Abstract
Carotenoid cleavage oxygenases (CCOs) are key enzymes that function in degrading carotenoids into a variety of apocarotenoids and some other compounds. In this study, we performed genome-wide identification and characterization analysis of CCO genes in Cerasus humilis. Totally, nine CCO genes could be classified into six subfamilies, including carotenoid cleavage dioxygenase 1 (CCD1), CCD4, CCD7, CCD8, CCD-like and nine-cis-epoxycarotenoid dioxygenase (NCED), were identified. Results of gene expression analysis showed that ChCCOs exhibited diverse expression patterns in different organs and in fruits at different ripening stages. To investigate the roles of ChCCOs in carotenoids degradation, enzyme assays of the ChCCD1 and ChCCD4 were performed in Escerichia coli BL21(DE3) that can accumulate lycopene, β-carotene and zeaxanthin. The prokaryotic expressed ChCCD1 resulted in obvious degradation of lycopene, β-carotene and zeaxanthin, but ChCCD4 did not show similar functions. To further determine the cleaved volatile apocarotenoids of these two proteins, headspace gas chromatography/mass spectrometer analysis was performed. Results showed that ChCCD1 could cleave lycopene at 5, 6 and 5′, 6′ positions to produce 6-methy-5-hepten-2-one and could catalyze β-carotene at 9, 10 and 9′, 10′ positions to generate β-ionone. Our study will be helpful for clarifying the roles of CCO genes especially ChCCD1 in regulating carotenoid degradation and apocarotenoid production in C. humilis.
Keywords: Cerasus humilis, carotenoid cleavage oxygenase, carotenoids, apocarotenoids, functional analysis
1. Introduction
Carotenoids, a subgroup of isoprenoids that typically contain 40 carbons and abundant conjugated double bounds, are the most conspicuous pigments and the most widely distributed secondary metabolites in plants [1,2]. They play vital roles in photosystem assembly, light harvesting and photoprotection and contribute greatly to the pigmentation, scents and flavors formation and stress responses of plants [1]. Carotenoids are important precursors of a large number of apocarotenoids and some other compounds, such as geranial, α-ionone, β-carotene, geranylacetone, farnesylacetone, pseudoionone, abscisic acids (ABA) and strigolactones (SL) [3]. It is noteworthy that these carotenoid-derived apocarotenoids have been proven to contribute greatly to diverse plant biological processes by acting as pigments, volatiles, signals and phytohormones, and so on [2].
Carotenoids can be cleaved into apocarotenoids by carotenoid cleavage oxygenases (CCOs), lipoxygenases, peroxidases and reactive oxygen species [2]. Among them, CCO-mediated carotenoid degradation is the main focus of attention for carotenoid degradation research. Depending on whether their substrates are epoxidized, CCOs can be divided into nine-cis-epoxycarotenoid dioxygenases (NCEDs) and carotenoid cleavage dioxygenases (CCDs) [4]. Ever since the first discovery of a maize CCO gene [5], a large number of plant CCOs have been identified and functionally studied. In the model plant Arabidopsis, the nine AtCCO members are divided into five subfamilies, including CCD1, CCD4, CCD7, CCD8 and NCED [4]. The classifications of CCOs in other plant species are mostly referred to as Arabidopsis or are based on sequence similarities, cleavage property, cleavage sites and substrate accessibility [3,6]. In addition to the five main CCO subfamilies, CCD-like, CCD2 and zaxinone synthase (ZAS) subfamily members were also identified in certain plant species [7,8,9].
Pieces of evidence have revealed that the functions of CCOs from different subfamilies varied a lot. For example, the CCD1, CCD2 and CCD4 subfamily members are reported to be involved in the biosynthesis of aroma-, flavor- and color formation-related apocarotenoids [10]; the CCD7 and CCD8 subfamily members are closely related to the SL biosynthesis [11]; the NCED members play a certain role in the growth and development of plants and are involved in ABA biosynthesis and [4] members from other subfamilies were found to be of diverse roles in the biosynthesis of some other carotenoids-derived compounds [6,12,13]. The functions of CCD1 and CCD4 subfamily members in carotenoid degradation and apocarotenoid production were the most widely studied in higher plants [14,15]. Research has revealed that CCD1 enzymes lack plastid localization peptides and are generally cytoplasmic [6]. Their cleaved products are usually responsible for the volatile formation [16,17], and their roles in controlling β-ionone generation have been reported in many plants [18,19,20,21]. Meanwhile, CCD4 enzymes are plastid-located, and their substrate specificity is generally higher than CCD1s [22]. Citrus CCD4 can cleave β-carotene, β,β-cryptoxanthin and zeaxanthin into apocarotenoids [23]. Grape VvCCD4 can cleave δ-carotene and lycopene to produce α-violonone and geranylacetone, respectively [24]. And Chrysanthemum CmCCD4a can cleave β-carotene into β-ionone, which is important for the flower pigmentation [25].
The Chinese dwarf cherry (Cerasus humilis) is a perennial woody fruit tree native to China [26]. Comparative genomic studies revealed that C. humilis shared a very close relationship with some fruit trees from the Prunus genus, such as P. persia and P. armeniaca [26]. The fruits of C. humilis contain a variety of carotenoids and their derived compounds [27]. Up until now, however, the CCO gene family in C. humilis has not been systematically studied. In this study, whole genome-wide identification and characterization of C. humilis CCO genes was performed. The expression patterns of ChCCO genes in different tissues and organs and in fruits at different ripening stages were studied using transcriptome data and quantitative real-time PCR analysis (qRT-PCR). Moreover, to uncover the functions of ChCCO genes in carotenoids degradation, ChCCD1 and ChCCD4 genes were cloned, inserted into prokaryotic expression vector pET-28a and subjected to enzyme assays in Escerichia coli BL21(DE3) that can accumulate lycopene, β-carotene and zeaxanthin. The results obtained in this study will provide a basis for understanding the roles of CCO genes in regulating carotenoid degradation and apocarotenoid production in C. humilis.
2. Results
2.1. Identification and Characterization of ChCCOs
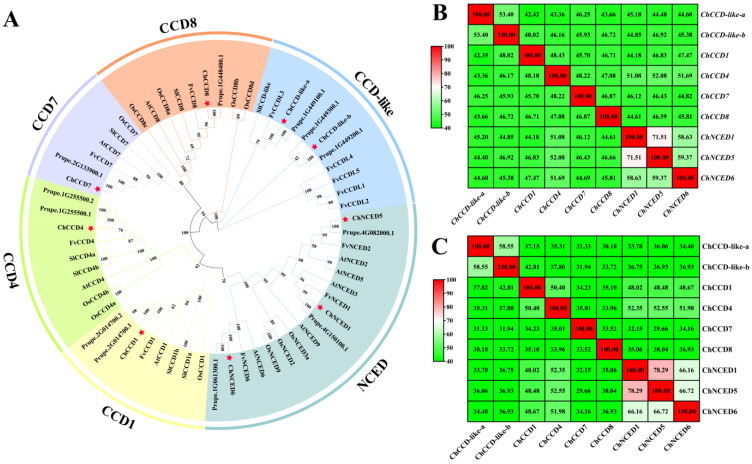
Totally, we identified nine CCOs from the C. humilis. Phylogenetic analysis revealed that they can be classified into six subfamilies, including CCD-like, CCD1, CCD4, CCD7, CCD8 and NCED (Figure 1A). Among these subfamilies, the NCED subfamily consisted of three members (ChNCED1, ChNCED5 and ChNCED6), the CCD-like subfamily contained two members and the other subfamilies each contained only one member. Sequence similarity analysis revealed high similarities among ChNCEDs (Figure 1B). The similarity between ChNCED1 and ChNCED5 was about 71.51%, and their similarities with ChNCED6 were about 58.63% and 59.37%, respectively. Similarities among their encoded proteins ranged from 66.16% to 78.29% (Figure 1C). Moreover, ChCCD-like-a and ChCCD-like-b shared a similarity of about 53.4%, and their encoded proteins shared a similarity of about 58.55%. Plant CCOs usually contained four conserved histidine active sites and three semi-conserved second shell glutamate residues [28]. Consistently, all the ChCCOs contained four conserved histidine and three conserved glutamate residues (Figure S1).
Figure 1.
Phylogenetic analysis results (A) of CCO proteins from Cerasus humilis (Ch), P. persica (Pp), Fragaria vesca (Fv), Solanum Lycopersicum (Sl), Oryza sativa (Os) and Arabidopsis thaliana (At), and nucleotides (B) and proteins (C) similarity analysis results of ChCCOs. CCD1, CCD4, CCD-like, CCD8, CCD7 and NCED are six subfamilies of CCOs. Red stars in A represent C. humilis CCD members. In B and C: the redder the color, the higher the similarity; the greener the color, the lower the similarity.
The coding sequence (CDS) length of ChCCOs ranged from 547 bp (ChCCD1) to 702 bp (ChCCD-like-a). Their encoded proteins consisted of 547~702 amino acids with their molecular weight ranging from 61777.14 Da (ChCCD1) to 78364.26 Da (ChCCD-like-a) and their theoretical isoelectric point (pI) ranging from 5.44 (ChCCD-like-a) to 6.97 (ChNCED6) (Table 1). All the ChCCOs were predicted to be hydrophilic proteins (GRAVY < 0), and, except for ChCCD7, ChNCED1 and ChNCED5 (with instability index > 40), all other ChCCOs were stable proteins (Table 1). Subcellular localization analysis revealed that ChCCOs were mainly located in the cytoplasm and chloroplast.
Table 1.
Physiochemical prosperities and subcellular location analysis results of ChCCOs. Chr: chromosome; CDS: coding sequence; bp: base pair; Chr: chromosome; AA: amino acid; pI: isoelectric point; GRAVY: grand average of hydropathicity.
| Gene Name | Chr | CDS/bp | Number of AA | Molecular Weight/Da | pI | Instability Index | GRAVY | Subcellular Location |
|---|---|---|---|---|---|---|---|---|
| ChCCD-like-a | Chr1 | 2109 | 702 | 78,364.26 | 5.44 | 36.82 | −0.196 | Chloroplast, Cytoplasm |
| ChCCD-like-b | Chr1 | 1677 | 558 | 62,470.54 | 5.49 | 28.40 | −0.292 | Cytoplasm |
| ChCCD1 | Chr5 | 1644 | 547 | 61,777.14 | 6.05 | 34.76 | −0.258 | Cytoplasm |
| ChCCD4 | Chr1 | 1794 | 597 | 65,717.9 | 6.10 | 38.51 | −0.229 | Chloroplast, Cytoplasm |
| ChCCD7 | Chr5 | 1848 | 615 | 68,499.76 | 5.67 | 46.35 | −0.219 | Cytoplasm |
| ChCCD8 | Chr1 | 1698 | 565 | 62,461.97 | 6.20 | 34.98 | −0.270 | Cytoplasm |
| ChNCED1 | Chr3 | 1899 | 632 | 70,288.74 | 6.73 | 45.34 | −0.435 | Chloroplast, Cytoplasm, Nucleus |
| ChNCED5 | Chr3 | 1854 | 617 | 68,027.19 | 6.45 | 46.67 | −0.335 | Cytoplasm |
| ChNCED6 | Chr1 | 1818 | 605 | 66,921.84 | 6.97 | 38.59 | −0.287 | Chloroplast |
2.2. Chromosome Location and Synteny Analysis of ChCCOs
Chromosome location analysis revealed that ChCCOs were located in three chromosomes of C. humilis (Table 1 and Figure S2), including five members (ChCCD-like-a, ChCCD-like-b, ChCCD4, ChCCD8 and ChNCED6) in Chr1, two members (ChNCED1 and ChNCED5) in Chr3 and two members (ChCCD1 and ChCCD7) in Chr5. Synteny analysis revealed that ChCCD-like-a and ChCCD-like-b were tandem duplicated genes (Figure S2).
2.3. Conserved Motifs in ChCCOs and Gene Structures of Their Corresponding Genes
Totally, we identified ten conserved motifs from the nine ChCCOs (Figure S3A). Among them, ChCCD-like-b, ChCCD1, ChCCD4, ChNCED1, ChNCED5 and ChNCED6 contained all the 10 conserved motifs. ChCCD-like-a did not contain Motif 1 and Motif 8, but it had two of Motif 10. ChCCD7 contained six motifs, including two of Motif 5 and one each of Motif 4, Motif 6, Motif 7 and Motif 8. And ChCCD8 only contained one each of Motif 2, Motif 3, Motif 4, Motif 6 and Motif 8.
Gene structure analysis results showed that, except ChNCED1 and ChNCED6, all ChCCOs had introns (Figure S3B). The number of introns in ChCCD-like-a was the largest (19), followed by ChCCD1 (14) and ChCCD-like-b (11). ChCCD8 and ChCCD7 had seven and five introns, respectively. And ChCCD4 and ChNCED5 both contained two introns.
2.4. Promoter Analysis of ChCCOs
The cis-acting elements in the promoter regions of ChCCOs were analyzed (Figure S4). Results showed that, in addition to the abundant light responsive, core promoter elements TATA-box and CAAT-box and growth and development-related elements, the ChCCOs’ promoters also contained a variety of phytohormone- and stress-responsive elements.
Ten types of phytohormone-responsive elements involving six phytohormones (ABA, MeJA, auxin, gibberellin (GA), ethylene and salicylic acid (SA)) were identified from the promoters of ChCCOs. Notably, the ABA-responsive element ABRE was found in promoters of all ChCCOs. The ChNCED6 promoter contained the largest number of the ABA-responsive element ABRE (18 in total), followed by ChNCED5 (8). Except for ChCCD4, the promoters of all other ChCCOs contained MeJA-related cis-acting elements (TGACG-motif and CGTCA-motif). The promoters of ChCCD-like-a, ChCCD-like-b, ChCCD1 and ChNCED6 contained auxin-responsive elements. The promoters of ChCCD-like-a, ChCCD-like-b, ChCCD4, ChNCED1 and ChNCED6 contained GA-responsive elements. The promoters of ChCCD4, ChCCD7, ChCCD8 and ChNCED6 contained the ethylene-responsive element ERE, and the ChCCD4, ChNCED1 and ChNCED5 promoters contained the SA-responsive TCA-element.
Among the stress-responsive elements, the defense and stress-related element MYB was identified in the promoters of all ChCCOs. The ChNCED5 promoter contained the largest number of MYB elements (a total of 7). However, except for ChNCED5, all the promoters of other ChCCOs contained the anaerobic inducible element ARE. Except for ChNCED6, all the promoters of ChCCOs contained the high-temperature response element STRE. Except for ChNCED1 and ChNCED6, the promoters of all other ChCCOs contained drought-inducibility-related elements. Moreover, the low-temperature responsive element LTRS was found in the promoters of ChCCD-like-a, ChCCD1 and ChCCD8.
Distributions of transcription factor binding sites (TFBS) on ChCCOs’ promoters were also analyzed. Totally, binding sites for 43 TFs were identified in the ChCCOs’ promoters (Figure S5). Among them, the total number of ERF binding sites identified in promoters of ChCCOs was the largest (584), followed by bHLH (410), Dof (396) and BBR-BPC (378). In the promoters of ChCCD1, ChCCD8, ChNCED1 and ChCCD7, binding sites for ERFs were found to be the most abundant, accounting for 127, 127, 126 and 103, respectively. The ChCCD7 promoter contained 103 binding sites for BCR-BPCs. In the promoter of ChCCD4, the binding site for TCP was the most abundant (113). In the promoter of ChCCD-like-a, the binding site for MYB was the largest (45). The ChCCD-like-b promoter contained 45 Dof binding sites and 44 MYB binding sites. The bHLH binding sites were the most abundant in the promoters of ChNCED6 (168) and ChNCED5 (73), respectively.
2.5. Protein–Protein Interaction Analysis of ChCCOs
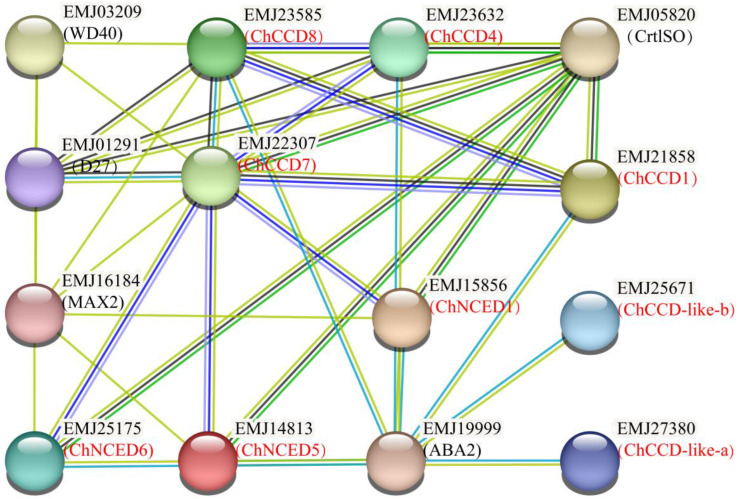
Based on the P. persica protein database, possible interacting proteins of ChCCOs were predicted. Results showed that all the ChCCOs were homologous proteins of P. persica CCOs (Figure 2). ChCCD-like-a, ChCCD-like-b, ChCCD1, ChCCD8, ChNCED1 and ChNCED5 were predicted to have the ability to interact with ABA2. ChCCD7 and ChCCD8 could interact with WD40, D27 (DWARF27) and MAX2 (MORE AXILLARY BRANCHING2). In addition, all the three ChNCEDs were predicted to be interacting proteins of MAX2. Moreover, ChCCD1, ChCCD4, ChCCD7 and all the three ChNCEDs were predicted to have the ability of interacting with CrtlSO.
Figure 2.
Protein–protein interaction network for ChCCOs based on the Prunus persica protein database.
2.6. Gene Expression Analysis of ChCCOs
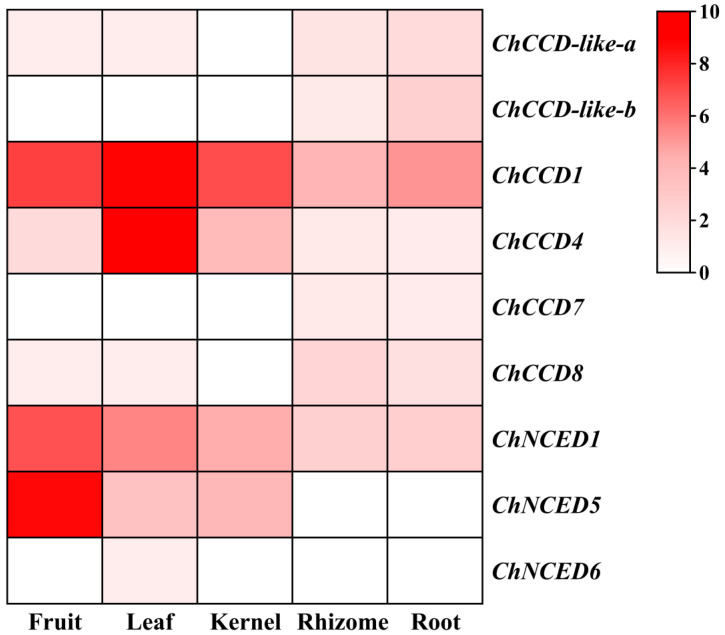
According to the transcriptome data of five C. humilis organs, including fruit, leaf, kernel, rhizome and root, we found that the expression levels of ChCCOs in different parts varied a lot (Figure 3). Of the nine ChCCOs, only ChCCD1, ChCCD4 and ChNCED1 expressed in all the five organs; ChCCD-like-a and ChCCD8 showed no expression in the kernel, and its expression in fruit, leaf, rhizome and root were all relatively low (FPKM < 2); ChNCED5 showed expression in fruit, leaf and kernel; ChCCD-like-b and ChCCD7 expressed in only rhizome and root; and the expression of ChNCED6 was leaf-specific.
Figure 3.
Heatmap for the transcriptome data-based expression analysis of ChCCOs in the fruit, leaf, kernel, rhizome and root of C. humilis. For heatmap drawing, log2(FPKM + 1) values of ChCCO genes were used. The redder the color, the higher the gene’s expression, and white represents no expression.
Among the six fruit-expressing ChCCOs (ChNCED5, ChCCD1, ChNCED1, ChCCD4, ChCCD8 and ChCCD-like-a), ChNCED5 expressed the highest (FPKM > 400), followed by ChCCD1 and ChNCED1 (both with FPKM > 100). The expression level of ChCCD4 ranked the fourth (with FPKM about 2) among the fruit-expressing ChCCOs. Although ChCCD8 and ChCCD-like-a showed expression in fruit, their expression levels were very low. There were seven ChCCOs expressed in the leaf of C. humilis. Among them, the expression of ChCCD1 and ChCCD4 ranked top two, and their FPKM values were both higher than 400. ChNCED1 and ChNCED5 also expressed relatively high in the leaf. However, the expression levels of the other three leaf-expressing ChCCOs (ChCCD-like-a, ChCCD8 and ChNCED6) were all very low (FPKM < 0.1). The expression levels of the four ChCCOs expressing in the kernel followed the order of ChCCD1 > ChNCED1 > ChNCED5 > ChCCD4. And the FPKM value of ChCCD1 in the kernel was more than 100. Except for ChNCED5 and ChNCED6, all other ChCCOs showed expression in C. humilis rhizome and root with ChCCD1 and ChNCED1 both ranking top two.
The expression of the same ChCCO gene also showed obvious spatial differences. For example, ChCCD1 expressed the highest in the leaf, followed by in the fruit and kernel; ChCCD4 expressed the highest in the leaf, followed by in the kernel and fruit; the expression levels of ChNCED1 and ChNCED5 in the fruit were much higher than that in the other four organs.
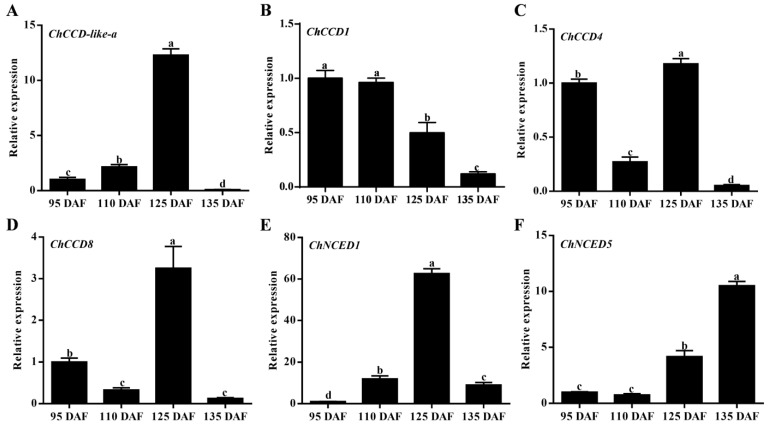
The C. humilis fruits are rich in carotenoids and carotenoid-derived compounds [27]. To analyze the expression patterns of fruit-expressing ChCCO genes in fruits at different ripening stages, quantitative real-time PCR (qRT-PCR) analysis was performed (Figure 4). Results showed that the expression levels of ChCCD-like-a and ChNCED1 increased sharply at 125 DAF (the color turning stage) but decreased at 135 DAF (the maturity stage). ChCCD4 and ChCCD8 exhibited a ‘fall-rise-fall’ expression change pattern during fruit ripening, and their expression levels both peaked at 125 DAF, followed by that in the fruit at 95 DAF. The expression of ChCCD1 in fruits at 95 DAF and 110 DAF were significantly higher than that in fruits at 125 DAF and 135 DAF, and its expression at 135 DAF was found to be the lowest. However, the expression of ChNCED5 in fruits at 125 DAF and 135 DAF was significantly higher than that in fruits at 95 DAF and 110 DAF, and its expression increased as fruit ripened. Except ChCCD1 and ChNCED5, the relative expression levels of other fruit-expressing ChCCOs were all the highest at 125 DAF. Moreover, the expression levels of all the fruit-expressing ChCCDs (including ChCCD-like-a, ChCCD1, ChCCD4 and ChCCD8) were the lowest at 135 DAF.
Figure 4.
Quantitative real-time PCR analysis results of ChCCOs in fruits at four different ripening stages. (A–F) represents expression analysis result for ChCCD-like-a, ChCCD1, ChCCD4, ChCCD8, ChNCED1 and ChNCED5, respectively. DAF: days after flowering. The different letters above the columns represent significant differences at p < 0.05 level.
2.7. Prokaryotic Expression and Enzyme Assay Analysis of ChCCD1 and ChCCD4 Proteins
The contributions of CCD1 and CCD4 in carotenoid degradation and apocarotenoid accumulation have been frequently demonstrated in many plant species [14,15]. To clarify the functions of ChCCD1 and ChCCD4, pET-ChCCD1 and pET-ChCCD4 prokaryotic expression vectors were constructed and individually transformed into E. coli BL21(DE3). After protein expression activation using IPTG, SDS-PAGE gel electrophoresis was used to detect the expression of ChCCD1 and ChCCD4 proteins. Results showed that E. coli BL21(DE3) carrying pET-ChCCD1 and pET-ChCCD4 could, respectively, express recombinant proteins with a molecular weight of about 61 kD and 65 kD (Figure S6), indicating that ChCCD1 and ChCCD4 proteins were correctly expressed.
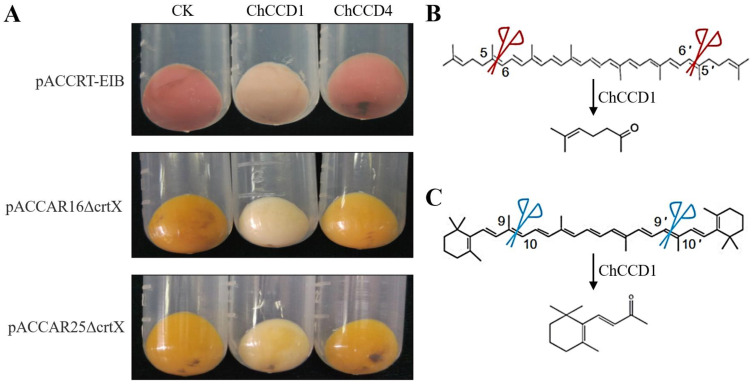
To reveal the roles of the ChCCD1 and ChCCD4 in carotenoid degradation and apocarotenoid accumulation, pET-ChCCD1/pET-ChCCD4 vectors were introduced into E. coli BL21(DE3) together with plasmid pACCRT-EIB/pACCAR16ΔcrtX/pACCAR25ΔcrtX. Before IPTG addition, the color of bacterial cultures carrying pET-ChCCD1/ChCCD4 and pACCRT-EIB, pET-ChCCD1/ChCCD4 and pACCAR16ΔcrtX and pET-ChCCD1/ChCCD4 and pACCAR25ΔcrtX was red, yellow-orange and yellow-orange (Figure 5), respectively. After IPTG induction, bacterial cultures expressing recombinant ChCCD1 exhibited remarkable color changes, varying from a lighter color (cultures expressing pET-ChCCD1 and pACCRT-EIB/pACCAR25ΔcrtX) to almost white (cultures expressing pET-ChCCD1 and pACCAR16ΔcrtX). However, the ChCCD4 expression did not result in significant bacterial culture color change. These results indicated that ChCCD1 could cleave lycopene, β-carotene and zeaxanthin. ChCCD4, however, could not cleave these substrates as efficiently as ChCCD1.
Figure 5.
Functional analysis results of ChCCD1 and ChCCD4 proteins. (A) The influences of ChCCD1 and ChCCD4 expression on the color changes of E. coli strains that can accumulate lycopene (carrying pACCRT-EIB vector), β-carotene (carrying pACCAR16ΔcrtX vector) and zeaxanthin (carrying pACCAR25ΔcrtX vector); CK: control bacteria with no IPTG addition. (B) ChCCD1 can cleave lycopene into 6-methyl-5-heptene-2-one. (C) ChCCD1 can cleave β-carotene into β-ionone.
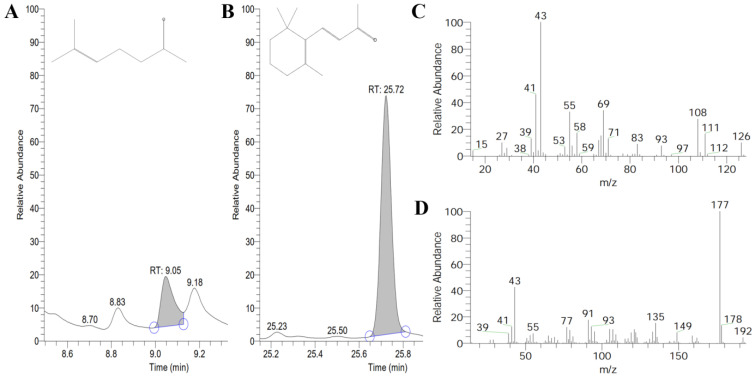
The volatile products of ChCCD1 and ChCCD4 in E. coli strains that can accumulate lycopene, β-carotene and zeaxanthin were further determined using headspace gas chromatography/mass spectrometer. Results showed that, after IPTG induction, among the volatiles released by the bacterial culture expressing pET-ChCCD1 and pACCRT-EIB, 6-methyl-5-heptene-2-one was detected (Figure 6). This indicated that ChCCD1 can cleave lycopene at 5, 6 and 5′, 6′ positions to produce 6-methyl-5-heptene-2-one. In the volatiles released by the bacterial culture expressing pET-ChCCD1 and pACCAR16ΔcrtX, β-ionone, an aroma substance produced by oxidative cleavage of β-carotene, was detected (Figure 6). This indicated that ChCCD1 can cleave β-carotene at 9, 10 and 9′, 10′ positions to produce β-ionone. No cleaved carotenoid products were detected in the volatiles released by other bacterial cultures.
Figure 6.
GC-MS detection results of the ChCCD1 cleaved volatile products of lycopene and β-carotene in E. coli. (A,B) for the cleaved volatile products of lycopene and β-carotene, respectively; (C,D) fragments pattern for 6-methy-5-hepten-2-one and β-ionone, respectively. Blue circles in (A) and (B) represent starting and ending time points of peak.
3. Discussion
In this study, for the first time, we performed whole genome-wide identification and characterization of the CCO genes in C. humilis. Totally, nine ChCCOs (including six ChCCDs and three ChNCEDs) belonging to six subfamilies were obtained. This classification was supported by their gene structures and conserved motifs in their encoded proteins. Most of the ChCCOs contained four histidine active sites, which might be closely related to their iron-binding abilities [28,29]. Subcellular localization analysis revealed that ChCCOs were mainly localized in cytoplasm and chloroplast, which was consistent with the CCOs from many other plant species [13,30,31,32]. Synteny analysis revealed that ChCCD-like-a and ChCCD-like-b were tandem duplicated genes, suggesting that the tandem duplication of CCD-like subfamily members contributed to the amplification of the CCO gene family in C. humilis.
Accumulated evidence demonstrated that CCOs might be involved in the plant responses to phytohormones and abiotic stresses [33,34,35,36]. In this study, we identified many phytohormone- and stress-responsive elements in the promoters of ChCCOs. The ABA-responsive element identified in the promoters of CCD genes from six Cucurbitaceae species was reported to be the most abundant among all the phytohormone-responsive elements [37]. Similarly, the abundance of an ABA-responsive element in the promoters of the litchi CCD1, CCD4, CCD7, CCD-like and NCED subfamily genes ranked the first among all the phytohormone-responsive elements [38]. Consistently, in our study, all the promoters of ChCCOs were predicted to contain the ABA-responsive element ABRE. There were nine, nine, nine and eight ChCCOs that contained MeJA-responsive elements, the anaerobic inducible element ARE, the high-temperature response element STRE and the drought-inducibility-related elements in their promoters, respectively. In addition, the promoters of ChCCD-like-a, ChCCD1 and ChCCD8 contained the low-temperature responsive element LTRS. These results suggested that ChCCOs might play roles in phytohormone and stress responses in C. humilis.
Transcription factors (TFs) play important roles in the biosynthesis of secondary metabolites including carotenoids. The expression of carotenoid metabolism-related genes has been continuously proven to be regulated by TFs [39,40]. In this study, we identified binding sites for 43 types of TFs in the promoters of ChCCOs, but the abundance of binding sites for different TFs varied a lot. For example, the binding site for ERF was the most abundant in the promoters of ChCCD1, ChCCD7, ChCCD8 and ChNCED1; ChCCD4 and ChCCD-like-a promoters had many binding sites for TCPs and MYBs, while ChNCED6 and ChNCED5 promoters were rich of bHLH binding sites. The distribution and abundance differences of TFBSs in their promoters suggested that the expression of ChCCOs might be regulated by different TFs.
The expression patterns of different CCO gene members varied a lot in different parts of plant species [3,37]. In this study, our transcriptome data-based gene expression analysis revealed that there were six, seven, four, seven and seven ChCCOs expressed in the fruit, leaf, kernel, rhizome and root, respectively. ChCCD1, ChCCD4 and ChNCED1 showed expression in all five organs, while the expression of ChNCED6 was found to be leaf-specific. ChCCD1 expressed much higher than other ChCCOs in the kernel, rhizome and root; ChCCD1 and ChCCD4 expressed highly in the leaf and ChCCD1, ChNCED1 and ChNCED5 showed high expression in the fruit. All this suggests that their roles in carotenoid degradation were spatially different in C. humilis. Moreover, our qRT-PCR analysis revealed that the expression patterns of ChCCOs in fruits at different ripening stages were also temporally different.
Our protein–protein interaction analysis also indicated that ChCCOs play different roles in C. humilis. Six ChCCOs (including ChCCD-like-a, ChCCD-like-b, ChCCD1, ChCCD8, ChNCED1 and ChNCED5) were predicted to interact with ABA2. In higher plants, ABA is derived from xanthophyll carotenoids via the C15 intermediate xanthoxin. ABA2, a xanthoxin dehydrogenase that catalyzes the conversion of xanthoxin to abscisic aldehyde, is a key protein function in ABA biosynthesis [41]. This indicates that these ChCCOs might play roles in ABA biosynthesis by interacting with ABA2. ChCCD7 and ChCCD8 were predicted to interact with WD40, D27 and MAX2. WD40 has been reported to function not only in the biosynthesis of flavonoids but also in the carotenoid-derived pigments [42]. D27 is a β-carotene isomerase that can catalyze the interconversion of all-trans- into 9-cis-β-carotene (the precursor of SLs) [43,44]. In saffron, CsD27-1 was found to be co-expressed with CCD7 and CCD8 in the mycorrhized roots [45]. MAX2 is a key regulatory gene in SL signal transduction [46]. The interactions of ChCCD7 and ChCCD8 with these proteins indicated that these two ChCCOs were involved in the carotenoid degradation and SL biosynthesis. All the three ChNCEDs were also predicted to be interacting proteins of MAX2, indicating that they also function in these processes. Additionally, ChCCD1, ChCCD4, ChCCD7 and the three ChNCEDs were identified to interact with CrtlSO, a carotenoid isomerase that can catalyze the cis-to-trans isomerization of poly-cis-isomer of lycopene into all-trans lycopenes [45], indicating again that these ChCCOs function in carotenoids degradation.
The roles of CCD1 in regulating the formation of apocarotenoid volatiles [17,18,19,20,31], and the function of the CCD4 gene in regulating the cleavage of carotenoids [47], have been confirmed in many plant species. The Rosa damascene RdCCD1 was reported to have the ability to cleave a variety of carotenoids at the 9, 10 and 9′, 10′ positions to produce a C14 dialdehyde and two C13 products, and it could also cleave lycopene at the 5, 6 and 5′, 6′ positions to produce 6-methyl-5-hepten-2-one [15]. The melon CmCCD1 could cleave a variety of carotenoids at 9, 10 and 9′, 10′ positions to generate several kinds of apocarotenoids [48]. In rice, CCD1 has been reported to have the ability to convert lycopene into volatiles, pseudoionone, 6-methyl-5-hepten-2-one and geranial [49]. In this study, we functionally analyzed the cleavage ability of ChCCD1 and ChCCD4 on lycopene and β-carotene and zeaxanthin by co-expressing them together with genes that can induce carotenoid accumulation in E. coli. Results showed that ChCCD1 can oxidize lycopene at 5, 6 and 5′, 6′ positions to produce 6-methyl-5-heptene-2-one and can cleave β-carotene at 9, 10 and 9′, 10′ positions to produce β-ionone. These results suggest that ChCCD1 plays a key role in the β-carotene accumulation in C. humilis and contributes greatly to the β-carotene-rich characteristics of C. humilis fruits [26,27]. Although ChCCD1 expression can also lead to the degradation of zeaxanthin, no volatile products were detected through GC/MS. This can be explained by the fact that the cleaved products of zeaxanthin by ChCCD1 are not volatiles. In addition, we also investigated the function of ChCCD4 in carotenoid degradation and apocarotenoid biosynthesis. Results showed that it did not show an obvious influence on the bacterial culture color change and volatiles release, which might be related to its higher substrate specificity [22].
4. Materials and Methods
4.1. Plant Materials
The two-year-old C. humilis cv. ‘Jinou No. 1’ materials were collected from the C. humilis resource nursery of Juxin Demonstration Park at Shanxi Agricultural University. At 95, 110, 125 and 135 days after flowering (DAF), fruits used for gene expression analysis were harvested, washed three times with sterile water, quick-frozen in liquid nitrogen and stored in a −80 °C freezer for further use.
4.2. Identification of Cerasus humilis CCO Genes
The C. humilis genome file [26] was provided by Dr. Pengfei Zhang from Shanxi Agricultural University. The Prunus persica CCO protein sequences were downloaded from NCBI (https://www.ncbi.nlm.nih.gov/, accessed on 3 March 2023) and used as queries to BLASTP against the C. humilis protein data to screen ChCCOs under the criterion of e-value ≤ 1 × 10−5. Meanwhile, the RPE65 (retinal pigment epithelial membrane protein 65) domain Markov model (PF03055) downloaded from Pfam (http://pfam.xfam.org/, accessed on 3 March 2023) was used to search the putative CCO proteins in C. humilis using HMMER 3.0 under the criterion of e-value ≤ 1 × 10−5. As some annotated ChCCOs do not have complete sequences, unigenes that were annotated as CCOs in our transcriptome data were subjected to gene cloning and sequencing confirmation to obtain their full-length CDSs. The obtained candidate CCOs were further subjected to conserved domain confirmation, and only proteins containing the RPE65 domain remained.
4.3. Bioinformatic Analysis of ChCCOs and Their Encoded Proteins
The physiochemical properties, subcellular localization and the existence of chloroplast transit peptide in ChCCOs were analyzed using ProtParam (https://web.expasy.org/protparam/, accessed on 5 March 2023), CELLO (http://cello.life.nctu.edu.tw/, accessed on 5 March 2023) and ChloroP (http://www.cbs.dtu.dk/services/ChloroP/, accessed on 5 March 2023), respectively. For gene structure analysis of ChCCOs, TBtools [50] was used. MEME (http://meme-suite.org/tools/meme, accessed on 5 March 2023) was applied to analyze the conservative motifs in each member of ChCCOs (the motif number was set as 10, and other parameters were set as default values). All the ChCCO genes were mapped to C. humilis chromosomes according to their location information, and synteny analysis was performed using MCscanX (Multiple Collinearity Scan Toolkit X version). MEGA7 software (Molecular Evolutionary Genetics Analysis Version 7.0) was applied for the multiple sequence alignments of CCO proteins from C. humilis, P. persica, Fragaria vesca, Solanum Lycopersicum, Oryza sativa and Arabidopsis thaliana and for phylogenetic tree construction by using the Neighbor Joining (NJ) method with default parameters (bootstrap = 1000). TBtools was used to extract the 2000 bp sequences upstream from the start codons (ATG) of ChCCOs from the C. humilis genome data. Additionally, the extracted sequences were used as promoter sequences of ChCCOs and were subjected to PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/, accessed on 6 March 2023) and PlantTFDB (http://plantregmap.gao-lab.org/binding_site_prediction.php, accessed on 6 March 2023) for the cis-acting element and transcription factor binding sites (TFBS) analysis, respectively. Given the close relationship between C. humilis and P. persica, the interacting proteins of ChCCOs were predicted using STRING (https://cn.string-db.org/, accessed on 7 March 2023) based on the P. persica protein database.
4.4. Gene Expression Analysis
Based on our transcriptome data, the expression patterns of ChCCO genes in the fruit, leaf, kernel, rhizome and root were analyzed. TBtools was used for drawing the heatmap of their expression levels.
Trizol (Invitrogen, CA, USA) and the PrimeScript RT Master Mix (Perfect Real Time) kit (Takara, Dalian, China) were used for isolating the total RNA from C. humilis fruits and for biosynthesizing the complementary DNA (cDNA) used for quantitative real-time PCR, respectively. Gene-specific primers used for quantitative real-time PCR were designed using Vector NTI (Table 2). The expression of ChCCO genes in fruits at different ripening stages was investigated on an ABI 7500 real-time PCR system. The amplification system contained 2 μL cDNA, 0.8 μL each of the forward and reverse primers (10 μM), 0.4 μL ROX Reference DyeⅡ, 10 μL SYBR solution and 6 μL ddH2O. The reaction procedure was set as follows: pre-denaturation at 95 °C for 3 min; denaturation at 95 °C for 15 s; annealing at 58.5 °C for 30 s; extension at 72 °C for 15 s; 40 cycles. Three biological replications were made for each gene. By using ChActin as an internal reference gene, the relative expression of ChCCOs in different samples was calculated using the 2−ΔΔCT method.
Table 2.
Information of primers used in this study. The nucleotide sequences underlined represent digestion site sequences of BamHI (GGATCC) and XhoI (CTCGAG).
| Gene Name | Forward Primer (5′–3′) | Reverse Primer (5′–3′) | Applications |
|---|---|---|---|
| ChActin | TTCAAAGACCAGCTCATCTGTGG | CAATGCCAGGGAACATAGTGGA | qRT-PCR |
| ChCCD-like-a | AAGTCAAGACCACCCTCTCCTCC | AACTCGTCTACGGGGCCAAAG | qRT-PCR |
| ChCCD1 | ATGGCGGAGGTTGAAGATGAGG | TTGGTGGGAGGAGTTTCATCAAG | qRT-PCR |
| ChCCD1 | CGGGATCCATGGCGGAGGTTGAAGATG | CCCTCGAGTTAGAGCTTTGCTTGTTCTTGC | Vector construction |
| ChCCD4 | GGATGCCTTCTCTTCCTCTTTCC | CGCGAGCTTTTGTTGTTAGTGG | qRT-PCR |
| ChCCD4 | CGGGATCCATGGATGCCTTCTCTTCCTC | CCCTCGAGCTACAACTTGTTGAGATCAC | Vector construction |
| ChCCD8 | ATGGCTTCCATAGCATTTTCCG | AGCCACTATTGCCGCTCTCTCT | qRT-PCR |
| ChNCED1 | CCTCTTCCTCTTCCAGCCCAA | GGCACTGGCTTTGAGGATTTAGA | qRT-PCR |
| ChNCED5 | GCTCTTCCAAAAGCACCCAATT | GGAAGTGAAGAACCGAGGGAGAT | qRT-PCR |
4.5. Expression of ChCCD1 and ChCCD4 in Escherichia coli
To clone the full-length CDSs of ChCCD1 and ChCCD4, gene-specific primer pairs with BamHI digestion site sequences (GGATCC) in the forward primers and XhoI digestion site sequences (CTCGAG) in the reverse primers were designed (Table 2). Amplified PCR products were double digested with BamHI and XhoI and introduced into the prokaryotic expression vector pET28, which had been digested using the same two enzymes to generate pET-ChCCD1 and pET-ChCCD4 vectors. Then, vectors were transformed into E. coli BL21(DE3) and incubated at 37 °C with gentle shaking at 125 rpm till OD600 of 0.5. The expression of recombinant ChCCD1 or ChCCD4 proteins was induced by the addition of isopropyl β-D-thiogalactopyranoside (IPTG, with a final concentration of 0.5 mM), after which the cultures were grown at 37 °C for an additional 5 h. SDS-PAGE gel electrophoresis was applied to detect the protein expression.
4.6. Enzyme Assays In Vitro and Volatile Compounds Detection
pET-ChCCD1/pET-ChCCD4 vectors were transformed into E. coli BL21(DE3) together with plasmids pACCRT-EIB (carrying crtE, crtB and crtI genes), pACCAR16ΔcrtX (carrying crtE, crtB, crtI and crtY genes) and pACCAR25ΔcrtX (carrying crtE, crtB, crtI, crtY and crtZ genes) [51], respectively. After color observation and PCR detection using gene cloning primers for vector construction (Table 2), positive colonies respectively carrying pET-ChCCD1 and pACCRT-EIB, pET-ChCCD1 and pACCAR16ΔcrtX, pET-ChCCD1 and pACCAR25ΔcrtX, pET-ChCCD4 and pACCRT-EIB, pET-ChCCD4 and pACCAR16ΔcrtX and pET-ChCCD4 and pACCAR25ΔcrtX, were inoculated into an LB liquid medium containing appropriate antibiotics and incubated at 37 °C till OD600 of 0.6. After IPTG addition, bacterial cultures were grown at 37 °C for an additional 5 h and gently shaken at 125 rpm to induce protein expression. Bacterial cultures that were not treated with IPTG were used as controls. The volatile compounds of bacterial cultures collected from the headspace were analyzed on a quadrupole GC/MS HP GCD (G1800A) coupled to an HP-5 silica capillary column (30 m × 0.25 mm) according to Huang et al. [14]. The oven temperature was held at 50 °C for 1 min and then increased to 200 °C at 4 °C/min intervals, with a helium flow rate of 1 mL/min. The EI-MS ionization voltage was 70 eV, and the ion source temperature was 280 °C. The mass range was recorded from 45 to 450 m/z, and spectra were evaluated with the Xcalibur software version 1.4.
5. Conclusions
In summary, for the first time, we identified and characterized the CCO gene family of C. humilis, investigated their expression patterns in different tissues and organs and in fruits at different ripening stages and functionally validated the roles of ChCCD1 in the degradation of lycopene, β-carotene and zeaxanthin. According to the results obtained in our study, it can be concluded that ChCCD1 plays a key role in carotenoid degradation and apocarotenoid accumulation in C. humilis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/plants12112114/s1, Figure S1: Multiple sequence alignment results of ChCCOs. Red, yellow and green shading represent 100%, ≥75% and ≥50% amino acid sequence similarity, respectively; Figure S2: Chromosomal locations of ChCCO genes; Figure S3: Conserved motifs in ChCCOs proteins (A) and gene structures (B) of their corresponding genes; Figure S4: The identified cis-acting elements in ChCCOs’ promoters. Red, yellow and green colors represent the high, moderate and low abundance of cis-acting elements in promoters, respectively. Figure S5: Heatmap for the transcription factor binding sites identified in promoters of ChCCOs. Red, yellow and green colors represent the high, moderate and low abundance of TFBS in promoters, respectively. Figure S6: SDS-PAGE gel electrophoresis detection results of ChCCD1 and ChCCD4 proteins. M: protein marker; 1–3: E. coli strains expressing ChCCD1; 4 and 5: E. coli strains carrying pET-ChCCD1 without IPTG induction; 6: E. coli strains carrying pET-ChCCD4 without IPTG induction; 7–9: E. coli strains expressing ChCCD4. Arrows represent target bands for recombinant ChCCD1 and ChCCD4.
Author Contributions
Conceptualization, C.C. and J.Z. (Jiancheng Zhang); methodology, R.Y., J.Z. (Jianying Zhang) and L.G.; software, R.Y., R.L., Y.Y., L.Y. and J.Z. (Jianying Zhang); validation, R.Y., Y.Y. and C.C.; formal analysis, J.Z. (Jianying Zhang), L.G. and R.Y.; investigation, J.Z. (Jianying Zhang), L.G. and R.Y.; resources, P.W., X.M., S.Z. and B.Z.; data curation, R.Y. and J.Z. (Jiancheng Zhang); writing—original draft preparation, C.C., R.Y., L.Y., L.G. and J.Z. (Jiancheng Zhang); writing—review and editing, C.C.; visualization, R.Y., L.G. and C.C.; supervision, J.Z. (Jiancheng Zhang); project administration, C.C. and J.Z. (Jiancheng Zhang); funding acquisition, C.C. and J.Z. (Jiancheng Zhang). All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
All data is available in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Natural Science Basic Research Program of Shanxi Province (202203021211274), the earmarked fund for Modern Agro-industry Technology Research System of Shanxi Province (SXFRS-2022 and 2023CYJSTX07-02), the Fund for High-level Talents of Shanxi Agricultural University (2021XG010), and the Reward Fund for PhDs and Postdoctors of Shanxi Province (SXBYKY2022004).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Yuan H., Zhang J., Nageswaran D., Li L. Carotenoid metabolism and regulation in horticultural crops. Hortic. Res. 2015;2:15036. doi: 10.1038/hortres.2015.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liang M.H., He Y.J., Liu D.M., Jiang J.G. Regulation of carotenoid degradation and production of apocarotenoids in natural and engineered organisms. Crit. Rev. Biotechnol. 2021;41:513–534. doi: 10.1080/07388551.2021.1873242. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Xu J., Liu A. Identification of the carotenoid cleavage dioxygenase genes and functional analysis reveal DoCCD1 is potentially involved in beta-ionone formation in Dendrobium officinale. Front. Plant Sci. 2022;13:967819. doi: 10.3389/fpls.2022.967819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan B.-C., Joseph L.M., Deng W.-T., Liu L., Li Q.-B., Cline K., McCarty D.R. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56. doi: 10.1046/j.1365-313X.2003.01786.x. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz S.H., Tan B.C., Gage D.A., Zeevaart J.A., McCarty D.R. Specific oxidative cleavage of carotenoids by VP14 of maize. Science. 1997;276:1872–1874. doi: 10.1126/science.276.5320.1872. [DOI] [PubMed] [Google Scholar]
- 6.Dhar M.K., Mishra S., Bhat A., Chib S., Kaul S. Plant carotenoid cleavage oxygenases: Structure-function relationships and role in development and metabolism. Brief. Funct. Genom. 2020;19:1–9. doi: 10.1093/bfgp/elz037. [DOI] [PubMed] [Google Scholar]
- 7.Wei Y., Wan H., Wu Z., Wang R., Ruan M., Ye Q., Li Z., Zhou G., Yao Z., Yang Y. A Comprehensive analysis of carotenoid cleavage dioxygenases genes in Solanum Lycopersicum. Plant Mol. Biol. Rep. 2015;34:512–523. doi: 10.1007/s11105-015-0943-1. [DOI] [Google Scholar]
- 8.Fang Q., Li Y., Liu B., Meng X., Yang Z., Yang S., Bao T., Kimani S., Gao X., Wang L. Cloning and functional characterization of a carotenoid cleavage dioxygenase 2 gene in safranal and crocin biosynthesis from Freesia hybrida. Plant Physiol. Biochem. 2020;154:439–450. doi: 10.1016/j.plaphy.2020.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Wang J.Y., Haider I., Jamil M., Fiorilli V., Saito Y., Mi J., Baz L., Kountche B.A., Jia K.P., Guo X., et al. The apocarotenoid metabolite zaxinone regulates growth and strigolactone biosynthesis in rice. Nat. Commun. 2019;10:810. doi: 10.1038/s41467-019-08461-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frusciante S., Diretto G., Bruno M., Ferrante P., Pietrella M., Prado-Cabrero A., Rubio-Moraga A., Beyer P., Gomez-Gomez L., Al-Babili S., et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc. Natl. Acad. Sci. USA. 2014;111:12246–12251. doi: 10.1073/pnas.1404629111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mishra S., Upadhyay S., Shukla R.K. The role of strigolactones and their potential cross-talk under hostile ecological conditions in plants. Front. Physiol. 2016;7:691. doi: 10.3389/fphys.2016.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahrazem O., Rubio-Moraga A., Berman J., Capell T., Christou P., Zhu C., Gomez-Gomez L. The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 2016;209:650–663. doi: 10.1111/nph.13609. [DOI] [PubMed] [Google Scholar]
- 13.Yao Y., Jia L., Cheng Y., Ruan M., Ye Q., Wang R., Yao Z., Zhou G., Liu J., Yu J., et al. Evolutionary origin of the carotenoid cleavage oxygenase family in plants and expression of pepper genes in response to abiotic stresses. Front. Plant Sci. 2021;12:792832. doi: 10.3389/fpls.2021.792832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang F.C., Molnar P., Schwab W. Cloning and functional characterization of carotenoid cleavage dioxygenase 4 genes. J. Exp. Bot. 2009;60:3011–3022. doi: 10.1093/jxb/erp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang F.C., Horvath G., Molnar P., Turcsi E., Deli J., Schrader J., Sandmann G., Schmidt H., Schwab W. Substrate promiscuity of RdCCD1, a carotenoid cleavage oxygenase from Rosa damascena. Phytochemistry. 2009;70:457–464. doi: 10.1016/j.phytochem.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Bouvier F., Suire C., Mutterer J., Camara B. Oxidative remodeling of chromoplast carotenoids: Identification of the carotenoid dioxygenase CsCCD and CsZCD genes involved in Crocus secondary metabolite biogenesis. Plant Cell. 2003;15:47–62. doi: 10.1105/tpc.006536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Auldridge M.E., Block A., Vogel J.T., Dabney-Smith C., Mila I., Bouzayen M., Magallanes-Lundback M., DellaPenna D., McCarty D.R., Klee H.J. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006;45:982–993. doi: 10.1111/j.1365-313X.2006.02666.x. [DOI] [PubMed] [Google Scholar]
- 18.Wei S., Hannoufa A., Soroka J., Xu N., Li X., Zebarjadi A., Gruber M. Enhanced β-ionone emission in Arabidopsis over-expressing AtCCD1 reduces feeding damage in vivo by the crucifer flea beetle. Env. Entomol. 2011;40:1622–1630. doi: 10.1603/EN11088. [DOI] [PubMed] [Google Scholar]
- 19.Simkin A.J., Schwartz S.H., Auldridge M., Taylor M.G., Klee H.J. The tomato carotenoid cleavage dioxygenase 1 genes contribute to the formation of the flavor volatiles β-ionone, pseudoionone, and geranylacetone. Plant J. 2004;40:882–892. doi: 10.1111/j.1365-313X.2004.02263.x. [DOI] [PubMed] [Google Scholar]
- 20.Simkin A.J., Underwood B.A., Auldridge M., Loucas H.M., Shibuya K., Schmelz E., Clark D.G., Klee H.J. Circadian regulation of the PhCCD1 carotenoid cleavage dioxygenase controls emission of β-ionone, a fragrance volatile of petunia flowers. Plant Physiol. 2004;136:3504–3514. doi: 10.1104/pp.104.049718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J., Wu B., Zhang N., Zhao M., Jing T., Wu Y., Hu Y., Yu F., Wan X., Schwab W., et al. Dehydration-induced carotenoid cleavage dioxygenase 1 reveals a novel route for β-ionone formation during tea (Camellia sinensis) withering. J. Agric. Food. Chem. 2020;68:10815–10821. doi: 10.1021/acs.jafc.0c04208. [DOI] [PubMed] [Google Scholar]
- 22.Us-Camas R., Aguilar-Espinosa M., Rodriguez-Campos J., Vallejo-Cardona A.A., Carballo-Uicab V.M., Serrano-Posada H., Rivera-Madrid R. Identifying Bixa orellana L. New carotenoid cleavage dioxygenases 1 and 4 potentially involved in bixin biosynthesis. Front. Plant Sci. 2022;13:829089. doi: 10.3389/fpls.2022.829089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latari K., Wust F., Hubner M., Schaub P., Beisel K.G., Matsubara S., Beyer P., Welsch R. Tissue-specific apocarotenoid glycosylation contributes to carotenoid homeostasis in Arabidopsis leaves. Plant Physiol. 2015;168:1550–1562. doi: 10.1104/pp.15.00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lashbrooke J.G., Young P.R., Dockrall S.J., Vasanth K., Vivier M.A. Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol. 2013;13:156. doi: 10.1186/1471-2229-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohmiya A., Kishimoto S., Aida R., Yoshioka S., Sumitomo K. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol. 2006;142:1193–1201. doi: 10.1104/pp.106.087130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang P., Yi S., Mu X., Zhang J., Du J. Chromosome-level genome assembly of Cerasus humilis using PacBio and Hi-C technologies. Front. Genet. 2020;11:956. doi: 10.3389/fgene.2020.00956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J., Wang P., Hao Y., Yang S., Zheng B., Niu Z., Du J. Overexpression of ChPSY gene from Cerasus humilis improved carotenoids synthesis in transgenic tomato. Acta Hortic. Sin. 2014;41:1563–1572. (In Chinese) [Google Scholar]
- 28.Harrison P.J., Bugg T.D. Enzymology of the carotenoid cleavage dioxygenases: Reaction mechanisms, inhibition and biochemical roles. Arch. Biochem. Biophys. 2014;544:105–111. doi: 10.1016/j.abb.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 29.Poliakov E., Gentleman S., Cunningham F.X., Jr., Miller-Ihli N.J., Redmond T.M. Key role of conserved histidines in recombinant mouse β-carotene 15,15’-monooxygenase-1 activity. J. Biol. Chem. 2005;280:29217–29223. doi: 10.1074/jbc.M500409200. [DOI] [PubMed] [Google Scholar]
- 30.Zhang S., Guo Y., Zhang Y., Guo J., Li K., Fu W., Jia Z., Li W., Tran L.P., Jia K.P., et al. Genome-wide identification, characterization and expression profiles of the CCD gene family in Gossypium species. 3 Biotech. 2021;11:249. doi: 10.1007/s13205-021-02805-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao X.L., Yang Y.L., Xia H.X., Li Y. Genome-wide analysis of the carotenoid cleavage dioxygenases gene family in Forsythia suspensa: Expression profile and cold and drought stress responses. Front. Plant Sci. 2022;13:998911. doi: 10.3389/fpls.2022.998911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu D., Qiu C., Lu X., Zeng Y., Zhang C., Li T., Zhu G., He J., Lin Q. Cloning and prokaryotic expression of carotenoid cleavage dioxygenases from mulberry (Morus notabilis) Evid. Based Complement Altern. Med. 2022;2022:4811144. doi: 10.1155/2022/4811144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su W., Zhang C., Feng J., Feng A., You C., Ren Y., Wang D., Sun T., Su Y., Xu L., et al. Genome-wide identification, characterization and expression analysis of the carotenoid cleavage oxygenase (CCO) gene family in Saccharum. Plant Physiol. Biochem. 2021;162:196–210. doi: 10.1016/j.plaphy.2021.02.041. [DOI] [PubMed] [Google Scholar]
- 34.Wang Y., Ding G., Gu T., Ding J., Li Y. Bioinformatic and expression analyses on carotenoid dioxygenase genes in fruit development and abiotic stress responses in Fragaria vesca. Mol. Genet. Genom. 2017;292:895–907. doi: 10.1007/s00438-017-1321-5. [DOI] [PubMed] [Google Scholar]
- 35.Chen H., Zuo X., Shao H., Fan S., Ma J., Zhang D., Zhao C., Yan X., Liu X., Han M. Genome-wide analysis of carotenoid cleavage oxygenase genes and their responses to various phytohormones and abiotic stresses in apple (Malus domestica) Plant Physiol. Biochem. 2018;123:81–93. doi: 10.1016/j.plaphy.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhao J., Li J., Zhang J., Chen D., Zhang H., Liu C., Qin G. Genome-wide identification and expression analysis of the carotenoid cleavage oxygenase gene family in five Rosaceae species. Plant Mol. Biol. Rep. 2021;39:739–751. doi: 10.1007/s11105-021-01284-9. [DOI] [Google Scholar]
- 37.Cheng D., Wang Z., Li S., Zhao J., Wei C., Zhang Y. Genome-wide identification of CCD gene family in six Cucurbitaceae species and its expression profiles in melon. Genes. 2022;13:262. doi: 10.3390/genes13020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yue X.Q., Zhang Y., Yang C.K., Li J.G., Rui X., Ding F., Hu F.C., Wang X.H., Ma W.Q., Zhou K.B. Genome-wide identification and expression analysis of carotenoid cleavage oxygenase genes in Litchi (Litchi chinensis Sonn.) BMC Plant Biol. 2022;22:394. doi: 10.1186/s12870-022-03772-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang J., He L., Dong J., Zhao C., Wang Y., Tang R., Wang W., Ji Z., Cao Q., Xie H., et al. Integrated metabolic and transcriptional analysis reveals the role of carotenoid cleavage dioxygenase 4 (IbCCD4) in carotenoid accumulation in sweetpotato tuberous roots. Biotechnol. Biofuels Bioprod. 2023;16:45. doi: 10.1186/s13068-023-02299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiong C., Luo D., Lin A., Zhang C., Shan L., He P., Li B., Zhang Q., Hua B., Yuan Z., et al. A tomato B-box protein SlBBX20 modulates carotenoid biosynthesis by directly activating PHYTOENE SYNTHASE 1, and is targeted for 26S proteasome-mediated degradation. New Phytol. 2019;221:279–294. doi: 10.1111/nph.15373. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez-Guzman M., Apostolova N., Belles J.M., Barrero J.M., Piqueras P., Ponce M.R., Micol J.L., Serrano R., Rodriguez P.L. The short-chain alcohol dehydrogenase ABA2 catalyzes the conversion of xanthoxin to abscisic aldehyde. Plant Cell. 2002;14:1833–1846. doi: 10.1105/tpc.002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meng Y., Wang Z., Wang Y., Wang C., Zhu B., Liu H., Ji W., Wen J., Chu C., Tadege M., et al. The MYB activator WHITE PETAL1 associates with MtTT8 and MtWD40-1 to regulate carotenoid-derived flower pigmentation in Medicago truncatula. Plant Cell. 2019;31:2751–2767. doi: 10.1105/tpc.19.00480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Y., Li J. Branching in rice. Curr. Opin. Plant Biol. 2011;14:94–99. doi: 10.1016/j.pbi.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Lopez-Jimenez A.J., Morote L., Niza E., Mondejar M., Rubio-Moraga A., Diretto G., Ahrazem O., Gomez-Gomez L. Subfunctionalization of D27 isomerase genes in Saffron. Int. J. Mol. Sci. 2022;23:10543. doi: 10.3390/ijms231810543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giuliano G., Giliberto L., Rosati C. Carotenoid isomerase: A tale of light and isomers. Trends Plant Sci. 2002;7:427–429. doi: 10.1016/S1360-1385(02)02329-4. [DOI] [PubMed] [Google Scholar]
- 46.Dong L., Ishak A., Yu J., Zhao R., Zhao L. Identification and functional analysis of three MAX2 orthologs in chrysanthemum. J. Integr. Plant Biol. 2013;55:434–442. doi: 10.1111/jipb.12028. [DOI] [PubMed] [Google Scholar]
- 47.Ma J., Li J., Zhao J., Zhou H., Ren F., Wang L., Gu C., Liao L., Han Y. Inactivation of a gene encoding carotenoid cleavage dioxygenase (CCD4) leads to carotenoid-based yellow coloration of fruit flesh and leaf midvein in peach. Plant Mol. Biol. Rep. 2013;32:246–257. doi: 10.1007/s11105-013-0650-8. [DOI] [Google Scholar]
- 48.Ibdah M., Azulay Y., Portnoy V., Wasserman B., Bar E., Meir A., Burger Y., Hirschberg J., Schaffer A.A., Katzir N., et al. Functional characterization of CmCCD1, a carotenoid cleavage dioxygenase from melon. Phytochemistry. 2006;67:1579–1589. doi: 10.1016/j.phytochem.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 49.Ilg A., Beyer P., Al-Babili S. Characterization of the rice carotenoid cleavage dioxygenase 1 reveals a novel route for geranial biosynthesis. FEBS J. 2009;276:736–747. doi: 10.1111/j.1742-4658.2008.06820.x. [DOI] [PubMed] [Google Scholar]
- 50.Chen C., Chen H., Zhang Y., Thomas H.R., Frank M.H., He Y., Xia R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020;13:1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 51.Misawa N., Satomi Y., Kondo K., Yokoyama A., Kajiwara S., Saito T., Ohtani T., Miki W. Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J. Bacteriol. 1995;177:6575–6584. doi: 10.1128/jb.177.22.6575-6584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data is available in this article.