Abstract
Objective
Evidence regarding the role of cellular immunity in protecting against COVID-19 is emerging. To better assess immune status, simple and robust assays measuring specific T-cell responses associated with humoral responses are needed. We aimed to evaluate the Quan-T-Cell SARS-CoV-2 test for measuring cellular immune responses in vaccinated healthy and immunosuppressed subjects.
Methods
T-cell responses were assessed in healthy vaccinated and unvaccinated and unexposed healthcare workers to determine the sensitivity and specificity of the EUROIMMUN SARS-CoV-2 Quan-T-Cell IGRA test performed on vaccinated kidney transplant recipients (KTRs).
Results
The EUROIMMUN SARS-CoV-2 Quan-T-Cell IGRA test showed good sensitivity (87.2%) and specificity (92.3%) at the calculated 147 mIU/mL cutoff, with an 88.33% accuracy. In KTRs, specific cellular immunity was lower than the antibody response; however, those with a positive IGRA result produced as much IFN-γ as healthy individuals.
Conclusions
The EUROIMMUN SARS-CoV-2 Quan-T-Cell IGRA test showed good sensitivity and specificity for the detection of specific T-cell responses against the SARS-CoV-2 spike protein. These results present an additional tool for better management of COVID-19, especially in vulnerable populations.
Keywords: COVID-19, SARS-CoV-2, Vaccine, Cellular immunity, Interferon-gamma release tests
1. Introduction
December 2020 marked the beginning of a worldwide vaccination campaign against SARS-CoV-2. Two years later, 13 billion doses had been administered worldwide, and 68.5% of the global population had received at least one dose of the COVID-19 vaccine. In Belgium, 78.6% of the population is fully vaccinated, and 98.7% has received booster doses [1]. SARS-CoV-2 vaccines have shown excellent efficacy in preventing severe COVID-19 and generating strong humoral and cellular immune responses [2,3].
Evidence regarding the role of cellular immunity in protection against COVID-19 is emerging. In patients with acute and convalescent COVID-19, SARS-CoV-2-specific T-cell responses are associated with mild disease, highlighting the importance of cellular immunity in SARS-CoV-2 infection [4]. SARS-CoV-2-specific CD4+ and CD8+ T lymphocytes are associated with better COVID-19 outcomes, whereas the absence of SARS-CoV-2-specific CD4+ T cells is correlated with severe or fatal COVID-19 [5]. IFN-γ is produced at high levels by SARS-CoV-2-specific CD4+ and CD8+ T cells in patients with COVID-19, and CD4+ T cells protect mice from lethal SARS-CoV-2 infections [5,6]. In contrast, neutralizing antibody (nAb) titers are not correlated with reduced COVID-19 severity [5]. Moreover, a SARS-CoV-2 cellular immune response with no detectable antibodies was observed in infected individuals, indicating that this immunity might be induced and maintained in the absence of a humoral response [7]. In a vaccinal setting, adapted T-cell immunity is detectable earlier than nAbs and is concomitant with the protective effect of vaccinations [[8], [9], [10]]. Moreover, T-cell responses do not seem to be affected by virus-variant mutations, whereas antibody responses are less effective [11,12]. Therefore, a vaccine must induce not only the production of nAbs but also T-cell immunity for the best clinical success [13]. Immunosuppressed patients, including kidney transplant recipients (KTRs), have lower humoral response rates than the general population [14,15].
Although serological assays have rapidly been developed and are now widely implemented in clinical laboratories, investigation of cellular immunity has lagged. To better assess immune status, monitor vaccinated individuals, and evaluate emerging vaccines and their ability to protect against multiplying SARS-CoV-2 variants, simple and robust assays that measure specific T-cell responses are needed.
In this study, we evaluated the Quan-T-Cell SARS-CoV-2 test for measuring cellular immune responses in vaccinated healthy and immunosuppressed subjects.
2. Materials and methods
2.1. Study design
This prospective study was conducted at the Cliniques Universitaires Saint-Luc (Brussels, Belgium) between 18th May and July 29, 2021. Blood samples were collected from 125 healthy healthcare workers (HCWs) and 61 KTRs. Among the 125 HCWs, 26 were unvaccinated and unexposed to SARS-CoV-2, based on negative anamnesis (no history of SARS-CoV-2 infection or close contact) and serology, and were, therefore, considered negative controls. The other 99 HCWs were unexposed (based on negative anamnesis), fully vaccinated for at least two weeks prior to inclusion and referred to as vaccinated HCWs. KTRs were included based on “full vaccination” (two doses) at least two weeks before inclusion and no history of SARS-CoV-2 exposition (Fig. 1). All participants completed a questionnaire that collected information on sex, age, COVID-19 history, coexistence of chronic diseases, current medications, transplant details, vaccine name, and injection date. Informed consent was obtained from all participants, and the study was approved by the IRD Board (B4032021000056). Clinical information forms and blood samples were anonymized.
Fig. 1.
Flowchart of study subject groups.
2.2. Subject samples
For each subject, blood was drawn by venipuncture in the antecubital vein and collected in a lithium-heparin and serum-gel 7.5 mL tube (S-Monovette, Sarstedt, Numbrecht, Germany). Serum gel tubes were centrifuged at room temperature for 10 min at 3500 rpm (Eppendorf 5810 benchtop centrifuge, Hamburg, Germany). SARS-CoV-2 serology testing was performed on the serum. Whole-blood lithium heparin was used to assess SARS-CoV-2 cell-mediated immunity.
2.3. Humoral immune response testing
Serology was assessed using two electrochemiluminescence immunoassay (ECLIA) kits detecting total antibodies directed against the receptor binding domain (RBD) and nucleocapsid (N) [Elecsys anti-SARS-CoV-2-S and Elecsys anti-SARS-CoV-2, Roche Diagnostics GmbH, Mannheim, Germany – positive threshold >0.8 binding antibody units (BAU)/mL (Anti-RBD), using the WHO international standard (NIBSC code 20/136), and >1.0 index (Anti-N), upper limit of detection 250 BAU/mL (anti-RBD)].
2.4. Cellular immune response testing
Quan-T-Cell SARS-CoV-2 stimulation tube set and Quan-T-Cell-ELISA (EUROIMMUN, Lübeck, Germany) were used to assess immunity due to CD4+ and CD8+ T lymphocytes. After drawing blood in a lithium heparin tube, 500 μL of whole blood was transferred within an hour into three specific tubes containing: 1) a SARS-CoV-2 IGRA BLANK with no T-cell activating component and reflecting background T-cell activity, 2) a SARS-CoV-2 IGRA TUBE coated with a pool of SARS-CoV-2 spike proteins able to stimulate specific CD4+ and CD8+ T lymphocytes, and 3) a SARS-CoV-2 IGRA STIM coated with a mitogen for non-specific T-cell stimulation and used as a control for the viability and stimulation ability of T-cells. After six inversions, these tubes were incubated at 37 °C for 20–24 h and then spun at room temperature for 10 min at 12000×g (Eppendorf centrifuge 5420). The supernatant was stored at −20 °C until measurement. For the IFN-γ ELISA, the supernatant was diluted 1:5 with a dilution buffer; additionally, six calibrators and two controls were used in each run. Specific IFN-γ concentrations were obtained after subtracting the BLANK value from the TUBE/STIM value, expressed in mIU/mL. Results <100 and >200 mIU/mL were considered negative and positive, respectively. The manufacturer defined a grey zone between 100 and 200 mIU/mL, and the results within this zone were interpreted as positive in the context of a high positive predictive value (exposed and vaccinated individuals) and immunosuppression. The upper limit of detection was 2500 mIU/mL. All the tests were CE-IVD marked.
2.5. Statistical analysis
Data were analyzed using GraphPad Prism version 9.2 (www.graphpad.com) and MedCalc version 14 (www.medcalc.com). We employed nonparametric tests because the data had a non-Gaussian distribution. For unpaired comparisons, we used the Mann–Whitney U and Kruskal–Wallis tests. Spearman's correlation test was used to measure the rank correlation. Fisher's exact test was used to compare categorical data, and McNemar's test was used to compare paired nominal data. We calculated the optimum cutoff value for the highest Youden index using receiver operating characteristic (ROC) analysis.
3. Results
3.1. Study group characteristics
Subjects with anti-N antibodies detected using Elecsys were excluded, as were those with indeterminate IGRA results (failure of non-specific T-cell stimulation) (Fig. 1). In the final analysis, 120 HCWs (94 vaccinated and 26 unvaccinated) and 48 KTRs were included, the characteristics of which are shown in Table 1.
Table 1.
Characteristics of vaccinated health care workers (HCWs), kidney transplant recipients (KTRs), and negative controls.
| Vaccinated HCWs (n = 94) | KTRs (n = 48) | Negative controls (n = 26) | |
|---|---|---|---|
| Age, (median range) in years | 40 (23–63) | 50 (25–78) | 37.5 (22–63) |
| Sex | |||
| Female (n [%]) | 73 (77.6%) | 25 (52%) | 17 (65.4%) |
| Male (n [%]) | 21 (22.4%) | 23 (48%) | 9 (34.6%) |
| Vaccine (n [%]) | – | ||
| BNT162b2 | 93 (98.9%) | 48 (100%) | – |
| ChadOx1 | 1 (1.1%) | – | – |
| Vaccination time | 5 (±3) * | 2 (±1) * | – |
| Median ± IQR (months) | |||
| Median time since transplantation (range) (months) | – | 114 (8–297) | – |
| Tacrolimus (Tac) | – | 44 (91.7%) | – |
| Mycophenolate (MPA) | – | 38 (79.1%) | – |
| Steroids (St) | – | 40 (83.3%) | – |
| Tac-MPA-St association | 27 (56.2%) | – | |
| Antimetabolite free regimen | – | 10 (20.8%) | – |
HCWs, healthcare workers; KTRs, kidney transplant recipients; anti-N Ab, antinucleocapsid antibodies; IGRA, interferon-release assay.
*p < 0.001.
3.2. Serology
All 94 vaccinated HCWs were positive for anti-RBD and negative for anti-N antibodies; anti-RBD values were ≥250 and between 84.5 and 228 BAU/mL in 89 and 5 patients, respectively. Unvaccinated HCWs had negative serology with Elecsys (anti-RBD and anti-N antibodies). Only 33/48 (68.7%) KTRs developed anti-RBD antibodies one month after the second BNT162b2 vaccine. The seroconversion rate of KTRs was significantly lower than that of vaccinated HCWs (p < 0.001). Positive anti-RBD antibodies ranged from 0.9 to ≥250 BAU/mL.
3.3. IGRA results in the negative control population
In our negative control population (n = 26), two patients (7.7%) had IFN-γ concentrations (after blank subtraction) between 100 and 200 mIU/mL, two had above 200 mIU/mL, and the rest had <100 mIU/mL. Negative values calculated after background subtraction were set at 0 mIU/mL. All patients had a negative serology with Elecsys (anti-N and anti-RBD) and no history of COVID-19 or close contact with infected individuals; therefore, they were considered true negatives. The specificity of the IGRA using cutoff values of 200 and 100 mIU/mL was 92.3 and 84.6%, respectively. ROC analysis showed an optimized cutoff value of 147 mIU/mL, with the highest Youden index.
3.4. IGRA results in vaccinated HCWs and cutoffs
Ten (10.6%) of the vaccinated HCWs yielded IGRA values below 100 mIU/mL, seven (7.5%) had between 100 and 200 mIU/mL (grey zone)—five (5.3%) of these had between 147 and 200 mIU/mL—and 77 (81.9%) had above 200 mIU/mL. Therefore, sensitivities after full vaccination in healthy individuals were 89.4, 87.2, and 81.9% using 100, 147, and 200 mIU/mL cutoffs, respectively. There was no statistical difference between individuals with an IGRA test result under 100 mIU/mL and above this value regarding the time after full vaccination (p = 0.118) and age (p = 0.108). The performances of the different cutoff values are listed in Table 2. For further analysis, we used a calculated cutoff value of 147 mIU/mL.
Table 2.
IGRA performance using different cutoffs.
| Cutoff ≥100 mIU/mL | Cutoff ≥147 mIU/mL | Cutoff ≥200 mIU/mL | |
|---|---|---|---|
| Sensitivity % (95% CI) | 89.4% (81.5–94.3) | 87.2% (79.0–92.5) | 81.9% (72.9–88.4) |
| Specificity (95% CI) | 84.6% (66.5–93.8) | 92.3% (75.9–97.9) | 92.3% (75.9–97.9) |
| Negative predictive value (95% CI) | 68.8% (51.4–82.1) | 66.7% (50.3–79.8) | 58.5% (43.4–72.2) |
| Positive predictive value (95% CI) | 95.5% (88,9–98.2) | 97.6% (91.7–99.3) | 97.4% (91.2–99.3) |
| Accuracy (95% CI) | 88.3% (81.4–92.9) | 88.3% (81.4–92.9) | 84.2% (76.6–89.6) |
3.5. IGRA results in KTRs
Nine (18.7%) KTRs had an IGRA test above 200 mIU/mL, three (6.3%) had IFN-γ values between 147 and 200 mIU/mL, and 36 (75%) had under 100 mIU/mL. None of the patients had levels between 100 and 147 mIU/mL. Using the calculated cutoff, 12 (25%) had positive IGRA results; nine IGRA-positive KTRs had a positive serology, and three were negative for anti-RBD antibodies. The three non-seroconverters had IGRA values of 176.7, 192.1, and 1501.0 mIU/mL. Positive IGRA values were not significantly different (p = 0.481) between seroconverter and non-seroconverter KTRs. There was a statistically significant difference in the percentage of IGRA positivity between vaccinated HCWs and KTRs (p < 0.001), where KTRs yielded fewer positive IGRA results.
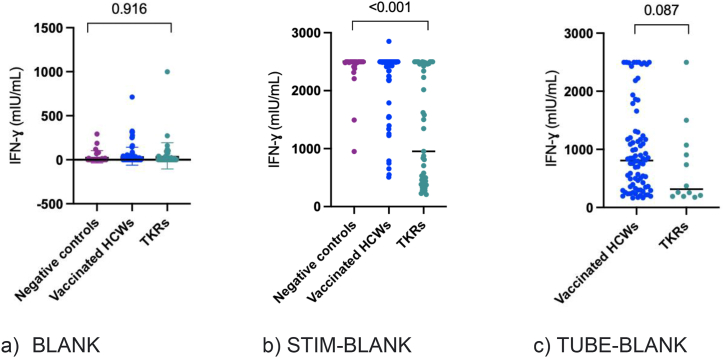
We compared the BLANK and calculated the IGRA STIM values (after blank subtraction) between the three populations (Fig. 2a and b). There was no difference in BLANK values among the three groups (p = 0.916); however, the calculated STIM IFN-γ values were significantly lower in KTRs than in the healthy population (p < 0.001). We also compared the positively calculated IGRA TUBE values between vaccinated HCWs and KTRs using the calculated cutoff value of 147 (Fig. 2c). Positive TUBE-BLANK IFN-γ values (≥147 mIU/mL) were not significantly different between vaccinated HCWs and KTRs (p = 0.087). The IFN-γ levels in the different groups are shown in Table 3. Negatively calculated values after background subtraction were set to 0 mIU/mL.
Fig. 2.
Comparison of IFN-γ BLANK values (a), STIM-BLANK (b), and TUBE-BLANK (c) in negative controls, vaccinated HCWs, and KTRs. There was no statistically significant difference in BLANK values between the three groups (p = 0.916) (2a). STIM-BLANK IFN-γ values were significantly lower in KTRs than in the healthy population (p < 0.001) (2b). TUBE-BLANK IFN-γ values over the cutoff of 147 mIU/mL were not significantly different between vaccinated HCWs and KTRs (p = 0.087) (2c). a) BLANK b) STIM-BLANK c) TUBE-BLANK.
Table 3.
Interferon-γ values in mIU/mL in the different study groups.
| Study groups | n | Mean | SD | Median | Min | Max |
|---|---|---|---|---|---|---|
| Negative controls | 26 | 49.1 | 108.3 | 0 | 0 | 423.9 |
| Vaccinated HCWs | 94 | |||||
| Positive IGRA at calculated cutoff | 82 | 998.8 | 758.5 | 808.8 | 164.5 | 2499 |
| Negative IGRA at calculated cutoff | 12 | 43.5 | 43.1 | 31.5 | 0 | 114.2 |
| KTRs | 48 | |||||
| Positive IGRA at calculated cutoff | 12 | 697,6 | 713.2 | 316.1 | 176.9 | 2499.5 |
| Negative IGRA at calculated cutoff | 36 | 21.6 | 27.7 | 9.7 | 0 | 84.1 |
Calculated cutoff: 147 mIU/mL.
Among IGRA-positive KTRs, 6/12 (50%) were under tacrolimus, mycophenolate, and steroids (Tac-MPA-St) tritherapy treatments; 3/12 (25%) received a combination of Tac and MPA, 1/12 (8,3%) was under ciclosporin, azathioprine, and MPA treatments, another was under ciclosporin, MPA, and steroid treatments, and a further individual received an antimetabolite-free regimen composed of tacrolimus and steroids. Among KTRs that did not develop vaccine T-cell immunity, 21/36 (58.3%) received the Tac-MPA-St treatment and 9/36 (25%) the antimetabolite-free treatment, with three treated with the mTOR inhibitor (sirolimus). In our cohort, neither the Tac-MPA-St treatment (p = 0.741) nor antimetabolites (p = 0.414) influenced cellular responses.
3.6. Serology vs IGRA
We performed McNemar's test to investigate the correlation between seroconversion and the development of adaptive cellular immunity following SARS-CoV-2 vaccination. The p-value was <0.001 for KTRs and vaccinated HCWs alone, indicating that there was no correlation between humoral and cellular adaptive immunity after vaccination.
3.7. Blank values
IFN-γ levels in the BLANK tube ranged from 0.5 to 999.0 mIU/mL. Mean (±SEM) and median (IC 95%) were 40.9 (±8.7) and 0.5 (0.5–5.05) mIU/mL, respectively. Fourteen of the 168 calculated IFN-γ values ranged from −163.15 to −1.22 [median (IQR) of −17.3 (−64.1 to −5.3) mIU/mL] owing to superior BLANK values than TUBE values. Three of the 14 samples had calculated IFN-γ levels lower than −100 mIU/mL. Inversion of the tubes was not excluded because we could not perform the test.
3.8. Indeterminate results
Twelve (20%) KTRs, all on steroids, had indeterminate IGRA results due to the failure of non-specific stimulation of T cells, indicating an inability to generate IFN-γ. One unexplained indeterminate IGRA test was observed in a vaccinated HCW, whereas KTRs showed more indeterminate results than HCWs (p < 0.001).
4. Discussion
In this study, the EUROIMMUN SARS-CoV-2 Quan-T-Cell IGRA demonstrated a good sensitivity and specificity for detecting cellular immune response to SARS-CoV-2 vaccines. It is easy to perform, amenable to automation, and suitable for large-scale applications. The IGRA test is widely used in clinical laboratories to diagnose tuberculosis [16].
The IGRA test showed good sensitivity and specificity of 87.2% and 92.3%, respectively, at calculated cutoffs of 147 mIU/mL, with an accuracy of 88.3%. Huzly et al. demonstrated sensitivity, specificity, and accuracy of 88.7, 98.1, and 97.1%, respectively, at a calculated cutoff using ROC analysis (≥135 mIU/mL). They proposed to adapt the cutoff to ≥200 mIU/mL in the case of BLANK ≥100 mIU/mL, which enhanced specificity to 100%. We did not encounter this result in our dataset. Interestingly, Huzly et al. also included HCWs with a history of COVID-19, which was not the case here [17].
Regarding the IGRA test evaluation after SARS-CoV-2 infection, Huzly et al. reported sensitivity to the test of 93.8 and 72.2% up to five months and more than five months post-infection, respectively [17]. Fernandez-Gonzalez et al. also showed a sensitivity of 100% three months post-infection and only 78% one-year post-infection using the same test [18].
The T-cell response to SARS-CoV-2 can also be evaluated using ELISpot or by measuring cell proliferation using flow cytometry. However, these tests are fastidious and not applicable to large-scale testing [19], whereas the EUROIMMUN SARS-CoV-2 Quan-T-Cell IGRA test is simple. After whole blood collection and transfer to the stimulation tubes, the separated plasma can be stored for four weeks at 2–8 °C and for at least three months at −20 °C [17].
Ten vaccinated HCWs had no specific SARS-CoV-2 T-cell responses, even though they exhibited high anti-RBD antibodies. Schiffner et al. reported similar observations using the same assay but after SARS-CoV-2 infection. However, low antibodies with high IFN-γ values were also described [20]. Huzly et al. did not show this discrepancy, as all vaccinated HCWs had positive IGRA test results [17].
We also assessed vaccine immunity in KTRs. As previously described, this was significantly lower than that observed in vaccinated healthy subjects as a consequence of immunosuppressive therapy administered to KTRs [[21], [22], [23]]. Altogether, 75% of KTRs developed either humoral or cellular immunity. Specific cellular immunity was lower than the antibody response, with only 25% having a positive IGRA test against 70% who seroconverted. Interestingly, 3/15 (20%) patients with no anti-RBD antibodies had a positive IGRA test, with Huzly et al. reporting similar results [17]. Stumpf et al. also observed lower cellular responses than humoral responses in KTRs using an IGRA ELISA test [24]. In contrast, Bertrand et al. reported a T-cell response rate of 57.8% after two injections of the BTN162b2 vaccine in KTRs using ELISpot immunoassay [21]. As SARS-CoV-2 reactive CD4+ T cells have been reported in more than 20% of unexposed individuals [[25], [26], [27]], this cross-reactive SARS-CoV-2 T-cell response is best explained by previous infections with the common cold human coronaviruses [28]. These studies used either the ELISpot assay or flow cytometry, whereas other studies that used the IGRA test did not detect specific IFN-γ production in unexposed subjects [17,29,30]. Because our negative controls were included during the pandemic, we cannot exclude the possibility of previously unknown exposure to the virus, even if they had negative serology and no history of COVID-19 contact or symptoms. The higher IGRA positivity rate with the ELISpot assay than that with the IGRA ELISA test in KTRs could be explained by the cross-reactivity observed with ELISpot.
Notably, although the positivity rate of the IGRA test was significantly different between KTRs and immunocompetent vaccinated HCWs, there was no difference in the median value of IFN-γ produced in positive tests between the two populations. When immunosuppressed patients respond, their response is as good as that of immunocompetent patients. Ramanathan et al. reported similar results using a commercial IGRA kit [15].
Calcineurin inhibitors and mycophenolates are associated with a failure to achieve humoral and cellular immunity compared to mTOR inhibitors and glucocorticoids [24]. In our KTR population, the Tac-MPA-St combination and antimetabolite prescription did not influence the IGRA test results. However, all three patients who received mTOR inhibitors had negative IGRA results.
KTRs also showed more indeterminate IGRA results than HCWs. Similar results were obtained using the IGRA tuberculosis test. Immunosuppression, especially with systemic steroids, leads to impairment of T-cell activity [31].
Impaired humoral immunity to SARS-CoV-2 vaccines has been reported in other immunocompromised populations, such as patients with hematologic malignancies, solid cancers, or severe autoimmunity [15]. Assessing both humoral and cellular responses together is of interest to patient management as it may help identify the best candidates for either revaccination or prophylactic monoclonal antibody therapy [32].
The assessment of cellular responses can also be useful for diagnosing post-infectious symptoms due to SARS-CoV-2 in seronegative patients who did not have access to RT-qPCR tests earlier during the pandemic [19] or who had negative RT-qPCR results. In post-vaccination settings, the IGRA test can be used to monitor the vaccination response, especially in immunocompromised patients who fail to develop the SARS-CoV-2 antibody. This can help identify patients who would benefit from a booster dose and can be used to evaluate vaccine efficacy against SARS-CoV-2 variants.
Our study had some limitations wherein we only included vaccinated participants and did not evaluate the performance of the test in the context of COVID-19. In addition, our negative control population was small and included patients during the pandemic; thus, previous contact with the virus cannot be excluded. Another limitation is that the immune response to the vaccine was not assessed simultaneously between the two groups. KTRs display a humoral response later than the general population [33]. Therefore, the cellular response may follow similar kinetics. A comparison between the two groups would have been more accurate if performed simultaneously. Finally, we did not assess nAbs; however, a recent paper shows a protective cutoff of anti-RBD antibody 264 (BAU)/mL is associated with 80% vaccine efficacy [34].
5. Conclusion
The EUROIMMUN SARS-CoV-2 Quan-T-Cell IGRA test showed good sensitivity and specificity for the detection of specific T-cell responses against the SARS-CoV-2 spike protein. It is easy to perform, amenable to automation, and applicable to a large number of samples, making it suitable for routine diagnostic laboratories. Together with serology, the IGRA represents an additional tool for better management and prevention of COVID-19, especially in vulnerable populations. However, larger studies are needed to confirm these results and establish the position of this test in assessing SARS-CoV-2 immunity.
Ethical approval statement and consent to participate
This study was approved by the Hospital and Department Ethics Committee of Saint-Luc-UCL (Brussels) on May 3, 2021 (B4032021000056). Written informed consent was obtained from all participants. We confirm that all methods were performed in accordance with the relevant guidelines and regulations.
Author contribution statement
Imane Saad Albichr; Benoît Kabamba-Mukadi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Samy Mzougui: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Arnaud Devresse; Eric Goffin; Nada Kanaan; Hector Rodriguez-Villalobos; Hélène Georgery: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Jean-Cyr Yombi; Leila Belkhir; Julien De Greef: Conceived and designed the experiments.
Anaïs Scohy: Performed the experiments; Analyzed and interpreted the data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e17186.
List of abbreviations
- ECLIA
electrochemiluminescence immunoassay
- HCWs
health care workers
- IFN-γ:
interferon gamma
- IGRA
interferon gamma release assay
- KTRs
kidney transplant recipients
- mTOR
mammalian target of rapamycin
- N
nucleocapsid
- nAb
neutralizing antibodies
- RBD
receptor binding domain
- Tac-MPA-St
tacrolimus, mycophenolate, and steroids
Appendix. ASupplementary data
The following are the supplementary data related to this article:
References
- 1.Ritchie H., Mathieu E., Rodés-Guirao L., et al. 2021. Coronavirus Pandemic.https://ourworldindata.org/covid-vaccinations (COVID-19). Our World Data [Internet]. 2020 [cited 2021 Aug 25] [Google Scholar]
- 2.Creech C.B., Walker S.C., Samuels R.J. SARS-CoV-2 vaccines. JAMA. 2021;325:1318–1320. doi: 10.1001/jama.2021.3199. [DOI] [PubMed] [Google Scholar]
- 3.Sahin U., Muik A., Vogler I., et al. 2020. BNT162b2 Induces SARS-CoV-2-Neutralising Antibodies and T Cells in Humans [Internet]. 2020 [cited 2021 Aug 25]https://www.medrxiv.org/content/10.1101/2020.12.09.20245175v1 [Google Scholar]
- 4.Sette A., Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861–880. doi: 10.1016/j.cell.2021.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012.e19. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhao J., Zhao J., Mangalam A.K., et al. Airway memory CD4(+) T cells mediate protective immunity against emerging respiratory coronaviruses. Immunity. 2016;44:1379–1391. doi: 10.1016/j.immuni.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ong D.S.Y., Fragkou P.C., Schweitzer V.A., et al. How to interpret and use COVID-19 serology and immunology tests. Clin. Microbiol. Infect. 2021;27:981–986. doi: 10.1016/j.cmi.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalimuddin S., Tham C.Y.L., Qui M., et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Méd. 2021;2:682–688.e4. doi: 10.1016/j.medj.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Painter M.M., Mathew D., Goel R.R., et al. Rapid induction of antigen-specific CD4+ T cells is associated with coordinated humoral and cellular immunity to SARS-CoV-2 mRNA vaccination. Immunity. 2021;54:2133–2142.e3. doi: 10.1016/j.immuni.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tarke A., Sidney J., Methot N., et al. 2021. Negligible Impact of SARS-CoV-2 Variants on CD4+ and CD8+ T Cell Reactivity in COVID-19 Exposed Donors and Vaccinees.https://www.biorxiv.org/content/10.1101/2021.02.27.433180v1 [Google Scholar]
- 12.Geers D., Shamier M.C., Bogers S., et al. SARS-CoV-2 variants of concern partially escape humoral but not T-cell responses in COVID-19 convalescent donors and vaccinees. Sci Immunol. 2021;6 doi: 10.1126/sciimmunol.abj1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sauer K., Harris T. An effective COVID-19 vaccine needs to engage T cells. Front. Immunol. 2020;11:2371. doi: 10.3389/fimmu.2020.581807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georgery H., Devresse A., Yombi J.-C., et al. Transplantation; 2021. Disappointing Immunization Rate after 2 Doses of the BNT162b2 Vaccine in a Belgian Cohort of Kidney Transplant Recipients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramanathan M., Murugesan K., Yang L.M., et al. 2021. Cell-Mediated and Humoral Immune Response to 2-Dose SARS-CoV2 mRNA Vaccination in Immunocompromised Patient Population.https://www.medrxiv.org/content/10.1101/2021.07.21.21260921v1 [Google Scholar]
- 16.Lu P., Chen X., Zhu L., et al. Interferon-gamma release assays for the diagnosis of tuberculosis: a systematic review and meta-analysis. Lung. 2016;194:447–458. doi: 10.1007/s00408-016-9872-5. [DOI] [PubMed] [Google Scholar]
- 17.Huzly D., Panning M., Smely F., et al. 2021. Validation and Performance Evaluation of a Novel Interferon-γ Release Assay for the Detection of SARS-CoV-2 Specific T-Cell Response [Internet]https://www.medrxiv.org/content/10.1101/2021.07.17.21260316v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández-González M., Agulló V., Padilla S., et al. Clinical performance of a standardized severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) interferon-γ release assay for simple detection of T-cell responses after infection or vaccination. Clin Infect Dis Off Publ Infect Dis Soc Am. 2022;75:e338–e346. doi: 10.1093/cid/ciab1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ameratunga R., Woon S.-T., Jordan A., et al. Perspective: diagnostic laboratories should urgently develop T cell assays for SARS-CoV-2 infection. Expet Rev. Clin. Immunol. 2021;17:421–430. doi: 10.1080/1744666X.2021.1905525. [DOI] [PubMed] [Google Scholar]
- 20.Schiffner J., Backhaus I., Rimmele J., et al. 2021. Long-term Course of Humoral and Cellular Immune Responses in Outpatients after SARS-CoV-2 Infection [Internet]https://www.medrxiv.org/content/10.1101/2021.06.24.21259218v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bertrand D., Hamzaoui M., Lemée V., et al. Antibody and T Cell response to SARS-CoV-2 messenger RNA BNT162b2 vaccine in kidney transplant recipients and hemodialysis patients. J. Am. Soc. Nephrol. 2021 doi: 10.1681/ASN.2021040480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rincon-Arevalo H., Choi M., Stefanski A.-L., et al. Impaired humoral immunity to SARS-CoV-2 BNT162b2 vaccine in kidney transplant recipients and dialysis patients. Sci Immunol [Internet] 2021 doi: 10.1126/sciimmunol.abj1031. https://immunology.sciencemag.org/content/6/60/eabj1031 [DOI] [PubMed] [Google Scholar]
- 23.Devresse A., Saad Albichr I., Georgery H., et al. T-Cell and antibody response after 2 doses of the BNT162b2 vaccine in a Belgian cohort of kidney transplant recipients. Transplantation. 2021;105:e142–e143. doi: 10.1097/TP.0000000000003892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stumpf J., Siepmann T., Lindner T., et al. Humoral and cellular immunity to SARS-CoV-2 vaccination in renal transplant versus dialysis patients: a prospective, multicenter observational study using mRNA-1273 or BNT162b2 mRNA vaccine. Lancet Reg Health Eur. 2021 doi: 10.1016/j.lanepe.2021.100178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Le Bert N., Tan A.T., Kunasegaran K., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 26.Grifoni A., Weiskopf D., Ramirez S.I., et al. Targets of T Cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501.e15. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Braun J., Loyal L., Frentsch M., et al. SARS-CoV-2-reactive T cells in healthy donors and patients with COVID-19. Nature. 2020;587:270–274. doi: 10.1038/s41586-020-2598-9. [DOI] [PubMed] [Google Scholar]
- 28.Mateus J., Grifoni A., Tarke A., et al. Selective and cross-reactive SARS-CoV-2 T cell epitopes in unexposed humans. Science. 2020;370:89–94. doi: 10.1126/science.abd3871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugesan K., Jagannathan P., Pham T.D., et al. Interferon-gamma release assay for accurate detection of SARS-CoV-2 T cell response. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa1537. ciaa1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Petrone L., Petruccioli E., Vanini V., et al. A whole blood test to measure SARS-CoV-2-specific response in COVID-19 patients. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2021;27:e7–e13. doi: 10.1016/j.cmi.2020.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Risk factors for indeterminate outcome on interferon gamma release assay in non-US-Born persons screened for latent tuberculosis infection. 2021. https://academic.oup.com/ofid/article/5/8/ofy184/5060271?login=true [DOI] [PMC free article] [PubMed]
- 32.Covid-19 . 2021. Asso - STF [Internet]https://www.transplantation-francophone.org/covid-19 [Google Scholar]
- 33.Georgery H., Devresse A., Saad Albichr I., et al. Transplantation; 2021. Delayed Humoral Response after 2 Doses of the BNT162b2 Vaccine in a Belgian Kidney Transplant Cohort. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Correlates of Protection against Symptomatic and Asymptomatic SARS-CoV-2 Infection | medRxiv [Internet] 2021. https://www.medrxiv.org/content/10.1101/2021.06.21.21258528v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.