Abstract
Key Clinical Message
Intrapleural streptokinase can be an option for loculated hemorrhagic pleural effusion among patients receiving CAPD and under DAPT. Its use can be individualized based on risk benefit analysis by the treating clinician.
Abstract
Pleural effusion is seen in up to 10 percent of patients on peritoneal dialysis (PD). A hemorrhagic pleural effusion is a diagnostic dilemma and a therapeutic challenge. We report a complicated case of 67 years old man with end stage renal disease, with coronary artery disease and stent in situ under dual antiplatelet therapy and continuous ambulatory peritoneal dialysis. The patient presented with left‐sided loculated hemorrhagic pleural effusion. He was managed with intrapleural streptokinase therapy. His loculated effusion resolved without any local and systemic bleeding manifestations. Therefore, in poor resource settings, Intrapleural streptokinase can be an option for loculated hemorrhagic pleural effusion among patients receiving CAPD and under DAPT. Its use can be individualized based on risk benefit analysis by the treating clinician.
Keywords: dual antiplatelet therapy, Hemorrhagic pleural effusion, Intrapleural streptokinase, peritoneal dialysis
Improvement of hemmorhagic pleural effusion with intrapleural streptokinase.

1. INTRODUCTION
Pleural effusion is seen in up to 10 percent of patients on peritoneal dialysis (PD). 1 The differential diagnosis of pleural effusion in PD patients is extensive. It includes general causes of pleural effusion and causes unique to PD patients. Transudative pleural effusions in PD patients may be commonly due to volume overload and cardiac failure, or rarely due to pleuroperitoneal leakage. 2 Exudative effusions could occur due to infection, inflammation, or hemorrhage. The presence of a hemorrhagic pleural effusion can narrow the differential diagnoses to trauma, tuberculosis, or tumor. Other causes include bleeding diatheses, pulmonary infarction, embolism, or vascular malformations. Uremia can also lead to a hemorrhagic pleural effusions in patients with chronic kidney disease. 3
Hemorrhagic pleural effusion is a diagnostic dilemma and a therapeutic challenge. Angioblastic and fibroblastic proliferation can lead to the formation of fibrin clots. 4 Thus, Intercostal chest tube drainage (ICT) becomes ineffective due to clots and septations in up to 40 percent of these cases. 5 The management of such septated effusion is debatable. Video‐assisted thoracoscopic surgery (VATS) is an effective modality of treatment. 6 Also, the administration of intrapleural enzymes is a reasonable cost‐effective approach, especially in resource‐poor settings. 7 Intrapleural enzymes like streptokinase (IPSK), urokinase, tissue plasminogen activator (tPa), or DNase lyse fibrin adhesions and allow free‐flow drainage. Side effects include chest pain, fever, chills, and allergic reactions. In addition, local pleural and systemic hemorrhages are reported adverse events of IPSK. 8
Nevertheless, the use of IPSK in ESRD patients on Continuous Ambulatory Peritoneal Dialysis (CAPD) has not been reported in the literature. We report a case of successful instillation of IPSK in a hemorrhagic pleural effusion in a patient on CAPD and dual antiplatelet therapy (DAPT). The effusion resolved without any local and systemic bleeding manifestations.
2. CASE REPORT
A 67 years old man, nonsmoker, with no contact history of tuberculosis, presented with chief complaints of gradually progressive shortness of breath for 7 days and left‐sided chest pain for 3 days. He had a past history of systemic hypertension, type 2 diabetes mellitus, chronic kidney disease under CAPD for 6 months and, coronary artery disease with a stent in situ for 3 months. He was under DAPT (aspirin 75 mg and clopidogrel 75 mg), statin, antihypertensive, hypoglycemic agents, and other supportive medications. Physical examination revealed pallor, tachycardia, and tachypnoea. The volume status of the patient assessed from jugular venous pressure was 6 cm H2O. Urine output was 200–300 mL/day. Chest X‐ray showed a left‐sided massive pleural effusion as shown in Figure 1. Ultrasonography (USG) chest and Computed Tomography (CT) chest showed effusion with clots with multiple septations as shown in Figure 2. There was no evidence of consolidation, cavitation or significant lymphadenopathy in CT chest. Pleural fluid analysis showed plenty of red blood cells, total leucocyte count of 200/microliters, with 40% granulocytes and 60% nongranulocytes. Pleural fluid protein level was 4.5 g/dL, and glucose was 70 mg/dL. Pleural fluid LDH level was 420 U/L. The overall picture was suggestive of exudative type of pleural effusion. We could not perform pleural fluid PH and CRP quantification in pleural fluid as those facilities were not available in our center. The adenosine deaminase (ADA) level was 46 IU/L. Pleural fluid gene‐xpert for mycobacterium tuberculosis was negative. The pleural fluid culture was sterile. The serum CRP level was 12 mg/dL. Sputum was negative for acid‐fast bacilli (AFB) on three consecutive sputum samples. There were no atypical cells on three malignant cytology samples.
FIGURE 1.

(A) Before ICT; (B) after ICT; (C) after 1st Dose STK; (D) after 2nd dose STK; (E) after 3rd dose STK; (F) at discharge.
FIGURE 2.
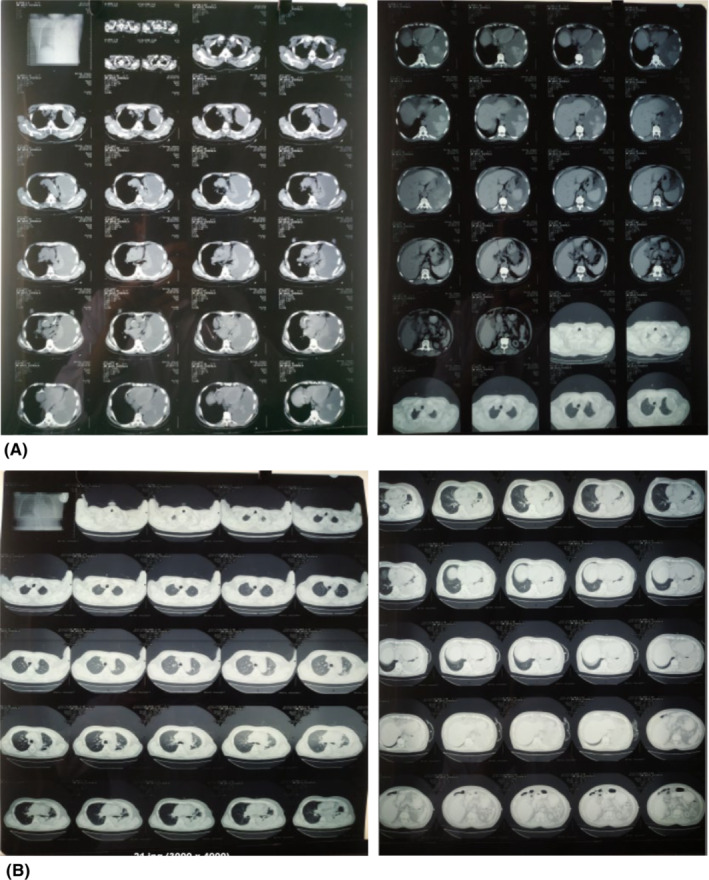
(A) Before ICT and IPSK; (B) after ICT and IPSK.
A tube thoracostomy was done. We noted 500 mL of dark red pleural fluid under the water seal bag. The ratio of blood hematocrit to pleural fluid hematocrit was less than 50 percent. On re‐evaluation with USG chest, multiple pockets of effusion were persistent. There was no expansion in the lung field. Hence, we administered intrapleural streptokinase 3 million units retained in the pleural cavity for 2 h, once daily for 3 days. Subsequently, we noted 700 mL, 800 mL, and 600 mL of dark red fluid on three consecutive days. The chest tube was in situ another 7 days and removed on the eighth day after no drainage for two consecutive days. We ensured adequate flushing and correct positioning of the tube on all days. Chest X‐ray and USG review after removal of the chest tube showed expansion of lung field as shown in Figure 1. The patient received intravenous Piperacillin and Tazobactam in renal modified dose for 7 days during the hospital stay. Throughout the hospital stay, the patient was on his routine peritoneal dialysis prescription with 1.5%, 2.5% and 4.25% dextrose on morning, day time and night dwell, respectively. The net ultrafiltrate (UF) was around 1300–1500 mL/day. Clopidogrel was on hold for the preceding 5 days of tube thoracostomy as per the pulmonology consultation. His DAPT was continued after tube thoracostomy considering the risk of stent thrombosis as per the cardiology consultation.
Apart from pain around the chest tube site, there were no other side effects or complications. There were no local and systemic bleeding manifestations during the hospital stay. The patient improved symptomatically and was hemodynamically stable at discharge. During follow‐up, the patient was asymptomatic, and chest X‐ray and USG review showed an expanded lung field.
3. DISCUSSION
Patients with chronic kidney disease on PD may develop hemorrhagic effusion due to various etiologies. Common causes such as parapneumonic effusion, tuberculosis, malignancy was ruled out in our patient as there were no features of consolidation, mass, cavitations or significant lymphadenopathy on CT chest and the pleural fluid gene‐xpert for mycobacterium tuberculosis was negative. Pleural fluid cytology also did not reveal any atypical cells. In addition, our patient did not have a history of smoking, connective tissue diseases, bleeding diathesis or a history of trauma to the chest.
Unique to ESRD, uremic pleuritis leads to an exudative fibrous hemorrhagic pleural fluid. 9 A study reported uremic pleural effusion as the most common cause of exudative effusion in ESRD patients 10 Reportedly; pleural effusions can occur with BUN ranging from 30 to 240 mg/dL. The pathogenesis of hemorrhagic fibrinous effusion in uremia is multifactorial. It is thought to be due to uremic toxins leading to serosal inflammation and pleuritis with increased capillary permeability, exudation of proteins and fibrin deposition. 11 In addition, altered hemostasis in patients with renal failure could contribute to hemorrhagic effusion in uremia. There is alteration in the platelet surface glycoprotein GPIIb/IIIa in patients with uremia, which is a receptor for von Willebrand factor and fibrinogen. It leads to decreased platelet function. Also nitric oxide, an inhibitor of platelet aggregation is increased in uremic pleuritis. 12 However, serositis in patients undergoing regular dialysis, improvement of effusion without any change to the dialysis regimen are not in favor of uremic pleuritis as a sole cause of the hemorrhagic effusion.
Similarly, our patient was under DAPT (aspirin and clopidogrel) for poststent coronary artery disease for 3 months. So, the hemorrhagic nature of fluid could be drug‐related. The association between hemorrhagic fluid and dual antiplatelet therapy has not been reported in literature yet. The UK‐HARP‐I study has shown that in patients with chronic kidney disease 100 mg of aspirin daily was not associated with an excess of major bleeds although there was a 3‐fold excess of minor bleeds. 13 Also, a metanalysis concluded glycoprotein IIb/IIIa inhibitors or clopidogrel may increase major bleeding in CKD. 14 In our patient, dual antiplatelet therapy could be major additive contributing factor to altered platelet function associated with renal failure. In addition, presence of anemia in our patient is an important clinical factor that predisposes uremic patients to bleed. 12 All these evidences support the cause of hemorrhagic effusion to be multifactorial. The improvement that occurred following drainage and intravenous antibiotics within few days and slightly raised serum CRP level supports infection as a probable etiology, however, fluid analysis, CT findings and sterile pleural fluid culture were not in favor of infection.
For the management of loculated hemorrhagic effusion, we used intrapleural streptokinase. A trial concluded that IPSK is beneficial after failed tube drainage and favors lung expansion in hemothorax. 15 Nevertheless, higher success rates have been observed with intrapleural tPA/DNase treatment with decreased likelihood of invasive interventions and shortened hospital stay., 16 , 17 In our patient, we used intrapleural streptokinase as tPA/DNase and video‐assisted thoracoscopic surgery were unavailable in our setting.
The safety of intrapleural streptokinase is also debatable. Studies document it to be safe., 18 While in a multicenter randomized control trial involving 427 participants, seven percent in the streptokinase group demonstrated local pleural or systemic bleeding. 8 Although systemic absorption of intrapleural administered streptokinase is low, cumulative doses of intrapleural streptokinase may cause systemic fibrinolysis. 19 , 20 Case reports of fatal hemorrhage from aortic dissection 21 and diffuse alveolar hemorrhage following intrapleural streptokinase have been reported. 22 Risk for hemorrhage is high in those on systemic anticoagulation. 16 Absolute contraindications to intrapleural fibrinolysis include any trauma, surgery, or major hemorrhage within 48 hours, a bronchopleural fistula and history of allergic reaction to the drug. 23
Our patient had chronic kidney disease on CAPD, a state of altered platelet function. Patients with CKD have increased risk of thrombosis. Paradoxically, these patients also have increased risk of hemorrhage. 24 Increased bleeding occurs due to platelet hypo reactivity to adenosine diphosphate (ADP) and increased platelet cyclic adenosine monophosphate (cAMP) and decreased thromboxane A2 formation. 25 Furthermore, our patient was under dual antiplatelet therapy (aspirin and clopidogrel). Hence, he was at a higher risk of severe bleeding post pleural procedures. 26 Although intrapleural fibrinolytics in setting of parapneumonic effusion and empyema to facilitate drainage is well documented in literature, its use for hemorrhagic effusion in a patient under DAPT and CAPD has not been reported in literature yet.
4. CONCLUSION
The etiology of hemorrhagic pleural effusion in our case is most likely multifactorial. Our patient was at increased risk of bleeding complications with pleural procedures due to the presence of anemia, renal failure, and dual antiplatelet therapy. Nevertheless, the use of intrapleural streptokinase (IPSK) was not contraindicated in our patient. Its administration was not associated with local or systemic bleeding manifestations in our patient. There was resolution of septations, which facilitated adequate drainage. Radiographically, the lung field expanded, and clinically, our patient improved. Therefore, in poor resource settings, Intrapleural streptokinase can be an option for loculated hemorrhagic pleural effusion among patients receiving CAPD and under DAPT. Its use can be individualized based on risk benefit analysis by the treating clinician.
AUTHOR CONTRIBUTIONS
Abhishek Thapaliya: Conceptualization; data curation; investigation; writing – original draft; writing – review and editing. Urza Bhattarai: Conceptualization; data curation; investigation; validation; writing – review and editing. Arun Gautam: Conceptualization; resources; supervision; validation; writing – review and editing. Deepak Dhakal: Data curation; investigation; resources. Bhupendra Shah: Supervision; validation; writing – review and editing. Sanjib Kumar Sharma: Conceptualization; investigation; resources; supervision; validation; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGMENTS
None.
Thapaliya A, Bhattarai U, Gautam A, Dhakal D, Shah B, Sharma SK. Management of hemorrhagic pleural effusion with intrapleural streptokinase in a patient on peritoneal dialysis and dual antiplatelet therapy. Clin Case Rep. 2023;11:e7517. doi: 10.1002/ccr3.7517
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Lew SQ. Hydrothorax: pleural effusion associated with peritoneal dialysis. Perit Dial Int. 2010;30(1):13‐18. [DOI] [PubMed] [Google Scholar]
- 2. Kennedy C, McCarthy C, Alken S, et al. Pleuroperitoneal leak complicating peritoneal dialysis: a case series. Int J Nephrol. 2011;2011:526753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Karki A, Riley L, Mehta HJ, Ataya A. Pleural effusions of urinary etiologies. Dis Mon. 2019;65(4):104‐108. [DOI] [PubMed] [Google Scholar]
- 4. Boersma WG, Stigt JA, Smit HJM. Treatment of haemothorax. Respir Med. 2010;104(11):1583‐1587. [DOI] [PubMed] [Google Scholar]
- 5. Ali HA, Lippmann M, Mundathaje U, et al. Spontaneous hemothorax: a comprehensive review. Chest. 2008;134(5):1056‐1065. [DOI] [PubMed] [Google Scholar]
- 6. Sorino C, Mondoni M, Lococo F, Marchetti G, Feller‐Kopman D. Optimizing the management of complicated pleural effusion: from intrapleural agents to surgery. Respir Med. 2022;191:106706. [DOI] [PubMed] [Google Scholar]
- 7. Shipe ME, Maiga AW, Deppen SA, et al. Cost‐effectiveness analysis of fibrinolysis vs Thoracoscopic decortication for early empyema. Ann Thorac Surg. 2021;112(5):1632‐1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Maskell NA, Davies CW, Nunn AJ, et al. UK controlled trial of intrapleural streptokinase for pleural infection. N Engl J Med. 2005;352(9):865‐874. [DOI] [PubMed] [Google Scholar]
- 9. Al Harby A, Al Furayh O, Al Dayel F, Al Mobeireek A. Pleural effusion in a patient with end‐stage renal disease. Ann Saudi Med. 2006;26(2):145‐146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakirci T, Sasak G, Ozturk S, Akcay S, Sezer S, Haberal M. Pleural effusion in long‐term hemodialysis patients. Transplantation Proceedings. Vol 39. Elsevier; 2007:889‐891. [DOI] [PubMed] [Google Scholar]
- 11. Krishnan M, Choi M. Fellows' Forum in Dialysis Edited by Mark A. Perazella: A Case of Uremia‐Associated Pleural Effusion in a Peritoneal Dialysis Patient. Seminars in dialysis. Wiley Online Library; 2001. [DOI] [PubMed] [Google Scholar]
- 12. Sohal AS, Gangji AS, Crowther MA, Treleaven D. Uremic bleeding: pathophysiology and clinical risk factors. Thromb Res. 2006;118(3):417‐422. [DOI] [PubMed] [Google Scholar]
- 13. Baigent C, Landray M, Leaper C, et al. First United Kingdom heart and renal protection (UK‐HARP‐I) study: biochemical efficacy and safety of simvastatin and safety of low‐dose aspirin in chronic kidney disease. Am J Kidney Dis. 2005;45(3):473‐484. [DOI] [PubMed] [Google Scholar]
- 14. Palmer SC, Di Micco L, Razavian M, et al. Effects of antiplatelet therapy on mortality and cardiovascular and bleeding outcomes in persons with chronic kidney disease: a systematic review and meta‐analysis. Ann Intern Med. 2012;156(6):445‐459. [DOI] [PubMed] [Google Scholar]
- 15. Bergh NP, Ekroth R, Larsson S, Nagy P. Intrapleural streptokinase in the treatment of haemothorax and empyema. Scand J Thorac Cardiovasc Surg. 1977;11(3):265‐268. [PubMed] [Google Scholar]
- 16. Gervais DA, Levis DA, Hahn PF, Uppot RN, Arellano RS, Mueller PR. Adjunctive intrapleural tissue plasminogen activator administered via chest tubes placed with imaging guidance: effectiveness and risk for hemorrhage. Radiology. 2008;246(3):956‐963. [DOI] [PubMed] [Google Scholar]
- 17. Piccolo F, Popowicz N, Wong D, Lee YC. Intrapleural tissue plasminogen activator and deoxyribonuclease therapy for pleural infection. J Thorac Dis. 2015;7(6):999‐1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Talib S, Verma G, Arshad M, et al. Utility of intrapleural streptokinase in management of chronic empyemas. J Assoc Physicians India. 2003;51:464‐469. [PubMed] [Google Scholar]
- 19. Cameron RJ, Davies HRH. Intra‐pleural fibrinolytic therapy versus conservative management in the treatment of adult parapneumonic effusions and empyema. Cochrane Database Syst Rev. 2008;16(2):CD002312. [DOI] [PubMed] [Google Scholar]
- 20. Davies CW, Lok S, Davies RJ. The systemic fibrinolytic activity of intrapleural streptokinase. Am J Respir Crit Care Med. 1998;157(1):328‐330. [DOI] [PubMed] [Google Scholar]
- 21. Srivastava P, Godden D, Kerr KM, et al. Fatal haemorrhage from aortic dissection following instillation of intrapleural streptokinase. Scott Med J. 2000;45(3):86‐87. [DOI] [PubMed] [Google Scholar]
- 22. Shah RM, Krochmal R, Pickering EM, Burrows W, Sachdeva A. Diffuse alveolar hemorrhage as a complication of Intrapleural fibrinolytic therapy. J Bronchology Interv Pulmonol. 2017;24(4):e54‐e56. [DOI] [PubMed] [Google Scholar]
- 23. Hamblin SE, Furmanek DL. Intrapleural tissue plasminogen activator for the treatment of parapneumonic effusion. pharmacotherapy: the journal of human pharmacology and drug. Therapy. 2010;30(8):855‐862. [DOI] [PubMed] [Google Scholar]
- 24. Baaten CC, Schröer JR, Floege J, et al. Platelet abnormalities in CKD and their implications for antiplatelet therapy. Clin J Am Soc Nephrol. 2022;17(1):155‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Escolar G, Díaz‐Ricart M, Cases A. Uremic platelet dysfunction: past and present. Curr Hematol Rep. 2005;4(5):359‐367. [PubMed] [Google Scholar]
- 26. Dangers L, Giovannelli J, Mangiapan G, et al. Antiplatelet drugs and risk of bleeding after bedside pleural procedures: a National Multicenter Cohort Study. Chest. 2021;159(4):1621‐1629. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


