Abstract
Treatment of head and neck squamous cell carcinoma (HNSCC) is a substantial clinical challenge due to the high local recurrence rate and chemotherapeutic resistance. This project seeks to identify new potential biomarkers of prognosis prediction and precision medicine to improve this condition. A synthetic data matrix for RNA transcriptome datasets and relevant clinical information on HNSCC and normal tissues was downloaded from the Genotypic Tissue Expression Project and The Cancer Genome Atlas (TCGA). The necrosis-associated long-chain noncoding RNAs (lncRNAs) were identified by Pearson correlation analysis. Then 8-necrotic-lncRNA models in the training, testing and entire sets were established through univariate Cox (uni-Cox) regression and Lasso-Cox regression. Finally, the prognostic ability of the 8-necrotic-lncRNA model was evaluated via survival analysis, nomogram, Cox regression, clinicopathological correlation analysis, and receiver operating characteristic (ROC) curve. Gene enrichment analysis, principal component analysis, immune analysis and prediction of risk group semi-maximum inhibitory concentration (IC50) were also conducted. Correlations between characteristic risk score and immune cell infiltration, immune checkpoint molecules, somatic gene mutations, and anti-cancer drug sensitivity were analyzed. Eight necrosis-associated lncRNAs (AC099850.3, AC243829.2, AL139095.4, SAP30L-AS1, C5orf66-AS1, LIN02084, LIN00996, MIR4435-2HG) were developed to improve the prognosis prediction of HNSCC patients. The risk score distribution, survival status, survival time, and relevant expression standards of these lncRNAs were compared between low- and high-risk groups in the training, testing and entire sets. Kaplan–Meier analysis showed the low-risk patients had significantly better prognosis. The ROC curves revealed the model had an acceptable predictive value in the TCGA training and testing sets. Cox regression and stratified survival analysis indicated that the 8 necrosis-associated lncRNAs were risk factors independent of various clinical parameters. We recombined the patients into 2 clusters through Consensus ClusterPlus R package according to the expressions of necrotic lncRNAs. Significant differences were found in immune cell infiltration, immune checkpoint molecules, and IC50 between clusters, suggesting these characteristics can be used to evaluate the clinical efficacy of chemotherapy and immunotherapy. This risk model may serve as a prognostic signature and provide clues for individualized immunotherapy for HNSCC patients.
Keywords: cold tumor, head and neck squamous cell carcinoma, hot tumor, lncRNA, necroptosis, prognosis
1. Introduction
Head and neck squamous cell carcinoma (HNSCC) is the most common malignant tumor and the seventh dominant cancer in the world. It is characterized by high treatment resistance rate.[1–3] The recurrence rate of locally advanced HNSCC is about 50% even after active treatments, including surgery, radiotherapy and chemotherapy.[4] The overall survival (OS) of metastatic HNSCC patients can be improved by using immune checkpoint inhibitor (ICI) antibodies against programmed cell death protein (PD)-1 or programmed death ligand (PD-L)-1, which have been approved by the US Food and Drug Administration as a first-line treatment in combination with chemotherapy. However, only 10% to 20% of patients benefit from the PD-1/PD-L1 blockade.[5–8] Researchers are studying the reasons for the failure of immunotherapy. In recent years, hot and cold tumors are considered to be related to immune resistance.[9] The common clinical “hot” and “cold” tumors refer to T-cell invasive, inflammatory tumors, and noninvasive, non-inflammatory tumors, respectively, which well reflect the high (I4) and low (I0) immune core categories.[10] This immune classification has been validated in other cancer types, such as melanoma.[11]
Because most tumors have innate resistance against apoptosis, induced necrosis and other death mechanisms have been gradually considered as promising treatment strategies.[12] Necroptosis is a recently-discovered programmed cell death and can enhance the anti-tumor immunity mediated by CD8 + leukocytes by activating RIPK1 and RIPK3 in the tumor microenvironment (TME).[13] Jingyuan Li et al confirmed that necrotic cells can promote the migration and invasion of normal HNSCC cells in vitro by releasing DAMPs.[14] Besides, necrosis was proved to be related to the invasive phenotype and tumor progression in breast cancer, lung cancer and cervical cancer.[15–17] Necrosis can also induce the immunosuppressive microenvironment of pancreatic ductal adenocarcinoma through CXCL1 and Mincle pathways, thereby promoting tumor invasion and metastasis.[18] Seehawer et al found that the necrotic microenvironment promoted the transformation of proto-carcinoma hepatocytes to highly malignant intrahepatic cholangiocarcinoma, while the apoptotic microenvironment facilitated the progression of mild hepatocellular carcinoma.[19]
Long-chain noncoding RNAs (lncRNAs) can control genes by affecting gene translation or directly interacting with proteins or other RNA species.[20] LncRNAs are involved in tumor development, including tumorigenesis and metastasis. Furthermore, lncRNAs play a vital role in the TME and are correlated with patient prognosis. However, the effects of necrosis-related lncRNAs in the TME and in predicting the prognosis of HNSCC remain unknown. Necrosis-associated lncRNAs as potential therapeutic targets for HNSCC have not been widely mentioned. Therefore, more knowledge about necrotic lncRNAs can help better understand the roles of necrosis and lncRNAs in immunotherapy.
Differentiating cold tumor from hot tumor and transforming cold tumor into hot tumor will improve the antitumor effect of immunotherapy.[21] A study about the effect of immune cell infiltration on the prognosis of esophageal squamous cell carcinoma patients showed that patients with grouped hot tumors had better OS and progression- free survival, while those with cold tumors had the opposite outcomes.[22] However, we still lack a simple and effective method to identify tumors.[9] Since lncRNAs are highly regarded as new cancer biomarkers in body fluids, we attempted to recombine patients according to necrosis-related lncRNAs to effectively identify thermal tumors and improve prognosis in clinical practice.
2. Materials and methods
2.1. Acquisition of information of HNSCC patients
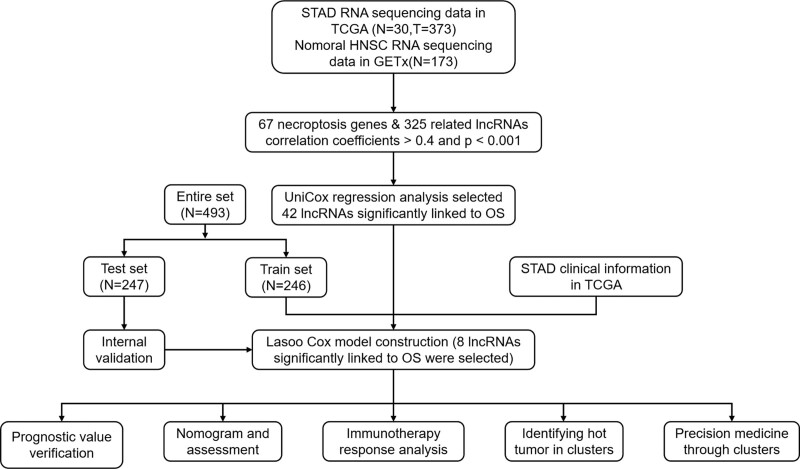
To get synthetic data matrices about HNSCC and normal tissues, we downloaded the RNA transcriptome datasets (HTSeq—Counts and HTSeq—FPKM) and relevant clinical information from Genotype-Tissue Expression Project (GTEx) (https://www.gtexportal.org/) and The Cancer Genome Atlas (TCGA) (https://portal.gdc.cancer.gov/). Then we converted the FPKM value to the TPM value of the synthetic matrix by using R packages data.table, tibble, dplyr, and tidyr. Finally, 2 synthetic matrices viz. the Counts and TPM matrices were obtained, which were used to identify differentially expressed lncRNAs, and for the other analyses, respectively. To reduce statistical bias in this analysis, we excluded HNSCC patients with missing OS or short OS (<30 days). With relevant clinical information, we retrieved 493 patients and randomly divided them into a training risk group and a testing risk group at the ratio of 1:1 by using Strawberry Perl and caret R package.
The study did not involve the patients and the animals, so this study was approved by the Ethics Committee of Jurong People Hospital.
2.2. Selection of necroptosis-related genes and lncRNAs
The necroptosis gene set M24779.gmt containing 8 necroptosis genes was downloaded from the Gene Set Enrichment Analysis (GSEA) (http://www.gsea-msigdb.org/gsea/index.jsp).[21] With previous reports about necroptosis, we finally obtained the profile of 67 necroptosis-related genes. Then we found 325 differentially expressed lncRNAs (Log2 fold change [FC] > 1, false discovery rate [FDR] < 0.05, and P < .05) after screening the synthetic data matrix on Strawberry Perl and limma R package.[23] Correlation analysis was performed between the 67 necroptotic genes and the differentially expressed lncRNAs in the combined matrices. Then 325 lncRNAs, with necroptosis-related genes, Pearson correlation coefficient > 0.4, and P < .001, were considered as necroptosis-related.
2.3. Establishment and validation of the risk signature
Based on the clinical data of the HNSCC cases in TCGA and GTEx, we conducted univariate Cox (uni-Cox) proportional risk regression analysis to screen out the necrosis-associated lncRNAs (P < .05). We then performed a 10-fold cross-validated Lasso regression with P of 0.05 and ran 1000 cycles.[24] To prevent overfitting, we set 1000 random stimuli for each cycle. Then a model was established, and the 1 -, 2 -, and 3-year receiver operating characteristic (ROC) curves of the model were drawn using computer programs. The risk score was calculated as follows:[24]
where lncRNA coefficient is the abbreviation of survival related lncRNA coefficient, exp (lncRNA) is lncRNA expression level, and coef (lncRNA) is the regression coefficient. Low- and high-risk groups were established according to the median risk score.[24] The prognostic value of the model was evaluated by analyzing its relationship with clinical factors through Chi-square test.
2.4. Independence factors and ROC
Uni-Cox and multi-Cox regression analyses were used to assess whether risk score and clinical characteristics were independent variables, and ROC was performed to compare the prognostic predictions of different factors.
2.5. Nomograms and survival analysis of different clinical features
Nomograms of 1-, 2-, and 3-year OS were established on the RMS R package using risk score, age, and tumor stage, and correction curves were established using Hosmer–Lemeshow test to clarify whether the predicted results were consistent with the actual results.[21]
2.6. Gene set enrichment analyses
With P < .05 and FDR < 0.25 as criteria, the pathways that were significantly enriched between the low-risk and high-risk groups were determined by planning the gene set (kegg.v7.4.symbols.GMT) and GSEA (https://www.gsea-msigdb.org/gsea/ login).
2.7. Investigation of TME and immune checkpoints
Based on the results of GSEA, we analyzed the immune cell factors in the risk population. We calculated the immune infiltration status of HNSCC patients from TCGA, including the TIMER, CIBERSORT, XCELL, QUANTISEQ, MCP counter, EPIC and CIBERSORT on TIMER 2.0 (http://timer.cistrome.org/).[21] Alternatively, we downloaded invasion estimates for all TCGA tumors from the same website. Differences in the contents of immune infiltrating cells were analyzed using Wilcoxon sign rank test, LIMma, SCALES, GGploT2, and GGTEXT R package, and the results were shown in bubble graphs. In addition, TME scores and immune checkpoint activation were compared between the low-risk and high-risk groups using the GGPUBR.
2.8. Exploration of model in the clinical treatment
In genomics of drug-sensitivity in cancers, we used r-pack Prrophtic to assess the treatment response of each HNSCC patient, which was determined by the semi-maximum inhibitory concentration (IC50) (https://www.Cancerrxgene.org/).[25]
2.9. Clusters based on 8 prognostic lncRNAs
To explore the immunotherapeutic response of HNSCC patients to, we explored potential molecular subsets through the consensus ClusterPlus (CC) R package based on prognostic lncRNA expressions.[26] Principal component analysis, T-distributed random neighbor embedding and Kaplan–Meier survival were conducted on the RTSSNE R package. Immunoassay and drug sensitivity were compared by GSA Base and Prrophtic R package.
3. Results
3.1. Necroptosis-related lncRNAs in HNSCC patients
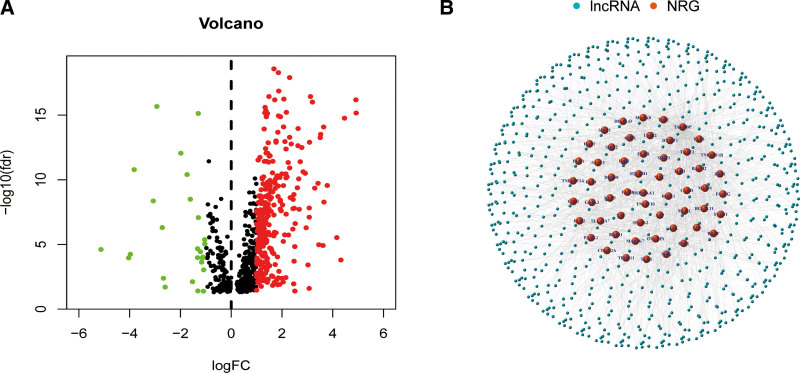
The flow of the study was exhibited in Figure 1. From the TCGA and GTEx matrices, we obtained 204 normal samples (173 samples from GTEx) and 373 tumor samples. According to the expressions of 67 necroptosis-related genes and the differentially expressed lncRNAs (|Log2FC| > 1 and P < .05) between normal and tumor samples, we finally got 325 necroptosis-related lncRNAs (correlation coefficient > 0.4 and P < .001).[23,27] Of them, 190 lncRNAs were upregulated, and the others were downregulated (Fig. 2A). The network and data between necroptosis-related genes (e.g., FLT3 and PLK2) and lncRNAs were shown in Figure 2B.
Figure 1.
Flow diagram of the study.
Figure 2.
Identification of necroptosis-related lncRNAs in patients with HNSCC. (A) The volcano plot of 325 differentially expressed necroptosis genes. (B) The network between necroptosis genes and lncRNAs (correlation coefficients >0.4 and P < .001). HNSCC = head and neck squamous cell carcinoma, lncRNAs = Long-chain noncoding RNAs.
3.2. Model construction and verification
Uni-Cox regression analysis revealed that 42 necroptosis-related lncRNAs were significantly correlated with OS (P < .05), which were plotted on a heat map (Fig. 3A and B). To avoid overfitting in the prognostic signature, we performed Lasso regression on these lncRNAs and extracted 42 necroptosis-related lncRNAs in HNSC when the first-rank value of Log (λ) was the minimum likelihood of deviance (Fig. 3C and D). Besides, we found 8 lncRNAs were upregulated in the Sankey diagram (Fig. 3E).
Figure 3.
Extraction of necroptosis-related lncRNAs prognostic signature in HNSC. (A) The prognostic lncRNAs extracted by univariate Cox regression analysis. (B) The expression profiles of 42 prognostic lncRNAs. (C) The 10-fold cross-validation for variable selection in the LASSO model. (D) The LASSO coefficient profile of 42 necroptosis-related lncRNAs. (E) The Sankey diagram of necroptosis genes and related lncRNAs. lncRNAs = long-chain noncoding RNAs.
The risk score was calculated as: .
With the risk score formula, the distribution of risk score, the survival status, survival time, and relevant expression standards of these lncRNAs were compared between low- and high-risk groups in the training, testing and entire sets. The results all indicated the low-risk group had better prognosis (Fig. 4A–D). Besides, the same results were found in conventional clinicopathologic characteristics, age, gender, grade, and stage (Fig. 4E).
Figure 4.
Prognosis value of the 8 necroptosis-related lncRNAs model in the train, test, and entire sets. (A) Exhibition of necroptosis related lncRNAs model based on risk score of the train, test, and entire sets, respectively. (B) Survival time and survival status between low and high-risk groups in the train, test, and entire sets, respectively. (C) The heat map of 8 lncRNAs expression in the train, test, and entire sets, respectively. (D) Kaplan–Meier survival curves of OS (survival probability) of patients between low- and high-risk groups in the train, test, and entire sets, respectively. (E) Kaplan–Meier survival curves of OS (survival probability) prognostic value stratified by age, gender, grade, stage between low- and high-risk groups in the entire set. lncRNAs = Long-chain noncoding RNAs, OS = overall survival.
3.3. Construction of nomograms
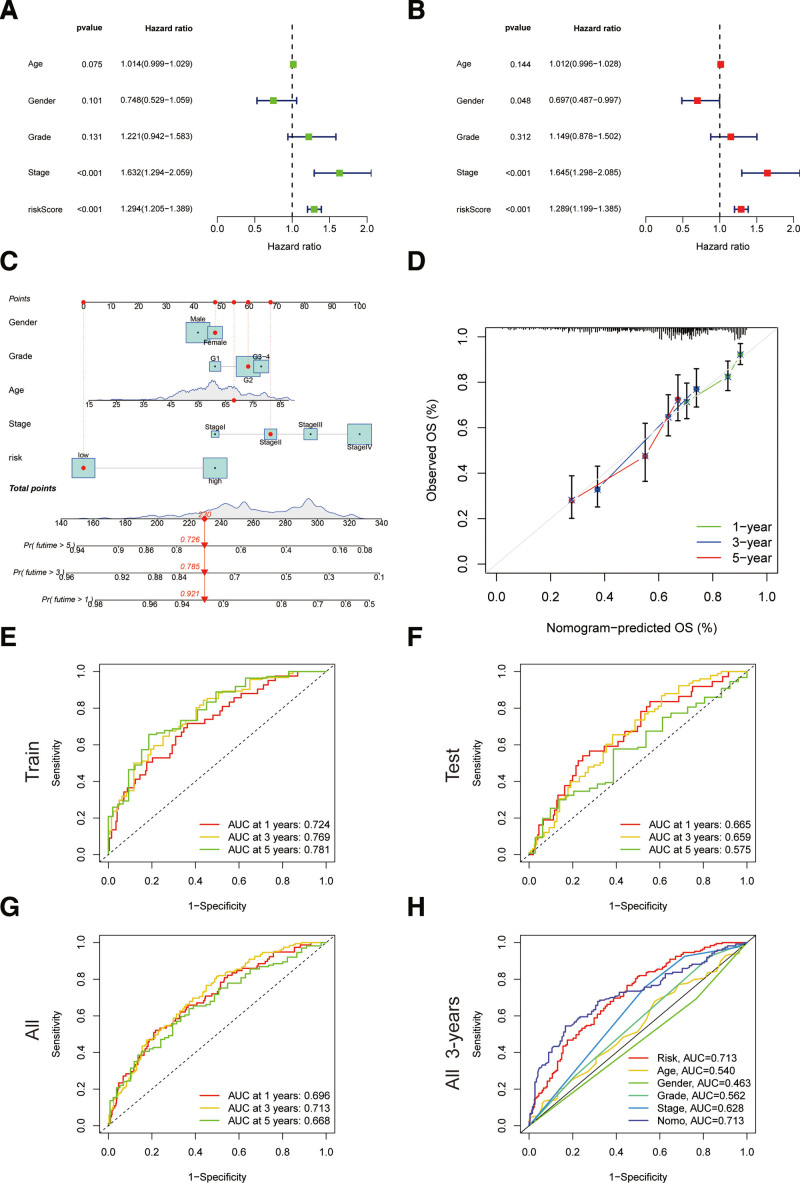
The hazard ratio of the risk score and 95% confidence interval were 1.294 and 1.205 to 1.389 (P < .001) respectively in uni-Cox regression, and 1.289 and 1.199 to 1.385 (P < .001) respectively in multi-Cox regression (Fig. 5A and B). In addition, we found another 2 independent prognostic parameters, including gender (0.697 and 0.487–0.997; P = .048) and tumor node metastasis (TNM) stage (1.645 and 1.298–2.065; P < .001) (Fig. 5B. With the 3 independent prognostic factors (risk score, gender, TNM stage, all P < .05 in multi-Cox), we built nomograms to predict the 1-, 2-, and 3-year OS incidences of HNSC patients (Fig. 5C). We also utilized the 1-, 2-, and 3-year calibration plots to attest that the nomograms well accorded with the prediction of 1-, 2-, and 3-year OS (Fig. 5D).
Figure 5.
Nomogram and assessment of the risk model. (A and B) Uni- and multi-Cox analyses of clinical factors and risk score with OS. (C) The nomogram that integrated the risk score, age, and tumor stage predicted the probability of the 1-, 2-, and 3-yr OS. (D) The calibration curves for 1-, 2-, and 3-yr OS. (E–G) The 1-, 2-, and 3-yr ROC curves of the train, test, and entire sets, respectively. (H) The 3-yr ROC curves of risk score, nomogram total score, and clinical characteristics. OS = overall survival, ROC = receiver operating characteristic.
3.4. Assessment of the risk model
Time-dependent ROC curves were utilized to evaluate the sensitivity and specificity of the model on the prognosis. We also illustrated the results of ROC with the area under the curve (AUC). The 1-, 2-, and 3-year AUCs were 0.724, 0.769, 0.781 with the training set, 0.665, 0.659, 0.575 with the testing set, 0.696, 0.713, and 0.668 with the entire set, respectively (Fig. 5E–G). The clinical factors and nomogram total score, and risk score (0.713) and nomogram (0.713) showed a predominant predictive ability on the 3-year ROC of the risk model (Fig. 5H).
3.5. GSEA
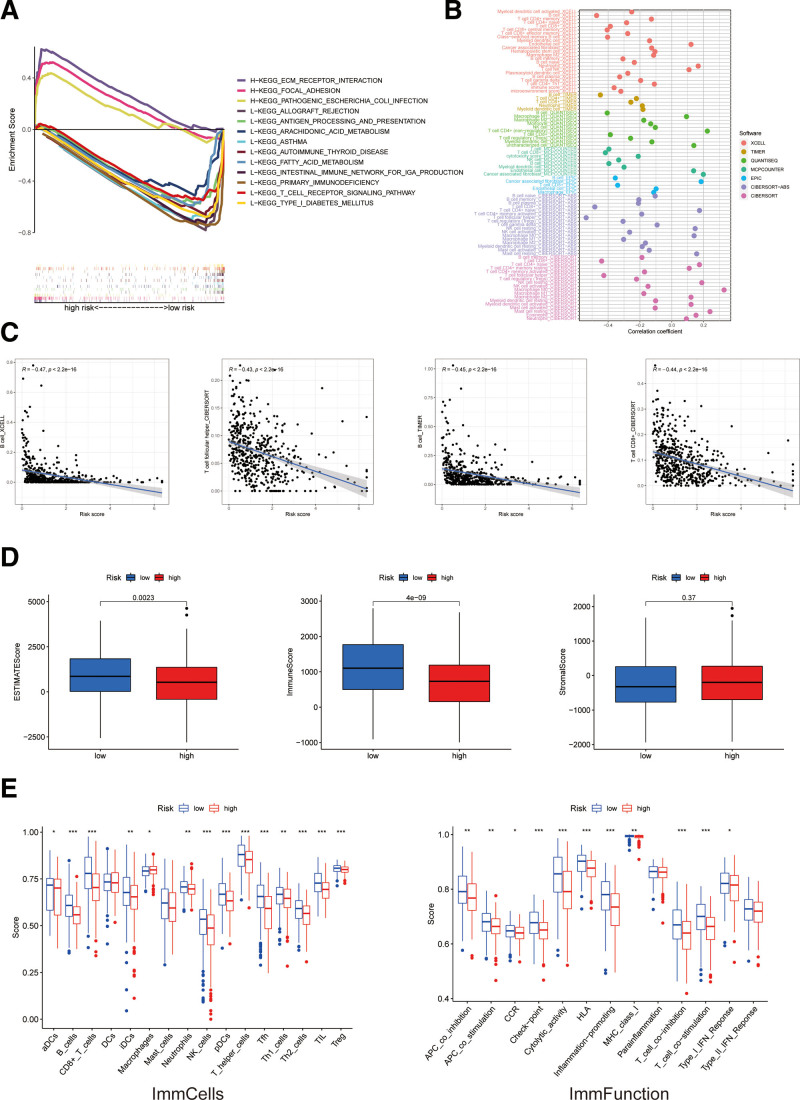
To investigate the differences in biological functions between the risk groups, we utilized the software GSEA to explore the high-risk group in the KEGG pathway in the entire set. Some cancer- and metabolism-related pathways were enriched by GSEA, including ECM receptor interaction and the focal adhesion pathway that were significantly associated with the high-risk group (all P < .05; FDR < 0.25; |NES| > 1.5) (Fig. 6A). Therefore, we made an immunity analysis in the model.
Figure 6.
The investigation of tumor immune factors and immunotherapy. (A) GSEA of the top 3 pathways significantly enriched in the high-risk group. (B) The immune cell bubble of risk groups. (C) The correlation between risk score and immune cells. (D) The comparison of immune-related scores between low- and high-risk groups. (E) The comparison of immune-cell scores and immune-function between low- and high-risk groups. GSEA = gene set enrichment analysis.
3.6. Investigation of immunity factors and clinical treatment in risk groups
More immune cells were associated with the low-risk group on different platforms exhibited at the immune cell bubble chart and in the document, such as B cell at TINEK, T cell CD8+ and macrophage M1 at QUANTISEQ, T cell CD8+ and T cell follicular helper at CIBERSORT-ABS, T cell CD8+ at MCPCOUNTER, and T cell CD8+ and T cell follicular helper at CIBERSORT (all P < .05) (Fig. 6B). Besides, the low-risk score was more associated with immune cells, such as T cell CD8+, B cells and T cell follicular helper (Fig. 6C). All of these results indicated the low-risk group had a higher immune infiltration status, a higher immune score and a higher ESTIMA. The microenvironment score of the low-risk group signified a different TME from the high-risk group (Fig. 6D). Most immune-cell scores and immune-function were also better (Fig. 6E) and most immune checkpoints were more activated (Fig. 7A) in the low-risk group. These results imply we can choose appropriate checkpoint inhibitors for HNSCC patients regrouped by the risk mode. We examined differences in HNSCC 50 chemotherapy or targeted drug IC50 between the 2 groups and found that 14 chemical or targeted drugs, which were applied to HNSCC therapy, showed lower IC50 in the low-risk group (Fig. 7B). These findings may be helpful for clinical treatment.
Figure 7.
The investigation of tumor immune factors and immunotherapy. (A) The difference of 34 checkpoints expression in risk groups. (B) The immunotherapy prediction of risk groups.
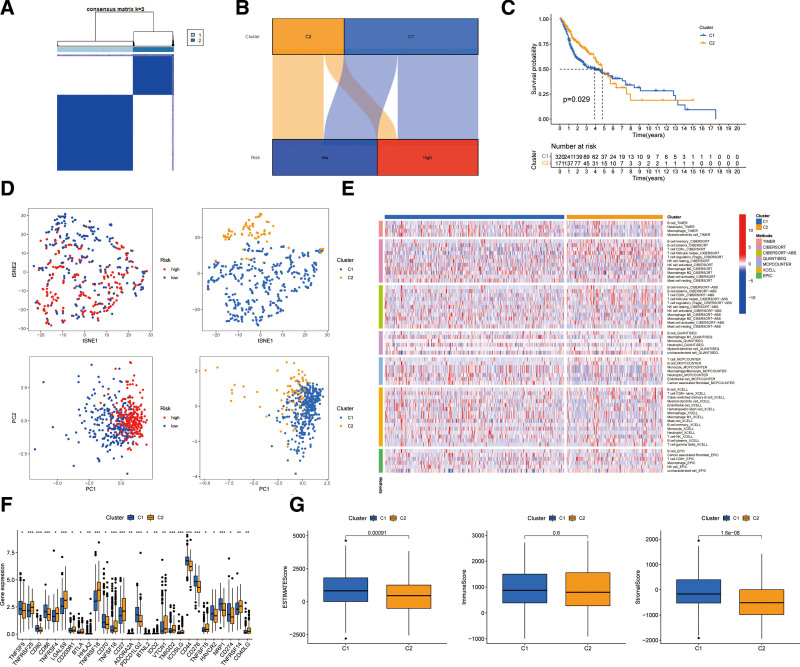
3.7. Distinguishing between cold and hot tumors and precision medicine in clusters
Different clusters, known as subtypes, usually show different immune microenvironments, leading to different immunotherapeutic responses.[28,29] We recombined the patients into 2 clusters through Consensus ClusterPlus R package based on the expressions of the 8 necrotic lncRNAs (Fig. 8A).[30] Cluster 1 was located partly in the low-risk group and partly in the high-risk group, and Cluster 2 was mainly located in the high-risk group (Fig. 8B). Besides, cluster 1 had better OS (P = .029) in the Kaplan–Meier analysis (Fig. 8C). T-distributed random neighbor embedding showed the 2 clusters can be clearly distinguished (Fig. 8D). In addition, we conducted principal component analysis to verify that the risk groups and clusters had different principal components (Fig. 8D). Cluster 1 was more highly infiltrated by immune cells according to analyses of the different platforms (Fig. 8E). Cluster 1 had a higher immune score and a higher ESTIMAT (microenvironment) score, indicating different TMESs (Fig. 8G). Almost all the immune checkpoints were more actively expressed in cluster 1, such as CD86, PDCD1LG2, CD276, CD44, HAVCR2, and CD274 (Fig. 8F). The CD8 + T cells with the pro-inflammation function and high immune score played vital roles in hot tumors.[21] Therefore, cluster 1 can be considered as the hot tumor and cluster 2 as the cold tumor. According to the notion of the cold and hot tumors, cluster 1 was more susceptible to immunotherapy. Then drug sensitivity comparison uncovered 14 immunotherapeutic chemical or targeted drugs (e.g., Imatinib and BMS536924) that showed different IC50 to systemic treatments in HNSCC (Fig. 9A). Because of these lncRNA clusters, we may further investigate immunotherapeutic responses and enhance precise medication in HNSCC patients.
Figure 8.
Distinction between cold and hot tumors and immunotherapy prediction. (A) Patients divided into 2 clusters by Consensus Cluster Plus. (B) The relationship between risk groups and clusters. (C) Kaplan–Meier survival curves of OS in clusters. (D) The t-SNE of 2 clusters and PCA of risk groups and clusters. (E) The heat map of immune cells in clusters. (F) The difference of 28 checkpoints expression in clusters. (G) The comparison of immune-related scores between clusters 1 and 2. OS = overall survival, PCA = principal component analysis, T-SNE = T-distributed random neighbor embedding.
Figure 9.
Fourteen immunotherapeutic drugs and chemical or targeted drugs showing significant IC50 difference. IC50 = semi-maximum inhibitory concentration.
4. Discussion
Treatment of HNSCC is a substantial clinical challenge owing to the high local recurrence rate and chemotherapeutic resistance.[31] Since the current TNM grading system is still unsuitable for predicting the survival outcomes of HNSCC patients,[31] there is a need to explore new HNSCC biomarkers. However, the existing studies on HNSCC-related biomarkers are insufficient to meet the clinical requirements for the diagnosis and prognosis of HNSCC. Necrosis is a common pathologic phenomenon of HNSCC.[32] It is strongly associated with an aggressive phenotype and poor prognosis in various cancers, including HNSCC,[14] but its mechanism has not been fully clarified. As a non-coding RNA, lncRNAs play an important role in tumor progression. LncRNAs can participate in tumor growth and metastasis through transcriptional and post-transcriptional mechanisms, and can affect the expression of oncogenic transcription factors.[33] In the immune system, LncRNAs are expressed at different levels in different environments, stages and cell types, which contributes to the coordination of immune function.[33] LncRNAs can be used as biomarkers or immunotherapy targets to predict and diagnose many different types of cancer, including HNSCC.[34] Since more attention is focused on the prognostic effect of necrosis in HNSCC, little is known about the use and internal interactions of necrosis-related lncRNAs as biomarkers in HNSCC. Therefore, further research about the influence of necrosis-related lncRNAs on HNSCC is necessary. We divided the patients into groups according to the necrotic lncRNA expression condition, constructed prognostic markers for the first time, and systematically studied the correlations of TME, immune cell infiltration and immune checkpoints with necrosis-related lncRNAs. We look forward to using these results to guide future clinical diagnosis and treatment.
With the necrosis-related lncRNAs identified from 493 HNSCC patients in TCGA, we found 8 necrosis-related lncRNAs (AC099850.3, AC243829.2, AL139095.4, SAP30L-AS1, C5orf66-AS1, LIN02084, LIN00996, MIR4435-2HG) to be independent prognostic factors of HNSCC in the training cohort. The 493 HNSCC patients were divided by the risk scores into low- and high-risk groups, and compared between groups in the training, testing and entire sets. The results all indicated the high-risk patients had significantly worse OS than the low-risk patients. This finding is consistent with the prognostic value of necrosis-related lncRNAs in colorectal cancer[35] and gastric cancer.[21]
To demonstrate the practical value of the risk score with the 493 HNSCC samples, we determined the 1-, 2-, and 3-year AUCs were 0.724, 0.769, 0.781 in the training set, 0.665, 0.659, 0.575 in the testing set, 0.696, 0.713, and 0.668 in the entire set, respectively. The ROC curves further verified the prognostic accuracy of risk scores. Results indicate risk scores can be used as a predictor of prognosis. On the 3-year ROC curve of the risk model, the clinical factors and nomogram total score, and risk score (0.713) and nomogram (0.713) showed the predominant predictive ability. The nomograms also predict the prognosis of HNSCC patients, and the prediction results are in good agreement. This finding is consistent with previous studies on breast cancer, gastric cancer and endometrial carcinoma.[21,36,37]
GSEA reveals that T cell receptor signaling pathways and intestinal immune network for iga production were enriched in the low-risk group, indicating that these pathways may be inhibited in the high-risk group. Reportedly, the T cell receptor signaling is crucial for T cell proliferation and adaptive immunity development, and abnormalities in this signaling can lead to immunodeficiency. Moreover, the enrichment of T cells is associated with a better prognosis,[38] which is consistent with our findings. We further found the degree of immune cell infiltration was negatively correlated with the risk score. Subsequently, we used CIBERSORT to further supplement 16 immune filtrating cells in the high- and low-risk groups. The relative proportions of B cells, aDCs, CD8 + T cells, iDCs, mast cells, neutrophils, NK-cells, PDcs, Tfh, T helper cells, Th1 cells, Th2 cells, TIL and Treg were all higher in the low-risk group. The high-risk group had higher relative proportions of activated dendritic cells and macrophages M0. These results suggest a significant difference in immune cell infiltration between high-risk and low-risk populations. Zhang J et al showed that neutrophils, activated mast cells, activated NK cells, resting memory CD4 + T cells, naïve CD4 + T cells, M2 macrophages and eosinophils were favorable factors for the OS of HNSCC patients.[39] Yang et al found the CD8 T cells, dormant but inactive mast cells, quiescent dendritic cells, and activated CD4 T cells were also associated with better survival of patients with cervical squamous cell carcinoma.[40] This finding is not completely consistent with our results. The reason for such difference was that Cluster 1 in our study was located in the low-risk group and partially in the high-risk group, while Cluster 2 was mainly located in the high-risk group. Therefore, further clinical practice is needed to verify our results. To further investigate the relationship between the model and immunity, we explored the correlation between risk score and 34 ICIs-related biomarkers. Results showed that the expressions of most biomarkers were up-regulated in the low-risk group (all P < .001), suggesting that the low-risk group benefited more from immunotherapy.
Different molecular subtypes, also known as clusters, are associated with tumor immunosuppression and the microenvironment. The differences in immune and TME scores among subtypes lead to different outcomes and immunotherapeutic responses.[29,41] Therefore, patients were divided into 2 clusters according to the expressions of these lncRNAs.[30] In this study, ConsensusClusterPlus method was adopted to classify patients, which is different from previous studies on glioma[42] and ovarian cancer.[33] The main reason is that, ConsensusClusterPlus enables quick and accurate visualization of cluster boundaries, which are not labeled in ConsensusCluster and ConsensusClusterPlus extends CC algorithm. ConsensusClusterPlus is an open source, bioconductor compatible software for different classifications of unsupervised discovery.[30] As expected, the 2 clusters have different immune microenvironments. Cluster 1 has immuno-suppressive TME, and has more highly infiltrated CD8 + T cells with strong pro-inflammatory activity, higher immune score, and higher activities, which can be identified as hot tumors.[9,43] Moreover, cluster 1 is more sensitive to immunotherapeutic drugs. Our results also suggested that patients with high risk and cluster2 belong to cold tumor subtype. The high-risk group and cluster2 are featured with immune infiltration reduction and T cells exhaustion, matching the definition of an “immune-desert” phenotype.[44] This means that the immune surveillance functions of patients with higher risk score were weakened, which is conducive to immune escape, and the effect of immunotherapy will be poor, this is consistent with the results of previous related studies on ovarian cancer.[33] So, Necroptosis-related lncRNAs not only can predict prognosis, but also act as a guide for individual therapy.
T-cell-targeted immunotherapy, microbiome regulation, or other immunotherapy drugs can be used to treat patients with hot tumors. For cold tumors, however, such treatment is not easy because it is unable to release the existing low-level immunity from T cells.[9] CD8 + T cells can kill cancer cells by releasing PRF1, GNLY or GZM, and break tolerance as a preexisting immune response, enhancing immunotherapy through the PD-1/PD-L1 immunosuppression axis. Therefore, it is wise to turn a cold tumor into a hot tumor, rather than just giving other treatments.[9,45] We constructed an 8-lncRNA model associated with necrosis and attempted to identify cold and hot tumors. The model was used to redivide the patients into low-risk and high-risk groups, which were then sent to Kaplan–Meier analysis, GSEA, IC50 prediction and other analyses. We found Cluster 1 was located partly in the low-risk group and partly in the high-risk group, and Cluster 2 was mainly located in the high-risk group. Although the risk groups can guide prognosis prediction and systemic treatments, we cannot precisely identify the hot tumors according to risk groups. This finding is similar to a previous study on gastric cancer.[21]
Nevertheless, our study has some limitations. First, our data and all analyses were based on TCGA, which can lead to bias. If we synthesized the data from other sources, we may get different results. Second, we did not experimentally verify the differences in molecular transcription and expression levels, which undoubtedly reduced the credibility of this study. Finally, we lack clinical follow-up data to justify the value of our prognostic model.
5. Conclusions
We systematically evaluated the value of necroptosis-related lncRNAs in survival prediction, the roles of the tumor microenvironment and immune cell infiltration, the potential regulatory mechanism of necroptotic lncRNAs, and the prediction of potential compounds for the treatment of HNSCC. Eight lncRNA features associated with necroptosis can predict the survival of HNSCC patients and may be helpful for individualized treatment of HNSCC in the future. The different groups composed of 8 lncRNA can be used as a tool to distinguish clinical hot and cold tumors.
Clinical medical science and technology development fund project of Jiangsu University (grant numbers: JLY2021097), supported by the Jurong Min sheng science and technology project of China (grant numbers: 2020SA00106), Jurong Social development science and technology project (grant numbers: ZA42112), supported by National Natural Science Foundation of China (grant numbers: 82003228), Jiangsu Science and technology project (grant numbers: BK20201080). Court-level Natural Science Foundation Project of Jurong People Hospital (grant numbers: JY20221001).
Author contributions
Conceptualization: Yujing Shi, Xiaoke Di, Yumeng Zhang, Liang Liang.
Data curation: Xiaoke Di, Yumeng Zhang, Nian Zuo, Lan Wang.
Formal analysis: Xiaoke Di, Yumeng Zhang, Liang Liang.
Investigation: Mengyang Ju, Xiaoke Di, Lan Wang.
Methodology: Yujing Shi, Mengyang Ju, Xiaoke Di.
Project administration: Yujing Shi, Lan Wang.
Resources: Mengyang Ju.
Software: Mengyang Ju.
Supervision: Xinchen Sun, Liang Liang.
Validation: Xiaoke Di, Nian Zuo, Xinchen Sun.
Visualization: Xiaoke Di.
Writing – original draft: Yujing Shi, Mengyang Ju, Xiaoke Di, Xinchen Sun, Liang Liang.
Writing – review & editing: Yujing Shi, Yumeng Zhang.
Abbreviations:
- AUC
- area under the curve
- FDR
- false discovery rate
- GSEA
- gene set enrichment analysis
- GTEx
- genotype-tissue expression project
- HNSCC
- head and neck squamous cell carcinoma
- IC50 =
- semi-maximum inhibitory concentration
- ICI
- immune checkpoint inhibitor
- lncRNAs
- Long-chain noncoding RNAs
- OS
- overall survival
- PD-1
- programmed cell death protein
- PD-L-1
- programmed death ligand
- ROC
- receiver operating characteristic
- TCGA
- The Cancer Genome Atlas
- TNM
- tumor node metastasis
- uni-Cox
- univariate cox
The authors have no conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
How to cite this article: Shi Y, Zhang Y, Zuo N, Wang L, Sun X, Liang L, Ju M, Di X. Necrotic related-lncRNAs: Prediction of prognosis and differentiation between cold and hot tumors in head and neck squamous cell carcinoma. Medicine 2023;102:23(e33994).
Contributor Information
Yujing Shi, Email: 1208519910@qq.com.
Yumeng Zhang, Email: zym20192021@163.com.
Nian Zuo, Email: zuo932431386@163.com.
Lan Wang, Email: 128871998@163.com.
Xinchen Sun, Email: sunxinchen2019@163.com.
Liang Liang, Email: lianglvlv@yeah.net.
Mengyang Ju, Email: drjmy2013@163.com.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Duhen R, Ballesteros-Merino C, Frye AK, et al. Neoadjuvant anti-OX40 (MEDI6469) therapy in patients with head and neck squamous cell carcinoma activates and expands antigen-specific tumor-infiltrating T cells. Nat Commun. 2021;12:1047. Published 2021 Feb 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Argiris A, Harrington KJ, Tahara M, et al. Evidence-based treatment options in recurrent and/or metastatic squamous cell carcinoma of the head and neck. Front Oncol. 2017;7:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Gillison ML, Blumenschein G, Fayette J, et al. Check-Mate 141: 1-year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist. 2018;23:1079–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seiwert TY, Burtness B, Mehra R, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65. [DOI] [PubMed] [Google Scholar]
- [8].Chow LQM, Haddad R, Gupta S, et al. Antitumor activity of pembrolizumab in biomarker unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J Clin Oncol. 2016;34:3838–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov. 2019;18:197–218. [DOI] [PubMed] [Google Scholar]
- [10].Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. [DOI] [PubMed] [Google Scholar]
- [11].Angelova M, Mlecnik B, Vasaturo A, et al. Evolution of metastases in space and time under immune selection. Cell. 2018;175:751–765.e16. [DOI] [PubMed] [Google Scholar]
- [12].Tang R, Xu J, Zhang B, et al. Ferroptosis, necroptosis, and pyroptosis in anticancer immunity. J Hematol Oncol. 2020;13:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Snyder AG, Hubbard NW, Messmer MN, et al. Intratumoral activation of the necroptotic pathway components RIPK1 and RIPK3 potentiates antitumor immunity. Sci Immunol. 2019;4:eaaw2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Li J, Huang S, Zeng L, et al. Necroptosis in head and neck squamous cell carcinoma: characterization of clinicopathological relevance and in vitro cell model. Cell Death Dis. 2020;11:391. Published 2020 May 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Pollheimer MJ, Kornprat P, Lindtner RA, et al. Tumor necrosis is a new promising prognostic factor in colorectal cancer. Hum Pathol. 2010;41:1749–57. [DOI] [PubMed] [Google Scholar]
- [16].Zhang L, Zha Z, Qu W, et al. Tumor necrosis as a prognostic variable for the clinical outcome in patients with renal cell carcinoma: a systematic review and meta-analysis. BMC Cancer. 2018;18:870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Leek RD, Landers RJ, Harris AL, et al. Necrosis correlates with high vascular density and focal macrophage infiltration in invasive carcinoma of the breast. Br J Cancer. 1999;79:991–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Seifert L, Werba G, Tiwari S, et al. The necrosome promotes pancreatic oncogenesis via CXCL1 and mincle-induced immune suppression. Nature. 2016;532:245–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Seehawer M, Heinzmann F, D'Artista L, et al. Necroptosis microenvironment directs lineage commitment in liver cancer. Nature. 2018;562:69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Quinn JJ, Chang HY. Unique features of long non-coding RNA biogenesis and function. Nat Rev Genet. 2016;17:47–62. [DOI] [PubMed] [Google Scholar]
- [21].Zhao Z, Liu H, Zhou X, et al. Necroptosis-related lncRNAs: predicting prognosis and the distinction between the cold and hot tumors in gastric cancer. J Oncol. 2021;2021:6718443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Diao P, Jiang Y, Li Y, et al. Immune landscape and subtypes in primary resectable oral squamous cell carcinoma: prognostic significance and predictive of therapeutic response. J ImmunoTher Cancer. 2021;9:e002434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Meng T, Huang R, Zeng Z, et al. Identification of prognostic and metastatic alternative splicing signatures in kidney renal clear cell carcinoma. Front Bioeng Biotechnol. 2019;7:270. Published 2019 Oct 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bunea F, She Y, Ombao H, et al. Penalized least squares regression methods and applications to neuroimaging. Neuroimage. 2011;55:1519–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Geeleher P, Cox NJ, Huang R. “Clinical drug response can be predicted using baseline gene expression levels and in vitro drug sensitivity in cell lines”. Genome Biol. 2014;15:R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Menares E, Gálvez-Cancino F, Cáceres-Morgado P, et al. Tissue-resident memory CD8+ T cells amplify anti-tumor immunity by triggering antigen spreading through dendritic cells. Nat Commun. 2019;10:4401. Published 2019 Sep 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Shen Y, Peng X, Shen C. “Identification and validation of immune-related lncRNA prognostic signature for breast cancer”. Genomics. 2020;112:2640–6. [DOI] [PubMed] [Google Scholar]
- [28].Das S, Camphausen K, Shankavaram U. “Cancerspecific immune prognostic signature in solid tumors and its relation to immune checkpoint therapies”. Cancers. 2020;12:2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].DeBerardinis RJ. “Tumor microenvironment, metabolism, and immunotherapy”. N Engl J Med. 2020;382:869–71. [DOI] [PubMed] [Google Scholar]
- [30].Wilkerson MD, Hayes DN. “ConsensusClusterPlus: a class discovery tool with confidence assessments and item tracking”. Bioinformatics. 2010;26:1572–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou LQ, Shen JX, Zhou JY, et al. The prognostic value of m6A-related lncRNAs in patients with HNSCC: bioinformatics analysis of TCGA database. Sci Rep. 2022;12:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ou D, Garberis I, Adam J, et al. Prognostic value of tissue necrosis, hypoxia-related markers and correlation with HPV status in head and neck cancer patients treated with bio or chemo-radiotherapy. Radiother Oncol. 2018;126:116–24. [DOI] [PubMed] [Google Scholar]
- [33].Liu J, Geng R, Ni S, et al. Pyroptosis-related lncRNAs are potential biomarkers for predicting prognoses and immune responses in patients with UCEC. Mol Ther Nucleic Acids. 2022;27:1036–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Jiang Y, Cao W, Wu K, et al. LncRNA LINC00460 promotes EMT in head and neck squamous cell carcinoma by facilitating peroxiredoxin-1 into the nucleus. J Exp Clin Cancer Res. 2019;38:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Liu L, Huang L, Chen W, et al. Comprehensive analysis of necroptosis-related long noncoding RNA immune infiltration and prediction of prognosis in patients with colon cancer. Front Mol Biosci. 2022;9:811269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Chen F, Yang J, Fang M, et al. Necroptosis-related lncRNA to establish novel prognostic signature and predict the immunotherapy response in breast cancer. J Clin Lab Anal. 2022;36:e24302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Liu J, Mei J, Wang Y, et al. Development of a novel immune-related lncRNA signature as a prognostic classifier for endometrial carcinoma. Int J Biol Sci. 2021;17:448–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang J, Li Z, Gao A, et al. The prognostic landscape of tumor-infiltrating immune cells in cervical cancer. Biomed Pharmacother. 2019;120:109444. [DOI] [PubMed] [Google Scholar]
- [39].Zhang J, Zhong X, Jiang H, et al. Comprehensive characterization of the tumor microenvironment for assessing immunotherapy outcome in patients with head and neck squamous cell carcinoma. Aging (Albany NY). 2020;12:22509–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang S, Wu Y, Deng Y, et al. Identification of a prognostic immune signature for cervical cancer to predict survival and response to immune checkpoint inhibitors. Oncoimmunology. 2019;8:e1659094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zeng D, Li M, Zhou R, et al. “Tumor microenvironment characterization in gastric cancer identifies prognostic and immuno-therapeutically relevant gene signatures”. Cancer Immunol Res. 2019;7:737–50. [DOI] [PubMed] [Google Scholar]
- [42].Cao K, Su F, Shan X, et al. Necroptosis-related lncRNAs: establishment of a gene module and distinction between the cold and hot tumors in glioma. Front Oncol. 2023;13:1087117. Published 2023 Apr 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zheng Y, Tian H, Zhou Z, et al. “A novel immune-related prognostic model for response to immunotherapy and survival in patients with lung adenocarcinoma”. Front Cell Dev Biol. 2021;9:651406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Zhang B, Wu Q, Li B, et al. m(6)A regulator-mediated methylation modification patterns and tumor microenvironment infiltration characterization in gastric cancer. Mol Cancer. 2020;19:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Hegde PS, Karanikas V, Evers S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin Cancer Res. 2016;22:1865–74. [DOI] [PubMed] [Google Scholar]