Abstract
Background and Objectives
Past studies on poststroke cognitive function have focused on the average performance or change over time, but few have investigated patterns of cognitive trajectories after stroke. This project used latent class growth analysis (LCGA) to identify clusters of patients with similar patterns of cognition scores over the first-year poststroke and the extent to which long-term cognitive outcome is predicted by the clusters (“trajectory groups”).
Methods
Data were sought from the Stroke and Cognition consortium. LCGA was used to identify clusters of trajectories based on standardized global cognition scores at baseline (T1) and at the 1-year follow-up (T2). One-step individual participant data meta-analysis was used to examine risk factors for trajectory groups and association of trajectory groups with cognition at the long-term follow-up (T3).
Results
Nine hospital-based stroke cohorts with 1,149 patients (63% male; mean age 66.4 years [SD 11.0]) were included. The median time assessed at T1 was 3.6 months poststroke, 1.0 year at T2, and 3.2 years at T3. LCGA identified 3 trajectory groups, which were characterized by different mean levels of cognition scores at T1 (low-performance, −3.27 SD [0.94], 17%; medium-performance, −1.23 SD [0.68], 48%; and high-performance, 0.71 SD [0.77], 35%). There was significant improvement in cognition for the high-performance group (0.22 SD per year, 95% CI 0.07–0.36), but changes for the low-performance and medium-performance groups were not significant (−0.10 SD per year, 95% CI −0.33 to 0.13; 0.11 SD per year, 95% CI −0.08 to 0.24, respectively). Factors associated with the low- (vs high-) performance group include age (relative risk ratio [RRR] 1.18, 95% CI 1.14–1.23), years of education (RRR 0.61, 95% CI 0.56–0.67), diabetes (RRR 3.78, 95% CI 2.08–6.88), large artery vs small vessel strokes (RRR 2.77, 95% CI 1.32–5.83), and moderate/severe strokes (RRR 3.17, 95% CI 1.42–7.08). Trajectory groups were predictive of global cognition at T3, but its predictive power was comparable with scores at T1.
Discussion
The trajectory of cognitive function over the first-year poststroke is heterogenous. Baseline cognitive function ∼3.6 months poststroke is a good predictor of long-term cognitive outcome. Older age, lower levels of education, diabetes, large artery strokes, and greater stroke severity are risk factors for lower cognitive performance over the first year.
Poststroke neurocognitive disorders affect nearly 1 in 2 stroke survivors,1,2 but the longitudinal changes in cognitive function after stroke are incompletely understood. Various studies have differentially shown that cognitive function after stroke on average improves, declines, or remains stable, and the mixed findings may be due to the length of study, the type of cognitive instruments used, and whether the study was hospital-based or population-based.3 These variable findings motivated us to examine long-term cognitive change after stroke in the Stroke and Cognition consortium (STROKOG) in a previous publication.4 We identified a turning point at approximately 1 year after stroke, with patients with stroke on average demonstrating an initial short period of small improvement followed by decline beginning at 1 year, with similar rates of cognitive change observed in global cognition and in individual cognitive domains except for executive function.
In this study, we turned our attention to the period between the baseline and 1-year poststroke assessments. Past studies on poststroke cognitive function, including ours, have focused on estimating the average change in cognition over time.4-7 This approach assumes that change is homogenous within the stroke population, but stroke is in fact a heterogenous condition, with variability in cognitive impairment and recovery after stroke. Identifying clusters of patients based on cognitive trajectory would benefit our understanding of the heterogeneity of poststroke cognitive outcomes.
Our project aims were to (1) use latent class growth analysis (LCGA) to identify naturally occurring clusters of patients with similar cognitive trajectories (“trajectory groups”) in the first year after the baseline assessment; (2) examine the risk factors for membership in the trajectory groups, including age, education, vascular risk factors, and stroke features; and (3) explore the extent to which the trajectory groups, cognitive performance at baseline and at 1 year, and change in performance are predictive of cognitive outcome at the long-term follow-up.
Methods
Sample
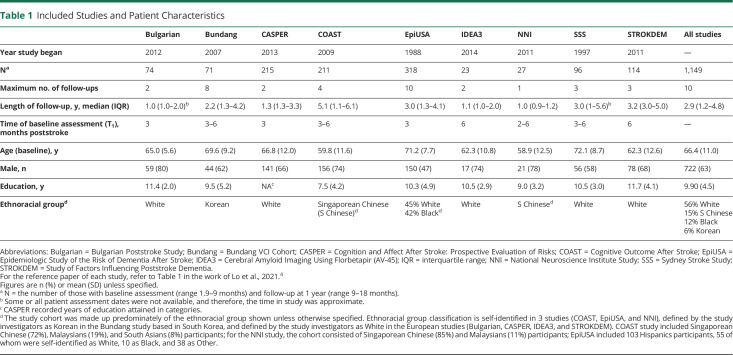
Nine STROKOG studies from Asia, Australia, Europe, and the United States that conducted detailed neuropsychological assessments at baseline (2, 3, or 6 months poststroke) and 1 year after stroke and recruited a control group or provided data from an appropriate comparison group contributed to this project (Table 1). All studies were hospital-based and included in our previous paper.4 As stated in the inclusion criteria of each study, all participants had sufficient knowledge of the language used in the assessments.
Table 1.
Included Studies and Patient Characteristics
Assessment Time Points
We defined T1 as the first detailed neuropsychological assessment (the “baseline” assessment), T2 as the 1-year poststroke follow-up assessment, and T3 as the third or final cognitive assessment if the patient had more than 3 assessments. Although wide ranges were set for T1 (1.9–9 months after stroke) and T2 (9 months–1.5 years after stroke) so that more patients could be included in the analyses, the assessment time points of many patients in certain studies did not fall within those ranges; therefore, it was necessary to exclude those patients so that we could map the cognitive trajectory between baseline and 1 year more precisely. We did not further expand the lower range for T1 because cognition is unstable during the acute phase.8 By definition, T3 must be >1.5 years poststroke. The narrower ranges of T1 = 2–6 months and T2 = 12–15 months were used in a sensitivity analysis. We compared patient characteristics between those who were included in the study vs those who were not eligible due to an assessment time point falling outside of the specified range or a lack of a qualifying follow-up assessment using χ2 or t test as appropriate.
Cognitive Outcomes
Because the neuropsychological tests varied between the studies (eTable 1, links.lww.com/WNL/C751), they were standardized and harmonized to form z-scores for 5 domains: attention and processing speed (attention), memory, language, perceptual motor, and executive function. Global cognition, the standardized average of the 5 domains, was considered as the primary outcome; the 5 domains were considered as the secondary outcome. Global cognition at T3 was defined as the long-term cognitive outcome.
Statistical Methods
Data Harmonization
Neuropsychological test scores from each assessment wave were harmonized by converting raw scores to standardized scores (z-scores) using an adaption of the “category-centered” method9 in which raw scores were standardized as z-scores using the means and SDs derived from each study's control group (or, if not recruited, a local stroke-free normative study). Predicted means and SDs were obtained using a regression model based on the control participants' raw test scores at common values of the covariates age, sex, and education. Neuropsychological tests were assigned to 1 of 5 cognitive domains based on previous work and common practice2,10 (eTable 1, links.lww.com/WNL/C751), and domain z-scores were derived as the standardized average of all tests in a domain. Global cognition was represented by the standardized average of the 5 domain z-scores. For details, refer to our previous publication.4
Identifying Patterns of Trajectories of Cognitive Function
LCGA was used to identify clusters of stroke patients with similar cognitive trajectories between T1 and T2.11 The model that fits the data best was chosen based on several parameters: Bayesian information criterion (BIC) and Akaike information criterion (AIC) which evaluate model fit; entropy, which indicates how well the model defines classes; and the average latent class posterior probability (ALCPP), which assesses the quality of classification. The best model has low BIC and AIC, high entropy (>0.8), high ALCPP (≥0.9), and >50 cases for each cluster.12 The chosen model was used to assign patients to their most likely group using the predicted probabilities of belonging to each class (i.e., trajectory group). The analysis included global cognition scores at T1 and T2. Only patients with qualifying baseline and follow-up assessments were included. In secondary analyses, we repeated the above using domain scores as the outcomes. The interpretation of the trajectory groups was based on their mean rate of change (the slope) in cognitive scores between T1 and T2 and their mean level of cognitive performance at T1 and T2. The Stata procedure “traj” was used.13
Risk Factors for Trajectory Groups
We examined the association of demographic, medical history, and stroke-related characteristics with trajectory groups using mixed-effects multinomial logistic regression.
Predictors of Long-term Cognitive Outcome
Separate linear mixed-effects models were used to examine the association of (1) trajectory groups, (2) cognitive scores at T1, and (3) change in cognition between T1 and T2 with the global cognition score at T3. The quadratic terms of scores at T1 and T2 were included to account for potential nonlinearity. We compared their predictive powers by examining the approximate adjusted R2, which is the proportion of variance explained after controlling for all other variables obtained from regression models with fixed effects.
All Analyses
One-stage individual participant data meta-analysis based on mixed models with study as the random effect was used in all analyses unless otherwise specified. We conducted the unadjusted analyses first and then the fully adjusted analyses, which included sex, age, education in years, ethnoracial groups, prior stroke, severe/moderate stroke, stroke subtype, a history of atrial fibrillation, diabetes, hypertension, and smoking as covariates in the models. APOE status was available in only 2 studies and was examined in a subgroup. We did not include recurrent stroke as a covariate in the risk factor analysis because it could be on the causal pathway, but in a sensitivity analysis, we examined recurrent stroke (a second stroke up until T1) as a potential risk factor by including it as a covariate in the model. Education in 4 categories (eTable 2, links.lww.com/WNL/C751) was used in place of education in years in the risk factor model in an additional analysis. Time in study for T1, T2, and T3 was included as a covariate as appropriate to account for differences in assessment times. The significance level was assessed at the 0.05 level (2-sided). All analyses were performed using Stata 15.1 (StataCorp, College Station, TX). The Strengthening the Reporting of Observational Studies in Epidemiology Statement was used for reporting.14
Standard Protocol Approvals, Registrations, and Patient Consents
Approval from the University of New South Wales Human Research Ethics Committee to conduct this project was received (reference HC210709). The ethics board had determined that participant consent was waived for all included studies.
Data Availability
Anonymized data will be shared on request from any qualified investigator.
Results
Nine studies contributed 2,280 patients with stroke, of whom 61% were followed up at T2. Seventeen percent (n = 239) of those patients were assessed outside of the ranges we specified for T1 and T2, so a total of 1,149 patients were included in our main analyses (see eFigure 1, links.lww.com/WNL/C751 for a flow diagram). Regarding significant differences between those who were included and excluded, there were higher proportions of smokers and patients with severe/moderate stroke excluded, and those excluded had significantly worse baseline global cognitive function than those included (−1.09 vs −0.96; p = 0.028; eTable 3). There were also a higher proportion of patients with small vessel strokes and a lower proportion of patients with cardioembolic strokes excluded from the analyses. Two studies had >50% loss to follow-up possibly because patients who were more mildly affected were not actively followed or were seen at primary care rather than study centers (eTable 4). We excluded the 2 studies with high proportions of loss to follow-up in a sensitivity analysis.
Of the 1,149 patients included, 63% were male, the mean age was 66.4 years (range: 23–94 years), and 54% did not complete high school (eTable 2, links.lww.com/WNL/C751). The median time between T1 and T2 was 9.0 months (interquartile range [IQR] 7.0–11.6). Of the 8 studies which conducted more than 1 follow-up assessments, 65% had at least 3 assessments and the median for the long-term follow-up (T3) was 3.2 years poststroke (IQR 2.1–4.7 years). Thirteen percent had a recurrent stroke before T3. Patient characteristics by study are summarized in Table 1 and eTable 2.
Trajectory Groups From T1 to T2
Based on our model selection criteria, the 3-class and 4-class models were in the acceptable range for good class delineation (eTable 5, links.lww.com/WNL/C751). We chose the 3-class model for parsimony, ease of interpretation, and greater power in the next set of analyses. Sensitivity analyses using stricter ranges for T1 and T2 and, secondly, excluding 2 studies with high percentage of loss to follow-up (n = 98) showed a similar pattern of results (eTable 6A).
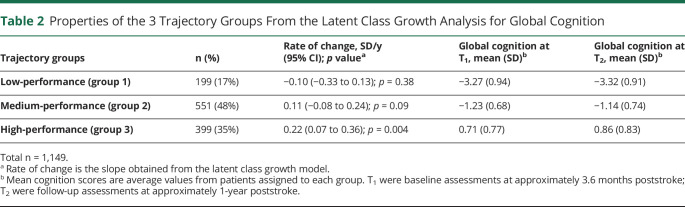
Table 2 summarizes the properties of the 3 trajectory groups regarding global cognition. Figure 1 indicates that there were large differences between the groups in the level of global cognition at T1 and T2. Group 1 (17%) was characterized by low levels of global cognition (on average −3.27 SD at T1) and declining cognitive function (−0.10 SD per year; 95% CI −0.33 to 0.13), although the slope was not significantly different from 0 (p = 0.38). Group 2 (48%) had medium levels of global cognition (−1.23 SD at T1) and showed a trend for small improvement (0.11 SD per year; 95% CI −0.08 to 0.24; p = 0.09). Group 3 (35%) had high levels of global cognition (0.71 at T1) and showed significantly improving cognitive function (0.22 SD per year; 95% CI 0.07–0.36). Since the overall level of global cognition at T1 and T2 rather than slope was the strongest distinguishing feature between the 3 trajectory groups, we labeled them low-performance, medium-performance, and high-performance groups. The rate of change for the low-performance group was significantly different from that of the high-performance group (t = 2.31; p = 0.021), but other pairwise comparisons for change were not significant. The 2 sensitivity analyses showed similar percentages and intercept values as the main results (eTable 6B, links.lww.com/WNL/C751).
Table 2.
Properties of the 3 Trajectory Groups From the Latent Class Growth Analysis for Global Cognition
Figure 1. Individual Changes in Global Cognition and the 3 Trajectory Groups Obtained From the Latent Class Growth Model.
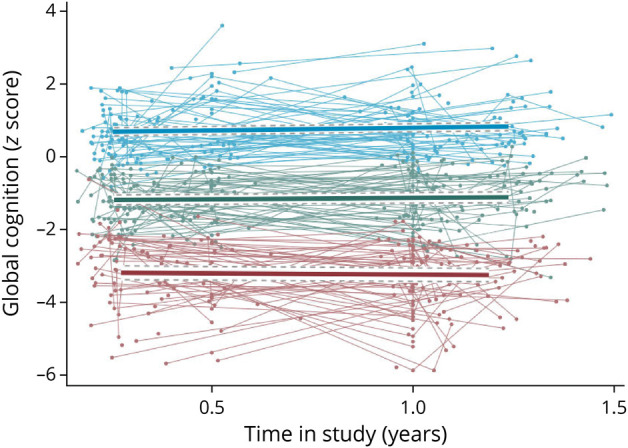
Blue = high-performance; green = medium-performance; red = low-performance. A random sample of 735 observations were used to plot the individual trajectories.
LCGA also identified 3-class models for all cognitive domains except for the perceptual motor, where a model with good separation of the latent classes could not be found (entropy values were <0.6). Similar to global cognition, the trajectory groups identified in each domain were distinguished by the level of cognitive performance at T1 and T2 (eFigure 2 and eTable 7, links.lww.com/WNL/C751). However, all of the trajectory groups for each domain showed stable or a trend for improving cognition except for the language domain, where patients with the poorest performance at T1 showed significant and large decline (−0.37 SD per year; 95% CI −0.68 to −0.05). Patients with medium and high memory scores at T1 or medium attention scores at T1 showed significant improvement. For the executive function domain, all patients regardless of performance at T1 showed stable cognition, with rates of change that were near 0.
Risk Factors for Trajectory Groups
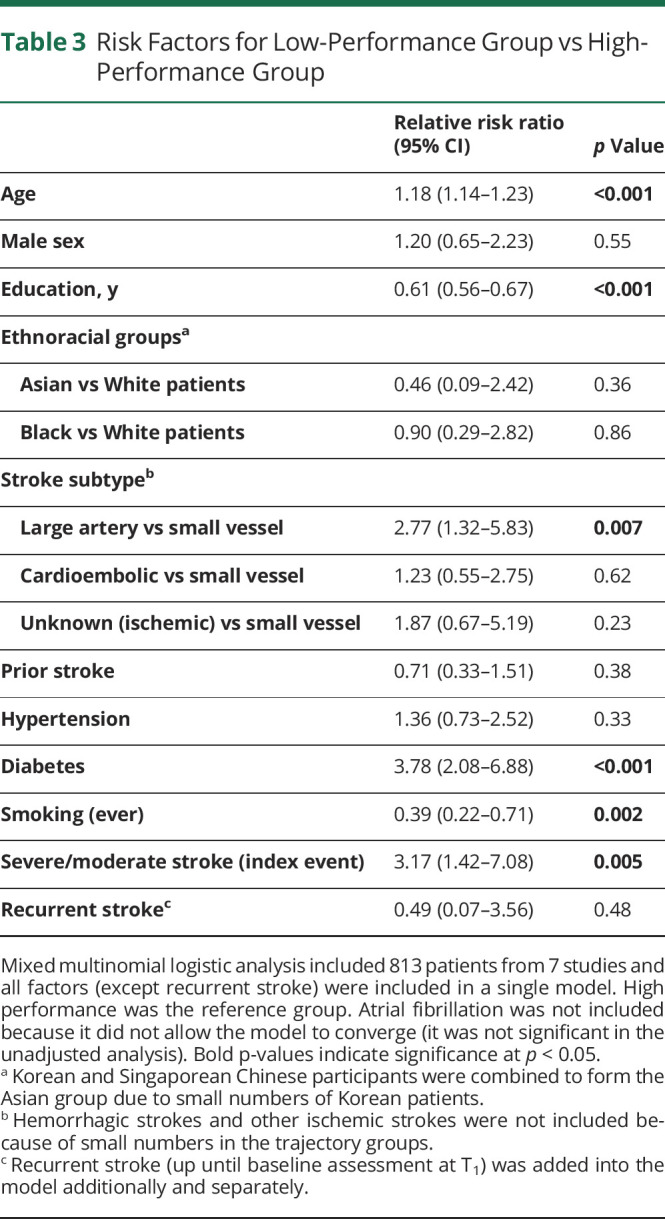
Patient characteristics of the trajectory groups are presented in eTable 8 (links.lww.com/WNL/C751). We examined the risk factors of belonging in the low-performance group using mixed-effects multinomial logistic regression. Results from the unadjusted analysis are given in eTable 9. APOE status was not significant (p = 0.36) and due to small numbers (n = 91), and it was not included in the multivariable model. The fully adjusted model, which adjusted for all factors simultaneously, showed that older age, a lower level of education, diabetes, large artery vs small vessel strokes, severe/moderate stroke, and, surprisingly, an absence of a history of smoking were associated with higher relative risk of membership in the low-performance group compared with high-performance over the first year after stroke (Table 3). The same factors except for large artery strokes were associated with the low-performance group compared with the medium-performance group (eTable 10). In the sensitivity analysis with 2 studies excluded, the results were nearly identical. Recurrent stroke was not significant when included as a covariate (unadjusted: p = 0.27; adjusted: p = 0.48); however, the number of patients with recurrent strokes before T1 is small (n = 6).
Table 3.
Risk Factors for Low-Performance Group vs High-Performance Group
The results of the analysis with education treated as a 4-category variable showed that for each increasing level of education, there was a decrease in the magnitude of the effect sizes, that is, having completed high school compared with not having completed high school had the greatest increase in benefit, but there was no significant additional protective effect of having completed a bachelor's degree compared with technical/college diploma (eTable 11, links.lww.com/WNL/C751).
In a complementary analysis, we examined the risk factors of cognitive function at T1 and T2. The fully adjusted models revealed the same set of factors except for smoking (eTables 12 and 13, links.lww.com/WNL/C751).
To make sense of the result relating to smokers having higher odds of belonging in the high-performance group, we examined the unadjusted association of smoking and cognition at T1, T2, and T3 and found that smokers had significantly higher global cognition scores at all 3 time points, indicating that our results were not due to chance multicollinearity.
Trajectory Groups as Predictors of Long-term Cognitive Outcome
The trajectory groups significantly predicted global cognition at the long-term follow-up in the unadjusted and adjusted analyses (Table 4, eTable 14, links.lww.com/WNL/C751). The trajectory groups identified for each domain were also significantly associated with global cognition at T3 (eTable 15).
Table 4.
Predictors of Global Cognition at the Long-term Follow-up
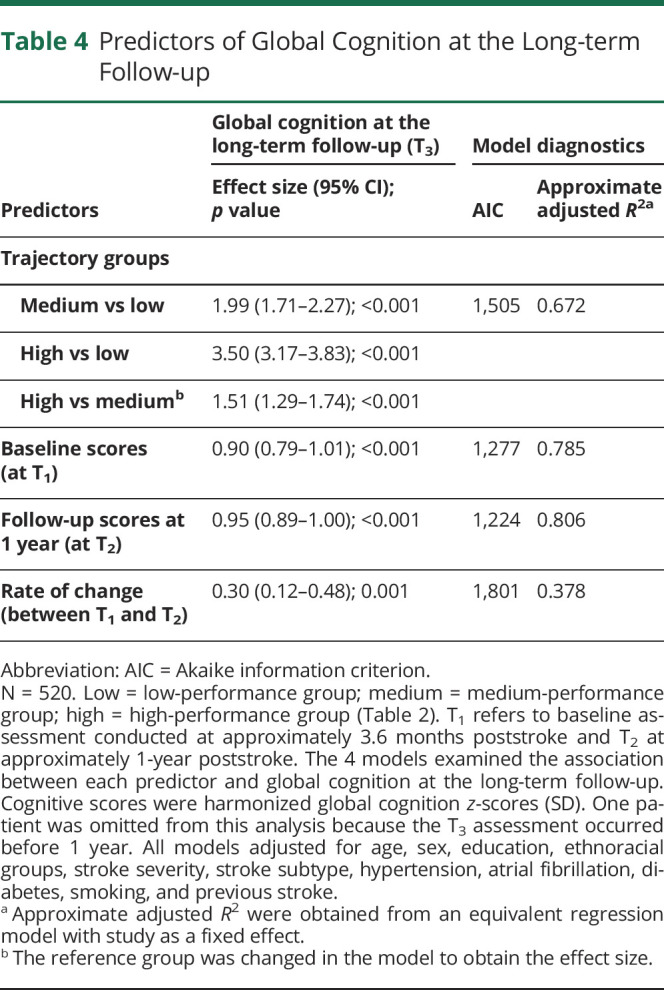
Cognition at T1 and T2 and Rate of Change Between T1 and T2, as Predictors of Long-term Cognitive Outcome
Linear mixed models showed strong prediction of global cognition at T3 by cognition scores at both T1 and T2, with the effect of cognition at T2 being only slightly stronger than that at T1 (1.1% higher; Table 4). The rate of change in cognition from T1 to T2 was also significant, but its level of prediction was much lower.
Post Hoc Analyses
We examined the extent to which the prediction of global cognition at T3 by trajectory groups can be accounted for by baseline scores at T1. Adding trajectory groups to the model with global cognition at T1 resulted in an increase in R2 of only 0.037 (eTable 16, links.lww.com/WNL/C751). Thus, controlling for global cognition at T1 reduced the variance explained by trajectory groups from an R2 of 0.718 to only 0.037, indicating that the prediction by trajectory groups of global cognition at T3 is largely explained by variation in performance at T1.
Discussion
In this collaborative study that included 9 international hospital-based stroke cohorts, we identified 3 groups in relation to cognitive trajectories between baseline and 1 year after stroke. Patients with high cognitive function at baseline, on average 3.6 months poststroke, experienced significant cognitive improvement, while those with medium and low cognitive function at baseline did not show a significant change in cognitive function. The predictive powers of global cognition scores at baseline and 1 year were very similar, while the rate of change was a significant but poorer predictor. Therefore, a stroke patient's prognosis relating to long-term cognitive outcome can be based on their cognitive assessment at around 3 months alone, with further assessment within the 1-year period adding little to the accuracy of the prediction.
A recent cohort study examined the different trajectories of cognitive function after strokes and similarly identified 3 trajectory groups differentiated by their levels of cognitive function over 3 time points.15 However, they did not find significant improvement from 3 to 12 months in any of the trajectory groups, which may be because only the Montreal Cognitive Assessment was used, which is not as sensitive as the tests used in our studies. Being a 1-year study, they were not able to examine long-term cognitive outcomes.
The importance of baseline cognitive function in predicting the cognitive trajectory and outcome was a noteworthy finding of our study. Some differences were also seen in the patterns related to the individual domain scores. The trajectory groups based on different domain scores were distinguished by their level of cognitive function across the 2 time points, but the rate of change was near zero in several trajectory groups. Although patients with the poorest performance in language at T1 showed significant decline, patients with the highest performance in memory at T1 showed significant improvement, and all patients showed a trend for small improvement, regardless of baseline scores in the attention domain. The reasons for these differences are not clear, and it is likely that the language domain differentially influenced the course of the group with initially low and then declining global cognitive function. We speculate that the differences may be related to the differential recovery patterns of brain regions and networks subserving these cognitive functions.
For a large proportion of the patients who experienced cognitive improvement over the first year after stroke, this may have been the result of a combination of genuine recovery and practice effects. The effects of drugs such as statins may have played a part, but we do not have sufficient data at present to examine this. For those with the poorest performance at baseline and declining cognition, baseline cognitive impairment at or before the time of stroke may reflect a reduction in neurologic reserve and neuroplastic potential, which may in turn reduce the potential for cognitive recovery.16 An acute stroke is followed by a period of enhanced plasticity in neural circuits during the period of recovery. This period has been divided into 4 phases: hyperacute, acute, subacute, and chronic.17 The hyperacute phase is characterized by extensive cell death, while in the acute phase, which lasts for about a week, there is delayed apoptosis, a prominent inflammatory response, and enhanced neuronal excitability. The inflammation is reduced in the subacute phase, and there is enhanced plasticity, with maximal recovery that continues until about 3 months. This is followed by the chronic phase, which is the period being investigated in this paper. Recovery is possible in this period, but previous research suggests that this is greatly dependent on neurorehabilitative therapy.18 However, this model of recovery has largely been developed using motor recovery in animal models of stroke,17 and its application to cognitive recovery in humans has not been demonstrated. Our study shows that improvement does occur in this period up to about a year, but not in all cases, and this is dependent on the baseline from which individuals begin the process following the subacute phase. We do not have data to explore whether neurorehabilitation played a role in our study, but most studies did not report any systematic cognitive rehabilitation in their patients.
We were unable to examine the cognitive function of patients with stroke before stroke onset because participants were recruited after being admitted to hospital for stroke. A recent population-based study from the Netherlands found that participants who had had a stroke compared with stroke-free participants had had steeper declines in cognitive function up to 10 years before stroke onset and that there was an acute decline in cognition at the time of stroke.19 One of the major limitations of that study and other population-based studies is that it did not examine stroke features. Although the Dutch study stratified results by sex, education, and APOE genotype, they presented average trajectories and did not examine the potentially heterogenous course of cognition before stroke. Our results call for more in-depth assessment of longitudinal change in cognitive function beginning before stroke and then during long-term follow-up that includes the examination of individual features such as stroke characteristics.
Our analyses of the risk factors for the trajectory groups and cognitive function at baseline and at 1 year revealed that those with poorer global cognition during the first-year poststroke had less education and they were older and more likely to have diabetes, large artery strokes rather than small vessel strokes, and a severe or moderately severe stroke.
Our results suggest a potential threshold effect of education, which has been reported in general population studies on the effect of education and cognitive decline. These studies found that after a certain level of educational attainment, additional schooling does not contribute to further reductions in risk of cognitive impairment.20,21 However, a recent systematic review22 and 2 stroke studies23,24 which examined the association of cognitive reserve and poststroke cognitive function did not report such an effect; further investigation is therefore warranted. The idea that cognitive reserve, operationalized as level of education, could delay the expression of the clinical manifestation of brain pathology has been discussed in the context of Alzheimer disease and could be extended to patients with stroke.16,24,25
Furthermore, our studies comprise older people recruited at different periods. Research data show that there has been a continuous increase in average years of schooling in the past century.26 The mean education level in our combined sample is low (9.9 years) compared with that in the current population. Although it is in keeping with the age of our cohorts (Table 1), it could bias our results for application to younger contemporary cohorts because education is a strong predictor of cognitive performance.
The higher proportion of men in our studies (63% overall) reflects the higher stroke risk in men compared with women during midlife.27 In addition, women are on average older at the time of stroke and have greater stroke severity and disability, which may affect their participation rates.28 However, we found no evidence that sex is associated with cognitive performance.
Several recent studies have linked diabetes mellitus with an increased risk of cognitive impairment, decline, and dementia.29-31 Our results further confirm diabetes as an important modifiable risk factor for poststroke cognitive impairment.
Several population-based studies have shown that the risk of dementia is elevated in patients with more severe stroke.32,33 However, the literature examining the longitudinal association of stroke severity and cognitive decline is limited. We found that moderate or severe stroke was associated with cognitive decline over the first year. It is likely that the brain lesions associated with severe strokes have direct effects on cognition and that the volume, location, and/or number of such lesions in these patients may hamper cognitive recovery.33
We found that large artery vs small vessel strokes were predictive of poorer cognitive performance over the first year. Research examining cognitive outcome by stroke subtype is sparse, and it is unclear whether cognitive impairment risk varies by stroke subtype. A recent stroke study found no significant difference in the severity of impairment between subtypes,34 while a population-based study found a higher prevalence of cognitive impairment with large artery strokes and a lower prevalence of impairment with small vessel strokes.35 Although large artery strokes tend to affect a larger area of the brain and thus increase the risk of cognitive impairment, smaller lacunar strokes are part of the spectrum of cerebral small vessel disease that can be a cause of vascular cognitive impairment in certain patients.36 More research is needed to examine short-term and long-term cognitive outcomes by stroke subtype.
Research has shown that smoking is a risk factor for cognitive decline and dementia.37 Surprisingly, we found that smoking was significantly associated with membership in the high-performance group over the first year after stroke, but the associations between smoking and cognitive scores at baseline and at the 1-year follow-up were not significant after covariate adjustment. We postulate that there is something inherently different about smokers that our cohort studies did not fully capture. Regardless, smoking is a well-established risk factor for stroke, cardiovascular diseases, and dementia and must be avoided.
The strengths of our work include the participation of 9 international studies which provided us with a diverse stroke cohort, the use of detailed neuropsychological tests for the assessment of global cognition and 5 cognitive domains, and our ability to adjust for several potential contributing and confounding factors, including stroke characteristics, vascular risk factors, and demographic variables. The sensitivity analyses produced consistent results suggesting that our conclusions are robust and not affected by slight variations in assessment schedule or by 2 studies with large proportion of loss to follow-up. Limitations include selective attrition due to patients with poorer baseline cognitive function or more severe stroke dropping out of the study or dying, and possible limited generalizability of findings to current stroke populations due to a lower mean education level in the sample. Our study is also limited by unmeasured or unknown confounding variables including medication use and stroke treatment, which could bias our results. Despite these limitations, our study provides a detailed examination of cognitive trajectory over the first year after stroke. Our results may help clinicians provide earlier guidance on prognosis to patients and their families and better determine early treatment and preventive strategies. An important future direction will be to study the effect of cognitive rehabilitation on cognitive outcomes through the first year after stroke.
Glossary
- AIC
Akaike information criterion
- ALCPP
average latent class posterior probability
- BIC
Bayesian information criterion
- IQR
interquartile range
- LCGA
latent class growth analysis
- STROKOG
Stroke and Cognition consortium
Appendix 1. Authors
Appendix 2. Coinvestigators

Study Funding
This work was supported by the National Health and Medical Research Council of Australia (APP1161858; 2019-2021) and (RG203943; 2021-2026).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Barbay M, Diouf M, Roussel M, Godefroy O; GRECOGVASC study group. Systematic review and meta-analysis of prevalence in post-stroke neurocognitive disorders in hospital-based studies. Dement Geriatr Cogn Disord. 2018;46(5-6):322-334. [DOI] [PubMed] [Google Scholar]
- 2.Lo JW, Crawford JD, Desmond DW, et al. Profile of and risk factors for poststroke cognitive impairment in diverse ethnoregional groups. Neurology 2019;93(24):e2257-e2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang EY, Amiesimaka O, Harrison SL, et al. Longitudinal effect of stroke on cognition: a systematic review. J Am Heart Assoc. 2018;7(2):e006443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lo JW, Crawford JD, Desmond DW, et al. Long-term cognitive decline after stroke: an individual participant data meta-analysis. Stroke. 2022;53(4):1318-1327. [DOI] [PubMed] [Google Scholar]
- 5.Levine DA, Galecki AT, Langa KM, et al. Trajectory of cognitive decline after incident stroke. JAMA. 2015;314(1):41-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng F, Yan L, Zhong B, Yang Z, Xie W. Progression of cognitive decline before and after incident stroke. Neurology. 2019;93(1):e20-e28. [DOI] [PubMed] [Google Scholar]
- 7.Zhao X, Chong EJY, Qi W, Pang T, Xu X, Chen C. Domain-specific cognitive trajectories among patients with minor stroke or transient ischemic attack in a 6-year prospective Asian cohort: serial patterns and indicators. J Alzheimers Dis. 2021;83(2):557-568. [DOI] [PubMed] [Google Scholar]
- 8.Kinsella G, Ford B. Acute recovery from patterns in stroke patients: neuropsychological factors. Med J Aust. 1980;2(12):663-666. [PubMed] [Google Scholar]
- 9.Griffith LE, van den Heuvel E, Raina P, et al. Comparison of standardization methods for the harmonization of phenotype data: an application to cognitive measures. Am J Epidemiol. 2016;184(10):770-778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lezak MD, Howieson DB, Loring DW. Neuropsychological Assessment, 4th ed. Oxford University Press; 2004. [Google Scholar]
- 11.Herle M, Micali N, Abdulkadir M, et al. Identifying typical trajectories in longitudinal data: modelling strategies and interpretations. Eur J Epidemiol. 2020;35(3):205-222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weller BE, Bowen NK, Faubert SJ. Latent class analysis: a guide to best practice. J Black Psychol. 2020;46(4):287-311. [Google Scholar]
- 13.Jones BL, Nagin DS. A note on a Stata plugin for estimating group-based trajectory models. Sociol Methods Res. 2013;42(4):608-613. [Google Scholar]
- 14.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ. 2007;335(7624):806-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Buvarp D, Rafsten L, Abzhandadze T, Sunnerhagen KS. A prospective cohort study on longitudinal trajectories of cognitive function after stroke. Sci Rep. 2021;11(1):17271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Umarova RM. Adapting the concepts of brain and cognitive reserve to post-stroke cognitive deficits: implications for understanding neglect. Cortex. 2017;97:327-338. [DOI] [PubMed] [Google Scholar]
- 17.Joy MT, Carmichael ST. Encouraging an excitable brain state: mechanisms of brain repair in stroke. Nat Rev Neurosci. 2021;22(1):38-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ward NS, Brander F, Kelly K. Intensive upper limb neurorehabilitation in chronic stroke: outcomes from the Queen Square programme. J Neurol Neurosurg Psychiatry. 2019;90(5):498-506. [DOI] [PubMed] [Google Scholar]
- 19.Heshmatollah A, Dommershuijsen LJ, Fani L, Koudstaal PJ, Ikram MA, Ikram MK. Long-term trajectories of decline in cognition and daily functioning before and after stroke. J Neurol Neurosurg Psychiatry. 2021;92(11):1158-1163. [DOI] [PubMed] [Google Scholar]
- 20.Makkar SR, Lipnicki DM, Crawford JD, et al. Education and the moderating roles of age, sex, ethnicity and apolipoprotein epsilon 4 on the risk of cognitive impairment. Arch Gerontol Geriatr. 2020;91:104112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hyun J, Hall CB, Katz MJ, et al. Education, occupational complexity, and incident dementia: a COSMIC collaborative cohort study. J Alzheimers Dis. 2022;85(1):179-196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Contador I, Alzola P, Stern Y, de la Torre-Luque A, Bermejo-Pareja F, Fernández-Calvo B. Is cognitive reserve associated with the prevention of cognitive decline after stroke? A Systematic review and meta-analysis. Ageing Res Rev. 2023;84:101814. [DOI] [PubMed] [Google Scholar]
- 23.Li F, Kong X, Zhu H, et al. The moderating effect of cognitive reserve on cognitive function in patients with Acute Ischemic Stroke. Front Aging Neurosci. 2022;14:1011510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin M, Sohn MK, Lee J, et al. Effect of cognitive reserve on risk of cognitive impairment and recovery after stroke: the KOSCO study. Stroke. 2020;51(1):99-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stern Y Cognitive reserve. Neuropsychologia. 2009;47(10):2015-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roser M, Ortiz-Ospina E. Global rise of education. Our World in Data. 2019. Accessed January 17, 2023. ourworldindata.org/global-rise-of-education. [Google Scholar]
- 27.Rexrode KM, Madsen TE, Yu AYX, Carcel C, Lichtman JH, Miller EC. The impact of sex and gender on stroke. Circ Res. 2022;130(4):512-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. 2010;12(1):6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biessels GJ, Despa F. Cognitive decline and dementia in diabetes mellitus: mechanisms and clinical implications. Nat Rev Endocrinol. 2018;14(10):591-604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes–systematic overview of prospective observational studies. Diabetologia. 2005;48(12):2460-2469. [DOI] [PubMed] [Google Scholar]
- 31.Lo JW, Crawford JD, Samaras K, et al. Association of prediabetes and type 2 diabetes with cognitive function after stroke: a STROKOG collaboration study. Stroke. 2020;51(6):1640-1646. [DOI] [PubMed] [Google Scholar]
- 32.Koton S, Pike JR, Johansen M, et al. Association of ischemic stroke incidence, severity, and recurrence with dementia in the Atherosclerosis Risk in Communities Cohort study. JAMA Neurol. 2022;79(3):271-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pendlebury ST, Rothwell PM; Oxford Vascular Study. Incidence and prevalence of dementia associated with transient ischaemic attack and stroke: analysis of the population-based Oxford Vascular Study. Lancet Neurol. 2019;18(3):248-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aam S, Einstad MS, Munthe-Kaas R, et al. Post-stroke cognitive impairment-impact of follow-up time and stroke subtype on severity and cognitive profile: the Nor-COAST study. Front Neurol. 2020;11:699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clery A, Bhalla A, Bisquera A, et al. Long-term trends in stroke survivors discharged to care homes: the South London Stroke Register. Stroke. 2020;51(1):179-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zanon Zotin MC, Sveikata L, Viswanathan A, Yilmaz P. Cerebral small vessel disease and vascular cognitive impairment: from diagnosis to management. Curr Opin Neurol. 2021;34(2):246-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Anstey KJ, von Sanden C, Salim A, O'Kearney R. Smoking as a risk factor for dementia and cognitive decline: a meta-analysis of prospective studies. Am J Epidemiol. 2007;166(4):367-378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared on request from any qualified investigator.