Abstract
Introduction:
Percutaneous endoscopic gastrostomy (PEG) is a common procedure performed world-wide on patients with different comorbidities, with many indications and overall low morbidity. However, studies showed an elevated early mortality in patients undergoing PEG placement. In this systematic review, we review the factors associated with early mortality after PEG.
Methods:
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. The methodological index for nonrandomized studies (MINORS) score system was used to perform qualitative assessment of all included studies. Recommendations were summarized for predefined key items.
Results:
The search found 283 articles. A refined total of 21 studies were included; 20 studies cohort studies and 1 case-control study. For the cohort studies, MINORS score ranged from 7 to 12 out of 16. The single case-control study scored 17 out of 24. The number of study patients ranged from 272 to 181,196. Thirty-day mortality rate varied from 2.4% to 23.5%. Albumin, age, body mass index, C-reactive protein, diabetes mellitus, and dementia were the most frequently associated factors to early mortality in patients undergoing PEG placement. Five studies reported procedure related deaths. Infection was the most commonly reported complication of PEG placement.
Conclusions:
PEG tube insertion is a fast, safe and effective procedure, but is not free of complications and can have a high early mortality rate as demonstrated in this review. Patient selection should be a key factor and the identification of factors associated with early mortality is important in the elaboration of a protocol to benefit patients.
Keywords: Critical illness, Deglutition disorders, Enteral nutrition, Malnutrition, PEG tube
INTRODUCTION
Gastrostomy is a well-established procedure to provide enteral nutrition in patients with dysphagia.1 Open gastrostomy placement has been associated with several complications, including surgical site infection, dehiscence, discomfort, and others.2,3 Gauderer et al. (1980) described a new endoscopic technique performed in 12 children and 19 adults that reduced procedure related complications.4
Advantages of utilizing percutaneous endoscopic gastrostomy (PEG) include performing the procedure without general anesthesia, use in patients with musculoskeletal deformities, reduced postoperative pain, and reduced risk of ileus. The patients are observed for 24 hours before starting feeding through the tube.5
PEG is now a very common procedure performed around the globe, performed on patients with different comorbidities, with many indications, and has overall low morbidity.6 However, many studies showed an elevated early mortality in patients undergoing PEG placement.2,3,7–26 We sought to perform a systematic review in order to review the factors associated with early mortality after PEG.
METHODS
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed during all stages of this systematic review. These guidelines use a checklist for reviewers and readers for reporting outcomes of systematic reviews based on observational case control and cohort studies. Furthermore, it specifies how to report background, methods, search strategy, results, discussion, and conclusion. This systematic review was approved by the PROSPERO registry under the number CRD42020184209.
Eligibility Criteria
The Population, Intervention, Comparison, Outcome, Study (PICOS) design strategy was used when considering studies for this analysis. We sought observational studies in which the outcome was to assess risk factors associated to mortality in patients undergoing PEG. Observational studies included cross-sectional, case control, and cohort study designs. Exclusion criteria: studies addressing other aspects of PEG, studies focused on prognostic or surgical technique, case series, editorials, or case reports. We considered studies published from 2000 and excluded conference abstracts studies with fewer than 200 patients or that did not have an appropriate statistical analysis. Manuscripts that were not in English, Portuguese, or Spanish were also excluded.
Information Sources
The following databases were used September 1, 2021 to September 30, 2021: ScieLO (Scientific Electronic Library Online), LILACS (Literatura Latino Americana em Ciências da Saúde), MEDLINE/PUBMED, Google Scholar, manual manuscripts search from references of other articles, and manuscripts from the grey literature.
Search Criteria
We conducted the search using Medical Subjects Heading (MeSH) terms: mortality AND percutaneous endoscopic gastrostomy AND factors.
Study Selection
The following steps were performed:
(1) Identification of titles of records from databases, (2) removal of duplicates, (3) screening and selection of abstracts, (4) assessment for inclusion through full-text articles, and (5) final inclusion in the study. Two reviewers (DL and RL) performed steps 1 to 5. Inclusion or exclusion of studies was decided unanimously. In cases of disagreement, a different reviewer had the final decision (LM).
Quality Assessment
The quality of all included studies was evaluated using the PRISMA guidelines27 and methodological Index for Nonrandomized Studies (MINORS) guidelines scoring system.28 MINORS is a validated instrument used to assess the quality of surgical studies. This score is based on an 8-item index (global ideal score of 16) for noncomparative studies and a 12-item index (global ideal score of 24) for comparative studies. Each manuscript had a MINORS score assessed by two authors (DL and RL).
Data Extraction
Two authors (DL and RL) extracted the data from the included studies and a third author (LM) checked the extracted data. Disagreements were resolved by discussion between the three authors. The following information was extracted from each included paper: authors, country, and year of publications; study design; risk factors for mortality after PEG placement; characteristics of study participants such as mean age, sex, number, and mortality rates.
RESULTS
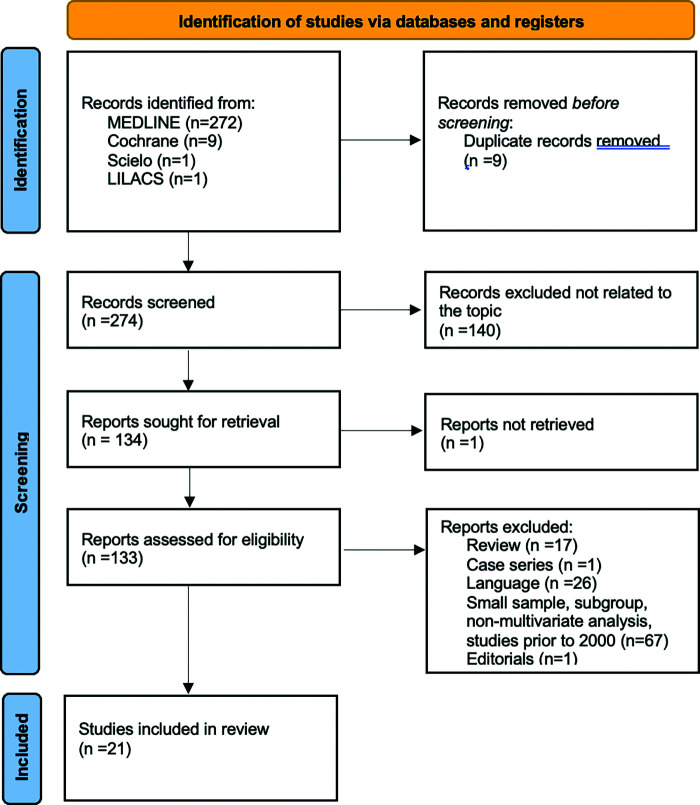
The systematic literature search found 283 articles, of which 9 articles were duplicates and were removed. The titles and abstracts from the remaining 274 articles were then assessed. After a careful evaluation, 140 articles did not meet study criteria and were excluded; the remaining 134 studies were thoroughly assessed within their full text. Case reports, editorials, letters to the editor, and general reviews were also removed. A refined total of 21 studies were included in the final review (Figure 1).
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses flowchart.
Twenty studies were cohort studies and one was a case-control study. For the cohort studies, MINORS scores ranged from 7 to 12 out of 16. The single case-control study had a 17 out of 24 score (Table 1). The number of patients in the above studies ranged from 272 to 181,196. Thirty-day mortality rate varied from 2.4% to 23.5%. Albumin, age, body mass index (BMI), C-reactive protein (CRP), diabetes mellitus, and dementia were the most frequently associated factors to early mortality in patients undergoing PEG placement (Table 2).
Table 1.
Literature on Percutaneous Endoscopic Gastrostomy Tube and Factors Associated with Mortality and Quality Scoring
| Authors | Year | Country | Study Design | Main Indications for PEG | Minors |
|---|---|---|---|---|---|
| Anderloni et al. | 2019 | Italy | Cohort | Dysphagia due to stroke | 11 |
| Arora et al. | 2013 | USA | Case-control | Stroke, other neurologic condition and malnutrition | 17 |
| Ayman et al. | 2017 | USA / Israel | Cohort | Dementia | 11 |
| Blomberg et al. | 2011 | Sweden | Cohort | Cancer, stroke and neurologic disease | 12 |
| Duzenli et al. | 2021 | Turkey | Cohort | Dementia | 10 |
| Gumaste et al. | 2014 | USA | Cohort | Stroke | 10 |
| Lang et al. | 2004 | Israel | Cohort | Dementia | 9 |
| Lee et al. | 2013 | Korea | Cohort | Stroke | 8 |
| Lima et al. | 2021 | Brazil | Cohort | Chronic neurologic dysphagia | 10 |
| Limpias et al. | 2021 | Japan | Cohort | Nononcological indication | 10 |
| Muratori et al. | 2017 | Italy | Cohort | Stroke | 12 |
| Pih et al. | 2018 | Korea | Cohort | Neurologic disease | 7 |
| Richter et al. | 2011 | Germany | Cohort | Neurogenic dysphagia | 11 |
| Sbeit et al. | 2019 | Israel | Cohort | Dementia | 9 |
| Smith et al. | 2008 | USA | Cohort | N/A | 9 |
| Suzuki et al. | 2010 | Japan | Cohort | Cerebrovascular diseases | 12 |
| Tabuenca et al. | 2019 | Spain | Cohort | Degenerative neurological diseases | 10 |
| Udd et al. | 2015 | Finland | Cohort | Neurologic disorders | 12 |
| Zopf et al. | 2011 | Germany | Cohort | Malignant disease | 10 |
| Leeds et al. | 2011 | UK | Cohort | Oropharyngeal malignancy | 12 |
| Macleod et al. | 2021 | UK | Cohort | Stroke | 10 |
Abbreviations: PEG, percutaneous endoscopic gastrostomy.
Table 2.
Clinical Outcomes and Factors Associated with Mortality
| Authors | N | Age | 30-Day Mortality | PEG Related Mortality | Factors Associated with Mortality |
|---|---|---|---|---|---|
| Neurologic disease | |||||
| Anderloni et al. | 557 | 72.9 (15.5) | 5.20% | 0 | Age, BMI, INR |
| Arora et al. | 181,196 | 71 years (18–116 y) | 10.8%* | N/A | Metastatic cancer, CHF, Renal failure, liver disease, pulmonary circulation disease, chronic pulmonary disease |
| Lima et al. | 277 | 73.3 (15.7) | 13% | N/A | Preoperative ICU hospitalization and low hemoglobin |
| Suzuki et al. | 931 | 81.4 ± 7.8 | 9.80% | 8 (0.8%) | Older age, higher CRP, higher BUN, lower albumin, male gender, and a previous history of ischemic heart disease***** |
| Macleod et al. | 808 | 69 (14–98 years) | 14% | N/A | Age > 60 years , low albumin, high CRP, low lymphocyte count |
| Muratori et al. | 438 | 77.3 (12.7) | 4% | 0 | Serum sodium ≥ 150 |
| Pih et al. | 401 | 68 (57 – 77) | 5% | 2 (0.4%) | Platelet count < 100,000/μL and CRP ≥ 5 mg/dL |
| Richter et al. | 1041 | 64 ± 14.65 (18 – 97) | 5.80% | 0 | Cancer |
| Gumaste et al. | 284 | 70.5 ± 16.4 | 6% | 0 | Female sex, positive urine cultures, and low serum albumin levels |
| Udd et al. | 401 | 64 ( ± 15) median | 11% | 2 (0.4%) | ≥ 75 years of age, ASA IV, CCI ≥ 4, BMI < 18.5 kg/m2, ongoing antibiotic therapy |
| Tabuenca et al. | 289 | 70.1 (13.6) | 13.20% | N/A | Older age, higher comorbidity and aspiration pneumonia***** |
| Lee et al. | 1,625 | 64.99 ± 14.51 | 2.40% | 2 (0.1%) | Low albumin and high CRP levels |
| Cancer | |||||
| Zopf et al. | 787 | 60.7 ± 14.2 | 6.50% | N/A | Higher age, lower BMI, diabetes mellitus |
| Leeds et al. | 403 | 200 (< 64 ) / 203 (> 64) | 12.70% | N/A | Age/ albumin |
| Dementia | |||||
| Ayman et al. | 392 | 82.9 (± 8.48)** | 9.40% | N/A | Dementia |
| Duzenli et al. | 309 | 78.1 ± 12.2 | 12.60% | 0 | Higher urea levels and higher CRP to albumin ratios |
| Lang et al. | 502 | 74 (15)*** | 8% | 0 | Albumin < 3, COPD, diabetes mellitus |
| Sbeit et al. | 272 | 77.3 ± 14**** | 23.50% | N/A | Older age, higher creatinine level, elevated CRP-to-albumin ratio |
| Others | |||||
| Blomberg et al. | 484 | 66 (±14) | 12% | 0 | Low albumin and high CRP levels |
| Limpias et al. | 388 | 72.04 (13.7) | 3.90% | 3 (0.7%) | Advanced cancer, low albumin, and high CRP levels |
| Smith et al. | 714 | 68 (16) | 22% | 0 | Older age, cancer, heart disease, nonwhite race, dialysis***** |
in hospital mortality.
dementia group.
hospitalized patients.
patients who died 30 days after PEG.
predictors of post-PEG death, not specifically 30-days.
Abbreviations: PEG, percutaneous endoscopic gastrostomy; INR, international normalized ratio blood test; CCI, craniocervical instability; ICU, intensive care unit; CRP, C-reactive protein; ASA, American Society of Anesthesiologists; CHF, chronic hearth failure; COPD, chronic obstructive pulmonary disease.
Five studies reported procedure related deaths. Infection was the most commonly reported complication of PEG placement (Table 3).
Table 3.
Complications Associated with PEG Placement
| Authors | Complications Related to PEG n (%) | Most Common Complications Related to PEG |
|---|---|---|
| Neurologic disease | ||
| Anderloni et al. | 28 (4.8) | Infection |
| Arora et al. | N/A | N/A |
| Lima et al. | 59 (21.3) | Tube avulsion |
| Suzuki et al. | N/A | N/A |
| Macleod et al. | N/A | N/A |
| Muratori et al. | 25 (5.7) | Peristomal cutaneous inflammation |
| Pih et al. | 38 (9.5) | Pneumoperitoneum |
| Richter et al. | 141 (13.5) | Local infection |
| Gumaste et al. | 8 (2.8) | Bleeding in PEG site and peristomal infection |
| Udd et al. | 110 (27.4) | Skin problems |
| Tabuenca et al. | 79 (27.3) | Digestive complications |
| Lee et al. | 215 (13.2) | Fever without evident infection |
| Cancer | ||
| Zopf et al. | N/A | N/A |
| Leeds et al. | N/A | N/A |
| Dementia | ||
| Ayman et al. | N/A | N/A |
| Duzenli et al. | 33 (13) | Tube leakage |
| Lang et al. | 25 (6)** | Wound infection |
| Sbeit et al. | N/A | N/A |
| Others | ||
| Blomberg et al. | 50 (11)* | Peristomal infection |
| Limpias et al. | 86 (22.2) | PEG site infection |
| Smith et al. | 9 (1.3) | Tube displacement |
Only reports peristomal infection.
Hospitalized patients.
DISCUSSION
Pioneers of the Endoscopic Feeding Tube
PEG tube placement was first described by Gauderer and Ponsky in the 1980s. Three key elements were needed to provide a safe long-term approach to the stomach without a laparotomy: reliable approximation of the stomach to the abdominal wall, protection of surrounding organs from injury, and control of placement site.29 Initially, the procedure was performed in 12 children (4 months to 18 years-old) and 19 adult patients.4,30 All patients had a neurologic syndrome which prevented them from swallowing. Three years later, they reported the results of PEG in 150 patients (50 children and 100 adults) with low morbidity (10%) and no deaths related to the procedure. The most common complication was wound infection, seen in seven patients.31 The procedure has been accepted worldwide and it was the second most common indication for upper-tract endoscopy in hospitalized patients in the United States at the end of the 20th century.6 The impact of PEG, in combination with the development of new tube feeding formulas, the production of PEG kits by the medical device industry has significantly increased. Additionally, the increase in the number publications related to PEG was remarkable.29
Indications
The classic indication for PEG tube placement is dysphagia secondary to neurologic disorders, head and neck or esophageal cancer, and dementia. In our review, 12 studies had neurologic disease as the main indication for PEG.2,7,9,10,12–14,16,18,19,21,23 Four studies had dementia as its main indication for PEG.8,11,20,24 Two recent reviews with meta-analysis showed no benefit in survival for patient with dementia and enteral tube feeding.32,33 The European Society for Clinical Nutrition and Metabolism guidelines on home enteral nutrition states that indication for PEG should not be used in advanced dementia or in patients with life expectancy shorter than 30 days.34
Factors Associated with Early Mortality After PEG
Despite being regarded as a safe, rapid, and effective in providing an enteral feeding in patients, PEG tube placement is not free of complications and is associated with high early mortality rates in some studies.7,11,12,18,24 Moreover, many studies have investigated factors associated with 30-day mortality after PEG in different populations.2,3,7–24 They identified heterogeneous factors that can be grouped in two large categories: factors associated with advanced signs of malnutrition and factors associated with chronic diseases.
Seven studies in our review identified low albumin as a factor associated with mortality.10,14,20–24 High levels of CRP was also independently associated with mortality in four studies.9,14,21,22 A prospective cohort by Blomberg et al. (2011) showed that the combination of low albumin and high CRP levels increases 30-day mortality by more than sevenfold after PEG insertion.14 Chronic inflammatory states negatively affect metabolism and the inflammatory system, causing appetite loss and cachexia. The combination of low albumin and high CRP levels may be an indication of a severely ill patient.14 Findings from Udd et al. (2015) corroborate this idea of frailty as an important factor associated with early mortality as characteristics associated characteristics included: age ≥ 75 years, ASA IV, craniocervical instability ≥ 4, BMI < 18.5 kg/m2, and ongoing antibiotic therapy.18
Most studies had patients with mean age varying from 60.7 to 82.9 years. Advanced age was one of the factors associated with mortality in several studies.3,7,10,11,13,18,33 Low BMI was also associated with mortality in different studies.3,13,18 Regarding gender, there is no consensus in the literature. Gumaste et al. showed female gender associated with early mortality.23 However, Suzuki et al. reported male sex as a factor associated with mortality.10
Our previous study with 277 patients showed intensive care unit hospitalization of two weeks before the procedure as a factor associated with 30-day mortality.2 Our first study had identified this factor associated with eight-week mortality. At the time, we did not have enough power to calculate factors associated with 30-day mortality and it was not included in this review.5 This was the first study with a large cohort to identify this factor which also can be explained by the frailty of the patient. Diabetes mellitus was also a risk factor in two studies.3,20 A multicenter retrospective cohort study by Muratori et al. (2017) with 438 patients identified hypernatremia (Na ≥ 150 mmol/L) independently related to one-month mortality (odds ratio 25.4; 95% confidence interval 7.4 – 86.8; P < 0.0001). They also found cancer, elevated CRP levels, and low albumin independently related to three-month mortality.19
In our review, 30-day mortality rate varied from 2.4% to an alarming 23.5%. Sbeit et al. (2019) reported a high mortality rate (23.5%) and its associated factors were older age, high creatinine level and elevated CRP-to-albumin level.11 Duzenli et al. (2021), found a 30-day mortality rate of 12.6%, and also found that an elevated CRP to albumin level as a predictor of mortality.24 Arora et al. (2013) performed a case-control study with more than 180,000 patients from the US Nationwide Inpatient Sample and found an in-hospital mortality of 10.8% showed metastatic cancer, chronic heart failure, renal failure, liver disease, pulmonary circulation disease, and chronic pulmonary disease as predictors of mortality.12
Complications
PEG tube placement is not free of complications, including death related to the procedure. In our review, complications related to PEG placement varied from 1.3% to 27.4%, wound infection being the most reported, followed by tube leakage and avulsion. Five studies reported death related to the procedure.9,10,18,21,22 Lee et al.21 reported two deaths due to peritonitis and septic shock within 48 hours after the procedure and no apparent perforation of the gastrointestinal tract or PEG dislodgment. Udd et al.18 and Pih et al.9 also reported two deaths. They considered an aspiration pneumonia as death-related to PEG and uncontrolled infection in a patient with cirrhosis that developed peritonitis after PEG placement.9 Udd et al. reported peritonitis as the reason for the PEG-related deaths. Limpias et al. reported three deaths: one due to sepsis and two due to aspiration pneumonia.22 Suzuki et al. did not report the reasons for PEG-related deaths.10
To PEG or Not to PEG
Several authors have addressed patient selection, indications, and timing for PEG tube insertion.35–37 The European Society of Gastrointestinal Endoscopy 2021 guidelines recommends early PEG tubes in patients with chronic degenerative diseases or select types of malignancy who have weight loss despite continued oral nutrition.38 These studies also suggest PEG tube insertion is contraindicated in patients with a life expectancy shorter than 30 days.
Dietrich et al. (2020) addresses the timing of PEG, suggesting that an early indication prior to catabolism and weight loss may benefit patients.39 It may be reasonable to initiate tube feeding when patients are still showing early signs of eating problems or malnutrition rather than starting after late signs of malnutrition have started, or holding enteral feeding all together.5 This is a challenging situation where a multidisciplinary team should give support to the family in the decision-making process. The use of a multidisciplinary team meeting decreased 30-day mortality from 10% to 6.6% in a study by Bond et al.40
A Mortality Predicting Score
Two studies tried to identify the best patients to benefit from PEG placement using the Sheffield Gastrostomy Score.25,26 Leeds et al. (2009) created this score in a study with 403 patients. This score utilized two variables, age and albumin. The authors used this score to estimate a 30-day mortality for patients. However, the score was not designed to decide if the patient should undergo PEG placement, but rather to help clinicians, patients, and their families with the informed consent.25 Macleod et al. (2021) applied the same score in a cohort of 808 patients and found that the score has a reasonable capacity to predict 30-day mortality after PEG. Furthermore, they suggested a revision and remodeling in the Sheffield score as they identified elevated CRP, low lymphocyte count and outpatient status as factors associated with increased risk of mortality.26
MINORS Qualitative Assessment
All studies lost points in the MINORS score system due to a lack of unbiased assessment.
Furthermore, none of the studies were blinded and only one study was a case-control study. None showed information about the prospective calculation of sample size and some studies were not clear about the inclusion of consecutive patients. Many were retrospective cohorts and data was not prospectively collected.
Strengths and Limitations
The limitations of our study are possible language and publication bias. We included only manuscripts published in English, Spanish, and Portuguese. Despite the broad literature search, we may not have identified all studies regarding this topic, or a viable study may be removed due to not meeting the inclusion criteria.
The main strength of this study lies in the rigor of our systematic review process. A comprehensive literature search was performed across a variety of databases. The review was registered on PROSPERO, an international database of prospectively registered systematic reviews covering many health-related outcomes. PROSPERO aims to provide a list of systematic reviews to help avoid duplication and reduce reporting bias by enabling comparison of the review with what was initially planned in the protocol.
We included studies with large samples (n >200) and where a proper statistical analysis was employed. Finally, PRISMA reporting guidelines were followed, and the well-validated MINORS was used to perform the qualitative analysis of all studies. PRISMA was developed to help systematic reviewers transparently report what was done, how it was done, and what the authors found.41 MINORS is a valid instrument designed to evaluate methodological quality of nonrandomized studies, comparative or noncomparative. It has been used extensively in the literature.28
CONCLUSIONS
PEG tube insertion although is a fast, safe, and effective procedure, is not free of complications and can have a high early mortality rate as demonstrated in this review. Patient selection should be a key factor when discussing this procedure with patients and their families, and the identification of factors associated with early mortality is important in the elaboration of a protocol to benefit patients.
Footnotes
Acknowledgements: none.
Disclosure: none.
Conflict of interests: PPS is a consultant for Asensus Surgical and he is a consultant, speaker, and receives research support from Heron Therapeutics.
Funding sources: none.
Informed consent: Dr. Diego Laurentino Lima declares that written informed consent was obtained from the patient/s for publication of this study/report and any accompanying images.
Contributor Information
Diego Laurentino Lima, Department of Surgery, Montefiore Medical Center, New York, NY..
Luiz Eduardo Correia Miranda, Oswaldo Cruz University Hospital, Faculty of Medical Science, University of Pernambuco, Recife, Brazil..
Raquel Nogueira Cordeiro Laurentino Lima, Department of Surgery, NYU Langone, New York, NY. (Dr. R. N. C. L. Lima).
Gustavo Romero-Velez, Department of Surgery, Montefiore Medical Center, New York, NY..
Ryan Chin, Department of Surgery, Montefiore Medical Center, New York, NY..
Phillip P. Shadduck, TOA Surgical Specialists and Duke Regional Hospital, Duke University, Durham, NC..
Prashanth Sreeramoju, Department of Surgery, Montefiore Medical Center, New York, NY..
References:
- 1.Chong VH, Vu C. Percutaneous endoscopic gastrostomy outcomes: can patient profiles predict mortality and weaning? Singapore Med J. 2006;47(5):383–387. [PubMed] [Google Scholar]
- 2.Lima DL, Miranda LEC, da Penha MRC, et al. Factors associated with 30-day mortality in patients after percutaneous endoscopic gastrostomy. JSLS. 2021;25(3):e2021.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zopf Y, Maiss J, Konturek P, Rabe C, Hahn EG, Schwab D. Predictive factors of mortality after PEG insertion: guidance for clinical practice. JPEN J Parenter Enteral Nutr. 2011;35(1):50–55. [DOI] [PubMed] [Google Scholar]
- 4.Gauderer MW, Ponsky JL, Izant RJ. Gastrostomy without laparotomy: a percutaneous endoscopic technique. J Pediatr Surg. 1980;15(6):872–875. [DOI] [PubMed] [Google Scholar]
- 5.Miranda LE, Da Penha MRC, Miranda ACG, Lima DL, Costa MWF, De Amorim AO. Risk factors associated with early mortality after percutaneous endoscopic gastrostomy in patients at a tertiary care center in Brazil: a retrospective single-center survival study. Arq Gastroenterol. 2019;56(4):412–418. [DOI] [PubMed] [Google Scholar]
- 6.Gauderer M. Twenty years of percutaneous endoscopic gastrostomy: origin and evolution of a concept and its expanded applications. Gastrointest Endosc. 1999;50(6):879–883. [DOI] [PubMed] [Google Scholar]
- 7.Agudo Tabuenca A, Altemir Trallero J, Gimeno Orna JA, Ocón Bretón MJ. Mortality risk factors after percutaneous gastrostomy: who is a good candidate? Clin Nutr. 2019;38(2):856–861. [DOI] [PubMed] [Google Scholar]
- 8.Ayman AR, Khoury T, Cohen J, et al. PEG insertion in patients with dementia does not improve nutritional status and has worse outcomes as compared with PEG insertion for other indications. J Clin Gastroenterol. 2017;51(5):417–420. [DOI] [PubMed] [Google Scholar]
- 9.Pih GY, Na HK, Ahn JY, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy insertion. BMC Gastroenterol. 2018;18(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suzuki Y, Tamez S, Murakami A, et al. Survival of geriatric patients after percutaneous endoscopic gastrostomy in Japan. World J Gastroenterol. 2010;16(40):5084–5091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sbeit W, Kadah A, Mari A, Mahamid M, Khoury T. Simple bedside predictors of survival after percutaneous gastrostomy tube insertion. Can J Gastroenterol Hepatol. 2019:1532918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora G, Rockey D, Gupta S. High in-hospital mortality after percutaneous endoscopic gastrostomy: results of a nationwide population-based study. Clin Gastroenterol Hepatol. 2013;11(11):1437–1444.e3. [DOI] [PubMed] [Google Scholar]
- 13.Anderloni A, Di Leo M, Barzaghi F, et al. Complications and early mortality in percutaneous endoscopic gastrostomy placement in lombardy: a multicenter prospective cohort study. Dig Liver Dis. 2019;51(10):1380–1387. [DOI] [PubMed] [Google Scholar]
- 14.Blomberg J, Lagergren P, Martin L, Mattsson F, Lagergren J. Albumin and C-reactive protein levels predict short-term mortality after percutaneous endoscopic gastrostomy in a prospective cohort study. Gastrointest Endosc. 2011;73(1):29–36. [DOI] [PubMed] [Google Scholar]
- 15.Barbosa M, Magalhaes J, Marinho C, Cotter J. Predictive factors of early mortality after percutaneous endoscopic gastrostomy placement: the importance of C-reactive protein. Clin Nutr Espen. 2016;14:19–23. [DOI] [PubMed] [Google Scholar]
- 16.Richter-Schrag HJ, Richter S, Ruthmann O, Olschewski M, Hopt UT, Fischer A. Risk factors and complications following percutaneous endoscopic gastrostomy: a case series of 1041 patients. Can J Gastroenterol. 2011;25(4):201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith BM, Perring P, Engoren M, Sferra JJ. Hospital and long-term outcome after percutaneous endoscopic gastrostomy. Surg Endosc. 2008;22(1):74–80. [DOI] [PubMed] [Google Scholar]
- 18.Udd M, Lindström O, Mustonen H, Bäck L, Halttunen J, Kylänpää L. Assessment of indications for percutaneous endoscopic gastrostomy–development of a predictive model. Scand J Gastroenterol. 2015;50(2):245–252. [DOI] [PubMed] [Google Scholar]
- 19.Muratori R, Lisotti A, Fusaroli P, et al. Severe hypernatremia as a predictor of mortality after percutaneous endoscopic gastrostomy (PEG) placement. Dig Liver Dis. 2017;49(2):181–187. [DOI] [PubMed] [Google Scholar]
- 20.Lang A, Bardan E, Chowers Y, et al. Risk factors for mortality in patients undergoing percutaneous endoscopic gastrostomy. Endoscopy. 2004;36(6):522–526. [DOI] [PubMed] [Google Scholar]
- 21.Lee C, Im JP, Kim JW, et al. Risk factors for complications and mortality of percutaneous endoscopic gastrostomy: a multicenter, retrospective study. Surg Endosc. 2013;27(10):3806–3815. [DOI] [PubMed] [Google Scholar]
- 22.Limpias Kamiya KJL, Hosoe N, Takabayashi K, et al. Factors predicting major complications, mortality, and recovery in percutaneous endoscopic gastrostomy. JGH Open. 2021;5(5):590–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gumaste VV, Bhamidimarri KR, Bansal R, Sidhu L, Baum J, Walfish A. Factors predicting early discharge and mortality in post-percutaneous endoscopic gastrostomy patients. Ann Gastroenterol. 2014;27(1):42–47. [PMC free article] [PubMed] [Google Scholar]
- 24.Duzenli T, Ketenci M, Akyol T, et al. Predictive factors of complications and 30-day mortality in patients undergoing percutaneous endoscopic gastrostomy: the utility of c-reactive protein to albumin ratio. Acta Gastroenterol Belg. 2021;84(2):283–288. [DOI] [PubMed] [Google Scholar]
- 25.Leeds JS, Morley SR, Robson HE, et al. Comparison of the sheffield gastrostomy score with an artificial neural network analysis. Gut. 2009;58:A124. [Google Scholar]
- 26.MacLeod CS, McKay R, Barber D, McKinlay AW, Leeds JS. Predicting 30-day mortality following PEG insertion: external validation of the Sheffield Gastrostomy Score and analysis for additional predictors. Clin Nutr Espen. 2021;42:227–232. [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712–716. [DOI] [PubMed] [Google Scholar]
- 29.Gauderer MWL. Gastrointestinal feeding access - from idea to application. J Pediatr Surg. 2019;54(6):1099–1103. [DOI] [PubMed] [Google Scholar]
- 30.Ponsky JL, Gauderer MW. Percutaneous endoscopic gastrostomy: a nonoperative technique for feeding gastrostomy. Gastrointest Endosc. 1981;27(1):9–11. [DOI] [PubMed] [Google Scholar]
- 31.Ponsky JL, Gauderer MW, Stellato TA. Percutaneous endoscopic gastrostomy. Review of 150 cases. Arch Surg. 1983;118(8):913–914. [DOI] [PubMed] [Google Scholar]
- 32.Lee YF, Hsu TW, Liang CS, et al. The efficacy and safety of tube feeding in advanced dementia patients: a systemic review and meta-analysis study. J Am Med Dir Assoc. 2021;22(2):357–363. [DOI] [PubMed] [Google Scholar]
- 33.Davies N, Barrado-Martín Y, Vickerstaff V, et al. Enteral tube feeding for people with severe dementia. Cochrane Database Syst Rev. 2021;8(8):CD013503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bischoff SC, Austin P, Boeykens K, et al. ESPEN guideline on home enteral nutrition. Clin Nutr. 2020;39(1):5–22. [DOI] [PubMed] [Google Scholar]
- 35.Wilcox CM, McClave SA. To PEG or not to PEG. Clin Gastroenterol Hepatol. 2013;11(11):1451–1452. [DOI] [PubMed] [Google Scholar]
- 36.Welbank T, Kurien M. To PEG or not to PEG that is the question. Proc Nutr Soc. 2021;80(1):1–8. [DOI] [PubMed] [Google Scholar]
- 37.Pennington C. To PEG or not to PEG. Clin Med (Lond). 2002;2(3):250–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arvanitakis M, Gkolfakis P, Despott EJ, et al. Endoscopic management of enteral tubes in adult patients - Part 1: Definitions and indications. European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2021;53(1):81–92. [DOI] [PubMed] [Google Scholar]
- 39.Dietrich CG, Schoppmeyer K. Percutaneous endoscopic gastrostomy. Too often? Too late? Who are the right patients for gastrostomy? World J Gastroenterol. 2020;26(20):2464–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bond A, Conley T, Fiske J, et al. Reducing 30-day post gastrostomy insertion mortality with a feeding issues multidisciplinary team meeting. Clin Nutr Espen. 2020;40:282–287. [DOI] [PubMed] [Google Scholar]
- 41.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]