Abstract
We examined the role of yeast transcription initiation factor IIE (TFIIE) in eukaryotic transcription-coupled repair (TCR), the preferential removal of DNA damage from the transcribed strands of genes over non-transcribed sequences. TFIIE can recruit the transcription initiation/repair factor TFIIH to the RNA polymerase II (RNA pol II) initiation complex to facilitate promoter clearance. Following exposure to UV radiation, the RNA pol II elongation complex is blocked at sites of UV-induced DNA damage, and may be recognized by nucleotide excision repair proteins, thus enabling TCR. The TFA1 gene encodes the large subunit of TFIIE. We determined how DNA repair is affected by TFA1 conditional mutations. In particular, we find proficient TCR in a heat-sensitive tfa1 mutant at the non-permissive temperature during which growth is inhibited and overall RNA pol II transcription is reported to be inhibited. We demonstrate that transcription of the RPB2 gene was reduced, but readily detectable, in the heat-sensitive tfa1 mutant at the non-permissive temperature and thereby prove that TCR does occur in an expressed gene in the absence of TFIIE in vivo. We demonstrate that TCR occurs even at low levels of transcription.
INTRODUCTION
Cellular DNA is under constant assault from both intrinsic and external sources of damage. A sophisticated network of responses has evolved to protect cells from this onslaught (for a comprehensive review see 1). An important component of this network is nucleotide excision repair (NER), a highly conserved pathway which removes an extensive array of DNA lesions including UV-induced cyclobutane pyrimidine dimers (CPD) and pyrimidine (6-4) pyrimidone photoproducts. The lesion is recognized, incisions are made in the damaged strand on either side of the lesion, the oligomer containing the lesion is removed and the resulting gap is filled and ligated. In eukaryotic cells the concerted action of ~30 proteins is required to complete these steps (2). NER does not occur uniformly throughout the genome. Genes that are undergoing transcription exhibit a faster rate of repair of the transcribed strand relative to the non-transcribed strand and the genome overall. This preferential repair of the transcribed strand is referred to as transcription-coupled repair (TCR). The mechanism(s) enabling TCR remains elusive. The following proposed mechanism of eukaryotic TCR is based upon the original model proposed by Mellon et al. (3) and is supported by studies in Escherichia coli (4,5). An elongating RNA pol II complex encounters a DNA lesion causing transcription to stop. A transcription-repair coupling factor (or factors) akin to E.coli Mfd protein, such as yeast Rad26 and the human homolog, Cockayne’s syndrome group B protein, recognizes and displaces the stalled RNA pol II transcription complex, while recruiting repair proteins to the DNA lesion site. This model is consistent with the DNA repair deficiencies observed for yeast rad26 mutants (6; K.S.Sweder, unpublished results) and cells from CS patients (7,8). In these mutants the efficiency of repair of CPDs in the transcribed strand of an expressed gene is reduced to a level similar to that of the non-transcribed strand, i.e. they are deficient in TCR while maintaining their ability to perform general NER.
It is highly probable that Rad26 works in conjunction with other, as yet unidentified, TCR proteins. In addition, it is likely that there are Rad26-independent mechanisms that enable TCR. Deletion of both RAD26 (responsible for TCR) and RAD7 (responsible for repair of non-transcribed DNA) is expected to eliminate all repair within the cell. However, a substantial amount of repair of the transcribed strand exists in a rad7rad26 double mutant (9,10). In an effort to understand the mechanism(s) of TCR in eukaryotic cells and the relationship between defects in TCR and human disease, we are identifying mutants with defective or altered TCR and NER. We have investigated DNA repair, cellular growth and transcription in yeast transcription factor IIE (TFIIE) mutants. TFIIE associates with the RNA pol II initiation complex through contacts with other initiation factors (TFIIF and TFIIB) and recruits TFIIH to the initiation complex. TFIIH functions both in transcription and DNA repair. The kinase activity of TFIIH phosphorylates the C-terminal domain (CTD) of the large subunit of RNA pol II to transform the initiation complex into the elongation complex. Additionally, TFIIH has been demonstrated in vivo to be required for all NER in the genome, be it transcribed or non-transcribed sequences (11,12). Reconstitution of NER in cell free systems has revealed that TFIIH interacts with one of the damage recognition proteins, Rad14, and many other factors to form a large repair complex at the site of DNA damage (13,14). The repair complex contains the endonuclease activities needed to incise upstream and downstream of the DNA damage. The association of TFIIE with TFIIH suggests that TFIIE may also be involved in DNA repair, perhaps specifically TCR.
TCR depends upon preferential recognition of or access to DNA damage in the transcribed strand of expressed genes over damage in the remainder of the genome. However, the nature of preferential recognition is not understood. The RNA pol II elongation complex is completely blocked at sites of UV-induced DNA damage and is a very stable entity. The previously described interaction between TFIIE and various transcription factors could promote the reassociation of TFIIH with the RNA polymerase stalled at the site of DNA damage (15). Supporting this idea, it has been shown that TFIIE binds RNA pol II stalled at a DNA pause site (15). Because TFIIE does not interact with the phosphorylated form of RNA pol II (15,16), the reassociation of TFIIE (and TFIIH) would presumably require that the stalled RNA polymerase be dephosphorylated first. Indeed, a type 2C phosphatase has been identified in yeast and mammals that specifically dephosphorylates the CTD of RNA pol II in an arrested complex (17–19). Therefore, reassociation of TFIIE and TFIIH with the stalled RNA polymerase complex could facilitate recruitment of the remaining repair proteins to promote preferential removal of transcription blocking DNA lesions, i.e. TCR.
Saccharomyces cerevisiae TFIIE is a heterotetrameric complex composed of 66 and 43 kDa subunits. The genes TFA1 and TFA2 encode the large and small subunits, respectively. The 66 kDa subunit contains a C2C2 zinc finger in its N-terminal half and its C-terminal portion is involved in binding the 43 kDa subunit. Mutations that alter the zinc finger of Tfa1p result in a heat-sensitive phenotype (20–22). Deletion mutations that remove portions of the C-terminus of Tfa1p result in cold sensitivity (20–22). However, these conditional mutations in TFA1 do not result in strains that are sensitive to UV radiation (20). It is important to note that in yeast (and bacteria) UV sensitivity is not a reliable indicator of defective TCR. For instance, yeast rad26 mutants are defective in TCR and yet are no more sensitive to UV radiation than their repair-proficient parent strain (6). Presently, defects in TCR can only be detected by in vivo repair assays. We examined cell growth and repair of UV-induced CPDs in the transcribed and non-transcribed strands of the RPB2 gene in a cold-sensitive and a heat-sensitive TFIIE mutant at the permissive and restrictive temperatures.
MATERIALS AND METHODS
Media, plasmids and strains
All media were prepared as described by Adams et al. (23). YPD medium was 1% yeast extract, 2% Bacto-peptone (Difco Laboratories) and 2% glucose. Selective medium was 2% glucose, 0.67% Bacto-yeast nitrogen base without vitamins (Difco Laboratories) supplemented with the appropriate amino acids and bases. Agar (2%) was added to media for plates. Yeast strains used in this study were YSB324 [MATa ura3-52 leu2-3 112 his3Δ200 tfa1Δ1::HIS3 (pRS315-TFA1)], YSB326 [MATa ura3-52 leu2-3 112 his3Δ200 tfa1Δ1::HIS3 (pRS315-tfa1(T218Δ))] and YSB331 [MATa ura3-52 leu2-3 112 his3Δ200 tfa1Δ1::HIS3 (pRS315-tfa1(C127F))]. The strains were a gift from the laboratory of S. Buratowski, Harvard Medical School. Plasmid pKS212 is a Bluescript vector (Stratagene) which contains the internal 1.0 kb EcoRI–XhoI from RPB2 (24). Plasmid pKS212 was linearized by cleaving with XhoI or EcoRI and incubated with [α-32P]CTP (Amersham), rNTPs and T7 RNA polymerase or T3 RNA polymerase, respectively, under conditions recommended by the manufacturer to generate strand-specific radioactive RNA probes for RPB2.
Growth and UV irradiation of yeast cells
The growth and irradiation of strains and purification of DNA was as previously described (11). Strains were maintained in selective medium and during repair experiments they were grown in YPD. Prior to UV irradiation, exponentially growing cultures at the permissive temperature were shifted to the non-permissive temperature until the cells stopped growing. All manipulations were performed under yellow light to preclude photoreactivation. Cultures were collected by centrifu-gation and resuspended in ice-cold phosphate-buffered saline at 1 × 107 cells/ml. Shaking cell suspensions were irradiated as a thin layer with predominantly 254 nm UV light using a germicidal lamp (American Ultraviolet Co.). The cells were collected by centrifugation after irradiation and either spheroplasted and lysed immediately or resuspended in their original growth media and incubated at the appropriate temperature for the specified repair times, and then spheroplasted and lysed. To generate the growth curves of YSB331, exponentially growing cultures at 24°C were either: UV irradiated or not then incubated in growth medium at 24°C for the times indicated; exponentially growing cultures at 24°C were shifted to 37°C for 6 h then UV irradiated or not and incubated in growth medium at 37°C for the times indicated; or exponentially growing cultures at 30°C were shifted to 15°C for 20 h then UV irradiated or not and incubated in growth medium at 15°C for the times indicated. To generate the growth curves of YSB324, exponentially growing cultures at 30°C were either UV irradiated or not then incubated in growth medium at 30°C for the times indicated; or exponentially growing cultures at 30°C were shifted to 37°C for 1 h then UV irradiated or not and incubated in growth medium at 37°C for the times indicated; or exponentially growing cultures at 30°C were shifted to 15°C for 20 h then UV irradiated or not and incubated in growth medium at 15°C for the times indicated.
Isolation and restriction of yeast DNA
Cells were digested with Zymolyase 100T (ICN Biochemicals) to generate spheroplasts (11). After digestion, spheroplasts were collected by centrifugation and resuspended in 0.2 ml of Zymolyase buffer lacking Zymolyase (0.25 M EDTA, 1 M sorbitol and 20 mM 2-mercaptoethanol). Spheroplasts were then diluted with the gradual addition of 2.8 ml of 0.05 M Tris–HCl pH 8.0, 0.05 M EDTA and lysed by the addition of 0.2 ml of 20% Sarkosyl (25). The mixture was incubated on ice for 10 min. Cellular debris and Sarkosyl were precipitated by the addition of 0.64 ml 5 M potassium acetate. Mixtures were incubated at 4°C overnight and centrifuged at 5000 r.p.m. in a Sorvall HL-6000 rotor at 4°C for 25 min. Supernatants containing chromosomal DNA were transferred to fresh tubes and further purified by phenol and chloroform extraction, ethanol precipitation (26), resuspension in 10 mM Tris–HCl pH 8.0, 1 mM EDTA (TE), followed by repeated washings with TE in Centricon 100 concentrators (Amicon). The samples were then treated with RNase (final concentration 50 µg/ml) and the DNA digested to completion with PvuI and PvuII restriction enzymes. The purified, restricted DNA was ethanol precipitated and resuspended in TE.
Repair analysis
The incidence of CPDs in each strand of a specific restriction fragment was determined by previously developed methods (3,11,27). Briefly, equal amounts of purified, restricted DNA was either mock treated (10 mM Tris–HCl pH 7.5, 0.1 M NaCl, 10 mM EDTA, 1 mg/ml BSA) or treated with the enzyme T4 endonuclease V in mock buffer for 20 min at 37°C. T4 endonuclease V is a CPD-specific DNA glycosylase/AP lyase which produces a single-stranded break specifically at each CPD. DNA was denatured, electrophoresed through 0.5% alkaline agarose gels to separate full-length restriction fragments from the digestion products of T4 endonuclease V, transferred to Hybond N+ (Amersham) membranes and hybridized with strand-specific 32P-labeled RNA probes generated by in vitro transcription of linearized pKS212. Autoradiograms were generated and signal intensities determined using a Hewlett-Packard Scanjet II and the application NIH Image 1.61. The Poisson expression was applied to calculate the number of CPDs per fragment from the ratio of intensities of bands from T4 endonuclease V treated and mock treated DNA samples.
Transcript run-on assay
Nascent transcripts in vivo were detected by elongation of nascent transcripts as described by Warner (28) with modifications. Cells were grown to early logarithmic growth at 24°C, shifted to 37°C for 6 h and irradiated with 254 nm light as described above. Following irradiation, 3 × 107 cells were mixed with an equal volume of ice or returned to their original medium to enable repair. Following the repair incubations, cells were mixed with an equal volume of ice and collected by centrifugation at 4500 r.p.m. in an Eppendorf 5804R centrifuge. Cells were resuspended in TMN buffer (10 mM Tris pH 7.5, 100 mM NaCl, 5 mM MgCl2) and incubated on ice for 5 min. Cells were collected by centrifugation at 4500 r.p.m. and resuspended in 0.95 ml of ice-cold water. Fifty microliters of sarcosine (10% w/v) was added and the cells were incubated on ice for an additional 15 min. Permeabilized cells were collected by centrifugation at 4500 r.p.m. and resuspended in 120 µl of reaction buffer (50 mM Tris pH 8.0, 100 mM KCl, 5 mM MgCl2, 1 mM MnCl2, 2 mM dithiothreitol, 250 mM ATP, 125 mM GTP, 125 mM CTP) and 1 µCi/µl [α-32P]UTP (Amersham) and incubated at room temperature for 15 min. Cells were collected by centrifugation at 4500 r.p.m., resuspended in 200 µl zymolyase buffer and stored at –20°C. Cells were lysed by digestion with zymolyase as described above and the cell extracts were added to the hybridization solution. Alternatively, permeabilized cells were directly added to the hybridization solution.
Hybond N+ nylon membranes containing immobilized PCR products or restriction enzyme-digested plasmids were hybridized with the radioactively labeled cellular RNA for >16 h at 50°C. Membranes were probed first with RNA from the heat-sensitive mutant YSB331. X-ray film was exposed to membranes hybridized with YSB331 RNA for 7 days. Membranes were stripped and then probed with RNA from the parent strain YSB324. X-ray film was exposed to membranes hybridized with YSB324 RNA for 5 days.
RESULTS
Repair in a heat-sensitive mutant of TFIIE large subunit
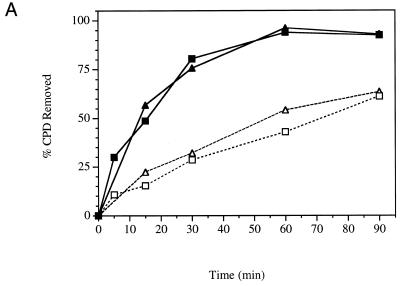
The removal of CPDs from each strand of the RPB2 gene was determined in the parental wild-type yeast strain (YSB324) and in a strain (YSB331) possessing a mutation in the zinc finger domain of the 66 kDa subunit of TFIIE. Replacing a cysteine with a phenylalanine at residue 127 results in a heat-sensitive phenotype (20). Figure 1A shows autoradiograms of membranes containing DNA from an experiment examining repair at the permissive temperature. The presence of CPDs immediately after irradiation is indicated by the relatively light band intensities in the lanes from the T4 endonuclease V treated DNA. Repair is observed as a reappearance of the band relative to the band in the lane from the mock treated DNA. Removal of CPDs from each strand of the RPB2 gene in YSB324 and YSB331 is represented graphically in Figure 2. At the permissive temperature YSB331 performed the same amount of repair of the transcribed strand as that observed in YSB324, the parental strain (Fig. 2A). Our results demonstrate that at the permissive temperature there is no significant defect in repair of the transcribed strand of the RPB2 gene in strain YSB331. The immobilized YSB324 and YSB331 DNA was also hybridized with radioactive RNA probes specific for the non-transcribed strand of RPB2. As shown in Figure 2A, at the permissive temperature, repair of the non-transcribed strand is proficient in both YSB324 and YSB331, but slower than that of the transcribed strand. Slower repair of the non-transcribed strand and the genome overall relative to the transcribed strand of active genes is characteristic of repair proficient strains. Our results demonstrate that at the permissive temperature there is no significant defect in repair of the non-transcribed strand of the RPB2 gene or in TCR in either the parental strain or in the TFIIE heat-sensitive mutant.
Figure 1.
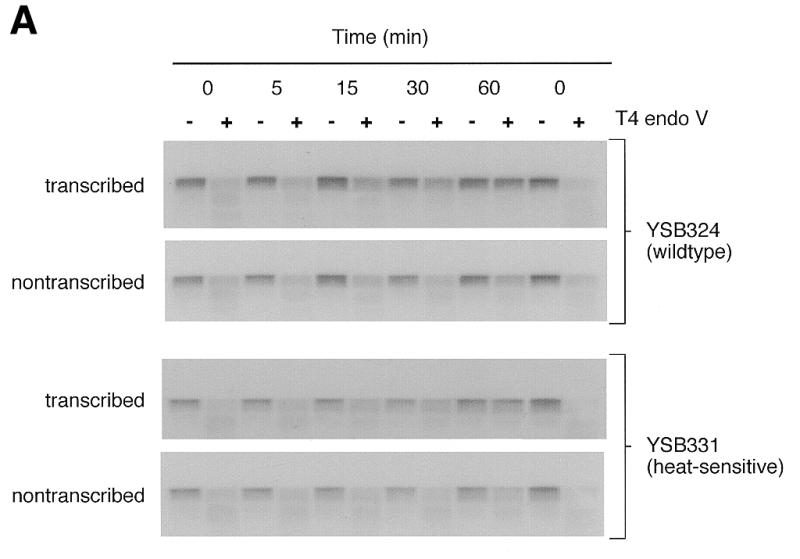
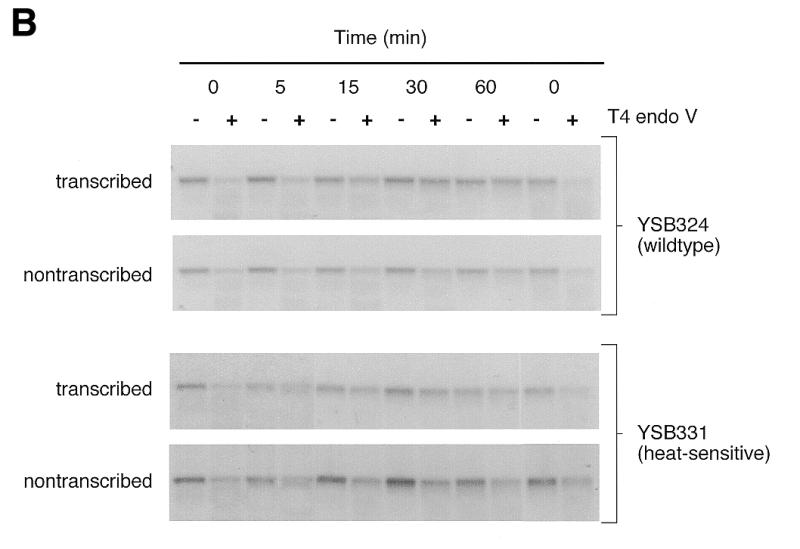
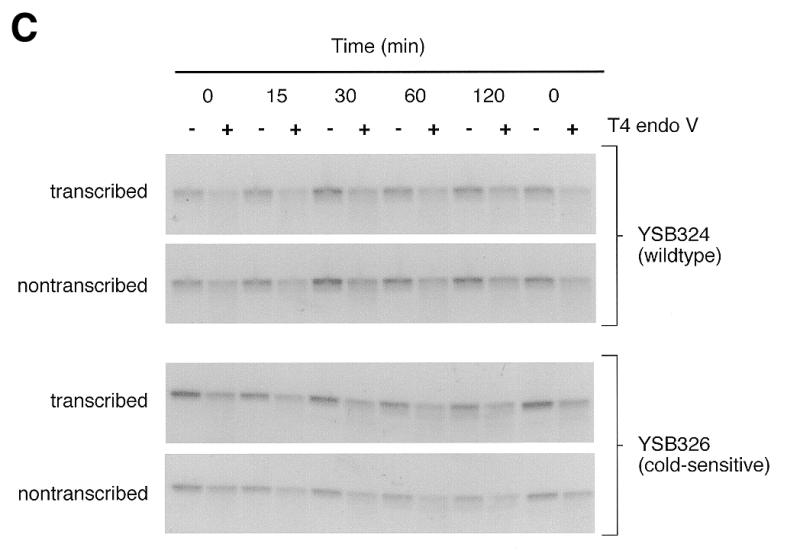
Autoradiograms illustrating removal of CPDs from each of the strands of the RPB2 gene in the wild-type parent strain (YSB324), a heat-sensitive TFIIE mutant (YSB331) and a cold-sensitive TFIIE mutant (YSB326) at permissive and non-permissive temperatures. Exponentially growing cultures of YSB324 and YSB331 at 30°C were UV irradiated and incubated in growth medium at 30°C for the times indicated (A). Exponentially growing cultures of YSB324 and YSB331 at 24°C were shifted to 37°C for 6 h then UV irradiated and incubated in growth medium at 37°C for the times indicated (B). Exponentially growing cultures of YSB324 and YSB326 at 30°C were shifted to 15°C for 20 h then UV irradiated and incubated in growth medium at 15°C for the times indicated (C). UV irradiations were with 60 J/m2. DNA purified from the cells was digested with PvuI and PvuII restriction endonucleases. Restricted DNA (0.2 µg) was digested with T4 endonuclease V or mock treated, then electrophoresed through 0.5% alkaline agarose. DNA was transferred to Hybond N+ membrane and hybridized with an RNA probe specific for the non-transcribed strand of the RPB2 gene and an autoradiogram was generated. The probe was stripped off and the immobilized DNA was then hybridized with an RNA probe specific for the transcribed strand. The autoradiograms show the 5.3 kb PvuI–PvuII restriction fragment.
Figure 2.
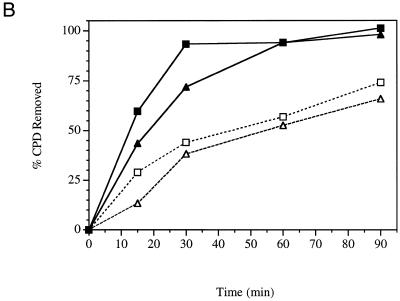
Time course for removal of CPDs from each of the two strands of RPB2 in a heat-sensitive TFIIE mutant (YSB331) and the wild-type parental strain (YSB324). Exponentially growing cultures at 30°C were UV irradiated with 60 J/m2 and incubated in growth medium at 30°C for the times indicated (A). Exponentially growing cultures at 24°C were shifted to 37°C for 6 h then UV irradiated with 60 J/m2 and incubated in growth medium at 37°C for the times indicated (B). Repair was determined from the measured incidences of CPDs in each strand of the PvuI–PvuII restriction fragment of the RPB2 gene. Data points represent the averages of four independent experiments. Transcribed strand, YSB324 (filled squares); non-transcribed strand, YSB324 (open squares); transcribed strand, YSB331 (filled triangles); non-transcribed strand, YSB331 (open triangles).
Repair experiments were repeated for YSB324 and YSB331 at 37°C, the non-permissive temperature for YSB331. Figure 1B shows autoradiograms of membranes containing DNA from an experiment examining repair at the non-permissive temperature. Removal of CPDs from each strand of the RPB2 gene in YSB324 and YSB331 is represented graphically in Figure 2B. Damage is removed significantly faster from the transcribed strand of RPB2 than from the non-transcribed strand in both YSB324 and YSB331, and repair of the non-transcribed strand of YSB331 is similar to repair of the non-transcribed strand of YSB324. In summary, the heat-sensitive mutation in the zinc finger domain of the large subunit of TFIIE results in cells which have proficient repair of the transcribed and non-transcribed strand. However, repair of both strands appears to be slightly slower in the mutant than in the parental strain. It is clear that the mutant cells are capable of proficient TCR, although at a slightly reduced rate.
Repair in a cold-sensitive mutant of TFIIE large subunit
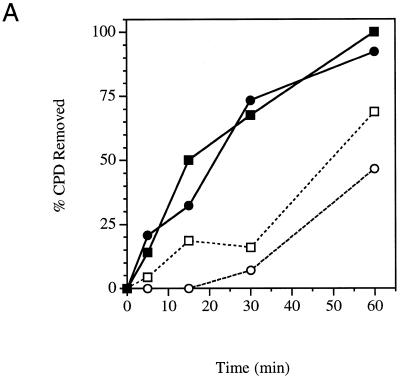
The removal of CPDs from each strand of the RPB2 gene was determined in the parental wild-type yeast strain (YSB324) and in a cold-sensitive strain (YSB326) containing a deletion of the 3′ end of the TFA1 gene, removing a portion of the C-terminus of the large subunit of TFIIE. At the permissive temperature repair of the transcribed strand in YSB326 was basically like that in the repair-proficient parent strain (Fig. 3A). In both strains by 60 min repair of the transcribed strand was essentially complete. Our results demonstrate that at the permissive temperature there is no significant defect in repair of the transcribed strand of the RPB2 gene in strain YSB326. Membranes were stripped and hybridized with radioactive RNA probes specific for the non-transcribed strand of RPB2. Repair of the non-transcribed strand in the cold-sensitive mutant is similar to that of the repair-proficient parent strain (Fig. 3A). Removal of CPDs remained low during the first 30 min following UV irradiation. However, by 60 min following UV irradiation ~70% of the CPDs had been removed from the non-transcribed strand in the parent strain, YSB324, and 45% of the CPDs had been removed in the cold-sensitive mutant, YSB326. We note that there is a slightly lower level of repair of the non-transcribed strand of RPB2 in the cold-sensitive mutant at all time points. Thus, at the permissive temperature there may be a slight defect in repair of the non-transcribed strand of the RPB2 gene in YSB326; however, there is no significant defect in TCR.
Figure 3.
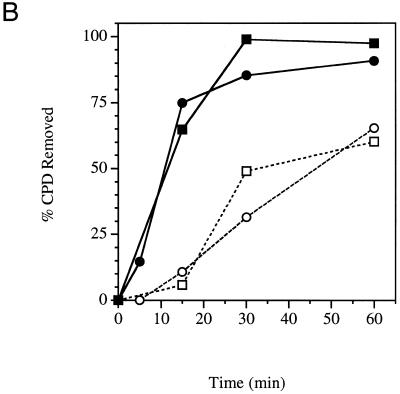
Time course for removal of CPDs from each of the two strands of RPB2 in a cold-sensitive TFIIE mutant (YSB326) and the wild-type parental strain (YSB324). Exponentially growing cultures at 30°C were UV irradiated with 60 J/m2 and incubated in growth medium at 30°C for the times indicated (A). Exponentially growing cultures at 30°C were shifted to 37°C for 1 h then UV irradiated with 60 J/m2 and incubated in growth medium at 37°C for the times indicated (B). Exponentially growing cultures at 30°C were shifted to 15°C for 20 h then UV irradiated with 60 J/m2 and incubated in growth medium at 15°C for the times indicated (C). Repair was determined from the measured incidences of CPDs in each strand of the PvuI–PvuII restriction fragment of the RPB2 gene. Data points represent the averages of three independent experiments. Transcribed strand, YSB324 (filled squares); non-transcribed strand, YSB324 (open squares); transcribed strand, YSB326 (filled circles); non-transcribed strand, YSB326 (open circles).
Experiments were repeated at 37°C, a temperature at which we find both wild-type and cold-sensitive strains grow, indicating that both strains are expressing functional TFIIE 66 kDa subunit. There was very efficient repair of the transcribed strand (Fig. 3B) in both YSB324 and YSB326. The immobilized DNA on the membranes was also hybridized with radioactive RNA probes specific for the non-transcribed strand of RPB2. Both strains also had efficient repair of the non-transcribed strand, but not as rapid as that of the transcribed strand (Fig. 3B). In summary, at 37°C both YSB324 and YSB326 are capable of efficient TCR and repair of the non-transcribed strand of the RPB2 gene.
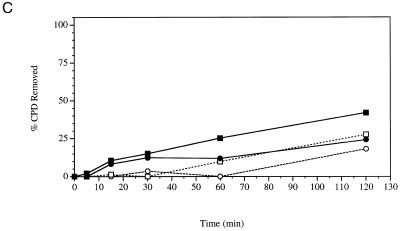
Repair experiments were repeated for YSB324 and YSB326 at 15°C, the non-permissive temperature for YSB326. Figure 1C shows autoradiograms of membranes containing DNA from an experiment examining repair at the non-permissive temperature. Removal of CPDs from each strand of the RPB2 gene in YSB324 and YSB326 is represented graphically in Figure 3C. Repair of both strands of RPB2 is very slow in both the repair-proficient parent strain YSB324 and the cold-sensitive mutant YSB326. Our results demonstrate that in the parental strain there exists low levels of TCR and repair of the non-transcribed strand at 15°C, and that in the cold-sensitive mutant repair of both the transcribed strand and non-transcribed strand is even more deficient than in the parental strain.
Cell growth of conditional mutants of TFIIE large subunit
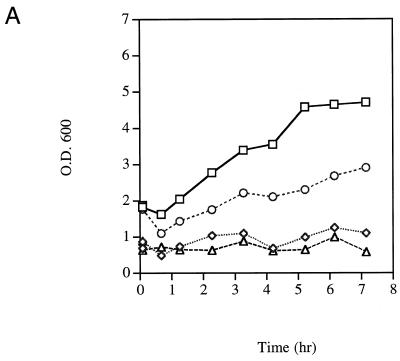
We examined growth of the heat-sensitive and cold-sensitive TFIIE mutants and the parental strain at several temperatures. In addition, we examined the effect of UV irradiation upon growth (Fig. 4). At 24°C the wild-type parental strain (YSB324) and the heat-sensitive mutant (YSB331) grew at the same rate, the cell numbers doubled about every 3 h. Following UV irradiation with 60 J/m2 the cell growth of both strains slowed to a doubling about every 5 h (data not shown). Similar results were obtained when the cells were grown at 30°C (data not shown). Six hours after the strains were shifted to 37°C, the non-permissive temperature for YSB331, the parental strain doubled about every 3 h, and consistent with the observations of others (20), the growth of YSB331 essentially ceased. We observed slower growth of YSB324 and no growth of YSB331 at 37°C following UV irradiation (Fig. 4A). At 15°C the parental strain and the heat-sensitive mutant grew at the same rate, doubling about every 8.5 h, UV irradiation further slowed the growth of both strains equally (data not shown). In summary, the heat-sensitive TFIIE mutant did not grow at 37°C, at all other temperatures it grew at essentially the same rate as the parental strain and UV irradiation slowed the growth of both strains at all temperatures examined.
Figure 4.
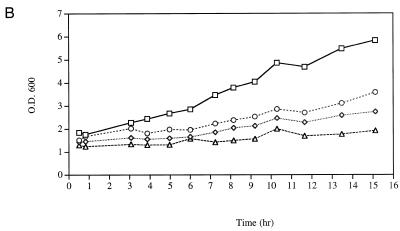
Growth in a heat-sensitive TFIIE mutant (YSB331), a cold-sensitive TFIIE mutant (YSB326) and the wild-type parental strain (YSB324). Exponentially growing cultures of YSB331 and YSB324 at 24°C were shifted to 37°C for 6 h then UV irradiated or not and incubated in growth medium at 37°C for the times indicated. YSB324 unirradiated (squares); YSB331 unirradiated (diamonds); YSB324 irradiated (circles); YSB331 irradiated (triangles) (A). Exponentially growing cultures of YSB326 and YSB324 at 30°C were shifted to 15°C for 20 h then UV irradiated or not and incubated in growth medium at 15°C for the times indicated. YSB324 unirradiated (squares); YSB326 unirradiated (diamonds); YSB324 irradiated (circles); YSB326 irradiated (triangles) (B). UV irradiations were with 60 J/m2. Growth was monitored by measuring the optical density of the cultures at 600 nm.
At 30°C the wild-type parental strain (YSB324) and the cold-sensitive mutant (YSB326) grew similarly with the cells doubling about every 2.4 h, following UV-irradiation cultures grew more slowly, doubling every 4.2 h (data not shown). When the cells were shifted to 37°C the parental strain and the cold-sensitive mutant grew at the same rate, UV irradiation slowed the growth of both strains slightly (data not shown). As reported (20), we observed that the growth rate of YSB326 at 15°C is significantly slower than that of YSB324. UV irradiation further reduced the growth rate at 15°C of both strains, irradiated YSB324 cultures took >12 h to double and YSB326 cultures >20 h (Fig. 4B). In summary, the cold-sensitive mutant grew more slowly than the parental cells at 15°C, at the other temperatures it grew at basically the same rate as the parental cells; and UV irradiation with 60 J/m2 slowed the growth of all strains at each temperature examined.
Ongoing transcription in a conditional mutant of TFIIE large subunit at the non-permissive temperature
We performed transcript run-on assays to determine whether or not the heat-sensitive tfa1 mutant was capable of transcription following UV irradiation at the non-permissive temperature. Using the method of Warner (28), we radioactively labeled nascent RNA transcripts in vivo at several time points over the first 90 min following UV irradiation. PCR products or restriction-digested plasmids were immobilized on nylon membranes and hybridized with radioactively labeled total cellular RNA. An autoradiograph is shown in Figure 5. The parental strain YSB324 shows robust hybridization of RNA to the RPB2 gene over the 90 min following UV irradiation. There is little or no hybridization of RNA to RPB1 (data not shown), LYS2 or RAD26 genes. The heat-sensitive tfa1 mutant YSB331 shows reduced, yet substantial, hybridization of RNA to the RPB2 gene. Like the parent strain, YSB331 shows little or no hybridization of RNA to RPB1 (data not shown), LYS2 or RAD26 genes. The heat-sensitive tfa1 mutant YSB331 is capable of transcribing the RPB2 gene at the non-permissive temperature.
Figure 5.
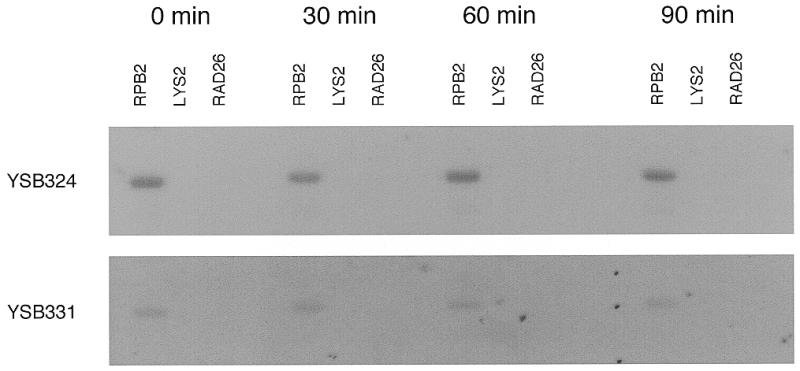
Time course for transcription of RPB2 in a heat-sensitive TFIIE mutant (YSB331) and the wild-type parental strain (YSB324). Exponentially growing cultures at 24°C were shifted to 37°C for 6 h then UV irradiated with 60 J/m2 and incubated in growth medium at 37°C for the times indicated. Nascent transcripts were radioactively labeled in vivo and hybridized to DNA immobilized on nylon membranes. The same membrane was used for YSB331 and then for YSB324. Top, YSB324; bottom, YSB331.
DISCUSSION
We investigated the influence of conditional mutations in the gene encoding the large subunit of TFIIE on NER, TCR and transcription. At the non-permissive temperatures, it has been reported that these conditional mutants do not express class II genes that are transcribed by RNA pol II. Active transcription is required for TCR (4,24,29); therefore, we expected to find a lack of TCR at the non-permissive temperatures. In addition, we examined repair at the permissive temperature to determine if the mutations leading to the conditional phenotypes might affect protein–protein interactions and lead to diminished TCR in transcriptionally competent cells. The mutation in TFA1 that leads to heat sensitivity is in the zinc-binding motif. The function of the Zn-binding motif is unknown, but it is thought to be necessary for the maintenance of proper protein structure (20). Mutation of the Zn-binding motif does not affect the ability of Tfa1p to associate with Tfa2p or to bind single-stranded DNA (20–22). In our experiments, the heat-sensitive mutant was maintained at the non-permissive temperature for 6 h to completely inhibit growth prior to UV irradiation. In the absence of functional TFIIE, transcription should be eliminated and growth ceases. Yet, we observed preferential repair of the transcribed strand within a class II gene, indicating that transcription is not completely shut off in the heat-sensitive mutant. In other words, in the absence of TFIIE we found TCR and, therefore, the requisite transcription by RNA pol II (4,24,29). Our results are at odds with the results of other laboratories studying TFIIE and transcription (20–22). Mutations in the TFA1 gene result in a rapid decline in total poly(A)+ mRNA and transcription of several different genes. We reconcile our results with their results by assuming that the level of transcription necessary to observe transcription-coupled DNA repair is below the level of transcription that can be observed by northern analysis or primer extension assays. Using an in vivo transcript run-on assay, we determined that transcription of the RPB2 gene does occur following UV irradiation in the heat-sensitive tfa1 mutant at the non-permissive temperature, although at a reduced rate relative to the parent strain. Several other genes did not appear to be transcribed at detectable levels at the non-permissive temperature. We conclude that the mutation leading to the heat-sensitive phenotype enables transcription to continue but at a level that is inadequate for growth. Transcription may also proceed if a low level of transcription initiation does not require TFIIE and, consequently, TFIIH or TFIIE is not needed for transcription initiation of a particular set of genes, including RPB2. Transcription initiation can occur in vivo in the absence of TFIIE (30). Transcription initiation can occur on supercoiled templates in vitro in the absence of TFIIE and TFIIH (31) and extracts from XPB and XPD (subunits of human TFIIH) cells contain reduced levels of TFIIH yet are competent for transcription initiation in vitro (32). Additionally, deletion of the CTD of the large subunit of RNA pol II, which is required for TFIIE and TFIIH binding, leads to defective RNA capping and processing, but does not abolish transcription of some genes (33,34).
Deletion of up to 293 residues from the C-terminus of Tfa1p results in functional TFIIE and a cold-sensitive growth. The remaining N-terminal 189 residues are sufficient for both basal transcription and activated transcription (21,22). We examined repair in wild-type and cold-sensitive strains. The cold-sensitive mutant was fully competent for TCR at the permissive temperature. Interestingly, the cold-sensitive mutant displayed a slight reduction in repair of the non-transcribed strand at the permissive temperature. On the other hand, both the parent strain and the cold-sensitive mutant were very deficient in NER at the non-permissive temperature. While repair of both strands of the reporter gene is greatly reduced in the wild-type strain at 15°C, we still observe some TCR. In the cold-sensitive mutant at 15°C, repair of the transcribed strand is slightly greater than repair of the non-transcribed strand at early times following UV irradiation. However, repair of the transcribed strand does not increase further and is not much greater than the repair of the non-transcribed strand at 2 h following UV irradiation. The slow repair observed in both strains was surprising in light of the different growth rates observed for the same strains at 15°C following UV irradiation. The growth rate of the mutant was significantly slower than that of the wild-type strain. Our results imply that there is limited transcription in both the wild-type and cold-sensitive mutant at 15°C.
TCR is dependent upon transcription of the transcribed DNA strand and is thought to be confined to genes transcribed by RNA pol II (24,29,35). Therefore, a great deal of research has focused on unique features of RNA pol II that may interact with repair proteins to enable TCR. However, the lack of TCR in genes transcribed by RNA pol II may reflect a lack of transcription rather than the absence of TCR. We present evidence showing that the heat-sensitive TFIIE mutant we examined was fully competent for transcription-coupled DNA repair of RPB2 at the non-permissive temperature and that transcription by RNA pol II is greatly reduced. We show that TCR occurs even at low levels of transcription, indicating that one pass of the RNA polymerase elongation complex may be sufficient to enable removal of DNA damage from the transcribed strand.
Acknowledgments
ACKNOWLEDGEMENTS
The authors thank members of the laboratory, Miriam Bucheli and Melvin Limson, as well as colleagues, Michael Hampsey, Marc Gartenberg and Kiran Madura, for helpful comments and critical reading of this manuscript. This work was supported in part by Public Health Service grant R29 GM53717 from the National Institutes of Health.
REFERENCES
- 1.Friedberg E.C., Walker,G.C. and Siede,W. (1995) DNA Repair and Mutagenesis. ASM Press, Washington, DC.
- 2.Aboussekhra A., Biggerstaff,M., Shivji,M.K., Vilpo,J.A., Moncollin,V., Podust,V.N., Protic,M., Hubscher,U., Egly,J.M. and Wood,R.D. (1995) Cell, 80, 859–868. [DOI] [PubMed] [Google Scholar]
- 3.Mellon I., Spivak,G. and Hanawalt,P.C. (1987) Cell, 51, 241–249. [DOI] [PubMed] [Google Scholar]
- 4.Mellon I. and Hanawalt,P.C. (1989) Nature, 342, 95–98. [DOI] [PubMed] [Google Scholar]
- 5.Selby C.P. and Sancar,A. (1993) Science, 260, 53–58. [DOI] [PubMed] [Google Scholar]
- 6.van Gool A.J., Verhage,R., Swagemakers,S.M., van de Putte,P., Brouwer,J., Troelstra,C., Bootsma,D. and Hoeijmakers,J.H.J. (1994) EMBO J., 13, 5361–5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Hoffen A., Natarajan,A.T., Mayne,L.V., van Zeeland,A.A., Mullenders,L.H.F. and Venema,J. (1993) Nucleic Acids Res., 21, 5890–5895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu Y., Bates,S. and Pfeifer,G.P. (1997) J. Biol. Chem., 272, 20747–20755. [DOI] [PubMed] [Google Scholar]
- 9.Sweder K.S., Verhage,R., Crowley,D.J., Brouwer,J. and Hanawalt,P.C. (1996) Genetics, 143, 1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verhage R.A., van Gool,A.J., de Groot,N., Hoeijmakers,J.H., van de Putte,P. and Brouwer,J. (1996) Mol. Cell. Biol., 16, 496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sweder K.S. and Hanawalt,P.C. (1994) J. Biol. Chem., 269, 1852–1857. [PubMed] [Google Scholar]
- 12.Sweder K.S., Chun,R.A., Mori,T. and Hanawalt,P.C. (1996) Nucleic Acids Res., 24, 1540–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guzder S.N., Habraken,Y., Sung,P., Prakash,L. and Prakash,S. (1995) J. Biol. Chem., 270, 12973–12976. [DOI] [PubMed] [Google Scholar]
- 14.Svejstrup J.Q., Wang,Z., Feaver,W.J., Wu,X., Bushnell,D.A., Donahue,T.F., Friedberg,E.C. and Kornberg,R.D. (1995) Cell, 80, 21–28. [DOI] [PubMed] [Google Scholar]
- 15.Maxon M.E., Goodrich,J.A. and Tjian,R. (1994) Genes Dev., 8, 515–524. [DOI] [PubMed] [Google Scholar]
- 16.Flores O., Lu,H. and Reinberg,D. (1992) J. Biol. Chem., 267, 2786–2793. [PubMed] [Google Scholar]
- 17.Chambers R.S. and Dahmus,M.E. (1994) J. Biol. Chem., 269, 26243–26248. [PubMed] [Google Scholar]
- 18.Chambers R.S., Wang,B.Q., Burton,Z.F. and Dahmus,M.E. (1995) J. Biol. Chem., 270, 14962–14969. [DOI] [PubMed] [Google Scholar]
- 19.Cho H., Kim,T.-K., Mancebo,H., Lane,W.S., Flores,O. and Reinberg,D. (1999) Genes Dev., 13, 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kuldell N.H. and Buratowski,S. (1997) Mol. Cell. Biol., 17, 5288–5298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai H. and Fukasawa,T. (1997) J. Biol. Chem., 272, 32663–32669. [DOI] [PubMed] [Google Scholar]
- 22.Tijerina P. and Sayre,M.H. (1998) J. Biol. Chem., 273, 1107–1113. [DOI] [PubMed] [Google Scholar]
- 23.Adams A., Gottschling,D.E., Kaiser,C.A. and Stearns,T. (1998) Methods in Yeast Genetics: A Cold Spring Harbor Laboratory Course Manual, 1997 Edn. Cold Spring Harbor Laboratory Press, Plainview, NY.
- 24.Sweder K.S. and Hanawalt,P.C. (1992) Proc. Natl Acad. Sci. USA, 89, 10696–10700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasmyth K.A. and Reed,S.I. (1980) Proc. Natl Acad. Sci. USA, 77, 2119–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J., Fritsch,E.F. and Maniatis,T. (1989) In Nolan,C. (ed.), Molecular Cloning: A Laboratory Manual, 2nd Edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 27.Bohr V.A., Smith,C.A., Okumoto,D.S. and Hanawalt,P.C. (1985) Cell, 40, 359–369. [DOI] [PubMed] [Google Scholar]
- 28.Warner J.R. (1991) Methods Enzymol., 194, 423–428. [DOI] [PubMed] [Google Scholar]
- 29.Leadon S.A. and Lawrence,D.A. (1992) J. Biol. Chem., 267, 23175–23182. [PubMed] [Google Scholar]
- 30.Sakurai H. and Fukasawa,T. (1999) Biochem. Biophys. Res. Commun., 261, 734–739. [DOI] [PubMed] [Google Scholar]
- 31.Holstege F.C., Tantin,D., Carey,M., van der Vliet,P.C. and Timmers,H.T. (1995) EMBO J., 14, 810–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Satoh M.S. and Hanawalt,P.C. (1997) Biochim. Biophys. Acta, 1354, 241–251. [DOI] [PubMed] [Google Scholar]
- 33.McCracken S., Fong,N., Yankulov,K., Ballantyne,S., Pan,G., Greenblatt,J., Patterson,S.D., Wickens,M. and Bentley,D.L. (1997) Nature, 385, 357–361. [DOI] [PubMed] [Google Scholar]
- 34.McNeil J.B., Agah,H. and Bentley,D. (1998) Genes Dev., 12, 2510–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christians F. and Hanawalt,P. (1992) Mutat. Res., 274, 93–101. [DOI] [PubMed] [Google Scholar]