PURPOSE
To report patient-reported outcomes (PROs) of a phase III trial evaluating total androgen suppression (TAS) combined with dose-escalated radiation therapy (RT) for patients with intermediate-risk prostate cancer.
METHODS
Patients with intermediate-risk prostate cancer were randomly assigned to dose-escalated RT alone (arm 1) or RT plus TAS (arm 2) consisting of luteinizing hormone-releasing hormone agonist/antagonist with oral antiandrogen for 6 months. The primary PRO was the validated Expanded Prostate Cancer Index Composite (EPIC-50). Secondary PROs included Patient-Reported Outcome Measurement Information System (PROMIS)-fatigue and EuroQOL five-dimensions scale questionnaire (EQ-5D). PRO change scores, calculated for each patient as the follow-up score minus baseline score (at the end of RT and at 6, 12, and 60 months), were compared between treatment arms using a two-sample t test. An effect size of 0.50 standard deviation was considered clinically meaningful.
RESULTS
For the primary PRO instrument (EPIC), the completion rates were ≥86% through the first year of follow-up and 70%-75% at 5 years. For the EPIC hormonal and sexual domains, there were clinically meaningful (P < .0001) deficits in the RT + TAS arm. However, there were no clinically meaningful differences by 1 year between arms. There were also no clinically meaningful differences at any time points between arms for PROMIS-fatigue, EQ-5D, and EPIC bowel/urinary scores.
CONCLUSION
Compared with dose-escalated RT alone, adding TAS demonstrated clinically meaningful declines only in EPIC hormonal and sexual domains. However, even these PRO differences were transient, and there were no clinically meaningful differences between arms by 1 year.
INTRODUCTION
The benefits versus risks of short-term hormones in addition to modern radiation therapy (RT) in patients with intermediate-risk prostate cancer has remained a vital question. NRG/Radiation Therapy Oncology Group (RTOG) 0815 was a phase III trial of dose-escalated RT alone with or without short-term total androgen suppression in intermediate-risk prostate cancer. Although there was no statistically significant improvement in overall survival in this trial, the addition of short-term androgen deprivation (STAD) to dose-escalated RT significantly reduced the rates of distant metastases, deaths from prostate cancer, and prostate-specific antigen (PSA)–defined relapses.1 Although there is some benefit in cancer control, a key question is: at what cost? From the clinician perspective, there was a significant increase in adverse events in the experimental arm, with 70% of patients receiving STAD experiencing any grade acute adverse event compared with 21% in the control arm (P < .001).1 Yet, how did the addition of STAD to RT affect patients from their own perspective? The primary patient-reported outcome (PRO) hypothesis in this study was that the quality-of-life (QOL) measurements would be worse in arm 2 than arm 1 because of the addition of STAD to radiation. The purpose of this analysis was to present these findings as reported directly by patients as PROs are essential to better understand the therapeutic ratio for interventions.2,3
CONTEXT
Key Objective
What is the impact on patient-reported outcomes (PROs) by adding 6 months of total androgen suppression (TAS) to dose-escalated radiation for patients with intermediate-risk prostate cancer?
Knowledge Generated
In this randomized study, adding 6 months of TAS to dose-escalated radiation was associated with some clinically meaningful declines related specifically to hormonal and sexual quality of life (QOL). However, this impact on PROs (using validated instruments) was short lived, and there were no clinically meaningful differences in QOL by 1 year after the initiation of treatment.
Relevance (M.A. Carducci)
This confirmatory large data set study of PROs in those undergoing dose-escalated radiotherapy/brachytherapy is useful when counseling patients receiving short-term androgen deprivation as to the probability of toxicities and how they evolve over treatment course and in survivorship.*
*Relevance section written by JCO Associate Editor Michael A. Carducci, MD, FACP, FASCO.
METHODS
Eligible patients had intermediate-risk prostate cancer defined by the presence of ≥1 of the following factors: clinical stage T2b-T2c, Gleason score 7, or pretreatment PSA 10-20 ng/mL. Further details are in the clinical paper.1 All institutions received approval from their institutional review boards. Informed consent was obtained from all study participants.
After registration, patients were stratified by the number of National Comprehensive Cancer Network intermediate-risk features, by pre-enrollment adult comorbidity evaluation-27 (ACE-27) score (<2 v ≥2) and the type of radiotherapy used to deliver their treatment: external beam radiotherapy (EBRT) alone or together with a brachytherapy boost. ACE-27 is a validated comorbidity instrument4 that, on the basis of the severity and number of medical illnesses, provides a score of 0-3 (none to severe).4 Patients were randomly assigned to receive either dose-escalated radiotherapy alone or in combination with STAD. Trial coordination, data collection, and statistical support were all conducted by NRG Oncology, a National Cancer Institute–funded cooperative oncology group. The most common RT was dose-escalated EBRT alone to a dose of 79.2 Gy in 44 fractions. Patients treated with a brachytherapy boost first underwent treatment with EBRT to a dose of 45 Gy in 25 fractions.
Low dose rate brachytherapy boost dose options with iodine-125 and palladium-103 isotopes were 110 Gy and 100 Gy, respectively, versus high dose rate boost dose option of 21 Gy in two fractions with iridium-192. Patients randomly assigned to receive STAD received both luteinizing hormone-releasing hormone agonist/antagonist therapy along with oral antiandrogen therapy for a total duration of 6 months beginning 8 weeks before the start of RT.
PRO Patient Assessments
The primary PRO was measured by the Expanded Prostate Cancer Index Composite (EPIC), which is reliable and valid.5 EPIC is a 50-item questionnaire using a Likert-like scale with responses transformed to a scale of 0-100. Higher scores correspond to better QOL. EPIC comprises four individually validated domains: urinary, bowel, sexual, and hormonal. Test-retest reliability and internal consistency are high for EPIC urinary, bowel, sexual, and hormonal domain scores.5
Secondary PROs included the Patient-Reported Outcome Measurement Information System (PROMIS) fatigue-short form (seven items).6 Participants' level of physical activity was assessed using the Godin Leisure-Time Exercise Questionnaire (GLTEQ), which has good test-retest reliability and validity.7,8 An indicator of sleep quality was also estimated by 1 item (Q3) of the Pittsburgh Sleep Quality Index (PSQI), which also has good reliability and validity.9 The EuroQOL five-dimensions scale questionnaire (EQ-5D), a two-part, self-assessment questionnaire, was also included.10 Overall scores are translated to a 0-1 scale with 0 indicating worst imaginable health state and 1 the best imaginable health state.10
To minimize missing QOL data, detailed instructions for collection of QOL were incorporated along with what to do if the patient missed a scheduled assessment, and NRG/RTOG provides individualized patient calendars readily available to Investigators and Research Associates (RAs) 24/7 on the RTOG web site. To further enhance compliance, automatic PRO reminders and past due notifications were emailed in real time to the RAs. If a PRO form could not be collected (either in clinic or by mail as needed), the RAs filled out a protocol form to document the reason for the missing form.
All protocol end points were measured from the date of random assignment.
Statistical Analysis
Pretreatment characteristics were summarized within each treatment arm using descriptive statistics. All eligible patients consenting to the QOL component of the study were analyzed according to the arm in which they were randomly assigned. The pretreatment characteristics of those who consented to the QOL component were compared with all other patients using chi-square tests for categorical variables and t tests for continuous data.
For the QOL outcomes, change scores, calculated as follow-up score minus baseline score, at the end of RT and at 6, 12, and 60 months, were compared between treatment arms using a two-sample t test. A negative change score indicates a decline in QOL for the EPIC, EQ-5D, and GLTEQ instruments, whereas a positive change indicates worsening for the PROMIS and PSQI scales. The differences between EPIC mean scores for each arm were also compared at each time point (using a two-sample t test). A significance level of 0.0125 to adjust for multiple comparisons with respect to the four EPIC domains was used to maintain an overall two-sided type 1 error of 0.05. Multiplicity adjustments were not applied to the other secondary QOL indices.
An effect size (ES), that is, a difference in group means at a given time point, of ≥0.50 standard deviation (SD) of the measure at that time point was considered clinically meaningful.2 For the PRO sample size, 200 patients per arm would provide 90% statistical power to detect an ES of 0.50 SD if the completion rate was only 60%, after Bonferroni adjustment.
Mixed effect regression models were also used to analyze the full set of longitudinal QOL outcomes. Fixed effects were treatment arm, time (modeled with four dummy variables using baseline as reference), treatment-by-time interactions, and the three stratification factors (number of risk factors, ACE-27 score, and RT modality). Random subject effects (intercepts) were included to allow for correlation within patients. With regard to missing data, the mixed-effects models are valid under a missing at random (MAR) assumption in which the missingness depends only on observed data, including observed values of the outcome variable and covariates. Although this is a plausible assumption, sensitivity analyses were conducted using pattern-mixture models.11,12 Patients were grouped according to whether they had complete data at all five time points (I1 = 0) versus incomplete data at one or more time points (I1 = 1). The grouping variable, I1, together with interactions between I1 and treatment arm, I1 and time, and I1, treatment arm, and time (three-way interaction) were added to the mixed-effects model to assess the impact of the missing data on the estimated treatment effects. A likelihood ratio test was performed to determine whether the added terms led to a significant improvement in model fit. Overall population estimates averaged over the two missing data patterns (weighted by the proportion of complete and incomplete data) were then derived for comparison against the original estimates obtained under the MAR assumption.
RESULTS
Baseline Characteristics and Completion Rates
A total of 1,538 patients from 223 centers were randomly assigned to receive dose-escalated radiotherapy alone (arm 1) or dose-escalated radiotherapy combined with STAD (arm 2) between September 2009 and March 2016.1 Forty-six patients were found to be ineligible post-random assignment and were excluded, both from the main analyses and those reported herein.
The initially planned subset of trial patients for the QOL component of the study was a minimum of 400 patients. Of the 436 patients who agreed to participate in the QOL component, 16 patients (3.7%) were ineligible for the reasons listed in the PRO CONSORT diagram, leaving n = 215 and n = 205 patients for analysis in arms 1 and 2, respectively (Fig 1). Completion rates for the QOL instruments over time are shown in Table 1. Denominators are the number of patients remaining alive (and who have not withdrawn consent) at each time point. Completion rates for the four EPIC domains were very similar, and, therefore, only the urinary domain is shown. For the primary PRO instrument (EPIC), the completion rates were ≥86% through the first year of follow-up and 70%-75% at 5 years.
FIG 1.

RTOG 0815 QOL CONSORT diagram. GS, Gleason score; PSA, prostate-specific antigen; QOL, quality of life; RT, radiation therapy; RTOG, Radiation Therapy Oncology Group; STAD, short-term androgen deprivation.
TABLE 1.
Patient Completion of QOL Assessment Measurements

Baseline patient and tumor characteristics were similar in the two treatment arms (Data Supplement [Table S1], online only). The pretreatment characteristics between these 420 patients and the remaining eligible patients on this trial (n = 1,072) were also similar (Data Supplement [Table S2]) with the exception of T-stage (40% T2 among QOL subset v 36% T2 in remaining patients; P = .049).
EPIC Domains
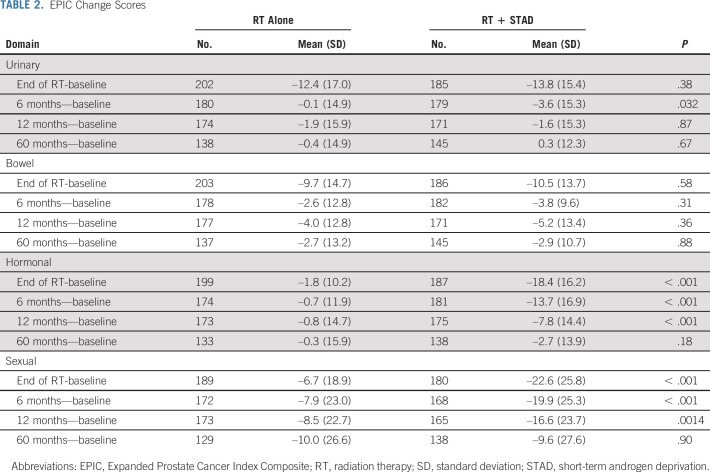
Analyses of changes from baseline in the EPIC measures are summarized in Table 2. Plots of mean scores with 95% confidence intervals over time for each treatment arm are given in Figure 2. Data Supplement [Table S3] provides descriptive statistics and 0.5 SD clinical thresholds, as well as P values from comparison of the mean scores at each time point. Although EPIC urinary and bowel scores decreased significantly by the end of RT in both arms, no clinically meaningful differences between arms were detected at any time point (Figs 2A and 2B). Moreover, within 6 months of RT, the EPIC urinary and bowel scores had essentially recovered.
TABLE 2.
EPIC Change Scores
FIG 2.
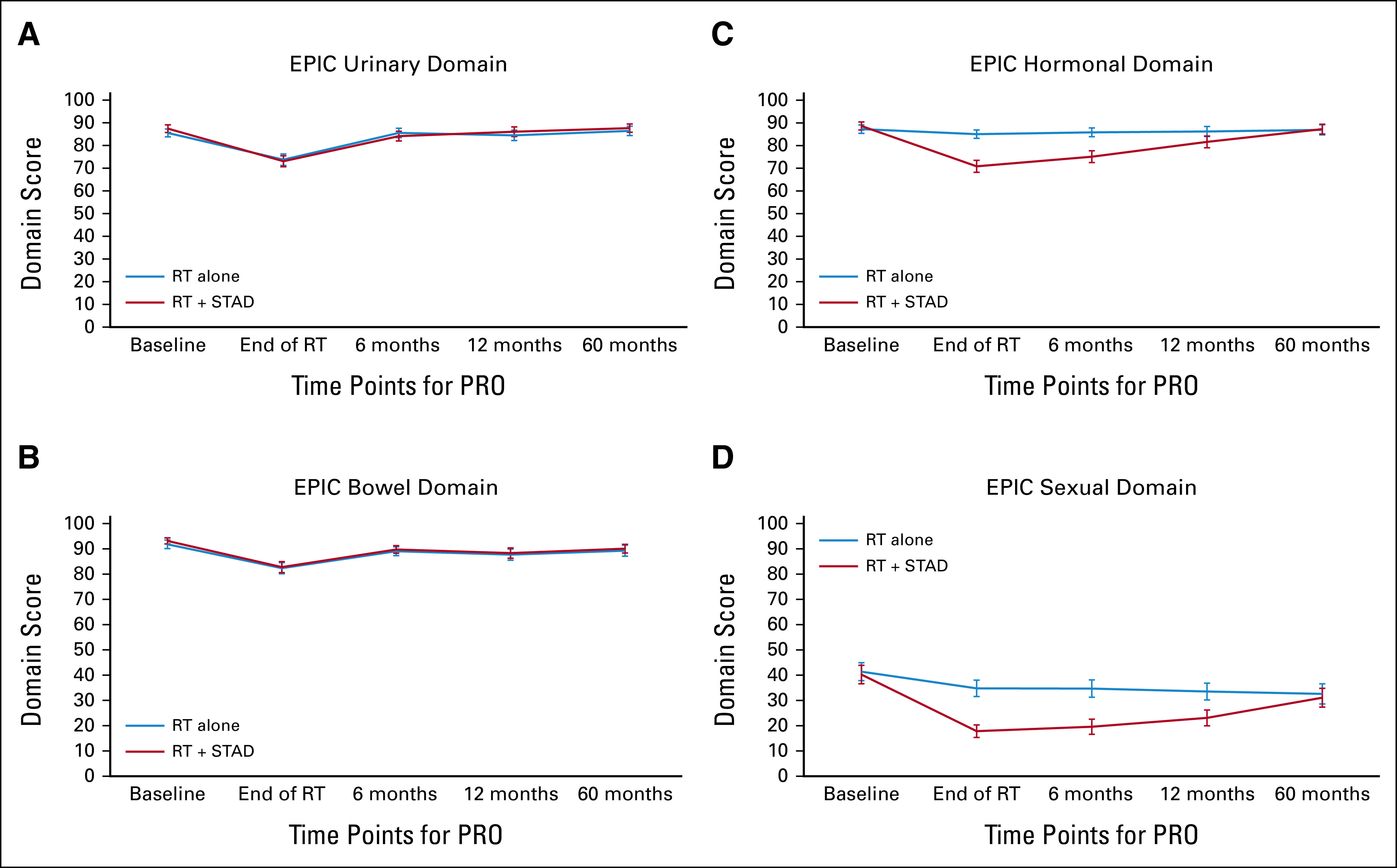
EPIC domain scores over time. (A) EPIC urinary domain, (B) EPIC bowel domain, (C) EPIC hormonal domain, and (D) EPIC sexual domain. Error bars represent 95% CIs. EPIC, Expanded Prostate Cancer Index Composite; PRO, patient-reported outcomes; RT, radiation therapy; STAD, short-term androgen deprivation.
For the EPIC hormonal and sexual domains, however, there were clinically meaningful differences between the two arms with significantly greater (P <.001) deficits in the RT + STAD arm. For both domains, the difference between arms remained statistically significant and clinically meaningful at the end of RT and at 6 months; at 1 year, the differences between arms for both domains were no longer clinically meaningful, although borderline for the hormone domain; at 5 years the scores for both domains had nearly returned to baseline in both arms (Figs 2C and 2D). These results were consistent on the basis of both the change score and the mean score comparisons at each time point (Table 2 and Data Supplement [Table S3]). The results of the mixed-effects models were also similar to those on the basis of the change and mean scores. Effect estimates are presented in the Data Supplement (Table S4).
EQ-5D, PROMIS, PSQI, and GLTEQ Measures
Changes from baseline for the EQ-5D, PROMIS, PSQI, and GLTEQ measures are summarized in the Data Supplement (Table S5), and Figure 3. EQ-5D scores showed little change from baseline (Fig 3A). PROMIS-fatigue scores increased (worsened) from baseline in both arms and were significantly (but not clinically meaningfully) higher in arm 2 at the end of RT, with similar scores at 6, 12, and 60 months (Fig 3B). Although there were statistically significant differences between the two arms at the end of RT and at 6 months in sleep quality favoring RT alone, there were no clinically meaningful differences at any time point (Fig 3C). Activity levels (per GLTEQ) remained relatively constant in both treatment arms (Fig 3D). Mixed-effects models yielded similar results (Data Supplement [Table S6]). Significant covariate effects were higher (better) EQ-5D and GLTEQ scores and lower (also better) PROMIS fatigue and PSQI scores in patients with less comorbidity (P < .0001, P = .0008, P <.0001, and P = .005, respectively). Patients receiving EBRT + LDR/HDR reported lower (better) PROMIS fatigue scores (P = .008).
FIG 3.
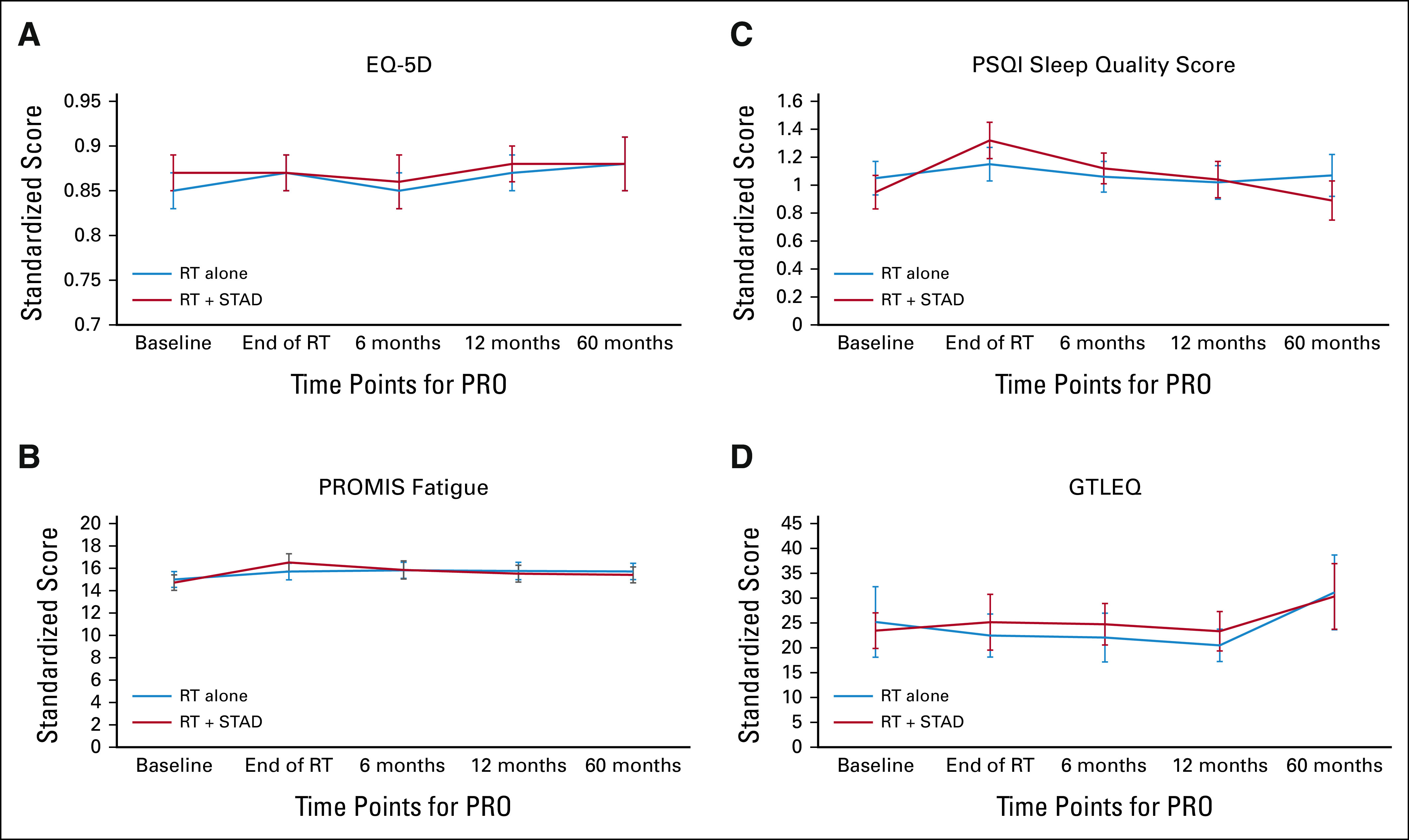
(A) Mean EQ-5D, (B) PROMIS fatigue, (C) PSQI sleep quality score, and (D) GTLEQ measures over time. Error bars represent 95% CIs. EQ-5D, EuroQoL five-dimension scale questionnaire; GLTEQ, Godin Leisure-Time Exercise Questionnaire; PRO, patient-reported outcomes; PROMIS, Patient-Reported Outcome Measurement Information System; PSQI, Pittsburgh Sleep Quality Index; RT, radiation therapy; STAD, short-term androgen deprivation.
Sensitivity Analyses
To assess the impact of missing data and the plausibility of the MAR assumption, pattern-mixture models were fit as described under Statistical Analysis. For each patient, we determined whether the QOL data were present or missing at each of the five visits. The percentage of patients with data at all five visits ranged from 24% (GLTEQ) to 44% (EPIC and PSQI). These complete data cases were contrasted with the patients who had missing data at one or more visits. Note that the latter includes patients who died before 60 months.
The results are summarized in the Data Supplement (Tables S7 and S8). Shown are the re-estimated effect estimates, obtained by incorporating the missing data pattern indicator (noncomplete v complete) and its interaction with other predictor variables into the model, and taking the weighted average of the effects over the two patterns, weighted by the relative size of each pattern. In general, no material effects on the results were seen, and the estimated effects were similar to those obtained previously. None of the likelihood ratio statistics was significant, indicating that the pattern mixture models did not improve fit over the original models assuming MAR. For example, for the EPIC hormonal domain, under MAR, the estimated differences between the STAD and control arms were –16.57, –13.68, –7.98, and –1.99 points at end of RT, 6 months, 1 year, and 5 years, respectively (Data Supplement [Table S4C]). On the basis of the pattern-mixture model, these were –15.57, –12.87, –7.39, and –2.01, respectively, with similar levels of statistical significance (Data Supplement [Table S7C]).
DISCUSSION
NRG 0815 is a landmark randomized phase III study that addresses the role of short-term hormones for patients with intermediate-risk prostate cancer receiving modern, dose-escalated radiation. This has been an ongoing question as most prior studies demonstrating a clinical benefit did so in the context of lower doses of RT and/or heterogeneous groups of patients (including high- and intermediate-risk patients). Although this study did not show a statistically significant survival benefit, it did demonstrate significant decreases in the rates of distant metastases, deaths from prostate cancer, and PSA-defined relapses.1 Yet, this finding only addresses the potential benefit in terms of cancer control. The current PRO analysis provides key information regarding the potential risks of this intervention directly from the patient perspective.
In this regard, the PRO results overall are reassuring. There were no clinically meaningful differences between arms for PROMIS-fatigue, EQ-5D, and EPIC bowel/urinary scores. Although the primary PRO hypothesis was correct, that the addition of hormones to dose-escalated RT demonstrated clinically meaningful declines in QOL, this was limited to the EPIC hormonal and sexual domains. Moreover, even these PRO differences were relatively short-lived, with no clinically meaningful differences between arms in these domains by 1 year after treatment initiation. By 5 years after treatment, the scores for all PRO measures had essentially returned to baseline in both arms. This key information can be shared with patients with intermediate-risk prostate cancer who are grappling with the decision between modern RT with or without short-term hormones. Indeed, these PRO results can be particularly helpful in counseling men regarding the expected timing of the impact of adding 6 months of hormones to radiation, such as the transient duration (ie, up to 1 year) of clinically meaningful reductions in sexual and hormonal QOL.
Our PRO results are overall consistent with those of Bolla et al13 who studied QOL using the EORTC instrument in their phase III study of 6 months of androgen suppression in patients with intermediate- or high-risk prostate cancer receiving lower versus modern doses of RT (70, 74, or 78 Gy). Of note, their PRO compliance rates were 76% and 37% at 1 and 5 years, respectively (compared with our EPIC compliance rates of 87% and 73%, respectively). Similar to our findings, the key QOL scales affected were hormonal symptoms and sexual activity/functioning, which were clinically significantly impaired by short-term hormones at 6 months. By year 1, these treatment differences in their study were still present, but less so (and only clinically relevant for the sexual scales) and by year 2, these PRO differences had essentially resolved.13
Our PRO analysis has several limitations. First, the PRO analysis is based on a sample size of approximately 400 patients in this trial. However, this was a preplanned statistical design with adequate power for the PRO analysis, and these 400 patients had similar characteristics to the rest of the patients on the study (Data Supplement [Table S2]). Second, although it would have been ideal to have a 2-year PRO time point, fortunately, the clinically meaningful PRO changes had essentially resolved in our study by 1 year. Furthermore, this study did not address the potential impact of other side effects of hormone therapy not directly captured in these QOL instruments, such as regarding bone or cardiovascular health. Strengths of our PRO study include that we had robust PRO compliance even out to 5 years, and our study focused specifically on intermediate-risk prostate patients all treated with modern doses of RT. In addition to the primary PRO (EPIC) domains for bowel, urinary, hormonal and sexual QOL, we also included key QOL issues related to fatigue, which is very important to patients, and assessed issues related to sleep and activity levels, as well as overall QOL using EQ-5D.
In conclusion, the addition of STAD to modern, dose-escalated RT demonstrated clinically meaningful declines in the EPIC hormonal and sexual domains compared with RT alone. However, these PRO differences were short-lived, with no clinically meaningful differences between arms in these domains by 1 year. There were no clinically meaningful differences at any time points between arms for PROMIS-fatigue, EQ-5D, and EPIC bowel/urinary scores. Beyond the clinical outcomes, these PROs provide added value to help individuals make fully informed decisions among treatment options.
Benjamin Movsas
Research Funding: Varian Medical Systems (Inst), Philips Healthcare (Inst), ViewRay (Inst)
Patents, Royalties, Other Intellectual Property: Lung phantom for image guidance, MR-CT imaging related patent for radiation oncology
Travel, Accommodations, Expenses: Varian Medical Systems, ViewRay, Alpha Tau
Gerard C. Morton
Employment: Odette Cancer Centre—Sunnybrook Hospital
Honoraria: Elekta
Deborah E. Citrin
Employment: Mid-Atlantic Permanente Medical Group
Jeff M. Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion Medical Systems, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Vivek S. Kavadi
Employment: US Oncology Network
Stock and Other Ownership Interests: McKesson/US Oncology Network
Adam C. Olson
Consulting or Advisory Role: RenovoRx
Research Funding: Reflexion Medical, Varian Medical Systems
Fabio L. Cury
Consulting or Advisory Role: Knight Pharmaceuticals, Sanofi/Aventis
Speakers' Bureau: Varian Medical Systems
Research Funding: Boston Scientific (Inst), Tolmar (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
Deborah W. Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society of Radiation Oncology (ASTRO), Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmont Cancer Center
Consulting or Advisory Role: Flatiron Health, Alliance for Clinical Trials in Oncology, University of Rochester
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part at the 2021 American Society for Radiation Oncology (ASTRO) Annual Meeting, Chicago, IL, October 24-27, 2021.
SUPPORT
Supported by grants U10CA180868 (NRG Oncology Operations), U10CA180822 (NRG Oncology SDMC), UG1CA189867 (NCORP), and U24CA180803 (IROC) from the National Cancer Institute (NCI).
AUTHOR CONTRIBUTIONS
Conception and design: Benjamin Movsas, Mohamed A. Elshaikh, Alvaro A. Martinez, Gerard C. Morton, Daniel J. Krauss, Deborah E. Citrin, Jeff M. Michalski, Rodney J. Ellis, Howard M. Sandler, Deborah W. Bruner
Administrative support: Di Yan, Bruce W. Hershatter
Provision of study materials or patients: Benjamin Movsas, Gerard C. Morton, Di Yan, Jeff M. Michalski, Rodney J. Ellis, Adam C. Olson, Fabio L. Cury, Michael A. Papagikos
Collection and assembly of data: Benjamin Movsas, Joseph P. Rodgers, Gerard C. Morton, Daniel J. Krauss, Di Yan, Bruce W. Hershatter, Rodney J. Ellis, Elizabeth M. Gore, Gary S. Gustafson, Adam C. Olson, Fabio L. Cury, Michael A. Papagikos, Deborah W. Bruner
Data analysis and interpretation: Benjamin Movsas, Joseph P. Rodgers, Alvaro A. Martinez, Gerard C. Morton, Di Yan, Deborah E. Citrin, Rodney J. Ellis, Vivek S. Kavadi, Vikram M. Velker, Adam C. Olson, Fabio L. Cury, Michael A. Papagikos, Theodore G. Karrison
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Dose-Escalated Radiation Alone or in Combination With Short-Term Total Androgen Suppression for Intermediate-Risk Prostate Cancer: Patient-Reported Outcomes From NRG/Radiation Therapy Oncology Group 0815 Randomized Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Benjamin Movsas
Research Funding: Varian Medical Systems (Inst), Philips Healthcare (Inst), ViewRay (Inst)
Patents, Royalties, Other Intellectual Property: Lung phantom for image guidance, MR-CT imaging related patent for radiation oncology
Travel, Accommodations, Expenses: Varian Medical Systems, ViewRay, Alpha Tau
Gerard C. Morton
Employment: Odette Cancer Centre—Sunnybrook Hospital
Honoraria: Elekta
Deborah E. Citrin
Employment: Mid-Atlantic Permanente Medical Group
Jeff M. Michalski
Stock and Other Ownership Interests: ViewRay
Consulting or Advisory Role: Mevion Medical Systems, Boston Scientific, Merck Sharp & Dohme, Blue Earth Diagnostics
Research Funding: Merck Sharp & Dohme (Inst)
Travel, Accommodations, Expenses: Boston Scientific, Merck Sharp & Dohme
Open Payments Link: https://openpaymentsdata.cms.gov/physician/221723
Vivek S. Kavadi
Employment: US Oncology Network
Stock and Other Ownership Interests: McKesson/US Oncology Network
Adam C. Olson
Consulting or Advisory Role: RenovoRx
Research Funding: Reflexion Medical, Varian Medical Systems
Fabio L. Cury
Consulting or Advisory Role: Knight Pharmaceuticals, Sanofi/Aventis
Speakers' Bureau: Varian Medical Systems
Research Funding: Boston Scientific (Inst), Tolmar (Inst)
Travel, Accommodations, Expenses: Varian Medical Systems
Howard M. Sandler
Consulting or Advisory Role: Janssen
Other Relationship: Caribou Publishing
Deborah W. Bruner
Employment: Emory University
Stock and Other Ownership Interests: AbbVie, Altria, Bristol Myers Squibb, GlaxoSmithKline, Johnson & Johnson, Pfizer, Procter & Gamble, Stryker, Viatris, Walgreens Boots Alliance
Honoraria: American Society of Radiation Oncology (ASTRO), Oncology Nursing Society, Memorial Sloan-Kettering Cancer Center, Alliance, Wilmont Cancer Center
Consulting or Advisory Role: Flatiron Health, Alliance for Clinical Trials in Oncology, University of Rochester
No other potential conflicts of interest were reported.
REFERENCES
- 1.Krauss DJ, Karrison T, Martinez AA, et al. : Dose‐escalated radiotherapy alone or in combination with short-term androgen deprivation for intermediate-risk prostate cancer: Results of a phase III multi-institutional trial. J Clin Oncol 41:3203-3216, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siddiqui F, Liu AK, Watkins-Bruner D, et al. : Patient-reported outcomes and survivorship in radiation oncology: Overcoming the cons. J Clin Oncol 32:2920-2927, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang SS, Movsas B: How vital are patient-reported outcomes? J Natl Cancer Inst 114:347-348, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallogjeri D, Piccirillo JF, Spitznagel EL Jr, et al. : Comparison of scoring methods for ACE-27: Simpler is better. J Geriatr Oncol 3:238-245, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wei JT, Dunn RL, Litwin MS, et al. : Development and validation of the Expanded Prostate Cancer Index Composite (EPIC) for comprehensive assessment of health-related quality of life in men with prostate cancer. Urology 56:899-905, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Cessna JM, Jim HSL, Sutton SK, et al. : Evaluation of the psychometric properties of the PROMIS Cancer Fatigue Short Form with cancer patients. J Psychosomatic Res 81:9-13, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Godin G, Jobin J, Bouillon J: Assessment of leisure time exercise behavior by self-report: A concurrent validity study. Can J Public Health 77:359-362, 1986 [PubMed] [Google Scholar]
- 8.Gionet NJ, Godin G: Self-reported exercise behavior of employees: A validity study. J Occup Environ Med 31:969-973, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Buysse DJ, Reynolds CF 3rd, Monk TH, et al. : The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res 28:193-213, 1989 [DOI] [PubMed] [Google Scholar]
- 10.Rabin R, de Charro F: EQ-SD: A measure of health status from the EuroQol Group. Ann Med 33:337-343, 2001 [DOI] [PubMed] [Google Scholar]
- 11.Little RJA: Pattern-mixture models for multivariate incomplete data. J Am Stat Assoc 88:125-133, 1993 [Google Scholar]
- 12.Hedeker D, Gibbons RD: Application of random-effects pattern-mixture models for missing data in longitudinal studies. Psychol Methods 2:64-78, 1997 [Google Scholar]
- 13.Bolla M, Maingon P, Carrie C, et al. : Short androgen suppression and radiation dose escalation for intermediate- and high-risk localized prostate cancer: Results of EORTC trial 22991. J Clin Oncol 34:1748-1756, 2016 [DOI] [PubMed] [Google Scholar]