Abstract
This study aimed to utilize wastewater surveillance for monitoring Mpox cases at a community level. Untreated wastewater samples were collected once a week from two wastewater treatment plants (A and B) in Baltimore City from July 27, 2022–September 22, 2022. The samples were concentrated via an adsorption–elution (AE) method and Polyethylene Glycol (PEG) precipitation method followed by quantitative polymerase chain reaction (qPCR). Monkeypox virus (MPXV) was detected in 89 % (8/9) samples from WWTP A and 55 % (5/9) samples from WWTP B with at least one concentration method. Higher detection rate in samples concentrated with PEG precipitation compared to AE method was observed, indicating that PEG precipitation is a more effective virus concentration method for MPXV. To our knowledge, this is the first study reporting the detection of MPXV in wastewater in Baltimore. The results highlight that wastewater surveillance could be used as a complementary early warning tool for monitoring future Mpox outbreaks.
Keywords: Adsorption-elution, Monkeypox, PEG precipitation, Wastewater-based epidemiology
Graphical abstract
1. Introduction
Mpox (formerly Monkeypox), a rare disease characterized by fever, intense, headache, lymphadenopathy, and rashes on the face and extremities that evolves into macules, papules, vesicles, pustules and crusts, is endemic in Central and West African countries (WHO, 2023). Mpox virus (MPXV), the causative agent of Mpox, is an enveloped, double-stranded DNA virus, approximately 197 kb long, belonging to Orthopoxvirus genus of the Poxviridae family. Previously, Mpox was limited to Africa only and sporadic cases outside of Africa were either travel-related or related to contact with infected-pets. However, recently in May 2022, Mpox cases were rapidly reported in regions that have historically never reported Mpox. This resulted in declaration of Mpox as a “public health emergency of international concern” by the WHO in July 2022. As of March 29, 2023, a total of 86,746 confirmed cases around the world (CDC, 2022) has been reported.
MPXV primarily spreads through direct contact with infected individuals with an active rash, open pustules or lesions, or by contaminated fomites or respiratory droplets or vertical transmission from an infected parent to child. In addition to infectious scabs and crusts, MPXV can also be released into wastewater due to shedding via urine, semen, saliva, and feces (Antinori et al., 2022; Towns et al., 2023; Peiró-Mestres et al., 2022; Maal-Bared et al., 2022; Tiwari et al., 2023). Based on this principle, wastewater-based epidemiology (WBE) has now been used to advance our understanding of the emergence and epidemiology of MPXV around the world (Wannigama et al., 2023). Recently, MPXV DNA has been detected in wastewater in Amsterdam, Netherlands (de Jonge et al., 2022), Poznan, Poland (Gazecka et al., 2023), Bangkok, Thailand (Wannigama et al., 2023), Paris, France (Wurtzer et al., 2022), Rome, Italy (La Rosa et al., 2023), and multiple cities of Spain (Girón-Guzmán et al., 2023). The USA was the hardest hit country with ~35 % of the total global cases with 38 deaths as of March 29, 2023 (CDC, 2022). Despite the outbreak of Mpox in 52 different locations, complementary WBE studies to clinical surveillance has only been carried out in San Francisco, California (Wolfe et al., 2023) and Miami-Dade County, Florida (Sharkey et al., 2023).
In the State of Maryland, the first case of Mpox was recorded on June 6, 2022. To date, 743 confirmed Mpox cases and 3 deaths have been reported with the highest number of cases (~33 %) in Baltimore City. Therefore, this study aimed to investigate the presence of MPXV DNA in wastewaters in Baltimore City, Maryland, USA using two concentration methods followed by quantitative polymerase chain reaction (qPCR). To our knowledge, this is the first study reporting the detection of MPXV in wastewater in Baltimore.
2. Materials and methods
A total of 18 wastewater samples were collected once a week from two wastewater treatment plants (WWTP) (A and B; 9 samples each), located in Baltimore from July 7 to September 22, 2022. The sampling dates corresponds to weeks 30–38 of 2022. Grab samples were collected in the morning (7–11 AM) from WWTP A whereas, 24 h composite samples were collected using an autosampler from WWTP B.. The WWTPs A and B serve a population of approximately 450,000 and 1.3 million, respectively. One liter of untreated raw sewage was collected in sterile 1 L Nalgene bottles, transported on ice to the laboratory, and processed within 6 h of sample collection.
Two virus concentration methods, namely adsorption–elution (AE) and polyethylene glycol (PEG) precipitation, were used to concentrate MPXV in wastewater. The two methods were selected because AE method involving filtration of wastewater treated with magnesium chloride through an electronegative membrane has been demonstrated to be very effective in concentrating enveloped virus surrogates (Ahmed et al., 2020) whereas, PEG precipitation is an economical method that is feasible for use in low and middle-income countries. AE method was performed as described previously (Sherchan et al., 2020) with use of Amicon Ultra-15 (Merck Millipore, Billerica, USA) filter instead of Centriprep YM-30 (Merck Millipore) as a slight modification. Briefly, 2.5 M MgCl2 was added to all samples (100 mL influent and 750 mL secondary treated and final effluent) to obtain a final concentration of 25 mM MgCl2. Samples were subsequently passed through an electronegative filter (90-mm diameter and 0.45-μm pore size; Merck Millipore, Billerica, USA; Catalog no. HAWP-09000) attached to a glass filter holder (Advantec, Tokyo, Japan). Magnesium ions were then removed by the passage of 200 mL of 0.5 mM H2SO4 (pH 3.0) through the filter, and the viruses were eluted with 10 mL of 1.0 mM NaOH (pH 10.8). The eluate was recovered in a tube containing 50 μL of 100 mM H2SO4 and 100 μL of 100× Tris-EDTA buffer for neutralization. 10 mL was then centrifuged and supernatant was processed using an Amicon filter (Merck Millipore) to obtain a final volume of approximately 650 μL. In PEG precipitation method, 40-mL of wastewater was mixed with 4.0 g of PEG8000 and 0.94 g of NaCl. to obtain the final concentrations of 10 % (w/v) and 0.4 M, respectively, followed by vortex mixing for 10 min (Malla et al., 2022). The mixture was centrifuged at 12,000 ×g for 100 min at 4 °C without incubation. The supernatant was discarded, leaving approximately 5 mL of the mixture in the tube. The mixture was recentrifuged at 12,000 ×g for 5 min at 4 °C. After carefully discarding the supernatant, the resulting precipitate was resuspended with 600 μL of PCR-grade water.
Viral DNA was extracted using Allprep PowerViral DNA/RNA kit (QIAGEN, MD, USA) to obtain a final volume of 100 μL DNA, according to the manufacturer's protocol. qPCR assay for MPXV was performed with a CFX96 Real-Time PCR Instrument (BioRad Laboratories, Hercules, CA). Reaction mixtures (25 μL) consisted of 12.5 μL of PerfecTa qPCR ToughMix (Quantabio, Beverly, MA), 500 nM each forward primer (5′- GGAAAATGTAAAGACAACGAATACAG), reverse primer (5′- GCTATCACATAATCTGGAAGCGTA) and 400 nM probe (/56-FAM/AAGCCGTAATCTATGTTGTCTATCGTGTCC/3BHQ_1/), and 2.5 μL of DNA template as described by the CDC assay (Li et al., 2010). The qPCR cycling conditions was 95 °C for 6 min followed by amplification consisting of 45 cycles of 95 °C for 5 s and at 60 °C for 20 s. Serial ten-fold dilutions of the gBlocks for MPXV, obtained from IDT (Coralville, IA) were used to produce standard curves. Molecular biology grade water was used as non-template controls. Negative and positive controls were included in each qPCR run and all samples were tested in duplicate.
3. Results and discussion
3.1. Detection of Mpox DNA in wastewater samples
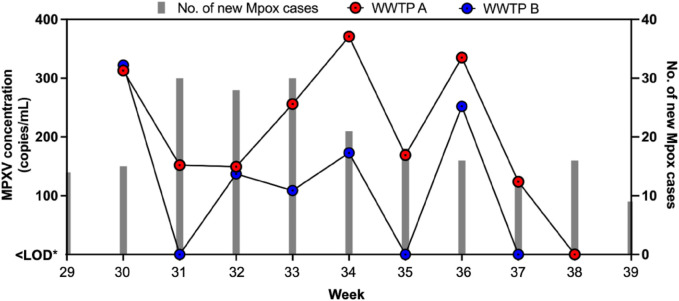
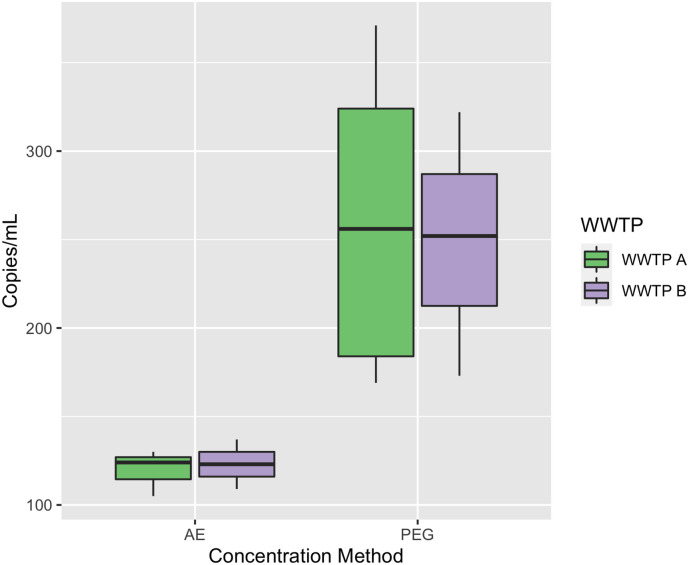
Fig. 1 summarizes the concentration of MPXV in wastewater collected in this study. Overall, MPXV was detected in 72 % (13/18) of the total wastewater samples analyzed by at least one virus concentrating method. Effective virus concentrating methods are critical to detect low viral levels in wastewater and our findings show the applicability of the two virus concentration methods for wastewater surveillance of Mpox. Recent studies have used several virus concentration methods to recover MPXV from wastewater. For instances, the research team in Thailand analyzed grab samples by using a 100 kDa Centricon® Plus-70 centrifugal ultrafiltration device to recover MPXV from diverse set of wastewaters while Wolfe et al., 2023 utilized magnetic hydrogel Nanotrap particles for concentrating viruses from wastewater solids. In the Netherlands, de Jonge et al. (2022) tested composite samples concentrated with centrifugation for detection of MPXV in wastewater. Sharkey et al. (2023) utilized the adsorption-extraction method using electronegative membrane and successfully recovered MPXV in grab and composite wastewater in Miami, USA. Girón-Guzmán et al. (2023), used an aluminum hydroxide adsorption-precipitation method for the detection of MPXV DNA in collected grab wastewaters in Spain. Similar to the concentration method used in this study, La Rosa et al. (2023) also utilized a modified PEG precipitation method to process 90 mL of wastewater and reported that 3 out of 20 samples tested positive for MPXV in Italy. Also, comparison between the two methods in this study revealed 10/18 and 5/18 samples to be positive for MPXV using PEG precipitation and AE methods, respectively. The high detection rate in samples concentrated with PEG precipitation indicates PEG precipitation is a more effective virus concentrating method compared to AE method for MPXV. This can be explained by the propensity of enveloped viruses such as MPXV to partition to solid fraction of wastewater and the ability of our protocol of PEG precipitation to analyze both solid and liquid fractions of wastewater. On a per mass basis, concentration of MPXV have also been shown to be higher in wastewater solids compared to wastewater liquid (Wolfe et al., 2023). It is also likely that MPXV loss might have occurred when pellet was discarded and only the supernatant fraction was used for ultrafiltration using an Amicon filter in AE method. At present, there is no standard concentration method yet for sensitive detection of MPXV in wastewater. Therefore, we strongly recommend the use of the PEG protocol as an economic alternative method for future WBE studies in endemic regions to identify Mpox outbreaks. Interestingly, WWTP A grab samples (8/9) had higher detection ratio compared to the WWTP B composite samples (5/9) in this study (Fig. 2 ). This finding indicates higher number of Mpox cases in regions covered by WWTP A compared to WWTP B and also shows that grab samplings when conducted in a particular timeframe that is likely to have high influx of wastewater can be equally effective for WBE of Mpox.
Fig. 1.
Concentration of MPXV in wastewater collected in different weeks and number of new Mpox cases in Baltimore.
Fig. 2.
Wastewater mpox viral DNA concentration.
Currently, most molecular assays for detection of MPXV in wastewater varies among testing laboratories. We used the CDC established qPCR assays (Li et al., 2010) following the two concentration methods for MPXV detection in wastewater. MPXV has been detected in wastewater using the same assays in Spain (Girón-Guzmán et al., 2023), Netherlands (de Jonge et al., 2022), USA (Wolfe et al., 2023), Thailand (Wannigama et al., 2023), Italy (La Rosa et al., 2023), Canada (Mejia et al., 2022), whereas commercially available qPCR TaqMan-specific probes and primers were utilized in France (Wurtzer et al., 2022) and Poland (Gazecka et al., 2023). Mejia et al. (2022) designed a novel qPCR assay and reported increased sensitivity in detecting MPXV. Further research must include testing the specificity, sensitivity, and accuracy of these MPXV assays as MPXV are prone to mutation at regions targeted by different assays.
3.2. Comparison between MPXV concentration and reported Mpox cases
Pearson's correlation analysis was performed to test for any relationship between wastewater concentration of MPXV and the number of new Mpox cases in Baltimore using Microsoft Office Excel 2019 (Microsoft Corporation, Redmond, WA, USA). The number of new Mpox cases per week was obtained from the Maryland Department of Health. No correlation was observed between MPXV concentration in wastewater collected from two WWTP and the number of new Mpox cases in Baltimore. This finding is in contrast to reports from France and USA (Wurtzer et al., 2022; Wolfe et al., 2023) that reported strong correlation between wastewater concentration of MPXV and new Mpox cases (Table 1 ). Due to stigma associated with getting infected with MPXV, it is highly likely that the number of Mpox cases are underreported. Furthermore, several factors such as decay of MPXV and differences in shedding pattern may have also impacted the concentration of MPXV in wastewater.
Table 1.
Published reports on MPXV detection in wastewater.
| Country | Sample Type | Concentration Method | Nucleic Acid Extraction kit | Molecular Assays | Findings | Reference |
|---|---|---|---|---|---|---|
| Baltimore,USA | Grab and 24-h composite | Polyethylene glycol precipitation and Adsorption-elution using an electronegative filter membrane | All prep PowerViral RNA/DNA kit | CDC assays G2R_G and G2R_WA. | MPXV was detected in 72 % (13/18) of the total wastewater samples. PEG precipitation method performed better compared to AE method. No correlation between wastewater concentration of MPXV and new Mpox cases was observed. |
This study |
| California, USA | 24-h composite influent and settled solids | For solids, suspends in buffer and homogenization For liquids, magnetic hydrogel Nanotrap particles |
Chemagic Viral DNA/RNA 300 Kit (PerkinElmer, Waltham, MA) and Zymo OneStep-96 PCR Inhibitor Removal kit (Zymo Research, Irvine, CA) | CDC assays G2R_G and G2R_WA using Droplet digital PCR |
Concentration of MPXV is significantly higher in wastewater solids compared to liquid fraction on a per mass basis. MPXV DNA was detected in wastewater samples across all sites. No difference in the ability to detect MPXV was observed between G2R_G and G2R-WA assays. Positive correlation between MPXV in wastewater solids and the incidence of reported cases at the sites was observed. |
Wolfe et al., 2023 |
| Miami USA |
Grab and composite samples | electronegative filtration | Zymo Research Quick-RNA Viral Kit | Assay targeting CrmB region of MPXV genome. | MPXV DNA was detected in concentrated wastewater collected weekly from the Miami-Dade Central District Wastewater Treatment Plant. It was first detected in July 2022 and positivity increased during the study period. | Sharkey et al., 2023 |
| Canada | 24-h composite samples | Centrifugation | MagAttract PowerMicrobiome DNA/RNA (Qiagen) |
CDC G2R assays (G2R_WA and G2R_G) and in-house-developed assay (G2R_NML). | Development and application of G2R_NML assay for Mpox surveillance. The positivity rate for G2R_G, G2R_WA, and G2R_NML assays was found to be 16 %, 28 % and 76 %, respectively. | Mejia et al., 2022 |
| France | 24-h composite samples | Centrifugation | PowerFecal Pro kit (#938036, QIAGEN, France) | MPXV TaqMan assay (20× concentrate) (#Vi07922155_s1, ThermoFisher Scientific, France) Qiacuity and Quantstudio |
MPXV concentrations ranged between ∼2000 and ∼40,000 copies/L in positive samples. Positive correlation between MPXV concentration in wastewater and the weekly new Mpox cases was observed. |
Wurtzer et al., 2022 |
| Netherlands | 24-h flow proportional | Centrifugation Processed volume: 25 mL |
DNeasy blood and tissue kit (Qiagen, Hilden, Germany) | CDC Assays (Li et al., 2010) | MPXV DNA was detected in 45/108 (42 %) wastewater samples between 16 May–3 July 2022. | de Jonge et al., 2022 |
| Italy | 24-h composite wastewater samples | Polyethylene glycol/sodium chloride precipitation protocol Processed volume 90 mL |
NucliSens miniMAG (bioMérieux, https://www.biomerieux.com) and OneStep PCR Inhibitor Removal Kit (Zymo Research) |
N3R, F3L, and CDC assay G2R_G. |
3/20 samples tested positive for MPXV. |
La Rosa et al., 2023 |
| Thailand | Grab | Centricon® Plus-70 centrifugal ultrafilter (100 kDa cut-off) (Merck Millipore) | DNeasy PowerSoil Pro Kit, Qiagen | CDC assays G2R_G and G2R_WA. |
4.76 % to 9.52 % sample positivity increase within a month | Wannigama et al., 2023 |
| Spain | Grab | Aluminum adsorption-precipitation method | Maxwell® RSC Instrument (Promega) using the Maxwell RSC Pure Food GMO and authentication kit (Promega) | CDC G2R_G and G2R_WA assays. |
56/312 samples tested positive for MPXV | Girón-Guzmán et al., 2023 |
| Poland | NA | NA | NA | Commercially available qPCR TaqMan-specific probes and primers (Vi07922155_s1; ThermoFisher) | MPXV was detected for several weeks at different treatment plants. | Gazecka et al., 2023 |
In summary, we detected MPXV in untreated wastewater samples from two WWTP in Baltimore. This is the first proof of concept study that reports the detection of MPXV in wastewater using PEG precipitation and AE concentration methods; however, PEG precipitation was deemed to be more effective in detecting MPXV. MPXV concentration in wastewater did not correlate with the number of new Mpox cases in this study. Further studies are needed to optimize concentration methods and qPCR assays to improve sensitivity in detecting MPXV in wastewater so that WBE approach can be utilized for sentinel surveillance of Mpox in non-endemic regions.
CRediT authorship contribution statement
Samendra P. Sherchan: Conceptualization, Methodology, Validation, Formal analysis, Writing – review & editing, Supervision, Funding acquisition. Tamunobelema Solomon: Methodology, Writing – review & editing. Oladele Idris: Methodology, Writing – review & editing. Daniel Nwaubani: Methodology, Writing – review & editing. Ocean Thakali: Data curation, Visualization, Formal analysis, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing interests.
Acknowledgments
This research was supported partially by the NIH grant R21AI157434 to Samendra Sherchan.
Editor: Damià Barceló
Data availability
Data will be made available on request.
References
- Ahmed W., Bertsch P.M., Bivins A., Bibby K., Farkas K., Gathercole A., Haramoto E., Gyawali P., Korajkic A., McMinn B.R., Mueller J.F., Simpson S.L., Smith W.J.M., Symonds E.M., Thomas K.V., Verhagen R., Kitajima M. Comparison of virus concentration methods for the RT-qPCR-based recovery of murine hepatitis virus, a surrogate for SARS-CoV-2 from untreated wastewater. Sci. Total Environ. 2020;739 doi: 10.1016/j.scitotenv.2020.139960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antinori A., Mazzotta V., Vita S., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27(22):2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC 2022. https://www.cdc.gov/poxvirus/mpox/response/2022/world-map.html
- Gazecka M., Sniezek J., Maciolek K., Kowala-Piaskowska A., Zmora P. Mpox virus detection in the wastewater and the number of hospitalized patients in Poznan metropolitan area, Poland. Int. J. Infect. Dis. 2023 doi: 10.1016/j.ijid.2023.05.014. [DOI] [PubMed] [Google Scholar]
- Girón-Guzmán, I., Díaz-Reolid, A., Truchado, P., Carcereny, A., García-Pedemonte, D., Hernáez, B., Bosch, A., Pintó, R. M., Guix, S., Allende, A., Alcamí, A., Pérez-Cataluña, A., & Sánchez, G. (2023). Spanish wastewater reveals the current spread of Monkeypox virus. Water Res., 231, 119621. Advance online publication. 10.1016/j.watres.2023.119621. [DOI] [PMC free article] [PubMed]
- de Jonge E.F., Peterse C.M., Koelewijn J.M., van der Drift A.-M.R., van der Beek R.F.H.J., Nagelkerke E., et al. The detection of monkeypox virus DNA in wastewater sam- ples in the Netherlands. Sci. Total Environ. 2022;852 doi: 10.1016/j.scitotenv.2022.158265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G., Mancini P., Veneri C., Bonanno Ferraro G., Lucentini L., Iaconelli M. Detection of monkeypox virus DNA in the wastewater of an airport in Rome, Italy: expanding environmental surveillance to emerging threats. Emerg. Infect. Dis. 2023;29:1. doi: 10.3201/eid2901.221311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Zhao H., Wilkins K., Hughes C., Damon I.K. Real-time PCR assays for the specific detection of Monkeypox virus West African and Congo Basin strain DNA. J. Virol. Methods. 2010;169:223–227. doi: 10.1016/J.JVIROMET.2010.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maal-Bared Rasha, Gerba Charles, Bibby Kyle, Munakata Naoko, Mehrotra Anna S., Brisolara Kari Fitzmorris, Haas Charles, Lee Gary, Nayak Bina, Swift Jay, Sherchan Samendra, Casson Leonard, Olabode Lola, Rubin Albert, Reimers Robert, Sobsey Mark. ACS ES&T Water; 2022. The Current Multicountry Monkeypox Outbreak: What Water Professionals Should Know. [Google Scholar]
- Malla B., Thakali O., Shrestha S., Segawa T., Kitajima M., Haramoto E. Application of a high-throughput quantitative PCR system for simultaneous monitoring of SARS-CoV-2 variants and other pathogenic viruses in wastewater. Sci. Total Environ. 2022;853 doi: 10.1016/j.scitotenv.2022.158659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia E., Hizon N., Dueck C., Lidder R., Daigle J., Wonitowy Q., Medina N., Mohammed U., Cox G., Safronetz D., Hagan M., Strong J., Nichani A., Mulvey M., Mangat C. 2022. Exploration of Wastewater Surveillance for Monkeypox Virus medRxiv 2022.11.10.22282091. [DOI] [Google Scholar]
- Peiró-Mestres, A., Fuertes, I., Camprubí-Ferrer, D., Marcos, M.Á., Vilella, A., Navarro, M., etal., 2022. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance 27 (28),2200503. 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed]
- Sharkey M.E., Babler K.M., Shukla B.S., Abelson S.M., Alsuliman B., Amirali A., Comerford S., Grills G.S., Kumar N., Laine J., Lee J., Lamar W.E., Mason C.E., Penso J., Reding B.D., Schürer S.C., Stevenson M., Vidović D., Solo-Gabriele H.M. Monkeypox viral nucleic acids detected using both DNA and RNA extraction workflows. Sci. Total Environ. 2023 doi: 10.1016/j.scitotenv.2023.164289. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherchan S.P., Shahin S., Ward L., Tandukar S., Aw T.G., Schmitz B., Ahmed W., Kitajima M. First detection of SARS-CoV-2 RNA in wastewater in North America: a study in Louisiana, USA. Sci. Total Environ. 2020;743 doi: 10.1016/j.scitotenv.2020.140621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari A., Adhikari S., Kaya D., Islam M.A., Malla B., Sherchan S.P., et al. Monkeypox outbreak: wastewater and environmental surveillance perspective. Sci. Total Environ. 2023;856(2) doi: 10.1016/j.scitotenv.2022.159166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towns J.M., Lim C.K., Chow E.P.F., et al. Persistence of monkeypox virus at oral and rectal sites. Lancet Microbe. 2023;4(4) doi: 10.1016/S2666-5247(22)00382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannigama D.L., Amarasiri M., Hongsing P., Hurst C., Modchang C., Chadsuthi S., Anupong S., Phattharapornjaroen P., S M A.H.R., Fernandez S., Huang A.T., Kueakulpattana N., Tanasatitchai C., Vatanaprasan P., Saethang T., Luk-In S., Storer R.J., Ounjai P., Ragupathi N.K.D., Kanthawee P.…Abe S. Multiple traces of monkeypox detected in non-sewered wastewater with sparse sampling from a densely populated metropolitan area in Asia. Sci. Total Environ. 2023;858(Pt 1) doi: 10.1016/j.scitotenv.2022.159816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Mpox Outbreak: Global Trends. Geneva: World Health Organization, 2023. 2023. https://worldhealthorg.shinyapps.io/mpx_global/ Available online:
- Wolfe M.K., Yu A.T., Duong D., et al. Use of wastewater for Mpox outbreak surveillance in California. N. Engl. J. Med. 2023 doi: 10.1056/NEJMc2213882. [DOI] [PubMed] [Google Scholar]
- Wurtzer S., Levert M., Dhenain E., Boni M., Tournier J.N., Londinsky N., Lefranc A., SIG O., Ferraris O., Moulin L. First detection of Monkeypox virus genome in sewersheds in France. Environ. Sci. Technol. Lett. 2022;9(11):991–996. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.