Abstract
Background and Objectives
Post-COVID condition (PCC) is common and often involves neuropsychiatric symptoms. This study aimed to use blood oxygenation level–dependent fMRI (BOLD-fMRI) to assess whether participants with PCC had abnormal brain activation during working memory (WM) and whether the abnormal brain activation could predict cognitive performance, motor function, or psychiatric symptoms.
Methods
The participants with PCC had documented coronavirus disease 2019 (COVID-19) at least 6 weeks before enrollment. Healthy control participants had no prior history of COVID-19 and negative tests for severe acute respiratory syndrome coronavirus 2. Participants were assessed using 3 NIH Toolbox (NIHTB) batteries for Cognition (NIHTB-CB), Emotion (NIHTB-EB), and Motor function (NIHTB-MB) and selected tests from the Patient-Reported Outcomes Measurement Information System (PROMIS). Each had BOLD-fMRI at 3T, during WM (N-back) tasks with increasing attentional/WM load.
Results
One hundred sixty-nine participants were screened; 50 fulfilled the study criteria and had complete and usable data sets for this cross-sectional cohort study. Twenty-nine participants with PCC were diagnosed with COVID-19 242 ± 156 days earlier; they had similar ages (42 ± 12 vs 41 ± 12 years), gender proportion (65% vs 57%), racial/ethnic distribution, handedness, education, and socioeconomic status, as the 21 uninfected healthy controls. Despite the high prevalence of memory (79%) and concentration (93%) complaints, the PCC group had similar performance on the NIHTB-CB as the controls. However, participants with PCC had greater brain activation than the controls across the network (false discovery rate–corrected p = 0.003, Tmax = 4.17), with greater activation in the right superior frontal gyrus (p = 0.009, Cohen d = 0.81, 95% CI 0.15–1.46) but lesser deactivation in the default mode regions (p = 0.001, d = 1.03, 95% CI 0.61–1.99). Compared with controls, participants with PCC also had poorer dexterity and endurance on the NIHTB-MB, higher T scores for negative affect and perceived stress, but lower T scores for psychological well-being on the NIHTB-EB, as well as more pain symptoms and poorer mental and physical health on measures from the PROMIS. Greater brain activation predicted poorer scores on measures that were abnormal on the NIHTB-EB.
Discussion
Participants with PCC and neuropsychiatric symptoms demonstrated compensatory neural processes with greater usage of alternate brain regions, and reorganized networks, to maintain normal performance during WM tasks. BOLD-fMRI was sensitive for detecting brain abnormalities that correlated with various quantitative neuropsychiatric symptoms.
Post-COVID condition (PCC) or long COVID syndrome is highly prevalent (∼42% based on a recent meta-analysis1), and the symptoms may persist for 2 years or longer.2–4 Neuropsychiatric symptoms are particularly common, including fatigue, inability to concentrate or brain fog, headaches, hyposmia/dysgeusia, sleep disorders, anxiety, and depression.3,5 Although the pathophysiology or mechanisms underlying these persistent symptoms remain unclear, neuropathology of patients with coronavirus disease 2019 (COVID-19) who died within 2 months of illness found microvascular injury with leakage of fibrinogen, along with activated microglia and hypertrophic astrocytes within the olfactory bulb and brain stem.6 Patients who died on average 28 days after hospitalization showed neuronal loss in the cerebellum, axonal swelling and neuronal degeneration in the pons, and widespread activation of glia and immune cells.7 Nonhuman primates infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) showed similar neuroinflammatory changes and Purkinje cell death in the cerebellum on necropsy.8 However, without postmortem brain tissue, whether evidence of brain injury persists in patients with PCC and ongoing neuropsychiatric symptoms after acute COVID-19 illness remains unknown.
Several neuroimaging studies evaluated brain abnormalities in patients with postacute COVID-19. PET studies showed decreased glucose metabolism in cortical and subcortical regions that correlated with poorer cognitive function and other functional complaints within the first few months of recovery from COVID-19.9–11 However, 6 months after acute COVID-19, glucose metabolism and cognition were normal despite memory and attention complaints.12 On MRI, 3 months after acute COVID-19, recovered participants showed enlarged gray matter volumes in bilateral olfactory cortices, hippocampi, insula, Heschl gyrus, Rolandic operculum, and cingulate gyri.13 In contrast, a larger longitudinal MRI study found that recovered participants with COVID-19 had greater decreases in cortical thickness than controls in multiple brain regions, including the orbitofrontal cortex and the parahippocampal gyrus, 2 brain regions related to olfaction.14 Several diffusion tensor imaging studies found variable abnormalities in brain diffusivity and fractional anisotropy,13,15–17 indicating disruption of microstructural integrity, which persisted in previously hospitalized patients at 1-year follow-ups.18 However, task-activated blood oxygenation level–dependent fMRI (BOLD-fMRI) has not been used to evaluate how brain function is affected in individuals with persistent neuropsychiatric symptoms after recovery from acute COVID-19.
We aimed to evaluate whether participants with long COVID symptoms at least 6 weeks after their acute illness have abnormal brain function on quantitative neurobehavioral measures and abnormal brain activation on task-activated BOLD-fMRI. We hypothesized that compared with controls, these recovered patients with long COVID syndrome would show persistent cognitive deficits and neuropsychiatric symptoms on 3 NIH Toolbox (NIHTB) batteries, including the Cognitive Battery (NIHTB-CB), Emotional Battery (NIHTB-EB), and Motor Battery (NIHTB-MB), and more symptoms for pain and poorer physical and mental health on the Patient-Reported Outcomes Measurement Information System (PROMIS). We also expected that participants with PCC would require greater brain activation than healthy controls, indicating greater usage of alternate brain regions to compensate for the residual brain injury or ongoing neuroinflammation. In addition, we expected that the greater brain activation in participants with PCC would be related to deficits on measures that were abnormal on the NIHTB batteries and PROMIS.
Methods
All participants were recruited through referrals from our medical center and flyers distributed locally. To minimize potential sources of bias or confounds, healthy controls were recruited to match the age range, self-reported gender, and racial/ethnic proportions of the PCC group. Self-reported race and ethnicity classifications were in accordance with US Census data.
Participant inclusion criteria for both groups included men or women of any race or ethnicity, 18–75 years of age, who were able and willing to provide informed consent. Participants with PCC were required to have a confirmed COVID-19 diagnosis from medical records, a documented positive PCR test for SARS-CoV-2 at least 6 weeks before enrollment (to minimize risk for ongoing infection and ensure that they no longer require hospitalization), and had at least 1 persistent post-COVID symptom. Healthy controls were required to have no history of COVID-19 symptoms or illness and a negative PCR test within 7 days or a negative SARS-CoV-2 antigen test on the study day. Exclusion criteria for both groups included (1) significant comorbid psychiatric illness that may confound study measures; (2) any confounding neurologic disorders, including significant prior head trauma with loss of consciousness >60 minutes; (3) taking medications that could significantly alter functional brain imaging studies; (4) any current or history (within the past 2 years) of severe substance use disorder (according to the Diagnostic and Statistical Manual of Mental Disorders-5); tobacco use was allowed; (5) positive urine toxicology screen on the day of assessments; (6) pregnancy or breastfeeding (self-report); and (7) contraindications for MR studies (e.g., metallic objects, electronic implants, or severe claustrophobia).
Each participant was assessed using a standardized neuropsychiatric evaluation, including substance use history, ECG, urine toxicology, and screening blood tests (complete blood count and comprehensive metabolic panel) from medical records within 1 year or collected at the screening visit. The participants with post–COVID-19 also completed a survey regarding their acute COVID-19 symptoms and treatments, any premorbid conditions, and current long COVID symptoms and severities (Table 1).
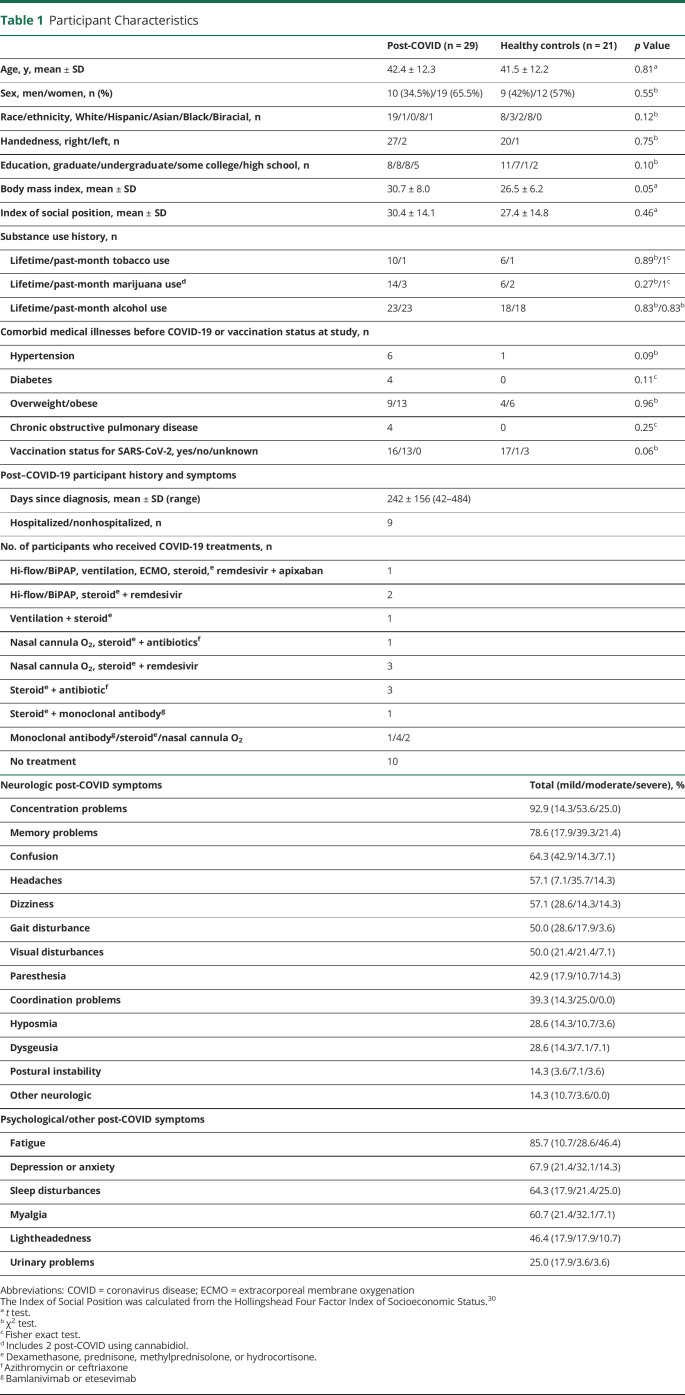
Table 1.
Participant Characteristics
Standard Protocol Approvals, Registrations, and Patient Consents
Written informed consent was obtained from all participants according to the Declaration of Helsinki using a protocol approved by the University of Maryland, Baltimore, Institutional Review Board (Human Research Protections Office at the University of Maryland, Baltimore; protocol number HP-00092062).
Quantitative Neurocognitive Tests and Psychiatric Symptom Assessments
Each participant was evaluated with 3 NIHTB batteries and selected PROMIS measures. The NIHTB-CB19 assessed 7 cognitive domains: (1) attention/executive functioning, (2) episodic memory, (3) working memory (WM), (4) language, (5) executive function, (6) processing speed, and (7) immediate recall. The NIHTB-EB20 assessed 4 domains: (1) negative affect, (2) psychological well-being, (3) stress and self-efficacy, and (4) social relationships. The NIH-MB21 assessed 5 domains: (1) dexterity, (2) strength, (3) balance, (4) endurance, and (5) locomotion. The PROMIS measures were selected from 4 domains to assess symptoms related to depression, anxiety, fatigue, and pain, which derived scores for Global Physical Health and Global Mental Health.22 Findings regarding these measures were reported previously in a larger cohort,23 but the current subsets of the participants' data are presented here.
Activation Paradigm, Image Acquisition, and Processing
Each participant performed 3 N-back WM tasks (0-back, 1-back, and 2-back) during BOLD-fMRI using a block design (30-second task period alternating with a 30-second rest period, 4 repeats over 4 minutes for each task, with 4 or 5 targets presented at random times per 30-second task block) as described.24,25 Briefly, during the 0-back task, participants pressed a button with their dominant hand thumb whenever a number flashed on the screen, and during the 1-back and 2-back tasks, the participant responded whenever the target letter was the same as the previous screen (1-back) or 2 screens previously (2-back). The rest period involved passive viewing of various symbols presented sequentially.
All scans were acquired on a 3T MR Scanner (Siemens Prisma). Structural MRI included a sagittal 3D magnetization-prepared rapid gradient echo (repetition time/echo time [TR/TE]/inversion time [TI] 2,200/4.47/1,000 milliseconds, 1-average, 256 × 256 × 160 matrix) and an axial fluid-attenuated inversion recovery sequence (TR/TE/TI 59,100/84/2,500 milliseconds, 1-average, 204 × 256 × 44 matrix). A board-certified neuroradiologist (E.H.H.) and an experienced board-certified neurologist (L.C.) both reviewed all structural MRIs, blinded to the COVID status of the participants, to exclude those with major structural abnormalities. BOLD-fMRI used a single-shot gradient-echo echo-planar sequence (TE/TR = 30/3,000 milliseconds, ∼42 axial 3-mm slices, 3-mm resolution, 80 excitations) with real-time motion correction.26 The MRI trigger pulse was synchronized to custom-stimulus software written in MATLAB (The MathWorks, Inc., Natick, MA). Participants responded with a button push during the task periods, providing reaction time and accuracy. Only fMRI data with <1.5 mm translations and <1.5° rotations during each scan and ≥70% performance accuracy were included in the final analyses.
fMRI scans were acquired from 53 participants, but scans from the 2-back task were excluded for 5 participants in the post-COVID group due to excess motion (n = 1) or <70% accuracy (n = 4), whereas scans from 6 controls were removed due to significant medical issues discovered after the scans (n = 2) or <70% accuracy (n = 4); see details in eFigure 1 (links.lww.com/WNL/C748). Data were processed using Statistical Parametric Mapping (SPM) 12. To avoid potential bias, all fMRI data were preprocessed by an engineer blinded to the participants' COVID status. For each task, a mask was created by combining activation maps (1-sample t test) for each group at p ≤ 0.05.
Sample Size Estimate and Statistical Analyses
We estimated that with 20 participants per group, assuming α = 0.05 and variability with an SD of 50% in each group, we had 89% power to detect a 1.5-fold change in outcome variables between groups. Of note, similar sample sizes per group yielded significant group effects in our prior fMRI studies.27–29
Statistical analyses were performed in R (version 4.1.2). Demographic data were compared between groups using t tests, χ2 tests, and Fisher exact test. Group differences on NIHTB and PROMIS measures were assessed using unpaired t tests for fully corrected T scores (adjusted for age, gender, education, and race/ethnicity) or analyses of covariance for raw scores (with adjustments for age, gender, and Index of Social Position [ISP] calculated from the Hollingshead Four Factor Index of Socioeconomic Status).30 Cohen d effect sizes with 95% CIs were calculated using the R package effsize.
Group differences in BOLD activation throughout the brain were compared in SPM12, using 2-sample t tests (with the appropriate mask), thresholded at ≥100 voxel clusters and T ≥1.7. Only corrected p values at the cluster level, with a false discovery rate (FDR) ≤0.05, were considered significant. Because portions of the WM network may also be involved in emotional regulation,31 linear regression was used to explore whether the abnormal brain activation predicted neurobehavioral outcome measures that were abnormal on the NIHTB or PROMIS. BOLD signals were also extracted at the 3 cluster maxima (6 × 6 × 6 mm = 216 mm3) for each task to perform post hoc analyses.
Data Availability
The data that support the findings of this study are available from the corresponding author on reasonable request.
Results
Participant Characteristics
Between February 2021 and February 2022, 169 potential participants were prescreened by telephone, 61/169 (36%) were enrolled for additional onsite screening, and 57/61 (93%) fulfilled the study criteria and completed the behavioral studies and 53/61 (87%) completed the fMRI scans (Table 1). Fifty participants (29 post-COVID and 21 controls) with usable fMRI scans are reported (see Participant Flow Diagram, eFigure 1, links.lww.com/WNL/C748).
The participants with PCC were diagnosed 219 ± 137 days (∼7 months) earlier and had similar age, gender proportion, racial and ethnic distribution, handedness, education, and socioeconomic status (from the ISP) as the healthy controls. Although the PCC group tended to have higher body mass indices than the controls, the proportions of participants in the overweight/obese categories were not different between groups. The 2 groups also had similar proportions that used tobacco, marijuana, or alcohol and similar prevalence of comorbid illnesses, including hypertension, diabetes, and chronic obstructive pulmonary disease. Vaccination status for SARS-CoV-2 was also not different between groups.
Among the participants with PCC, 9/29 were hospitalized, and 19/29 required 1 or more treatments during their acute illness, which included supplemental oxygen (n = 11, nasal cannula O2, Hi-Flow/BiPAP, ventilation, or extracorporeal membrane oxygenation), steroids (n = 15, dexamethasone, prednisone, methylprednisolone, or hydrocortisone), remdesivir (n = 5), monoclonal antibodies (n = 3, bamlanivimab and etesevimab), antibiotics for secondary infections (n = 5, azithromycin and ceftriaxone), and/or apixaban for deep vein thrombosis (n = 1). See Table 1 for the number of participants who received 1 or more of these treatments.
Of the 50 usable fMRI datasets, 9 participants (6 post-COVID and 3 controls) had minor abnormalities on their structural MRIs that were not exclusionary. Five (3 participants with PCC and 2 controls) had slightly more than age-related white matter lesions, 2 (1 in each group) had lacunar infarcts, 1 control had microhemorrhages, and 1 control had both a small (6-mm) old infarct and a microhemorrhage.
Neuropsychiatric Symptoms in Participants With Post–COVID-19 Condition
The participants with PCC endorsed a high prevalence of cognitive complaints (concentration problems [92.9%], memory problems [78.6%], and confusion [64.3%]) and neurologic symptoms, including headaches [57.1%], visual disturbances [50%], gait disturbance [50%], paresthesia [42.9%], and coordination problems [39.3%]) (Table 1, Figure 1A). The common acute symptoms of hyposmia and dysgeusia persisted in 28.6% of participants with PCC. They also had a high prevalence of new-onset psychiatric and other symptoms, including fatigue (85.7%), depression/anxiety (67.9%), sleep disturbance (64.3%), myalgia (60.7%), lightheadedness (46.4%), and urinary problems (27.6%, including frequency, dysautonomia, and recurrent urinary tract infections).
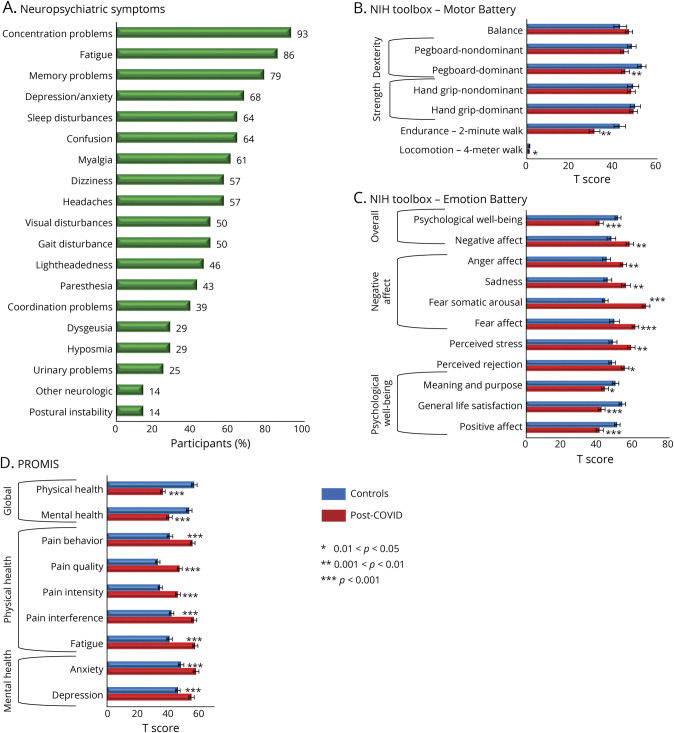
Figure 1. Prevalence of Neuropsychiatric Symptoms and the Assessment Scores From the NIHTB Batteries and the PROMIS.
(A) Self-reported neuropsychiatric symptoms by the participants with COVID-19. (B) NIH Toolbox Motor Battery. (C) NIH Toolbox Emotion Battery. (D) PROMIS. COVID-19 = coronavirus disease 2019; PROMIS = Patient-Reported Outcomes Measurement Information System.
NIH Toolbox Batteries and PROMIS Measures
Despite the high prevalence of neurocognitive complaints, the participants with PCC had normal performance on all 7 cognitive domains of the NIHTB-CB (data not shown, but similar to those reported in the larger cohort23). However, on the NIHTB-MB, the participants with PCC performed poorer than controls on locomotion (4-m walk, Cohen d = −0.74, 95% CI −0.14 to −1.34, p = 0.01), endurance (2-Minute Walk, d = −0.93, 95% CI −0.31 to −1.55, p = 0.003), and dominant hand dexterity (9-Hole Pegboard test, d = −0.79, 95% CI −0.19 to −0.38, p = 0.007) (Figure 1B). The 2 groups' performance was similar on the Standing Balance Test and the nondominant hand Pegboard.
In addition, on the NIHTB-EB (Figure 1C), the participants with PCC showed markedly poorer psychological well-being than controls, including lower positive affect (d = −1.02, 95% CI −0.41 to −1.63, p = 9 × 10−4) and general life satisfaction (d = −1.20, 95% CI −0.57 to −1.82, p = 1.3 × 10−4) and much higher perceived stress (d = 0.95, 95% CI 0.34–1.56, p = 0.002) and negative affect measures, including anger (d = 0.93, 95% CI 0.33–1.54, p = 0.002), sadness (d = 0.85, 95% CI 0.25–1.56, p = 0.004), fear somatic arousal (d = 2.23, 95% CI 1.50–2.96, p = 5 × 10−10), and fear affect (d = 1.04, 95% CI 0.43–1.65, p = 7 × 10−4).
PROMIS measures corroborated with the NIHTB-EB. The participants with PCC had much higher T scores than controls for mental health symptoms, including depression and anxiety, as well as for physical health symptoms, with more fatigue and pain measures, leading to poorer overall mental (d = −1.37, 95% CI −0.73 to −2.00, p = 2 × 10−5) and physical health (d = −2.59, 95% CI −1.81 to −3.37, p = 6 × 10−12) (Figure 1D).
BOLD-fMRI During WM Tasks
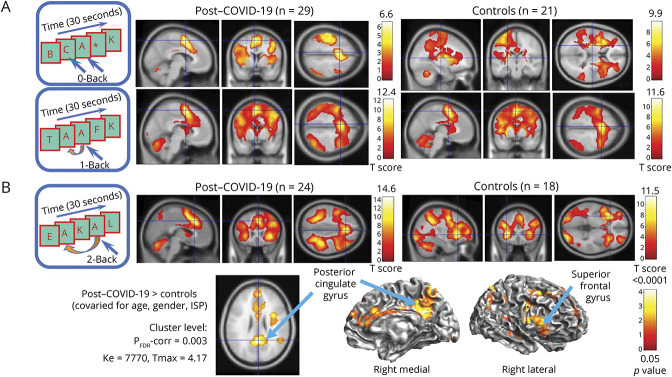
Across all participants, brain activation showed the typical load-dependent increases in the WM network with increasing task difficulty.24,25 Participants with PCC and controls showed no group differences in brain activation during the 0-back and 1-back tasks (Figure 2A). However, participants with PCC had greater brain activation than controls (T = 4.17, FDR-corrected p = 0.003) on the 2-back task for a large cluster in the WM network, with 3 subcluster maxima centered at the right posterior cingulate gyrus (PCG: 2, −50, 26 and 8, −56, 28) and right superior frontal gyrus (SFG: 36, 2, 28) (Figures 2B, 3, and 4).
Figure 2. Group fMRI Activation Maps and Group Comparison During the N-Back Tasks.
(A) The 0-back and 1-back task paradigms, as shown sequentially on the presentation computer during the fMRI, are illustrated on the left, and the typical activation maps for each group are shown in 3 orientations. No group differences were found on these tasks (hence, the group comparison data are not shown). (B) The 2-back task paradigm is illustrated on the left, and the group activation maps show the typical WM activation patterns, including activation in the bilateral dorsolateral or inferior prefrontal cortices, the anterior cingulate cortex, the precuneus and bilateral posterior parietal and occipital regions, and the cerebellum. Note the higher Tmax scores on the color scale for the 2-back task than the 0-back and 1-back tasks shown above. Cortical surface maps and an axial image below show the brain regions with greater activation in participants with post–COVID-19 compared with healthy controls on the 2-sample t tests (covaried for age, gender, and Index of Social Position). Clusters with corrected p ≤ 0.05 and ≥100 voxels are shown, with cluster maxima shown in the posterior cingulate gyrus and the superior frontal gyrus (see also Figure 3). COVID-19 = coronavirus disease 2019; WM = working memory.
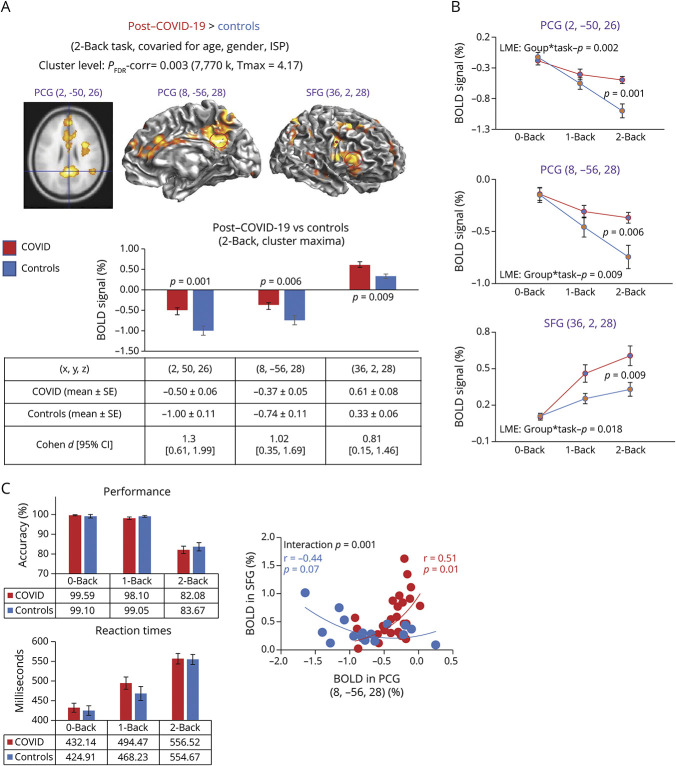
Figure 3. Brain Activation Differences (and %BOLD Signals) on the 2-Back Task and Performance During the N-Back Tasks.
(A) On the 2-back task, participants with post-COVID-19 condition had greater BOLD signals than uninfected controls, and subcluster maxima are shown in 2 subregions of the PCG and the SFG. Group comparisons were evaluated with analysis of covariance (covaried for age, gender, and ISP) at the cluster level p-corrected = 0.003, 7,770 voxels, and Tmax = 4.17. The bar graphs below show the % BOLD signal extracted from a 27-voxel region of interest (red circles) center at each of the 3 cluster maxima shown on the map, showing less deactivation in the PCG regions but greater activation in the SFG. (B) In each of the cluster maxima shown in A, BOLD signals from 0-back, 1-back, and 2-back tasks were extracted to illustrate that the 2 groups were different (on the linear mixed-effects model), and the differences are maximal and significant only with the 2-back task (post hoc p values are shown). (C) Performance with % accuracy and reaction times during the 3 N-back tasks show no group differences. Bottom graph: greater deactivation (more negative % BOLD signals) in the PCG predicted greater activation in the SFG only in the controls, whereas participants with post-COVID condition showed only minimal deactivation and greater activation in PCG was related to greater activation in PCG. BOLD = blood oxygenation level dependent; COVID-19 = coronavirus disease 2019; PCG = posterior cingulate gyrus; SFG = superior frontal gyrus.
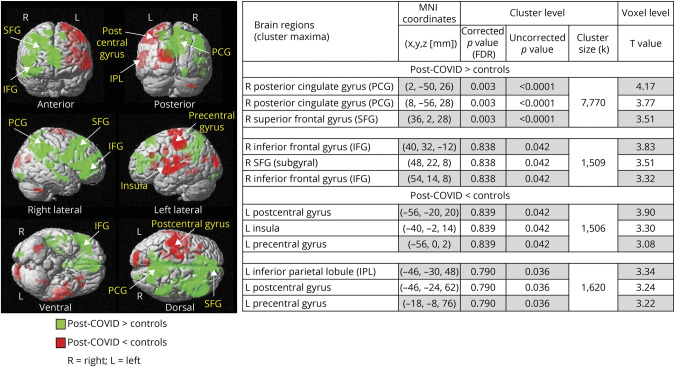
Figure 4. Regional Group Differences on Activation During the 2-Back Task.
The activation maps show both brain regions that are greater (green) or lesser (red) in the post-COVID condition group compared with the controls. The table on the right shows the MNI coordinates of where the group differences are located both with the corrected p values with FDR and the uncorrected p values at the cluster level, as well as the maximum T scores in each of the subclusters. The 3 significant clusters (at the FDR-corrected level) that are greater in post-COVID than controls are also shown in Figures 2 and 3. BOLD = blood oxygenation level dependent; COVID-19 = coronavirus disease 2019; FDR = false discovery rate; MNI = Montreal Neurological Institute; PCG = posterior cingulate gyrus; SFG = superior frontal gyrus.
Using extracted BOLD signals at the cluster maxima, we delineated that the greater BOLD signals in the participants with PCC than the controls were due to greater activation in the SFG region, but lesser deactivation in the PCG regions (Figure 3A). The extracted BOLD signals across the 3 tasks further demonstrate that the group differences increased parametrically (linear mixed-effects model p values = 0.002–0.018), with only the 2-back task showing significant group differences on post hoc analyses (post hoc p = 0.001–0.009) (Figure 3B). Despite these group differences in brain activation, the 2 groups had similar accuracy and reaction times for each task (Figure 3C). Furthermore, an anticorrelation between deactivation on the 2-back task in the PCG (the default mode region) and activation in the SFG was observed only for the control but not the post-COVID group (Figure 3C, bottom).
Several brain regions showed lesser activation on the 2-back task at the uncorrected cluster level in participants with post-COVID than controls (Figure 4). The 2 large clusters with lesser activation included the subcluster maxima in the left hemisphere for the postcentral gyrus, insula, precentral gyrus, and inferior parietal lobule (red regions, Figure 4). By contrast, all brain regions with greater activation on the 2-back task in the post-COVID group than controls were in the right hemisphere (green regions, Figure 4).
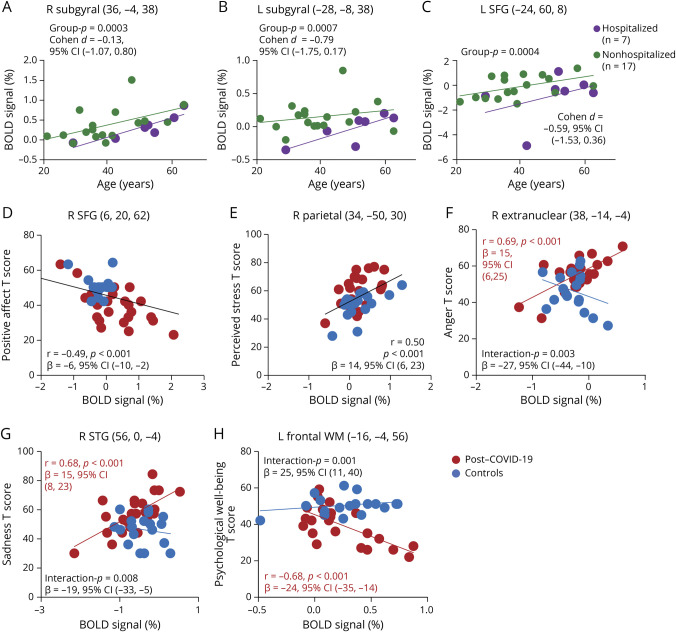
For the 2-back task, when comparing COVID-19 severity during the acute phase, subjects with PCC who were hospitalized with more severe illness showed lesser activation than the nonhospitalized individuals in several frontal regions (right and left subgyral and left SFG, FDR-corrected p = 0.001 [cluster level]). The extracted BOLD signals from these cluster maxima are shown (Figure 5, A–C). We further evaluated for possible gender-specific differences (COVID-19-by-gender, covaried for age and ISP) but found no gender-specific effects on brain activation.
Figure 5. Hospitalized Effect on Regional % BOLD Signals and Regional Brain Activation Predicted Emotional Symptoms on the NIHTB-Emotion Battery.
(A–C) Comparison of COVID-19 severity on brain activation during the 2-back task. The scatterplots show the %BOLD signals extracted from the cluster maxima in brain regions showing significantly less activation (FDR-corrected p = 0.001, cluster level) in hospitalized participants with post–COVID-19 (purple) than those who were not hospitalized (green), in the right and left subgyral region, and the left SFG across the age range. (D and E) Across all participants, those with higher % BOLD signals in the right SFG had lower scores for positive affect, and those with higher % BOLD signals in the right parietal region endorsed more perceived stress. (F–H) Lower regional activation predicted higher T scores on anger and sadness and lower level of psychological well-being only in the post–COVID-19 group. BOLD = blood oxygenation level dependent; COVID-19 = coronavirus disease 2019; FDR = false discovery rate; NIHTB = NIH Toolbox Battery; SFG = superior frontal gyrus; WM = working memory.
Furthermore, BOLD signals in the WM network on the 2-back task predicted T scores on the NIHTB-EB measures that showed significant group differences (eTable 1, links.lww.com/WNL/C750). Specifically, we found similar relationships for both groups in the right SFG and right parietal regions, where higher BOLD signals correlated with lower positive affect and more perceived stress (right SFG: r = 0.49, p < 0.001, β = −6, 95% CI −10 to −2; right parietal: r = 0.50, p < 0.001, β = 14, 95% CI 6–23; Figure 5, D and E). However, we also found differences (interactions) in the correlations between the 2 groups: only the COVID-19 group with higher BOLD signals at the right extranuclear region had greater anger affect (r = 0.69, p < 0.001, β = 15, 95% CI 6–25), higher BOLD signals at the right superior temporal gyrus correlated with more sadness (r = 0.68, β = 15, 95% CI 8–23, p < 0.001), and higher BOLD signals at the left frontal white matter correlated with lower psychological well-being (r = −0.68, β = −24, 95% CI −35 to −14, p < 0.001) (Figure 5, F–H).
Similarly, BOLD signals in the WM network on the 2-back task predicted the scores on PROMIS measures that showed significant group differences (eTable 1, links.lww.com/WNL/C750). Greater brain activation in the left frontal lobe on the 2-back task predicted more psychiatric symptoms and poorer mental health on the PROMIS (eFigure 2, links.lww.com/WNL/C749). Specifically, across all participants, greater BOLD signals in the left anterior cingulate gyrus predicted higher levels of anxiety (r = 0.51, p = 0.001, β = 18, 95% CI 10–27, eFigure 2A), greater BOLD signals in the left insular-subgyral region predicted more depressive symptoms (r = 0.78, p < 0.001, β = 31, 95% CI 20–42, eFigure 2B), and greater BOLD signals in the left inferior frontal gyrus predicted poorer global mental health (r = −0.51, p < 0.001, β = −7, 95% CI −10 to −3, eFigure 2C).
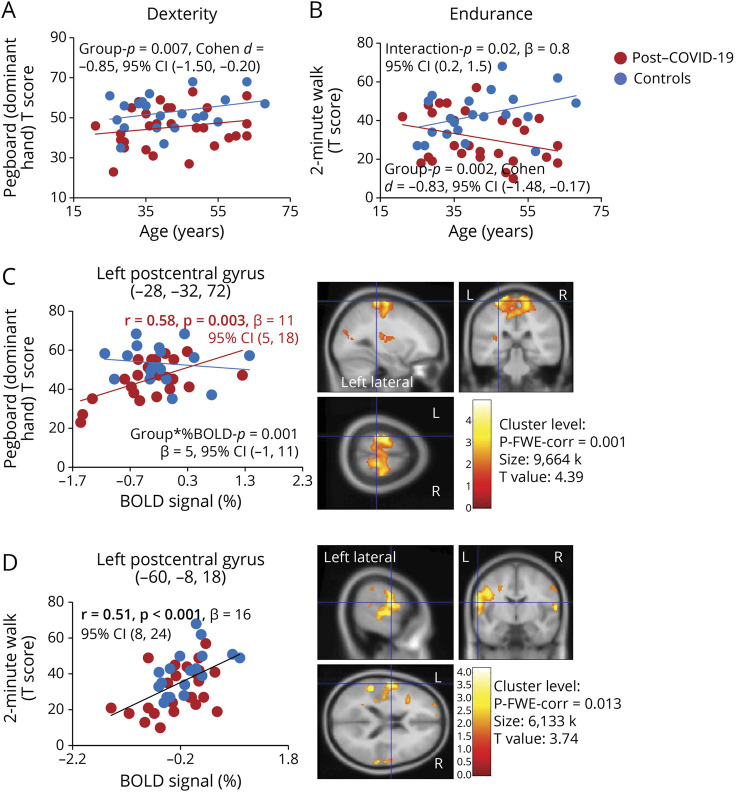
Last, on the NIHTB-MB, participants with prior COVID-19 had poorer dexterity than controls across the age spectrum (dominant hand, d = −0.85, group p = 0.007, 95% CI −1.50 to −0.20, Figure 6A) and poorer endurance (2-Minute Walk, d = −0.83, 95% CI 1.48 to −0.17, group p = 0.002), especially in the older participants (β = 0.8, 95% CI 0.2–1.5, group-by-age p = 0.02, Figure 6B). In brain regions that showed nonsignificant lesser activation in the participants with PCC than controls, lower BOLD signals in the left postcentral gyrus predicted poorer dexterity on the Pegboard dominant hand T scores only for the Participants with PCC (r = 0.58, p = 0.003, β = 11, 95% CI 5–18, Figure 6C), whereas lower BOLD signals in another region in the left postcentral gyrus predicted poorer endurance on the 2-Minute Walk across all participants (r = 0.51, p < 0.001, β = 16, 95% CI 8–24, Figure 6D). Although participants with PCC also had slower locomotion on 4-Meter Walk than the controls, none of the activated brain regions showed a correlation with the performance on this test.
Figure 6. Poorer Motor Performance Was Related to Lower % BOLD Signals in the Left Postcentral Gyrus (the Sensorimotor Cortex).
(A and B) Participants with post–COVID-19 (red dots) had slower performance on manual dexterity (9-Hole Pegboard dominant hand) and poorer endurance (2-Minute Walk) as shown by their lower T scores on these tasks compared with the healthy controls (blue dots) across the age spectrum, especially for the older participants on the 2-Minute Walk task. (C) Only participants with post–COVID-19 who had lesser brain activation in the left postcentral gyrus had poorer dexterity in their dominant hand; no such relationship was found in the healthy controls. (D) Across all participants, those with lesser brain activation in another region of the left postcentral gyrus had poorer endurance. BOLD = blood oxygenation level dependent; COVID-19 = coronavirus disease 2019; FWE = family-wise error.
Discussion
Participants with PCC, on average 7 months after their COVID-19 diagnosis, reported a high prevalence of neuropsychiatric symptoms, including brain fog and concentration or memory problems. Despite these subjective complaints, the participants with PCC had relatively normal performance on objective cognitive testing with the NIHTB-CB, including attention and WM, and during the fMRI tasks. However, they had poorer dexterity and endurance on the NIHTB-MB relative to both the healthy controls and the normative database, especially the older individuals with PCC. In addition, the greater emotional distress and negative affect endorsed on the NIHTB-EB and the mental health symptoms assessed on the PROMIS concurred with the post-COVID participants' complaints. Furthermore, our participants with postacute COVID-19 had poorer global physical health, attributed by significantly more fatigue and pain symptoms.
This task-activated BOLD-fMRI study evaluated recovered patients with COVID-19. Although the participants with PCC had normal and similar accuracy and reaction times as the controls, they had greater brain activation, with lesser deactivation in the right PCG and greater activation in the right SFG within the WM network on the more difficult 2-back task. These findings indicated a reorganized WM network, with greater or compensatory usage of the nondominant brain regions, but less usage of the parietal default mode resources, to maintain normal performance. Furthermore, greater brain activation predicted more severe neuropsychiatric symptoms in these recovered patients.
The normal performance in our participants with post-COVID assessed objectively in 7 cognitive domains on the NIHTB-CB is consistent with the normal Mini-Mental State Examination in 443 mainly nonhospitalized individuals, ∼9 months after their SARS-CoV-2 infection, relative to 1,328 matched controls.32 Another study evaluated 31 participants with long COVID, 202 ± 58 days after acute illness, who had neurocognitive complaints of impaired attention, memory, and multitasking abilities; these participants also had normal scores on neuropsychological tests and on the Montreal Cognitive Assessment.12 More sensitive tests that can assess fatigue and cognitive endurance are needed.
However, the post-COVID participants' higher T scores on psychological and emotional symptoms, perceived stress, and negative affect and their lower T scores on psychological well-being and general life satisfaction on the NIHTB-EB are consistent with their subjective complaints and were further corroborated by the higher levels of depressive and anxiety symptoms and poorer mental health on the PROMIS. These findings concur with a meta-analysis report that evaluated 10,530 patients and found a high prevalence of these symptoms in those with post-COVID syndrome at mid (3–6 months) to long-term (>6 months) follow-ups, which suggested that these symptoms might become even more prevalent over time.33
In addition, the elevated T scores on pain measures (behavior, quality, intensity, and interference) in the participants with PCC are also consistent with a comprehensive review of 54 reports that found a high prevalence of pain in 21,668 patients with COVID-19, including 33.9% who had headaches, 47.1% had a sore throat, 61.0% had myalgia or arthralgia, 17.7% had chest pain, and 14.5% had abdominal pain.34
Relative to the controls, our participants with post-COVID had poorer dexterity in their dominant hands across the age spectrum and lower endurance on the 2-Minute Walk test. The slower performance on the 2-Minute Walk test is similar to that reported in 37/66 (56%) hospitalized patients with COVID at 3–6 months of follow-up,35 but the abnormality persisted in only 14/161 (8.7%) hospitalized patients 1 year later.35 Similarly, lower endurance on the 6-Minute Walking Test was reported in 79% of hospitalized patients with COVID at 6-week follow-up36 and in only 29% of those at 6-month follow-up.37 Although two-thirds of our participants with PCC were not hospitalized, they showed relatively lower endurance, especially the older participants.
During WM tasks, men typically activate bilaterally or predominantly the right hemisphere network, whereas women typically show greater activation lateralized to the left hemisphere.27 However, although two-thirds of our participants with PCC were women, they had greater activation in the nondominant right hemisphere, which indicates a reorganized neural network, with suboptimal activation in the normal network but greater activation in alternate brain regions, to maintain normal performance during the N-back tasks, and likely on the NIHTB-CB. Such a reorganized neural network was also observed in people with HIV, who showed similar adaptation of their neural network during task-activated BOLD-fMRI, even in cognitively asymptomatic individuals.28,29
The cluster maxima in the PCG coincided with the well-described medial parietal node in the default mode network (DMN), showing that greater activation in this region resulted from lesser deactivation in the COVID-19 group than the control group. Such diminished or deficits in task-induced deactivation in the DMN were also found in individuals with mild Alzheimer disease (AD),38 those with only the APOE-ε4 genetic risk for AD,39 and middle-aged individuals with subclinical cognitive decline.40 Other brain disorders with lower than normal deactivation in the DMN include schizophrenia,41 idiopathic generalized epilepsy,42 attention deficit/hyperactivity disorder,43 and obsessive-compulsive disorders.44 Less deactivation in the DMN suggests less brain reserve because the deactivation might provide reallocation of neural resources for other brain regions or networks. The relatively greater SFG activation in our participants with PCC is consistent with greater attentional modulation of the top-down dorsal attentional network. Greater activation in this region of the brain is often observed with brain injury in those with chronic neuroinflammation, such as that seen in persons with HIV infection25,28,29 or mild traumatic brain injury.45 Because the anti-correlation between greater deactivation in the DMN and the greater activation in SFG was seen only in the controls, the participants with post-COVID might have deficits in the DMN due to COVID-19–induced brain injury, requiring compensatory usage of alternative brain regions in the contralateral right hemisphere, such as the greater activation in the SFG.
Greater activation in the WM network predicted less positive affect and more perceived stress across all participants, but more symptoms of anger and sadness, and poorer psychological well-being only in the participants with post-COVID. The associations across all participants might be due to the greater stress from the pandemic, whereas the associations between greater left frontal activation and the psychological symptoms might reflect the greater severity of these symptoms in the participants with PCC. Although the exact pathophysiology of PCC remains unclear, peripheral immune markers such as C-reactive protein and peripheral inflammatory markers including lymphocytes and platelets correlated with greater severity of depressive symptoms in patients with PCC.46,47 Future studies evaluating both peripheral and CSF immune markers may provide further insights into these relationships. The altered brain activation was observed only with the higher attentional/WM load, suggesting that higher cognitive demand in their daily lives could further contribute to the negative psychological and psychiatric symptoms in participants with PCC.
Last, lesser brain activation on the 2-back task within the left dominant postcentral sensorimotor gyrus predicted poorer dominant hand dexterity in the participants with PCC and lower endurance with the 2-Minute Walk across all participants. These findings further delineate the relationships between the sensorimotor cortex and motor function.
Our study has several limitations. First, the moderate sample size precluded the evaluation of further subgroup analyses, such as gender-specific effects, group differences in age-dependent changes, or more detailed evaluation of disease severity, in relation to the symptoms associated with PCC. Second, this study was conducted primarily during the Delta variant phase in the United States; therefore, how the new variants might affect the brain could not be assessed. Third, without SARS-CoV-2 antibody testing, we cannot exclude the remote possibility of prior asymptomatic infections in the controls. Furthermore, longitudinal follow-ups are needed because some long COVID symptoms decreased at 2-year follow-up,48 whereas psychiatric or cognitive function may worsen over time,33 especially in older survivors of COVID-19.49 Last, due to the study's cross-sectional design, the abnormal brain activation in the post-COVID group cannot be causally attributed to COVID-19.
In summary, the NIHTB and PROMIS measures quantified the post-COVID participants' neuropsychiatric symptoms, including high levels of fatigue, pain and emotional symptoms, and motor deficits, but demonstrated normal cognitive function. On BOLD-fMRI, these participants with post–COVID-19 had a reorganized network with less activation indicating suboptimal functioning in the normal network, but greater brain activation in the contralateral hemisphere, likely to maintain normal performance during the WM tasks and on the NIHTB-CB. The abnormal brain activation predicted poorer motor dexterity and endurance, as well as neuropsychiatric symptoms, which might support the WM network's involvement in emotional regulation.31 Task-activated BOLD-fMRI is sensitive and objective for evaluating post-COVID brain abnormalities, especially with a parametric design using increasing cognitive load (i.e., a brain stress test). Future longitudinal follow-ups using these quantitative measures and task-activated BOLD-fMRI may allow us to delineate whether or when these altered brain activation patterns and the neuropsychiatric symptoms will normalize. Finally, correlations between immune or neuronal injury markers and other brain imaging biomarkers (e.g., MR spectroscopy50) are needed to further delineate the possible relationship between immune activation and persistent brain injury in participants with post-COVID condition.
Acknowledgment
The authors thank their research participants for their participation. They also thank Dr. Andrea Levine for referring some of the participants with post-COVID condition to the study.
Glossary
- AD
Alzheimer disease
- BOLD-fMRI
blood oxygenation level–dependent fMRI
- COVID-19
coronavirus disease 2019
- DMN
default mode network
- FDR
false discovery rate
- ISP
Index of Social Position
- NIHTB
NIH-Toolbox Battery
- NIHTB-CB
NIHTB for Cognition Battery
- NIHTB-EB
NIHTB for Emotion Battery
- NIHTB-TB
NIHTB for Motor Function Battery
- PCC
post-COVID condition
- PCG
posterior cingulate gyrus
- PROMIS
Patient-Reported Outcomes Measurement Information System
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SFG
superior frontal gyrus
- SPM
Statistical Parametric Mapping
- TE
echo time
- TI
inversion time
- TR
repetition time
- WM
working memory
Appendix. Authors

Study Funding
This work was supported by a grant from the National Institute of Neurological Disorders and Stroke (R21-NS121615).
Disclosure
S. Kottilil is a member of the Scientific Advisory Board at Merck, Regeneron, Silverback Therapeutics, Zhuhai Yufan Biotechnologies, and The Liver Company and has received grants paid to the institution from Gilead Sciences and Arbutus Pharmaceuticals. L. Chang, M.C. Ryan, H. Liang, X. Zhang, E. Cunningham, J. Wang, E. Wilson, E.H. Herskovits, and T. Ernst report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Chen C, Haupert SR, Zimmermann L, Shi X, Fritsche LG, Mukherjee B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a meta-analysis and systematic review. J Infect Dis. 2022;226(9):1593-1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carfi A, Bernabei R, Landi F, Group GAC-P-ACS. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324(6):603-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021;12:698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. 2022;9(10):815-827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Leon S, Wegman-Ostrosky T, Perelman C, et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021;11(1):16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee MH, Perl DP, Nair G, et al. Microvascular injury in the brains of patients with Covid-19. N Engl J Med. 2021;384(5):481-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colombo D, Falasca L, Marchioni L, et al. Neuropathology and inflammatory cell characterization in 10 autoptic COVID-19 brains. Cells. 2021;10(9):2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutkai I, Mayer MG, Hellmers LM, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. 2022;13(1):1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hosp JA, Dressing A, Blazhenets G, et al. Cognitive impairment and altered cerebral glucose metabolism in the subacute stage of COVID-19. Brain. 2021;144(4):1263-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donegani MI, Miceli A, Pardini M, et al. Brain metabolic correlates of persistent olfactory dysfunction after SARS-CoV-2 infection. Biomedicines. 2021;9(3):287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guedj E, Campion JY, Dudouet P, et al. 18F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. 2021;48(9):2823-2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dressing A, Bormann T, Blazhenets G, et al. Neuropsychologic profiles and cerebral glucose metabolism in neurocognitive long COVID syndrome. J Nucl Med. 2022;63(7):1058-1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu Y, Li X, Geng D, et al. Cerebral micro-structural changes in COVID-19 patients: an MRI-based 3-month follow-up study. EClinicalMedicine. 2020;25:100484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douaud G, Lee S, Alfaro-Almagro F, et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604(7907):697-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qin Y, Wu J, Chen T, et al. Long-term microstructure and cerebral blood flow changes in patients recovered from COVID-19 without neurological manifestations. J Clin Invest. 2021;131(8):e147329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang L, Zhou M, Li L, et al. Characteristics of mental health implications and plasma metabolomics in patients recently recovered from COVID-19. Transl Psychiatry. 2021;11(1):307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang H, Ernst T, Oishi K, et al. Abnormal brain diffusivity in participants with persistent neuropsychiatric symptoms after COVID-19. Neuroimmun Pharmacol Ther. 2023;2(1):37-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang S, Zhou Z, Yang D, et al. Persistent white matter changes in recovered COVID-19 patients at the 1-year follow-up. Brain. 2021;145(5):1830-1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weintraub S, Dikmen SS, Heaton RK, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80(11 suppl 3):S54-S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Babakhanyan I, McKenna BS, Casaletto KB, Nowinski CJ, Heaton RK. National Institutes of Health Toolbox Emotion Battery for English- and Spanish-speaking adults: normative data and factor-based summary scores. Patient Relat Outcome Meas. 2018;9:115-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reuben DB, Magasi S, McCreath HE, et al. Motor assessment using the NIH Toolbox. Neurology. 2013;80(11 suppl 3):S65-S75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cella D, Riley W, Stone A, et al. The Patient-Reported Outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005-2008. J Clin Epidemiol. 2010;63(11):1179-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ryan MC, Liang H, Wilson E, et al. Quantifying the neuropsychiatric symptoms in post-acute sequelae of COVID-19 (PASC) using the NIH Toolbox and PROMIS. Neuroimmun Pharmacol Ther. Epub 15 Aug 2022. [DOI] [PMC free article] [PubMed]
- 24.Tomasi D, Ernst T, Caparelli EC, Chang L. Common deactivation patterns during working memory and visual attention tasks: an intra-subject fMRI study at 4 Tesla. Hum Brain Mapp. 2006;27(8):694-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang L, Lohaugen GC, Andres T, et al. Adaptive working memory training improved brain function in human immunodeficiency virus-seropositive patients. Ann Neurol. 2017;81(1):17-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44(3):457-465. [DOI] [PubMed] [Google Scholar]
- 27.Speck O, Ernst T, Braun J, Koch C, Miller E, Chang L. Gender differences in the functional organization of the brain for working memory. Neuroreport. 2000;11:2581-2585. [DOI] [PubMed] [Google Scholar]
- 28.Ernst T, Chang L, Jovicich J, Ames N, Arnold S. Abnormal brain activation on functional MRI in cognitively asymptomatic HIV patients. Neurology. 2002;59(9):1343-1349. [DOI] [PubMed] [Google Scholar]
- 29.Chang L, Tomasi D, Yakupov R, et al. Adaptation of the attention network in human immunodeficiency virus brain injury. Ann Neurol. 2004;56(2):259-272. [DOI] [PubMed] [Google Scholar]
- 30.Hollingshead A. Four-Factor Index of Social Status. Yale University; 1975. [Google Scholar]
- 31.Lee TW, Xue SW. Does emotion regulation engage the same neural circuit as working memory? A meta-analytical comparison between cognitive reappraisal of negative emotion and 2-back working memory task. PLoS One. 2018;13(9):e0203753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petersen EL, Goßling A, Adam G, et al. Multi-organ assessment in mainly non-hospitalized individuals after SARS-CoV-2 infection: the Hamburg City Health Study COVID programme. Eur Heart J. 2022;43(11):1124-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Premraj L, Kannapadi NV, Briggs J, et al. Mid and long-term neurological and neuropsychiatric manifestations of post-COVID-19 syndrome: a meta-analysis. J Neurol Sci. 2022;434:120162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weng LM, Su X, Wang XQ. Pain symptoms in patients with coronavirus disease (COVID-19): a literature review. J Pain Res. 2021;14:147-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baricich A, Borg MB, Cuneo D, et al. Midterm functional sequelae and implications in rehabilitation after COVID-19: a cross-sectional study. Eur J Phys Rehabil Med. 2021;57(2):199-207. [DOI] [PubMed] [Google Scholar]
- 36.Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID-19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pihlajamaki M, DePeau KM, Blacker D, Sperling RA. Impaired medial temporal repetition suppression is related to failure of parietal deactivation in Alzheimer disease. Am J Geriatr Psychiatry. 2008;16(4):283-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Persson J, Lind J, Larsson A, et al. Altered deactivation in individuals with genetic risk for Alzheimer's disease. Neuropsychologia. 2008;46(6):1679-1687. [DOI] [PubMed] [Google Scholar]
- 40.Hansen NL, Lauritzen M, Mortensen EL, et al. Subclinical cognitive decline in middle-age is associated with reduced task-induced deactivation of the brain's default mode network. Hum Brain Mapp. 2014;35(9):4488-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anticevic A, Cole MW, Murray JD, Corlett PR, Wang XJ, Krystal JH. The role of default network deactivation in cognition and disease. Trends Cogn Sci. 2012;16(12):584-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Parsons N, Bowden SC, Vogrin S, D'Souza WJ. Default mode network dysfunction in idiopathic generalised epilepsy. Epilepsy Res. 2020;159:106254. [DOI] [PubMed] [Google Scholar]
- 43.Metin B, Krebs RM, Wiersema JR, et al. Dysfunctional modulation of default mode network activity in attention-deficit/hyperactivity disorder. J Abnorm Psychol. 2015;124(1):208-214. [DOI] [PubMed] [Google Scholar]
- 44.Goncalves OF, Soares JM, Carvalho S, et al. Patterns of default mode network deactivation in obsessive compulsive disorder. Sci Rep. 2017;7(1):44468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAllister TW, Sparling MB, Flashman LA, Guerin SJ, Mamourian AC, Saykin AJ. Differential working memory load effects after mild traumatic brain injury. Neuroimage. 2001;14(5):1004-1012. [DOI] [PubMed] [Google Scholar]
- 46.Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guo Q, Zheng Y, Shi J, et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: a mixed-method study. Brain Behav Immun. 2020;88:17-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huang L, Li X, Gu X, et al. Health outcomes in people 2 years after surviving hospitalisation with COVID-19: a longitudinal cohort study. Lancet Respir Med. 2022;10(9):863-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu YH, Chen Y, Wang QH, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol. 2022;79(5):509-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Saleh MG, Chang L, Liang H, et al. Ongoing oxidative stress in individuals with post-acute sequelae of COVID-19. Neuroimmun Pharmacol Ther. Epub 15 Aug 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author on reasonable request.