased risk of congenital malformations affecting the nervous system, eye/ear/face and neck, circulatory system and musculoskeletal system. When a woman with lupus becomes pregnant, careful fetal ultrasound and newborn screening can be helpful in identifying the risk of potential malformations.
Acknowledgments
The authors would like to thank S. Oh (Department of Biostatistics, Seoul Metropolitan Government Seoul National University Boramae Medical Center, Korea) for statistical advice.
Footnotes
Contributors: Conceptualisation and study design— YMJ, JKP, SML and GJC. Data collection and curation— YMJ, JKP, SML and GJC. Data analysis— YMJ, JKP, SML and GJC. Data interpretation— YMJ, JKP, C-WP, M-JO, JSP, JKJ, SML and GJC. All authors reviewed and revised the manuscript draft for scientific content, read and approved the final draft, and had the final decision to submit the manuscript for publication. Guarantor— SML and GJC.
Funding: This research was supported by the grant from the Seoul National University Hospital (SNUH) research fund (0420210560) and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2021R1F1A1046707).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. Raw data were generated at Health Insurance Review and Assessment of the National Health Insurance Service. Derived data supporting the findings of this study are available from the corresponding author on request.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study was approved by the Institutional Review Board of the Korean University Guro Hospital (Approval No. 2020GR0105). Informed consent from participants was waived because of the retrospective nature of the study and the analysis used anonymous and public data.
References
- 1.Clark CA, Spitzer KA, Nadler JN, et al. Preterm deliveries in women with systemic lupus erythematosus. J Rheumatol 2003;30:2127–32. [PubMed] [Google Scholar]
- 2.Gimovsky ML, Montoro M, Paul RH. Pregnancy outcome in women with systemic lupus erythematosus. Obstet Gynecol 1984;63:686–92. [PubMed] [Google Scholar]
- 3.Nossent HC, Swaak TJ. Systemic lupus erythematosus. VI. Analysis of the interrelationship with pregnancy. J Rheumatol 1990;17:771–6. [PubMed] [Google Scholar]
- 4.Petri M. Hopkins lupus pregnancy center: 1987 to 1996. Rheum Dis Clin North Am 1997;23:1–13. 10.1016/s0889-857x(05)70311-2 [DOI] [PubMed] [Google Scholar]
- 5.Yasmeen S, Wilkins EE, Field NT, et al. Pregnancy outcomes in women with systemic lupus erythematosus. J Matern Fetal Med 2001;10:91–6. 10.1080/714904302 [DOI] [PubMed] [Google Scholar]
- 6.Moroni G, Ponticelli C. Pregnancy in women with systemic lupus erythematosus (SLE). Eur J Intern Med 2016;32:7–12. 10.1016/j.ejim.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 7.Tan Y, Yang S, Liu Q, et al. Pregnancy-related complications in systemic lupus erythematosus. J Autoimmun 2022;132:102864. 10.1016/j.jaut.2022.102864 [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Han X, Liu W, et al. Pregnancy in patients with systemic lupus erythematosus: a systematic review. Arch Gynecol Obstet 2023;308:63–71. 10.1007/s00404-022-06718-7 [DOI] [PubMed] [Google Scholar]
- 9.Vinet É, Pineau CA, Scott S, et al. Increased congenital heart defects in children born to women with systemic lupus erythematosus: results from the offspring of systemic lupus erythematosus mothers registry study. Circulation 2015;131:149–56. 10.1161/CIRCULATIONAHA.114.010027 [DOI] [PubMed] [Google Scholar]
- 10.Clark CA, Spitzer KA, Laskin CA. Decrease in pregnancy loss rates in patients with systemic lupus erythematosus over a 40-year period. J Rheumatol 2005;32:1709–12. [PubMed] [Google Scholar]
- 11.Andreoli L, Bertsias GK, Agmon-Levin N, et al. EULAR recommendations for women’s health and the management of family planning, assisted reproduction, pregnancy and Menopause in patients with systemic lupus erythematosus and/or antiphospholipid syndrome. Ann Rheum Dis 2017;76:476–85. 10.1136/annrheumdis-2016-209770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Öztürk HNO, Türker PF. Fetal programming: could Intrauterin life affect health status in adulthood. Obstet Gynecol Sci 2021;64:473–83. 10.5468/ogs.21154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hauser-Cram P, Warfield ME, Shonkoff JP, et al. Children with disabilities: a longitudinal study of child development and parent well-being. Monogr Soc Res Child Dev 2001;66:i–viii. [PubMed] [Google Scholar]
- 14.Poley MJ, Stolk EA, Tibboel D, et al. Short term and long term health related quality of life after congenital anorectal malformations and congenital diaphragmatic hernia. Arch Dis Child 2004;89:836–41. 10.1136/adc.2002.016543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hochberg MC. Updating the American college of rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. 10.1002/art.1780400928 [DOI] [PubMed] [Google Scholar]
- 16.Petri M, Orbai A-M, Alarcón GS, et al. Derivation and validation of the systemic lupus international collaborating clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. 10.1002/art.34473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zahid S, Mohamed MS, Wassif H, et al. Analysis of cardiovascular complications during delivery admissions among patients with systemic lupus erythematosus, 2004-2019. JAMA Netw Open 2022;5:e2243388. 10.1001/jamanetworkopen.2022.43388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daliri S, Safarpour H, Bazyar J, et al. The relationship between some neonatal and maternal factors during pregnancy with the prevalence of congenital malformations in Iran: a systematic review and meta-analysis. J Matern Fetal Neonatal Med 2019;32:3666–74. 10.1080/14767058.2018.1465917 [DOI] [PubMed] [Google Scholar]
- 19.Chen L, Yang T, Chen L, et al. Risk of congenital heart defects in offspring exposed to maternal diabetes mellitus: an updated systematic review and meta-analysis. Arch Gynecol Obstet 2019;300:1491–506. 10.1007/s00404-019-05376-6 [DOI] [PubMed] [Google Scholar]
- 20.Ramakrishnan A, Lee LJ, Mitchell LE, et al. Maternal hypertension during pregnancy and the risk of congenital heart defects in offspring: a systematic review and meta-analysis. Pediatr Cardiol 2015;36:1442–51. 10.1007/s00246-015-1182-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics 1986;42:121–30. [PubMed] [Google Scholar]
- 22.Vinet É, Bernatsky S. Outcomes in children born to women with rheumatic diseases. Rheum Dis Clin North Am 2017;43:263–73. 10.1016/j.rdc.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 23.Wallenius M, Salvesen KÅ, Daltveit AK, et al. Systemic lupus erythematosus and outcomes in first and subsequent births based on data from a national birth registry. Arthritis Care Res (Hoboken) 2014;66:1718–24. 10.1002/acr.22373 [DOI] [PubMed] [Google Scholar]
- 24.Ko HS, Kwak DW, Oh S-Y, et al. Clinical significance of soft markers in second trimester Ultrasonography for pregnant Korean women: a multicenter study and literature review. Obstet Gynecol Sci 2022;65:145–55. 10.5468/ogs.21216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Chen S, Zühlke L, et al. Global birth prevalence of congenital heart defects 1970-2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455–63. 10.1093/ije/dyz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akuka A, Ben-Shabat N, Watad A, et al. Association of anti-Ro Seropositivity with cardiac rhythm and conduction disturbances. Eur Heart J 2022;43:4912–9. 10.1093/eurheartj/ehac516 [DOI] [PubMed] [Google Scholar]
- 27.De Carolis S, Garufi C, Garufi E, et al. Autoimmune congenital heart block: a review of biomarkers and management of pregnancy. Front Pediatr 2020;8:607515. 10.3389/fped.2020.607515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mofors J, Eliasson H, Ambrosi A, et al. Comorbidity and long-term outcome in patients with congenital heart block and their siblings exposed to Ro/SSA Autoantibodies in utero. Ann Rheum Dis 2019;78:696–703. 10.1136/annrheumdis-2018-214406 [DOI] [PubMed] [Google Scholar]
- 29.Gryka-Marton M, Szukiewicz D, Teliga-Czajkowska J, et al. An overview of neonatal lupus with anti-Ro characteristics. Int J Mol Sci 2021;22:17. 10.3390/ijms22179281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lamers WH, Moorman AFM. Cardiac septation: a late contribution of the embryonic primary myocardium to heart Morphogenesis. Circ Res 2002;91:93–103. 10.1161/01.res.0000027135.63141.89 [DOI] [PubMed] [Google Scholar]
- 31.Rauen T, Grammatikos AP, Hedrich CM, et al. cAMP-responsive element modulator Α (CREMα) contributes to decreased Notch-1 expression in T cells from patients with active systemic lupus erythematosus (SLE). J Biol Chem 2012;287:42525–32. 10.1074/jbc.M112.425371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirchler C, Husar-Memmer E, Rappersberger K, et al. Type I interferon as cardiovascular risk factor in systemic and cutaneous lupus erythematosus: a systematic review. Autoimmun Rev 2021;20:102794. 10.1016/j.autrev.2021.102794 [DOI] [PubMed] [Google Scholar]
- 33.Arthur HM, Bamforth SD. TGFβ signaling and congenital heart disease: insights from mouse studies. Birth Defects Res A Clin Mol Teratol 2011;91:423–34. 10.1002/bdra.20794 [DOI] [PubMed] [Google Scholar]
- 34.Boezio GL, Bensimon-Brito A, Piesker J, et al. Endothelial TGF-Β signaling instructs smooth muscle cell development in the cardiac outflow tract. Elife 2020;9:e57603. 10.7554/eLife.57603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards RJ, Allport TD, Stoodley NG, et al. External hydrocephalus and Subdural bleeding in infancy associated with transplacental anti-Ro antibodies. Arch Dis Child 2012;97:316–9. 10.1136/adc.2010.194746 [DOI] [PubMed] [Google Scholar]
- 36.Cabañas F, Pellicer A, Valverde E, et al. Central nervous system Vasculopathy in neonatal lupus erythematosus. Pediatr Neurol 1996;15:124–6. 10.1016/0887-8994(96)00159-2 [DOI] [PubMed] [Google Scholar]
- 37.Nakayama-Furukawa F, Takigawa M, Iwatsuki K, et al. Hydrocephalus in two female siblings with neonatal lupus erythematosus. Arch Dermatol 1994;130:1210–2. [PubMed] [Google Scholar]
- 38.Park-Wyllie L, Mazzotta P, Pastuszak A, et al. Birth defects after maternal exposure to corticosteroids: prospective cohort study and meta‐analysis of epidemiological studies. Teratology 2000;62:385–92. [DOI] [PubMed] [Google Scholar]
- 39.Schmidt AB, Lund M, Corn G, et al. Oral corticosteroids during pregnancy and offspring risk of congenital heart defects: a nationwide cohort study. Int J Epidemiol 2020;49:638–47. 10.1093/ije/dyz213 [DOI] [PubMed] [Google Scholar]
- 40.Bay Bjørn A-M, Ehrenstein V, Hundborg HH, et al. Use of corticosteroids in early pregnancy is not associated with risk of oral clefts and other congenital malformations in offspring. Am J Ther 2014;21:73–80. 10.1097/MJT.0b013e3182491e02 [DOI] [PubMed] [Google Scholar]
- 41.Lateef A, Petri M. Managing lupus patients during pregnancy. Best Pract Res Clin Rheumatol 2013;27:435–47. 10.1016/j.berh.2013.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parke AL, Rothfield NF. Antimalarial drugs in pregnancy–the North American experience. Lupus 1996;5 Suppl 1:S67–9. [PubMed] [Google Scholar]
- 43.Parke A, West B. Hydroxychloroquine in pregnant patients with systemic lupus erythematosus. J Rheumatol 1996;23:1715–8. [PubMed] [Google Scholar]
- 44.Huybrechts KF, Bateman BT, Zhu Y, et al. Hydroxychloroquine early in pregnancy and risk of birth defects. Am J Obstet Gynecol 2021;224:290. 10.1016/j.ajog.2020.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howley MM, Werler MM, Fisher SC, et al. Maternal exposure to hydroxychloroquine and birth defects. Birth Defects Res 2021;113:1245–56. 10.1002/bdr2.1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costedoat-Chalumeau N, Amoura Z, Huong DLT, et al. Safety of hydroxychloroquine in pregnant patients with connective tissue diseases. Autoimmun Rev 2005;4:111–5. 10.1016/j.autrev.2004.11.009 [DOI] [PubMed] [Google Scholar]
- 47.Kirk EP. Organ transplantation and pregnancy. A case report and review. Am J Obstet Gynecol 1991;164:1629–33. 10.1016/0002-9378(91)91447-5 [DOI] [PubMed] [Google Scholar]
- 48.Kossoy LR, Herbert CM, Wentz AC. Management of heart transplant recipients: guidelines for the obstetrician-gynecologist. Am J Obstet Gynecol 1988;159:490–9. 10.1016/s0002-9378(88)80116-9 [DOI] [PubMed] [Google Scholar]
- 49.Haugen G, Fauchald P, Sødal G, et al. Pregnancy outcome in renal allograft recipients: influence of Ciclosporin A. Eur J Obstet Gynecol Reprod Biol 1991;39:25–9. 10.1016/0028-2243(91)90137-a [DOI] [PubMed] [Google Scholar]
- 50.Coscia LA, Armenti DP, King RW, et al. Update on the Teratogenicity of maternal mycophenolate mofetil. J Pediatr Genet 2015;4:42–55. 10.1055/s-0035-1556743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dolk H, Loane M, Garne E. The prevalence of congenital anomalies in Europe. Rare Diseases Epidemiology 2010:349–64. 10.1007/978-90-481-9485-8 [DOI] [PubMed] [Google Scholar]
- 52.World Health Organization . Birth defects in South-East Asia: a public health challenge: situation analysis; 2013.
- 53.Lee LA, Coulter S, Erner S, et al. Cardiac immunoglobulin deposition in congenital heart block associated with maternal anti-Ro Autoantibodies. Am J Med 1987;83:793–6. 10.1016/0002-9343(87)90918-1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2022-002916supp001.pdf (141.7KB, pdf)
Data Availability Statement
Data are available upon reasonable request. Raw data were generated at Health Insurance Review and Assessment of the National Health Insurance Service. Derived data supporting the findings of this study are available from the corresponding author on request.