Abstract
Cost-effectiveness analyses of weight loss programs for university students can inform administrator decision-making. This study quantifies and compares the costs and cost-effectiveness of implementing two digitally-delivered weight loss interventions designed for university populations. Healthy Body Healthy U (HBHU) was a randomized controlled trial comparing TAILORED (personalized) versus TARGETED (generic) weight loss interventions adapted specifically for young adults to a CONTROL intervention. Participants (N = 459; 23.3 ± 4.4 years; mean BMI 31.2 ± 4.4 kg/m2) were recruited from two universities. Implementation costs were examined from a payer (i.e., university) perspective, comparing both the average cost effectiveness ratio (ACER) and the incremental cost effectiveness ratio (ICER) of the two interventions. Cost-effectiveness measures were calculated for changes in body weight, abdominal circumference, HDL cholesterol, systolic and diastolic blood pressure, and HbA1c. The overall 6-month implementation costs were $105.66 per person for the TAILORED intervention and $91.44 per person for the TARGETED intervention. The ACER for weight change was $107.82 for the TAILORED and $179.29 for the TARGETED interventions. The ICER comparing TAILORED with TARGETED for change in body weight was $5.05, and was even lower ($2.28) when including only those with overweight and not obesity. The ICERs for change in abdominal circumference, HDL cholesterol, systolic and diastolic blood pressure, and HbA1c were $3.49, $59.37, $1.57, $2.64, and $47.49, respectively. The TAILORED intervention was generally more cost-effective compared with the TARGETED intervention, particularly among those with overweight. Young adults with obesity may require more resource-intensive precision-based approaches.
Keywords: cost, cost effectiveness, tailored, weight loss, intervention, young adult
Tailored weight loss interventions designed for university students may be more cost effective when compared with generic interventions.
Implications.
Researchers: This is the first cost effectiveness study to examine cardiometabolic outcomes among young adults within a university setting.
Practitioners: Personalized technology-based programs are generally cost effective for student weight loss and cardiometabolic outcomes, relative to pharmaceutical or commercial programs.
Policymakers: Establishing cost-effective methods for delivering programming to young adults within educational settings can provide important skills for lifelong chronic disease prevention.
INTRODUCTION
The prevalence of obesity continues to rise in the U.S. and globally [1, 2]. This is concerning because obesity contributes to heart disease, cancer, and type 2 diabetes as well as all-cause mortality [3]. In fact, these diseases are increasingly occurring among younger age groups [4, 5]. In the U.S., the current prevalence of obesity is 40% among young adults (ages 20–39 years) [1], and the annual population-level incremental costs of obesity-related comorbidities assumes a considerable economic burden among communities [6]. These data indicate a need for effective programs designed to promote weight loss and maintenance early in life before comorbidities are established. Of particular importance for establishing healthy weight management behaviors is the transition from youth to young adulthood [7–9], a developmental period that coincides with university enrollment.
College and university campuses represent a community through which chronic disease prevention programming can be implemented. In the U.S. in 2019–2020, 20 million students were enrolled in postsecondary education [10], with 3.1 million enrolled in post-baccalaureate (or graduate/professional study) [11], representing an opportune location for weight-related behavioral interventions. Weight loss programming, however, remains relatively scarce on college/university campuses [9], particularly in comparison with other student services (e.g., drug and alcohol use, sexual violence prevention) [12]. This may be due, in part, to a combination of institutional barriers (e.g., resources, coordination across departments, competing student health issues taking priority) and participant barriers (e.g., campus built-environment, busy schedules, stigma) [13].
Technology-based interventions are a promising mechanism for providing weight loss programming for young adults, though more research among this population is needed [14]. Delivery options for technology-based interventions are diverse, including websites, apps, and text-messaging [15]. Intervention components range from tools to assist with self-monitoring and feedback to connecting participants with healthcare specialists in real-time. However, a systematic review of 47 technology-based weight loss interventions found the mean age of participants was 41 years, suggesting that these programs are largely designed for adult populations [15]. Technology-based weight loss services may be especially salient to young, university student populations, given that 99% of those ages 18–29 use the internet [16] and 48% say they are online “almost constantly” [17]. However, while previous cost-effectiveness studies of technology-based physical activity promotion [18, 19] and behavioral weight loss [20] programs have been conducted with technology or intermediate communication (i.e., phone or in-person) [19], print-based interventions have been considered more cost-effective than counselor-delivered, phone-based interventions among sedentary adults [18]. On the other hand, when accounting for participant time costs, internet-based weight loss services for adults with overweight/obesity are viewed as more cost-effective than in-person programs [20]. To date, a knowledge gap appears to exist with regard to the cost-effectiveness of digital behavioral weight loss programs that: (i) are specifically designed for college/university students; or (ii) compare treatments disseminated via the same technology channels and differ only in the type of messaging used (i.e., personalized vs. generic). Furthermore, few studies have incorporated improvements in cardiometabolic risk factors into the calculated costs of weight-loss programs [21]. We previously observed that digital therapy was effective at promoting weight loss in the Healthy Body Healthy U (HBHU) randomized controlled trial [22]. In the current analysis, we compared the cost-effectiveness of the TAILORED (personalized) versus TARGETED (generic) treatment arms of the program.
METHODS
Overview of the main trial and participants
The HBHU randomized controlled trial examined the efficacy of two digital weight loss programs for students (undergraduate or graduate) with overweight/obesity recruited from two university sites from 2015–2018 [22, 23]. Participants were young adults aged 18–35 years, meeting the following eligibility criteria: BMI of 25–45 kg/m2; enrolled in a college or university in the greater DC/Boston areas; an active Facebook user (identified by at least one log-in within the past month); having regular access to text messages; and fluent in English. Participants were recruited using a social marketing approach for promotional outreach, including branding of study materials, positioning flyers high-trafficked residential areas, circulating outreach messages via digital channels such as emails from professors and student groups, in-person events, as well as social media postings. See Whiteley et al. for more details [24]. Exclusion criteria included trying to gain weight or participating in other weight loss or physical activity studies. For full inclusion and exclusion criteria, please see Napolitano et al. [23].
Participants were randomly assigned to one of three conditions: (i) TAILORED (i.e., personalized) treatment (n = 150); (ii) TARGETED (i.e., generic) treatment (n = 152); and (iii) CONTACT CONTROL (n = 157). All participants received program content via Facebook, text messaging, and weekly reports. Weight loss content for the TAILORED and TARGETED groups was based on the Diabetes Prevention Program [25, 26] and adapted for young adults. The CONTROL group received general healthy body content (e.g., body image, sleep) via the same channels. Similar to the Diabetes Prevention Program, the intervention was more intensive (i.e., weekly delivery) during the first 6 months, followed by a tapered intervention dose through month 18. Participants provided clinic-based assessments of height, body weight, abdominal circumference, blood pressure, and a fasting blood sample at baseline, 6-, 12-, and 18-months to determine cardiometabolic risk factors. As mentioned above, for the purpose of the current paper, we included only the results from the TAILORED and TARGETED treatments, as those arms were focused on physical activity promotion and weight reduction and can best inform university payer decisions regarding costs of reducing weight and chronic disease. All study procedures were approved by the Institutional Review Boards at both university campuses, and all participants provided informed consent prior to their enrollment in the study.
Cost analyses framework
The time horizon for the cost analyses was 6 months (comprising the most intensive phase of intervention delivery in the HBHU study) from a university payer perspective. Similar to Daumit et al. [27], this approach was based on the following assumptions: (i) the costs of developing the trial were considered sunk costs (i.e., they were not expected to be repeated if the intervention were to be adopted broadly) [28], and were not included in the cost analyses; (ii) calculated estimates reflected costs of implementing the programs in a real-world application (i.e., research recruitment and testing costs were not included); and (iii) the costs of the control arm of the study were not included in the analyses. We also tested the sensitivity of our analysis to variations in the number of participants enrolled to provide university decision-makers with data regarding costs and benefits of implementing this program on campuses with varying enrollment sizes.
Body weight and cardiometabolic outcomes
Body weight was measured in duplicate and consistently in the morning using a digital scale (Seca Model 769). Participants were asked to remove bulky outer clothing and shoes prior to measurements with averages calculated and recorded [23]. At the same clinic visit and following an overnight fast of at least 8 h, blood samples were obtained for determination of HDL-C and hemoglobin A1c (HbA1c; A1cNow+, PTS Diagnostics). Abdominal circumference was measured at the umbilicus using a cloth tape on participant exhale and rounded to the nearest 0.1 cm. Blood pressure was taken after participants sat quietly for 5 min using a digital blood pressure monitor (OMRON HEM-907XL). Abdominal circumference and blood pressure (systolic and diastolic) measurements were recorded in triplicate and averaged. Weight loss at 6-months was reported previously [22]; all other cardiometabolic outcomes are primary findings from the current analysis.
Measures used to calculate costs
Payer
Costs for the payer from a university perspective were calculated. The total payer cost was calculated by summing all direct costs associated with intervention delivery. Costs required to duplicate a program of this nature were personnel costs, program materials, intervention delivery costs, and fixed costs for space, hardware, and software. Payer costs for personnel and space reflect the difference in staff time allocations of the TAILORED and TARGETED treatment programs, given the different allocation of time devoted to delivering each. Costs per participant per month were also calculated, with all costs being converted to a 6-month metric.
Personnel
Personnel costs were estimated by analyzing staff activity logs completed by multiple members of the research team throughout the study. Team members were asked to track both research- and intervention-related activities during both active engagement, active enrollment, and study implementation weeks. For the cost effectiveness analyses, only intervention-related hours were utilized for each staff member. Intervention-based hours included time spent reviewing participant safety, discussing and contacting participants as well as participant communication and general office tasks. The average hours spent on each activity was multiplied by each staff member’s hourly wage to calculate the average cost per hour for intervention activities. Personnel costs that differed based on treatment group were scaled to calculate the average weekly cost per participant for both treatment groups. Given the time to deliver the tailored intervention, personnel cost was allocated at 80% for TAILORED, 15% for TARGETED, with the final 5% allocated to CONTROL.
Materials
Material costs consist of recruitment items since recruitment efforts would occur in real-world application of this program regardless of setting. Recruitment costs included flyers, giveaways, and advertisements for print, social media, and online newspapers. Recruitment costs were summed to determine a total and divided by the number of participants enrolled in the trial to determine a cost per participant. Recruitment costs did not differ based on group assignment; however, the totals reflect the number of individuals assigned to each treatment group.
Intervention costs
We included costs to support the delivery of the program content, including automated text messaging and materials. These intervention costs are estimated in 2015 dollars based on number and price of outgoing and incoming text messages per week, along with fees for programmable messaging and phone number allocation. The quantity of outgoing and incoming text messages accounts for differences in the treatment groups for the intervention-related task of self-monitoring. Other intervention costs included digital bathroom scales, which were distributed to participants to track their weight throughout the program (see Table 2). Intervention development costs were not included in our analyses, as the platform and code for the HBHU trial are readily available and previously developed at least five years ago.
Table 2.
Intervention cost schedule through month 6
| Activities | Tailored (N = 150) | Targeted (N = 152) |
|---|---|---|
| Personnel | $1,960.11 | $1,369.33 |
| Recruitment materials | ||
| Tabling materials | $16.05 | $16.15 |
| Giveaways | $728.14 | $732.96 |
| Flyers | $82.63 | $83.18 |
| Print Ads | $551.48 | $555.13 |
| Social media Ad | $71.84 | $72.32 |
| Online newspaper | $41.30 | $41.03 |
| Intervention | ||
| Text message fees | $654.01 | $307.05 |
| Bathroom scale | $2,263.49 | $2,278.48 |
| Fixed | ||
| Space/facilities | $9,369.14 | $8,328.13 |
| Hardware | $50.01 | $50.01 |
| Software | $61.57 | $61.57 |
| Total payer cost | $15,849.78 | $13,895.34 |
| Cost per participant at 6 months | $105.66 | $91.44 |
| Average monthly cost/participant | $17.61 | $15.24 |
| Average monthly incremental cost/participant (Tailored relative to Targeted) |
$2.37 | – |
Fixed costs
Fixed costs included space/facilities, hardware, and software. Space and utilities were calculated using an estimated 64 square feet of office space at a monthly rate of $50 per square foot [29] in Year 2015 dollars, or $3200 per month. Weekly costs for space and utilities were adjusted for group differences, and a total group cost was calculated. As the space and utilities were shared, the value was estimated as a proportion with TAILORED and TARGETED allocated at 45% and 40%, respectively, with the final 15% allocated to CONTROL.
Hardware cost included one computer at $1,408.26. This price was annuitized over the 5-year study period. We assumed a 5% discount rate, with a 10% scrap value after a 5-year useful life. Hardware cost did not differ based on treatment group and was transformed into a total price per treatment group (based on number of participants in each group). Software included accounts required for cloud hosting, registration and renewal of domain name, and SSL certificate. These costs were averaged per participant per time point, accounting for group assignment differences.
Statistical analysis
Cost-effectiveness ratios
Microsoft Excel 2021 (Microsoft) was used to calculate the cost measures. The average cost effectiveness ratio (ACER) was calculated by dividing the average cost by the average heath benefit within each treatment arm. The incremental cost effectiveness ratio (ICER) was calculated by dividing the incremental cost to implement the TAILORED versus the TARGETED treatment by the incremental health benefit. In our case, the health benefit is operationalized as unadjusted weight change (kg), abdominal circumference (cm), and individual cardiometabolic risk factors: HDL (mg/dL), systolic blood pressure (SBP [mmHg]), diastolic blood pressure (DBP [mmHg]), and HbA1c (%).
Cost-effectiveness may depend on the size of a university. A sensitivity analysis therefore was conducted based on a small (< 5,000), medium (5,000–15,000), or large (> 15,000) student body size and assumed that 10% of students among the respective university sizes would take part in the program. This resulted in sample sizes of 95 (small), 380 (medium), and 570 (large) for each university size-type.
RESULTS
The final analytic sample included 150 TAILORED and 152 TARGETED participants. Participants (23.5 ± 4.4 years) were 80.5% female and 48.3% non-Hispanic White, with an average BMI of 31.3 ± 4.5 kg/m2. Significant (unadjusted) weight loss at 6-months was achieved in the TAILORED intervention arm (−0.98 [−1.76, −0.20] kg), but not in the TARGETED arm [22]. However, abdominal circumference was significantly reduced in both arms (TAILORED: −1.98 [−2.87, −1.09] cm; TARGETED: −1.30 [−2.19, −0.41] cm). Systolic blood pressure significantly decreased among TAILORED only (−1.86 [−3.35, −0.38] mmHg) and diastolic blood pressure significantly increased among TARGETED only (1.97 [0.58, 3.36] mmHg). HDL-C and HbA1c did not significantly change for either group at 6-months.
When the analyses were stratified by weight status, among participants with overweight (BMI 25–29.9 kg/m2), similar patterns for weight loss and the other cardiometabolic outcomes were observed. Among participants with obesity (BMI ≥ 30 kg/m2), however, there was no difference in weight loss between intervention arms (0 [−1.63, 1.64] kg) and both the TAILORED and TARGETED groups had a significant reduction in abdominal circumference (TAILORED: −1.63 [−3.08, −0.18] cm and TARGETED: −2.01 [−3.34, −0.69] cm). HDL-C increased among the TARGETED group only (2.16 [0.49, 3.82] mg/dL), while blood pressure and HbA1c did not significantly change for either group (Table 1).
Table 1.
Effectiveness measures: 6-month change in cardiometabolic clustering outcomes among Tailored vs. Targeted participants
| Tailored | Targeted | Diff. in change, Tailored–Targeted | |||||
|---|---|---|---|---|---|---|---|
| Baseline mean (N) | 6-month follow-up (N) | Change, baseline to f/up | Baseline mean (N) | 6-month follow-up (N) | Change, baseline to f/up | ||
| (95% CI) | (95% CI) | (95% CI) | |||||
| Full sample (N = 302 | |||||||
| Weight (kg) | 87.12 (150) | 87.05 (110) | −0.98 (−1.76, −0.20) | 86.28 (152) | 84.96 (119) | −0.51 (−1.29, 0.27) | −0.47 (−1.56, 0.63) |
| Ab circ. (cm) | 100.48 (150) | 99.34 (108) | −1.98 (−2.87, −1.09) | 99.09 (152) | 97.26 (117) | −1.30 (−2.19, −0.41) | −0.68 (−1.93, 0.57) |
| HDL (mg/dL) | 50.26 (140) | 49.63 (94) | 0.66 (−0.92, 2.24) | 48.06 (141) | 48.80 (104) | 0.62 (−0.67, 1.92) | 0.04 (−2.17, 1.78) |
| SBP (mmHg) | 114.25 (150) | 113.23 (106) | −1.86 (−3.35, −0.38) | 113.72 (152) | 113.85 (115) | −0.35 (−1.89, 1.18) | −1.51 (−3.63, 0.61) |
| DBP (mmHg) | 72.39 (150) | 73.73 (107) | 1.07 (−0.40, 2.54) | 72.98 (152) | 74.90 (116) | 1.97 (0.58, 3.36) | −0.90 (−2.91, 1.11) |
| HbA1c (%) | 5.34 (134) | 5.24 (95) | −0.08 (−0.18, 0.01) | 5.28 (136) | 5.29 (101) | −0.03 (−0.13, 0.07) | −0.05 (−0.18, 0.08) |
| Sample with BMI 25–30 kg/m2 (N = 147 | |||||||
| Weight (kg) | 76.36 (72) | 74.85 (52) | −1.54 (−2.49, −0.59) | 77.28 (74) | 77.04 (59) | 0.50 (−1.62, 0.63) | 1.04 (−2.51, 0.41) |
| Ab circ. (cm) | 92.65 (72) | 90.17 (51) | −2.37 (−3.36, −1.38) | 92.16 (74) | 91.32 (58) | −0.55 (−1.76, 0.66) | −1.82 (−3.37, −0.28) |
| HDL (mg/dL) | 52.59 (70) | 53.38 (45) | 1.56 (−0.75, 3.86) | 49.32 (72) | 48.81 (53) | −0.17 (−1.94, 1.59) | 1.73 (−1.14, 4.60) |
| SBP (mmHg) | 112.73 (72) | 112.57 (50) | −1.91 (−3.68, −0.13) | 113.57 (74) | 112.93 (56) | −1.16 (−3.32, 1.00) | −0.75 (−3.51, 2.01) |
| DBP (mmHg) | 70.23 (72) | 72.28 (50) | 1.10 (−0.83, 3.02) | 71.14 (74) | 74.13 (57) | 3.04 (1.11, 4.95) | −1.94 (−4.62, 0.75) |
| HbA1c (%) | 5.33 (67) | 5.18 (48) | −0.06 (−0.20, 0.07) | 5.23 (67) | 5.23 (49) | −0.02 (−0.16, 0.13) | −0.04 (−0.25, 0.15) |
| Sample with BMI > 30 kg/m2 (N = 155 | |||||||
| Weight (kg) | 97.05 (78) | 97.99 (58) | −0.47 (−1.69, 0.75) | 95.21 (77) | 93.28 (59) | −0.47 (−1.59, 0.64) | 0 (−1.63, 1.64) |
| Ab circ. (cm) | 107.71 (78) | 107.54 (57) | −1.63 (−3.08, −0.18) | 105.97 (77) | 103.52 (58) | −2.01 (−3.34, −0.69) | 0.38 (−1.56, 2.33) |
| HDL (mg/dL) | 46.89 (70) | 45.76 (50) | −0.18 (−2.36, 2.01) | 46.23 (69) | 49.21 (48) | 2.16 (0.49, 3.82) | −2.34 (−5.05, 0.37) |
| SBP (mmHg) | 115.65 (78) | 113.82 (56) | −1.82 (−4.20, 0.56) | 113.80 (77) | 114.78 (58) | 0.54 (−1.71, 2.78) | −2.36 (−1.82, 0.54) |
| DBP (mmHg) | 74.38 (78) | 75.00 (57) | 1.04 (−1.20, 3.28) | 74.75 (77) | 75.57 (58) | 0.84 (−1.20, 2.88) | 0.20 (−2.80, 3.20) |
| HbA1c (%) | 5.41 (67) | 5.31 (47) | −0.10 (−0.24, 0.04) | 5.33 (68) | 5.36 (51) | −0.04 (−0.18, 0.09) | −0.06 (−0.25, 0.13) |
95% CI are unadjusted. Significant confidence intervals are shown in boldface.
BMI Body Mass Index; Ab circ abdominal circumference; SBP systolic blood pressure; DBP diastolic blood pressure.
Costs
The total payer cost for the TAILORED arm at month 6 was $15,849.78 for an average monthly cost per participant of $17.61, and 6-month cost per participant of $105.66 (Table 2). The total payer cost for the TARGETED arm at month 6 was $13,895.34 for an average monthly cost per participant of $15.24 and 6-month cost of $91.44. The average monthly incremental cost per participant of the TAILORED arm relative to the TARGETED arm was $2.37.
The average cost effectiveness ratio (ACER) over 6-months for weight loss in the TAILORED arm was $107.82, and for the TARGETED arm was $179.29. In the TAILORED intervention arm, the ACERs for abdominal circumference, HDL-C, systolic and diastolic blood pressure, and HbA1c were $53.36, $160.09, $56.81, $−98.75, and $1,320.75 per unit change, respectively. In the TARGETED arm the ACERs for abdominal circumference, HDL, systolic and diastolic blood pressure, and HbA1c were $70.34, $147.48, $261.26, $−46.42, and $3,048.00 per unit change, respectively (Table 3).
Table 3.
Average cost effectiveness ratios for TAILORED and TARGETED intervention arms
| Intervention arm | Health outcome | Monthly cost per participant ($) Mean |
Cost per participant at 6M ($) Mean |
Monthly effectiveness per participant Mean (95% CI) |
ACER ($) Mean |
|---|---|---|---|---|---|
| TAILORED | Weight (kg) | 17.61 | 105.66 | 0.98 (0.20, 1.76) | 107.82 |
| Abdominal circ. (cm) | 1.98 (1.09, 2.87) | 53.36 | |||
| HDL (mg/dL) | 0.66 (−0.92, 2.24) | 160.09 | |||
| SBP (mmHg) | 1.86 (0.38, 3.35) | 56.81 | |||
| DBP (mmHg) | −1.07 (−2.54, 0.40) | −98.75 | |||
| HbA1c (%) | 0.08 (−0.01, 0.18) | 1320.75 | |||
| TARGETED | Weight (kg) | 15.24 | 91.44 | 0.51 (−0.27, 1.29) | 179.29 |
| Abdominal circ. (cm) | 1.30 (0.41, 2.19) | 70.34 | |||
| HDL (mg/dL) | 0.62 (−0.67, 1.92) | 147.48 | |||
| SBP (mmHg) | 0.35 (−1.18, 1.89) | 261.26 | |||
| DBP (mmHg) | −1.97 (−3.36, 0.58) | −46.42 | |||
| HbA1c (%) | 0.03 (−0.07, 0.13) | 3048.00 |
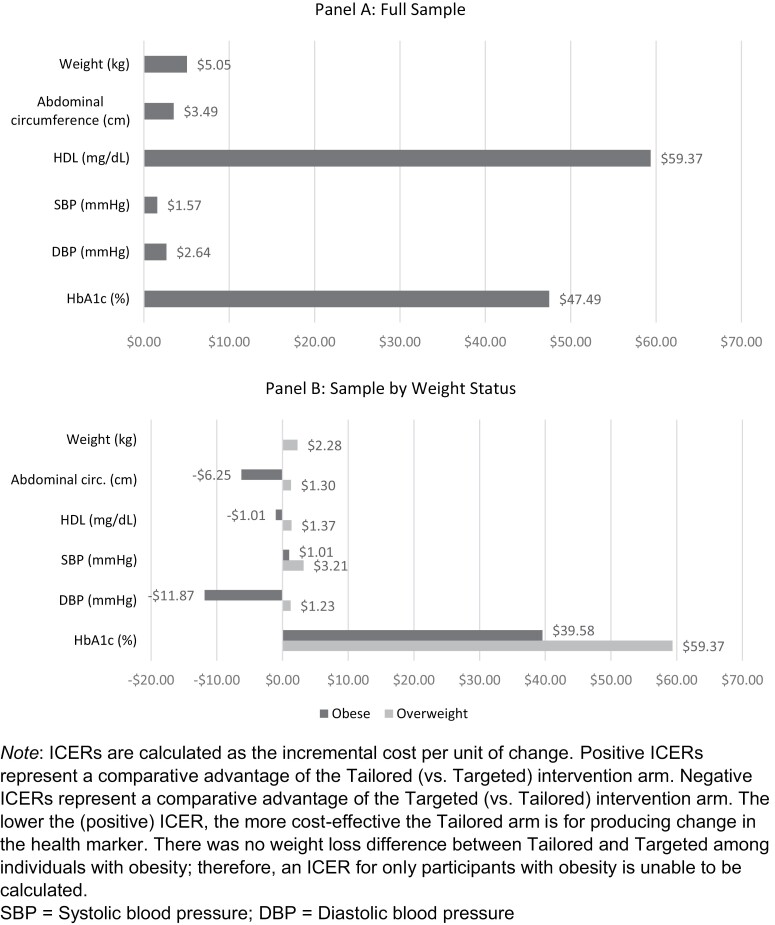
For the full analytic sample, the incremental cost effectiveness ratio (ICER) over 6 months for weight loss in the TAILORED versus TARGETED intervention arm was $5.05 per kg lost. For abdominal circumference, HDL-C, systolic and diastolic blood pressure, and HbA1c, the ICERs were $3.49, $59.37, $1.57, $2.64, and $47.49 per unit change, respectively (Fig. 1A). Among participants who had overweight, the ICER for weight loss in the TAILORED versus TARGETED intervention arm was even lower than that for the full sample ($2.28 per kg lost). The ICERs for abdominal circumference, HDL-C, systolic and diastolic blood pressure, and HbA1c were $1.30, $1.37, $3.21 $1.23, and $59.37 per unit change, respectively (Fig. 1B). Among participants who had obesity, the ICER for weight loss in the TAILORED versus TARGETED arms could not be calculated because there was no difference in weight loss. The ICERs for abdominal circumference, HDL-C, systolic and diastolic blood pressure, and HbA1c were −$6.25, −$1.01, $1.01, −$11.87, and $39.58 per unit change, respectively. Negative values indicate that the TARGETED intervention outperformed the TAILORED arm for those outcomes.
Fig 1.
Incremental cost-effectiveness ratios (ICERs) for Tailored (vs. Targeted) intervention arm participants for the full sample (panel A) and by weight status (panel B). ICERs are calculated as the incremental cost per unit of change. Positive ICERs represent a comparative advantage of the Tailored (vs. Targeted) intervention arm. Negative ICERs represent a comparative advantage of the Targeted (vs. Tailored) intervention arm. The lower the (positive) ICER, the more cost-effective the Tailored arm is for producing change in the health marker. There was no weight loss difference between Tailored and Targeted among individuals with obesity; therefore, an ICER for only participants with obesity is unable to be calculated. SBP systolic blood pressure; DBP diastolic blood pressure.
Sensitivity analyses
As university size (i.e., number of program participants) increased, both the ICER and ACER for each health outcome decreased (Table 4). For example, at a small university where it is estimated that 95 students would participate in such a program, the ICER for weight loss is $6.15 (TARGETED ACER = $256.24). At a medium-sized university (N = 380) the ICER for weight loss is $3.27 (TARGETED ACER = $102.35), and at a large university (N = 570) the ICER for weight loss is $2.95 (TARGETED ACER = $85.18). These findings indicate that an intervention such as ours becomes more cost effective as the size of the student population within a university becomes larger.
Table 4.
Sensitivity analyses for university student population size
| ICER | ACER (TAILORED) |
ACER (TARGETED) |
|
|---|---|---|---|
| Weight (kg) | |||
| HBHU Sample | 5.05 | 107.82 | 179.29 |
| Small university | 6.15 | 151.04 | 256.24 |
| Medium university | 3.27 | 62.63 | 102.35 |
| Large university | 2.95 | 52.84 | 85.18 |
| Abdominal circumference reduction (cm) | |||
| HBHU Sample | 3.49 | 53.36 | 70.34 |
| Small university | 4.25 | 74.76 | 100.52 |
| Medium university | 2.26 | 31.00 | 40.15 |
| Large university | 2.04 | 26.15 | 33.42 |
| HDL increase (mg/dL) | |||
| HBHU Sample | 59.37 | 160.09 | 147.48 |
| Small university | 72.33 | 224.27 | 210.77 |
| Medium university | 38.39 | 93.00 | 84.19 |
| Large university | 34.62 | 78.84 | 70.06 |
| SBP (mmHg) | |||
| HBHU Sample | 1.57 | 56.81 | 261.26 |
| Small university | 1.92 | 79.58 | 373.37 |
| Medium university | 1.02 | 33.00 | 149.14 |
| Large university | 0.92 | 27.84 | 124.11 |
| DBP (mmHg) | |||
| HBHU Sample | 2.64 | −98.75 | −46.42 |
| Small university | 3.21 | 138.34 | −66.34 |
| Medium university | 1.71 | −57.36 | −26.50 |
| Large university | 1.54 | −48.39 | −23.23 |
| HbA1c reduction (%) | |||
| HBHU Sample | 47.49 | 1320.75 | 3048.00 |
| Small university | 57.86 | 1850.25 | 4356.00 |
| Medium university | 30.71 | 767.25 | 1740.00 |
| Large university | 27.70 | 647.25 | 1448.00 |
Table presents sensitivity analyses per variations in the number of participants estimated to enroll at a small (N = 95/arm), medium (N = 380/arm), or large (N = 570/arm) university.
ICER incremental cost effectiveness ratio (incremental cost [$]/unit change in outcome); SBP systolic blood pressure; DBP diastolic blood pressure.
DISCUSSION
Our digitally-delivered personalized (i.e., TAILORED) intervention resulted in significant changes over 6 months in body weight [22] among the full sample, as well as for those who were overweight. Furthermore, those in the TAILORED intervention arm experienced significant improvements in abdominal circumference and systolic blood pressure, and this was true among the full sample and in those who were overweight. For those with obesity, the TAILORED intervention resulted in significant reductions in abdominal circumference only. Thus, it appears that the more personalized messaging delivered via the TAILORED treatment was necessary for reducing body weight and systolic blood pressure, but only in those without obesity. On the other hand, even the content-only (TARGETED) intervention was sufficient in significantly reducing abdominal circumference, regardless of weight status. Excess abdominal adiposity is a significant risk factor of cardiovascular disease and mortality [30]. Data from the CARDIA study demonstrate that early onset of abdominal adiposity in young adulthood is linked to increased risk of type 2 diabetes in middle-to-late adulthood. In fact, each year with abdominal adiposity was associated with a 4% increase in diabetes risk [31]. Lifestyle interventions, such as diet and physical activity can help in the management of abdominal adiposity [32, 33]. One meta-analysis concluded that internet-based interventions resulted in an average decrease in abdominal circumference of 2.99 cm compared with 0.81 cm for minimal interventions (e.g., information only) [34]. We observed significant reductions in abdominal circumference ranging from 1.63 to 2.37 cm in the TAILORED arm and from 1.30 to 2.01 cm in the TARGETED arms, which are within the range highlighted in the meta-analysis [34]. For other risk factors such as body weight, blood pressure, and glucose regulation, young adults with obesity may require even more intensive interventions [22].
The magnitude of weight loss necessary to induce improvements in other cardiometabolic risk factors is an important factor for payers. Among middle-aged adults, weight loss thresholds as low as 2% are associated with improvements in systolic blood pressure, HbA1c, glucose, and triglycerides [35]. In the current study, participants in the TAILORED arm experienced a 6-month weight loss of 1 kg (1.1%) and 1.5 kg (2.0%) for the full sample and those with overweight, respectively. A meta-analysis examining the effects of weight reduction blood pressure changes indicated that each kilogram of weight lost resulted in an average systolic blood pressure drop of −1.05 mmHg [36]. Our observed decreases of −1.86 mmHg for the full sample and −2.37 mmHg for those with overweight are comparable to these findings, suggesting that our TAILORED intervention (delivered digitally and at a dose likely comparable to other types of interventions) is favorable for risk reduction.
We observed no significant changes in HbA1c, diastolic blood pressure, or HDL-C in the current study. Markers of cardiometabolic disease manifest differently among younger adults compared with their older counterparts, as younger adults tend to have a higher prevalence of HDL-C [37, 38] and triglycerides [37] dysregulation and a lower prevalence of hyperglycemia and hypertension [38]. At baseline, 19% of this cohort had HbA1c concentrations ≥ 5.7%, 11% had isolated diastolic hypertension, and 47% for HDL-C concentrations < 40 mg/dL (men) or < 50 mg/dL (women) [39]. It is possible that early display of these cardiometabolic markers might indicate more physiological resistance to treatment and the need for more intensive interventions, relative to middle- or older-aged adults.
The cost of delivering the interventions in the HBHU study is comparable to that of similar trials. For example, Krukowski and colleagues [20] reported a cost of $373 per participant for an internet-based intervention, with the difference in cost relative to our digital interventions mainly attributed to the personnel time needed to conduct online groups. Daumit et al. [27]. reported a cost of $101 per participant study for a remote intervention, which is similar to our 6-month costs of $105.66 per participant and $91.44 per participant for the TAILORED and TARGETED arms, respectively. These same authors observed a cost-benefit of $99 per each kg lost [27], compared with our estimate of about $108 per kg of lost weight within the TAILORED intervention. Moreover, the average monthly cost per participant in the TAILORED treatment arm was approximately $18, which is comparable to the costs of commercially-available programs such as Noom and WWI (about $10 to $40 per month) [40, 41].
Although the TAILORED intervention was more costly to deliver ($105.66) than the TARGETED arm ($91.44), when examining the average cost-benefit ratio, the TAILORED arm was more cost-effective over 6 months with regard to weight loss ($107.82 per kg vs. $179.29 per kg for the TAILORED and TARGETED arms, respectively). An older study by Finkelstein and Kruger [42], reported an ACER of $155 per kg (annualized) for a commercial program (Weight Watchers) vs. $232 for a pharmaceutical intervention.
Sensitivity analyses were conducted to provide an estimate for a payer (University) regarding the cost per unit change for each of the cardiometabolic outcomes examined. For example, if the 6-month TAILORED intervention were delivered to 10% of the students from a large sized university (> 15,000 students), it would cost an additional $2.95 per student to produce a 1 kg reduction in body weight compared with the TARGETED intervention, and this is relative to other programmatic health needs of students in which universities invest. For example, more than 1100 colleges and universities have required completion of programs targeting alcohol (i.e., Electronic Check-up to Go [e-CHUG] [43] and AlcoholEdu) [44], with an average cost of approximately $2.60 per student [45, 46]. Universities have prioritized alcohol and substance use reduction programs, with investments of $2 per student being returned in terms of reductions in health costs, injuries, and death [47]. A call-to-action for universities is to also invest in weight loss programming for similar cost returns. This is particularly important, given that BMI and academic performance are negatively correlated [48–50], indicating that universities can benefit student learning outcomes by implementing weight management programs.
Few studies in behavioral medicine report on the cost-effectiveness of interventions [51]. This study was the first to compare the cost of weight loss interventions delivered via the same modalities but differing in intensity and personalization. The strengths of this study include the randomized controlled trial design, a large racially diverse young adult sample recruited from two university sites, as well as the measurement of several cardiometabolic outcomes. We also note the limitations to this work. First, we did not include participant costs in the analysis, as our focus was on the university payer. Given that the TAILORED intervention required more of the participant’s time in terms of self-monitoring and reviewing personalized feedback, it may be more costly when the participant time is factored into the estimates. Further, those who were more engaged [52] lost more weight and participant time was not accounted for relative to engagement. Additionally, it is difficult to compare the costs of the current intervention to previous investigations given different implementation years, and methods. Finally, the analysis does not disentangle diet and physical activity in relation to changes in cardiometabolic outcomes or the costs of engaging in physical activity or eating a healthier diet.
Nonetheless, our findings have important implications for student health practice and implementation. Cardiovascular health in early adulthood is related to reduced costs and health care utilization later in life [53], suggesting the importance of early intervention to address excess body weight and cardiovascular derangements in young adulthood. Tailored, personalized programs may be especially necessary for weight loss and reversal of early cardiometabolic impairments. These personalized interventions can also help to identify the drivers of weight loss success and maintenance, although further investigation and resources may be needed for those young adults with obesity. As universities are examining student health and overall wellness programming, the investment of five additional dollars per student over a 6-month period in order to deliver a personalized program resulting in weight loss and improved cardiometabolic biomarkers seems like a sound return on investment.
Contributor Information
Melissa A Napolitano, Department of Prevention and Community Health, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA; Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Caitlin P Bailey, Department of Prevention and Community Health, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Meghan N Mavredes, Department of Prevention and Community Health, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Charles J Neighbors, Department of Population Health, Grossman School of Medicine, New York University, New York, NY, USA.
Jessica A Whiteley, Departmen of Exercise and Health Sciences, College of Nursing and Health Sciences, The University of Massachusetts at Boston, Boston, MA, USA.
Michael W Long, Department of Prevention and Community Health, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Laura L Hayman, Department of Nursing, College of Nursing and Health Sciences, The University of Massachusetts at Boston, Boston, MA, USA.
Steven K Malin, Department of Kinesiology and Division of Endocrinology, Metabolism and Nutrition, Rutgers University, New Brunswick, NJ, USA.
Loretta DiPietro, Department of Exercise and Nutrition Sciences, Milken Institute School of Public Health, The George Washington University, Washington, DC, USA.
Funding
Research reported in this manuscript was supported by National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under award number R01DK100916 to MA Napolitano. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Compliance with Ethical Standards
Conflicts of interest: The authors declare no conflicts of interest.
Ethics approval: The study was reviewed and approved by the Institutional Review Boards of The George Washington University (#121325) and the University of Massachusetts Boston (#2014046).
Consent to participate: Informed consent was obtained from all individual participants included in the study.
Author Contributions: MAN, MNM, CJN, JAW, LLH, and LDP conceptualized the study. MAN, MNM, JAW, and LDP collected the data. MAN, CPB, and MNM conducted the analyses. MAN, CPB, and MNM drafted the manuscript. CJN, JAW, MWL, LLH, SKM, and LDP provided consultation on aspects of study design, data collection, analysis, and reporting. All authors reviewed and approved the final manuscript.
Transparency Statements: Study registration: The study was registered at Clinical Trials.gov https://clinicaltrials.gov/ct2/show/NCT02342912. Analytic plan pre-registration: While the analysis plan was pre-specified by the Investigative team, the analysis plan was not formally pre-registered in a central repository. Data availability: De-identified data from this study are not available in a public archive. De-identified data that support the findings of this study will be made available (as allowable according to institutional IRB standards) 6 months following publication for 5 years to researchers submitting a specific request and data sharing agreement to the corresponding author. Analytic code availability: Analytic code used to conduct the analyses presented in this study are not available in a public archive. They may be available by emailing the corresponding author. Materials availability: Some of the materials used to conduct the study including intervention protocols, and examples of intervention content are available in [22].
REFERENCES
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL.. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. NCHS Data Brief. 2020; 2020(360):1–8. [PubMed] [Google Scholar]
- 2. World Health Organization. Obesity and Overweight. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. Accessed November 11, 2020. [Google Scholar]
- 3. Centers for Disease Control and Prevention. Leading Causes of Death; 2021. https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm. Accessibility verified July 28, 2021. [Google Scholar]
- 4. Nolan PB, Carrick-Ranson G, Stinear JW, Reading SA, Dalleck LC.. Prevalence of metabolic syndrome and metabolic syndrome components in young adults: a pooled analysis. Prev Med Rep. 2017; 7:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koroukian SM, Dong W, Berger NA.. Changes in age distribution of obesity-associated cancers. JAMA Netw Open. 2019; 2(8):e199261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Q, Blume SW, Huang JC, Hammer M, Ganz ML.. Prevalence and healthcare costs of obesity-related comorbidities: evidence from an electronic medical records system in the United States. J Med Econ. 2015; 18(12):1020–1028. [DOI] [PubMed] [Google Scholar]
- 7. Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Am Psychol. 2000; 55(5):469–480. [PubMed] [Google Scholar]
- 8. Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA.. Emerging adulthood and college-aged youth: AN overlooked age for weight-related behavior change. Obesity. 2008; 16(10):2205–2211. [DOI] [PubMed] [Google Scholar]
- 9. Bailey CP, Sharma S, Economos CD, Hennessy E, Simon C, Hatfield DP.. College campuses’ influence on student weight and related behaviours: a review of observational and intervention research. Obes Sci Pract. 2020; 6(6):694–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Center for Education Statistics. Total Fall Enrollment in Degree-Granting Postsecondary Institutions, by Attendance Status, Sex of Student, and Control of Institution: Selected Years, 1947 Through 2023. U.S. Department of Commerce, Census Bureau, American Community Survey Web site; 2019. https://nces.ed.gov/programs/digest/d19/tables/dt19_303.10.asp Accessed verified October 10, 2022. [Google Scholar]
- 11. National Center for Education Statistics. Postbaccalaureate Enrollment. U.S. Department of Education. Postsecondary Education Web site; 2021. https://nces.ed.gov/programs/coe/indicator/chb. Access verified August, 5, 2021. [Google Scholar]
- 12. Lynch S, Hayes S, Napolitano M, Hufnagel K.. Availability and accessibility of student-specific weight loss programs and other risk prevention health services on college campuses. JMIR Public Health Surveill. 2016; 2(1):e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bennett M, Whiteley JA, Gu J, Gaminian A, Napolitano MA.. A qualitative investigation of the need for and feasibility of weight loss programs on university campuses. Obes Res Clin Pract. 2022; 16(1):72–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Willmott TJ, Pang B, Rundle-Thiele S, Badejo A.. Weight management in young adults: systematic review of electronic health intervention components and outcomes. J Med Internet Res. 2019; 21(2):e10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rumbo-Rodríguez L, Sánchez-SanSegundo M, Ruiz-Robledillo N, Albaladejo-Blázquez N, Ferrer-Cascales R, Zaragoza-Martí A.. Use of technology-based interventions in the treatment of patients with overweight and obesity: a systematic review. Nutrients. 2020; 12(12):3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pew Research Center. Internet/Broadband Fact Sheet; 2021. https://www.pewresearch.org/internet/fact-sheet/internet-broadband/. Accessibility verified August 5, 2021. [Google Scholar]
- 17. Pew Research Center. About Three-in-Ten U.S. Adults Say They Are “Almost Constantly” Online; 2021. https://www.pewresearch.org/fact-tank/2021/03/26/about-three-in-ten-u-s-adults-say-they-are-almost-constantly-online/. Accessibility verified August 5, 2021. [Google Scholar]
- 18. Sevick MA, Napolitano MA, Papandonatos GD, Gordon AJ, Reiser LM, Marcus BH.. Cost-effectiveness of alternative approaches for motivating activity in sedentary adults: results of Project STRIDE. Prev Med. 2007; 45(1):54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis BA, Williams DM, Neighbors CJ, Jakicic JM, Marcus BH.. Cost analysis of Internet vs. print interventions for physical activity promotion. Psychol Sport Exerc. 2010; 11(3):246–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Krukowski RA, Tilford JM, Harvey-Berino J, West DS.. Comparing behavioral weight loss modalities: incremental cost-effectiveness of an internet-based versus an in-person condition. Obesity. 2011; 19(8):1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Finkelstein EA, Khavjou O, Will JC.. Cost-effectiveness of WISEWOMAN, a program aimed at reducing heart disease risk among low-income women. J Womens Health. 2006; 15(4):379–389. [DOI] [PubMed] [Google Scholar]
- 22. Napolitano MA, Whiteley JA, Mavredes M, et al. Effect of tailoring on weight loss among young adults receiving digital interventions: an 18 month randomized controlled trial. Transl Behav Med. 2021; 11(4):970–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Napolitano MA, Whiteley JA, Mavredes MN, et al. Using social media to deliver weight loss programming to young adults: Design and rationale for the Healthy Body Healthy U (HBHU) trial. Contemp Clin Trials. 2017; 60:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Whiteley JA, Faro JM, Mavredes M, et al. Application of social marketing to recruitment for a digital weight management intervention for young adults, Transl Behav Med. 2021; 11(2):484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. The Diabetes Prevention Program Research Group. The diabetes prevention program (DPP): description of lifestyle intervention, Diabetes Care. 2002; 25(12):2165–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. The Diabetes Prevention Program Research Group. The Diabetes Prevention Program, Design and methods for a clinical trial in the prevention of type 2 diabetes, Diabetes Care. 1999; 22(4):623–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Daumit GL, Janssen EM, Jerome GJ, et al. Cost of behavioral weight loss programs implemented in clinical practice: the POWER trial at Johns Hopkins. Transl Behav Med. 2020; 10(1):103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tate DF, Finkelstein EA, Khavjou O, Gustafson A.. Cost effectiveness of internet interventions: review and recommendations. Ann Behav Med. 2009; 38(1):40–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Offices.net. Washington DC Office Market: 2016 Forecast; 2016. https://offices.net/news/washington-dc-office-market-2016-forecast/. Accessibility verified August 5, 2021. [Google Scholar]
- 30. Zhang C, Rexrode KM, van Dam RM, Li TY, Hu FB.. Abdominal obesity and the risk of all-cause, cardiovascular, and cancer mortality: sixteen years of follow-up in US women. Circulation. 2008; 117(13):1658–1667. [DOI] [PubMed] [Google Scholar]
- 31. Reis JP, Hankinson AL, Loria CM, et al. Duration of abdominal obesity beginning in young adulthood and incident diabetes through middle age: the CARDIA study. Diabetes Care. 2013; 36(5):1241–1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Paley CA, Johnson MI.. Abdominal obesity and metabolic syndrome: exercise as medicine? BMC Sports Sci Med Rehabil. 2018; 10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Romaguera D, Norat T, Mouw T, et al. Adherence to the Mediterranean diet is associated with lower abdominal adiposity in European men and women. J Nutr. 2009; 139(9):1728–1737. [DOI] [PubMed] [Google Scholar]
- 34. Seo DC, Niu J.. Evaluation of internet-based interventions on waist circumference reduction: a meta-analysis. J Med Internet Res. 2015; 17(7):e181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011; 34(7):1481–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM.. Influence of weight reduction on blood pressure: a meta-analysis of randomized controlled trials. Hypertension. 2003; 42(5):878–884. [DOI] [PubMed] [Google Scholar]
- 37. Devers MC, Campbell S, Simmons D.. Influence of age on the prevalence and components of the metabolic syndrome and the association with cardiovascular disease. BMJ Open Diabetes Res Care. 2016; 4(1):e000195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sumner AD, Sardi GL, Reed IJ.. Components of the metabolic syndrome differ between young and old adults in the US population. J Clin Hypertens. 2012; 14(8):502–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dipietro L, Zhang Y, Mavredes M, et al. Physical activity and cardiometabolic risk factor clustering in young adults with obesity. Med Sci Sports Exerc. 2020; 52(5):1050–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Noom. Noom membership options. https://www.noom.com/blog/how-much-does-noom-cost/?msclkid=5f80aebe0a0417ced198cdfa3c51d314&upv=3&sp=microsoft&utm_source=o&utm_medium=paidsearch&utm_campaign=267504658&utm_content=&utm_term=kwd-71812267178355:loc-190|noom&gid=1148990098158091|7284312581953&type=branded|intent|e&cid=&pos=&step=pros&lang=en&device=c&group=brand-intent&msclkid=5f80aebe0a0417ced198cdfa3c51d314. 2022. Accessed April, 4, 2022. [Google Scholar]
- 41. WW International. weightwatchers.com; 2022. https://www.weightwatchers.com/us/. Access verified April 4, 2022. [Google Scholar]
- 42. Finkelstein EA, Kruger E.. Meta- and cost-effectiveness analysis of commercial weight loss strategies. Obesity. 2014; 22(9):1942–1951. [DOI] [PubMed] [Google Scholar]
- 43. Walters ST, Vader AM, Harris TR.. A controlled trial of web-based feedback for heavy drinking college students. Prev Sci. 2007; 8(1):83–88. [DOI] [PubMed] [Google Scholar]
- 44. Carey KB, Carey MP, Henson JM, Maisto SA, DeMartini KS.. Brief alcohol interventions for mandated college students: comparison of face-to-face counseling and computer-delivered interventions. Addiction. 2011; 106(3):528–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chesley K. Success of AlcoholEdu Results in Four More Years of Funding. Stanford Report; 2010. https://news.stanford.edu/news/2010/july/alcohol-education-survey-070710.html. Accessibility verified. [Google Scholar]
- 46. Stanford University. Common Data Set 2010–2011. Stanford University; 2010. [Google Scholar]
- 47. Ramsey E. Economies of Prevention: The Financial Impact of Alcohol Use on Colleges and Universities. Partners in Prevention; 2013. [Google Scholar]
- 48. Brignac A, Bellar D, Judge LW, et al. The relationship of BMI to grade point average, age and multiple fitness tests. J Strength Cond Res. 2011; 25(4):S121. [Google Scholar]
- 49. Aimé A, Villatte A, Cyr C, Marcotte D.. Can weight predict academic performance in college students? An analysis of college women’s self-efficacy, absenteeism, and depressive symptoms as mediators. J Am Coll Health. 2017; 65(3):168–176. [DOI] [PubMed] [Google Scholar]
- 50. Anderson AS, Good DJ.. Increased body weight affects academic performance in university students. Prev Med Rep. 2016; 5:220–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kaplan RM, Gold M, Duffy SQ, et al. Economic analysis in behavioral health: toward application of standardized methodologies. Health Psychol. 2019; 38(8):672–679. [DOI] [PubMed] [Google Scholar]
- 52. Whiteley JA, Tjaden AH, Bailey CP, et al. Engagement with digital weight loss intervention components and weight outcomes. In review.
- 53. Schiman C, Liu L, Shih YT, et al. Cardiovascular health in young and middle adulthood and medical care utilization and costs at older age—The Chicago Heart Association Detection Project Industry (CHA). Prev Med. 2019; 119:87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]