OBJECTIVES:
Studies evaluating inhaled prostacyclins for the management of acute respiratory distress syndrome (ARDS) have produced inconsistent results regarding their effect on oxygenation. The purpose of this systematic review and meta-analysis was to evaluate the change in the Pao2/Fio2 ratio after administration of an inhaled prostacyclin in patients with ARDS.
DATA SOURCES:
We searched Ovid Medline, Embase, Cumulative Index to Nursing and Allied Health Literature, Cochrane, Scopus, and Web of Science.
STUDY SELECTION:
We included abstracts and trials evaluating administration of inhaled prostacyclins in patients with ARDS.
DATA EXTRACTION:
Change in the Pao2/Fio2 ratio, Pao2, and mean pulmonary artery pressure (mPAP) were extracted from included studies. Evidence certainty and risk of bias were evaluated using Grading of Recommendations Assessment, Development, and Evaluation and the Cochrane Risk of Bias tool.
DATA SYNTHESIS:
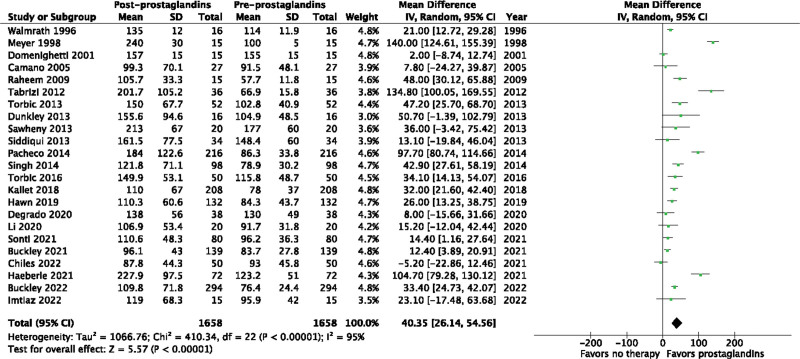
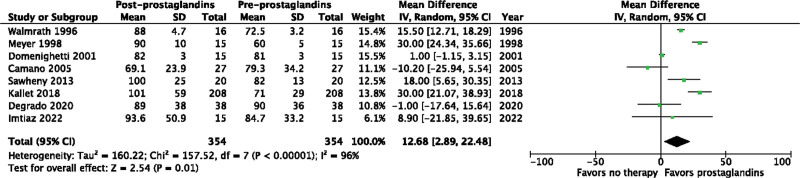
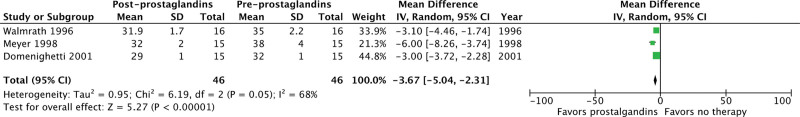
We included 23 studies (1,658 patients) from 6,339 abstracts identified by our search strategy. The use of inhaled prostacyclins improved oxygenation by increasing the Pao2/Fio2 ratio from baseline (mean difference [MD], 40.35; 95% CI, 26.14–54.56; p < 0.00001; I2 = 95%; very low quality evidence). Of the eight studies to evaluate change in Pao2, inhaled prostacyclins also increased Pao2 from baseline (MD, 12.68; 95% CI, 2.89–22.48 mm Hg; p = 0.01; I2 = 96%; very low quality evidence). Only three studies evaluated change in mPAP, but inhaled prostacyclins were found to improve mPAP from baseline (MD, –3.67; 95% CI, –5.04 to –2.31 mm Hg; p < 0.00001; I2 = 68%; very low quality evidence).
CONCLUSIONS:
In patients with ARDS, use of inhaled prostacyclins improves oxygenation and reduces pulmonary artery pressures. Overall data are limited and there was high risk of bias and heterogeneity among included studies. Future studies evaluating inhaled prostacyclins for ARDS should evaluate their role in ARDS subphenotypes, including cardiopulmonary ARDS.
Keywords: acute respiratory distress syndrome, mechanical ventilation, meta-analysis, oxygenation, prostacyclin, pulmonary vasodilator
KEY POINTS
Question: The purpose of this systematic review and meta-analysis was to evaluate the change in the Pao2/Fio2 ratio after administration of an inhaled prostacyclin in patients with acute respiratory distress syndrome (ARDS).
Findings: In patients with ARDS, inhaled prostacyclins improve the Pao2/Fio2 ratio, Pao2, and mean pulmonary artery pressure compared with baseline.
Meaning: In patients with ARDS, use of inhaled prostacyclins improves oxygenation and reduces pulmonary artery pressures, but overall data are limited and of low quality with significant heterogeneity among studies.
Acute respiratory distress syndrome (ARDS) is an acute inflammatory process that damages alveoli and precipitates hypoxic respiratory failure. Initial management should be aimed at treating the underlying cause of ARDS to minimize ongoing injury (1–3). Nonpharmacologic treatment strategies such as lung-protective ventilation, minimizing inflation pressures, and use of early prone positioning have resulted in the greatest mortality benefit in patients with ARDS. Use of higher positive end-expiratory pressure (PEEP) and conservative fluid management have been associated with better oxygenation and a higher number of ventilator-free days, respectively (1, 2).
With the exception of neuromuscular blocking agents, pharmacologic agents, either as adjuncts or rescue therapies have not demonstrated mortality benefit to patients (4–6). Inhaled pulmonary vasodilators are selectively delivered to the ventilated part of the lung and thought to provide benefit in ARDS by improving Pao2, pulmonary vascular resistance, ventilation-perfusion mismatch, right ventricle (RV) dysfunction, and pulmonary artery pressures (7, 8). The two most frequently studied and prescribed inhaled pulmonary vasodilators are inhaled nitric oxide and inhaled epoprostenol, a prostacyclin (9). Although there are limited comparative studies, inhaled nitric oxide and inhaled prostacyclins are accepted as interchangeable in clinical practice, with similar clinical outcomes and inhaled epoprostenol often favored due to decreased cost (9–11).
Data evaluating inhaled prostacyclins has been primarily observational and low quality demonstrating transient improvements in oxygenation without sustained clinical improvements resulting in decreased duration of mechanical ventilation or mortality (12, 13). Given the renewed interest in inhaled prostacyclins due to the COVID-19 pandemic and increased prevalence of ARDS cases, we sought to complete a systematic review and meta-analysis including this new data to evaluate patients with ARDS receiving inhaled pulmonary prostacyclins. We hypothesized that inhaled prostacyclins would improve oxygenation and pulmonary artery pressures from baseline.
MATERIALS AND METHODS
Data Sources
A systematic search of existing, relevant literature was performed by the authors, including an experienced medical information specialist, in the databases Medline, Embase, Web of Science, Scopus, Cumulative Index to Nursing and Allied Health Literature, and Cochrane. The databases were searched from inception to January 26, 2023. Three elements were used in the search strategies: adult respiratory distress syndrome, prostaglandin/prostacyclin, and no case reports. These three elements were searched using controlled vocabulary, when available in the databases, and text word searching to obtain results from PubMed and “text word only” databases. The complete search strategy can be found in the supplementary material (Table S1, http://links.lww.com/CCX/B205). The articles were imported in the reference software Endnote and then exported to the systematic review management software Covidence and checked for duplicates. Our systematic review and meta-analysis is registered in International Prospective Register of Systematic Reviews (CRD42021278376). The systematic review is reported per the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Table S2, http://links.lww.com/CCX/B205).
Study Selection
The titles/abstracts of identified studies were screened for full-text review by two independent study investigators (H.T., A.S.). Studies, regardless of published language, were included for full-text review if they evaluated the use of inhaled prostacyclins in patients greater than or equal to 18 years old with ARDS. Full-text studies and abstracts were then independently reviewed by two study investigators (H.T., A.S.). Studies were included in the meta-analysis if they reported baseline and post-prostacyclin Pao2/Fio2 ratio in adult patients with ARDS according to any ARDS definition in any published language and study design. No restrictions were placed on type/duration of inhaled prostacyclin evaluated. We excluded case reports and studies missing statistical data required to run the meta-analysis and case reports, which included fewer than 10 patients.
Data Extraction
Relevant information from each study was selected and entered into a database in duplicate by two independent investigators (H.T., A.S.). We collected important study characteristics including study design, sample size, study location, inclusion and exclusion criteria, and methodology. We also extracted data for all predefined endpoints of interest including change in Pao2/Fio2 ratio (primary outcome), change in Pao2, and change in mean pulmonary artery pressure (mPAP). We chose change in Pao2/Fio2 ratio as the primary outcome, as it is the most common primary outcome used in observational studies (12, 13). Additionally, we extracted mortality, duration of mechanical ventilation, hospital and ICU length of stay, and adverse effects from all studies reporting these outcomes.
Risk of Bias and Quality Assessment
The Cochrane Risk of Bias 2 tool (14) was used for each randomized trial to evaluate the methodology for randomization, concealment, blinding, completeness of data, and selection outcome reporting. Each of these domains were assessed for low risk of bias, high risk of bias, or some concerns. The Cochrane Risk of Bias in Non-Randomized Studies of Interventions tool (15) was used for each nonrandomized trial to evaluate the methodology for confounding, patient selection, interventions, missing data, outcome measurements, and reported results. Each of these domains was assessed for low, moderate, serious, or critical risk of bias. Two independent authors (H.T., A.S.) assessed the methodological quality of articles. Based upon study design and methodological quality each individual study received an overall risk of bias according to the appropriate Cochrane Risk of Bias tool.
The two independent authors (H.T., A.S.) also used the Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework (16) to evaluate the quality of evidence included in our pooled analysis across the domains of risk of bias, inconsistency, indirectness, imprecision, and publication bias and overall quality of evidence was assigned to outcomes of interest after consensus between the two reviewing authors.
Statistical Analysis
RevMan 5.3 (Cochrane Review Manager Software; Nordic Cochrane Center, Copenhagen, Denmark) was used to pool data and the DerSimonian-Laird methods for random effects models (17) were applied. Mean differences (MDs) were calculated for continuous outcomes, with 95% CIs. The Mantel-Haenszel χ2 statistic was used to assess for heterogeneity between studies, where p value of less than 0.01 indicated significant heterogeneity, and the I2 statistic, where I2 greater than 75% indicated significant heterogeneity. We were unable to assess for publication bias by using a funnel plot or other statistical methods due to the number of studies included in our meta-analysis (18).
RESULTS
Study Selection
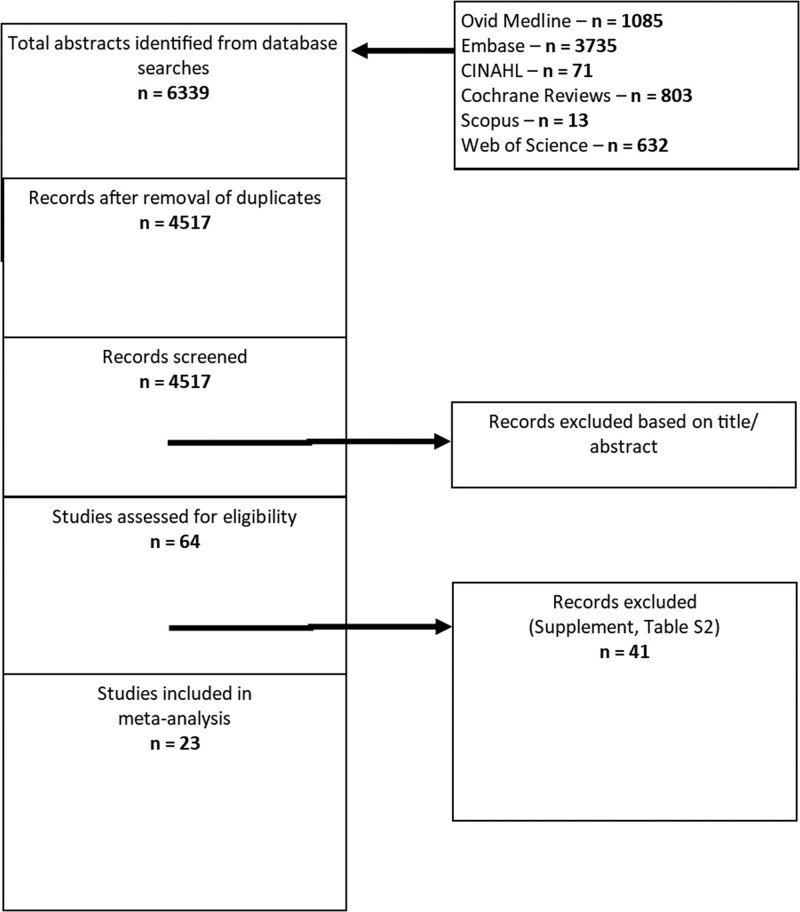
Our search strategy identified 6,339 possible references for inclusion in our analysis. After the removal of duplicate references, 4,517 references were available for screening. Ultimately, we identified 23 studies (10, 19–40) that met our inclusion criteria (Fig. 1; and Table S3, http://links.lww.com/CCX/B205).
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart. CINAHL = Cumulative Index to Nursing and Allied Health Literature.
Study Description
Characteristics of the included studies are listed in Table S4 (http://links.lww.com/CCX/B205). Of the 23 included studies, seven were prospective studies and 16 were retrospective chart reviews. A total of 1,658 patients with ARDS were included in the 23 studies. Of the 23 included studies, six studies evaluated inhaled prostacyclin therapy in patients with COVID-19 ARDS (33, 35–38, 40). Inhaled epoprostenol was evaluated in 19 studies (10, 19, 21–25, 28–36, 38–40), inhaled alprostadil was evaluated in three studies (20, 22, 27), and inhaled iloprost was evaluated in two studies (26, 37). There was variability in dosing strategies and duration of therapy. Pao2 was reported in eight studies (19–22, 26, 31, 33, 40) and mPAP was reported in three studies (19–21). The mean baseline Pao2/Fio2 ratio for the included patients was 90.50 ± 41.46 (n = 1,658) and the mean Acute Physiology and Chronic Health Evaluation II score was 31.35 ± 28.97 (n = 809). The mean duration of therapy was 3.86 ± 6.47 days (n = 798). The change in the Pao2/Fio2 ratio over time is reported in Figure S1 (http://links.lww.com/CCX/B205).
Risk of Bias and Study Quality
Risk of bias of the included studies was determined using the appropriate Cochrane Risk of Bias tool (14, 15) and listed in Tables S5 and S6 (http://links.lww.com/CCX/B205). Of the included studies, only seven were prospective studies (19–21, 23, 26, 27, 37) and only one trial used blinding (27). Overall, there was high risk of bias across all included trials based on their methodology, concern for confounders, and data analyses. A total of three studies (n = 51) were rated as low overall risk of bias (19, 21, 26) and only one study (n = 15) was rated as overall moderate risk of bias (20), with the remainder of the studies rated with overall serious risk of bias.
Quality of the evidence for outcomes of interest was determined using the GRADE assessment tool (16) and listed in Table S7 (http://links.lww.com/CCX/B205). All three outcomes of interest were rated as having very low quality evidence to support findings.
Outcomes
Inhaled prostacyclins may improve the Pao2/Fio2 ratio from baseline (MD, 40.35; 95% CI, 26.14–54.56 mm Hg; p < 0.00001; I2 = 95%) in patients with ARDS (Fig. 2). The use of inhaled prostacyclins may also increase Pao2 from baseline (MD, 12.68; 95% CI, 2.89–24.48 mm Hg; p = 0.01; I2 = 96%) (Fig. 3). Finally, inhaled prostacyclins may decrease mPAP from baseline (MD, –3.67; 95% CI, –5.04 to –2.31 mm Hg; p < 0.00001; I2 = 68%) (Fig. 4). The Pao2/Fio2 ratio was also evaluated separately in non-COVID-19 ARDS (Fig. S2, http://links.lww.com/CCX/B205) and COVID-19 ARDS (Fig. S3, http://links.lww.com/CCX/B205). Inhaled prostacyclins may improve the Pao2/Fio2 ratio from baseline in both patients with non-COVID-19 ARDS (MD, 33.83; 95% CI, 30.48–37.18 mm Hg; p < 0.00001; I2 = 95%) and COVID-19 ARDS (MD, 19.45; 95% CI, 11.06–27.84 mm Hg; p < 0.00001; I2 = 90%).
Figure 2.
Effect of prostacyclins on Pao2/Fio2 ratio. df = degrees of freedom.
Figure 3.
Effect of prostacyclins on Pao2. df = degrees of freedom.
Figure 4.
Effect of prostacyclins on mean pulmonary artery pressure. df = degrees of freedom.
Mortality was reported in 20 studies for an overall mortality rate of 56.2% (906/1,612 patients). Duration of mechanical ventilation was reported in 10 studies (861 patients) with a mean duration of 14.85 ± 16.47 days. Hospital length of stay was reported in six studies (416 patients) with a mean length of stay of 20.45 ± 16.07 days and ICU length of stay was reported in eight studies (552 patients) with a mean length of stay of 18.51 ± 16.35 days. Adverse effects were reported in 11 studies with low rates of tachycardia, hypotension, thrombocytopenia, and need for RBC transfusions (10, 25–27, 29–34, 37).
DISCUSSION
In this meta-analysis, we found that inhaled prostacyclins improve oxygenation and pulmonary artery pressures in patients with ARDS, as assessed by improvements in the Pao2/Fio2 ratio, Pao2, and mPAP. This is the first meta-analysis to evaluate inhaled prostacyclins in patients with both non-COVID-19 and COVID-19-related ARDS and the largest meta-analysis evaluating these therapies to date. Previous meta-analyses also found inhaled prostacyclins to improve oxygenation and pulmonary artery pressures and agreed that the data evaluating these therapies is limited and of low quality (12, 13).
Despite demonstrating consistent improvements in oxygenation in published literature, these improvements have been transient and have not resulted in decreased clinical outcomes like hospital length of stay, need for mechanical ventilation, and mortality (12, 13). Available literature evaluating inhaled prostacyclins in ARDS is primarily single-center and observational, which limits the generalizability of the results and often does not appropriately control for interventions, which may confound the results of the study. In one of the largest studies included in our meta-analysis by Pacheco et al (28), 216 ARDS patients received inhaled epoprostenol. The authors found a statistically significant increase in the Pao2/Fio2 ratio from baseline to the time of inhaled epoprostenol discontinuation, but mortality remained high in this study at 63%. Interestingly, patients who had a more robust response to inhaled epoprostenol therapy were more likely to survive, potentially offering insight into a patient population that may be more likely to benefit from inhaled epoprostenol therapy. Kallet et al (31) further investigated the response to inhaled epoprostenol and found 60% of patients with severe ARDS had improvements in oxygenation with administration of inhaled epoprostenol. Higher baseline Pao2/Fio2 ratio, increasing lung compliance, and trauma as the ARDS etiology were associated with a better response to inhaled epoprostenol therapy. This further supports the proposed mechanism of benefit for inhaled prostacyclins in ARDS with their benefit relying on higher functional residual capacity and greater lung surface area being oxygenated. Therefore, the benefit of inhaled pulmonary vasodilators is likely limited in patients with low functional residual capacity not receiving high PEEP, recruitment maneuvers, or prone positioning.
As Kallet et al (31) demonstrated the majority of ARDS patients treated with inhaled prostacyclins are likely to experience transient benefits in oxygenation, but there may be ARDS subphenotypes more likely to respond to and experience most sustained benefit from inhaled prostacyclins. We included three studies in our meta-analysis that evaluated the impact of inhaled prostacyclins on mPAP and demonstrated a decrease in patients who received inhaled prostacyclins (19–21). Additionally, Walmrath et al (19) and Domenighetti et al (21) also reported improvements in pulmonary vascular resistance in patients with ARDS who received inhaled prostacyclins suggesting that patients with subphenotypes with cardiopulmonary involvement including RV dysfunction, acute cor pulmonale, or underlying cardiopulmonary comorbidities may also benefit from inhaled prostacyclins.
Although inhaled prostacyclins are local therapies and should have limited systemic effects, adverse effects can occur. The most common adverse effects include hypotension, thrombocytopenia, bleeding, rebound hypoxemia, and bronchospasm (8). The meta-analysis by Fuller et al (12) found adverse effects to be inconsistently reported across studies. Adverse effects reported across included studies were thrombocytopenia, anemia, transfusion requirements, and hypotension. The rates of hypotension were vastly different between prospective studies and retrospective studies (0.69% vs 17.4%, respectively) and the high rates of hypotension in retrospective studies should be cautiously interpreted due to the likelihood of confounders.
Our systematic review and meta-analysis has a few limitations. First, we did not contact authors for studies that did not report changes in Pao2 and mPAP and the meta-analysis was done using pooled data and not individual patient data. Additionally, 70% of the included studies were retrospective and we were unable to account for the many confounders that may exist in these studies. There was observed heterogeneity across studies, which could not be accounted for in our meta-analysis due to missing data related to medication dose response and timing, ARDS severity stratification, and use of adjunctive agents. We did not stratify results based on risk of bias level to further evaluate heterogeneity, but only 66 of 1,658 patients included in the meta-analysis were evaluated in a low-moderate risk of bias study. Additionally, changes in practice and ARDS management over time could have also impacted outcomes. Overall, the quality of evidence from the included studies was low with many risks of biases. Despite these limitations, our meta-analysis has many strengths compared with prior meta-analyses. First, we completed a thorough literature search to identify applicable trials and this is the largest meta-analysis to date including the greatest number of patients with ARDS. We also included patients with ARDS pre- and during the COVID-19 pandemic and patients with cardiopulmonary ARDS in an attempt to identify ARDS subphenotypes, which may benefit from inhaled prostacyclins.
It is clear that nonpharmacologic interventions like lung-protective ventilation, optimal titration of PEEP, prone positioning, and conservative fluid management should be implemented in patients with ARDS (1, 2). To date, ARDS guidelines do not mention the use of inhaled prostacyclins, even as an adjunct, rescue therapy (2). Use of inhaled prostacyclins help improve oxygenation in hypoxemic patients, but this does not translate into long-term benefits. Therefore, inhaled prostacyclins should not be used as a frontline adjunctive therapy in all patients, but rather a case-by-case evaluation by clinicians should be conducted to assess whether inhaled prostacyclins are physiologically indicated. Future studies should evaluate homogenous patient populations, dose response and timing of inhaled prostacyclins, consider more clinically meaningful outcomes, and account for confounders to better clarify the role of inhaled prostacyclin therapy in ARDS.
CONCLUSIONS
In patients with ARDS, use of inhaled prostacyclins improves oxygenation and reduces pulmonary artery pressures. Overall, data are limited and of low quality with significant heterogeneity among studies. Future studies evaluating inhaled prostacyclins for ARDS should evaluate dose response and their role in ARDS subphenotypes, including cardiopulmonary ARDS, to better understand their role in ARDS.
Supplementary Material
Footnotes
The authors have disclosed that they do not have any potential conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (http://journals.lww.com/ccejournal).
REFERENCES
- 1.Thompson BT, Chambers RC, Liu KD: Acute respiratory distress syndrome. N Engl J Med 2017; 377:562–572 [DOI] [PubMed] [Google Scholar]
- 2.Fan E, Del Sorbo L, Goligher EC, et al. ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine: An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 2017; 195:1253–1263 [DOI] [PubMed] [Google Scholar]
- 3.Brower RG, Matthay MA, Morris A, et al. ; Acute Respiratory Distress Syndrome Network: Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342:1301–1308 [DOI] [PubMed] [Google Scholar]
- 4.Duggal A, Ganapathy A, Ratnapalan M, et al. : Pharmacological treatments for acute respiratory distress syndrome: Systematic review. Minerva Anestesiol 2015; 81:567–588 [PubMed] [Google Scholar]
- 5.Papazian L, Forel JM, Gacouin A, et al. ; ACURASYS Study Investigators: Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med 2010; 363:1107–1116 [DOI] [PubMed] [Google Scholar]
- 6.Moss M, Huang DT, Brower RG, et al. ; National Heart, Lung, and Blood Institute PETAL Clinical Trials Network: Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med 2019; 380:1997–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Levy SD, Alladina JW, Hibbert KA, et al. : High-flow oxygen therapy and other inhaled therapies in intensive care units. Lancet 2016; 387:1867–1878 [DOI] [PubMed] [Google Scholar]
- 8.Dzierba AL, Abel EE, Buckley MS, et al. : A review of inhaled nitric oxide and aerosolized epoprostenol in acute lung injury or acute respiratory distress syndrome. Pharmacotherapy 2014; 34:279–290 [DOI] [PubMed] [Google Scholar]
- 9.Bosch NA, Law AC, Vail EA, et al. : Inhaled nitric oxide vs epoprostenol during acute respiratory failure: An observational target trial emulation. Chest 2022; 162:1287–1296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Torbic H, Szumita PM, Anger KE, et al. : Inhaled epoprostenol vs inhaled nitric oxide for refractory hypoxemia in critically ill patients. J Crit Care 2013; 28:844–848 [DOI] [PubMed] [Google Scholar]
- 11.Ammar MA, Bauer SR, Bass SN, et al. : Noninferiority of inhaled epoprostenol to inhaled nitric oxide for the treatment of ARDS. Ann Pharmacother 2015; 49:1105–1112 [DOI] [PubMed] [Google Scholar]
- 12.Fuller BM, Mohr NM, Skrupky L, et al. : The use of inhaled prostaglandins in patients with ARDS: A systematic review and meta-analysis. Chest 2015; 147:1510–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khokher W, Malhas SE, Beran A, et al. : Inhaled pulmonary vasodilators in COVID-19 infection: A systematic review and meta-analysis. J Intensive Care Med 2022; 37:1370–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterne JAC, Savović J, Page MJ, et al. : RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clin Res Ed) 2019; 366:l4898. [DOI] [PubMed] [Google Scholar]
- 15.Sterne JA, Hernán MA, Reeves BC, et al. : ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (Clin Res Ed) 2016; 355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balshem H, Helfand M, Schunemann HJ, et al. : GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 2011; 64:401–406 [DOI] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N: Meta-analysis in clinical trials. Control Clin Trials 1986; 7:177–188 [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. : Bias in meta-analysis detected by a simple, graphical test. BMJ (Clin Res Ed) 1997; 315:629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walmrath D, Schneider T, Schermuly R, et al. : Direct comparison of inhaled nitric oxide and aerosolized prostacyclin in acute respiratory distress syndrome. Am J Respir Crit Care Med 1996; 153:991–996 [DOI] [PubMed] [Google Scholar]
- 20.Meyer J, Theilmeier G, Van Aken H, et al. : Inhaled prostaglandin E1 for treatment of acute lung injury in severe multiple organ failure. Anesth Analg 1998; 86:753–758 [DOI] [PubMed] [Google Scholar]
- 21.Domenighetti G, Stricker H, Waldispuehl B: Nebulized prostacyclin (PGI2) in acute respiratory distress syndrome: Impact of primary (pulmonary injury) and secondary (extrapulmonary injury) disease on gas exchange response. Crit Care Med 2001; 29:57–62 [DOI] [PubMed] [Google Scholar]
- 22.Camamo JM, McCoy RH, Erstad BL: Retrospective evaluation of inhaled prostaglandins in patients with acute respiratory distress syndrome. Pharmacotherapy 2005; 25:184–190 [DOI] [PubMed] [Google Scholar]
- 23.Raheem S: Aerosolized epoprostenol as adjunct therapy for acute respiratory distress syndrome. Crit Care Med 2009; 37:A195 [Google Scholar]
- 24.Tabrizi MB, Schinco MA, Tepas JJ, 3rd, et al. : Inhaled epoprostenol improves oxygenation in severe hypoxemia. J Trauma Acute Care Surg 2012; 73:503–506 [DOI] [PubMed] [Google Scholar]
- 25.Dunkley KA, Louzon PR, Lee J, et al. : Efficacy, safety, and medication errors associated with the use of inhaled epoprostenol for adults with acute respiratory distress syndrome: A pilot study. Ann Pharmacother 2013; 47:790–796 [DOI] [PubMed] [Google Scholar]
- 26.Sawheny E, Ellis AL, Kinasewitz GT: Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 2013; 144:55–62 [DOI] [PubMed] [Google Scholar]
- 27.Siddiqui S: Use of inhaled PGE1 to improve diastolic dysfunction, LVEDP, pulmonary hypertension and hypoxia in ARDS - a randomized clinical trial. Open J Anethesiol 2013; 3:7 [Google Scholar]
- 28.Pacheco J, Arnold H, Skrupky L, et al. : Predictors of outcome in 216 subjects with ARDS treated with inhaled epoprostenol. Respir Care 2014; 59:1178–1185 [DOI] [PubMed] [Google Scholar]
- 29.Singh A: Impact of inhaled epoprostenol on oxygenation in acute respiratory distress syndrome. Am J Respir Crit Care Med 2014; 189:A3087 [Google Scholar]
- 30.Torbic H, Szumita PM, Anger KE, et al. : Clinical and economic impact of formulary conversion from inhaled Flolan to inhaled Veletri for refractory hypoxemia in critically ill patients. Ann Pharmacother 2016; 50:106–112 [DOI] [PubMed] [Google Scholar]
- 31.Kallet RH, Burns G, Zhuo H, et al. : Severity of hypoxemia and other factors that influence the response to aerosolized prostacyclin in ARDS. Respir Care 2017; 62:1014–1022 [DOI] [PubMed] [Google Scholar]
- 32.Hawn JM, Wanek M, Bauer SR, et al. : Effectiveness, safety, and economic comparison of two inhaled epoprostentol products (Flolan and Veletri) in cardiothoracic surgery patients. Ann Pharmacother 2018; 52:956–964 [DOI] [PubMed] [Google Scholar]
- 33.DeGrado JR, Szumita PM, Schuler BR, et al. : Evaluation of the efficacy and safety of inhaled epoprostenol and inhaled nitric oxide for refractory hypoxemia in patients with coronavirus disease 2019. Crit Care Explor 2020; 2:e0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buckley MS, Agarwal SK, Garcia-Orr R, et al. : Comparison of fixed-dose inhaled epoprostenol and inhaled nitric oxide for acute respiratory distress syndrome in critically ill adults. J Intensive Care Med 2021; 36:466–476 [DOI] [PubMed] [Google Scholar]
- 35.Li J, Fink JB, Augustynovich AE, et al. : Effects of inhaled epoprostenol and prone positioning in intubated coronavirus disease 2019 patients with refractory hypoxemia. Crit Care Explor 2020; 2:e0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonti R, Pike CW, Cobb N: Responsiveness of inhaled epoprostenol in respiratory failure due to COVID-19. J Intensive Care Med 2021; 36:327–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haeberle HA, Calov S, Martus P, et al. : Inhaled prostacyclin improves oxygenation in patients with COVID-19-induced acute respiratory distress syndrome. medRxiv Preprint posted online November 16, 2021. doi: 10.1101/2021.11.15.21266343 [Google Scholar]
- 38.Chiles JW, 3rd, Vijaykumar K, Darby A, et al. : Letter to the Editor: “Use of inhaled epoprostenol with high flow nasal oxygen in non-intubated patients with severe COVID-19.” J Crit Care 2022; 69:153989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buckley MS, Mendez A, Radosevich JJ, et al. : Comparison of 2 different inhaled epoprostenol dosing strategies for acute respiratory distress syndrome in critically ill adults: Weight-based vs fixed-dose administration. Am J Health Syst Pharm 2023; 80(Suppl 1):S11–S22 [DOI] [PubMed] [Google Scholar]
- 40.Imtiaz K, Jodeh W, Sudekum D, et al. : The use of inhaled epoprostenol for acute respiratory distress syndrome secondary due to COVID-19: A case series. J Crit Care Med (Targu Mures) 2022; 8:33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.