Abstract
In this research article, Zr-MOFs based copper complex as a novel heterogeneous and porous catalyst was designed and prepared. The structure of catalyst has verified by various techniques such as FT-IR, XRD, SEM, N2 adsorption–desorption isotherms (BET), EDS, SEM-elemental mapping, TG and DTG analysis. UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was used as an efficient catalyst in the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitrile derivatives. The aromatization of titled molecules is performed via a cooperative vinylogous anomeric-based oxidation both under air and inert atmospheres. The unique properties of the presented method are short reaction time, high yield, reusability of catalyst, synthesis of desired product under mild and green condition.
Subject terms: Catalyst synthesis, Heterogeneous catalysis, Organocatalysis
Introduction
Nowadays, metal–organic frameworks as high surface areas materials are a new group of porous materials with potential applications such as gas storage and separation, drug delivery, sensors, batteries, supercapacitors as well as catalytic applications1,2. This framework is a class of organic–inorganic hybrid crystalline materials consisting of metallic nucleus that are linked by strong coordination bonds to organic ligands3,4. The different properties of these porous materials make them a good catalytic candidate for cross coupling, oxidation/reduction, and multicomponent reactions5–10. The post-modification method enhances catalytic performance and their variability. According this method, our research team reported a number of catalysts in the synthesis of organic compounds as biological active candidates11–16. Copper complex is widely used as catalysts in many organic reactions such as oxidation, cross coupling and catalytic organic reactions17–19. Recently, multicomponent reactions have investigated in the presence of palladium, nickel, copper, Fe, and Zr based catalytic systems20–22. In this report, a porous and heterogeneous catalyst based on Zr-MOFs with a copper complex is prepared. The simultaneous presence of copper and zirconium will enhance the catalytic application. This new system of porous complexes will lead to a new approach in the design and synthesis of catalysts. Figure 1 shows the final structure of the copper complex based on Zr-MOFs as well as the topology and structure of the UiO-66(Zr) grid.
Figure 1.
Structure and morphology of UiO-66(Zr)-NH2 as well as the final structure of a copper complex based on Zr-MOFs.
Diversity of fused N-heterocycles such as pyrazolo[3,4-b]pyridine and 1,2-dihydropyridine-3-carbonitrile containing of indole and pyrazole moieties may be suitable candidates for biological and pharmacological studies23–26. These materials are suitable candidate for antimicrobial, anticancer, anticonvulsant, antifungal, HIV, anti-tumor, antioxidant, antihypertension and urinary incontinence treatment (Fig. 2a)27–32. The target synthesized molecules in this paper may be show biological properties due to the simultaneous presence of indole and pyrazole moieties (Fig. 2b).
Figure 2.
(a) The structure of compounds with medicinal and biological properties includes pyrazolo[3,4-b]pyridine, 1,2-dihydropyridine-3-carbonitrile, indole and pyrazole nucleus. (b) Target synthesized molecules with indole and pyrazole moieties.
Anomeric effect (AE) as a fundamental example of stereoelectronic interactions has great educational and research applications33–35. It was discovered in 1955 by J. T. Edward in his studies on the carbohydrate chemistry36. The reported theory for the development of anomeric effect (AE) concept had been proposed that sharing the lone pair’s electrons of heteroatoms (X: N, O) to the anti-bonding orbital C–Y (nX → σ*C–Y) weakened it (Fig. 3a). Stereoelectronic effects have also a major role in the oxidation–reduction of susceptible biological compounds such as NADPH/NADP+ (Fig. 3b)37–39. Recently, we and our coworkers have reviewed the role of the above-mentioned fundamental concepts comprehensively34,35.
Figure 3.
(a) The geminal versus vinylogous anomeric effect in organic synthesis. (b) The structures of NADPH/NADP+.
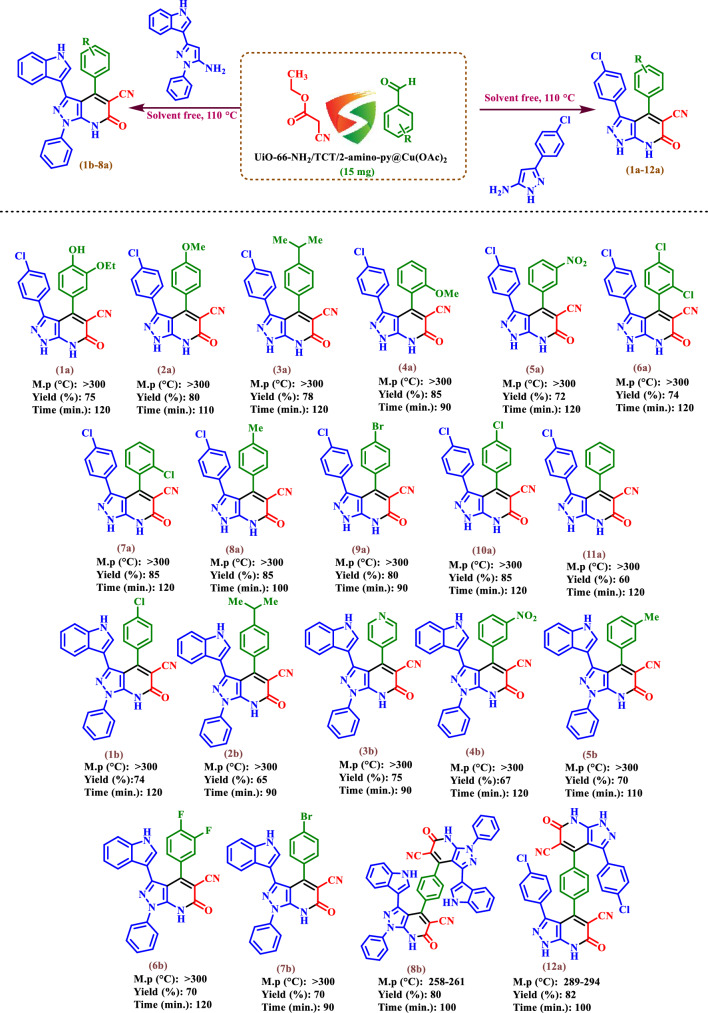
According to the above-mentioned idea, we have architectonic and synthesis of a copper complex based on Zr-MOFs as a novel heterogeneous and porous catalyst. This porous catalyst was applied for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles by reaction of various aromatic aldehydes (bearing electron-donating and electron-withdrawing groups), ethyl cyanoacetate, 3-(1H-indol-3-yl)-1-phenyl-1H-pyrazol-5-amine or 3-(4-chlorophenyl)-1H-pyrazol-5-amine under solvent-free at 110 °C via a cooperative vinylogous anomeric-based oxidation (Fig. 4).
Figure 4.
Preparation of pyrazolo[3,4-b]pyridine-5-carbonitriles using UiO-66-NH2/TCT/2-Amino-Py@Cu(OAc)2 as heterogeneous and porous catalyst.
Experimental section
Materials
All materials and solvents used in this work such as zirconium chloride (ZrCl4, Merck, 99%), 2-amino terephthalic acid (NH2-BDC, Merck, 95%), 2-amino-pyridine (Merck, 95%), 2,4,6-trichloro-1,3,5-triazine (TCT, Merck, 98%), Cu(CH3COO)2) Merck, 95%), N(Et)3) Merck), EtOH (Merck, 99%), ethyl cyanoacetate (Merck, 98%), acetonitrile (Merck, 99% ), p-toluene sulfonic acid (Merck, 98.5%), aldehyde derivatives (Merck), hydrazine (Merck, 80% in H2O), phenyl hydrazine (Merck, 97%) and N, N-dimethylformamide (DMF, Aldrich, 99%) were obtained from commercial sources without further purification.
Preparation of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as heterogeneous and porous catalyst
Firstly, UiO-66-NH2 and UiO-66-NH2/TCT were synthesized according to the previously reports45. In a 50 mL round-bottom flask, UiO-66-NH2/TCT (0.5 g), 2-aminopyridine (7 mmol, 0.658 g), N(Et)3 (20 mol%, 0.02 g) and dry THF (25 mL) as a solvent were refluxed for 24 h. In the next step, solid mixture was separated by centrifuge (3000 rpm/min) and washed three times with ethanol and dried in a vacuum oven at 60 °C for 12 h46,47. In the following, in a 25 mL round-bottom flask, a mixture of UiO-66-NH2/TCT/2-amino-Py (0.5 g) and Cu(CH3COO)2 (0.2 mmol, 0.036 g) were stirred in ethanol (20 mL) as a solvent in room temperature for 2 h. Then, UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was filtered using a centrifuge (3000 rpm/min) and dried under vacuum at 60 °C to grow a copper complex based on Zr-MOFs as a novel heterogeneous and porous catalyst (Fig. 5).
Figure 5.
Synthesis Zr-MOFs based copper complex as a novel heterogeneous and porous catalyst.
General method for the preparation of new pyrazolo[3,4-b]pyridine-5-carbonitriles
The first, raw materials such as 3-(1H-indol-3-yl)-1-phenyl-1H-pyrazol-5-amine (1) and 3-(4-chlorophenyl)-1H-pyrazol-5-amine (2) were prepared according to the previously reported (Fig. 4)48–52. In the following, in a 10 mL round-bottomed flask, a mixture of aromatic aldehydes (1 mmol), ethyl cyanoacetate (1 mmol, 0.113 g) and (1) or/and(2) in percent of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 (15 mg) as catalyst were stirred under solvent-free condition at 110 °C. The progress and completion of the reaction was monitored by using TLC technique. Then the reaction mixture was allowed to cooled up to room temperature. The reaction mixture was dissolved in hot ethanol (20 mL) to separate the catalyst by using centrifugation (4000 rpm/min). The desired products (1a–12a) were washed with acetone/ethanol and collected by simple filtration and (1b–8b). Finally, the crude products were purified by column chromatography (Fig. 4).
Results and discussion
Since the role of the anomeric effect can be found in the course of synthesis of various organic compounds53,54, herein, we decided to synthesize new compounds via an anomeric supporting mechanism. On the other hand, the importance of developing new catalysts for chemical reactions increased our motivation to produce new porous catalysts. Creating a copper complex based on metal–organic frameworks creates a new approach to the preparation of heterogeneous catalysts. The structure of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as a porous and heterogeneous catalyst was completely identified using various techniques such as FT-IR, XRD, SEM, N2 adsorption–desorption isotherms (BET), BJH, EDS, SEM-elemental mapping, TG and DTG. The UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was used for prepareing new pyrazolo[3,4-b]pyridine-5-carbonitriles. These compounds may be had biological and medicinal applications due to the presence of indole and pyrazole moieties. The structure of the synthesized compounds was confirmed using FT-IR, 1H-NMR, 13C-NMR and melting point techniques. This report describes all the experiments package, including the synthesis of catalyst, the optimization and mechanism of the reaction via anomeric-based oxidation pathway for the aromatization of the mentioned molecules under air and neutral atmosphere.
The FT-IR spectra of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as a catalyst and starting materials were shown in Fig. 6. The two peaks at 3475 and 3357 cm−1 of NH2 functional groups are represented synthesis of UiO-66-NH245. Also, the absorption peaks at 2800–3000 cm−1 are related to aromatic C–H and C=C stretches bands. The addition of different compounds during the catalyst synthesis steps results in changes in the spectra that indicate a change in structure.
Figure 6.

FT-IR spectra of catalyst and starting materials.
The XRD pattern of different stages of materials and catalyst synthesis was compared (Fig. 7). The XRD pattern of UiO-66-NH2 was identical to the previously reported data45. The last stage of copper complex based on Zr-MOFs has been proved by appearing of peaks. Also, below the peak at 2θ < 10, indicating the structure of the crystal plates of the various phases has suitable stability.
Figure 7.
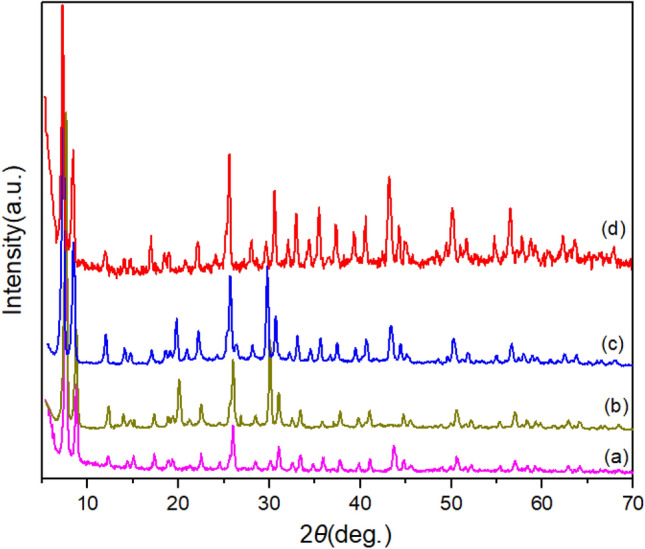
Comparison XRD pattern of (a) UiO-66-NH2, (b) UiO-66-NH2/TCT (c) UiO-66-NH2/TCT/2-amino-Py and (d) UiO-66-NH2/TCT/2-Amino-Py@Cu(OAc)2 a copper complex based on Zr-MOFs as a novel heterogeneous and porous catalyst.
The morphology of UiO-66-NH2 and UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was also studied by scanning electron microscopy (SEM) technique (Fig. 8a). As shown in Fig. 8a, morphology of catalyst particles is tetrahedral which is in good condition and not completely stacked. Also, the morphology of UiO-66-NH2 is stable after post-modification. Elemental mapping analysis shows Zr, N, O, C and Cu atoms which were confirmed in the structure of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 (Fig. 8b). Furthermore, the well-dispersed distribution of elements in the UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was determined and verified by elemental mapping analysis (Fig. 8b).
Figure 8.
(a) Scanning electron microscope (SEM) images of UiO-66-NH2 (a,b) and UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 (c,d). (b) EDX spectroscopy and elemental mapping analysis of UiO-66-NH2/TCT/2-amino-Py@ Cu(OAc)2 a copper complex based on Zr-MOFs as a novel heterogeneous and porous catalyst.
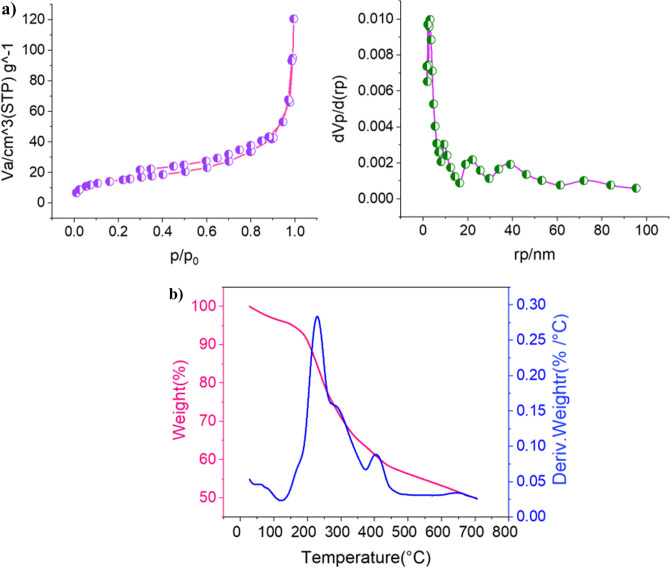
In another searching, the textural properties of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 were studied by N2 adsorption–desorption isotherms (Fig. 9a). Based on the obtained results, the area calculated based on the BET equation, the total pore volume 115 m2 g−1 and 0.1523 cm3 g−1 respectively. The pore size distribution of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 based on BJH method is shown in (Fig. 9a). The mean pore diameter for the catalyst is 8.48 nm. The presence of a suitable surface area, as well as the size of catalyst cavities can be a major reason for the high efficiency at the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles. The thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was shown in Fig. 9b. According to this diagram, several failures due to the separation of the copper complex and organic compounds of Zr-MOFs are shown. The diagram shows that the synthesized catalyst is stable up to 240 °C.
Figure 9.
(a) N2 adsorption–desorption isotherms and the pore size distribution of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2. (b) Thermal gravimetric (TG) and derivative thermal gravimetric (DTG) analysis of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as a novel heterogeneous and porous catalyst.
After confirming the structure of a copper complex based on Zr-MOFs, we attempted to evaluate its catalytic performance for the synthesis of various new pyrazolo[3,4-b]pyridine-5-carbonitriles. For this purpose, we selected the reaction between 4-chloro-benzaldehyde (1 mmol, 0.140 g), ethyl cyanoacetate (1 mmol, 0.113 g) and 3-(4-chlorophenyl)-1H-pyrazol-5-amine (1 mmol, 0.193 g) as a model reaction. In order to select suitable conditions, the model of reaction was evaluated using various solvents, different temperatures and amounts of catalysts. The results are shown in Table 1. According to the presented data in Table 1, the best of choice for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles was achieved in percent of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 (15 mg) as catalyst under solvent-free conditions at 110 °C.
Table 1.
Effect of different amounts of catalyst, temperature and solvent on the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles.
| Entry | Catalyst (mg) | Temp. (°C) | Solvent | Time (min.) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 20 | 110 | – | 120 | 85 |
| 2 | 15 | 110 | – | 120 | 85 |
| 3 | 10 | 110 | – | 120 | 76 |
| 4 | 5 | 110 | – | 120 | 70 |
| 5 | – | 110 | – | 120 | 43 |
| 6 | 15 | Reflux | EtOAc | 300 | 45 |
| 7 | 15 | Reflux | EtOH | 240 | 60 |
| 8 | 15 | Reflux | MeOH | 240 | 45 |
| 9 | 15 | Reflux | CH3CN | 300 | 55 |
| 10 | 15 | Reflux | H2O | 300 | 20 |
| 11 | 15 | Reflux | H2O/EtOH | 300 | 60 |
| 12 | 15 | Reflux | H2O/CH3CN | 360 | Trace |
| 13 | 15 | Reflux | CHCl3 | 360 | 30 |
| 14 | 15 | Reflux | Acetone | 300 | Trace |
| 15 | 15 | 70 | – | 120 | 60 |
| 16 | 15 | 90 | – | 120 | 73 |
| 17 | 15 | 110 | – | 30 | 40 |
| 18 | 15 | 110 | – | 60 | 73 |
Significant values are in [bold].
After selecting the optimal conditions for the synthesis of 3,4-bis(4-chlorophenyl)-6-oxo-6,7-dihydro-1H-pyrazolo[3,4-b]pyridine-5-carbonitrile (1a), a wide range of aromatic aldehydes including electron withdrawing, electron releasing and heterocyclic rings were tested for obtaining of desired products (Fig. 10). As shown in Fig. 10, the obtained results indicated that UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 is appropriate for the preparation of target molecules in high to excellent yields (60–85%) with relatively short reaction times (90–120 min.).
Figure 10.
Synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles using UiO-66-NH2/TCT/2-amino-Py@Cu a copper complex based on Zr-MOFs as a novel heterogeneous and porous catalyst.
To evaluate the performance of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as a catalyst in comparison to those other catalysts for the preparation pyrazolo[3,4-b]pyridine-5-carbonitriles, we have used various homogeneous and heterogeneous catalysts and previous stages of the final catalyst for the condensation reaction 4-chloro-benzaldehyde (1 mmol, 0.140 g), ethyl cyanoacetate (1 mmol, 0.113 g) and 3-(4-chlorophenyl)-1H-pyrazol-5-amine (1 mmol, 0.193 g) as a model reaction in Table 2. As shown the obtained data in the Table 2, UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 is the best catalyst for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitrile derivatives.
Table 2.
Evaluation of various catalyst for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles with UiO-66-NH2/TCT/2-Amino-py@cu.
| Entry | Catalyst | Amount of catalyst | Time (min.) | Yield (%) |
|---|---|---|---|---|
| 1 | UiO-66-NH2/TCT/2-amino-py@Cu(OAc)2 (This work) | 15 (mg) | 120 | 85 |
| 2 | UiO-66-NH245 | 15 (mg) | 180 | 22 |
| 3 | UiO-66-NH2/TCT | 15 (mg) | 180 | Trace |
| 4 | UiO-66-NH2/TCT/2-amino-py | 15 (mg) | 150 | 38 |
| 5 | Cu(OAc)2 | 15 (mol%) | 180 | 35 |
| 6 | ZrCl4 | 15 (mol%) | 180 | – |
| 7 | GTBSA55 | 15 (mg) | 120 | 74 |
| 8 | N(Et)3 | 15 (mol%) | 180 | 76 |
| 9 | [PVI-SO3H]Cl56 | 15 (mol%) | 150 | 65 |
| 10 | p-TSA | 15 (mol%) | 120 | 45 |
| 11 | NaOH | 15 (mol%) | 100 | 66 |
| 12 | Pipyridine | 15 (mol%) | 120 | 80 |
| 13 | MIL(100)(Cr)/NHEtN(CH2PO3H2)211,12 | 15 (mg) | 100 | 74 |
| 14 | KOH | 15 (mol%) | 120 | 70 |
| 15 | [Py-SO3H]Cl57 | 15 (mol%) | 120 | 63 |
| 16 | SSA58,59 | 15 (mol%) | 180 | 56 |
Significant values are in [bold].
Suggested mechanism for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles using UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as a heterogeneous and porous catalyst was shown in Fig. 11. At the first step, ethyl cyanoacetate is converted to enolate form and react with activated aldehyde to produce intermediate (I) by losing one molecule of H2O. In the following, (3-(1H-indol-3-yl)-1-phenyl-1H-pyrazol-5-amine (1) and/or 3-(4-chlorophenyl)-1H-pyrazol-5-amine (2) attack to intermediate (I) as a Michael acceptor created intermediate (II). In the next step, intermediate (II) is converted to intermediate (III) through tautomerization and intramolecular cyclization. Finally, the intermediate (III) converts to their corresponding derivatives via a cooperative vinylogous anomeric based oxidation and releases one molecule of hydrogen (–H2) and/or hydrogen peroxide (–H2O2) molecules26,60,61. The obtained results of the reaction model under argon, nitrogen and oxygen atmospheres are similar which are verified the presented mechanism. The term cooperative is used when more than one lone pair of electrons and other donors are sharing the anti-bonding orbitals of one acceptor bond (nN → σ*C–H). The simultaneous cooperative sharing of electrons from donors into the anti-bonding orbitals of the C–H bond is a major driving force for hydride releasing (nN → σ*C–X).
Figure 11.
The proposed mechanism for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles using UiO-66-NH2/TCT/2-Amino-Py@Cu(OAc)2.
To prove the recyclability of the presented catalyst, we tested model reaction under the optimal reaction conditions in the previously section. The results of Fig. 12a show that the UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 as a catalyst can be reused up to 4 times without noticeable changes in its catalytic activity. This performance indicates the high stability of the copper complex created on the Zr-MOFs as a heterogeneous and porous catalyst. To prove the stability of the catalyst structure, the recovered catalyst was evaluated by FT-IR and XRD analysis. The results are shown in Fig. 12b and c. According to the results, there have been not many changes in the catalyst structure, indicating the stability of the catalyst. Also, to investigate the heterogeneous nature of the protocols and Cu leaching, ICP results proved that no Zr and Cu leaching was detected in the filtrate (Zr: 2.41 × 10−6 and Cu: 2.03 × 10−5 mol/g respectively) upon reaction completion, which indicates the high stability of the prepared catalyst.
Figure 12.
(a) Recyclability of catalyst for the synthesis of pyrazolo[3,4-b]pyridine-5-carbonitriles. Comparison (b) XRD, (c) FT-IR of reused and fresh catalyst.
Conclusions
In summary, Zr-MOFs based copper complex was introduced. At this catalyst, copper was supported on the surface of metal–organic frameworks as a new porous complex. Proper stability and morphology of the presented catalyst can create a new approach in the preparation of porous and heterogeneous catalysts. Catalytic performance of UiO-66-NH2/TCT/2-amino-Py@Cu(OAc)2 was demonstrated in the synthesis of new pyrazolo[3,4-b]pyridine-5-carbonitriles via anomeric based oxidation concept. These compounds can have biological and medicinal applications due to the presence of indole and pyrazole nucleus. High efficiency of products and gentle green conditions are other features of the products synthesized using this new porous and heterogeneous catalyst.
Supplementary Information
Acknowledgements
We thank the Bu-Ali Sina University and Iran Science Elites Federation (INSF) for financial support.
Author contributions
E.T., and H.S.; methodology, validation, investigation. M.Z. investigation and writing the original draft. M.A.Z.; supervision, resources, project administration, funding acquisition, conceptualization, writing-review. A.K. supervision. M.A.A. Performing Mass-spectroscopy of synthesized products.
Data availability
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mahmoud Zarei, Email: mahmoud8103@yahoo.com.
Mohammad Ali Zolfigol, Email: mzolfigol@yahoo.com.
Ardeshir Khazaei, Email: Khazaei_1326@yahoo.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-34172-1.
References
- 1.Millward AR, Yaghi OM. Metal–organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005;127:17998–17999. doi: 10.1021/ja0570032. [DOI] [PubMed] [Google Scholar]
- 2.Kalmutzki MJ, Diercks CS, Yaghi OM. Metal–organic frameworks for water harvesting from air. Adv. Mater. 2018;30:1704304. doi: 10.1002/adma.201704304. [DOI] [PubMed] [Google Scholar]
- 3.Kitagawa S. Metal–organic frameworks (MOFs) Chem. Soc. Rev. 2014;43:5415–5418. doi: 10.1039/C4CS90059F. [DOI] [PubMed] [Google Scholar]
- 4.Masoomi MY, Morsali A, Dhakshinamoorthy A, Garcia H. Mixed-metal MOFs: Unique opportunities in metal–organic framework (MOF) functionality and design. Angew. Chem. 2019;131:15330–15347. doi: 10.1002/ange.201902229. [DOI] [PubMed] [Google Scholar]
- 5.Farrusseng D, Aguado S, Pinel C. Metal–organic frameworks: Opportunities for catalysis. Angew. Chem. Int. Ed. Engl. 2009;48:7502–7513. doi: 10.1002/anie.200806063. [DOI] [PubMed] [Google Scholar]
- 6.Sepehrmansourie H. Spotlight: Metal organic frameworks (MOFs): As multi-purpose catalysts. Iran. J. Catal. 2021;11:207–215. [Google Scholar]
- 7.Jiang H, Cheng H, Zang C, Tan J, Sun B, Bian F. Photocatalytic aldehydes/alcohols/toluenes oxidative amidation over bifunctional Pd/MOFs: Effect of Fe-O clusters and Lewis’s acid sites. J. Catal. 2021;401:279–287. doi: 10.1016/j.jcat.2021.08.008. [DOI] [Google Scholar]
- 8.Ghasemzadeh MA, Mirhosseini-Eshkevari B, Tavakoli M, Zamani F. Metal–organic frameworks: Advanced tools for multicomponent reactions. Green Chem. 2020;22:7265–7300. doi: 10.1039/D0GC01767A. [DOI] [Google Scholar]
- 9.Sepehrmansourie H, Zarei M, Zolfigol MA, Babaee S, Rostamnia S. Application of novel nanomagnetic metal–organic frameworks as a catalyst for the synthesis of new pyridines and 1,4-dihydropyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 2021;11:5279. doi: 10.1038/s41598-021-84005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng X, Song Y, Lin W. Dimensional reduction of Lewis acidic metal–organic frameworks for multicomponent reactions. J. Am. Chem. Soc. 2021;143:8184–8192. doi: 10.1021/jacs.1c03561. [DOI] [PubMed] [Google Scholar]
- 11.Sepehrmansouri H, Zarei M, Zolfigol MA, Moosavi-Zare AR, Rostamnia S, Moradi S. Multilinker phosphorous acid anchored En/MIL-100(Cr) as a novel nanoporous catalyst for the synthesis of new N-heterocyclic pyrimido [4,5-b] quinolines. Mol. Catal. 2020;481:110303. doi: 10.1016/j.mcat.2019.01.023. [DOI] [Google Scholar]
- 12.Tavakoli E, Sepehrmansourie H, Zarei M, Zolfigol MA, Khazaei A, Hosseinifard M. Applications of novel composite UiO-66-NH2/melamine with phosphorous acid tags as a porous and efficient catalyst for the preparation of novel spiro-oxindoles. New J. Chem. 2022;46:19054–19061. doi: 10.1039/D2NJ03340B. [DOI] [Google Scholar]
- 13.Kalhor S, Zarei M, Sepehrmansourie H, Zolfigol MA, Shi H, Wang J, Arjomandi J, Hasani M, Schirhagl R. Novel uric acid-based nano organocatalyst with phosphorous acid tags: Application for synthesis of new biologically-interest pyridines with indole moieties via a cooperative vinylogous anomeric based oxidation. Mol. Catal. 2021;507:111549. doi: 10.1016/j.mcat.2021.111549. [DOI] [Google Scholar]
- 14.Rasooll MM, Zarei M, Zolfigol MA, Sepehrmansourie H, Omidi A, Hasani M, Gu Y. Novel nano-architectured carbon quantum dots (CQDs) with phosphorous acid tags as an efficient catalyst for the synthesis of multisubstituted 4H-pyran with indole moieties under mild conditions. RSC Adv. 2021;11:25995–26007. doi: 10.1039/D1RA02515E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Babaee S, Zarei M, Sepehrmansourie H, Zolfigol MA, Rostamnia S. Synthesis of metal–organic frameworks MIL-101(Cr)-NH2 containing phosphorous acid functional groups: Application for the synthesis of N-amino-2-pyridone and pyrano [2,3-c] pyrazole derivatives via a cooperative vinylogous anomeric-based oxidation. ACS Omega. 2020;5:6240–6249. doi: 10.1021/acsomega.9b02133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jalili F, Zarei M, Zolfigol MA, Rostamnia S, Moosavi-Zare AR. SBA-15/PrN(CH2PO3H2)2 as a novel and efficient mesoporous solid acid catalyst with phosphorous acid tags and its application on the synthesis of new pyrimido [4,5-b] quinolones and pyrido [2,3-d] pyrimidines via anomeric based oxidation. Microporous Mesoporous Mater. 2020;294:109865. doi: 10.1016/j.micromeso.2019.109865. [DOI] [Google Scholar]
- 17.Zhong M, Pannecoucke X, Jubault P, Poisson T. Recent advances in photocatalyzed reactions using well-defined copper (I) complexes. Beilstein J. Org. Chem. 2020;16:451–481. doi: 10.3762/bjoc.16.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nasrollahzadeh M, Motahharifar N, Nezafat Z, Shokouhimehr M. Copper (II) complex anchored on magnetic chitosan functionalized trichlorotriazine: An efficient heterogeneous catalyst for the synthesis of tetrazole derivatives. Colloids Interface Sci. Commun. 2021;44:100471. doi: 10.1016/j.colcom.2021.100471. [DOI] [Google Scholar]
- 19.Xia J, Hirai T, Katayama S, Nagae H, Zhang W, Mashima K. Mechanistic study of Ni and Cu dual catalyst for asymmetric C–C bond formation; asymmetric coupling of 1,3-dienes with C-nucleophiles to construct vicinal stereocenters. ACS Catal. 2021;11:6643–6655. doi: 10.1021/acscatal.1c01626. [DOI] [Google Scholar]
- 20.Qi J, Wei F, Tung CH, Xu Z. Modular synthesis of α-quaternary chiral β-lactams by a synergistic copper/palladium-catalyzed multicomponent reaction. Angew. Chem. Int. Ed. Engl. 2021;60:13814–13818. doi: 10.1002/anie.202100601. [DOI] [PubMed] [Google Scholar]
- 21.Gawande MB, Bonifácio VD, Varma RS, Nogueira ID, Bundaleski N, Ghumman CAA, Branco PS. Magnetically recyclable magnetite-ceria (Nanocat-Fe-Ce) nanocatalyst-applications in multicomponent reactions under benign conditions. Green Chem. 2013;15:1226–1231. doi: 10.1039/c3gc40375k. [DOI] [Google Scholar]
- 22.McGrath KP, Hoveyda AH. A multicomponent Ni, Zr, and Cu-catalyzed strategy for enantioselective synthesis of alkenyl-substituted quaternary carbons. Angew. Chem. Int. Ed. Engl. 2014;53:1910–1914. doi: 10.1002/anie.201309456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jiang B, Ye Q, Fan W, Wang SL, Tu SJ, Li G. Four-component strategy for selective synthesis of azepino [5, 4, 3-cd] indoles and pyrazolo [3, 4-b] pyridines. Chem. Commun. 2014;50:6108–6111. doi: 10.1039/C4CC00740A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torabi M, Yarie M, Zolfigol MA, Azizian S, Gu Y. A magnetic porous organic polymer: Catalytic application in the synthesis of hybrid pyridines with indole, triazole and sulfonamide moieties. RSC Adv. 2022;12:8804–8814. doi: 10.1039/D2RA00451H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mohamed LW, Shaaban MA, Zaher AF, Alhamaky SM, Elsahar AM. Synthesis of new pyrazoles and pyrozolo [3, 4-b] pyridines as anti-inflammatory agents by inhibition of COX-2 enzyme. Bioorg. Chem. 2019;83:47–52. doi: 10.1016/j.bioorg.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Torabi M, Zolfigol MA, Yarie M, Gu Y. Application of ammonium acetate as a dual rule reagent-catalyst in synthesis of new symmetrical terpyridines. Mol. Catal. 2021;516:111959. doi: 10.1016/j.mcat.2021.111959. [DOI] [Google Scholar]
- 27.Faria JV, Vegi PF, Miguita AGC, Dos Santos MS, Boechat N, Bernardino AMR. Recently reported biological activities of pyrazole compounds. Bioorg. Med. Chem. 2017;25:5891–5903. doi: 10.1016/j.bmc.2017.09.035. [DOI] [PubMed] [Google Scholar]
- 28.Charris-Molina A, Castillo JC, Macías M, Portilla J. One-Step synthesis of fully functionalized pyrazolo [3, 4-b] pyridines via isobenzofuranone ring opening. J. Org. Chem. 2017;82:12674–12681. doi: 10.1021/acs.joc.7b02471. [DOI] [PubMed] [Google Scholar]
- 29.Abnous K, Manavi H, Mehri S, Alibolandi M, Kamali H, Ghandadi M, Hadizadeh F. In vitro evaluation of dihydropyridine-3-carbonitriles as potential cytotoxic agents through PIM-1 protein kinase inhibition. Res. Pharm. Sci. 2017;12:196. doi: 10.4103/1735-5362.207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amer MM, Aziz MA, Shehab WS, Abdellattif MH, Mouneir SM. Recent advances in chemistry and pharmacological aspects of 2-pyridone scaffolds. J. Saudi Chem. Soc. 2021;25:101259. doi: 10.1016/j.jscs.2021.101259. [DOI] [Google Scholar]
- 31.Abdelaziz ME, El-Miligy MM, Fahmy SM, Mahran MA, Hazzaa AA. Design, synthesis and docking study of pyridine and thieno [2, 3-b] pyridine derivatives as anticancer PIM-1 kinase inhibitors. Bioorg. Chem. 2018;80:674–692. doi: 10.1016/j.bioorg.2018.07.024. [DOI] [PubMed] [Google Scholar]
- 32.Ling Y, Hao ZY, Liang D, Zhang CL, Liu YF, Wang Y. The expanding role of pyridine and dihydropyridine scaffolds in drug design. Drug Des. Dev. Ther. 2021;15:4289. doi: 10.2147/DDDT.S329547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Alabugin IV. Stereoelectronic Effects: A Bridge Between Structure and Reactivity. 1. Wiley; 2016. [Google Scholar]
- 34.Alabugin IV, Kuhn L, Medvedev MG, Krivoshchapov NV, Vil VA, Yaremenko IA, Mehaffy P, Yarie M, Terent’ev AO, Zolfigol MA. Stereoelectronic power of oxygen in control of chemical reactivity: The anomeric effect is not alone. Chem. Soc. Rev. 2021;50:10253–10345. doi: 10.1039/D1CS00386K. [DOI] [PubMed] [Google Scholar]
- 35.Alabugin V, Kuhn L, Krivoshchapov NV, Mehaffy P, Medvedev MG. Anomeric effect, hyperconjugation and electrostatics: Lessons from complexity in a classic stereoelectronic phenomenon. Chem. Soc. Rev. 2021;50:10212–10252. doi: 10.1039/D1CS00564B. [DOI] [PubMed] [Google Scholar]
- 36.Juaristi E, Cuevas G. Recent studies of the anomeric effect. Tetrahedron. 1992;48:5019–5087. doi: 10.1016/S0040-4020(01)90118-8. [DOI] [Google Scholar]
- 37.Bai CB, Wang NX, Xing Y, Lan XW. Progress on chiral NAD(P)H model compounds. Synlett. 2017;28:402–414. [Google Scholar]
- 38.He T, Shi R, Gong Y, Jiang G, Liu M, Qian S, Wang Z. Base-promoted cascade approach for the preparation of reduced knoevenagel adducts using hantzsch esters as reducing agent in water. Synlett. 2016;27:1864–1869. doi: 10.1055/s-0035-1562099. [DOI] [Google Scholar]
- 39.Hamasaka G, Tsuji H, Uozumi Y. Organoborane-catalyzed hydrogenation of unactivated aldehydes with a Hantzsch ester as a synthetic NAD(P)H analogue. Synlett. 2015;26:2037–2041. doi: 10.1055/s-0034-1378846. [DOI] [Google Scholar]
- 40.Babaee S, Zarei M, Zolfigol MA. MOF-Zn-NHC as an efficient N-heterocyclic carbene catalyst for aerobic oxidation of aldehydes to their corresponding carboxylic acids via a cooperative geminal anomeric based oxidation. RSC Adv. 2021;11:36230–36236. doi: 10.1039/D1RA05494E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sepehrmansourie H, Zarei M, Zolfigol MA, Gu Y. A new approach for the synthesis of bis (3-Indolyl) pyridines via a cooperative vinylogous anomeric based oxidation using ammonium acetate as a dual reagent-catalyst role under mild and green condition. Polycycl. Aromat. Compd. 2022 doi: 10.1080/10406638.2022.2128830. [DOI] [Google Scholar]
- 42.Sepehrmansourie H, Zarei M, Zolfigol MA, Babaee S, Azizian S, Rostamnia S. Catalytic synthesis of new pyrazolo [3, 4-b] pyridine via a cooperative vinylogous anomeric-based oxidation. Sci. Rep. 2022;12:14145. doi: 10.1038/s41598-022-17879-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmadi H, Zarei M, Zolfigol MA. Catalytic application of a novel basic alkane-sulfonate metal–organic frameworks in the preparation of pyrido [2,3-d] pyrimidines via a cooperative vinylogous anomeric-based oxidation. ChemistrySelect. 2022;47:e202202155. doi: 10.1002/slct.202202155. [DOI] [Google Scholar]
- 44.Kalhor S, Zarei M, Zolfigol MA, Sepehrmansourie H, Nematollahi D, Alizadeh S, Arjomandi J. Anodic electrosynthesis of MIL-53(Al)-N(CH2PO3H2)2 as a mesoporous catalyst for synthesis of novel (N-methyl-pyrrol)-pyrazolo [3,4-b] pyridines via a cooperative vinylogous anomeric based oxidation. Sci. Rep. 2021;11:19370. doi: 10.1038/s41598-021-97801-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen M, Tu Y, Wu S. Preparation of UiO-66-NH2@PDA under water system for chemical warfare agents degradation. Materials. 2021;14:2419. doi: 10.3390/ma14092419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moghaddam FM, Jarahiyan A, Heidarian Haris M, Pourjavadi A. An advancement in the synthesis of nano Pd@magnetic amine-functionalized UiO-66-NH2 catalyst for cyanation and O-arylation reactions. Sci. Rep. 2021;11:11387. doi: 10.1038/s41598-021-90478-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadeghi S, Jafarzadeh M, Abbasi AR, Daasbjerg K. Incorporation of CuO NPs into modified UiO-66-NH2 metal–organic frameworks (MOFs) with melamine for catalytic C–O coupling in the Ullmann condensation. New J. Chem. 2017;41:12014–12027. doi: 10.1039/C7NJ02114C. [DOI] [Google Scholar]
- 48.Pandey G, Vaitla J. Desulfonylative methenylation of β-keto sulfones. Org. Lett. 2015;17:4890–4893. doi: 10.1021/acs.orglett.5b02455. [DOI] [PubMed] [Google Scholar]
- 49.Sun L, Bera H, Chui WK. Synthesis of pyrazolo [1, 5-a][1, 3, 5] triazine derivatives as inhibitors of thymidine phosphorylase. J. Med. Chem. 2013;65:1–11. doi: 10.1016/j.ejmech.2013.03.063. [DOI] [PubMed] [Google Scholar]
- 50.Afsar J, Zolfigol MA, Khazaei A, Zarei M, Gu Y, Alonso DA, Khoshnood A. Synthesis and application of melamine-based nano catalyst with phosphonic acid tags in the synthesis of (3-indolyl) pyrazolo [3,4-b] pyridines via vinylogous anomeric based oxidation. Mol. Catal. 2020;482:110666. doi: 10.1016/j.mcat.2019.110666. [DOI] [Google Scholar]
- 51.Slaett J, Romero I, Bergman J. Cyanoacetylation of indoles, pyrroles and aromatic amines with the combination cyanoacetic acid and acetic anhydride. Synthesis. 2004;16:2760–2765. [Google Scholar]
- 52.Ahmad I, Mishra NK, Ghosh T. 5-(1H-Indol-3-yl)-pyrazolyl derivatives as colorimetric sensor for anions. J. Incl. Phenom. Macrocycl. 2013;76:183–191. doi: 10.1007/s10847-012-0188-7. [DOI] [Google Scholar]
- 53.Yarie M. Catalytic anomeric based oxidation. Iran. J. Catal. 2017;7:85–88. [Google Scholar]
- 54.Yarie M. Catalytic vinylogous anomeric based oxidation. Iran. J. Catal. 2020;10:79–83. doi: 10.1039/d0ra04461j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zarei M, Sepehrmansourie H, Zolfigol MA, Karamian R, Farida SHM. Novel nano-size and crab-like biological-based glycoluril with sulfonic acid tags as a reusable catalyst: Its application to the synthesis of new mono-and bis-spiropyrans and their in vitro biological studies. New J. Chem. 2018;42:14308–14317. doi: 10.1039/C8NJ02470G. [DOI] [Google Scholar]
- 56.Sepehrmansourie H, Zarei M, Taghavi R, Zolfigol MA. Mesoporous ionically tagged cross-linked poly (vinyl imidazole) s as novel and reusable catalysts for the preparation of N-heterocycle spiropyrans. ACS Omega. 2019;4:17379–17392. doi: 10.1021/acsomega.9b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moosavi-Zare AR, Zolfigol MA, Zarei M, Zare A, Khakyzadeh V, Hasaninejad A. Design, characterization and application of new ionic liquid 1-sulfopyridinium chloride as an efficient catalyst for tandem Knoevenagel–Michael reaction of 3-methyl-1-phenyl-1H-pyrazol-5 (4H)-one with aldehydes. Appl. Catal. A Gen. 2013;467:61–68. doi: 10.1016/j.apcata.2013.07.004. [DOI] [Google Scholar]
- 58.Zolfigol MA. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for production of thionitrites and disulfides under mild conditions. Tetrahedron. 2001;57:9509–9511. doi: 10.1016/S0040-4020(01)00960-7. [DOI] [Google Scholar]
- 59.Sepehrmansourie H. Silica sulfuric acid (SSA): As a multipurpose catalyst. Iran. J. Catal. 2020;10:175–179. [Google Scholar]
- 60.Torabi M, Zolfigol MA, Yarie M, Notash B, Azizian S, Azandaryani MM. Synthesis of triarylpyridines with sulfonate and sulfonamide moieties via a cooperative vinylogous anomeric-based oxidation. Sci. Rep. 2021;11:16846. doi: 10.1038/s41598-021-95830-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Naseri AM, Zarei M, Alizadeh S, Babaee S, Zolfigol MA, Nematollahi D, Shi H. Synthesis and application of [Zr-UiO-66-PDC-SO3H]Cl MOFs to the preparation of dicyanomethylene pyridines via chemical and electrochemical methods. Sci. Rep. 2021;11:16817. doi: 10.1038/s41598-021-96001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.