Abstract
Background:
Low-intensity pulsed ultrasound with microbubbles (LIPU/MB) can be used to open the blood-brain barrier (BBB). We aimed to determine the safety and pharmacokinetics of LIPU/MB to enhance the delivery of albumin-bound paclitaxel (ABX) to the peri-tumoral brain of recurrent glioblastoma (GBM) patients.
Methods:
We conducted a dose-escalation phase 1 clinical trial (NCT04528680). Adults (>18 years old) with recurrent GBM, tumor diameter <70 mm, and Karnofsky-performance ≥70 were eligible. A nine-emitter ultrasound device was implanted into a skull-window after tumor resection. LIPU/MB with intravenous ABX infusion was performed every 3 weeks for up to 6 cycles. Six dose levels (40-260 mg/m2) were evaluated. We did pharmacokinetic analyses of LIPU/MB in a subgroup of patients from the current study, and a subgroup who received carboplatin as part of a similar trial (NCT03744026). The primary endpoint was dose-limiting toxicity (DLT). Safety was assessed in all treated patients (n=17). Analysis was performed as per protocol. BBB opening was investigated by pre and post-sonication MRI.
Findings:
9 males and 8 females were enrolled between October 29th 2020 and February 21st 2022. Median follow-up was 11.89 months (IQR 11.12, 12.78). At a dose level of 260 mg/m2, grade 3 encephalopathy occurred in 1/12 patients (8%) during the first cycle (considered DLT), and on a second patient on cycle 2 (grade 2). In both cases, it resolved, and treatment continued at a lower ABX dose. Imaging analysis showed BBB opening in the brain regions targeted by LIPU/MB which diminished over the first 1 hr. after sonication. LIPU/MB led to increase in brain parenchymal concentrations of ABX by 3.7-times (non-sonicated brain mean [ABX] 0.0373 uM, 95% CI 0.0223 - 0.0625 uM vs. sonicated brain mean [ABX] 0.1386 uM, 95% CI 0.0828 – 0.2319 uM, p<0.0001) and of carboplatin by 5.9-times (non-sonicated brain mean [carboplatin] 0.9914 uM, 95% CI 0.5624 - 1.747 uM vs. sonicated brain mean [carboplatin] 5.878 uM, 95% CI 3.4622 - 9.98 uM, p=0.00012 on pharmacokinetic study done on a similar trial (NCT03744026).
Interpretations:
LIPU/MB using a skull-implantable ultrasound transiently opens the BBB allowing for safe, repeated penetration of cytotoxic drugs into the brain. This study has prompted a subsequent phase 2 study, which is ongoing.
Funding:
NIH/NCI 1R01CA245969-01A1, P50CA221747, and philanthropic support. In-kind support from Carthera and BMS.
Introduction
The majority of drugs do not cross the blood-brain barrier (BBB), limiting the agents available for treatment of brain diseases.(1) In the case of infiltrative high-grade gliomas, the BBB remains intact in the peri-tumoral brain where tumor cells migrate and infiltrate into the parenchyma while protected from exposure to drugs.(2) Consequently, 80-90% of glioblastoma (GBM) recur within the 2-cm margin of peri-tumoral brain around the resection cavity. (3, 4)
Paclitaxel (PTX) is a chemotherapeutic agent approximately 1400-times more potent than temozolomide (TMZ), the standard chemotherapeutic used for gliomas. PTX exhibits similar activity for glioma cell lines as for other cancers for which this agent is part of the standard regimen.(5, 6) In contrast to TMZ, PTX does not cross the BBB (7) and failed to show efficacy in Phase 1/2 trials when systemically administrated for malignant gliomas.(8, 9)
Low-intensity pulsed ultrasound with concomitant administration of intravenous (IV) microbubbles (MB) (LIPU/MB) can be used to open the BBB. In brain capillaries, MB oscillate upon stimulation by ultrasound, generating mechanical stress on the endothelial wall that opens the BBB. The effect of LIPU/MB on BBB permeability has been demonstrated in animal models and in clinical trials. (10-13)
Early phase 1/2 clinical trials of LIPU/MB in patients with GBM, brain metastases, Alzheimer's disease, and amyotrophic lateral sclerosis, have shown the safety of this approach.(12, 14-17) The opening of the BBB has been demonstrated indirectly on magnetic resonance imaging (MRI) or by SPECT with radio-labeled antibodies.(10, 12) Yet the magnitude of the effect of LIPU/MB-based BBB opening on drug levels shortly after LIPU/MB has not been quantified, and the timing of BBB closure after the procedure remains poorly understood.
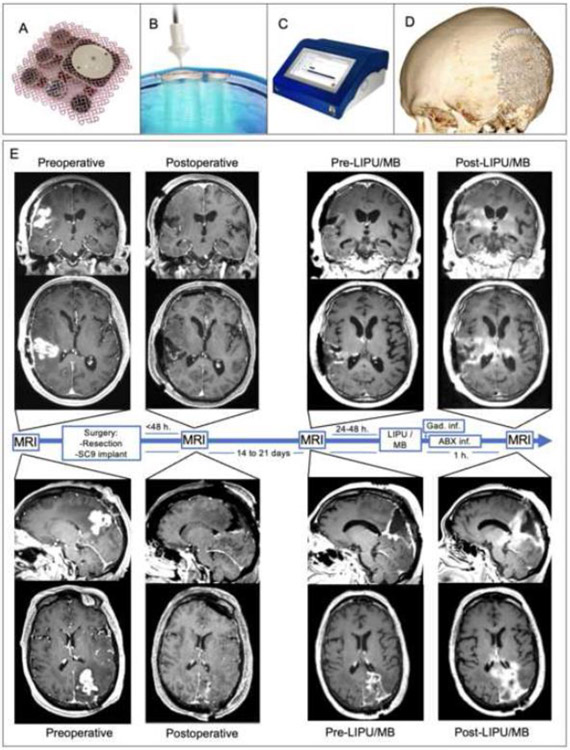
We previously showed that LIPU/MB enhances the penetration of PTX across the BBB in mice, and that an FDA-approved, cremophor-free, albumin-bound paclitaxel formulation (ABX, Abraxane®, Bristol Myers Squibb, New York, NY, USA) is well-tolerated in this setting.(6) Here we report results of a phase 1 clinical trial in which ABX was administered in conjunction with LIPU/MB-based BBB opening in patients with recurrent GBM [NCT04528680]. We used a novel device composed of nine ultrasound emitters (SC9, SonoCloud-9, Carthera, Lyon, France) implanted into a skull window at (Figure 1A) thus allowing ultrasound waves to bypass the skull. The device can be activated by connecting a transdermal needle to an external power supply/pulse generator at the time of chemotherapy administration.
Figure 1. Skull-implantable device composed of 9 ultrasound emitters achieves large, reproducible volume of BBB opening in peri-tumoral brain.
The SC9 system consists of an implantable device (A) with nine, 1 MHz ultrasound emitters that is implanted in a window in the skull during resection surgery and (B) a single-use transdermal needle that is used to connect the implantable ultrasound to a (C) pulse-generator with a touchscreen interface. (D) 3D reconstruction of post-operative computer tomography showing the implant of SC9 on a window in the skull. (E) MRI T1 with contrast sequences of two patients as representative examples of BBB opening determined by gadolinium leaking into the peri-tumoral brain after sonication, but not before sonication. From left to right, preoperative, postoperative, pre-LIPU/MB, and post-LIPU/MB MR images are provided. Brain enhancement (seen as hyper-intensity), on post-LIPU/MB that is not seen in the pre-LIPU/MB represents BBB opening with permeation of gadolinium elicited by SC9.
To investigate the effects of LIPU/MB on the brain parenchymal drug concentrations, we administered chemotherapy in conjunction with sonication (LIPU/MB) in the operating room prior to tumor resection in select patients being treated on clinical trials using the SC9 device in conjunction with ABX [NCT04528680] or carboplatin (CBDCA) [NCT03744026], respectively.
Methodology
Study design and participants:
We conducted a phase 1 clinical trial for recurrent GBM [NCT04528680] to evaluate LIPU/MB-based opening of the BBB and concomitant administration of ABX conducted at Northwestern University Feinberg School of Medicine, Chicago, IL. The primary objectives were to i) evaluate the safety and maximal tolerated dose (MTD) of ABX after LIPU/MB-based opening of the BBB in patients with recurrent GBM; ii) determine the effect of LIPU/MB-based BBB opening on PTX concentrations in the peri-tumoral brain._The study was approved by IRB (STU00212298), and all patients provided written informed consent. Eligibility criteria included age ≥ 18 years, diagnosis of recurrent GBM (IDH wild-type) after failure of 1 or 2 lines of prior therapy (interval since end of radiation ≥ 12 weeks), WHO performance status ≤ 2. Patients were required to be amenable to tumor resection, with enhancing tumor size ≤ 70 mm maximal diameter, or expected residual peri-tumoral brain (after resection) of ≤ 70 mm (see appendix p. 2 for anatomical considerations).
LIPU/MB was performed using SC9, a novel skull-implantable device composed of nine ultrasound emitters (Figure 1). Appendix p. 18,19 includes the protocol and summary of its amendments.
Procedures:
To implant the SC9 device, under neuronavigation guidance we created a 6x6 cm cranial window (Figure 1A). The implant was fixed to the bone using standard surgical screws (appendix p. 2, example shown on Figure 1D).
To perform BBB opening, the SC9 was activated by connecting the implanted device to the pulse generator through percutaneous access using a single-use sterile transdermal needle and cable (Figure 1B). The pulse generator was controlled using a touchscreen interface (Figure 1C, appendix p. 2). Simultaneous with intravenous (IV) injection of microbubbles (perflutren lipid microsphere, Definity® 10 μl/kg, Lantheus, N. Billerica, MA) over 30 seconds, the pulse generator activated the SC9 device for 4 ½ minutes, immediately followed by IV administration of the chemotherapy.
The first cycle of sonication and chemotherapy was scheduled within 1-3 weeks from surgery, preceded by a new baseline MRI obtained 1-2 days pre-sonication. Immediately following the sonication procedure, ABX was administered IV over 30 minutes. For cycle 1, a bolus of gadolinium was injected either within minutes of conclusion of LIPU/MB, or at the time of acquisition of a post-sonication MRI that was obtained after completion of ABX infusion (approximately 60 minutes from LIPU/MB, see appendix p. 14), similar to what has been done before.(18) The same procedure (without gadolinium injection and post-sonication MRI) was repeated every 3 weeks as clinically indicated until disease progression or for up to 6 cycles. All treatments were delivered in the outpatient setting, and patients were monitored for acute toxicities for 4-6 hours after the procedure.
We evaluated ABX dose-levels (DL) 40 mg/m2, 80 mg/m2, 135 mg/m2, 175 mg/m2, 215 mg/m2 and 260 mg/m2 with concomitant LIPU/MB every 3 weeks. Upon occurrence of DLT or upon reaching highest DL, the cohort was expanded to a total of 12 treated and evaluable patients.
Pharmacokinetic studies were conducted in a subset of patients for which tumor location justified the resection of peri-tumoral brain as per standard neurosurgical technique. For this, we performed intraoperative LIPU/MB of peri-tumoral brain with concomitant ABX (IV over 30 minutes), or CBDCA (IV over 30 minutes) in the context of a site-specific amendment of a separate clinical trial (NCT03744026). Biopsy of sonicated and non-sonicated peri-tumoral brain and collection of blood samples was performed for quantification of drug levels and hemoglobin, to determine the effect of BBB opening on drug concentrations in the brain, and on brain/plasma ratios. For pharmacokinetic studies, the intraoperative ABX dose was 80 mg/m2 for all patients (except for patients in DL1: 40 mg/m2); intraoperative CBDCA dose was AUC 3.5. All patients provided written informed consent for this translational study. For further details see appendix p. 4-5. All patients included in the pharmacokinetic analysis had visual confirmation of BBB opening by fluorescein, availability of paired sonicated and non-sonicated peri-tumoral brain specimens that were at least 1 cm from the enhancing tumor determined by stereotaxic coordinates and had sufficient tissue for paired measurement of hemoglobin.
After completing of study treatment, follow-up visits occurred as clinically indicated. Follow-up until progression usually included a clinical visit at least every 2 months, and an MRI every 2-3 months. All patients were followed for survival either clinically or by regular telephone follow-up at least every 2 months.
Outcomes:
The primary endpoint was dose-limiting toxicity (DLT) occurring during cycle 1 of sonication and ABX chemotherapy. Treatment-emergent toxicities were independently reviewed by the Lurie Cancer Center’s Data Safety Monitoring Board and who had to approve each patient’s DL assignment or dose escalation.
DLT was defined as toxicity that is treatment-emergent and possibly, probably or definitely related/attributable to LIPU/MB or to the LIPU/MB plus ABX infusion procedure (excluding intraoperative procedure) occurring during the DLT period (defined as 21 days from the first SC9 sonication procedure associated with ABX treatment). DLT included any related toxicity ≥ grade 3 that does not respond to optimal medical management (including steroids) within 10 days, exceptions are enumerated here below. CNS toxicity of ≥ grade 2 that does not revert to grade ≤ 1 within 21 days, i.e., time for next treatment cycle. Grade 4 CNS toxicity Any treatment-emergent and related toxicity (except hematotoxicity, nausea/vomiting, fatigue and hypersensitivity to ABX or MB injections) > grade 2 that has not reverted to a grade ≤ 2 by day 22 of the first cycle. Treatment-emergent toxicity/events that are unequivocally not related to the sonication or ABX (e.g. attributed to disease progression) will not be considered a DLT. Further information on DLT definition and examples of DLT are found on the protocol (appendix p. 19).
Patients were closely monitored for both acute and late/cumulative toxicities and were clinically examined at least once per cycle prior to the next administration (weekly during cycle 1). During cycle 1, complete blood counts were drawn at least once a week. Treatment emergent symptoms and toxicity were recorded and scored according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 5. MRIs for disease evaluation were performed every 3 cycles or as clinically indicated. Pre-specified exploratory endpoints were quantification of the BBB opening by comparison of pre-sonication to post-sonication contrast MRI (reported here). Additional exploratory endpoints to be reported separately, were drug levels in the enhancing tumor tissue, pattern of treatment failure relative to regions of sonicated brain, objective response rate, and the effect of LIPU/MB on circulating cell-free DNA, RNA and/or exosomes, as well as analyses to characterize the effect LIPU/MB-based BBB opening on the brain. These analyses include single-cell RNA-seq, and different microscopy techniques among other approaches.
Statistical analyses:
The trial was conducted with an adaptive Bayesian Optimal Interval Design (BOIN) design with a target DLT rate for the MTD of <20%. Up to 17 patients were deemed necessary to test this hypothesis. If a patient dropped out of the study before the end of the first cycle (DLT evaluation period) for any reason other than treatment related toxicity, replacement of this subject was allowed. As per the BOIN design, interim analysis for DLT rate was performed after every patient completed the DLT period. The predetermined threshold for significance was p value < 0.05. Safety / DLT determination, and MRI assessment of BBB opening was performed in all treated patients (n=17). For pharmacokinetics, analysis was done in 7 patients where resection of peri-tumoral brain was clinically justified. For the pharmacokinetic studies, to examine the effect of sonication on PTX concentrations, the significance calculation for the single-patient analysis presented in Figure 4A was obtained with the Wilcoxon rank sum exact test. For the aggregate analysis of patients presented in Figure 4B and C, we fit a mixed-effects model with random intercept to account for correlation of within patient repeated measures. These analyses were conducted in R version 4.0.5.
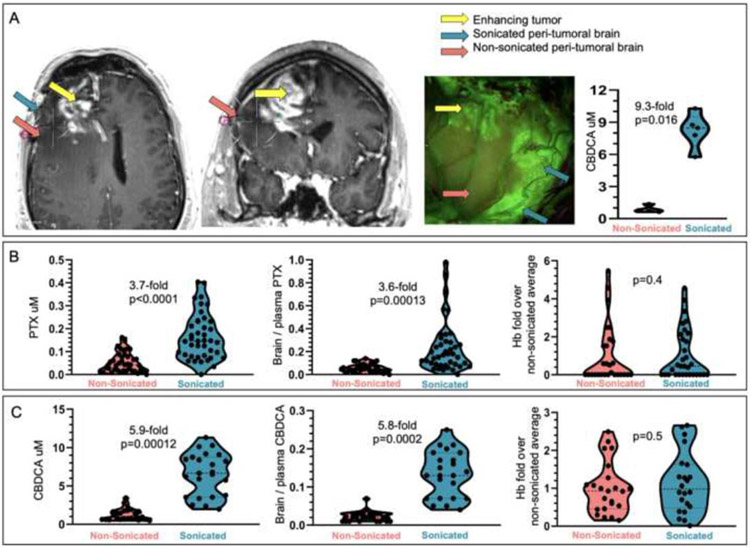
Figure 4. Analysis of the effect of LIPU/MB on the concentration of PTX and CBDCA in the peri-tumoral brain.
(A) Example of a case where intraoperative LIPU/MB was performed in the peri-tumoral brain for pharmacokinetic analysis of CBDCA concentrations. Stereotactic coordinates for each biopsy site were recorded on the pre-operative MRI are indicated by differently colored arrows in axial and coronal planes, and on the photo from surgical microscope. Violin plots show absolute CBDCA concentrations for biopsies corresponding to the sonicated and non-sonicated peri-tumoral brain (red arrow for non-sonicated, green for sonicated brain, and yellow for tumor) (n=5 biopsy samples for sonicated brain, and n=4 for non-sonicated brain). (B) Violin plots showing absolute drug concentrations (left), brain / plasma ratios using plasma levels at 45 minutes after LIPU/MB) (center), and corresponding % hemoglobin (Hb) content (right) (presented as ratio of Hb % for sonicated/non-sonicated tissue) following IV administration of albumin-bound paclitaxel (ABX) (n=7 patients, 81 biopsy samples, 41 sonicated and 40 non-sonicated, out of which 28 non-sonicated and 32 sonicated were also analyzed for Hb) and (C) Carboplatin (CBDCA) (n=3 patients, 48 biopsy samples, 23 sonicated and 25 non-sonicated, out of which 22 sonicated and 23 non-sonicated biopsies were also analyzed for Hb). For A, p value was calculated using the Wilcoxon rank sum exact test. For B and C, p values and fold/times changes of means were calculated using mixed effects model.
Post-hoc statistical analyses:
The relationship between PTX or CBDCA concentrations with fluorescein was investigated through Spearman correlations using Prism version 9.3.1. For imaging analysis of BBB closure, enhancement between two different cycles was compared by two-sample t-test. A linear mixed-effect model was used to describe the relationship between time of sonication to gadolinium injection versus enhancement on post-sonication MRI and between time of gadolinium injection to MRI acquisition versus enhancement on post-sonication MRI (appendix p. 7). Progression-free survival was calculated from date of registration to date of unequivocal progression. We defined the later as the date when clinical and/or imaging-based assessment led to determination of progression, leading to changes in the management of the patient (e.g. discontinuation of treatment, introduction of a new treatment, etc.) Overall survival was calculated from date of registration to date of death. Progression-free and overall survival estimates were also obtained via the Kaplan-Meier method and computed by R version 4.0.5
Role of the funding source:
The funders of the study had no role in study design, data collection, and data interpretation. The manufacturer of the device provided technical input and assistance, and contributed to the imaging analysis presented. Interpretation of the data and writing of the manuscript, and the decision to submit for publication was performed by the investigators.
Results:
Between October 29th 2020 and February 21st 2022, we screened 18 patients for trial participation and 17 patients were enrolled and treated (one consented patient was excluded due to presence of leptomeningeal disease on the preoperative MRI). Baseline patient and tumor characteristics are summarized in Table 1. A total of 6 ABX dose levels were explored, one patient was treated per DL for DL 1-5, and 12 patients treated at DL 6 (260 mg/m2). Data cutoff for analysis was done on September 6th, 2022. At this date, the median follow-up for the trial cohort was 11.89 (IQR 11.12, 12.78) months.
Table 1:
Patient and Tumor Characteristics:
| Patient Characteristics (at inclusion) | n = 17 (%) | |
|---|---|---|
| Age median (range) | 57 years (33-72) | |
| IQR | 52; 63 | |
| Male / Female | 9 (53%) / 8 (47%) | |
| Race; white / not reported | 12 (71%) / 5 (29%) | |
| Ethnicity | ||
| Hispanic | 1 (6%) | |
| Non-hispanic | 13 (76%) | |
| Not reported/refused | 3 (18%) | |
| WHO Performance Status; median (range) | 1 (0 - 1) | |
| Time since initial diagnosis; median (range) | 12 months (7 - 51) | |
| Prior Treatments: | ||
| Radiotherapy 60 Gy | 17 (100%) | |
| Temozolomide | 16 (94%)† | |
| # of prior lines of treatment | ||
| 1 | 16 (94%) | |
| 2 | 1 (6%) | |
| Corticosteroid therapy (<6 mg/day) | 2 (12%) | |
| Anti-epileptic therapy | 12 (71%) | |
| Tumor Characteristics (prior to implant surgery) | ||
| Mean largest enhancing tumor diameter (mm) | 28.7 (range 20-41) | |
| Tumor location | ||
| Left | frontal | 1 (6%) |
| parietal | 4 (24%) | |
| temporal | 1 (6%) | |
| Right | frontal | 4 (24%) |
| parietal | 3 (18%) | |
| temporal | 2 (12%) | |
| occipital | 2 (12%) | |
| Pathology on resected specimen | ||
| Glioblastoma (IDH wild-type, sequenced) | 17 100% | |
| MGMT gene promoter | ||
| methylated/unmethylated | 5 / 12 (29 / 71%) | |
| Treatment Characteristics | ||
| Number of sonication/chemotherapy cycles | 68 | |
| Number of patients treated dose levels 1-5 | 5 | |
| Number of patients treated at MTD (dose level 6) | 12 | |
| # pts receiving ≥ 5 cycles | 6 (50%) | |
One patient with an MGMT gene promoter unmethylated tumor was treated on a protocol that omitted TMZ in favor of an investigational agent
We did not observe surgical complications or infections attributed to the SC9 implant. Patient 108 suffered small wound dehiscence grade 2 at a remote location from the implant that was repaired, and the patient was able to continue study treatments.
We performed a total of 68 cycles of LIPU/MB-based BBB opening. Steroids were weaned off postoperatively and no patients were on any dexamethasone during the sonication procedures. The median time between surgery and beginning of cycle 1 was 17 days (IQR 14,18 days). The median number of cycles per patient was 3 (range 2 – 6). We did not observe progressive neurological deficits attributed to LIPU/MB (appendix p. 9). LIPU/MB-based BBB opening was most commonly associated with immediate yet transient grade 1 headache, and other grade 1 neurological deficits. These acute treatment-emergent adverse events (e.g. paresthesia, weakness, dysphasia, dysarthria, dysesthesia, blurred vision, or facial weakness) correlated anatomically with the brain region sonicated (e.g. left temporal LIPU/MB leading to transient grade 1 dysphasia. Appendix p. 11 describes LIPU/MB-related neurological adverse events per patient and per cycle.
No DLT was observed on escalating DL up to 215 mg/m2. At DL 6 (260 mg/m2), one patient experienced grade 3 encephalopathy 2 hours post administration of cycle 1, considered DLT. Another patient experienced grade 2 encephalopathy also 2 hours after sonication and ABX administration on cycle 2. In both patients, encephalopathy completely resolved within 1-2 days. With appropriate dose reductions, these patients subsequently completed a total of 5 and 3 cycles respectively, without further occurrence of encephalopathy. Appendix p. 13 describes grade 2 or greater treatment-emergent adverse events associated for cycle 1 (DLT period) and for all cycles. Grade 2 peripheral neuropathy was observed in 1 patient treated at 260 mg/m2 on cycle 3, and subsequent cycles were dose reduced to 215 mg/m2, yet the neuropathy (a known side effect of PTX) persisted at grade 2. Treatment-emergent adverse events are reported in Table 2.
Table 2.
Treatment Emergent Adverse Events (TEAE) per patient (N = 17 patients, >10% of patients (and all grade ≥ 3 AE), all cycles) sequence in order of frequency.
| Treatment Emergent Adverse Events (TEAE) | ||||
|---|---|---|---|---|
| AE | All Grades | Grade 1-2 | Grade 3 | Grade 4 |
| Anemia | 15 (88.24%) | 15 (88.24%) | 0 (0%) | 0 (0%) |
| Headache | 15 (88.24%) | 15 (88.24%) | 0 (0%) | 0 (0%) |
| Leukopenia | 15 (88.24%) | 10 (58.82%) | 5 (29.41%) | 0 (0%) |
| Hypertension | 14 (82.35%) | 9 (52.94%) | 5 (29.41%) | 0 (0%) |
| Lymphopenia | 14 (82.35%) | 11 (64.71%) | 3 (17.65%) | 0 (0%) |
| Hyperglycemia | 12 (70.59%) | 12 (70.59%) | 0 (0%) | 0 (0%) |
| Neutropenia | 12 (70.59%) | 4 (23.53%) | 7 (41.18%) | 1 (5.88%) |
| Fatigue | 11 (64.71%) | 10 (58.82%) | 1 (5.88%) | 0 (0%) |
| Seizure | 8 (47.06%) | 5 (29.41%) | 3 (17.65%) | 0 (0%) |
| Bradycardia | 8 (47.06%) | 8 (47.06%) | 0 (0%) | 0 (0%) |
| Alopecia | 7 (41.18%) | 7 (41.18%) | 0 (0%) | 0 (0%) |
| Dysphasia | 7 (41.18%) | 6 (35.29%) | 1 (5.88%) | 0 (0%) |
| Nausea | 7 (41.18%) | 7 (41.18%) | 0 (0%) | 0 (0%) |
| ALAT increased | 6 (35.29%) | 6 (35.29%) | 0 (0%) | 0 (0%) |
| ASAT increased | 6 (35.29%) | 6 (35.29%) | 0 (0%) | 0 (0%) |
| CNS abnormality, other |
6 (35.29%) | 6 (35.29%) | 0 (0%) | 0 (0%) |
| Thrombocytopenia | 6 (35.29%) | 6 (35.29%) | 0 (0%) | 0 (0%) |
| Scalp pain | 6 (35.29%) | 6 (35.29%) | 0 (0%) | 0 (0%) |
| Blurred vision | 5 (29.41%) | 5 (29.41%) | 0 (0%) | 0 (0%) |
| Dysesthesia | 5 (29.41%) | 5 (29.41%) | 0 (0%) | 0 (0%) |
| Insomnia | 4 (23.53%) | 4 (23.53%) | 0 (0%) | 0 (0%) |
| Muscle weakness upper limb |
4 (23.53%) | 4 (23.53%) | 0 (0%) | 0 (0%) |
| Sinus tachycardia | 4 (23.53%) | 4 (23.53%) | 0 (0%) | 0 (0%) |
| Weight loss | 4 (23.53%) | 4 (23.53%) | 0 (0%) | 0 (0%) |
| Alkaline phosphatase ↑ |
3 (17.65%) | 3 (17.65%) | 0 (0%) | 0 (0%) |
| Anorexia | 3 (17.65%) | 3 (17.65%) | 0 (0%) | 0 (0%) |
| Dizziness | 3 (17.65%) | 3 (17.65%) | 0 (0%) | 0 (0%) |
| Facial muscle weakness |
3 (17.65%) | 3 (17.65%) | 0 (0%) | 0 (0%) |
| Arthralgia | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Cognitive disturbance | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Depression | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Dysarthria | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Encephalopathy | 2 (11.76%) | 1 (5.88%) | 1 (5.88%) | 0 (0%) |
| Fever | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Hypercalcemia | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Hypokalemia | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Hyponatremia | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Hypophosphatemia | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Memory impairment | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Optic nerve disorder | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Paresthesia | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
| Somnolence | 2 (11.76%) | 2 (11.76%) | 0 (0%) | 0 (0%) |
BBB opening was demonstrated by comparing enhancement on pre-sonication (1-2 days before cycle 1) versus post-sonication contrast-enhanced MRI. This allowed identification of regions of the peri-tumoral brain where LIPU/MB led to BBB opening as evidenced by gadolinium-based enhancement seen in the brain parenchyma (Figure 1E and appendix p. 14). The SC9 can target an approximate brain volume of 53 ml, corresponding to 9 cylinders, each 10 mm in diameter and 75 mm in depth. The volume of peritumoral brain targeted that showed enhancement attributed to sonication (post-sonication MRI) ranged from 3.5 mL to 20.9 mL (median: 11.9 mL, interquartile range: 11. mL - 13.9 mL). This volume is variable as the region targeted by the ultrasound can contain resection cavity or tissue that enhances pre-sonication (tumor tissue or scar), which we subtracted from in the calculation of volume of brain with BBB opening.
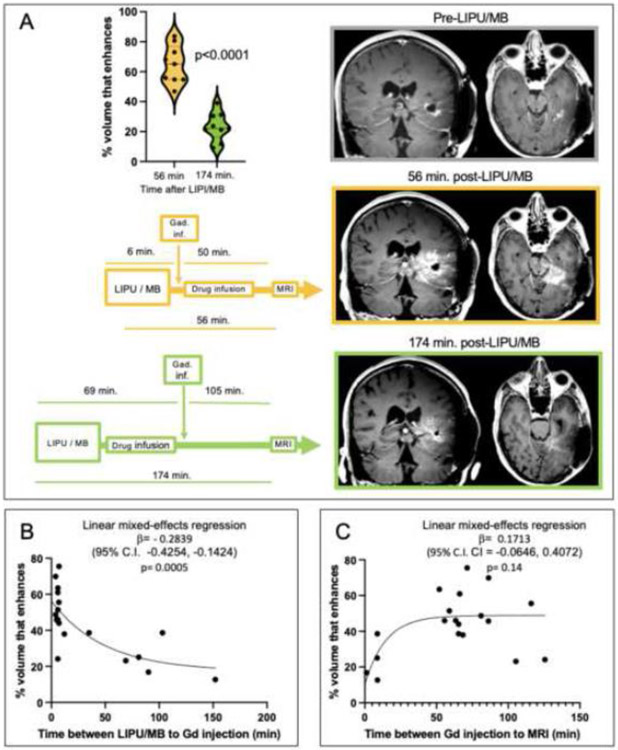
We investigated the timing of BBB restoration. Initially, post-sonication contrast MRI occurred after finishing ABX infusion, approximately 1 hour after LIPU/MB, and gadolinium was injected at the time of MRI acquisition (n= 4), leading to faint contrast enhancement of the area with BBB opening (appendix p.16). For subsequent patients (n= 13), gadolinium was injected within minutes after LIPU/MB, before MRI acquisition (appendix p.16). For a patient who received gadolinium infusion within 2 minutes of sonication and whose MRI showed robust enhancement of the sonicated brain in cycle 1, a post-sonication MRI after cycle 2 was repeated with a delay in gadolinium infusion (69 minutes) and MRI acquisition (174 minutes) from the time of LIPU/MB, which resulted in a decrease in the enhancement related to BBB opening (Figure 2A).
Figure 2. Effect of timing of brain sonication, gadolinium infusion and MRI on post-LIPU/MB brain enhancement.
(A) Schematic of timing of gadolinium infusion (Gad. inf) and MRI relative to LIPU/MB, MR images and enhancement quantification through a violin plot comparing enhancement of peri-tumoral brain that was targeted by each of the SC9 ultrasound probes (n=9) on images obtained with gadolinium infusion early (6 minutes after the beginning / 2 minutes after finishing LIPU/MB) after sonication, and subsequent MRI done after drug infusion (top) versus with deliberate delay of gadolinium infusion and MRI (bottom) on the same patient. P value was calculated using student’s two-tailed unpaired t-test. Scatter plot demonstrating relationship between time between sonication and Gd infusion (B) or time between Gd infusion and beginning of MRI (C) vs. enhancement of peri-tumoral brain targeted by SC9 ultrasound probes in 19 sonication cycles conducted in 17 patients. A one-phase exponential decay model was fitted to the data and strength of correlation was determined using a linear mixed-effects regression model. To quantify the % of sonicated brain volume with BBB opening i.e. enhancement after sonication, a region of interest within the brain that was targeted by each emitter that was not enhancing prior to sonication was used as the denominator. Images of LIPU/MB based BBB opening for all patients are available on appendix p. 14.
To characterize the rate of closure of the BBB after LIPU/MB (i.e. the loss of brain permeability to gadolinium over time), we evaluated time interval of sonication to gadolinium administration compared to the amount of enhancement of peri-tumoral brain that was targeted by the SC9 ultrasound emitters. We demonstrated an inverse correlation suggesting rapid restauration of the BBB within an hour of LIPU/MB. (Figure 2B). We investigated whether these results are influenced by the clearance of gadolinium from the brain, or time of gadolinium infusion to MRI versus enhancement of peri-tumoral brain that was targeted by the SC9 ultrasound probes. Yet these variables did not correlate (Figure 2C). The statistical analyses of these correlations were not pre-specified.
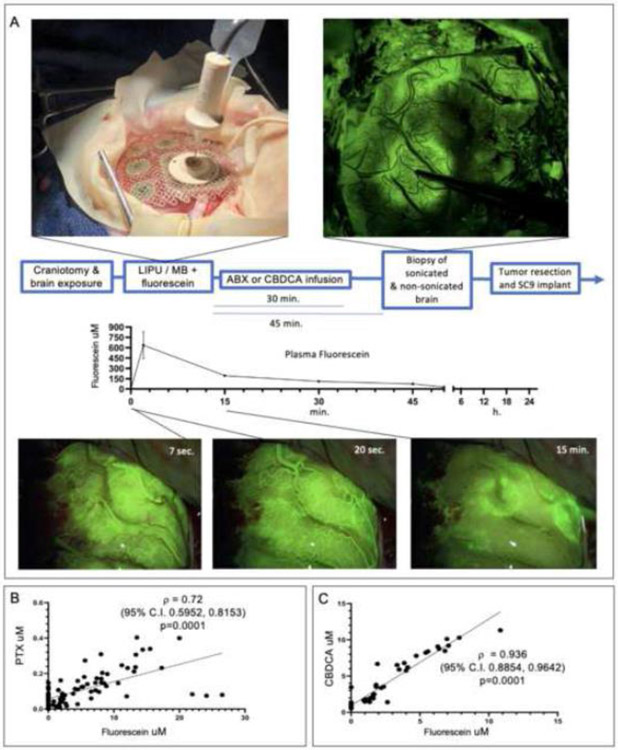
We performed pharmacokinetic studies that included sonication of non-enhancing peri-tumoral brain in 7 patients receiving intraoperative ABX, and in 3 patients receiving CBDCA. Concomitant administration of fluorescein allowed for dynamic visualization of LIPU/MB-based BBB opening (Supplementary Video and Figure 3A and appendix p. 15). Biopsies of sonicated and non-sonicated peri-tumoral brain for drug quantification were obtained approximately 45 minutes after sonication, after the peak plasma concentration of PTX or CBDCA (appendix p. 16). The brain parenchyma concentration of fluorescein correlated with that of ABX (Figure 3B), and with CBDCA (Figure 3C), similar to what we reported in pre-clinical models.(6)
Figure 3. Visualization of LIPU/MB-based BBB opening for intraoperative pharmacokinetic experiment in the peri-tumoral human brain.
(A) Schematic, representative intraoperative fluorescent-based microsurgical photographs illustrating how the LIPU/MB procedure was done for visualization of BBB opening using sodium fluorescein, and plasma clearance of this agent (n=10 patients). Scatter plots show correlation between PTX/ABX (n=7 patients, 81 biopsies, 41 sonicated and 40 non-sonicated) (B) and CBDCA (n=3 patients, 48 biopsies, 23 sonicated and 25 non-sonicated) (C) with fluorescein 45 minutes after LIPU/MB across biopsies of peri-tumoral brain. For A, error bars represent standard error of the mean. For B and C, Spearman correlation was computed, with two-tail p value reported.
LIPU/MB led to a several-times increase in chemotherapy concentration in the brain parenchyma (Figure 4A illustrates an example). For this patient, LIPU/MB led to a 9.3-times increase in the CBDCA concentration in the peri-tumoral brain (non-sonicated brain mean [CBDCA] 0.88 uM, standard deviation 0.38 uM, vs. sonicated brain mean [CBDCA] 8.21 uM, standard deviation 1.65 uM, Wilcoxon rank sum exact test p=0.016). LIPU/MB increased the absolute brain PTX concentration 3.7-times (non-sonicated brain mean [PTX] 0.0373 uM, 95% CI 0.0223 - 0.0625 uM vs. sonicated brain mean [PTX] 0.1386 uM, 95% CI 0.0828 - 0.2319 uM, p<0.0001, Figure 4B left). The brain/plasma ratio (B/P ratio) showed a 3.6-times increase for PTX by LIPU/MB (non-sonicated brain mean PTX B/P ratio 0.0485, 95% CI 0.0303 - 0.0775 vs. sonicated brain mean PTX B/P ratio 0.1735, 95% CI 0.1087 - 0.277, p=0.00013, Figure 4B center). LIPU/MB increased the absolute brain CBDCA concentration 5.9-times (non-sonicated brain mean [CBDCA] 0.9914 uM, 95% CI 0.5624 - 1.747 uM vs. sonicated brain mean [CBDCA] 5.878 uM, 95% CI 3.4622 - 9.98 uM, p=0.00012, Figure 4C left) and the CBDCA B/P ratio by 5.8-times (non-sonicated brain mean [CBDCA] B/P ratio 0.0204, 95% CI 0.0116 - 0.0360 vs. sonicated brain mean [CBDCA] B/P ratio 0.1177, 95% CI 0.0695 - 0.1994, p=0.0002, Figure 4C center).
We compared the PTX concentration in sonicated samples obtained from the subcortical white matter (brain mean [PTX] 0.15556 uM, 95% C.I. 0.06693, 0.36155 uM) versus sonicated superficial/ cortical biopsy sites (brain mean [PTX] 0.13363 uM, 95% C.I. 0.07702, 0.23186 uM) from 3 patients, but found no significant difference between these sites (data not shown, p=0.9).
To rule out that the differences in concentration between sonicated and non-sonicated brain samples could relate to blood-contamination, in a subset of the samples we compared the percentage of hemoglobin content between sonicated and non-sonicated peri-tumoral brain but found no significant differences between these samples in the case of PTX (Figure 4B right) or CBDCA (Figure 4C right).
As of the date of data cutoff, 10 of the 17 patients died of disease progression. In a post-hoc descriptive analysis, the median progression-free survival was 2.9 months (95% C.I. 2.7, 4.6 months) and overall survival was 11 months (95% C.I. 7.95, not reached). Most patients discontinued treatment due to progression except patients 110 and 111 Kaplan-Meier and swimmer’s plots summarizing per-patient timeline of treatment and outcomes are included in the appendix p. 17.
Discussion:
Our study shows that LIPU/MB can effectively enhance the delivery of ABX and CBDCA across the BBB into the human brain, and in the case of ABX, that this can be done safely. The safety of repeated LIPU/MB with skull implantable systems has been reported, yet in our study the brain sonication field is larger 9-times larger than initial pilot studies using a single 1 ultrasound emitter.(10) We achieved BBB opening in deep, critical brain structures such as the thalamus and basal ganglia. We sonicated every 3 weeks as per the established schedule for ABX 260 mg/m2. Whereas LIPU/MB has been done every 2 weeks in Alzheimer’s,(15) too frequent administration (e.g. daily) could lead to skin breakdown at puncture site. The reproducibility of BBB opening over cycles by LIPU/MB has been previously reported.(14) Moreover, the safety of LIPU/MB-based BBB opening using transcranial devices has also been demonstrated (12, 13, 19) supporting the feasibility of this approach.
We escalated the ABX dose to 260 mg/m2, the approved regimen for metastatic breast cancer.(20) While we observed dose-dependent encephalopathy, a known rare side effect of ABX reported in its label, this was reversible and treatments were continued. Overall, we confirmed our preclinical observation that enhancing the brain delivery of ABX with LIPU/MB is well tolerated.(6)
Pharmacokinetic studies performed shortly after LIPU/MB demonstrated the effect of BBB opening on drug concentrations in the human brain. Our results are in line with preclinical studies reporting that brain drug penetration following LIPU/MB is influenced by the molecular weight (MW).(21) LIPU/MB increased CBDCA (MW 371 g/mol) brain/plasma ratio 5.8-times, while the increase seen with PTX (MW 853 g/mol) was 3.6-times. We observed a tighter correlation between CBDCA and fluorescein than with between PTX and fluorescein brain concentrations. In line with this, the MW of fluorescein (412 g/mol) is similar to CBDCA.
Preclinical studies cannot inform whether LIPU/MB would lead to meaningful concentrations of circulating drugs in the human brain, as dosing of drugs and MB, infusion rates, biodistribution, sonication parameters, as well as drug clearance vary across species. Measurement of absolute drug concentrations in the human brain following LIPU/MB is particularly important in gliomas, as the peri-tumoral brain where the BBB is intact BBB, is infiltrated by tumor glioma cells.
We recently reported an analysis of human glioma susceptibility to PTX.(5) In this study, half of the cell lines were resistant to PTX (mean IC50 = 1.6 uM) and half were susceptible (mean IC50=0.025 uM), offering an approximation of meaningful PTX concentration. Our pharmacokinetic studies were performed with ABX doses of 40-80 mg/m2, leading to a mean parenchymal concentration of PTX of 0.1386 uM in the sonicated brain. Considering that 260 mg/m2 is 3 to 6-times higher than the intraoperative doses we used, and that the PTX plasma concentrations increase proportional to ABX dose(22), our results indicate that LIPU/MB with concomitant ABX infusion leads to concentrations that are cytotoxic for half of human glioma cell lines.
Our results shed light into the rate of restoration of BBB integrity after LIPU/MB. Previous human studies reported the restoration of the BBB integrity by 24 hours after sonication.(23-25) Preclinical studies showed that BBB repair starts shortly after LIPU/MB, and is completed within 6 hours.(26, 27) In contrast, our analyses suggest that most of BBB integrity is restored within the first hour after LIPU/MB. This is important as delay in drug administration after LIPU/MB will lead to peak plasma levels when the BBB is largely restored, limiting agent penetration into the brain. The temporal dynamics for BBB repair are complex, and vary depending on the LIPU/MB technology i.e. sonication parameters used,(27) as well as the molecular characteristics of the drug.(28) Thus, animal modeling might be unreliable to optimize the timing of LIPU/MB procedure relative to drug infusion in patients.
There are limitations of our image-based temporal analysis of BBB closure. Enhancement might not exhibit a linear relationship to gadolinium concentration, and permeability of gadolinium might not be representative of that for other molecules. In addition, we did not characterize the decay in post-sonication enhancement past 150 minutes.
The SC9 can target a brain volume of approximately 53 ml. While this is considerably larger than previous skull implantable devices,(10) this volume might not be sufficient to achieve efficacy for large tumors, as sonication of a large portion of peri-tumoral brain coverage is required. Other limitations of the SC9 device include the fixed field of sonication and the need for percutaneous connection of the device, which might limit the frequency of LIPU/MB.
Our study has several limitations, and important pharmacokinetic questions remain unanswered. The temporal and spatial dynamics of drug accumulation, dispersion and clearance in the human brain following LIPU/MB, as well as characterization of the effect of this procedure on drug concentrations in tumor tissue remain largely unexplored. Preclinical studies suggest that LIPU/MB can enhance the delivery of drugs into the tumor core, and stabilize drug levels for longer in this compartment.(29) Our trial results have led to the investigation of LIPU/MB to deliver ABX plus CBDCA for GBM in an ongoing phase II clinical trial we are conducting (NCT04528680). Along with several other reports, our findings support the feasibility of LIPU/MB to effectively bypass the BBB and treat diseases in the brain, an organ that is beyond the reach for many pharmacological agents.
Supplementary Material
Supplementary Video. Video shows human brain (right frontal lobe) following intraoperative LIPU/MB, at time of fluorescein injection, illustrating BBB opening.
Research in context.
Evidence before this study
The blood-brain barrier (BBB) remains a major challenge for treatment of malignant gliomas. This disease is characterized by the presence of unresectable clusters of tumor cells that infiltrate into the peri-tumoral human brain, where the BBB limits the penetration of most chemotherapeutic drugs. Low-intensity pulsed ultrasound, combined with circulating microbubbles (LIPU/MB), is an emerging approach to transiently open the BBB for drug delivery. We performed a search in PubMed using the terms “ultrasound”, “blood-brain barrier” and "clinical trial" within the title or abstract, leading to 28 articles published between 2003 and February 16th, 2023. We also performed a search on clinicaltrials.gov using the terms “glioma” for condition, “ultrasound” for intervention and “drug” as other term, to identify trials evaluating this approach registered as of February 9th, 2023. We found 21 clinical trials, some of which reported outcomes supporting the safety of LIPU/MB for BBB opening in humans. These studies demonstrated BBB opening by MRI or single-photon emission computerized tomography (SPECT) on a limited volume of brain sonication. The direct effect of LIPU/MB on drug concentrations in the human brain shortly after LIPU/MB procedure has not yet been described. Moreover, the rate of BBB integrity restoration within the first few hours after LIPU/MB, information that is critical for delivering systemic drugs to the brain with this approach, was not reported in humans.
Added value of this study
Our study provides data on the safety of performing LIPU/MB-based on large areas of the brain, in the context of delivery of albumin-bound paclitaxel. This drug has poor distribution in the human brain and is associated with (peripheral) neurotoxicity. In contrast, we report that the delivery of this drug across the BBB is safe and overall, well tolerated. The results of pharmacokinetic studies are direct evidence of drug penetration into the human brain following this procedure and provide an insight into the magnitude drug brain permeability achieved for two different cytotoxic agents, allowing initial observations on how drug size might exhibit distinct brain permeability following LIPU/MB. We also characterize the timing of BBB integrity restoration following LIPU/MB, elucidating a critical time window for delivery of systemic drugs to the brain using this approach.
Implications of all the available evidence
This study provides the first direct evidence that LIPU/MB increases the brain concentration of systemically administered drugs by multiple fold. We report that large volume BBB opening is safe, reproducible, and can be repeated over multiple cycles of chemotherapy. Thus, large size drugs that previously were not used for gliomas, can now be considered for the treatment of diseases in the brain, in this case glioblastoma. Whereas the approach of opening the BBB with ultrasound-activated microbubbles is under investigation using various technologies, our study indicates that the BBB closes rapidly after LIPU/MB, a factor that must be considered to optimize timing of drug infusion relative to LIPU/MB to accomplish robust drug penetration into the human brain.
Acknowledgments:
This work is funded by the NIH/NCI 1R01CA245969-01A1 (A.M. Sonabend and R. Stupp), NIH/NCI P50CA221747 SPORE for Translational Approaches to Brain Cancer (M.S. Lesniak), as well as generous philanthropic support from the Moceri Family Foundation and the Panattoni family. In-kind support was provided by Carthera and BMS for devices and drug respectively. We are grateful for the work of Blake Nichting and Mark Senesac from Brainlab for the kind assistance related to intraoperative stereotaxic registration of brain biopsies related to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures:
A.M. Sonabend and R. Stupp have received in-kind (drug) support from BMS, in kind (ultrasound devices) and research support from and Carthera, and in kind (drug) and research support from Agenus. A.M. Sonabend, D.Y. Zhang, V.A. Arrieta and R. Stupp are co-authors of IP filed by Northwestern University related to therapeutic ultrasound. R. Stupp has acted or is acting as a scientific advisor or has served on advisory boards for the following companies: Alpheus Medical (formerly Craniovation), AstraZeneca, Boston Scientific, Carthera, Celularity, GT Medical, Insightec, Lockwood (BlackDiamond), Northwest Biotherapeutics, Novocure, Inc., Syneos Health (Boston Biomedical), TriAct Therapeutics, Varian Medical Systems. R.V. Lukas is on the Scientific Advisory Board and Speakers' bureau for Merck, on the Speakers’ bureau for Novocure, and has obtained research support from BMS. He received honoraria for editing for EBSCO, Medlink, Neurology and Elsevier. P. Kumthekar participates in advisory boards for Novocure, Janssen, SDP Oncology, Affinia, Sintetica, Mirati. She has done consulting for Biocept, Enclear Therapies, Affinia Therapeutics and Bioclinica. She has received research support from Genentech and Novocure. M. Canney, C. Dessaux, G. Bouchoux, and A. Carpentier are employees of Carthera, inventors of patents related to the technology and/or have stock ownership in Carthera. A. Carpentier has received funding support from H2020 European Innovation council, is a paid consultant of Carthera, and is part of the Board of Directors of Carthera. J. Bebawy is Vice Chair of the Neuro Education Track Subcommittee from the American Society of Anesthesiologists.
Data sharing:
The trial protocol is provided in the appendix. De-identified data from this study can be made available upon request and approval by the study management committee and subject to appropriate data transfer agreements. Requests should be directed to AMS.
References
- 1.Pitz MW, Desai A, Grossman SA, Blakeley JO. Tissue concentration of systemically administered antineoplastic agents in human brain tumors. J Neurooncol. 2011;104(3):629–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldape K, Brindle KM, Chesler L, Chopra R, Gajjar A, Gilbert MR, et al. Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rapp M, Baernreuther J, Turowski B, Steiger HJ, Sabel M, Kamp MA. Recurrence Pattern Analysis of Primary Glioblastoma. World Neurosurg. 2017;103:733–40. [DOI] [PubMed] [Google Scholar]
- 4.Wick W, Stupp R, Beule AC, Bromberg J, Wick A, Ernemann U, et al. A novel tool to analyze MRI recurrence patterns in glioblastoma. Neuro Oncol. 2008;10(6):1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dmello C, Sonabend A, Arrieta VA, Zhang DY, Kanojia D, Chen L, et al. Translocon-associated protein subunit SSR3 determines and predicts susceptibility to paclitaxel in breast cancer and glioblastoma. Clin Cancer Res. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang DY, Dmello C, Chen L, Arrieta VA, Gonzalez-Buendia E, Kane JR, et al. Ultrasound-mediated Delivery of Paclitaxel for Glioma: A Comparative Study of Distribution, Toxicity, and Efficacy of Albumin-bound Versus Cremophor Formulations. Clin Cancer Res. 2020;26(2):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heimans JJ, Vermorken JB, Wolbers JG, Eeltink CM, Meijer OW, Taphoorn MJ, et al. Paclitaxel (Taxol) concentrations in brain tumor tissue. Ann Oncol. 1994;5(10):951–3. [DOI] [PubMed] [Google Scholar]
- 8.Chang SM, Kuhn JG, Robins HI, Schold SC Jr., Spence AM, Berger MS, et al. A Phase II study of paclitaxel in patients with recurrent malignant glioma using different doses depending upon the concomitant use of anticonvulsants: a North American Brain Tumor Consortium report. Cancer. 2001;91(2):417–22. [DOI] [PubMed] [Google Scholar]
- 9.Chamberlain MC, Kormanik P. Salvage chemotherapy with paclitaxel for recurrent primary brain tumors. J Clin Oncol. 1995;13(8):2066–71. [DOI] [PubMed] [Google Scholar]
- 10.Idbaih A, Canney M, Belin L, Desseaux C, Vignot A, Bouchoux G, et al. Safety and Feasibility of Repeated and Transient Blood-Brain Barrier Disruption by Pulsed Ultrasound in Patients with Recurrent Glioblastoma. Clin Cancer Res. 2019;25(13):3793–801. [DOI] [PubMed] [Google Scholar]
- 11.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. MRI-guided targeted blood-brain barrier disruption with focused ultrasound: histological findings in rabbits. Ultrasound Med Biol. 2005;31(11):1527–37. [DOI] [PubMed] [Google Scholar]
- 12.Meng Y, Reilly RM, Pezo RC, Trudeau M, Sahgal A, Singnurkar A, et al. MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases. Sci Transl Med. 2021;13(615):eabj4011. [DOI] [PubMed] [Google Scholar]
- 13.Sonabend AM, Stupp R. Overcoming the Blood-Brain Barrier with an Implantable Ultrasound Device. Clin Cancer Res. 2019;25(13):3750–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carpentier A, Canney M, Vignot A, Reina V, Beccaria K, Horodyckid C, et al. Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med. 2016;8(343):343re2. [DOI] [PubMed] [Google Scholar]
- 15.Epelbaum S, Burgos N, Canney M, Matthews D, Houot M, Santin MD, et al. Pilot study of repeated blood-brain barrier disruption in patients with mild Alzheimer's disease with an implantable ultrasound device. Alzheimers Res Ther. 2022;14(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. 2019;10(1):4373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park SH, Kim MJ, Jung HH, Chang WS, Choi HS, Rachmilevitch I, et al. Safety and feasibility of multiple blood-brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg. 2020:1–9. [DOI] [PubMed] [Google Scholar]
- 18.Asquier N, Bouchoux G, Canney M, Martin C, Law-Ye B, Leclercq D, et al. Blood-brain barrier disruption in humans using an implantable ultrasound device: quantification with MR images and correlation with local acoustic pressure. J Neurosurg. 2019;132(3):875–83. [DOI] [PubMed] [Google Scholar]
- 19.Rezai AR, Ranjan M, D'Haese PF, Haut MW, Carpenter J, Najib U, et al. Noninvasive hippocampal blood-brain barrier opening in Alzheimer's disease with focused ultrasound. Proc Natl Acad Sci U S A. 2020;117(17):9180–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gradishar WJ, Tjulandin S, Davidson N, Shaw H, Desai N, Bhar P, et al. Phase III trial of nanoparticle albumin-bound paclitaxel compared with polyethylated castor oil-based paclitaxel in women with breast cancer. J Clin Oncol. 2005;23(31):7794–803. [DOI] [PubMed] [Google Scholar]
- 21.Choi JJ, Wang S, Tung YS, Morrison B, 3rd, Konofagou EE. Molecules of various pharmacologically-relevant sizes can cross the ultrasound-induced blood-brain barrier opening in vivo. Ultrasound Med Biol. 2010;36(1):58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ibrahim NK, Desai N, Legha S, Soon-Shiong P, Theriault RL, Rivera E, et al. Phase I and pharmacokinetic study of ABI-007, a Cremophor-free, protein-stabilized, nanoparticle formulation of paclitaxel. Clin Cancer Res. 2002;8(5):1038–44. [PubMed] [Google Scholar]
- 23.Mainprize T, Lipsman N, Huang Y, Meng Y, Bethune A, Ironside S, et al. Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study. Sci Rep. 2019;9(1):321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gasca-Salas C, Fernandez-Rodriguez B, Pineda-Pardo JA, Rodriguez-Rojas R, Obeso I, Hernandez-Fernandez F, et al. Blood-brain barrier opening with focused ultrasound in Parkinson's disease dementia. Nat Commun. 2021;12(1):779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, et al. Blood-brain barrier opening in Alzheimer's disease using MR-guided focused ultrasound. Nat Commun. 2018;9(1):2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Reilly MA, Hough O, Hynynen K. Blood-Brain Barrier Closure Time After Controlled Ultrasound-Induced Opening Is Independent of Opening Volume. J Ultrasound Med. 2017;36(3):475–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu PC, Chai WY, Tsai CH, Kang ST, Yeh CK, Liu HL. Focused Ultrasound-Induced Blood-Brain Barrier Opening: Association with Mechanical Index and Cavitation Index Analyzed by Dynamic Contrast-Enhanced Magnetic-Resonance Imaging. Sci Rep. 2016;6:33264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marty B, Larrat B, Van Landeghem M, Robic C, Robert P, Port M, et al. Dynamic study of blood-brain barrier closure after its disruption using ultrasound: a quantitative analysis. J Cereb Blood Flow Metab. 2012;32(10):1948–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park J, Aryal M, Vykhodtseva N, Zhang YZ, McDannold N. Evaluation of permeability, doxorubicin delivery, and drug retention in a rat brain tumor model after ultrasound-induced blood-tumor barrier disruption. J Control Release. 2017;250:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video. Video shows human brain (right frontal lobe) following intraoperative LIPU/MB, at time of fluorescein injection, illustrating BBB opening.
Data Availability Statement
The trial protocol is provided in the appendix. De-identified data from this study can be made available upon request and approval by the study management committee and subject to appropriate data transfer agreements. Requests should be directed to AMS.