Background:
Hypoxic-ischemic brain injury (HIBI) is a disabling consequence of cardiopulmonary resuscitation, which has no direct treatment except supportive care. Many studies have used pharmacological agents to reduce or stop this disability. MLC901 is a traditional Chinese medicine showing neuroprotective and regenerative effects on focal and global ischemia in previous animal and human studies. We designed an experimental, randomized, double-blind, placebo-controlled study to analyze MLC901 efficacy in HIBI patients.
Methods:
In a randomized, placebo-controlled trial, 35 patients with HIBI were randomly designated to receive either MLC901 or placebo capsules 3 times per day over 6 months. We assessed the 2 groups by modified Rankin Scale and Glasgow Outcome Scale at baseline, and follow-up visits in 3rd month, and 6th-month after injury.
Results:
Thirty-one patients completed this study. There was no significant difference in baseline characteristics between the 2 groups as regards age, gender, time of resuscitation, the interval between injury and start of the intervention, and the length of intensive care unit stay. Both the placebo and intervention groups improved during the investigation. However, the Glasgow Outcome Scale and modified Rankin Scale scales were significantly improved in the MLC901 group compared to the placebo after 6 months (P < .05) with close to no adverse effects. No major side effect was reported.
Conclusion:
MLC901 has shown, compared to placebo, a statistically better improvement at 6 months in neurological functions of patients with HIBI.
Keywords: Glasgow Outcome Scale, hypoxic-ischemic brain injury, MLC901, modified Rankin Scale, NeuroAiD II
1. Introduction
One of the consequences of successful cardiopulmonary resuscitation is hypoxic-ischemic brain injury (HIBI) which can occur due to cerebral edema and micro-hemorrhages.[1] Anoxic neurological injury is the leading cause of mortality and everlasting disability among patients who have survived the acute phase of cardiac arrest.[2] Currently, there is no direct treatment for patients with HIBI, but supportive care can minimize secondary injury to the brain.[3] Optimizing cerebral perfusion can be controlled by adjusting the mean arterial pressure,[2] and targeted temperature management can decrease cerebral oxygen demand by induced hypothermia.[3] Other parts of supportive care are oxygenation and ventilation to avoid hyperoxia and hypoxia and maintain PaCO2 levels between 35 and 45 mm Hg in HIBI patients.[4] Many studies have evaluated various pharmacological agents as single or multidrug combination therapies. They showed that neuroprotection of combination therapies is more effective due to the synergistic effects of these drugs on the multitude of altered pathways.[5]
MLC901 is a multiherbal medicine recommended to improve the central nervous system’s functional state, helping restore sensory, cognitive, and motor functions in patients with brain damage.[6] The mode of action of MLC901 has been described as hyperpolarization of ATP-sensitive potassium channels, especially in neurons that have been injured by oxygen-glucose deprivation, thus enabling a neuroprotective effect on ischemic consequences.[7] Additionally, published in vitro studies have investigated the impact of MLC901 in focal[8] and global ischemia.[9] The evaluation of MLC901 on global ischemia in rats showed results that support its use in patients after a cardiac arrest.
Based on all these data, the present study aimed to evaluate the therapeutic effectiveness of MLC901 on neurological function and clinical outcomes in patients with HIBI.
2. Material and methods
2.1. Study design and participants
This randomized, double-blind, placebo-controlled study was conducted between March 2020 and August 2022 in a university-affiliated hospital in Tehran, Iran. The study objective was to assess the efficacy of MCL901 in HIBI patients. To be eligible for this study, patients should satisfy the following inclusion criteria: age superior to 14 years with an initial cardiac arrest leading to coma, that is, Glasgow Coma Scale 8 or less, the successful return of spontaneous circulation, and persistent coma after the return of spontaneous circulation. The exclusion criteria were pregnancy, cardiogenic shock (systolic blood pressure <90 mm Hg despite epinephrine infusion), possible causes of coma other than cardiac arrest (drug overdose, head trauma, or cerebrovascular accident), an underlying background of known acute or chronic disease including renal failure, liver or pulmonary disorders, previous stroke, dementia, and brain injury.
MLC901, also known as NeuroAiDII, is a capsule form of drug that should be taken orally and combines 9 herbal components (0.114g radix Paeoniae rubrae, 0.57g radix Astragali, 0.114g radix Salviae miltiorrhizae, 0.114g rhizoma Chuanxiong, 0.114g radix Angelicae Sinensis, 0.114g radix Polygalae, 0.114g Prunus persica, 0.114g Carthamus tinctorius, and 0.114g rhizoma Acori tatarinowii).[10]
After the return of spontaneous circulation, 35 eligible patients were randomly assigned to MLC901 (18 patients) and placebo (17 patients) groups within 24 hours after cardiac arrest.
The randomization has been classified based on gender, age, and Glasgow Coma Scale. MLC901 or matching placebo were taken orally with 2 capsules (vegetable capsules filled with <2 grams of stevia-sweetened powder) 3 times daily for 6 months. The patients have been assessed at baseline, 1, 3, and 6 months after admission.
2.2. Outcome measures
We examine participants at baseline and at 1, 3, and 6-month follow-up visits with a modified Rankin Scale (mRS) and Glasgow Outcome Scale (GOS). In patients with neurological damage, a commonly used scale to determine their disability, especially physical disability and dependence is mRS, which portrays symptoms, incapacity, reliance, and mortality results.[11,12] It ranges from zero to 6, with a lower score indicating a lower degree of disability (Table 1).[13] Another widely used scale to investigate patients with brain injury is GOS which ranges from 1 to 5, with a higher score indicating a higher recovery (Table 1).[14] GOS is one of the most recommended measurements in studies concerning brain injury and trauma due to its reliability, accessibility, and ease of use. It provides an overview of patient outcomes after injury.[15]
Table 1.
Modified Rankin Scale (mRS) and Glasgow Outcome Scale (GOS) subscales.
| mRS | Score | GOS | Score |
|---|---|---|---|
| Asymptomatic | 0 | Death | 1 |
| Symptoms in the absence of significant disability | 1 | ||
| Slight disability. Not able to perform all of their previous activities but do not need help with daily activities | 2 | Persistent vegetative state: minimal responsiveness | 2 |
| Moderate disability. Requires some help but is able to walk unassisted | 3 | Severe disability: conscious but disabled; dependent on others for daily support | 3 |
| Moderately severe disability. Impossibility of independent existence without requiring constant attention | 4 | Moderate disability: disabled but independent; can work in a sheltered setting | 4 |
| Severe disability. Requires constant attention night and day | 5 | Good recovery: resumption of everyday life despite minor deficits | 5 |
| Death | 6 |
GOS = Glasgow Outcome Scale, mRS = modified Rankin Scale.
Liver, renal function, and coagulation profile were checked regularly. A list of possible side effects of medication was used for assessment.
2.3. Statistical analysis
Our statistical analysis was done by SPSS for Windows version 26 (IBM, Armonk, NY) software. Descriptive data were reported as a mean ± standard deviation for continuous values and frequency for categorical values. Standard distributions of values were determined by the Kolmogorov–Smirnov test. The chi-square test was used to compare nonparametrical data, and the Kruskal–Wallis was used to compare parametric data without normal distribution between the 2 study groups. The independent t test was used to compare the mean in 2 groups, and the repeated measures t test was applied to compare 2 groups’ data over 6-month periods. The level of significance for the statistical test was 0.05.
2.4. Ethical consideration
This study had been ethically approved by the ethics committee of the Shahid Beheshti University of Medical Science, Tehran, Iran, and each patient’s legal surrogate had written consent according to Helsinki Declaration and as permitted by local regulations (ethical number: IR.SBMU.RETECH.REC.1400.510). Our recovered participants who were able to decide were asked to consent to stay in our study.
3. Results
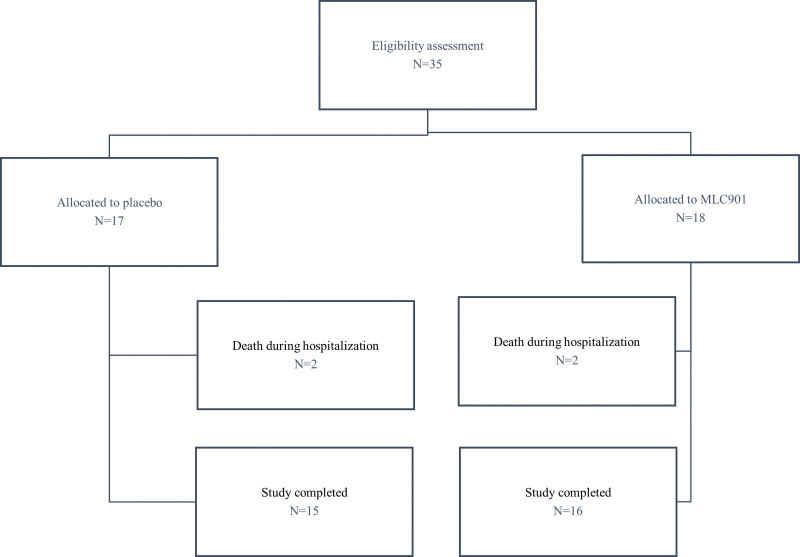
A total of 35 participants were entered and randomized into the study. In each group, 2 patients from each group died during hospitalization unrelated to the study treatment. The first patient from the MLC901 group died due to cardiac arrest on day 4, and the second patient due to septicemia on day 11. The first patient from the control group died due to cardiac arrest on day 4, and the second patient died of septicemia on day 16 (Fig. 1). The analyzed population for efficacy includes all 31 patients who completed the trial.
Figure 1.
CONSORT flow diagram of the study.
Baseline patients’ characteristics, time of resuscitation, the interval between admission to the intensive care unit, and length of intensive care unit stay are shown in Table 2. These data were not significantly different between the 2 study groups. On the other hand, the causes of the patient’s cardiac arrest were almost similar in both groups.
Table 2.
Baseline characteristics.
| Parameter | MLC901 [n] | Placebo [n] | P value |
|---|---|---|---|
| Age | 74.92 (±11.39) [13] | 76 (±9.73) [12] | .80 |
| Gender (male) | 10 (62.5%) | 11 (73.33%) | .42 |
| Onset of arrest to the end of resuscitation (min)* | 86.81 (±52) [16] | 106.33 (±50.23) [15] | .3 |
| Injury to intervention (hr)† | 21.54 (±17.9) [14] | 18.04 (±16.02) [13] | .6 |
| Length of ICU stay (d) | 9.08 (±3.84) [13] | 11.83 (±2.82) [12] | .06 |
ICU = intensive care unit.
From onset of cardiac arrest to termination of successful resuscitation.
Interval between injury and start of the intervention.
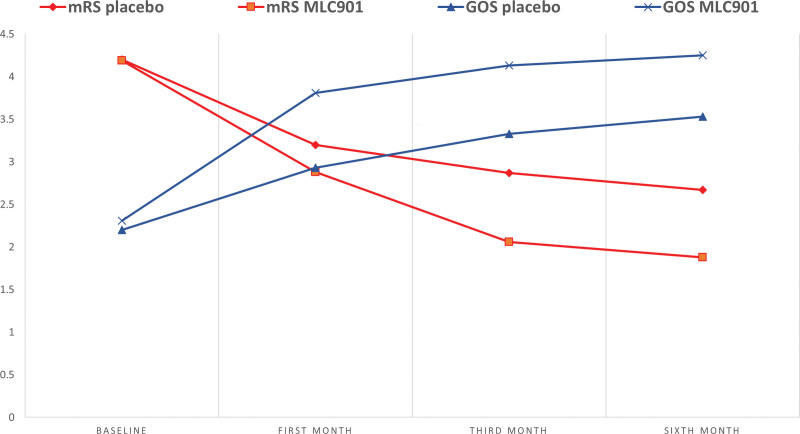
The mRS and GOS scores are compared in Figure 2 and Tables 3 and 4. A significantly higher improvement in the MLC901 group compared to the placebo group is observed on both scales at months 3 and 6 on mRS and from months 1 to 6 with GOS. Overall, the functional improvement over time was significantly different between the 2 groups (repeated measures t test: mRS P value: 0.021 and GOS P value: 0.018).
Figure 2.
Differences in GOS and mRS scores during the follow-ups. GOS = Glasgow Outcome Scale, mRS = modified Rankin Scale.
Table 3.
The modified Rankin Scale of patients with HIBI treated with MLC901 (n = 15) versus placebo (n = 16).
| mRS | Mean (SD) | No Symptoms | No significant disability | Slight disability | Moderate disability | Moderately severe disability | Severe disability | Dead | P value* | |
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | MLC901 | 4.19 (0.65) |
0 | 0 | 0 | 2 (12.5%) | 9 (56.25%) | 5 (31.25%) | 0 | .96 |
| Placebo | 4.2 (0.77) |
0 | 0 | 1 (6.66%) | 0 | 9 (60%) | 5 (33.34%) | 0 | ||
| First month | MLC901 | 2.88 (0.61) | 0 | 0 | 4 (25%) | 10 (62.5%) | 2 (12.5%) | 0 | 0 | .21 |
| Placebo | 3.2 (0.77) | 0 | 0 | 3 (20%) | 6 (40%) | 6 (40%) | 0 | 0 | ||
| Third month | MLC901 | 2.06 (0.68) | 0 | 3 (18.75%) | 9 (56.25%) | 4 (25%) | 0 | 0 | 0 | .004 |
| Placebo | 2.87 (0.74) | 0 | 0 | 5 (33.34%) | 7 (46.66%) | 3 (20%) | 0 | 0 | ||
| Sixth month | MLC901 | 1.88 (0.61) | 0 | 4 (25%) | 10 (62.5%) | 2 (12.5%) | 0 | 0 | 0 | .001 |
| Placebo | 2.67 (0.61) | 0 | 0 | 6 (40%) | 8 (53.34%) | 1 (6.66%) | 0 | 0 | ||
HIBI = hypoxic-ischemic brain injury, mRS = modified Rankin Scale.
Independent t test.
Table 4.
The Glasgow Outcome Scale of patients with HIBI treated with MLC901 (n = 15) versus placebo (n = 16).
| GOS | Mean (SD) | Dead | Persistent vegetative | Severe disability | Moderate disability | Good recovery | P value* | |
|---|---|---|---|---|---|---|---|---|
| Baseline | MLC901 | 2.31 (0.8) | 0 | 10 (62.5%) | 5 (31.25%) | 1 (6.25%) | 0 | .69 |
| Placebo | 2.2 (0.77) | 0 | 11 (73.34%) | 3 (20%) | 1 (6.66%) | 0 | ||
| First month | MLC901 | 3.81 (0.66) | 0 | 0 | 5 (31.25%) | 9 (52.94%) | 2 (12.5%) | .002 |
| Placebo | 2.93 (0.8) | 0 | 5 (33.34%) | 6 (40%) | 4 (26.66%) | 0 | ||
| Third month | MLC901 | 4.13 (0.71) | 0 | 0 | 3 (18.75%) | 8 (50%) | 5 (31.25%) | .003 |
| Placebo | 3.33 (0.62) | 0 | 1 (6.66%) | 8 (53.34%) | 6 (40%) | 0 | ||
| Sixth month | MLC901 | 4.25 (0.68) | 0 | 0 | 2 (12.5%) | 8 (50%) | 6 (37.5%) | .009 |
| Placebo | 3.53 (0.74) | 0 | 1 (6.66%) | 6 (40%) | 7 (46.66) | 1 (6.66%) | ||
GOS = Glasgow Outcome Scale, HIBI = hypoxic-ischemic brain injury.
Independent t test.
There were no major adverse events related to the study treatment. Liver, renal function, and coagulation profile remained within the normal range in all patients in both groups. The most common adverse events experienced by patients in the MLC901 group were nausea (3 patients), and abdominal discomfort (3 patients), dry mouth (1 patient) which were minor and tolerable. There was no treatment withdrawal for adverse events.
Four patients died during hospitalization (2 in each group). The most common adverse events experienced by patients in the MLC901 group were dry mouth, nausea, and abdominal discomfort, which were tolerable for them.
4. Discussion
In this study, we evaluated the effect of MLC901 on HIBI patients who had successful resuscitation due to cardiac arrest. Our results show an improvement of mRS and GOS scores in both MLC901 and placebo groups at 6 months, the improvement starting as early as the first month in the MLC901 group compared to the placebo, and this improvement was sustained for 6 months. A significant improvement in mRS and GOS scores was observed in the MLC901 group compared to the placebo group, as shown on 2 different functional scales at follow-up visits, supporting the use of MLC901 as a convenient medication for HIBI patients. Right after cardiac arrest, hypoxia and loss of blood flow can cause irreversible brain damage in a period of seconds; therefore, it demands a treatment that could help reperfusion by antiinflammation and antioxidant effects at the acute phase.[16] In addition, after the acute phase, treatment with neurorestorative properties could help to repair cerebral injuries. It has been proven that MLC901 has such properties in patients with traumatic brain injuries.[17]
Both scales, GOS, and mRS, were used to investigate HIBI patients’ neurorehabilitation in a previous cohort study.[18] Many studies have used these scales to evaluate pharmacological agents in HIBI patients. A previous clinical trial study in German showed that Coenzyme Q10 plus hypothermia improved neurological function in 3-month follow-ups with the GOS scale in cardiac arrest survivors.[19] Another paper published in 2016 assessed Xenon combined with hypothermia and hypothermia alone in comatose patients who survived cardiac arrest during 6 months visits that used the mRS scale for their evaluation. This study didn’t significantly differ between the 2 groups’ neurological outcomes.[20]
Following resuscitation, numerous pathways can cause brain damage containing acidosis, ionic balance, peri-infarct depolarization, oxidative stress excitotoxicity, inflammation, and apoptosis which need medication with multiple effects. MLC901 is a multiherbal medicine with mostly multitarget effects such as inducing neurogenic processes in cortical neurons, stimulating brain-derived neurotrophic factor secretion (a growth factor), and neuroproliferation.[21] A study in 2011 used MLC901 in rats with global brain ischemia; this study showed a significant positive effect of this medicine on neurological function, particularly in the first 3 hours after starting MLC901. Also, this study can provide scientific support for evaluating MLC901 in HIBI patients.[9] In another study, the recovery of patients after an acute ischemic stroke was followed for 24 months by Barthel Index and mRS tests. The study showed that receiving MLC601 added to the rehabilitation program increased functional recovery, and this clinical improvement persisted over 2 years.[8] A study by Murie-Fernandez et al, conducted in Spain on 127 patients over 3 months after moderate to severe ischemic stroke using MLC901 as a dietary supplement, showed a significant association between MLC901 and poststroke recovery, notably in severe stroke patients assessed by Barthel Index, mRS, and the National Institute of Health Stroke Scale.[22]
The cognitive recovery ability of MLC601/901 was suggested in multiple studies, A randomized clinical trial performed on 125 patients with mild to moderate Alzheimer Disease displayed the potential of MLC901 to slow down the cognitive impairment and disease progression while being safe and free of side effects as an add-on therapy compared to placebo.[23] Another randomized clinical trial published in 2017 evaluated the effect of MLC601 on 70 mild cognitive impairment patients over 6 months; this study also showed the effectiveness of MLC601 among mild cognitive impairment patients.[24] On the other hand, the investigation of MLC901 on vascular cognitive impairment in no dementia patients showed insignificant effects on executive function and verbal fluency after 3 and 6 months.[25] in 2017, the effect and side effects of MLC601 were investigated among 82 vascular dementia patients for 2 years; in the end, this study displayed the efficacy of MLC601 was superior to that of placebo, without adverse events related to MLC601.[26] The use of MLC901 has also been tested on 81 patients with moderate to Severe traumatic brain injury (TBI) over 6 months to determine its effectiveness assessed with GOS and mRS scales; results provided evidence that MLC901 can be used as a valid treatment for these patients,[27] consistent with the report of Theadom et al, showing improvement most commonly in complex attention and executive functioning recovery in mild to moderate TBI patients using MLC901 based on computerized neurocognitive screening vital signs.[17] The 2 studies suggested the accepted basis that spontaneous functional recovery occurs due to brain tissue plasticity days to months after a brain injury such as stroke or TBI.[28,29] Still, the MLC901 group’s improvements in these studies were significant.
Also, in addition to the positive effect of MLC901 on brain functions, a study had shown the critical effect of this drug on reducing vascular events such as recurrent stroke, acute coronary event, and vascular death in 3 months follow-up of patients.[30]
There are advantages and limitations to this study. The period of 6 months was insufficient to investigate the long-term effects of MLC901 and its possible adverse effects. Our study could have also benefited from a larger number of participants and applying self-reporting questionnaires such as Cognitive Failure Questioner to evaluate perception, memory, and motor function.[31] Nowadays, extended GOS is recommended for assessing the disability of patients with a brain injury which is more sensitive than GOS in minor changes of functional activity.[32] As far as we know, this is the first human study on MLC901 in HIBI patients. To facilitate the process, we suggest that future studies could prolong the investigation while reducing the number of pills daily and increasing the number of patients to come to a better conclusion.
5. Conclusion
We provided evidence of the effect of MLC901 in improving the state of disability after successful cardiopulmonary resuscitation in patients suffering from HIBI. However, future studies are necessary to investigate different aspects of MLC901’s long-term effects on brain function in HIBI patients.
Author contributions
Conceptualization: Hossein Pakdaman, Akram Esfandani, Mohammad Hossein Hosseini, Ali Amini Harandi.
Data curation: Koroush Gharagozli, Mohammad Hossein Hosseini.
Formal analysis: Faezeh Karamiani.
Investigation: Hossein Pakdaman, Maryam Shamsi Goushki, Saman Moini.
Methodology: Koroush Gharagozli, Faezeh Karamiani, Akram Esfandani, Mohammad Hossein Hosseini.
Project administration: Ali Amini Harandi.
Resources: Ali Sobhanian, Faeze Maghsoudlu.
Supervision: Ali Amini Harandi.
Validation: Ali Sobhanian.
Visualization: Faeze Maghsoudlu.Writing – original draft: Faezeh Karamiani, Maryam Shamsi Goushki, Saman Moini.
Writing – review & editing: Ali Amini Harandi.
Abbreviations:
- GOS
- Glasgow Outcome Scale
- HIBI
- hypoxic-ischemic brain injury
- mRS
- modified Rankin Scale
- TBI
- traumatic brain injury
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Clinical trial protocol registration code (clinicaltrials.gov): NCT05621590.
How to cite this article: Pakdaman H, Gharagozli K, Karamiani F, Shamsi Goushki M, Moini S, Sobhanian A, Maghsoudlu F, Esfandani A, Hosseini MH, Amini Harandi A. MLC901 in hypoxic-ischemic brain injury patients: A double-blind, randomized placebo-controlled pilot study. Medicine 2023;102:23(e33914).
Contributor Information
Hossein Pakdaman, Email: Hpakdaman20@gmail.com.
Koroush Gharagozli, Email: gharagozli@yahoo.com.
Faezeh Karamiani, Email: faeze.karamiani@gmail.com.
Maryam Shamsi Goushki, Email: m.shamsi92@gmail.com.
Saman Moini, Email: samanmoini97@gmail.com.
Ali Sobhanian, Email: ali.amini.harandi@gmail.com.
Faeze Maghsoudlu, Email: faezemaghsoodloo1988@yahoo.com.
Akram Esfandani, Email: esfandani_a@gmail.com.
Mohammad Hossein Hosseini, Email: hooman199728@gmail.com.
References
- [1].Busl KM, Greer DM. Hypoxic-ischemic brain injury: pathophysiology, neuropathology and mechanisms. NeuroRehabilitation. 2010;26:5–13. [DOI] [PubMed] [Google Scholar]
- [2].Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47:1393–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. [DOI] [PubMed] [Google Scholar]
- [4].Nolan JP, Sandroni C, Böttiger BW, et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: post-resuscitation care. Intensive Care Med. 2021;47:369–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Choudhary RC, Shoaib M, Sohnen S, et al. Pharmacological approach for neuroprotection after cardiac arrest-a narrative review of current therapies and future neuroprotective cocktail. Front Med (Lausanne). 2021;8:636651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Han SY, Hong ZY, Xie YH, et al. Therapeutic effect of Chinese herbal medicines for post stroke recovery: a traditional and network meta-analysis. Medicine (Baltim). 2017;96:e8830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Moha Ou Maati H, Borsotto M, Chatelain F, et al. Activation of ATP-sensitive potassium channels as an element of the neuroprotective effects of the traditional Chinese medicine MLC901 against oxygen glucose deprivation. Neuropharmacology. 2012;63:692–700. [DOI] [PubMed] [Google Scholar]
- [8].Widmann C, Gandin C, Petit-Paitel A, et al. The traditional Chinese medicine MLC901 inhibits inflammation processes after focal cerebral ischemia. Sci Rep. 2018;8:18062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Quintard H, Borsotto M, Veyssiere J, et al. MLC901, a traditional Chinese medicine protects the brain against global ischemia. Neuropharmacology. 2011;61:622–31. [DOI] [PubMed] [Google Scholar]
- [10].Chen CL, Ikram K, Anqi Q, et al. The NeuroAiD II (MLC901) in vascular cognitive impairment study (NEURITES). Cerebrovasc Dis. 2013;35(Suppl 1):23–9. [DOI] [PubMed] [Google Scholar]
- [11].Lee SY, Kim DY, Sohn MK, et al. Determining the cut-off score for the modified barthel index and the modified Rankin Scale for assessment of functional independence and residual disability after stroke. PLoS One. 2020;15:e0226324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kongsawasdi S, Klaphajone J, Wivatvongvana P, et al. Prognostic factors of functional outcome assessed by using the modified Rankin Scale in subacute ischemic stroke. J Clin Med Res. 2019;11:375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Farrell B, Godwin J, Richards S, et al. The United Kingdom transient ischaemic attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54:1044–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hall K, Cope DN, Rappaport M. Glasgow outcome scale and disability rating scale: comparative usefulness in following recovery in traumatic head injury. Arch Phys Med Rehabil. 1985;66:35–7. [PubMed] [Google Scholar]
- [15].McMillan T, Wilson L, Ponsford J, et al. The Glasgow outcome scale – 40 years of application and refinement. Nat Rev Neurol. 2016;12:477–85. [DOI] [PubMed] [Google Scholar]
- [16].Nutma S, le Feber J, Hofmeijer J. Neuroprotective treatment of postanoxic encephalopathy: a review of clinical evidence. Front Neurol. 2021;12:614698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Theadom A, Barker-Collo S, Jones KM, et al. MLC901 (NeuroAiD II™) for cognition after traumatic brain injury: a pilot randomized clinical trial. Eur J Neurol. 2018;25:1055–e82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jöhr J, Halimi F, Pasquier J, et al. Recovery in cognitive motor dissociation after severe brain injury: a cohort study. PLoS One. 2020;15:e0228474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Damian MS, Ellenberg D, Gildemeister R, et al. Coenzyme Q10 combined with mild hypothermia after cardiac arrest: a preliminary study. Circulation. 2004;110:3011–6. [DOI] [PubMed] [Google Scholar]
- [20].Laitio R, Hynninen M, Arola O, et al. Effect of inhaled xenon on cerebral white matter damage in comatose survivors of out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2016;315:1120–8. [DOI] [PubMed] [Google Scholar]
- [21].Heurteaux C, Gandin C, Borsotto M, et al. Neuroprotective and neuroproliferative activities of NeuroAid (MLC601, MLC901), a Chinese medicine, in vitro and in vivo. Neuropharmacology. 2010;58:987–1001. [DOI] [PubMed] [Google Scholar]
- [22].Murie-Fernández M, Marzo MM. Predictors of neurological and functional recovery in patients with moderate to severe ischemic stroke: the EPICA study. Stroke Res Treat. 2020;2020:1419720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chen CLH, Lu Q, Moorakonda RB, et al. Alzheimer’s Disease THErapy With NEuroaid (ATHENE): a randomized double-blind delayed-start trial. J Am Med Dir Assoc. 2022;23:379–386.e3. [DOI] [PubMed] [Google Scholar]
- [24].Pakdaman H, Amini Harandi A, Abbasi M, et al. Efficacy and safety of MLC601 in the treatment of mild cognitive impairment: a pilot, randomized, double-blind, placebo-controlled study. Dement Geriatr Cogn Dis Extra. 2017;7:136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chen CLH, Nguyen TH, Marasigan S, et al. NEURoaid II (MLC901) in cognitively Impaired not demenTEd patientS (NEURITES): a pilot double blind, placebo-controlled randomized trial. Alzheimers Dement (N Y). 2021;7:e12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Pakdaman H, Amini Harandi A, Gharagozli K, et al. MLC601 in vascular dementia: an efficacy and safety pilot study. Neuropsychiatr Dis Treat. 2017;13:2551–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Hossein Pakdaman A, Esfandani A, Yousefi A. MLC901 for moderate to severe traumatic brain injury: pilot, randomized, double-masked, placebo-controlled trial. Open Access J Complement Altern Med. 2020;2:257–62. [Google Scholar]
- [28].Hara Y. Brain plasticity and rehabilitation in stroke patients. J Nippon Med Sch. 2015;82:4–13. [DOI] [PubMed] [Google Scholar]
- [29].Chen H, Epstein J, Stern E. Neural plasticity after acquired brain injury: evidence from functional neuroimaging. PM R. 2010;2(12 Suppl 2):S306–12. [DOI] [PubMed] [Google Scholar]
- [30].Chen CL, Venketasubramanian N, Lee CF, et al. Effects of MLC601 on early vascular events in patients after stroke: the CHIMES study. Stroke. 2013;44:3580–3. [DOI] [PubMed] [Google Scholar]
- [31].Wallace JC, Kass SJ, Stanny CJ. The cognitive failures questionnaire revisited: dimensions and correlates. J Gen Psychol. 2002;129:238–56. [DOI] [PubMed] [Google Scholar]
- [32].Weir J, Steyerberg EW, Butcher I, et al. Does the extended Glasgow outcome scale add value to the conventional Glasgow outcome scale? J Neurotrauma. 2012;29:53–8. [DOI] [PMC free article] [PubMed] [Google Scholar]