Abstract
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus's worldwide pandemic has highlighted the urgent need for reliable, quick, and affordable diagnostic tests for comprehending and controlling the epidemic by tracking the world population. Given how crucial it is to monitor and manage the pandemic, researchers have recently concentrated on creating quick detection techniques. Although PCR is still the preferred clinical diagnostic test, there is a pressing need for substitutes that are sufficiently rapid and cost-effective to provide a diagnosis at the time of use. The creation of a quick and simple POC equipment is necessary for home testing. Our review's goal is to provide an overview of the many methods utilized to identify SARS-CoV 2 in various samples utilizing portable devices, as well as any potential applications for smartphones in epidemiological research and detection. The point of care (POC) employs a range of microfluidic biosensors based on smartphones, including molecular sensors, immunological biosensors, hybrid biosensors, and imaging biosensors. For example, a number of tools have been created for the diagnosis of COVID-19, based on various theories. Integrated portable devices can be created using loop-mediated isothermal amplification, which combines isothermal amplification methods with colorimetric detection. Electrochemical approaches have been regarded as a potential substitute for optical sensing techniques that utilize fluorescence for detection and as being more beneficial to the Minimizing and simplicity of the tools used for detection, together with techniques that can amplify DNA or RNA under constant temperature conditions, without the need for repeated heating and cooling cycles. Many research have used smartphones for virus detection and data visualization, making these techniques more user-friendly and broadly distributed throughout nations. Overall, our research provides a review of different novel, non-invasive, affordable, and efficient methods for identifying COVID-19 contagious infected people and halting the disease's transmission.
Keywords: Smartphone, Point-of-care testing, SARS-CoV-2, Review, COVID-19
Introduction
The past 20 years have witnessed the advent of multiple pandemics, including severe acute respiratory syndrome (SARS), Ebola, and MERS, which have raised questions about the effectiveness and legitimacy of health systems and medical breakthroughs. Coronavirus disease has arisen as a severe pandemic, which has resulted in substantial economic and social damage around the world. However, progress in the field of artificial intelligence (AI), cloud technology and the network of physical objects known as ‘Internet of Things’ offer a unique possibility to better medical care [1,2]. China, where COVID-19 was originally identified during the latter months of 2019, used successfully technology in its healthcare management systems to tackle the widespread occurrence of the disease and maintain daily life as usual [3].
The etiology of Coronavirus disease is attributed to SARS-CoV-2, a beta coronavirus with a single-strand RNA genome. The virus has spike proteins that act as entry points for the virus to invade the cells, by binding to the membrane receptors on the surface of human cells, specifically, angiotensin-converting enzyme 2 or ACE2 receptors [4,5]. Due to mutations affecting its protein, the virus demonstrates remarkable immune evasion and makes mutant variants. Typical signs of SARS-CoV-2 disease manifests abnormally high body temperature, coughing, fatigue, and lung infection, which can progress to severe inflammation and congestion with fluids [6]. Recent strains like Omicron and Delta have dominated the viral species with variations in symptoms, posing a challenge for diagnosing the virus relying on traditional signs alone. Additionally, asymptomatic cases have increased, adding further difficulty to confirming infections [4,5]. In spite of the efforts exerted to allow close contact tracing of SARS-CoV-2 infections, reliable fast diagnostic tools are essential.
Current diagnostic techniques for Coronavirus in 2019 involve amplifying and detecting one or more viral ribonucleic acid (RNA), computed tomography, serologic testing, and antigen [7]. SARS-CoV-2 is an RNA virus, so reverse transcription is needed. Real-time polymerase chain reaction (RT-PCR) allows the detection and quantification of the amplified DNA fragments in real-time [8]. However, RT-qPCR has certain drawbacks such as the requirement of temperature cycling and specialists, Laboratory methods that involve isolating the virus, breaking it down, and eliminating any material of inhibition [9]. Although PCR remains the primary clinical diagnostic method, alternative techniques is an immediate requirement, especially those offering sufficient speed and affordable cost to detect the virus right at the point of use [9].
Several alternatives have been developed to address these limitations. Loop-mediated isothermal amplification (RT-LAMP), and recombinase polymerase amplification (RT-RPA) do not need thermal cycling and can generate fast results. The Cas13a integrated lateral-flow readout detects the virus without the need to extract the RNA, reducing complexity and the need for costly materials [10]. Protein-based techniques employing antibodies targeting viral proteins as spikes can also quickly identify SARS-CoV-2. Such tests can be developed from current lateral-flow test kits for quick home testing that have been authorized by the FDA. Microfluidics has been employed to handle these tasks at a cost-effective rate, with the microfluidic biomedical sensory systems based on smartphones offering a portable, compact, and user-friendly solution [11].
Innovative developments in microfluidics, nanotechnology, microelectron mechanical systems (MEMS) technology, 3D printing, and data analytics have resulted in the wide dissemination and commercialization of point-of-care testing (POCT) instruments. [12]. Devices of Lateral flow, isothermal nucleic acid amplification assays (INAA), lab-on-a-disc or chip (LOAD/ LOC) devices, and microfluidic paper-based analytical devices (PADs) are among the POCT devices developed for COVID diagnosis. In addition, several studies have used smartphones for the detection of the virus and data visualization [13]. These devices offer a novel, non-invasive, economical, and efficient method for preventing the spread of coronavirus disease 2019 and curb its dissemination [14]. The aim of this work article is to give a summary of the various approaches employed for identifying the presence of severe acute respiratory syndrome coronavirus 2 in different specimens, particularly through the utilization of portable devices. Additionally, this work seeks to investigate the possibility of using smartphones as a diagnostic tool in data visualization and epidemiological studies.
Methods
The authors independently reviewed the literature using Scopus and PubMed, two scholarly databases. Only peer-reviewed articles in English were included in the search process. Methods like mesh and search phrases were employed. In order to cover a broader range of literature, search terms like COVID-19, smartphone, and point-of-care were combined with various Boolean operators, "AND" or "OR" operators. The keywords were searched within article title, abstract and keywords. The studies were first filtered by the authors based on their titles, after which the abstracts were filtered. Papers that were abstract only, reviews, duplicated, not applicable in SAR-Cov-2 virus, not employing smartphone technology or unrelated have been removed. The references of some research were manually searched in order to provide additional pertinent studies for further screening. Eventually, 49 studies in total were included. The following data were retrieved when an article was chosen for inclusion: sample, sample preparation, target part of the virus for detection, type of smart phones and sensors, study principle, sensitivity, accuracy, and time taken by the devices to interpret the results of the test. Fig. 1 shows the PRISMA of the systematic review.
Fig. 1.
A flow diagram following PRISMA guidelines to depict the studies that were incorporated in the review.
Discussion
In the era of COVID-19, there is an increasing demand for diagnostic techniques that are portable and provide quick results. The traditional method of diagnosing this virus using PCR has been associated with several limitations, such as high cost, the complexity of the testing process, and the need for skilled personnel to operate the equipment. Multiple studies have been conducted in an effort to develop alternative diagnostic methods that overcome these drawbacks.
The aim of researchers globally is to continuously explore innovative solutions to combat the ongoing COVID-19 epidemic. Among these solutions, quick viral detection assays have been continually improved and expanded. POCT offers ongoing and efficient testing of people who have been infected with SARS-CoV-2, while utilizing statistical methods for the analysis of large sets of COVID-19 data in relation to both location and timeframe. The potential applications of point-of-care devices are diverse, including testing at home to monitoring patients in medical settings, to tracking the virus's geographical spread by public health organizations. These devices not only enable early diagnosis of viral infections but also offer valuable opportunities for disease surveillance and control. Table 1 summarizes the applications of POCT devices, and the performance data. The sample type is crucial to select the sensor that satisfies the need. The steps involved in preparing the sample for testing must also be considered, which may include RNA or DNA extraction. The specific viral component being targeted by the test is another key parameter, with options ranging from detecting viral RNA to antigens or antibodies. Sensitivity and accuracy are essential parameters that can influence the overall quality of the test, with highly sensitive tests having lower detection limits. Time required to perform the test from sample collection to obtaining results is another important consideration. The type of smartphone used for the test is also critical, as compatibility with the testing system can vary. Finally, the role of the smartphone in the testing process should be defined, whether it simply displays the result or is fully integrated with the testing system to provide automated analysis and reporting.
Table 1.
Applications of smartphone-based POCT of the SARS-CoV-2.
| Sensor type | Sample | Sample preparation | Target | Sensitivity | Accuracy | Time (min) | Smartphone type | Smartphone role | Refs. |
|---|---|---|---|---|---|---|---|---|---|
| Electro-chemical | Nasopharyngeal swabs | Sample dilution and incubation for 2 h. | the peptidase of ACE2 domain | 5.14 ng/mL for S1 and 2.09 ng/mL for S2 | 99.37% | 1 | NR | Mobile application. | [15] |
| Electrochemical | Nasopharyngeal swab | NR | a SARS-CoV-2 S protein | 1 pF up to 3 µF | NR | <2 | Android smartphone | Mobile application readout the results remotely | [7] |
| Electrochemical | Blood | Exposure for piranha solution for 1 h at 100°C | SARS-CoV-2 antibody | ∼0.3 pM | 99.2% | NR | NR | Imaging processing | [16] |
| Electrochemical | Blood | centrifugation storage at −80°C till analysis | SARS-CoV-2 S1 spike antigen | 1 fg/mL | 92.8% | <3 | Not Reported | Result readout by a mobile application | [17] |
| electrochemical | saliva | RNA extraction and nucleic acid isolation thermally | Viral antigen | Not Reported | 100% | 60 | Not Reported | Mobile application | [18] |
| electrochemical | blood | Catalyzation to amplifies immune reaction. | antigen of SARS-CoV-2 | 0.1 ng/mL, | Not Reported | 16 | Not Reported | Imaging | [19] |
| electrochemical | saliva | Dilution Incubation at room temperature for 30 min | Streptavidin protein | 64 nM = 5.12 pmole | Not Reported | <10 | The device can be connected to Android and IOS systems | Mobile application. | [20] |
| electrochemical | Sputum, Throat swab, Urine, Feces, blood | Heating for30 min at 56 °C | RNA of COVID-19 Au@Fe3O4 particles | 200 copies/mL | 85.5% | 30 | Not Reported | detect the viral RNA without amplification | [21] |
| electrochemical | Blood | sonication followed by a 3 min treatment with O2 plasma, 70% ethanol, and thermal exposure." | N and S genes | 1 copy number/μL | Not Reported | 6 min | HM-300S, Hotmine 3D, Korea | connected to a micro-controller, serves as a switch, receiving the command from the micro-controller To activate and deactivate the two boost converter circuits | [22] |
| Electrochemical | Blood | No sample preparation | anti-SARS-CoV-2 N protein specific antibodies | 5 ng/mL in low-volume (10 μL) | 96.6% | 20 min | a Samsung Galaxy S9 smartphone with a 12.2-megapixel camera | Operate the electrochemical capillary-flow device and the potentiostat | [23] |
| Electrochemical | Saliva, Nasopharyngeal swab | Hybridization Wash /remove excess | streptavidin-avidin | 30 TCID50 /mL | 80.5% | 18 | Not Reported | Result readout by a mobile application | [24] |
| Electro-chemical | blood serum samples | Centrifugation incubation dilutions | antibody for SARS-CoV-2 spike protein | 2.9 ng mL−1 | NR | NR | NR | Mobile application with a diagnostic platform | [25] |
| Electro-chemical | nasopharyngeal, throat, and nose swabs | RNA extraction,incubation, purification, thermal exposure | nucleocapsid (N) gene of SARS-cov2 | 10 copies per reaction | 100 | <20 | Android device | smartphone application | [6] |
| Electro-chemical | Serum | NR | SARS-CoV-2 nucleocapsid protein | 50 pg/mL | 97 | <1 hour | Google Pixel 2 | A diagnostic tool capable of identifying the nucleocapsid protein of SARS-CoV-2 | [26] |
| Electro-chemical | Nasopharyngeal swab | Incubation centrifugation | SARS-CoV-2 rna | 102–103 copies per reaction | 100% | 1 hour | OnePlus 6T | Detection of enhanced florescence by smartphone camera | [27] |
| Electro-chemical | Nasopharyngeal & human saliva samples | RNA extraction and amplification then incubated for 30 min. | SARS-CoV-2 nucleic acid SARS-CoV-2 (N gene | 1.0 pM | 100% | 42 | NR | The smartphone equipped with electrochemical workstation and mobile application readout. | [28] |
| Fluorescence | Nasopharyngeal swab | the swab is thermally lysed (95°C, 1 min). and the nucleic acid amplification with intercalating fluorescent dye occurs at 65°C | RNA | 50 RNA copies per μL | 100% | 30 min | Huawei P30 Pro | smartphone-based reader and for image analysis | [4] |
| Fluorescence | nasopharyngeal swab and saliva | Not mentioned | SARS-CoV-2 virus particles | 370 vp/mL | NR | 15 min | NR | smartphone App | [29] |
| Fluorescence | Serum samples | plasma stabilized in 4% sodium citrate, diluted and incubated for 10−20 min then three washes, anti-human IgG conjugated with alkaline phosphatase then addition of fluorescence substrate. | SARS-CoV-2 IgG and IgM titers & S1antigen | 50 μg/mL | NR | 5 min | iPhone 6S | Recording Enzyme AttoPhos fluorescence with a camera | [30] |
| Fluorescence | synthesized SARS-CoV-2 RNA by Gene Pharma | irradiation with a UV lamp (302 nm) in a transmission reflectance analyzer incubated 5 min at 50°C for | SARS-CoV-2 RNA | 3.77 aM (∼2 copies/μL) | NR | 40 min | iPhone 12 | application for fluorescence readout | [31] |
| Fluorescence | RNA sample | diluted in nuclease-free water, amplification, reverse transcription, denaturation and extension by thermal exposure. | RNA | 1 copy per μL, | >95% | 40 min | NR CMOS camera (IMX477, Sony, Japan) | image sensor, smartphone application readout and transmitting the quantitative results to a server computer | [32] |
| Fluorescence | Saliva and nasal swab | Lysis condition | CRISPR-Cas12a | 0.38 copies/μl | 96.6% | 15 min | Samsung Galaxy S9 smartphone with a 12.2-megapixel camera | Source of laser diode of excitation | [33] |
| Fluorescence | Nasopharyngeal swab | Incubated for 15 min at 95°C | As1e, N, E genes | 2 × 101 genome copies/μL | Not Reported | 1 min | CMOS camera upon excitation with a 488 nm LED light source | processing application | [34] |
| Fluorescence | Saliva and nasopharyngeal samples | RNA capture and concentration, using a single-syringe infusion pump. | RNA virus + detection of the B.1.1.7, B.1.351, or P.1 variants | threshold of ∼2000 relative fluorescence units (RFUs) for the detection of SARS-CoV-2 | NR | 1 hour | NR | The app uses the embedded camera in a smartphone in combination with a color segmentation algorithm | [35] |
| Fluorescence | respiratory swab samples | mineral oil was added to cover the extracted nucleic acids with amplification | RNA virus | 20 copies RNA of SARS-CoV-2 | 100% | 40 min | NR | Enable detection of fluorescence by naked eyes | [36] |
| Fluorescence | nasal swab and saliva | diluting the swab in a nontoxic buffer, and mixing with reagents | SARS-CoV-2 nucleocapsid phosphoprotein & SARS-CoV-2 RNA | 0.38 SARS-CoV-2 RNA copies/μl 0.5 to 2.5 copies/μL | 95% | 17 min | NR | software to guide for setting up the test. | [37] |
| Fluorescence | Serum and saliva samples | Human serum or saliva was stained for 6 min with the SYBR Green dye | SARS-CoV-2′s nucleocapsid gene (N-protein) | 0.1 nM | NR | NR | NR | Imaging, Processing by application | [38] |
| Flourescence. | Blood specimens | interleukin-6 antibodies and thrombin binding aptamers | 50 μg/ml for the IL-6 and 1 μM for the thrombin | Not Reported | NR | Huawei P30 Pro smartphone using a 3Dprinted dark box. the smartphone imager were analyzed using ImageJ | Imaging, Processing by application, 3D printed smartphone imager with a built in UV-LED light source was used. | [39] | |
| Fluorescence and colorimetric | Saliva samples | isothermal RPA | E gene and RdRP gene | 9.5 RNA copies per reaction for the E gene and 17 RNA copies per reaction for the RdRP gene. the dipstick method was 130 RNA copies per reaction. | NR | 20–30 min | Galaxy Nexus API 28 | “CovidApp” smartphone application | [40] |
| Colorimetric | Nasopharyngeal swab samples | Not mentioned | intact viruses | viral loads of 1 × 103 copies/mL | 100% accuracy | - | smartphones with 12-megapixel image sensors (Motorola MotoX and Apple iPhone 8) | Image analysis | [41] |
| Colorimetric | blood | Dilution of Sample incubation at 37°C for 1 h. Wash the plate 5 times, add 90 μL substrate solution to each well | neutralizing antibodies produced from vaccinations | 160 ng/mL | 95% | Not mentioned | HUAWEI P30 Pro | a portable smartphone-based reader using Lego blocks to image the LFIA results | [42] |
| Colorimetric | Blood | centrifugation. and incubation for 30 min at room temperature. | neutralizing antibodies for covid-19 | Not Reported | 83.52% | 45 min | Not Reported | Application reader, designed for sensing signals through the catalyzed substrate | [43] |
| Colorimetric | Saliva | incubated at 37°C with a 5% CO2 atmosphere. | Au nanoparticles functionalized with polyclonal antibodies (f-AuNP | 0.28 PFU mL-1 | 100% | a Samsung smartphone (Galaxy J8, 16 megapixels camera, with Android 10). | processing the images collected from the smartphone camera, measuring the concentration of SARS-CoV-2 | [44] | |
| Colorimetric | Saliva, tissue, nasopharyngeal swabs | Extraction of RNA or DNA + DNA amplification | RNA virus | <5 copies/reaction | 97% (Ct < 36.8) and 98% (Ct < 30) | < 30 min | The electronics layer of the qcLAMP device consists of three main components: a Raspberry Pi Zero W board (RPi), a Camera Pi module and a custom made PCB PRi Hat. | The sample exposed to a mini camera able to take snapshots of the color change in real time during LAMP amplification. | [45] |
| Colorimetric | Saliva | Not reported | SARS-CoV-2 spike protein | 1 ng/mL | Not reported | Not reported | Not reported | smartphone images were used to assess the sensor output by using the red chromatic shift (RCS) of the signal response | [46] |
| Colorimetric | Nasopharyngeal swab | Lysis and target RNA enrichment, | Orf1a gene and the N gene | 5 copies per µL of the sample | NR | less than one hour | Redmi 7 and iPhone | freely available “camera color picker” smartphone apps | [47] |
| Colorimetric | Blood | freeze-dried and mixed for 40 min at 37°C. The magnets were used to capture the complexes catalyzed for 10 min at 37°C | RNA virus | 10 pg/mL | 75.7% | 1 hour | NR | telemedicine | [48] |
| Colorimetric | Saliva | optimized chemical treatment and heat inactivation | SARS-CoV-2 nucleic acid | ∼100 copies | 98% | <1 hour | NR | mobile application for detection and quantification of lateral-flow strips. | [10] |
| Colorimetric | Saliva or blood | NR | SARS-CoV-2 nucleic acid and their variants viral N and S genes | ∼400 copies of starting RNA substrate | 100% | Within 1 hour | NR | smartphone app TOPSE, to assist detection by returning a predictive score based on background correction | [49] |
| Colorimetric | Serum sample | Sample deposition over the nitrocellulose membrane and kept at room temperature over-night, Thermal exposure for 30 min at 37°C, Silver enhancement | spike protein (S) and/or the nucleoprotein (N) | 0.11 ng/mL | NR | Xiaomi Redmi Note 9 and Huawei Y7 prime 2018 | ImageJ software for processing and result readout and analysis | [50] | |
| Colorimetric | blood samples | incubation for 30 min at room temperature | nucleocapsid protein (NP) | 7.5 pg/mL | 100% | 45 min | Huawei Mate 20 Pro | Image processing and data analysis via a mobile application | [51] |
| Colorimetric | nose and throat swabs | dissolve in PBS, extraction. RNA was eluted out in molecular-grade water | SARS- CoV-2 cDNA and RNA based on the viral N-gene | 3.9 × 103 RNA copies/ml | 81.66% | 15 | iPhone cameras | The quantification of LFA test and control lines was performed by using a smartphone-based in vitro diagnostics (IVD) device | [52] |
| Fluorescent, colorimetric, or electrochemical | Saliva | diluted 10-fold Isothermal incubation at 65°C for 35 min | viral RNA | less than 35 × 104 viral particles /mL | NR | 35 min | iPhone6 | A smartphone device is used to image the samples containing fluorescent beads post-RT-LAMP and correlates decreased diffusivity to positive sample | [53] |
| Thermal | Thermal images of the participants’ exposed upper backs | NO sample | lungs | 92% | 62% | up to 3 min | FLIR ONE thermal camera device (both iOS- and Android) | image processing using a portable thermal camera connected to the phone | [54] |
| Thermal | droplets | Thermal exposure | detection of SARS-CoV-2 RNA | 30 fM of target ssRNA | NR | 20min | NR | application to avoid user errors in readout | [55] |
| Ultrasound | No sample | - | Lung imaging | lung ultrasound for COVID has demonstrated an extremely high sensitivity for detecting such abnormalities | Depend on the lung expert. | - | Samsung Galaxy Tablet A | portable smartphone support ultrasound probes for communicating with a remote expert using Zoom Teleconferencing (telemedicine) | [56] |
| Ultrasound | lung ultrasound | NR | Lung imaging | a minimum 720p video | Depends on a remote clinical ultrasound expert | NR | NR | smartphone-supported tele-ultrasound system using online video conferencing | [57] |
| Flow-meter | Saliva | viruses were cultured and the cell lysates were collected and centrifuged | antigen antibody reaction | 1 fg/μL SARS-CoV-2 from 1% saliva and 10 fg/μL from simulated saline gargle | 89% | <15 | NR | A smartphone camera automatically measured the flow profile | [58] |
| light sensor | Nasal or oropharyngeal swab | cultured in Vero cells and the RNA was extracted | nucleocapsid (N) gene | 11.46 fM | 100% | within 20 min | NR | Presenting diagnostic test results to the user via Bluetooth | [59] |
Electrochemical biosensors
The use of electroanalytical tools for point-of-care (POC) rapid testing of severe acute respiratory syndrome coronavirus 2 is an area of significant interest. However, there are several nurturing challenges that should be considered in order to enable the effective utilization of these novel bioanalytical devices [1]. Traditional methods of COVID-19 detection require several complicated, and laborious steps. The ultimate goal of these POCTs is to be reagent-free with minimal or no sample treatment, without sacrificing the selectivity. The integration of electrochemical amplification and recognition can be effective but expensive, whereas molecular amplification necessitates the use of costly and highly purified biochemicals. By utilizing CRISPR-based biosensing, it is possible to conduct an extremely sensitive assay without the need for amplification, which offers a novel biosensing mechanism that is much more selective, sensitive and rapid than Monitoring of amplicons through either ELISA or fluorometry.
Although advancements have been made in the technologies of m-health, IoT, and machine learning, there is a lack in uniform databases or Systems used for communication to provide real-time information on completed tests that were used for diagnosis and their findings, as well as exposure risk, clinical, and other information. Different methods have been recorded for electrochemical detection of SARS-CoV-2 using nasopharyngeal swabs, saliva, or blood. Soares et al. developed a portable centrifugal microfluidic platform that utilizes beads, as well as a LAMP-based technique for detecting viral RNA from nasopharyngeal swab specimens that have been heat-inactivated. The accuracy was perfect, and the evaluation took one hour. [27]. Manazo et al. used an isothermal amplification (RT-LAMP) reaction to create a quick POC test that performs diagnosis and uses the technology of semiconductors to identify Severe acute respiratory syndrome coronavirus 2 in RNA samples that have been extracted. It is possible to detect nucleic acid by observing alterations in pH levels that arise as a result of the amplification of nucleic acid using the isothermal method. The accuracy was 99.37%, and within 20 min, results were visualized on a smartphone [6].
Beduk et al. combined gold nanoparticles (AuNPs) and laser-scribed graphene (LSG) sensors as reliable, promising biosensing systems. ACE2, an enzymatic receptor, was selected as the target, it has the ability to strongly bind with spike proteins through a mechanism that can be likened to a key-lock model. The device has effectively been utilized to test 63 nasopharyngeal swabs that were obtained from individuals who had been diagnosed with COVID-19, and the exceptional time efficiency was 1 min with an accuracy of 99.37% [15]. Georgas et al. created a SARS-CoV-2 electrochemical biosensor using the virus' structural spike (S) protein's affinity for the ACE2 protein. A conductive electrode transducer has ACE2 fixed inside of it, and When angiotensin converting enzyme 2 is bound by the S protein of the virus, the transducer's electrical characteristics alter, increasing its effective capacitance. Differentiation between positive and negative samples is achievable by having a measured response that is decreasing in shape and a maximum alteration in capacitance that exceeds two percent. The experiments could be repeated with both genuine virus samples and S protein samples [7].
These studies have shown promising results for the use of electroanalytical tools that enable the swift identification of SARS-CoV-2. However, numerous obstacles must still be considered to enable their effective application as POC devices. These challenges include poor selectivity, high cost, lack of uniform databases and communication systems, and limited information on how these biosensors can be turned into POC devices. Additional investigation is required to overcome these obstacles and develop more accurate, efficient, and user-friendly electroanalytical tools for the Swift identification of SARS-COV-2.
The COVID-19 pandemic has led to an unparalleled demand for quick, low-cost, and accurate diagnostic methods. In response, researchers have developed electrochemical biosensors and lateral flow strip-integrated nanozyme-based chemiluminescence assays To detect the existence of the SARS-CoV-2 antigens in human saliva.
Singh et al developed a technique of detecting SARS-CoV-2 antigens in saliva based on aptamers using commercially available glucometers and low-cost chemicals per test [18]. This method is scalable due to its reliance on a widely used diagnostic tool, the glucometer, and its ability to integrate with electronic health data systems via smartphone connectivity. In clinical trials, this assay identified SARS-CoV-2 infection in patient saliva in a very short period of time, as one hour and with a sensitivity of 100%, while also effectively distinguishing between infected specimens and controls. However, limitations include aptamer selection based on literature rather than custom SELEX, lack of virion lysis potentially leaving complex mixtures of intact virus and protein, retrospective and limited clinical dataset, and greater variability at higher antigen concentrations attributed to the heterogeneity of saliva.
A rapid and precise SARS-CoV-2 spike antigen detection assay was developed by Liu et al, which integrated a lateral flow strip with a paper-based chemiluminescence assay using nanozymes [19]. By using Co-Fe@hemin-peroxidase nanozyme, which can produce chemiluminescence similar to natural peroxidase HRP the signal of an immune response is amplified. The test is capable of identifying SARS-CoV-2 antigen with high sensitivity, with a detection threshold of 0.1 ng/mL and with range of linearity of 0.2–100 ng/mL, and it does not generate false positives by reacting with influenza A subtypes or other coronaviruses. The test can be conducted within a duration of 16 min and signal identification possible with a smartphone camera. Although this research demonstrated the sensitivity of the test for recombinant antigen and pseudovirus, further confirmatory research is needed for actual clinical samples like saliva or nasal swab samples. Additionally, the accuracy of this method is not reported. Electrochemical biosensors and lateral flow strip-integrated nanozyme-based chemiluminescence assays offer low-cost, quick, and precise diagnostic techniques for detecting SARS-CoV-2 antigens. However, both methods have limitations that need to be addressed, such as aptamer selection and heterogeneity of saliva, and further research is needed to confirm their accuracy for clinical samples. These innovations propose a promising solution for early identifying of the infection of SARS-COV-2 and reducing the Economic burden on healthcare facilities.
Wu et al. [20] presented a bioassay platform that is affordable, portable, and speedy called MagiCoil that uses magnetic particle spectrometry (MPS) to enable sensitive and quantitative bioassays. The device utilizes the harmonics from oscillating magnetic nanoparticles as metrics for identifying the dynamic magnetic responses of these particles. The authors have shown that the present device can detect streptavidin with a limit of detection of 64 nM, while by modifying the surface on magnetic nanoparticles, the device's flexibility enables the detection of different diseases. Although the MPS-based bioassays exhibit high levels of sensitivity, the portable device presently faces challenges related to inadequate sensitivity and necessitates further improvements. The device's features, however, make it suitable for testing in real-world, non-clinical settings such as classroom, home, and office.
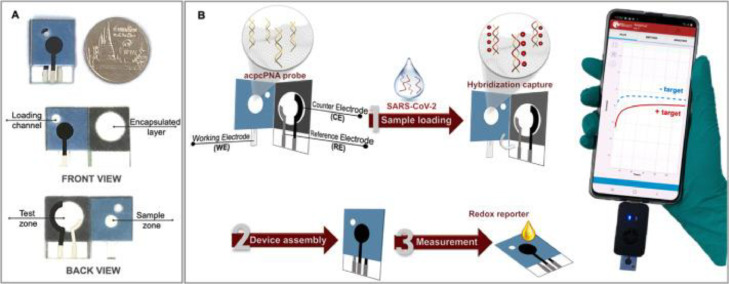
In other studies, Lomae et al. [28] utilized a smartphone-assisted paper-based electrochemical sensor and Sensit Smart potentiostat to identify SARS-COV-2 in nasopharyngeal and salivary specimen. The accuracy of this method was reported to be 100%, and the duration of measurement was 42 min. The proposed PNA-based ePAD is illustrated in Fig. 2 , which details the detection mechanism and process for identifying SARS-CoV-2 using a PNA-based electrochemical paper-based analytical device (ePAD) sensor that is linked to a potentiostat controlled by a smartphone.
Fig. 2.
(A) The PNA-based ePAD displaying its anterior and rear aspects. (B) The process of detection for SARS-CoV-2 using the PNA-based ePAD sensor, linked to a potentiostat based on smartphones. With permission from [28].
An immunosensor was created by Li et al. [26] to measure the nucleocapsid protein of SARS-CoV-2 in serum with exceptional sensitivity and speed, the device is linked to a smartphone. The device involves the use of magnetic nanobeads that for amplifying the signal and carrying out immunomagnetic enrichment, and it was shown to produce results within less than 60 min with an accuracy of 97%. These studies highlight the potential of portable diagnostic devices that use novel approaches to accurately detect SARS-CoV-2 in nonclinical settings. While some methods require further improvements, they have the potential to reduce the strain on local healthcare systems and increase access to testing in areas lacking adequate medical resources.
In this context, Samper et al. [23] created an electrochemical capillary-flow device capable of measuring IgG antibodies against SARS-COV-2 nucleocapsid proteins (anti-N antibody) with outstanding sensitivity and accuracy. A membrane for filtering blood is integrated into the device, allowing for plasma extraction on-site, which removes the requirement for preparing the sample beforehand. The passive microfluidic circuit (ELISA) consisting of layers of double-sided adhesive films and hydrophilic polyester layers automates the stages of an enzyme-linked immunosorbent assay. The membrane was coated with a recombinant nucleocapsid protein to receive the sample, and the anti-N antibodies were identified using chronoamperometry on a screen-printed carbon electrode.
The cost-effective and simple design of the device allows for its potential use at POC by untrained users. Furthermore, the apparatus is capable of being linked to a smartphone-operated near-field communication potentiostat, demonstrating its real POC potential. The cutting-edge electrochemical capillary-flow device may help with the diagnosis of communicable illnesses, particularly at the point of treatment.
Similarly, Nguyen et al. [22] proposed using a CE analyzer in a handheld-style is integrated, automated, and smartphone-controlled to detect SARS-COV-2. The smartphone's electricity was utilized as a light source for the blue dot laser, and Python codes were used for automation. The entire procedure was completed within six minutes, and the smartphone's built-in web app was used for data processing. The setup includes a microcontroller, a smartphone, a laser, a CE chip, two booster converter circuits, and a relay. Both the operation of the CE and the laser turn-on procedure are powered by the smartphone, and the fluorescence signature of the RT-PCR using the smartphone's CMOS camera equipped with a filter for fluorescence detection (Fig. 3 ).
Fig. 3.
An overview showcasing the entirety of the system. (B) The internal components of the system. (C) A top-down view of the μCE system. With permission from [22].
In another study, Mavrikou et al. [17] presented a proof-of-concept creation of a novel bioelectric biosensor to rapidly detect of IgG antibodies against the spike (S) protein antigen of SARS-COV-2 that utilizes immobilized Vero cells that express the corresponding antigen (HBsAg). The biosensor consists of a one-use collection of screen-printed electrodes that have been designed to be immobilized and express the S1 protein. When antibodies against S1 are administered to the engineered cell population, a quick, focused, and specific modification in the potential of the cellular membrane takes place, which is then detected by a specially designed handheld potentiometer. The finished product is wirelessly sent to a smartphone using Bluetooth technology with a personalised graphical user interface. The detection limit for anti-S1 antibodies was demonstrated to be 5 ng/mL, and the accuracy of this method was 92.8%. Clinical study outcomes came from patients who had received the vaccine or had already contracted the virus. The selectivity of the test was showcased by the absence of cross-reactivity with antibodies against other respiratory viruses. Their approach aims to make it easier to accurately identify antibodies in the initial phases post-vaccination and to keep track of how long and to what extent immunity develops both in a medical testing and self-administered tests after additional clinical verification and expansion to test for IgM, IgA, and potentially neutralizing antibodies.
In the current COVID-19 pandemic, point-of-care (POC) testing using microfluidic devices and smartphones has emerged as a promising approach to bridge the gap between laboratory-based diagnosis and rapid clinical decision-making. Nguyen et al. [16] developed an integrated, miniaturized capillary electrophoresis (CE) system that can be controlled by a smartphone. The fluorescence detection of reverse transcription-polymerase chain reaction (RT-PCR) amplicons was carried out on-board by the system, which used 2 boost converters, an excited laser, and a CMOS camera. In addition, they designed an online application that displays typical capillary electropherograms on a smartphone using the fluorescence intensity and its running duration data. The authors were able to correctly analyze the S and N genes of SARS-COV-2 in six samples using their integrated smartphone-associated CE system. Similarly, Beduk et al. [25] designed a portable POC detection tool that can be used to operate a custom smartphone application. By employing Laser-Scribed Graphene Biosensor, they developed an electrochemical immunoassay that used blood serum or plasma without any prior preparation. Using commercial RT-PCR, all patient specimens were clinically specified whether being COVID-19 negative or positive. The efficiency of the gold-modified LSG sensors was also evaluated using antibody and ELISA IgG and IgA assays. Fig. 4 shows the portable device and the reaction of patients with COVID-19 positive (+) and negative (-) is measured.
Fig. 4.
(a) A PoC device. (b) Display of DPVs (differential pulse voltammograms) (c) DPVs response of the AuNPs-LSG sensor (With permission from [25])
Zhao et al. [21] reported a technology for electrochemical detection With a high level of sensitivity. which targeted the RNA of SARS-CoV-2 using graphene oxide functionalized with calixarene. Utilizing a portable electrochemical device with smartphone connectivity, the technology adopted a supersandwich-type recognition strategy and was able to detect SARS-CoV-2 RNA without the need for nucleic acid amplification or reverse-transcription, thus demonstrating practicality. Thus, the biosensor exhibited exceptional specificity and selectivity in both in silico analysis and experimental testing, where it was tested against 88 RNA extracts from 25 patients confirmed to have SARS-CoV-2 and 8 patients in recovery. The biosensor demonstrated better detectable ratios compared to RT-qPCR, with a limit of detection (LOD) of 200 copies/mL, which is the lowest reported among RNA measurements of SARS-CoV-2. Furthermore, the assay required only two copies (10 μL) of the virus.
The authors carried out differential pulse voltammetry (DPV) with the help of a Smartphone equipped with a Sensit Smart electrochemical sensor. They conducted JEM 2100 transmission electron microscopy to describe the morphologies of the processed samples, FTIR research, and TGA analysis using, respectively, a Nicolet IS10 Fourier transform infrared (FTIR) spectrophotometer Impact 410 from Thermo Fisher Scientific and a Q50 thermogravimetric analysis (TGA) device from New Castle, USA. The X-ray photoelectron spectroscopy (XPS) research was conducted utilizing an ESCALAB 250 photoelectron spectrometer, while the X-ray powder diffraction (XRD) investigation was conducted using a Bruker D8-advance X-ray diffractometer. Finally, zeta potential observations were conducted using an electrochemical workstation called a Malvern Zetasizer Nano. For the qPCR work, a QuantStudio 5 Real-Time PCR System was used.
These studies showcase the capabilities of innovative technological advancements in the identification or diagnosis of communicable diseases, particularly COVID-19, through POC devices that allow for quick and easy diagnosis outside of traditional laboratory settings. By utilizing smartphones and microfluidic circuits, these devices may have a notable influence on the accurate and widespread identification of antibodies during the initial phase after vaccination and in tracking the strength and persistence of the acquired immune response. As we move towards the fourth industrial revolution, it is clear that the technology related to information and communication will be essential to developing a state-of-the-art system for monitoring health.
These papers showed that the integration of miniaturized CE systems and electrochemical immunoassays with smartphones has enabled rapid and accurate diagnosis outside of traditional laboratory settings. These technologies have enormous potential to enable widespread and dependable identification of antibodies during the initial phases post-vaccination and to track the duration and degree of acquired immunity. Their implementation should be expedited, providing a possible way forward for point-of-care testing during pandemics.
Fluorescence biosensors
The outbreak of COVID-19 has emphasized the necessity for prompt and precise diagnostic equipment for point-of-care testing that can be used outside of traditional laboratory settings. Chen et al. [36] created an extremely sensitive diagnostic method utilizing a smartphone and a portable 3D printer to visualize the fluorescence signal created without the use of special equipment. The study was based on respiratory swab samples, where nucleic acids and RT-LAMP solution were covered with mineral oil, and CRISPR/Cas12a reagents were added within the tube cover. After the RT-LAMP reaction, the CRISPR/Cas12a reagents were combined with the amplification reagents using manual shaking, preventing contamination from amplicon aerosol by not opening the lid twice throughout the entire operation. A green fluorescence could be detected at concentrations of 2 × 104, 2 × 103, 2 × 102, 2 × 101 copies of RNA samples (ORF gene as the target fragments). No green fluorescence signal was detected for the sample of 2 × 10° copies and there was no template control group. The fluorescence produced could be seen without the aid of specialized equipment by using a portable 3D printer and a smartphone, enabling visual detection, which holds promise for POC detection. The whole process of amplification and detection could be finished in forty minutes with a sensitivity of 20 RNA copies of SARS-CoV-2, and the results of detection showed complete accuracy for Seven samples collected from patients who tested positive for respiratory illness, as well as three samples collected from patients who tested negative, were provided by the Zhejiang Provincial Center for Disease Control and Prevention.
Similarly, Ganguli et al. [4] utilized a portable diagnostic method based on a 3D cartridge made additively, and an optical reader built into a smartphone to establish RT-LAMP for identifying the presence of the SARS-CoV-2 POC. The authors demonstrated the POC detection model by detecting SARS-CoV-2 in ten clinical samples containing viral transport medium (VTM). The POC instrument was built for affordability, accessibility, and scalability, utilizing components that were easily created on a consumer 3D printer and a housing that was readily accessible in the marketplace. With a smartphone, optical detection could be done. The disposable cartridge was produced using high-speed, production-grade additive manufacturing machinery, which does not require any additional labor when produced in large quantities. The basic idea behind how it works is relatively straightforward and requires very little training since the whole assay can be completed inside the cartridge. Nasopharyngeal samples were placed in the chip to detect the fluorescent intensity arising from the sample with 100% accuracy in detection within 30 min.
These studies show the capability of innovative technological advancements in the identification of infectious diseases, particularly COVID-19, through portable and easy-to-use POC devices that allow for quick and easy diagnosis outside of traditional laboratory settings. By utilizing smartphones, 3D printing, and optical detection, these devices have enormous potential to aid in the widespread and dependable identification of antibodies during the initial phases after vaccination, and to monitor the strength and persistence of immunity obtained.
Both methods reported here are highly sensitive due to their utilization of CRISPR/Cas12a and RT-LAMP technologies, allowing quick and accurate identification of SARS-CoV-2. These technologies offer promise for creating of low-cost POC diagnostic instruments that could potentially be utilized for use in resource-limited areas. Their implementation should be expedited, providing a possible way forward for POC testing during pandemics. The integration of CRISPR/Cas12a and RT-LAMP technologies with smartphones and 3D printing has enabled rapid and accurate diagnosis outside of traditional laboratory settings. These technologies have enormous potential to assist in the widespread and dependable identification of antibodies during the initial stages following vaccination and in tracking the strength and persistence of acquired immunity. Their implementation should be expedited, providing a possible way forward for POC testing during pandemics.
Nguyen et al. [34] developed a cutting-edge POC device utilizing the Internet of Things (IoT) for real-time, isothermal amplification-mediated, direct reverse transcription-loop assay to identify SARS-CoV-2. The testing device was compact, lightweight, and powered by a smartphone and a portable battery. The integrated microfluidic chip had four reaction chambers designed to address the As1e, N, and E genes as well as a negative control, enabling the concurrent analysis of several SARS-CoV-2 genes. The CMOS camera captured the fluorescence intensities of every compartment after being stimulated by a 488 nm LED light emitter. The IoT-based POC device's microprocessor processed the recorded data before transmitting it wirelessly and displaying it on the linked smartphone in real-time. With three SARS-CoV-2 primer sets and the lowest concentration that can be reliably detected is 2 × 101 genome copies/µL, positive results could be obtained with high accuracy and high detection capability. This platform offers a cutting-edge molecular diagnostic instrument to check for SARS-CoV-2 wherever and whenever required.
Chen et al. [36], Ganguli et al. [4], and Nguyen et al. [34] all utilized nasopharyngeal specimens for the identification of SARS-CoV-2 virus with 100% accuracy, making the devices used in these studies not competitive in terms of specificity. However, the detection process time can distinguish between the devices used in the different studies, with Chen et al. [36] taking 40 min, Ganguli et al. [4] taking 30 min, and Nguyen et al. [34] taking only one minute to detect the virus. Therefore, the device developed by Nguyen et al. [34] is advantageous for the bedside detection of the virus.
De Puig et al. designed a special smartphone application for the identification of SARS-CoV-2 virus [35]. The study utilized specimen from the nasopharynx and saliva, employing an Aladdin infusion pump with a single syringe to demonstrate the capture and concentration of RNA on paper. An optical filter, LEDs, and fluorescent readout were present in the low-heat section. The authors created miSHERLOCK, a cost-effective point-of-care diagnostic system based on CRISPR that uses raw patient utilizing saliva as a means to obtain, refine, and intensify viral RNA, can perform amplification and detection protocols and generate a visual fluorescent output with just three user interactions in under an hour, from the time of sample input to output reply. The miSHERLOCK tool detected SARS-CoV-2 with 96% sensitivity and 95% specificity when the threshold was set at 2000 relative fluorescence units (RFUs) for clinical saliva samples with varying viral loads. At the same time, RT-qPCR testing validated the miSHERLOCK device's ability to detect SARS-CoV-2 in clinical saliva samples with 96% sensitivity and 95% particularly using a threshold of 2000 RFUs.
Panpradist et al. [37] utilized reverse transcription loop-mediated isothermal amplification (RT-LAMP), which enables the simultaneous fluorescence detection of several targets, to develop the Harmony assay. The four-plexed test amplified the SARS-CoV-2 nucleocapsid phosphoprotein (N gene) and an engineered IAC simultaneously. Fluorescence was detected by a detector linked to mobile software. Nasal swabs and saliva samples were used, and any of the three SARS-CoV-2 targets had to be present for a test to be positive. The results showed the potential of the Harmony assay for rapid and accurate POC diagnosis of COVID-19
The work of Beduk et al and Huang et al was similar to the work of Georgas et al. [7] where gold nanoparticles (AuNPs) and laser-scribed graphene (LSG) sensors were combined as reliable, promising biosensing systems [15,29]. However, Huang et al created a straightforward, inexpensive tool that uses a nano-plasmonic biosensor integrated with a typical 96-well plate or chip cartridge to quickly and accurately identify the SARS-CoV-2 virus. Their research proved that the nano-plasmonic sensor device has the Outstanding speed (15 min) and accuracy (LOD = 370 vp/mL) needed to directly identify the entire Severe acute respiratory syndrome coronavirus 2. Besides that, similar quick and sensitive detection abilities are shown using inexpensive handheld optical equipment that can be managed using a smartphone application. The SARS-CoV-2 pseudovirus could only be quantified to a maximum of 4000 particles in the smart phone within a 15 min period by fluorescence detection [29].
Ning et al outlined the creation of a handheld, COVID-19 test using saliva samples. The test provides results within 15 min and does not require extracting RNA molecules from a biological sample or specialized laboratory equipment. In this assay, the smartphone-based fluorescence microscope's laser diode instrument stimulates the viral amplicon signal by increasing CRISPR-Cas12a activity. the new tool demonstrated that the smallest quantity of viral particles that can be reliably detected by an assay was lower than the RT-PCR reference assay (0.38 copies/l) and accurately measured the concentration of virus particles in a sample, the tool has a wide dynamic range and can quantitatively detect from 1 copy/L up to 105 copies/L. SARS-CoV-2 is detected by CRISPR (severe acute respiratory syndrome coronavirus 2). The prospective application of this portable test for COVID-19 diagnosis at the point of care using saliva samples is supported by similar RNA levels in samples of saliva from patients and swabs taken from the nasal area, excellent correlation between quantities of virus detected using RT-PCR and the CRISPR assay read by the smartphone, and similar RNA levels in both samples. This assay platform has a number of characteristics that should make it appropriate for application in diverse point-of-care testing settings. For example, it analyses saliva specimen that can be obtained directly by the individual undergoing testing, thereby alleviating the requirements on healthcare personnel, it displays strong and reliable performance when faced with significant fluctuations in sample dilution, denaturation, as well as varying CRISPR-FDS reaction temperatures and durations, and it utilizes a low-cost, compact smartphone-linked reader [33].
Panpradist et al. [37], de Puig et al. [35] and Huang et al. [29] had all created their point-of-care devices to detect SARS-CoV-2 virus using nasopharyngeal and saliva specimens with detection accuracy 95 and 96.6% for Panpradist et al and Ning et al [33] devices respectively. Comparing the time taken for the detection process for the 4 studies, it revealed that the process takes 60 min, 17 min, 15 min,15 min, respectively making Ning et al device the most accurate and least time consuming bedside diagnostic device for diagnosis of COVID-19 by fluorescent detection from nasopharyngeal and saliva human samples.
Cherkaoui et al. [40] designed a fast molecular diagnostic tool for the detection of SARS-CoV-2 using reverse transcription-recombinase polymerase amplification (RT-RPA). The study utilized saliva samples and detected two gene targets simultaneously. The assay employed real-time fluorescence and dipstick methods and could detect the virus within 20–30 min. The authors also created a smartphone app for capturing and analyzing the output. By providing two detection approaches, the assay will be more available in diverse environments, depending on their available means. Although the authors did not report the accuracy of their device, the short detection duration makes it a viable option for rapid, near-patient virus detection.
Yoo et al. [38] aimed to design a diagnostic assay that would allow quantification of SARS-CoV-2 in serum or saliva samples. The study used "lead-start isothermal amplification," which allowed DNA polymerases to be activated or deactivated at room temperature on demand using lead ion (Pb2+) activation. The authors linked GR5 DNAzyme-responsive strand cleavage in response to Pb2+ with DNA polymerase inhibition by the TQ30 aptame, resulting in either starter or terminator of isothermal DNA amplification. The authors used a smartphone camera to collect the fluorescence signals of the resultant amplicons and used mobile software for image analysis. The authors did not report the accuracy of their device, but the technique is sensitive, efficient, and accurate, addressing the needs of POC virus RNA detection while requiring little equipment.
Reis et al. [30] carried out a four-plex sandwich enzyme-linked immunosorbent assay (ELISA) for the determination of serotype-specific levels of the dengue NS1 protein using serum samples obtained from human patients. and a single-plex measurement of SARS-CoV-2 IgM and IgG antibodies in simulated samples and recovered COVID-19 human samples. The authors used syphon cassettes, and with the aid of a smartphone camera and a cheap LED light source, the AttoPhos fluorescence signal was captured, facilitating the on-site or non-laboratory-based efficient immunoassays required for the diagnosis and monitoring of significant illnesses like dengue and SARS-CoV-2. The authors reported that the syphon tools are appropriate for serological COVID-19 testing, including measuring antibody titers.
Hang et al. [31] demonstrated that the end-point fluorescence signal output of RTF-EXPAR may be used in conjunction with CRISPR-Cas12a to diagnose SARS-CoV-2 in 40 min, achieving an extremely low detection threshold of 3.77 aM (less than 2 copies/L). They employed the smartphone in conjunction with a smartphone application for interpreting fluorescence outcomes, which is able to identify viral RNA up to a sensitivity level of 4.81 aM (3 copies/L), further streamlining the test and providing a user-friendly diagnostic platform. Although Hang et al. did not report on the accuracy of their device, the technique is sensitive, efficient, and accurate, addressing the needs of POC virus RNA detection while requiring little equipment.
Choi et al. [32] offered a portable, energy-efficient, and user-friendly multiplexed digital PCR (IM-dPCR) system that is connected with the Internet of Things. The study used synthetic RNA samples to assess the effectiveness of their apparatus. The authors integrated a 9-plexed RT-dPCR experiment with a fluorescence imaging technique with multiple colors and created a smartphone app to operate the dPCR gadget and transmit the numerical results to a remote server computer. The authors reported that both the IM-dPCR and commercial real-time PCR systems accurately identified SARS-CoV-2 virus, but IM-dPCR method was more sensitive at low concentrations (10 copies per µL), suggesting that it was able to identify the virus accurately and more sensitively. In comparison to the commercial system, the IM-dPCR system also demonstrated a 2-fold quicker turnaround time (40 min), while maintaining portability, repeatability, and flexibility of use.
These studies offer a cutting-edge molecular diagnostic instrument to check for SARS-CoV-2 wherever and whenever needed. Future research should continue to focus on developing affordable and east to use devices for mass testing in resource-limited areas.
Colorimetric biosensors
Colorimetric diagnostic tools for the identification of SARS-CoV-2 have become increasingly crucial amid the COVID-19 pandemic. Numerous research efforts have been dedicated to the creation of innovative technologies to address this need. An example of such a study by Colbert et al. developed a portable device that combines reverse transcription loop mediated isothermal amplification (RT-LAMP) with particle diffusometry (PD) to effectively detect SARS-CoV-2 within a time frame of 35 min [53]. The study utilized saliva samples and found capable of detecting to 35 × 104 viral particles in every milliliter of saliva utilizing colorimetric, fluorescence or electrochemical signals. RNA or heat-treated viral samples were diluted in a consecutive 10-fold manner, and the samples underwent isothermal heating at a temperature of 65 °C for a duration of 35 min using a QuantStudio 5 Real-Time PCR instrument. An iPhone6-powered specialized microscope gadget was used to record 30 s movies of experimental data, which were then processed by a proprietary smartphone programme. The researchers correlated decreased diffusivity with good sample results by viewing samples that contain fluorescent beads after using RT-LAMP [53].
Another study by Prainito et al. utilized smartphone photos to evaluate a plasmonic biosensor's signal response following exposure to the spike protein of the SARS-CoV-2 virus [46]. Once the images captured by the smartphone camera, a smartphone app was employed to diagnose the condition by determining the level of SARS-CoV-2. The authors depended on saliva with a plaque-forming unit, the minimum detectable amount of 0.28 plaque-forming units per milliliter (PFU/mL) in human saliva and the range of concentrations from 1 to 100 nanograms per milliliter (ng/mL). To gather precise quantitative data, smartphone photos of the polydopamine (PDA)-coated polyvinylidene fluoride (PVDF) membranes were analyzed with the assistance of a computer application. The experimental findings indicated that the manufactured PDA sensors had great selectivity and were only weakly affected by pH and temperature. The affordable price, exceptional sensitivity, and user-friendly structure of this rapid, color-based sensing device make it applicable in impoverished nations [46].
Azmi et al. solved the problem of the need for pretest RNA extraction by using CASSPIT (Cas13 Assisted Saliva-based and Smartphone Integrated Testing) with the utilization of saliva samples directly and the absence of a separate RNA extraction process for the identification of SARS-CoV-2 [60]. Lateral flow assay (LFA) and a semi-quantitative method of detection were integrated to deliver highly precise testing output. The test strip result was linked to a smartphone application to allow data visualization and home testing.
Finally, Materón et al. created employing a mobile application following the image processing from the smartphone's camera to ascertain the amount of SARS-CoV-2 [34]. Employing a Samsung mobile device (Galaxy J8, 16 megapixel camera, Android 10), images were obtained from the microcentrifuge tube for sensing using a smartphone. The RGB (red, green, blue) mean qualities were considered as a logical sign progressively through the free application.
Materón et al. [44] created an electrochemical device for the identification of SARS-CoV-2 in human saliva using a pyrrolidinyl peptide nucleic acid (acpcPNA) probe. The biological recognition component of the working electrode was changed to acpcPNA to capture the target complementary DNA (cDNA). Hybridization with the acpcPNA probe prevented redox conversion of the redox reporter in the existence of the desireded cDNA, reducing the electrochemical response to SARS-CoV-2 concentration. The researchers successfully identified SARS-CoV-2 in human saliva with a detection threshold of 0.28 mL-1 plaque-forming units (PFU) with 100% accuracy.
Papadakis et al. [45] created a colorimetric biosensor for LAMP amplification-based detection of SARS-CoV-2 in various specimens, such as nasopharyngeal swabs, saliva, and tissue. The device employs a plastic tube positioned with 90 degree on a heated platform with a small camera exposed on the side walls to capture real-time images of the color shift occurring during LAMP amplification. The electronics layer consists of a Raspberry Pi Zero W board, a Camera Pi module, and a specially designed PCB RPi Hat. While the Camera Pi is used for picture acquisition, the Raspberry Pi serves as the system's main computer. The PCB Hat is a specific interface to regulate the electrical input and the functioning of the heating element, sensor, and light-emitting diodes (LEDs). The authors were able to detect SARS-CoV-2 in saliva samples within less than 30 min.
Azmi et al. [60] designed a fast, non-invasive device of giagnosis for SARS-CoV-2 detection in saliva based on electrochemical impedance spectroscopy (EIS). The authors used gold nanoparticles (AuNPs) as a sensing platform with excellent sensitivity and specificity for SARS-CoV-2 detection. The device demonstrated the ability to detect SARS-CoV-2 in saliva samples with 98% accuracy within an hour.
Prainito et al. [46] designed a colorimetric biosensor for detection of the SARS-CoV-2 virus in saliva specimens using aptamers. The device utilizes aptamers using as a capturing element to trap SARS-CoV-2 virus particles. The researchers successfully identified SARS-CoV-2 in saliva samples, achieving a detection limit of 0.17 ng/μL.
Colbert et al. [53] developed a diagnostic device for SARS-CoV-2 detection in saliva samples using fluorescence, colorimetric, and electrochemical biosensors. The diagnosis process takes only 35 min to reveal the results on a smartphone. The researchers successfully identified the presence of SARS-CoV-2 in saliva samples with a minimum detectable amount of 5 nM.
The clinical value of biosensors in SARS-CoV-2 detection has been established through several studies. Papadakis et al. [45] demonstrated the clinical value of their biosensor in detecting SARS-CoV-2 in various specimens, such as nasopharyngeal swabs, saliva, and biological tissue. The researchers successfully identified the presence of SARS-CoV-2 within less than 30 min in saliva samples, establishing the clinical value of their biosensor for rapid SARS-CoV-2 detection.
Furthermore, Materón et al. [44] showed the high accuracy of their electrochemical sensor in detecting SARS-CoV-2 was identified in saliva from human individuals with complete accuracy. Azmi et al. [60] also demonstrated the high accuracy of their EIS-based diagnostic device in detecting SARS-CoV-2 within samples of saliva with 98% accuracy within an hour.
Moreover, the low detection limit of biosensors has been demonstrated by several studies. Papadakis et al. [45] successfully identified the presence of SARS-CoV-2 in various specimens, such as nasopharyngeal swabs, saliva, and tissue. With a low detection limit of less than 5 copies/reaction. Materón et al. [44] were able to detect SARS-CoV-2 in human saliva with a threshold of detection as low as 0.28 mL-1 plaque-forming units (PFU) with 100% accuracy.
Agarwal et al. [52] utilized Reverse Transcription Loop-Mediated Isothermal Amplification (RTLAMP) in addition to Lateral Flow Assay (LFA) to identidy SARS-CoV-2 in nasopharyngeal swabs. The team introduced some modifications to minimize the lack of specificity in the LFA output, including the employment of helicase and reverse transcriptase (RTase) enzymes to increase polymerase activity. They also utilized an in vitro diagnostic (IVD) that is based on a smartphone system to quantify the LFA test and control lines, achieving an accuracy rate of 81.66% within 15 min.
Bokelmann et al. [47] developed Cap-iLAMP (capture and improved loop-mediated isothermal amplification) by combining an enhanced color-based RT-LAMP assay with a RNA extraction based on hybridization capture for samples obtained from gargling with a liquid solution. Smartphone-based color grading was used to identify the N gene and Orf1a gene. With the use of the iPhone and Redmi 7, free smartphone apps were used to convert RGB values into hue, allowing the quantification of the number of viral genomes present in a sample ranging from 100 to 500. The study achieved a minimum amount that can be reliably detected of 5–25 viral genome copies per l when using RNA concentrated from 500 l of gargle lavage to 25 l of the final volume.
Shokr et al. [41] standardized an assay that when detecting certain viral particles or genetic material, the microfluidic chip generates a visual result which can be detected with a smartphone camera. The signal is produced by coupling metal nanocatalysts with target-specific antibodies (nanoprobes). The team created accurate image recognition algorithms that can distinguish between positive and negative microchip images using a smartphone camera and adversary machine learning techniques. This study achieved a detection rate of 100% accuracy.
Ghorbanizamani et al. [61] developed a diagnostic test that uses fuchsine dye-loaded polymersomes for a COVID-19 diagnostic test that utilizes a paper-based dot blot assay and smartphone technology to detect spike proteins through changes in color. The objective of this platform is to offer a versatile alternative to traditional nanoparticle-based assays that use gold and silver, with comparable performance and capabilities. The team used serum through biomedical paper-based colorimetric biosensors. A smartphone was used to take pictures, and the analysis of them was done by special software.
Liu et al. [48] produced 37 serum samples by collecting blood from twenty patients who had a prior positive COVID-19 test result with NAT and employed a nanozyme-linked immunosorbent assay (NLISA) that can be operated using a smartphone (SP-NLISA) to detect the unique nucleocapsid phosphoprotein (NP) of SARS-CoV-2. The results were transmitted through Bluetooth to a smartphone, which improved data transmission. The assay utilized a colorimetric method on blood samples, achieving a sensitivity rate of 75.7% for NP antigens.
The clinical value of smartphone-based biosensors in SARS-CoV-2 detection has been established through several studies. Agarwal et al. [52], Bokelmann et al. [47], and Shokr et al. [41] demonstrated high levels of accuracy in using smartphones as a tool for detecting the presence of SARS-CoV-2, with Agarwal et al. achieving an accuracy rate of 81.66% within 15 min. Similarly, Ghorbanizamani et al. [61] created a versatile platform that can rival traditional nanoparticle-based assays that use gold and silver.
Moreover, the low detection limit of smartphone-based biosensors has been demonstrated by several studies. Bokelmann et al. successfully identified concentrations as low as 5–25 viral genome copies per l when using RNA extracted from 500 l of gargle lavage was concentrated down to a final volume of 25 l. Liu et al. achieved a sensitivity rate of 75.7% for NP antigens, demonstrating the capability of SP-NLISA in detecting SARS-CoV-2 in serum samples.
Tong et al. [42] created a lateral flow immunoassay (LFIA) platform that utilizes polydopamine nanoparticles (PDAs) to produce a colorimetric signal with artificial intelligence (AI) assistance designed to measure the levels of neutralizing antibodies present in serum samples. The platform combines a smartphone reader and a PDA-based LFIA, along with an AI algorithm to enable precise and quantitative examination of the findings. With a detection limit of 160 ng/mL and a detection range of 625–10,000 ng/mL, the developed platform showed a strong level of agreement with a commercial ELISA kit. Furthermore, the PDA-based LFIA technology showed greater accuracy in quantifying clinical serum, as the test was compared to a commercial LFIA that uses gold nanoparticles clinical serum. The platform's straightforward operation, quick detection time, and low cost make it a promising tool for evaluating the effectiveness of vaccines on a large scale.
Similarly, Wang et al. [43] developed a surrogate viral neutralization test (TEMFIS-sVNT) utilizing a system that combines an immunosensing smartphone platform known as TEMFIS with a track-etched microporous membrane filtration microplate (TEM) for the one-step testing of neutralizing antibodies to SARS-CoV-2. EMFIS-sVNT was able to identify NAP in 92.68% of individuals who had been diagnosed with COVID-19 and 76% of vaccine recipients and found that all blood donors tested negative for neutralizing antibodies, demonstrating high specificity. TEMFIS-sVNT exhibited intra-assay and inter-assay precisions with coefficient variations less than 9 and 14%, in order. TEMFIS-sVNT thus of neutralizing antibodies against SARS-CoV-2 present in blood samples at the point of care.
Wu et al. [51] proposed an improved smartphone-based high-throughput fiber-integrated immunosensing system (HFIS) to detect the presence of SARS-CoV-2 nucleocapsid protein (NP) in serum samples. The immunoassay was conducted on a PTEM-coated 100-well microplate, using microspheres that are coupled with immunoglobulins and absorbent paper being used for washing. A device that can read microplates and is integrated with optical fibers and smartphone technology was developed, and an application was created to enable on-site detection. By means of a smartphone, the output can be instantly transmitted to the terminal, along with feedback on the results and any required medical guidance.
Azhar et al. [49] developed the FnCas9 Editor Linked Uniform Detection Assay (FELUDA), Utilizing a Cas9 enzyme-based enzymatic readout, the method directly identifies nucleotide sequences without requiring the trans-cleavage of reporter molecules. FELUDA has the potential to substitute for PCR in settings where resources are limited. FELUDA enables such diagnosis using low-cost paper strips through the chemical process of capturing substrate molecules that are biotinylated and bound to ribonucleoproteins (RNPs) on a separate test line of the paper strip, utilizing a chimeric gRNA labeled with FAM. The smartphone app TOPSE (True Outcome Predicted by Strip Evaluation) provides a predicted score based on background correction for identification.
Blood samples have also been employed in point-of-care devices for detecting the SARS-CoV-2 virus [42,43]. The detection accuracy was as high as 95%, 100%, and 100% for Tong et al. [42], Azhar et al. [49], and Wu et al. [51] devices, respectively. However, the detection process time distinguishes Azhar et al. from Wu et al.'s devices, with Azhar et al. taking 60 min and Wu et al. taking 45 min for point-of-care testing.
Colorimetric smartphone-based biosensors have shown great potential in detecting SARS-CoV-2 in serum samples. These devices are highly sensitive, accurate, and cost-effective, making them an excellent tool for the detection of COVID-19. The PDA-based LFIA platform developed by Tong et al. [42] showed promising results in quantitatively detecting neutralizing antibodies, while TEMFIS-sVNT by Wang et al. [43] provides a quantitative assessment of SARS-CoV-2 neutralizing antibodies.
Miscellaneous biosensors
Kirkpatrick et al. [56,57] developed a novel method called tele-mentored self-performed ultrasound was created to perform medical imaging on the International Space Station, with remote guidance and supervision by medical experts. The examination is more thorough than routine examinations that concentrate solely on the front and side of the chest since it encompasses 14 different places. The accuracy of the ultrasound devices depends on the remote clinical lung ultrasound expert interpretation of the data transmitted to them by online communication. This technique takes only a few minutes for the expert to interpret the lung result.
Xu et al. [55] indicated that films made of liquid crystal (LC) that are patterned with cationic surfactant and a complementary 15-mer single-stranded DNA (ssDNA) probe undergo ordering changes at femtomolar concentrations of single-stranded ribonucleic acid (ssRNA) of SARS-CoV-2. To offer early identification of SARS-CoV-2 ssRNA, diagnostic kit that utilizes liquid crystal (LC) technology was created along with an accompanying smartphone application (app). These products may be utilised for dependable at-home self-tests of SARS-CoV-2 without the use of complicated tools or methods.
Brzezinski et al. [54] created a new point-of-care, non-contact thermal imaging instrument to detect COVID-19 based on trimming image processing techniques. The authors used a thermal camera that can be carried attaches directly to cellphones to take thermal pictures of the backs of those who had COVID-19 and those who did not. The innovative image processing algorithms successfully identified COVID-19 with an area under the curve (AUC) of 0.85 and up to 92% sensitivity using several texture and shape cues that were automatically derived from the thermal pictures. Clinical factors linked to COVID-19 disease development had an inverse correlation with thermal imaging scores.
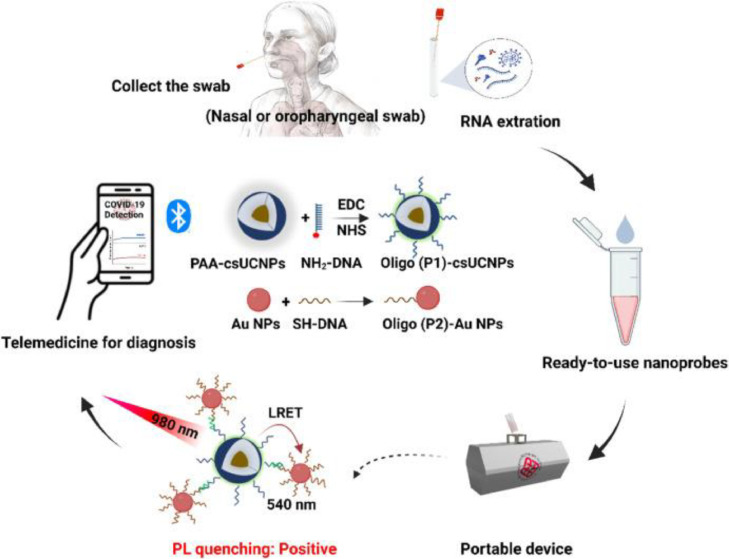
Song et al. [59] developed a diagnostic device that does not need amplification of the target to detect nucleic acid in the specimen. They present a platform of diagnosis that utilizes upconversion luminescence (PULD) technology, and a portable device that can be controlled using a smartphone provides an on-site, quick, and ultrasensitive identification of SARS-CoV-2 using an upconversion LRET-based assay. The system uses a unique current-to-frequency conversion-based signal detection and processing technique that may considerably boost the sensor's sensitivity. Additionally, the N gene of SARS-CoV-2 is captured using two ready-to-use probes, oligo-modified lanthanide-doped core-shell UCNPs (csUCNPs) and Au NPs, without the use of additional restricted reagents or target amplification in a single assay, demonstrating the system's ease of use, high selectivity, and sensitivity. Fig. 5 shows a schematic diagram of designed POCT setup.
Fig. 5.
A simplified design concept for a COVID-19 diagnostic platform, controlled using a mobile phone at the point-of-care. The platform incorporates a one-pot upconversion luminescence sandwich assay, which is ready-to-use and does not require target amplification. With permission from [59].
Akarapipad et al. [58] provided a Python script for examining the flow profile on a paper microfluidic chip using a smartphone camera unaffected by changes in ambient light. The study depended on human saliva from a pool of volunteers made either 1% or 10% of the simulated saline gargle samples. Although the authors created a diagnostic device depending on lateral flow immunoassay technique that takes only less than 15 min in detecting the virus in saliva samples, it has only 89% accuracy.
These studies demonstrate that smartphone-based biosensors and other diagnostic devices have great potential in detecting SARS-CoV-2. These devices are highly sensitive, accurate, and cost-effective, making them an excellent tool for point-of-care diagnosis of COVID-19. The self-performed ultrasound developed by Kirkpatrick et al. [56,57] is a promising method for medical imaging, while the LC-based diagnostic kit and smartphone app developed by Xu et al. [55] can provide at-home self-tests without the use of complicated tools or methods. Similarly, the non-contact thermal imaging instrument developed by Brzezinski et al. [54] may be used to screen for COVID-19 outside of hospitals, and the PULD platform developed by Song et al. [59] provides quick and ultrasensitive detection of SARS-CoV-2. However, the accuracy and detection time vary among these devices. Therefore, further studies are needed to evaluate the diagnostic accuracy, sensitivity, specificity, and feasibility of these devices in clinical settings.
Future perspective
Smartphone-based point-of-care testing for SARS-CoV-2 has the potential to revolutionize COVID-19 screening and address the current bottlenecks faced worldwide. One of the significant advantages of using readily available samples such as blood, respiratory mucosa, saliva, or exhaled breath is that they can be self-administered, minimizing the need for healthcare workers and reducing the risk of exposure. The integration of electroanalytical devices with IoT modules and smartphones can enable real-time monitoring of test results, providing patients and healthcare professionals with timely information to make informed decisions.
Another bottleneck in COVID-19 screening is the disproportionate international standards enforced by regulatory bodies such as the International Electrotechnical Commission Technical Committee, FDA, WHO, and ISO. As smartphone-based POC testing becomes more prevalent, there needs to be a concerted effort to harmonize international standards and ensure that new diagnostic instruments meet established guidelines. This will require collaboration between governments, regulatory bodies, healthcare professionals, and technology companies to streamline the approval process and reduce t-he time to market for new diagnostic tools.
Finally, inadequate testing coordination has been a significant challenge in COVID-19 screening. Increasing the availability of testing locations, including office spaces, in-car testing, and self-testing, can help minimize exposure while ensuring that patients and susceptible samples are monitored effectively. The integration of smartphone-based POC testing with these testing locations can further enhance coordination and improve patient outcomes. For these reasons, the future of COVID-19 screening looks promising with the advent of smartphone-based POC testing, but it requires collaborative efforts to overcome the current bottlenecks and ensure widespread adoption. In spite of the efforts exerted to allow close contact tracing of SARS-CoV-2 infections, reliable fast diagnostic tools are essential [62], [63], [64].
Conclusion
The COVID-19 pandemic has emphasized the importance of prompt and precise point-of-care diagnostic devices that can be used by patients without the help of a physician. With the development of smartphone-based POC testing for SARS-CoV-2, significant progress has been made in addressing this need. These devices offer several advantages, including quick detection times, high accuracy, and sensitivity to low concentrations of the virus. In addition to their diagnostic capabilities, these devices can also be used for remote patient monitoring, allowing patients to isolate themselves at home while still receiving medical attention. The integration of smartphone-based POC testing with other testing locations can further improve coordination and ensure widespread adoption of these devices. It is now essential to take this study into consideration as a comparison between different devices to standardize one of them as a point-of-care tool for COVID-19. Moreover, the principles used in developing these devices can be applied to the development of other tools to detect different infectious viruses, limiting the transmission of infectious diseases. Smartphone-based POC testing for SARS-CoV-2 signifies a notable progress in the fight against COVID-19 and offers promising possibilities for the future of infectious disease diagnosis and management.
Availability of data
All data are available upon reasonable request.
Ethics approval and consent to participate
Not required.
Consent for publication
Not Applicable.
Availability of data and materials
The datasets generated and analyzed during the current study are available in the manuscript and in the supplementary material.
Code availability (software application or custom code)
Not applicable.
Funding
No fund was received for this work.
CRediT authorship contribution statement
Berlanty A. Zayed: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Ahmed N. Ali: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Alaa A. Elgebaly: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Nourhan M. Talaia: Conceptualization, Data curation, Formal analysis, Methodology, Writing – original draft. Mahmoud Hamed: Conceptualization, Data curation, Methodology, Formal analysis, Writing – original draft. Fotouh R. Mansour: Conceptualization, Methodology, Project administration, Supervision, Data curation, Formal analysis, Writing – original draft.
Declaration of Competing Interest
The authors declare no competing interest.
Acknowledgments
Not Applicable.
Editor: Benjamin Gyampoh
References
- 1.Hamed M., El-Hasab M., Mansour F.R. Direct acting anti-hepatitis C combinations as potential COVID-19 protease inhibitors. VirusDisease. 2021;32:279–285. doi: 10.1007/s13337-021-00691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zayed B.A., Talaia A.M., Gaaboobah M.A., Amer S.M., Mansour F.R. Google trends as a predictive tool in the era of COVID-19: a scoping review. Postgrad. Med. J. 2023 doi: 10.1093/postmj/qgad012. [DOI] [PubMed] [Google Scholar]
- 3.Khaloufi H., Abouelmehdi K., Beni-Hssane A., Rustam F., Jurcut A.D., Lee E., et al. Deep learning based early detection framework for preliminary diagnosis of covid-19 via onboard smartphone sensors. Sensors. 2021;21:1–21. doi: 10.3390/s21206853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ganguli A., Mostafa A., Berger J., Aydin M.Y., Sun F., Stewart de Ramirez S.A., et al. Rapid isothermal amplification and portable detection system for SARS-CoV-2. Proc. Natl. Acad. Sci. U. S. A. 2020;117:22727–22735. doi: 10.1073/pnas.2014739117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang D.M., Chang T.J., Hung K.F., Wang M.L., Cheng Y.F., Chiang S.H., et al. Smart healthcare: a prospective future medical approach for COVID-19. J. Chin. Med. Assoc. 2023;86:138–146. doi: 10.1097/JCMA.0000000000000824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rodriguez-Manzano J., Malpartida-Cardenas K., Moser N., Pennisi I., Cavuto M., Miglietta L., et al. Handheld point-of-care system for rapid detection of SARS-CoV‑2 extracted RNA in under 20 min. ACS Cent. Sci. 2021;7:307–317. doi: 10.1021/acscentsci.0c01288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Georgas A., Agiannis K., Papakosta V., Priftis P., Angelopoulos S., Ferraro A., et al. A biosensor platform for point-of-care SARS-CoV-2 screening. Biosensors. 2022;12:487. doi: 10.3390/bios12070487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bedair A., Okasha K., Mansour F.R. Spectroscopic methods for COVID-19 detection and early diagnosis. Virol. J. 2022;19:152. doi: 10.1186/s12985-022-01867-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alkhodari M., Khandoker A.H. Detection of COVID-19 in smartphone-based breathing recordings: a pre-screening deep learning tool. PLoS One. 2022;17:1–25. doi: 10.1371/journal.pone.0262448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azmi I., Faizan M.I., Kumar R., Yadav S.R., Chaudhary N., Singh D.K., et al. A saliva-based RNA extraction-free workflow integrated with Cas13a for SARS-CoV-2 Detection. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.632646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Young R.M., Solis C.J., Barriga-Fehrman A., Abogabir C., Thadani A.R., Labarca M., et al. Smartphone screen testing, a novel pre-diagnostic method to identify SARS-CoV-2 infectious individuals. eLife. 2021;10:371–373. doi: 10.7554/eLife.70333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Young R.M., Solis C.J., Barriga-Fehrman A., Abogabir C., Thadani Á.R., Labarca M., et al. Smartphone screen testing, a novel pre-diagnostic method to identify sars-cov-2 infectious individuals. eLife. 2021;10:1–13. doi: 10.7554/eLife.70333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao M., Tian F., Liu X., Zhou Q., Pan J., Luo Z., et al. Virus detection: from state-of-the-art laboratories to smartphone-based point-of-care testing. Adv. Sci. 2022;9 doi: 10.1002/advs.202105904. (Weinheim, Baden-Wurttemberg, Ger. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Q., Li Y., Gao Q., Jiang C., Tian Q., Ma C., et al. Real-time monitoring of isothermal nucleic acid amplification on a smartphone by using a portable electrochemical device for home-testing of SARS-CoV-2. Anal. Chim. Acta. 2022;1229 doi: 10.1016/j.aca.2022.340343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beduk D., Ilton de Oliveira Filho J., Beduk T., Harmanci D., Zihnioglu F., Cicek C., et al. All In One” SARS-CoV-2 variant recognition platform: machine learning-enabled point of care diagnostics. Biosens. Bioelectron. X. 2022;10 doi: 10.1016/j.biosx.2022.100105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.W.S. Jingjie Nan, Thickness-Sensing Sandwiched Plasmonic Biosensors Enable Label-Free Naked-Eye Antibody Quantificationme, chemistry journal, ACS puplications, n.d. [DOI] [PMC free article] [PubMed]
- 17.Mavrikou S., Papaioannou G.M., Tsekouras V., Hatziagapiou K., Tatsi E.B., Filippatos F., et al. Ultra-fast and sensitive screening for antibodies against the SARS-CoV-2 S1 spike antigen with a portable. Bioelectr. Biosens. Chemosens. 2022;10:254. doi: 10.3390/chemosensors10070254. [DOI] [Google Scholar]
- 18.Singh N.K., Ray P., Carlin A.F., Magallanes C., Morgan S.C., Laurent L.C., et al. Hitting the diagnostic sweet spot: Point-of-care SARS-CoV-2 salivary antigen testing with an off-the-shelf glucometer. Biosens. Bioelectron. 2021;180 doi: 10.1016/j.bios.2021.113111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu D., Ju C., Han C., Shi R., Chen X., Duan D., et al. Nanozyme chemiluminescence paper test for rapid and sensitive detection of SARS-CoV-2 antigen. Biosens. Bioelectron. 2020;173 doi: 10.1016/j.bios.2020.112817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu K., Chugh V.K., di Girolamo A., Liu J., Saha R., Su D., et al. A portable magnetic particle spectrometer for future rapid and wash-free bioassays. ACS Appl. Mater. Interfaces. 2021;13:7966–7976. doi: 10.1021/acsami.0c21040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhao H., Liu F., Xie W., Zhou T.-C., OuYang J., Jin L., et al. Ultrasensitive supersandwich-type electrochemical sensor for SARS-CoV-2 from the infected COVID-19 patients using a smartphone. Sens. Actuators. B. Chem. 2021;327 doi: 10.1016/j.snb.2020.128899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen V.D., Nguyen H.Q., Bui K.H., Ko Y.S., Park B.J., Seo T.S. A handheld-type total integrated capillary electrophoresis system for SARS-CoV-2 diagnostics: Power, fluorescence detection, and data analysis by smartphone. Biosens. Bioelectron. 2022;195 doi: 10.1016/j.bios.2021.113632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Samper I.C., Sánchez-Cano A., Khamcharoen W., Jang I., Siangproh W., Baldrich E., et al. Electrochemical capillary-flow immunoassay for detecting anti-SARS-CoV-2 nucleocapsid protein antibodies at the point of care. ACS Sensors. 2021;6:4067–4075. doi: 10.1021/acssensors.1c01527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ardalan S., Ignaszak A. Innovations and challenges in electroanalytical tools for rapid biosurveillance of SARS-CoV-2. Adv. Mater. Technol. 2022;7 doi: 10.1002/admt.202200208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beduk T., Beduk D., de Oliveira Filho J.I., Zihnioglu F., Cicek C., Sertoz R., et al. Rapid point-of-care COVID-19 diagnosis with a gold-nanoarchitecture-assisted laser-scribed graphene biosensor. Anal. Chem. 2021;93:8585–8594. doi: 10.1021/acs.analchem.1c01444. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Lillehoj P.B. Microfluidic magneto immunosensor for rapid, high sensitivity measurements of SARS-CoV-2 nucleocapsid protein in serum. ACS Sens. 2021;6:1270–1278. doi: 10.1021/acssensors.0c02561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soares R.R.G., Akhtar A.S., Pinto I.F., Lapins N., Barrett D., Sandh G., et al. Sample-to-answer COVID-19 nucleic acid testing using a low-cost centrifugal microfluidic platform with bead-based signal enhancement and smartphone read-out. Lab Chip. 2021;21:2932–2944. doi: 10.1039/d1lc00266j. [DOI] [PubMed] [Google Scholar]
- 28.Lomae A., Preechakasedkit P., Hanpanich O., Ozer T., Henry C.S., Maruyama A., et al. Label free electrochemical DNA biosensor for COVID-19 diagnosis. Talanta. 2023;253 doi: 10.1016/j.talanta.2022.123992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang L., Ding L., Zhou J., Chen S., Chen F., Zhao C., et al. One-step rapid quantification of SARS-CoV-2 virus particles via low-cost nanoplasmonic sensors in generic microplate reader and point-of-care device. Biosens. Bioelectron. 2021;171 doi: 10.1016/j.bios.2020.112685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reis N.M., Needs S.H., Jegouic S.M., Gill K.K., Sirivisoot S., Howard S., et al. Gravity-driven microfluidic siphons: fluidic characterization and application to quantitative immunoassays. ACS Sensors. 2021;6:4338–4348. doi: 10.1021/acssensors.1c01524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hang X.M., Wang H.Y., Liu P.F., Zhao K.R., Wang L. Cas12a-assisted RTF-EXPAR for accurate, rapid and simple detection of SARS-CoV-2 RNA. Biosens. Bioelectron. 2022;216 doi: 10.1016/j.bios.2022.114683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi J.W., Seo W.H., Lee Y.S., Kim S.Y., Kim B.S., Lee K.G., et al. Development of an IoT-integrated multiplexed digital PCR system for quantitative detection of infectious diseases. Lab Chip. 2022;22:3933–3941. doi: 10.1039/d2lc00726f. [DOI] [PubMed] [Google Scholar]
- 33.Ning B., Yu T., Zhang S., Huang Z., Tian D., Lin Z., et al. A smartphone-read ultrasensitive and quantitative saliva test for COVID-19. Sci. Adv. 2021;7:eabe3703. doi: 10.1126/sciadv.abe3703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nguyen H.Q., Bui H.K., Phan V.M., Seo T.S. An internet of things-based point-of-care device for direct reverse-transcription-loop mediated isothermal amplification to identify SARS-CoV-2. Biosens. Bioelectron. 2022;195 doi: 10.1016/j.bios.2021.113655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Puig H., Lee R.A., Najjar D., Tan X., Soekensen L.R., Angenent-Mari N.M., et al. Minimally instrumented SHERLOCK (miSHERLOCK) for CRISPR-based point-of-care diagnosis of SARS-CoV-2 and emerging variants. Sci. Adv. 2021;7:23–26. doi: 10.1126/sciadv.abh2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen Y., Shi Y., Chen Y., Yang Z., Wu H., Zhou Z., et al. Contamination-free visual detection of SARS-CoV-2 with CRISPR/Cas12a: A promising method in the point-of-care detection. Biosens. Bioelectron. 2020;169 doi: 10.1016/j.bios.2020.112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panpradist N., Kline E.C., Atkinson R.G., Roller M., Wang Q., Hull I.T., et al. Harmony COVID-19: a ready-to-use kit, low-cost detector, and smartphone app for point-of-care SARS-CoV-2 RNA detection. Sci. Adv. 2021;7:1–18. doi: 10.1126/sciadv.abj1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoo H., Lee J.Y., Park K.S., Oh S.S. Lead-start isothermal polymerase amplification controlled by DNAzymatic switches. Nanoscale. 2022;14:7828–7836. doi: 10.1039/d1nr07894a. [DOI] [PubMed] [Google Scholar]
- 39.Mahmoud M., Ruppert C., Rentschler S., Laufer S., Deigner H.P. Combining aptamers and antibodies: Lateral flow quantification for thrombin and interleukin-6 with smartphone readout. Sens. Actuators B Chem. 2021;333 doi: 10.1016/j.snb.2020.129246. [DOI] [Google Scholar]
- 40.Cherkaoui D., Huang D., Miller B.S., Turbé V., McKendry R.A. Harnessing recombinase polymerase amplification for rapid multi-gene detection of SARS-CoV-2 in resource-limited settings. Biosens. Bioelectron. 2021;189 doi: 10.1016/j.bios.2021.113328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shokr A., Pacheco L.G.C., Thirumalaraju P., Kanakasabapathy M.K., Gandhi J., Kartik D., et al. Mobile health (mHealth) viral diagnostics enabled with adaptive adversarial learning. ACS Nano. 2021;15:665–673. doi: 10.1021/acsnano.0c06807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tong H., Cao C., You M., Han S., Liu Z., Xiao Y., et al. Artificial intelligence-assisted colorimetric lateral flow immunoassay for sensitive and quantitative detection of COVID-19 neutralizing antibody. Biosens. Bioelectron. 2022;213 doi: 10.1016/j.bios.2022.114449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang C., Wu Z., Liu B., Zhang P., Lu J., Li J., et al. Track-etched membrane microplate and smartphone immunosensing for SARS-CoV-2 neutralizing antibody. Biosens. Bioelectron. 2021;192 doi: 10.1016/j.bios.2021.113550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Materón E.M., Gómez F.R., Almeida M.B., Shimizu F.M., Wong A., Teodoro K.B.R., et al. Colorimetric detection of SARS-CoV-2 using plasmonic biosensors and smartphones. ACS Appl. Mater. Interfaces. 2022;14:54527–54538. doi: 10.1021/acsami.2c15407. [DOI] [PubMed] [Google Scholar]
- 45.Papadakis G., Pantazis A.K., Fikas N., Chatziioannidou S., Tsiakalou V., Michaelidou K., et al. Portable real-time colorimetric LAMP-device for rapid quantitative detection of nucleic acids in crude samples. Sci. Rep. 2022;12:1–15. doi: 10.1038/s41598-022-06632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Prainito C.D., Eshun G., Osonga F.J., Isika D., Centeno C., Sadik O.A. Colorimetric detection of the SARS-CoV-2 virus (COVID-19) in artificial saliva using polydiacetylene paper strips. Biosensors. 2022;12:1–14. doi: 10.3390/bios12100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bokelmann L., Nickel O., Maricic T., Pääbo S., Meyer M., Borte S., et al. Point-of-care bulk testing for SARS-CoV-2 by combining hybridization capture with improved colorimetric LAMP. Nat. Commun. 2021;12:1–8. doi: 10.1038/s41467-021-21627-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu B., Wu Z., Liang C., Lu J., Li J., Zhang L., et al. Development of a smartphone-based nanozyme-linked immunosorbent assay for quantitative detection of SARS-CoV-2 nucleocapsid phosphoprotein in blood. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.692831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azhar M., Phutela R., Kumar M., Ansari A.H., Rauthan R., Gulati S., et al. Rapid and accurate nucleobase detection using FnCas9 and its application in COVID-19 diagnosis. Biosens. Bioelectron. 2021;183 doi: 10.1016/j.bios.2021.113207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ghorbanizamani F., Moulahoum H., Zihnioglu F., Evran S., Cicek C., Sertoz R., et al. Quantitative paper-based dot blot assay for spike protein detection using fuchsine dye-loaded polymersomes. Biosens. Bioelectron. 2021;192 doi: 10.1016/j.bios.2021.113484. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z., Wang C., Liu B., Liang C., Lu J., Li J., et al. Smartphone-based high-throughput fiber-integrated immunosensing system for point-of-care testing of the SARS-CoV-2 nucleocapsid protein. ACS Sens. 2022;7:1985–1995. doi: 10.1021/acssensors.2c00754. [DOI] [PubMed] [Google Scholar]
- 52.Agarwal S., Warmt C., Henkel J., Schrick L., Nitsche A., Bier F.F. Lateral flow–based nucleic acid detection of SARS-CoV-2 using enzymatic incorporation of biotin-labeled dUTP for POCT use. Anal. Bioanal. Chem. 2022;414:3177–3186. doi: 10.1007/s00216-022-03880-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Colbert A.J., Lee D.H., Clayton K.N., Wereley S.T., Linnes J.C., Kinzer-Ursem T.L. PD-LAMP smartphone detection of SARS-CoV-2 on chip. Anal. Chim. Acta. 2022;1203 doi: 10.1016/j.aca.2022.339702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brzezinski R.Y., Rabin N., Lewis N., Peled R., Kerpel A., Tsur A.M., et al. Automated processing of thermal imaging to detect COVID-19. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-96900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu Y., Rather A.M., Song S., Fang J.C., Dupont R.L., Kara U.I., et al. Ultrasensitive and selective detection of SARS-CoV-2 using thermotropic liquid crystals and image-based machine learning. Cell Rep. Phys. Sci. 2020;1 doi: 10.1016/j.xcrp.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kirkpatrick A.W., McKee J.L., Conly J.M. Longitudinal remotely mentored self-performed lung ultrasound surveillance of paucisymptomatic Covid-19 patients at risk of disease progression. Ultrasound J. 2021;13:27. doi: 10.1186/s13089-021-00231-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kirkpatrick A.W., McKee J.L., Moeini S., Conly J.M., Ma I.W.Y., Baylis B., et al. Pioneering remotely piloted aerial systems (drone) delivery of a remotely telementored ultrasound capability for self diagnosis and assessment of vulnerable populations—the sky is the limit. J. Digit. Imaging. 2021;34:841–845. doi: 10.1007/s10278-021-00475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akarapipad P., Kaarj K., Breshears L.E., Sosnowski K., Baker J., Nguyen B.T., et al. Smartphone-based sensitive detection of SARS-CoV-2 from saline gargle samples via flow profile analysis on a paper microfluidic chip. Biosens. Bioelectron. 2022;207 doi: 10.1016/j.bios.2022.114192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Song M., Wong M.C., Li L., Guo F., Liu Y., Ma Y., et al. Rapid point-of-care detection of SARS-CoV-2 RNA with smartphone-based upconversion luminescence diagnostics. Biosens. Bioelectron. 2023;222 doi: 10.1016/j.bios.2022.114987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Azmi I., Faizan M.I., Kumar R., Yadav S.R., Chaudhary N., Singh D.K., et al. A saliva-based rna extraction-free workflow integrated with Cas13a for SARS-CoV-2 detection. Front. Cell. Infect. Microbiol. 2021;11:1–14. doi: 10.3389/fcimb.2021.632646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ghorbanizamani F., Moulahoum H., Zihnioglu F., Evran S., Cicek C., Sertoz R., et al. Quantitative paper-based dot blot assay for spike protein detection using fuchsine dye-loaded polymersomes. Biosens. Bioelectron. 2021;192 doi: 10.1016/j.bios.2021.113484. [DOI] [PubMed] [Google Scholar]
- 62.Zaw T.O.K., Muthaiyah S., Anbananthen K.S.M., Soe M.T. A novel approach on covid-19 contact tracing – utilization of low calibrated transmission power & signal captures in BLE. Emerg. Sci. J. 2022;6:181–192. doi: 10.28991/esj-2022-SPER-013. [DOI] [Google Scholar]
- 63.Mitra A., Soman B., Gaitonde R., Singh G., Roy A. Data science methods to develop decision support systems for real-time monitoring of COVID-19 outbreak. J. Hum. Earth Futur. 2022;3:223–236. doi: 10.28991/HEF-2022-03-02-08. [DOI] [Google Scholar]
- 64.Zaw T.O.K., Muthaiyah S., Anbananthen K.S.M., Soe M.T., Park B., Kim M.J. The necessity of close contact tracing in combating COVID-19 infection – a systemic study. Emerg. Sci. J. 2022;6:275–284. doi: 10.28991/esj-2022-SPER-019. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available upon reasonable request.
The datasets generated and analyzed during the current study are available in the manuscript and in the supplementary material.