Abstract
To exhaustively explore the association of infant genetic polymorphisms of methionine synthase (MTR) gene with the risk of non-syndromic congenital heart disease (CHD). A hospital-based case–control study involving 620 CHD cases and 620 health controls was conducted from November 2017 to March 2020. Eighteen SNPs were detected and analyzed. Our date suggested that the genetic polymorphisms of MTR gene at rs1805087 (GG vs. AA: aOR = 6.85, 95% CI 2.94–15.96; the dominant model: aOR = 1.77, 95% CI 1.35–2.32; the recessive model: aOR = 6.26, 95% CI 2.69–14.54; the addictive model: aOR = 1.81, 95% CI 1.44–2.29) and rs2275565 (GT vs. GG: aOR = 1.52, 95% CI 1.15–1.20; TT vs. GG: aOR = 4.93, 95% CI 1.93–12.58; the dominant model: aOR = 1.66, 95% CI 1.27–2.17; the recessive model: aOR = 4.41, 95% CI 1.73–11.22; the addictive model: aOR = 1.68, 95% CI 1.32–2.13) were significantly associated with the higher risk of CHD. And three haplotypes of G-A-T (involving rs4659724, rs95516 and rs4077829; OR = 5.48, 95% CI 2.58–11.66), G-C-A-T-T-G (involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993; OR = 0.78, 95% CI 0.63–0.97) and T-C-A-T-T-G (involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993; OR = 1.60, 95% CI 1.26–2.04) were observed to be significantly associated with risk of CHD. Our study found that genetic polymorphisms of MTR gene at rs1805087 and rs2275565 were significantly associated with higher risk of CHD. Additionally, our study revealed a significant association of three haplotypes with risk of CHD. However, the limitations in this study should be carefully taken into account. In the future, more specific studies in different ethnic populations are required to refine and confirm our findings.
Trial registration: Registration number: ChiCTR1800016635; Date of first registration: 14/06/2018.
Subject terms: Genetics, Cardiology
Introduction
Congenital heart disease (CHD) refers to a gross structural abnormality in heart or intrathoracic great vessels occurring in the embryonic period1. It is the most common human birth defect and the primary non-infectious cause of infant mortality2,3. The incidence of CHD worldwide is estimated to be approximately 8.22 per 1000 live births and rising4,5. Over the past few decades, rapid advances in surgical treatments and interventional therapies decreased the mortality of CHD6. Nevertheless, its associated complications (such as arrhythmia, heart failure and sudden cardiac death) and neurodevelopmental disorders may still occur after effective correction of cardiac abnormalities7–9.
CHD is a complicated pathological progress which can be influenced by environmental factors or genetic factors, or a combination of two factors10,11. Researches show maternal extrinsic and intrinsic factors may increase CHD risk, such as folate deficiency, diabetes, obesity and so on12,13. However, not all pregnant women who are exposed to risk factors will bear CHD infants, which demonstrates that individual’s susceptibility to CHD may vary depending on genetic factors. Mounting evidence supports that genetic risk factors play a crucial role in the etiology of CHD14. Further exploration of the hereditary etiology of CHD will likely be an essential step to improve treatments for patients with CHD.
Methionine synthase (MTR) gene, encoding methionine synthase, map to chromosomes 1q43. MTR, the major regulatory enzyme involved in folate/homocysteine (Hcy) metabolic pathway, which can transfer the methyl group from 5-methyltetrahydrofolic acid to Hcy and produce methionine and tetrahydrofolic acid by means of remethylation15. It is the only way to decrease the concentration of plasma Hcy in the early stage of embryo development. When MTR is insufficient or inactive, homocysteine will accumulate in the body resulting in hyperhomocysteinemia (HHcy). Published literature have found that HHcy might be a risk factor of birth defects including CHD16,17. Hence a presumption was raised that the genetic variants of MTR gene may alter the susceptibility to CHD by affecting the folate/Hcy metabolism.
Presently, some studies focused on 1–2 loci of the MTR gene when assessing the association of MTR genetic polymorphisms with the risk of CHD. The present study is the first to comprehensively evaluate the 18 single nucleotide polymorphisms (SNPs) of MTR gene and the risk of CHD in Han Chinese populations.
Materials and methods
Recruitments of study participants
From November, 2017 to March, 2020, all consenting children in the study were recruited from the Hunan Children's Hospital, Hunan Province, China. Children recruited from Department of Cardiothoracic Surgery with CHD were selected as the case group. The diagnosis and classification of the CHD were confirmed by echocardiography and/or surgery. During the same period. children recruited from Department of Child Healthcare without any congenital malformations after medical examination were selected as the control group.
Ethical approval was given by the Ethics Committee of Xiangya School of Public Health, Central South University (No. XYGW-2018–36). Additionally, it had been registered in Chinese Clinical Trial Registry Center with registration number ChiCTR1800016635 (date of first registration: 14/06/2018) and is available at http://www.chictr.org.cn/listbycreater.aspx. Information and biological samples were collected from participants after obtaining written informed consent from their parents.
Inclusion and exclusion criteria
In the present study, CHD was the outcome of interest which included ventricular septal defect (VSD), atrial septal defect (ASD), atrioventricular septal defect (AVSD), patent ductus arteriosus (PDA), aorto-pulmonary window (APW), tetralogy of Fallot (TOF) and complete transposition of great arteries (TGA). Of note, this study concerned on non-syndromic CHD. Patients with other organ malformations or known chromosomal abnormalities were excluded. We required that all case and controls were Han Chinese descent to reduce residual confounding factors from genetic and cultural differences owing to different ethnics. To minimize potential recall bias of exposure by mothers during the pre-pregnancy to the early stage of this pregnancy, we only included the study subjects who were less than 1 year old. Additionally, the case and controls should meet the following inclusion criteria: 1) child whose mother was spontaneous pregnancy; 2) singleton pregnancy; 3) completed the questionnaire and provided the blood sample; 4) child whose mother was unreported history of depression or other psychiatric disorders.
Information collection
Considering the influence of potential confounding factors in the later analysis, the trained investigators used self-designed questionnaire to collect the corresponding information through one-to-one interview. The covariates for mothers were pre-selected based on literature review as followings: age at this pregnancy (< 35 or ≥ 35), residence (rural or urban), body mass index (BMI) before pregnancy(< 18.5, 18.5–24.9, 24.9–29.9 or ≥ 30.0), history of gestational diabetes mellitus (yes or no), history of gestational hypertension(yes or no), history of consanguineous marriage(yes or no), family history of congenital malformations(yes or no), cold before pregnancy(yes or no), smoking before pregnancy(yes or no), alcohol drinking before pregnancy(yes or no), folic acid consumption before or during pregnancy(yes or no). We defined folic acid consumption as any use of folic acid in 3 months before pregnancy and/or during the first-trimester pregnancy. To reduce recall bias to some extent, the exposure information was further confirmed by consulting their Maternal and Child Health Manual and medical records.
When mothers completed the questionnaires mentioned above, three milliliters of peripheral venous blood were provided from their children and collected in EDTA treated (ethylenediamine tetra acetic acid) anticoagulant tubes. And then blood samples immediately centrifuged into plasma and blood cells. Blood cells were separated and stored at − 80 °C until genotyping analysis. Genomic DNA was isolated from peripheral blood cells using the QIAamp DNA Mini Kit (Qiagen, Valencia, CA), and dissolved in sterile TBE buffer based on the manufacturer’s protocol.
SNP selection and genotyping
MTR gene was the candidate gene for this study. We selected the corresponding candidate loci of the MTR gene based on a previously published study18. Briefly, SNP tags were selected using the SNPBrowser™ (version 3.0). This program was provided by AppliedBiosystems Inc which allowed selection of SNP markers from the HapMap database (http://www.hapmap.org/). For each target gene, tagging SNPs were selected based on the pairwise r2 ≥ 0.8. We excluded these SNPs with minor allele frequencies lower than 10% in Asians. Finally, these genetic loci (rs1266164, rs3768139, rs6676866, rs4077829, rs955516, rs1050993, rs2229276, rs4659743, rs12060570, rs1806505, rs3768142, rs4659724, rs6668344, rs1805087, rs2275565, rs3754255, rs10925252, rs3820571) of MTR gene, were considered as candidate loci in our study.
We used the matrix-assisted laser desorption and ionization time-of-flight mass spectrometry Mass Array system (Agena iPLEXassay, San Diego, CA, USA) to genotype the polymorphisms of MTR gene. The laboratory technician, who performed the genotyping, retyped and double-checked each sample, and recorded the genotype data, was blinded to whether the samples were from cases or controls. We set the minimum call rate of SNP genotyping at the level of 50% to ensure data integrity of the participant’s genotypes that had been called.
Statistical analysis
Categorical variables were described as frequencies and percentages. The Chi-square Test was used to compare the differences in qualitative demographic features between the case group and the control group. Hardy–Weinberg equilibrium (HWE) was tested using the goodness-of-fit Chi-square Test in the controls. In the present study, we comprehensively analyzed the association of genotype and three genetic models (i.e., dominant model, recessive model and additive model) for every SNP with the risk of CHD. Odds ratio (OR) and 95% confidence intervals (CIs) were calculated using logistic regression analysis to evaluate the associations between MTR gene polymorphism and CHD risk. Adjusted OR (aOR) was calculated by multivariable logistic regression to adjust statistically significant variables of maternal characteristics between case and control groups, which aims to further evaluate independent association of SNPs of the MTR gene on the susceptibility of CHD. Besides, to get a more precise P value, the false discovery rate (FDR_P) was applied to multiple test corrections. Linkage disequilibrium test was used to evaluate whether there was a strong association between the two SNPs. Associations between haplotype and the risk of CHD were estimated by haplotype analysis. Linkage disequilibrium test and haplotype analysis was conducted using Haploview 4.2 software. Other statistical analyses were conducted using R software (version 3.5.0). All tests were two-tailed, with P < 0.05 set as the statistically significant difference.
Ethics approval and consent to participate
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Xiangya School of Public Health of Central South University (No. XYGW-2018-36), and written informed consent was obtained from all mothers. The protocol of this study was registered at the Chinese Clinical Trial Registry with registration number ChiCTR1800016635 and is available at http://www.chictr.org.cn/listbycreater.aspx.
Results
Sociodemographic characteristics
Based on inclusion criteria, a total of 620 children with CHD were recruited into the case group, 620 healthy children into the control group. Among 620 CHD cases, 139 were diagnosed with ASD, 448 with VSD, 25 with AVSD, 168 with PDA, 32 with TOF, 7 with APW, and 3 with TGA. Considering that some cases have been diagnosed with multiple subtypes of CHD, the sum of the various subtypes was not equal to 620. Comparison of maternal characteristics in cases and controls were summarized in Table 1. Significant differences were found in case and control groups for BMI before pregnancy, history of gestational diabetes mellitus, history of gestational hypertension, history of consanguineous marriage, family history of congenital malformations, cold before pregnancy, smoking before pregnancy, alcohol drinking before pregnancy, folic acid consumption before or during pregnancy. These potential confounding factors were adjusted when estimating the association between MTR gene polymorphisms with the risk of CHD.
Table 1.
Comparison of maternal characteristics in cases and controls a.
| Variables | Case (n = 620) | Control (n = 620) | Univariate analysisb |
|---|---|---|---|
| Age at this pregnancy (Years) | – | – | χ2 = 0.993; P = 0.319 |
| < 35 | 546(88.1%) | 557(89.8%) | – |
| ≥ 35 | 74(11.9%) | 63(10.2%) | |
| Residence | – | – | χ2 = 36.153; P < 0.001 |
| Rural | 444(71.6%) | 342(55.2%) | – |
| Urban | 176(28.4%) | 278(44.8%) | |
| BMI before pregnancy | – | – | χ2 = 18.353; P < 0.001 |
| < 18.5 | 112(18.1%) | 156(25.2%) | |
| 18.5–24.9 | 425(68.5%) | 382(61.6%) | |
| 24.9–29.9 | 62(10.0%) | 43(6.9%) | |
| ≥ 30.0 | 21(3.4%) | 39(6.3%) | |
| History of gestational diabetes mellitus(yes) | 63(10.2%) | 17(2.7%) | χ2 = 28.274; P < 0.001 |
| History of gestational hypertension(yes) | 43(6.9%) | 9(1.5%) | χ2 = 23.204; P < 0.001 |
| History of consanguineous marriage(yes) | 21(3.4%) | 3(0.5%) | χ2 = 13.766; P < 0.001 |
| Family history of congenital malformations(yes) | 36(5.8%) | 5(0.8%) | χ2 = 24.241; P < 0.001 |
| Cold before pregnancy(yes) | 121(19.5) | 72(11.6%) | χ2 = 14.734; P < 0.001 |
| Smoking before pregnancy(yes) | 36(5.8) | 13(2.1%) | χ2 = 11.240; P = 0.001 |
| Alcohol drinking before pregnancy(yes) | 81(13.1%) | 43(6.9%) | χ2 = 12.939; P < 0.001 |
| Folic acid consumption before or during pregnancy(yes) | 526(84.8%) | 577(93.1%) | χ2 = 21.344; P < 0.001 |
a Data presented as number (percentage) unless otherwise indicated;
b P < 0.05 was considered to indicate a statistically significant difference.
Genetic variants of MTR gene and risk of CHD
MTR genotype frequencies in infant and the results of HWE test were shown in Table 2. The genotype frequencies of the 18 SNPs of MTR gene in the control group all conformed to HWE (all P values > 0.05).
Table 2.
Genotype frequencies of MTR gene and HWE test of the control groupa.
| Genetic loci | Chromosome | Major allele | Minor allele | MAF | Group | Genotype frequencies | Pb | ||
|---|---|---|---|---|---|---|---|---|---|
| AA | AB | BBc | |||||||
| rs955516 | 1:236,980,504 | T | A | 0.500 | Control | 196 (31.6%) | 314(50.6%) | 110(17.8%) | 0.414 |
| Case | 206(33.2%) | 298(48.1%) | 116(18.7%) | ||||||
| rs1266164 | 1:237,050,951 | C | T | 0.167 | Control | 427(68.9%) | 168(27.1%) | 25(4.0%) | 0.106 |
| Case | 411(66.3%) | 185(29.8%) | 24(3.9%) | ||||||
| rs3768139 | 1:237,027,668 | C | G | 0.183 | Control | 424(68.4%) | 171(27.6%) | 25(4.0%) | 0.146 |
| Case | 411(66.3%) | 185(29.8%) | 24(3.9%) | ||||||
| rs6676866 | 1:237,064,626 | G | T | 0.358 | Control | 221(35.6%) | 285(46.0%) | 114(18.4%) | 0.192 |
| Case | 190(30.6%) | 314(50.6%) | 116(18.8%) | ||||||
| rs1050993 | 1:237,062,305 | G | A | 0.189 | Control | 418(67.5%) | 177(28.5%) | 25(4.0%) | 0.257 |
| Case | 408(65.8%) | 185(29.8%) | 27(4.4%) | ||||||
| rs2229276 | 1:237,054,569 | A | G | 0.484 | Control | 175(28.2%) | 307(49.5%) | 138(22.3%) | 0.879 |
| Case | 182(29.4%) | 302(48.7%) | 136(21.9%) | ||||||
| rs4659743 | 1:237,059,387 | T | A | 0.173 | Control | 420(67.8%) | 175(28.2%) | 25(4.0%) | 0.215 |
| Case | 406(65.5%) | 183(29.5%) | 31(5.0%) | ||||||
| rs1806505 | 1:236,996,575 | C | T | 0.490 | Control | 190(30.6%) | 320(51.6%) | 110(17.8%) | 0.216 |
| Case | 219(35.3%) | 293(47.3%) | 108(17.4%) | ||||||
| rs3768142 | 1:237,028,564 | T | C | 0.294 | Control | 260(41.9%) | 269(43.4%) | 91(14.7%) | 0.119 |
| Case | 248(40.0%) | 273(44.0%) | 99(16.0%) | ||||||
| rs4659724 | 1:236,974,124 | G | A | 0.437 | Control | 192(31.0%) | 316(51.0%) | 112(18.0%) | 0.362 |
| Case | 239(38.5%) | 279(45.0%) | 102(16.5%) | ||||||
| rs6668344 | 1:237,001,326 | C | T | 0.484 | Control | 174(28.1%) | 310(50.0%) | 136(21.9%) | 0.925 |
| Case | 188(30.3%) | 305(49.2%) | 127(20.5%) | ||||||
| rs1805087 | 1:237,048,500 | A | G | 0.181 | Control | 486(78.4%) | 127(20.5%) | 7(1.1%) | 0.685 |
| Case | 408(65.8%) | 175(28.2%) | 37(6.0%) | ||||||
| rs4077829 | 1:236,987,790 | G | T | 0.440 | Control | 194(31.3%) | 316(51.0%) | 110(17.7%) | 0.339 |
| Case | 201(32.4%) | 308(49.7%) | 111(17.9%) | ||||||
| rs2275565 | 1:237,048,676 | G | T | 0.213 | Control | 475(76.6%) | 139(22.4%) | 6(1.0%) | 0.230 |
| Case | 412(66.5%) | 181(29.1%) | 27(4.4%) | ||||||
| rs3754255 | 1:237,009,857 | T | C | 0.440 | Control | 172(27.7%) | 301(48.6%) | 147(23.7%) | 0.494 |
| Case | 179(28.9%) | 286(46.1%) | 155(25.0%) | ||||||
| rs3820571 | 1:237,060,433 | T | G | 0.189 | Control | 417(67.3%) | 175(28.2%) | 28(4.5%) | 0.086 |
| Case | 416(67.1%) | 180(29.0%) | 24(3.9%) | ||||||
| rs10925252 | 1:237,022,362 | C | T | 0.490 | Control | 145(23.4%) | 298(48.1%) | 177(28.5%) | 0.368 |
| Case | 156(25.2%) | 276(44.5%) | 188(30.3%) | ||||||
| rs12060570 | 1:236,989,069 | G | C | 0.500 | Control | 193(31.1%) | 317(51.1%) | 110(17.8%) | 0.304 |
| Case | 224(36.1%) | 285(46.0%) | 111(17.9%) | ||||||
MAF minimum allele frequency.
a Data presented as number (percentage) unless otherwise indicated;
b P < 0.05 was considered to indicate a statistically significant difference;
c AA, Homezygous with minor allele; AB, Heterozygous; BB, Homezygous with major allele.
Associations between each SNP of MTR gene and the risk of CHD based on univariate and multivariate logistic regression analysis were summarized in Table 3. After adjustment, the genetic polymorphism of MTR gene at rs1805087 was significantly associated with the higher risk of CHD (GG vs. AA: aOR = 6.85, 95% CI 2.94–15.96; the dominant model: aOR = 1.77, 95% CI 1.35–2.32; the recessive model: aOR = 6.26, 95% CI 2.69–14.54; the addictive model: aOR = 1.81, 95% CI 1.44–2.29). Additionally, the genetic polymorphism of MTR gene at rs2275565 was significantly associated with the higher risk of CHD (GT vs. GG: aOR = 1.52, 95% CI 1.15–1.20; TT vs.GG: aOR = 4.93, 95% CI 1.93–12.58; the dominant model: aOR = 1.66, 95% CI 1.27–2.17; the recessive model: aOR = 4.41, 95% CI 1.73–11.22; the addictive model: aOR = 1.68, 95% CI 1.32–2.13). However, statistically significant associations between the risk of CHD and genetic polymorphisms at other loci of MTR gene were not observed.
Table 3.
Genetic variants of MTR gene and risk of CHD.
| Genotype | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| P | OR (95% CI) | FDR_P | P | aOR (95% CI) b | FDR_Pa | |
| rs955516 | ||||||
| TT | 1 | 1 | 1 | 1 | 1 | 1 |
| TA | 0.427 | 0.90(0.70–1.16) | 0.834 | 0.384 | 0.89(0.67–1.16) | 0.864 |
| AA | 0.984 | 1.00(0.72–1.39) | 0.993 | 0.586 | 1.10(0.78–1.56) | 0.884 |
| Dominant modelc | 0.544 | 0.93(0.73–1.18) | 0.703 | 0.641 | 0.94(0.73–1.22) | 0.869 |
| Recessive modeld | 0.659 | 1.07(0.80–1.42) | 0.941 | 0.276 | 1.19(0.87–1.62) | 0.954 |
| Additive modee | 0.871 | 0.99(0.84–1.16) | 0.990 | 0.767 | 1.03(0.86–1.22) | 0.860 |
| rs1266164 | ||||||
| CC | 1 | 1 | 1 | 1 | 1 | 1 |
| CT | 0.289 | 1.14(0.89–1.47) | 0.834 | 0.285 | 1.16(0.89–1.51) | 0.864 |
| TT | 0.993 | 0.10(0.56–1.78) | 0.993 | 0.684 | 0.88(0.47–1.65) | 0.884 |
| Dominant modelc | 0.332 | 1.13(0.89–1.43) | 0.703 | 0.387 | 1.12(0.87–1.45) | 0.697 |
| Recessive modeld | 0.884 | 0.96(0.54–1.70) | 0.941 | 0.583 | 0.84(0.45–1.57) | 0.954 |
| Additive modee | 0.445 | 1.08(0.86–1.32) | 0.801 | 0.590 | 1.06(0.86–1.32) | 0.860 |
| rs3768139 | ||||||
| CC | 1 | 1 | 1 | 1 | 1 | 1 |
| GC | 0.386 | 1.12(0.87–1.43) | 0.834 | 0.373 | 1.13(0.87–1.47) | 0.864 |
| GG | 0.974 | 0.99(0.56–1.76) | 0.993 | 0.667 | 0.87(0.47–1.63) | 0.884 |
| Dominant modelc | 0.431 | 1.10(0.87–1.40) | 0.703 | 0.487 | 1.10(0.85–1.41) | 0.797 |
| Recessive modeld | 0.884 | 0.96(0.54–1.70) | 0.941 | 0.583 | 0.84(0.45–1.57) | 0.954 |
| Additive modee | 0.541 | 1.06(0.87–1.30) | 0.819 | 0.692 | 1.05(0.84–1.30) | 0.860 |
| rs6676866 | ||||||
| GG | 1 | 1 | 1 | 1 | 1 | 1 |
| GT | 0.053 | 1.28(0.10–1.65) | 0.273 | 0.083 | 1.27(0.97–1.67) | 0.383 |
| TT | 0.307 | 1.18(0.86–1.64) | 0.834 | 0.806 | 1.05(0.74–1.48) | 0.884 |
| Dominant modelc | 0.062 | 1.25(0.99–1.59) | 0.227 | 0.153 | 1.20(0.93–1.55) | 0.424 |
| Recessive modeld | 0.884 | 1.02(0.77–1.36) | 0.941 | 0.542 | 0.91(0.67–1.24) | 0.954 |
| Additive modee | 0.183 | 1.11(0.95–1.31) | 0.672 | 0.532 | 1.06(0.89–1.25) | 0.860 |
| rs1050993 | ||||||
| GG | 1 | 1 | 1 | 1 | 1 | 1 |
| GA | 0.587 | 1.07(0.84–1.37) | 0.879 | 0.546 | 1.09(0.83–1.42) | 0.884 |
| AA | 0.724 | 1.11(0.63–1.94) | 0.916 | 0.901 | 1.04(0.57–1.91) | 0.954 |
| Dominant modelc | 0.547 | 1.08(0.85–1.36) | 0.703 | 0.556 | 1.08(0.84–1.39) | 0.834 |
| Recessive modeld | 0.777 | 1.08(0.62–1.89) | 0.941 | 0.965 | 1.01(0.56–1.85) | 0.970 |
| Additive modee | 0.546 | 1.06(0.87–1.30) | 0.819 | 0.611 | 1.06(0.85–1.31) | 0.860 |
| rs2229276 | ||||||
| AA | 1 | 1 | 1 | 1 | 1 | 1 |
| AG | 0.676 | 0.95(0.73–1.23) | 0.901 | 0.976 | 1.00(0.76–1.34) | 0.976 |
| GG | 0.738 | 0.95(0.69–1.30) | 0.916 | 0.628 | 1.09(0.77–1.53) | 0.884 |
| Dominant modelc | 0.661 | 0.95(0.74–1.21) | 0.709 | 0.830 | 1.03(0.79–1.35) | 0.934 |
| Recessive modeld | 0.891 | 0.98(0.75–1.28) | 0.941 | 0.578 | 1.08(0.82–1.44) | 0.954 |
| Additive modee | 0.719 | 0.97(0.83–1.14) | 0.990 | 0.642 | 1.04(0.88–1.23) | 0.860 |
| rs4659743 | ||||||
| TT | 1 | 1 | 1 | 1 | 1 | 1 |
| TA | 0.535 | 1.08(0.84–1.39) | 0.875 | 0.480 | 1.10(0.84–1.44) | 0.884 |
| AA | 0.370 | 1.28(0.74–2.21) | 0.834 | 0.461 | 1.25(0.70–2.23) | 0.884 |
| Dominant modelc | 0.399 | 1.11(0.87–1.40) | 0.703 | 0.384 | 1.12(0.87–1.44) | 0.697 |
| Recessive modeld | 0.413 | 1.25(0.73–2.15) | 0.941 | 0.519 | 1.21(0.68–2.15) | 0.954 |
| Additive modee | 0.320 | 1.10(0.91–1.34) | 0.799 | 0.340 | 1.11(0.90–1.37) | 0.860 |
| rs1806505 | ||||||
| CC | 1 | 1 | 1 | 1 | 1 | 1 |
| CT | 0.072 | 0.79(0.62–1.02) | 0.288 | 0.116 | 0.80(0.61–1.06) | 0.464 |
| TT | 0.339 | 0.85(0.61–1.18) | 0.834 | 0.646 | 0.92(0.65–1.31) | 0.884 |
| Dominant modelc | 0.080 | 0.81(0.64–1.03) | 0.240 | 0.165 | 0.83(0.65–1.08) | 0.424 |
| Recessive modeld | 0.881 | 0.98(0.73–1.31) | 0.941 | 0.751 | 1.05(0.77–1.44) | 0.970 |
| Additive modee | 0.205 | 0.90(0.77–1.06) | 0.672 | 0.448 | 0.94(0.79–1.11) | 0.860 |
| rs3768142 | ||||||
| TT | 1 | 1 | 1 | 1 | 1 | 1 |
| GT | 0.616 | 1.06(0.84–1.36) | 0.879 | 0.575 | 1.08(0.83–1.40) | 0.884 |
| GG | 0.440 | 1.14(0.82–1.59) | 0.834 | 0.939 | 0.99(0.69–1.42) | 0.966 |
| Dominant modelc | 0.488 | 1.08(0.86–1.36) | 0.703 | 0.676 | 1.05(0.83–1.34) | 0.869 |
| Recessive modeld | 0.528 | 1.11(0.81–1.51) | 0.941 | 0.761 | 0.95(0.68–1.33) | 0.970 |
| Additive modee | 0.421 | 1.07(0.91–1.25) | 0.801 | 0.889 | 1.01(0.85–1.20) | 0.889 |
| rs4659724 | ||||||
| GG | 1 | 1 | 1 | 1 | 1 | 1 |
| GA | 0.007 | 0.71(0.55–0.91) | 0.050 | 0.017 | 0.72(0.55–0.942) | 0.122 |
| AA | 0.062 | 0.73(0.53–1.02) | 0.279 | 0.235 | 0.81(0.57–1.15) | 0.846 |
| Dominant modelc | 0.005 | 0.72(0.57–0.90) | 0.030 | 0.022 | 0.74(0.58–0.96) | 0.132 |
| Recessive modeld | 0.452 | 0.89(0.67–1.20) | 0.941 | 0.906 | 0.98(0.72–1.34) | 0.970 |
| Additive modee | 0.021 | 0.83(0.71–0.97) | 0.126 | 0.106 | 0.87(0.73–1.03) | 0.636 |
| rs6668344 | ||||||
| CC | 1 | 1 | 1 | 1 | 1 | 1 |
| CT | 0.480 | 0.91(0.70–1.18) | 0.857 | 0.789 | 0.96(0.73–1.28) | 0.884 |
| TT | 0.368 | 0.86(0.63–1.19) | 0.834 | 0.810 | 0.96(0.68–1.35) | 0.884 |
| Dominant modelc | 0.382 | 0.90(0.70–1.15) | 0.703 | 0.771 | 0.96(0.74–1.26) | 0.925 |
| Recessive modeld | 0.532 | 0.92(0.70–1.20) | 0.941 | 0.908 | 0.98(0.74–1.32) | 0.970 |
| Additive modee | 0.355 | 0.93(0.79–1.09) | 0.799 | 0.799 | 0.98(0.83–1.16) | 0.860 |
| rs1805087 | ||||||
| AA | 1 | 1 | 1 | 1 | 1 | 1 |
| AG | < 0.001 | 1.64(1.26–2.14) | < 0.001 | 0.006 | 1.49(1.12–1.98) | 0.054 |
| GG | < 0.001 | 6.30(2.78–14.27) | < 0.001 | < 0.001 | 6.85(2.94–15.96) | < 0.001 |
| Dominant modelc | < 0.001 | 1.89(1.46–2.43) | < 0.001 | < 0.001 | 1.77(1.35–2.32) | < 0.001 |
| Recessive modeld | < 0.001 | 5.56(2.46–12.57) | < 0.001 | < 0.001 | 6.26(2.69–14.54) | < 0.001 |
| Additive modee | < 0.001 | 1.88(1.51–2.34) | < 0.001 | < 0.001 | 1.81(1.44–2.29) | < 0.001 |
| rs4077829 | ||||||
| GG | 1 | 1 | 1 | 1 | 1 | 1 |
| GT | 0.635 | 0.94(0.73–1.21) | 0.879 | 0.771 | 0.96(0.73–1.26) | 0.884 |
| TT | 0.875 | 0.97(0.70–1.35) | 0.993 | 0.733 | 1.06(0.75–1.52) | 0.884 |
| Dominant modelc | 0.670 | 0.95(0.75–1.21) | 0.709 | 0.918 | 0.99(0.76–1.28) | 0.964 |
| Recessive modeld | 0.941 | 1.01(0.76–1.35) | 0.941 | 0.583 | 1.09(0.80–1.49) | 0.954 |
| Additive modee | 0.805 | 0.98(0.83–1.15) | 0.990 | 0.812 | 1.02(0.86–1.22) | 0.860 |
| rs2275565 | ||||||
| GG | 1 | 1 | 1 | 1 | 1 | 1 |
| GT | 0.002 | 1.50(1.16–1.94) | 0.018 | 0.003 | 1.52(1.15–1.20) | 0.036 |
| TT | < 0.001 | 5.19(2.12–12.69) | < 0.001 | 0.001 | 4.93(1.93–12.58) | 0.018 |
| Dominant modelc | < 0.001 | 1.65(1.29–2.12) | < 0.001 | < 0.001 | 1.66(1.27–2.17) | < 0.001 |
| Recessive modeld | 0.001 | 4.66(1.91–11.37) | < 0.001 | 0.002 | 4.41(1.73–11.22) | 0.018 |
| Additive modee | < 0.001 | 1.68(1.34–2.10) | < 0.001 | < 0.001 | 1.68(1.32–2.13) | < 0.001 |
| rs3754255 | ||||||
| TT | 1 | 1 | 1 | 1 | 1 | 1 |
| TC | 0.500 | 0.91(0.70–1.19) | 0.857 | 0.302 | 0.86(0.65–1.14) | 0.864 |
| CC | 0.934 | 1.01(0.75–1.38) | 0.993 | 0.565 | 0.91(0.65–1.27) | 0.884 |
| Dominant modelc | 0.659 | 0.95 (0.74–1.21) | 0.709 | 0.327 | 0.88(0.67–1.14) | 0.697 |
| Recessive modeld | 0.597 | 1.07(0.84–1.39) | 0.941 | 0.970 | 0.10(0.75–1.32) | 0.970 |
| Additive modee | 0.969 | 1.00(0.86–1.17) | 1.000 | 0.523 | 0.95(0.80–1.12) | 0.860 |
| rs3820571 | ||||||
| TT | 1 | 1 | 1 | 1 | 1 | 1 |
| TG | 0.809 | 1.03(0.80–1.32) | 0.971 | 0.726 | 1.05(0.80–1.37) | 0.884 |
| GG | 0.597 | 0.86(0.49–1.51) | 0.879 | 0.357 | 0.75(0.41–1.38) | 0.864 |
| Dominant modelc | 0.952 | 1.01(0.80–1.28) | 0.952 | 0.964 | 1.01(0.78–1.30) | 0.964 |
| Recessive modeld | 0.571 | 0.85(0.49–1.49) | 0.941 | 0.329 | 0.74(0.40–1.36) | 0.954 |
| Additive modee | 0.880 | 0.99(0.81–1.20) | 0.990 | 0.757 | 0.97(0.78–1.20) | 0.860 |
| rs10925252 | ||||||
| CC | 1 | 1 | 1 | 1 | 1 | 1 |
| CT | 0.293 | 0.86(0.65–1.14) | 0.834 | 0.085 | 0.77(0.57–1.04) | 0.383 |
| TT | 0.934 | 0.99(0.73–1.34) | 0.993 | 0.357 | 0.86(0.62–1.19) | 0.864 |
| Dominant modelc | 0.466 | 0.91(0.70–1.18) | 0.703 | 0.117 | 0.80 (0.61–1.06) | 0.424 |
| Recessive modeld | 0.493 | 1.09(0.85–1.39) | 0.941 | 0.909 | 1.02(0.78–1.33) | 0.970 |
| Additive modee | 1.000 | 1.00(0.86–1.16) | 1.000 | 0.390 | 0.93(0.79–1.10) | 0.860 |
| rs12060570 | ||||||
| GG | 1 | 1 | 1 | 1 | 1 | 1 |
| GC | 0.046 | 0.78(0.60–0.99) | 0.273 | 0.061 | 0.77(0.59–1.01) | 0.366 |
| CC | 0.401 | 0.87(0.63–1.21) | 0.834 | 0.775 | 0.95(0.67–1.35) | 0.884 |
| Dominant modelc | 0.063 | 0.80 (0.63–1.01) | 0.227 | 0.121 | 0.82(0.63–1.06) | 0.424 |
| Recessive modeld | 0.941 | 1.01(0.76–1.35) | 0.941 | 0.508 | 1.11(0.81–1.51) | 0.954 |
| Additive modee | 0.224 | 0.91(0.77–1.06) | 0.672 | 0.499 | 0.94(0.79–1.12) | 0.860 |
aOR = adjusted odds ratio; CI = confidence interval.
a P < 0.05 was considered to indicate a statistically significant difference;
b Adjusted for residence, BMI before pregnancy, history of gestational diabetes mellitus, history of gestational hypertension, history of consanguineous marriage, family history of congenital malformations, cold before pregnancy, smoking before pregnancy, alcohol drinking before pregnancy, and folate supplement before or during pregnancy.
c Dominant model means homozygous variant + heterozygous variant versus homozygous wild-type.
d Recessive model means homozygous variant versus heterozygous variant + homozygous wild-type.
e Additive model means homozygous variant versus heterozygous variant versus homozygous wild-type.
Significant values are in [bold].
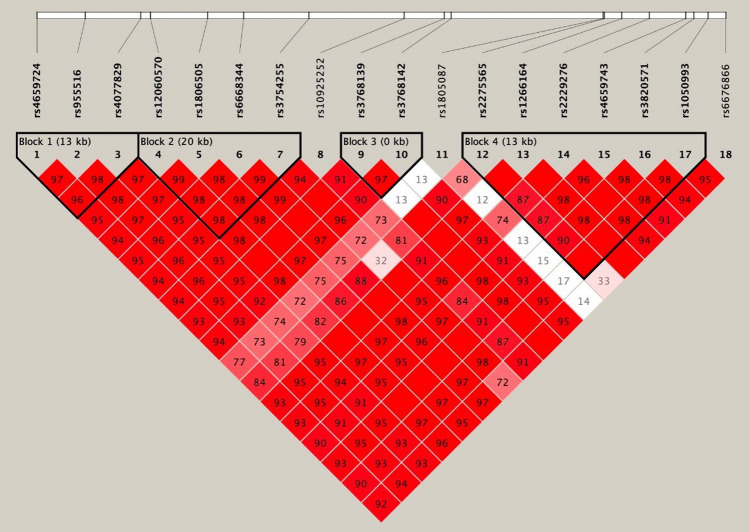
Linkage disequilibrium test and haplotype analysis
As shown in Fig. 1, these SNPs constructed four potential linkage disequilibrium blocks. The haplotype frequencies of MTR genetic polymorphisms were shown in Table 4. For the risk of CHD, three haplotypes of G-A-T (involving rs4659724, rs95516 and rs4077829; OR = 5.48, 95% CI 2.58–11.66), G-C-A-T-T-G (involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993; OR = 0.78, 95% CI 0.63–0.97) and T-C-A-T-T-G (involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993; OR = 1.60, 95% CI 1.26–2.04) were identified.
Figure 1.
Linkage disequilibrium (LD) analysis of the MTHFR SNPs in both populations.
Table 4.
Haplotype frequencies of MTR genetic polymorphisms.
| Haplotypes | Case (%) | Control (%) | aOR (95%CI) b | P | FDR_Pa |
|---|---|---|---|---|---|
| rs4659724-rs95516-rs4077829 | |||||
| GTG | 700.9(56.7%) | 689.8(55.8%) | 1 | ||
| AAT | 477.9(38.7%) | 521.8(42.2%) | 0.90 (0.77–1.06) | 0.072 | 0.144 |
| GAT | 45.1(3.7%) | 8.1(0.7%) | 5.48(2.58–11.66) | < 0.001 | < 0.001 |
| rs12060570-rs1806505-rs6668344-rs3754255 | |||||
| GCCC | 588.9(47.6%) | 591.9(47.7%) | 1 | ||
| CTTT | 501.9(40.6%) | 533.9(43.1%) | 0.95(0.80–1.12) | 0.217 | 0.362 |
| GCCT | 85.7(6.9%) | 60.0(4.8%) | 1.44(1.01–2.03) | 0.027 | 0.068 |
| GCTT | 51.4(4.2%) | 48.1(3.9%) | 1.07(0.71–1.62) | 0.721 | 0.801 |
| rs3768139-rs3768142 | |||||
| CT | 768.3(62.0%) | 782.4(63.1%) | 1 | ||
| CG | 238.7(19.3%) | 236.6(19.1%) | 1.03(0.84–1.26) | 0.916 | 0.916 |
| GG | 232.3(18.7%) | 214.4(17.3%) | 1.10(0.89–1.36) | 0.349 | 0.499 |
| rs2275565-rs1266164-rs2229276-rs4659743-rs3820571-rs1050993 | |||||
| GCGTTG | 554.9(45.0%) | 579.3(46.9%) | 1 | ||
| GCATTG | 212.1(17.2%) | 282.7(22.9%) | 0.78(0.63–0.97) | < 0.001 | < 0.001 |
| GTAAGA | 228.0(18.5%) | 213.0(17.2%) | 1.12(0.90–1.39) | 0.420 | 0.525 |
| TCATTG | 213.9(17.3%) | 139.3(11.3%) | 1.60(1.26–2.04) | < 0.001 | < 0.001 |
aOR ,adjusted odds ratio; CI, confidence interval.
a P < 0.05 was considered to indicate a statistically significant difference;
b The aOR values and 95% CIs were calculated using binary logistic regression and adjusted for residence, BMI before pregnancy, history of gestational diabetes mellitus, history of gestational hypertension, history of consanguineous marriage, family history of congenital malformations, cold before pregnancy, smoking before pregnancy, alcohol drinking before pregnancy, and folate supplement before or during pregnancy.
Significant values are in [bold].
Discussion
This study explored the association between 18 SNPs of MTR gene with the risk of CHD. After adjustment for potential confounders, we found a small number of significant positive associations among our SNP analyses which seemed consistent with hypothesis that mutant genotypes increased the risk of CHD. Besides, the haplotype analysis showed that G-A-T (OR = 5.48, 95% CI 2.58–11.66), G-C-A-T-T-G (OR = 0.78, 95% CI 0.63–0.97) and T-C-A-T-T-G (OR = 1.60, 95% CI 1.26–2.04) were significantly associated with risk of CHD.
Convincing evidence implied that folate deficiency and HHcy were associated with an increased risk of CHD19. Accordingly, genes related to folate/Hcy metabolism were attractive candidates for understanding genetic susceptibility factors for CHD. To data, numerous maternal genes associated with folate/Hcy metabolism pathway had been assessed to the genetic risk factors of CHD, such as the genes of methylene-tetrahydrofolate reductase (MTHFR) and cystathionine beta synthase (CBS)20,21. It was demonstrated that periconceptional folic acid supplementation was associated with a decreased risk of CHD but this association could be modified by variants of the infant MTR gene22. Thus, the present study was the first time to investigate the 18 SNPs of MTR gene in infant and the risk of CHD. Our study indicated that MTR SNPs at rs1805087 and rs2275565 may be associated with the risk of CHD. After controlling for the confounding factors, polymorphisms of MTR gene at rs1805087 and rs2275565 trended to increase the risk of CHD in the mutant genotypes (GG vs. AA at rs1805087, aOR = 1.812; TT vs. GG at rs2275565, aOR = 1.679). Published literature had already reported some gene polymorphisms of MTR on susceptibility to CHD. They declared that rs28372871 (+ 186 T > G) and rs1131450 (+ 905G > A) variants of MTR were significantly associated with the higher risk of CHD and an increase in plasma Hcy concentration23,24. In addition, Deng et al.22 suggested that mutant alleles of MTR gene at rs1770449 and rs1050993 in infant were associated with an increased risk of CHD. Among the SNP mentioned above, only rs1050993 was included in our study and no association between rs1050993 and the risk of CHD was observed. As far as our knowledge, this present study was the first time to find that polymorphisms of MTR gene at rs1805087 and rs2275565 were associated with the higher risk of CHD, and it was also the first time that the association between infant MTR gene polymorphism and the susceptibility of CHD was comprehensively evaluated. It could help to provide new candidate loci of MTR gene when exploring the genetic susceptibility factor of CHD.
The polymorphism rs1805087 (A2756G) in MTR gene had become a hot topic in the study of genetic susceptibility factors for birth defects. The A2756G mutation contributed to the substitution of aspartic acid (A allele) to glycine (G allele)25. It was located in a protein region interacting with S-adenosyl methionine (SAM) and auxiliary proteins which were significant for the reducing methylation and reactivating the vitamin B12 cofactor, which can be oxidatively inactivated in the catalytic process26. Thus, mutants might weaken the binding of SAM and/or auxiliary proteins and increased the level of plasma homocysteine27. Concerning associations between the polymorphism MTR rs1805087 and the plasma homocysteine level was still controversial28,29. Previous studies reported the association between rs1805087 and MTR. Dr. Shi30 and Dr. Galdieri’s results31 suggested that maternal MTR gene at rs1805087 could not affect the susceptibility to CHD in offspring. With respect to some subtypes of CHD, a case–control study in Chinese population also showed that MTR SNPs at rs1805087 was not associated with the occurrence of VSD32. Nevertheless, the polymorphism of MTR at rs1805087 were identified to be significantly associated with the higher risk of other diseases, such as preeclampsia and breast cancer33,34. Our data suggested that infant MTR gene at rs1805087 were associated with the higher risk of CHD which was not reported yet. But these observations will need to be replicated in future studies.
Taking the possible interactions between different SNPs into account, we analyzed the haplotypes of MTR gene in CHD. Although in single locus analysis, statistically significant associations between genetic polymorphisms at rs1050993 and the risk of CHD were not observed in our research. Interestingly, our research found that the haplotype block formed by rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993 was associated with risk of CHD. A previous study focusing on the relationship of haplotypes of MTR genetic polymorphisms with conotruncal heart defects has provided evidence that haplotypes involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993 were associated with the increased risk of conotruncal heart defects. On the other hand, associations of haplotypes including the above-mentioned loci with the decreased risk of spina bifida were observed, whose difference may be related to the haplotype frequency18. However, given the different subtypes of congenital defects and physiopathologic pathways, further research is still needed to determine the potential mechanism of the protective effect of the haplotypes on the susceptibility of CHD. Previously, only one study22, found that haplotype of C-A-A formed by rs1770449, rs1805087 and rs1050993 in MTR gene was associated with the occurrence of CHD. Thus, rs1050993 may affect the risk of CHD through gene interaction. However, more researches and elucidations for rs1050993 in the future are needed to figure out its mechanism.
This case–control study had some limitations. Firstly, the data of this study were mainly from the same hospital, and the sample source may be concentrated in a certain type of population, which may affect the representativeness of the sample and lead to selection bias. Secondly, considering the obvious ethnic and regional differences in MTR gene polymorphisms, we recruited the participants restricted to the Han Chinese ethnicity. Thirdly, although adjusting for a variety of potential confounding factors, we still cannot completely exclude the involvement of the possibility of residual confounding. Because confounding factors involved in this research did not account for all the risk factors of CHD.
Conclusions
The present study is the first to exhaustively estimate the association of 18 SNPs of MTR gene with the risk of CHD, which suggests that genetic polymorphisms of MTR gene at rs1805087 and rs2275565 are significantly associated with CHD. In addition, our study supports a significant association of three haplotypes of G-A-T (involving rs4659724, rs95516 and rs4077829), G-C-A-T-T-G (involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993) and T-C-A-T-T-G (involving rs2275565, rs1266164, rs2229276, rs4659743, rs3820571 and rs1050993) with risk of CHD. However, the limitations in this study should be carefully taken into consideration. In the future, more specific studies in different ethnic populations are required to refine and confirm our findings.
Acknowledgements
The authors would like to thank the editors and reviewers for their suggestions and all colleagues working in Maternal and Child Health Promotion and Birth Defect Prevention Group.
Abbreviations
- MTR
Methionine synthase
- CHD
Congenital heart disease
- SNPs
Single nucleotide polymorphisms
- Hcy
Homocysteine
- HHcy
Hyperhomocysteinemia
- HWE
The Hardy–Weinberg equilibrium
- FDR_P
False discovery rate P value
- OR
Odds ratio
- CIs
Confidence intervals
- aOR
Adjusted OR
- BMI
Body mass index
- MTHFR
Methylene-tetrahydrofolate reductase
- CBS
Cystathionine beta synthase
- ASD
Atrial septal defect
- VSD
Ventricular septal defect
- AVSD
Atrioventricular septal defect
- PDA
Patent ductus arteriosus
- APW
Aorto-pulmonary window
- TOF
Tetralogy of Fallot
- TGA
Complete transposition of great arteries
- SAM
S-adenosyl methionine
Author contributions
Y.P.L. and T.W.Z. wrote the main manuscript text; Y.P.L., T.T.W. and T.W.Z. analyzed the data; J.B.Q. and T.B.Y. reviewed and revised the manuscript; Y.P.L., T.W.Z., X.L.S., T.T.W., S.M.Z., M.T.S., J.H.W. and J.S. have collected the data. All authors have read and approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation Program of China (82073653 and 81803313), Hunan Outstanding Youth Fund Project (2022JJ10087), National Key Research and Development Project (2018YFE0114500), China Postdoctoral Science Foundation (2020M682644), Hunan Provincial Science and Technology Talent Support Project (2020TJ-N07), Hunan Provincial Key Research and Development Program (2018SK2063), Open Project from NHC Key Laboratory of Birth Defect for Research and Prevention (KF2020006), Natural Science Foundation of Hunan Province (2018JJ2551), Natural Science Foundation of Hunan Province of China (Grant Numbers 2022JJ40207), and Changsha Municipal Natural Science Foundation (Grant Numbers kq2202470).
Data availability
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: [European Variation Archive (EVA) repository and Project: PRJEB54935, Analyses: ERZ12298562], [http://ftp.ebi.ac.uk/pub/databases/eva/PRJEB54935/].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yiping Liu and Taowei Zhong.
References
- 1.Zhao B, Lin Y, Xu J, Ni B, Da M, Ding C, Hu Y, Zhang K, Yang S, Wang X, et al. Replication of the 4p16 susceptibility locus in congenital heart disease in Han Chinese populations. PLoS One. 2014;9(9):e107411. doi: 10.1371/journal.pone.0107411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zaidi S, Brueckner M. Genetics and genomics of congenital heart disease. Circ. Res. 2017;120(6):923–940. doi: 10.1161/CIRCRESAHA.116.309140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurvitz M, Burns KM, Brindis R, Broberg CS, Daniels CJ, Fuller SM, Honein MA, Khairy P, Kuehl KS, Landzberg MJ, et al. Emerging research directions in adult congenital heart disease: A report from an NHLBI/ACHA working group. J. Am. Coll. Cardiol. 2016;67(16):1956–1964. doi: 10.1016/j.jacc.2016.01.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu Y, Chen S, Zühlke L, Black GC, Choy MK, Li N, Keavney BD. Global birth prevalence of congenital heart defects 1970–2017: Updated systematic review and meta-analysis of 260 studies. Int. J. Epidemiol. 2019;48(2):455–463. doi: 10.1093/ije/dyz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao L, Chen L, Yang T, Wang T, Zhang S, Chen L, Ye Z, Luo L, Qin J. Birth prevalence of congenital heart disease in China, 1980–2019: A systematic review and meta-analysis of 617 studies. Eur. J. Epidemiol. 2020;35(7):631–642. doi: 10.1007/s10654-020-00653-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van der Bom T, Zomer AC, Zwinderman AH, Meijboom FJ, Bouma BJ, Mulder BJ. The changing epidemiology of congenital heart disease. Nat. Rev. Cardiol. 2011;8(1):50–60. doi: 10.1038/nrcardio.2010.166. [DOI] [PubMed] [Google Scholar]
- 7.Verheugt CL, Uiterwaal CS, van der Velde ET, Meijboom FJ, Pieper PG, van Dijk AP. Mortality in adult congenital heart disease. European heart journal. 2010;31(10):1220–1229. doi: 10.1093/eurheartj/ehq032. [DOI] [PubMed] [Google Scholar]
- 8.Gaynor JW, Stopp C, Wypij D, Andropoulos DB, Atallah J, Atz AM, Beca J, Donofrio MT, Duncan K, Ghanayem NS, et al. Neurodevelopmental outcomes after cardiac surgery in infancy. Pediatrics. 2015;135(5):816–825. doi: 10.1542/peds.2014-3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang T, Chen L, Yang T, Huang P, Wang L, Zhao L, Zhang S, Ye Z, Chen L, Zheng Z, et al. Congenital heart disease and risk of cardiovascular disease: A meta-analysis of cohort studies. J. Am. Heart Assoc. 2019;8(10):e12030. doi: 10.1161/JAHA.119.012030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aburawi EH, Aburawi HE, Bagnall KM, Bhuiyan ZA. Molecular insight into heart development and congenital heart disease: An update review from the Arab countries. Trends Cardiovasc. Med. 2015;25(4):291–301. doi: 10.1016/j.tcm.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, Li P, Chen S, Xi L, Guo Y, Guo A, Sun K. Influence of genes and the environment in familial congenital heart defects. Mol. Med. Rep. 2014;9(2):695–700. doi: 10.3892/mmr.2013.1847. [DOI] [PubMed] [Google Scholar]
- 12.Fung A, Manlhiot C, Naik S, Rosenberg H, Smythe J, Lougheed J, Mondal T, Chitayat D, Mccrindle BW, Mital S. Impact of prenatal risk factors on congenital heart disease in the current era. J. Am. Heart Assoc. 2013;2(3):e64. doi: 10.1161/JAHA.113.000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalisch-Smith JI, Ved N, Sparrow DB. environmental risk factors for congenital heart disease. Cold Spring Harb. Perspect. Biol. 2020;12(3):a037234. doi: 10.1101/cshperspect.a037234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blue GM, Kirk EP, Giannoulatou E, Sholler GF, Dunwoodie SL, Harvey RP, Winlaw DS. Advances in the genetics of congenital heart disease: A clinician's guide. J. Am. Coll. Cardiol. 2017;69(7):859–870. doi: 10.1016/j.jacc.2016.11.060. [DOI] [PubMed] [Google Scholar]
- 15.Zhao JY, Yang XY, Gong XH, Gu ZY, Duan WY, Wang J, Ye ZZ, Shen HB, Shi KH, Hou J, et al. Functional variant in methionine synthase reductase intron-1 significantly increases the risk of congenital heart disease in the Han Chinese population. Circulation. 2012;125(3):482–490. doi: 10.1161/CIRCULATIONAHA.111.050245. [DOI] [PubMed] [Google Scholar]
- 16.Feng Y, Wang S, Chen R, Tong X, Wu Z, Mo X. Maternal folic acid supplementation and the risk of congenital heart defects in offspring: A meta-analysis of epidemiological observational studies. Sci. Rep. 2015;5:8506. doi: 10.1038/srep08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blom HJ, Smulders Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011;34(1):75–81. doi: 10.1007/s10545-010-9177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw GM, Lu W, Zhu H, Yang W, Briggs FB, Carmichael SL, Barcellos LF, Lammer EJ, Finnell RH. 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med. Genet. 2009;10:49. doi: 10.1186/1471-2350-10-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Driel LM, de Jonge R, Helbing WA, van Zelst BD, Ottenkamp J, Steegers EA, Steegers-Theunissen RP. Maternal global methylation status and risk of congenital heart diseases. Obstet. Gynecol. 2008;112(2 Pt 1):277–283. doi: 10.1097/AOG.0b013e31817dd058. [DOI] [PubMed] [Google Scholar]
- 20.Sun M, Wang T, Huang P, Diao J, Zhang S, Li J, Luo L, Li Y, Chen L, Liu Y, et al. Association analysis of maternal MTHFR gene polymorphisms and the occurrence of congenital heart disease in offspring. BMC Cardiovasc. Disord. 2021;21(1):298. doi: 10.1186/s12872-021-02117-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li Y, Diao J, Li J, Luo L, Zhao L, Zhang S, Wang T, Chen L, Yang T, Chen L, et al. Association of maternal dietary intakes and CBS gene polymorphisms with congenital heart disease in offspring. Int. J. Cardiol. 2021;322:121–128. doi: 10.1016/j.ijcard.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 22.Deng C, Deng Y, Xie L, Yu L, Liu L, Liu H, Dai L. Genetic polymorphisms in MTR are associated with non-syndromic congenital heart disease from a family-based case-control study in the Chinese population. Sci. Rep. 2019;9(1):5065. doi: 10.1038/s41598-019-41641-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tanaka T, Scheet P, Giusti B, Bandinelli S, Piras MG, Usala G, Lai S, Mulas A, Corsi AM, Vestrini A, et al. Genome-wide association study of vitamin B6, vitamin B12, folate, and homocysteine blood concentrations. Am. J. Hum. Genet. 2009;84(4):477–482. doi: 10.1016/j.ajhg.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao JY, Qiao B, Duan WY, Gong XH, Peng QQ, Jiang SS, Lu CQ, Chen YJ, Shen HB, Huang GY, et al. Genetic variants reducing MTR gene expression increase the risk of congenital heart disease in Han Chinese populations. Eur. Heart J. 2014;35(11):733–742. doi: 10.1093/eurheartj/eht221. [DOI] [PubMed] [Google Scholar]
- 25.Olteanu H, Munson T, Banerjee R. Differences in the efficiency of reductive activation of methionine synthase and exogenous electron acceptors between the common polymorphic variants of human methionine synthase reductase. Biochemistry-Us. 2002;41(45):13378–13385. doi: 10.1021/bi020536s. [DOI] [PubMed] [Google Scholar]
- 26.Li WX, Lv WW, Dai SX, Pan ML, Huang JF. Joint associations of folate, homocysteine and MTHFR, MTR and MTRR gene polymorphisms with dyslipidemia in a Chinese hypertensive population: A cross-sectional study. Lipids Health Dis. 2015;14:101. doi: 10.1186/s12944-015-0099-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Biselli JM, Goloni-Bertollo EM, Haddad R, Eberlin MN, Pavarino-Bertelli EC. The MTR A2756G polymorphism is associated with an increase of plasma homocysteine concentration in Brazilian individuals with Down syndrome. Braz. J. Med. Biol. Res. 2008;41(1):34–40. doi: 10.1590/S0100-879X2006005000195. [DOI] [PubMed] [Google Scholar]
- 28.Fillon-Emery N, Chango A, Mircher C, Barbé F, Bléhaut H, Herbeth B, Rosenblatt DS, Réthoré MO, Lambert D, Nicolas JP. Homocysteine concentrations in adults with trisomy 21: Effect of B vitamins and genetic polymorphisms. Am. J. Clin. Nutr. 2004;80(6):1551–1557. doi: 10.1093/ajcn/80.6.1551. [DOI] [PubMed] [Google Scholar]
- 29.Fredriksen A, Meyer K, Ueland PM, Vollset SE, Grotmol T, Schneede J. Large-scale population-based metabolic phenotyping of thirteen genetic polymorphisms related to one-carbon metabolism. Hum. Mutat. 2007;28(9):856–865. doi: 10.1002/humu.20522. [DOI] [PubMed] [Google Scholar]
- 30.Shi H, Yang S, Liu Y, Huang P, Lin N, Sun X, Yu R, Zhang Y, Qin Y, Wang L. Study on environmental causes and SNPs of MTHFR, MS and CBS genes related to congenital heart disease. PLoS One. 2015;10(6):e128646. doi: 10.1371/journal.pone.0128646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galdieri LC, Arrieta SR, Silva CM, Pedra CA, D'Almeida V. Homocysteine concentrations and molecular analysis in patients with congenital heart defects. Arch. Med. Res. 2007;38(2):212–218. doi: 10.1016/j.arcmed.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 32.Su J, Li Z. Analysis of MTR and MTRR gene polymorphisms in Chinese patients with ventricular septal defect. Appl. Immunohistochem. Mol. Morphol. 2018;26(10):769–774. doi: 10.1097/PAI.0000000000000512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osunkalu VO, Taiwo IA, Makwe CC, Quao RA. Methylene tetrahydrofolate reductase and methionine synthase gene polymorphisms as genetic determinants of pre-eclampsia. Pregnancy Hypertens. 2020;20:7–13. doi: 10.1016/j.preghy.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Sangrajrang S, Sato Y, Sakamoto H, Ohnami S, Khuhaprema T, Yoshida T. Genetic polymorphisms in folate and alcohol metabolism and breast cancer risk: A case-control study in Thai women. Breast Cancer Res. Treat. 2010;123(3):885–893. doi: 10.1007/s10549-010-0804-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The name of the repository and accession number can be found below: [European Variation Archive (EVA) repository and Project: PRJEB54935, Analyses: ERZ12298562], [http://ftp.ebi.ac.uk/pub/databases/eva/PRJEB54935/].