Abstract
Background and Objectives
Motoric cognitive risk (MCR) syndrome is a type of pre-dementia. It is defined as the co-occurrence of subjective cognitive complaints and a slow gait speed. A recent study found that handgrip strength (HGS) asymmetry is associated with an increased risk of neurodegenerative disorders. We aimed to investigate the associations of HGS weakness and asymmetry separately and together with MCR incidence among older Chinese adults.
Methods
Data from the 2011 and 2015 waves of the China Health and Retirement Longitudinal Study were used. HGS values <28 kg for male participants and <18 kg for female participants were considered HGS weaknesses. HGS asymmetry was assessed by the ratio of nondominant to dominant HGS. We used 3 different cutoff values of HGS ratio to define asymmetry, including 10%, 20%, and 30%. Specifically, HGS ratios <0.90 or >1.10 (10%), <0.80 or >1.20 (20%), and <0.70 or >1.30 (30%) were classified as asymmetry. The participants were classified into 4 groups: neither weakness nor asymmetry (neither), asymmetry only, weakness only, and weakness and asymmetry (both). The association between baseline HGS status and 4-year incidence of MCR was examined using logistic regression analyses.
Results
A total of 3,777 participants 60 years and older were included in the baseline analysis. The prevalence of MCR at the baseline was 12.8%. Participants with asymmetry only, weakness only, and both showed significantly increased risk of MCR. After excluding participants with MCR at baseline, 2,328 participants were included in the longitudinal analysis. There were 111 MCR cases (4.77%) over the 4-year follow-up period. Participants with HGS weakness and asymmetry together at baseline had increased odds of incident MCR (HGS ratio at 10%: odds ratio [OR] 4.48, p < 0.001; HGS ratio at 20%: OR 5.43, p < 0.001; HGS ratio at 30%: OR 6.02, p < 0.001).
Discussion
These results show that the presence of both HGS asymmetry and weakness is associated with MCR incidence. The early recognition of HGS asymmetry and weakness may be helpful in the prevention and treatment of cognitive dysfunction.
Motoric cognitive risk (MCR) syndrome is an intermediate state between normal aging and dementia, characterized by the presence of both objective slow gait (SG) speed and subjective cognitive complaints (SCCs).1 MCR can predict incident cognitive impairment2 and dementia.1,2 In previous studies, MCR could be used to independently predict a wide range of adverse outcomes, including falls,3 disability,4 hospitalization,3 and mortality.5 The prevalence of MCR was approximately 10% in older adults worldwide.6 SCCs and SG last approximately 12–15 years before the subsequent mild cognitive impairment stage.7,8 Therefore, targeting MCR and its associated risk factors is important for developing early effective intervention to cognitive impairment.
Handgrip strength (HGS) is a convenient and common assessment of muscle function9 and is an indicator for the diagnosis of sarcopenia and frailty.10,11 Reduced HGS strongly predicts falls,12 hospitalization,13 poorer cognitive functioning,14 and mortality.15 Of particular relevance to this study, HGS is related to the individual components of MCR syndrome. Lower HGS predicts cognitive complaints16 and steeper declines in gait speed over time.17 However, muscle function cannot be fully assessed using maximal HGS because it only reflects the function of one hand. Recently, HGS asymmetry, characterized by a large difference in strength of both hands, has emerged as another dimension of impaired muscle function.18,19 HGS asymmetry is associated with future functional deficits, including functional disability,20 decline in cognitive function,21 falls and fractures,22,23 and accelerated mortality.24
Studies on the association between HGS weakness and asymmetry alone and in combination with MCR are limited among older adults. Regarding the mechanism, the grip strength generated in the HGS evaluation process is partially regulated by the nervous system that mediates the coordination of motor control.25 HGS asymmetry is associated with neurodegenerative disorders.25 Therefore, we hypothesized that HGS weakness and asymmetry together are associated with an increased risk of MCR compared with individuals with HGS weakness or asymmetry only or neither. This study investigated the cross-sectional and longitudinal associations between HGS weakness only, HGS asymmetry only, and the presence of both HGS weakness and asymmetry and MCR syndrome in older adults using a large sample from the China Health and Retirement Longitudinal Study (CHARLS) to examine the role of incorporating HGS asymmetry assessment on MCR risk.26 This may lead to early identification of MCR and improvement in the prevention and treatment of MCR to slow the process of cognitive decline.
Methods
Study Population
This study used wave1 (2011) and wave2 (2015) data from the CHARLS data set. CHARLS is a national longitudinal study on middle-aged and elderly people (45 years and older) in China. Information on demographics, health, economic, and social circumstances was collected. The study design and survey instruments have been published elsewhere.26
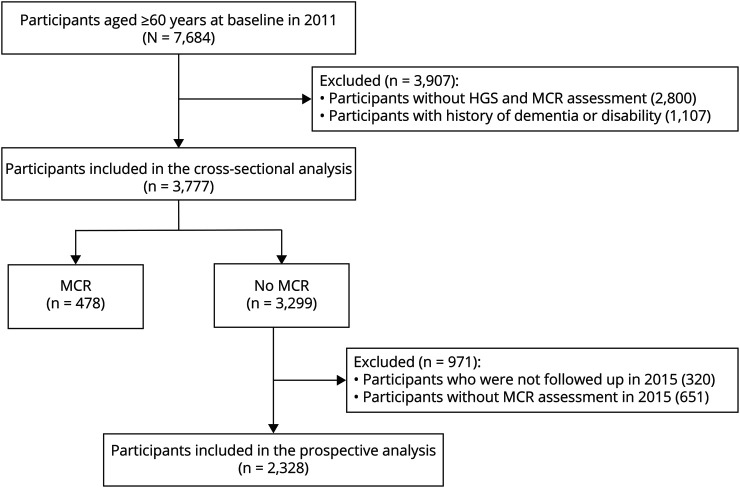
In this study, 3,777 participants 60 years and older in 2011 were included. HGS was measured in 2011 (baseline) while SCCs and gait speed were assessed at baseline and during the follow-up survey in 2015. Participants with a history of dementia or disability at baseline were excluded from this study. In addition, people who had MCR at baseline, were not successfully followed up, and did not complete MCR assessment in 2015 were also excluded from the longitudinal analyses. Finally, 2,328 participants were included in the prospective analyses. A detailed selection of study population is described in Figure 1.
Figure 1. Study Flow.
HGS = handgrip strength; MCR = motoric cognitive risk.
Standard Protocol Approvals, Registrations, and Patient Consents
CHARLS was approved by the Biomedical Ethics Review Committee of Peking University (IRB00001052–11015). All participants signed informed consent before data collection.
HGS Weakness and Asymmetry
HGS was tested using a mechanical dynamometer (Yuejian WL-1000, Nantong, China).26 The dominant hand of each participant was recorded. Participants started the test in a standing position with the elbow flexed at a 90° angle. The participants were asked to perform the task twice with each hand. The highest HGS value for the dominant hand was used in our analysis. Weakness was defined using the Asian Work Group for Sarcopenia 2019 consensus (maximal HGS <28 kg in male participants and <18 kg in female participants).27 The HGS ratio was calculated as the maximal HGS of the nondominant hand to the maximal HGS of the dominant hand. Following previous studies, the 10% rule was used to define HGS asymmetry, which indicated that the HGS of the dominant hand was generally 10% greater than that of the nondominant hand.24,28 Therefore, asymmetry was defined by an HGS ratio of <0.90 or >1.10. To further explore the relationship between different HGS ratios and MCR, we also defined HGS asymmetry as 20% and 30% rule. Specifically, asymmetry was defined by the HGS ratio of <0.80 or >1.20 (20%) or HGS ratio <0.70 or >1.30 (30%). Figure 2 depicts the proportions of persons with different HGS ratios.
Figure 2. Percentages of Different HGS Ratios.
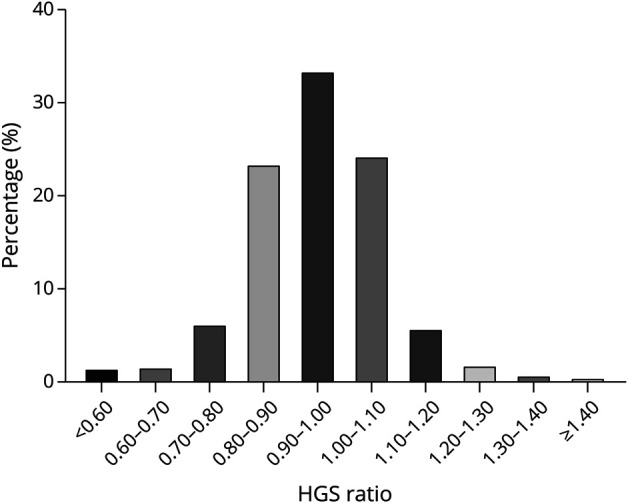
HGS = handgrip strength.
MCR Syndrome
MCR was defined using the criteria proposed by Verghese et al.2,29 and included diagnosis with SCC and SG, but without dementia or mobility disability. The analytical sample included 3,777 participants 60 years and older in 2011 after excluding (1) participants with missing data on both HGS and MCR assessments, (2) participants with self-reported dementia or probable dementia based on a cognitive function score less than 6 in the CHARLS,30 and (3) participants with self-reported physical disability or mobility disability defined as needing assistance when performing activities of daily living. SCCs were measured based on a “fair” or “poor” response to the following question: “How would you rate your memory at the present time?” Finally, infrared sensors were used to measure the speed of usual-pace walking over a 4-m distance.31 SG was defined by gait speed greater than or equal to 1 standard deviation below the average of age and sex-specific values.31 The cutoff values of SG for participants younger than 75 years and those 75 years and older were 0.50 and 0.39 m/s, respectively, in men and 0.46 and 0.39 m/s, respectively, in women (eTable 1, links.lww.com/WNL/C772), which were similar to those in previous studies.29
Covariates
Potential confounders were controlled for by evaluating the following participant characteristics: sociodemographic, lifestyle, and health characteristics at baseline, including age, sex (male/female), residence (urban/rural), marital status (married/widowed/others), education (illiteracy/unfinished primary school/primary school/middle school/high school and above), smoking status (never/former/current smoke), drinking status (never/former/current drink), body mass index (BMI), and history of diabetes, heart disease, hypertension, and stroke (yes/no).
Statistical Analysis
The HGS status was used to classify participants into 4 groups at baseline: (1) neither weakness nor asymmetry (neither), (2) asymmetry only, (3) weakness only, and (4) weakness and asymmetry (both). Analysis of variance and the χ2 test were used to evaluate the differences in the baseline sociodemographic, lifestyle, and health characteristics across the 4 groups. Univariate and multivariate logistic regression analyses were performed to calculate odds ratios (ORs) and 95% CIs of the association between baseline HGS status and MCR in cross-sectional analyses, as well as cumulative incident cases over the 4 years. The covariates adjusted in the multivariate logistic regression model included age, sex, residence, marital status, education, smoking status, drinking status, and history of chronic diseases at baseline. In longitudinal analyses, follow-up time was also adjusted. All analyses were performed using Stata 15.1 software (StataCorp, College Station, TX), and p < 0.05 was considered statistically significant.
Data Availability
The data for CHARLS are available at charls.ccer.edu.cn/charls/.
Results
Baseline Characteristics of Study Population
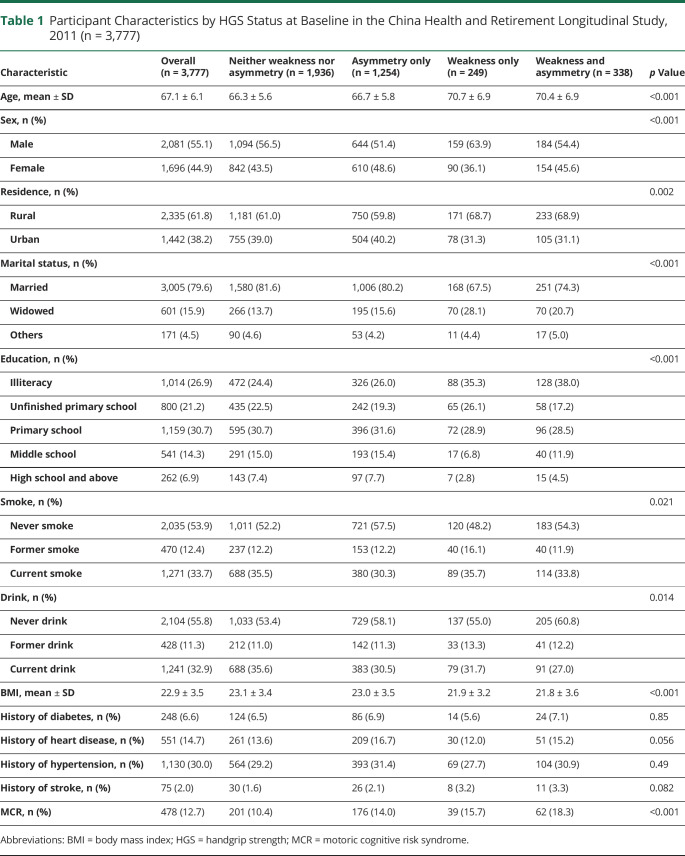
The baseline characteristics of the participants are listed in Table 1. Among the 3,777 participants 60 years and older, 478 (12.7%) were diagnosed with MCR syndrome. The mean age was 67.1 ± 6.1 years, and 44.9% were female. The prevalence of HGS asymmetry only (HGS ratio at 10%), weakness only, and asymmetry and weakness together were 33.2%, 6.6%, and 10.3%, respectively. Participants with HGS asymmetry only, weakness only, and asymmetry and weakness together had a higher MCR prevalence than those with neither weakness nor asymmetry (14.0%, 15.7%, and 18.3% vs 10.4%, respectively). Participant characteristics were compared according to their HGS status. Participants with HGS asymmetry and weakness were older than those with normal HGS. Except for a history of chronic diseases, all other characteristics, including sociodemographic, lifestyle, and BMI, were significantly different among the 4 groups (Table 1).
Table 1.
Participant Characteristics by HGS Status at Baseline in the China Health and Retirement Longitudinal Study, 2011 (n = 3,777)
Cross-sectional Association Between HGS Status and MCR
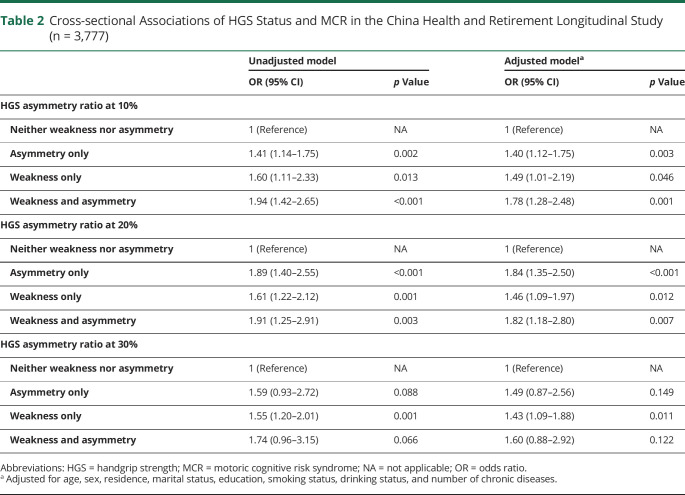
The cross-sectional associations between the HGS status and MCR are shown in Table 2. Individuals with HGS asymmetry only, weakness only, and both had a higher prevalence of MCR in the crude and adjusted models when the HGS asymmetry ratio was at 10% and 20% (all, p < 0.05). After adjustment, compared with the neither group, individuals with both experienced the greatest odds for MCR (10% rule: OR 1.78, 95% CI 1.28–2.48; 20% rule: OR 1.82, 95% CI 1.18–2.80), with weakness only being associated with 1.49-fold (10% rule) and 1.46-fold (20% rule) higher odds of MCR and asymmetry only being associated with 1.40-fold (10% rule) and 1.84-fold (20% rule) higher odds of MCR. When the HGS ratio was at 30%, only participants with weakness only had higher odds of MCR.
Table 2.
Cross-sectional Associations of HGS Status and MCR in the China Health and Retirement Longitudinal Study (n = 3,777)
Longitudinal Association Between HGS Status and MCR
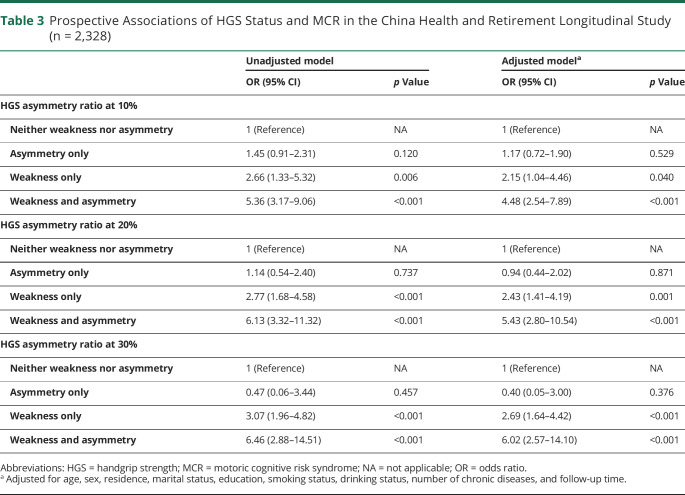
The longitudinal associations between HGS status and MCR over 4 years of follow-up (mean follow-up time was 47.8 months) are presented in Table 3. The 4-year incidence of MCR was 4.77% for all participants without MCR at baseline. After adjusting all covariates, HGS weakness only and asymmetry and weakness together were significantly associated with an increased risk of MCR (weakness only: OR 2.15, 95% CI 1.04–4.46; both: OR 4.48, 95% CI 2.54–7.89). The results remained similar with the HGS ratio at 20% and 30%. The higher the degree of asymmetry, the higher the risk of MCR in participants with weakness and asymmetry together. We also adjusted baseline gait speed in the longitudinal analyses to investigate whether handgrip could provide incremental validity for incident MCR over measuring gait, and the results remained robust (eTable 2, links.lww.com/WNL/C772). The results were similar when adjusting for baseline MCR status (eTable 3).
Table 3.
Prospective Associations of HGS Status and MCR in the China Health and Retirement Longitudinal Study (n = 2,328)
Discussion
This study revealed that the presence of both HGS weakness and asymmetry was robustly associated with MCR in a nationally representative sample of older Chinese adults. This study indicated that HGS and asymmetry are both associated with MCR risk, in an additive manner.
Consistent with a previous study in China,16 the prevalence of MCR at baseline in the current study was 12.7%, which was higher than that in Europe.6 Our findings were consistent with previous studies that found that HGS decline was associated with higher odds of prevalent MCR and incident MCR. A previous cross-sectional study conducted in Chinese community-dwelling elderly individuals showed that muscle weakness was associated with MCR in male participants.16 Another study conducted by Zhang et al.32 suggested that sarcopenia was independently associated with higher odds of MCR among older Chinese individuals. These 2 studies were based on cross-sectional analyses. Our study further confirmed these findings through longitudinal analysis.
Furthermore, our results show that the presence of both HGS asymmetry and weakness are more strongly associated with risk of MCR than either HGS asymmetry or HGC weakness alone. In this study, nearly one-third of participants had asymmetric HGS (HGS ratio at 10%). The risk of incident MCR increases with the increase of asymmetry degree. Although few studies have investigated the relationship between HGS weakness and asymmetry together and MCR, the link between HGS weakness and asymmetry and cognitive decline has been reported in a previous study.21 MCR and cognitive decline are involved in the pathogenesis of dementia. Maximum grip strength requires complex coordination behavior, including the participation of a large number of motor units and the activation of brain networks.33 Dysfunction of the nervous system or brain may lead to decreased coordination of various tasks, such as difficulty in getting things, more time required to complete tasks, decreased strength, and HGS asymmetry.25,34 HGS asymmetry may indicate different activation of the cerebral hemisphere and imbalance of neural function, which may explain our results and why previous studies have found that elderly people with HGS asymmetry and weakness have a higher risk of neurodegenerative diseases and why HGS asymmetry may indicate25 disability20 and cognitive decline.21 However, the precise mechanism of the relationship between HGS asymmetry and weakness and MCR remains unclear and requires further study. Our results indicated that the presence of both HGS weakness and asymmetry may exacerbate the cognitive decline. We recommend collecting maximal HGS data and examining HGS asymmetry in clinical and epidemiologic settings. We can evaluate the risk of MCR by measuring HGS and asymmetry before the SCCs or declined physical function occurs.
Our study has several strengths. First, this study investigated the separate and combined effects of HGS asymmetry and weakness on the risk of MCR in older adults. Second, the prospective study design and large sample size made the results more reliable. Third, we not only investigated asymmetry defined by a HGS ratio at 10% according to previous studies18 but also HGS at 20% and 30%. Our study has some limitations. First, participants who were unable to perform the HGS evaluation were excluded in this study. Therefore, our conclusion might not be generalized to the general older population. Second, there may be selection bias because we excluded participants who were lost to follow-up or had missing data on MCR and HGS during the 2 waves. Finally, HGS may naturally vary between hands.35 A standardized HGS asymmetry cutoff value could be warranted.18
Older adults with HGS weakness and asymmetry had a higher risk of future MCR, thereby suggesting that having both HGS weakness and asymmetric strength could reveal whether individuals are at a greater risk of MCR than others. Handgrip and asymmetry, a safe and quick method of assessing muscle function, are associated MCR in an additive manner. The HGS asymmetry assessment may be considered as an addition to HGS programs for early screening of individuals with higher risk of MCR and other diseases, as well as interventions for improving muscle strength and correcting strength asymmetry.
Acknowledgment
We thank the CHARLS team for collecting and providing data publicly to researchers. We thank all participants and staffs involved in this research.
Glossary
- BMI
body mass index
- CHARLS
China Health and Retirement Longitudinal Study
- HGS
handgrip strength
- MCR
motoric cognitive risk
- OR
odds ratio
- SCC
subjective cognitive complaint
- SG
slow gait
Appendix. Authors
Study Funding
This work was partially supported by the National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Y2022JC004); 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD20010); the National Key R&D Program of China (2018YFC2002400); and the Chengdu Science and Technology Bureau Major Science and Technology Application Demonstration Project (2019YF0900083SN).
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Verghese J, Wang C, Lipton RB, Holtzer R. Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci. 2013;68(4):412-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verghese J, Annweiler C, Ayers E, et al. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology. 2014;83(8):718-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beauchet O, Sekhon H, Schott AM, et al. Motoric cognitive risk syndrome and risk for falls, their recurrence, and postfall fractures: results from a prospective observational population-based cohort study. J Am Med Dir Assoc. 2019;20(10):1268-1273. [DOI] [PubMed] [Google Scholar]
- 4.Doi T, Shimada H, Makizako H, Tsutsumimoto K, Verghese J, Suzuki T. Motoric cognitive risk syndrome: association with incident dementia and disability. J Alzheimers Dis. 2017;59(1):77-84. [DOI] [PubMed] [Google Scholar]
- 5.Ayers E, Verghese J. Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimers Dement. 2016;12(5):556-564. [DOI] [PubMed] [Google Scholar]
- 6.Maggio M, Lauretani F. Prevalence, incidence, and clinical impact of cognitive-motoric risk syndrome in Europe, USA, and Japan: facts and numbers update 2019. J Cachexia Sarcopenia Muscle. 2019;10(5):953-955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J. The trajectory of gait speed preceding mild cognitive impairment. Arch Neurol. 2010;67(8):980-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reisberg B, Prichep L, Mosconi L, et al. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer's disease. Alzheimers Dement. 2008;4(1 suppl 1):S98-S108. [DOI] [PubMed] [Google Scholar]
- 9.Lee SY, Jin H, Arai H, Lim JY. Handgrip strength: should repeated measurements be performed in both hands? Geriatr Gerontol Int. 2021;21(5):426-432. [DOI] [PubMed] [Google Scholar]
- 10.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146-M157. [DOI] [PubMed] [Google Scholar]
- 12.Hiraoka A, Tamura R, Oka M, et al. Prediction of risk of falls based on handgrip strength in chronic liver disease patients living independently. Hepatol Res. 2019;49(7):823-829. [DOI] [PubMed] [Google Scholar]
- 13.Gil S, Jacob Filho W, Shinjo SK, et al. Muscle strength and muscle mass as predictors of hospital length of stay in patients with moderate to severe COVID-19: a prospective observational study. J Cachexia Sarcopenia Muscle. 2021;12(6):1871-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrath R, Robinson-Lane SG, Cook S, et al. Handgrip strength is associated with poorer cognitive functioning in aging Americans. J Alzheimers Dis. 2019;70(4):1187-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rijk JM, Roos PR, Deckx L, van den Akker M, Buntinx F. Prognostic value of handgrip strength in people aged 60 years and older: a systematic review and meta-analysis. Geriatr Gerontol Int. 2016;16(1):5-20. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Feng BL, Wang CY, et al. Prevalence and factors associated with motoric cognitive risk syndrome in community-dwelling older Chinese: a cross-sectional study. Eur J Neurol. 2020;27(7):1137-1145. [DOI] [PubMed] [Google Scholar]
- 17.Fragala MS, Alley DE, Shardell MD, et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J Am Geriatr Soc. 2016;64(1):144-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mahoney SJ, Hackney KJ, Jurivich DA, Dahl LJ, Johnson C, McGrath R. Handgrip strength asymmetry is associated with limitations in individual basic self-care tasks. J Appl Gerontol. 2022;41(2):450-454. [DOI] [PubMed] [Google Scholar]
- 19.Go YJ, Lee DC, Lee HJ. Association between handgrip strength asymmetry and falls in elderly Koreans: a nationwide population-based cross-sectional study. Arch Gerontol Geriatr. 2021;96:104470. [DOI] [PubMed] [Google Scholar]
- 20.McGrath R, Vincent BM, Jurivich DA, et al. Handgrip strength asymmetry and weakness together are associated with functional disability in aging Americans. J Gerontol A Biol Sci Med Sci. 2021;76(2):291-296. [DOI] [PubMed] [Google Scholar]
- 21.McGrath R, Cawthon PM, Cesari M, Al Snih S, Clark BC. Handgrip strength asymmetry and weakness are associated with lower cognitive function: a panel study. J Am Geriatr Soc. 2020;68(9):2051-2058. [DOI] [PubMed] [Google Scholar]
- 22.McGrath R, Clark BC, Cesari M, Johnson C, Jurivich DA. Handgrip strength asymmetry is associated with future falls in older Americans. Aging Clin Exp Res. 2021;33(9):2461-2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrath R, Blackwell TL, Ensrud KE, Vincent BM, Cawthon PM. The associations of handgrip strength and leg extension power asymmetry on incident recurrent falls and fractures in older men. J Gerontol A Biol Sci Med Sci. 2021;76(9):e221-e227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGrath R, Tomkinson GR, LaRoche DP, Vincent BM, Bond CW, Hackney KJ. Handgrip strength asymmetry and weakness may accelerate time to mortality in aging Americans. J Am Med Dir Assoc. 2020;21(12):2003-2007.e1. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Ho M, Chau PH. Handgrip strength asymmetry is associated with the risk of neurodegenerative disorders among Chinese older adults. J Cachexia Sarcopenia Muscle. 2022;13(2):1013-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Y, Hu Y, Smith JP, Strauss J, Yang G. Cohort profile: the China Health and Retirement Longitudinal Study (CHARLS). Int J Epidemiol. 2014;43(1):61-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L-K, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Direct Assoc. 2020;21(3):300-307.e2. [DOI] [PubMed] [Google Scholar]
- 28.Armstrong CA, Oldham JA. A comparison of dominant and non-dominant hand strengths. J Hand Surg. 1999;24(4):421-425. [DOI] [PubMed] [Google Scholar]
- 29.Xu W, Bai A, Liang Y, Lin Z. Association between depression and motoric cognitive risk syndrome among community-dwelling older adults in China: a 4-year prospective cohort study. Eur J Neurol. 2022;29(5):1377-1384. [DOI] [PubMed] [Google Scholar]
- 30.Li C, Jin S, Cao X, et al. Catastrophic health expenditure among Chinese adults living alone with cognitive impairment: findings from the CHARLS. BMC Geriatr. 2022;22(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun X, Harris KE, Hou L, et al. The prevalence and associated factors of motoric cognitive risk syndrome in multiple ethnic middle-aged to older adults in west China: a cross-sectional study. Eur J Neurol. 2022;29(5):1354-1365. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, Zhang Y, Lv Z, Xiang J. Sarcopenia and motoric cognitive risk syndrome: a moderated mediation model. BMC Geriatr. 2022;22(1):141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carson RG. Get a grip: individual variations in grip strength are a marker of brain health. Neurobiol Aging. 2018;71:189-222. [DOI] [PubMed] [Google Scholar]
- 34.Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol A Biol Sci Med Sci. 2003;58(2):146-152. [DOI] [PubMed] [Google Scholar]
- 35.Petersen P, Petrick M, Connor H, Conklin D. Grip strength and hand dominance: challenging the 10% rule. Am J Occup Ther. 1989;43(7):444-447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for CHARLS are available at charls.ccer.edu.cn/charls/.