Abstract
Background
To reduce the harmful health effects of combustible cigarette smoke (CS), some (CS) users attempt to substitute CS with electronic cigarettes (ECIG) and/or heated tobacco products (HTP). In this animal study, we evaluated the acute effects of substituting CS consumption with ECIG or HTP thus mimicking the dual users’ approach, on the lungs of a mouse model.
Methods
C57BL/6 mice were divided into Control, ECIG, HTP, CS, ECIG + CS, HTP + CS, and HTP + ECIG groups. Animals were exposed for 3 hours in AM and PM sessions to either air, CS, ECIG, or HTP for seven days. Lung injury was assessed by: wet to dry (W/D) ratio, albumin concentration in bronchoalveolar lavage fluid, expression of IL-1β, IL-6, and TNF-α, histopathology examination, reactive oxygen species (ROS) production, and assessment of cellular apoptosis.
Results
W/D ratio was significantly increased in mice exposed to CS only. Albumin leak and expression of IL-1β, IL-6, and TNF-a were elevated in CS, ECIG + CS, and HTP + CS. Histological examination revealed significant inflammatory cells infiltration, as well as collagen deposit in CS, ECIG + CS, HTP + CS. ROS production was significantly increased in CS, ECIG + CS, HTP + CS. Finally, cell death was also significantly increased in CS, ECIG + CS, and HTP + CS.
Conclusion
In this animal model, substituting 50% of daily CS exposure by either ECIG or HTP exposure did not result in significant attenuation of acute lung injury.
Implications (What does this study add?):
Substituting 50% of combustible tobacco nicotine exposure with ECIG or HTP does not reduce lung injury compared to 100% CS.
Even in a best-case scenario where smokers are able to partially substitute rather than augment cigarette use with ECIG or HTP products, we find no evidence of pulmonary disease risk reduction.
Introduction
Novel tobacco and nicotine products, such as heated tobacco products (HTP) and electronic cigarettes (ECIGs) have been introduced into the global markets with a claim of reduced harm relative to combustible cigarettes. ECIG products electrically heat and vaporize a nicotine-containing liquid to produce an inhalable aerosol, while HTP products electrically heat and vaporize engineered tobacco sheets to do the same.1 Smoke from combustible cigarettes (CS) contains numerous toxicants and carcinogens and is strongly associated with many cardiovascular and respiratory diseases and cancer.2 ECIGs can deliver high quantities of nicotine which are associated with nicotine toxicity and enhanced risk of addiction.3 ECIG use was recently associated with acute lung injury that can be fatal.4 HTP effects are not well documented, though animal studies have recently described lung injury in vulnerable groups.5 In their marketing literature, manufacturers indicate that compared to CS, ECIG, and HTP can reduce exposure to toxic and carcinogenic chemicals.6–8
Influenced by such claims,9 some conventional cigarette smokers have adopted a “dual user” approach in which they attempt to partially substitute CS with ECIG or HTP in an attempt to reduce harm; others do so also as part of a strategy to quit smoking.10 In England, a recent study, however, failed to find an association between quitting smoking and ECIG or HTP consumption.11 In the United States, dual users – people who use both combustibles and ECIG or HTP – account for approximately 16% of total smokers and smoking cessation success rates are not substantially higher in the dual users’ group.12 The latest systemic global review by the Australian Department of Health noted limited evidence that ECIGs is an effective aid for quitting smoking when used in a clinical setting.13
The health effects of combining conventional CS with other ECIG or HTP products are not well investigated. We note that while some dual users attempt to substitute CS with ECIG or HTP, in the real world, daily dual-users tend to have greater daily nicotine intake than CS-only smokers. The end result is some degree of nicotine augmentation rather than perfect substitution, as CS smokers may also seek to use ECIG or HTP in life situations where smoking is not feasible.13,14 In this study, we examined the acute effects of ECIG, HTP, the combined use of CS with either HTP or ECIG, as well as the combined use of non-combustible products (HTP + ECIG) to have a better understanding of the relationships in this animal model. We selected the Vapor4life ECIG system and the IQOS HTP for the study because of their wide availability.
Methods
This work was approved by the institutional animal care and use committee at the American University of Beirut and animal procedures were conducted according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Two-month-old C57BL/6 male mice were kept in a temperature-controlled room and on a 12 h/12 h-dark/light cycle. Mice were provided with standard chow and water except when mice were placed in the tobacco smoke exposure apparatus. The CS, ECIG, or HTP exposure apparatus (ONARES, CH Technologies, USA) consisted of a smoke generator, mixing/conditioning chamber, and a twelve “nose only” rodent exposure carousel.15 One port was dedicated to sampling analysis and the remaining 11 ports were used for animal smoke exposure. Animals were acclimated to retainers for one week before placement in the carousel. Throughout the experiments, animals were evaluated daily for signs of distress that included decreased activity, ruffled hair, hyperventilation, and decreased food intake.
Mice were divided into seven groups and each group included 8 animals: Control, CS, ECIG, HTP, ECIG + CS, HTP + CS, and HTP + ECIG. As previously described,15 mice were positioned in retainers and placed into the holes of the carousel. Mice were exposed to a morning and an afternoon session for seven consecutive days. During the session, mice received a continuous flow of fresh air at a flow rate of 3LPM. When puffing, the fresh air was mixed with the puffed smoke from the CS, HTP, and ECIG at a flow rate of 1.2LPM. The flow rate at the sampling port was constant at 1LPM. Each session lasted for three hours. ECIG + CS and HTP + CS groups received CS in the morning and ECIG or HTP in the afternoon session, respectively. The HTP + ECIG group received HTP in the morning session and ECIG in the afternoon session.
As described previously, CS was generated from 3R4F cigarettes (University of Kentucky, Lexington, KY). ECIG aerosol was generated using pre-filled V4L (3.5 Ohm, 18 mg/ml labeled nicotine concentration) cartomizer cartridges, connected to a flow-activated 4.2 V Vapor Titan Soft Touch battery (Vapor4life, Illinois, USA). Cartridges and batteries were replaced every 30 min to ensure steady aerosol generation during the three-hour exposure sessions. HTP aerosols were generated utilizing the “I quit smoking system” (IQOS). The tobacco sticks, holders, and batteries were purchased from the manufacturer. Tobacco sticks were placed into a holder which heated the tobacco and generated aerosols. Tobacco sticks were replaced every 6 min. The holder battery was electrically charged through a charger before every use.
Given that nicotine and TPM emissions from IQOS tobacco are comparable to combustible cigarettes, the smoking session for HTP was programmed similarly to that of CS exposure with a puff duration of 2 s, and the inter-puff interval was set to 58 s generating a volume of 35 ml per puff.15 For ECIG, puff duration was set to 4 s and the inter-puff interval was 14 s generating a volume of 80 ml per puff.
Total particulate matter (TPM) emissions from the sessions were characterized under these puffing conditions using the procedures and apparatus previously reported resulting in an average TPM concentration of 112 mg/m3 for CS, 114 for HTP, and 283 for ECIG.15 Nicotine concentrations were calculated from the TPM concentrations based on the average Nicotine yield for the given device3,16,17 resulting in nicotine concentrations of 8.9 mg/m3 for CS, 6.8 mg/m3 for HTP, and 2.9 mg/m3 for ECIG (Fig. 4S).
Figure 4.
Cell death assessment in the lungs. A: Ctrl; B: ECIG; C: HTP; D: CS; E: ECIG + CS; F: HTP + CS; and G: HTP + ECIG. Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining of lung sections. A significant increase in apoptotic activity was demonstrated in CS, ECIG + CS, and HTP + CS when compared to the control.
At the end of the experiment, animals were anesthetized, and the trachea was cannulated with polyethylene tubing. Animals were exsanguinated by severing the aorta. The diaphragm was dissected to allow free lung expansion. The left lung was clipped, and the right lung was then washed three times by slowly instilling 0.5 ml of PBS 1× (Ca2+ and Mg2+ free, 37°C) and then gradually aspirating the washed fluid. The lower lobe of the left lung was excised for pulmonary water content evaluation. The upper lobe of the left lung was fixed in formalin for histopathology examination, ROS production, and TUNEL assay. The remaining right lung lobes were individually frozen in liquid nitrogen for RNA extraction.
Wet-to-dry Lung Weight Ratio
The left lower lobes (LLL) were weighed and recorded as the wet weight. LLLs were then placed in a 95°C oven to dry for two days. Dried tissues were weighed, and the wet-to-dry ratios (W/D) were then calculated.
Albumin Level
The concentration of albumin in the bronchoalveolar lavage fluid (BALF) was determined by an immune turbidimetric assay. Agglutination with albumin was created by antigen/antibody complex reactions which were measured turbidimetrically at the Clinical Chemistry Laboratory of the American University of Beirut Medical Center using a Hitachi 912 Auto-analyzer (Roche Diagnostics, Basel, Switzerland).
Transcriptional Expression Profile of Inflammatory Mediators IL-1β, IL-6, and TNF-α
The quantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) method was used to assess inflammatory mediators’ transcriptional levels. RNA was extracted from the lungs using the TRIzol method (Invitrogen, Carlsbad, CA, USA). Briefly, 1 ml of TRIzol reagent, was used per 50–100 mg of a tissue sample, followed by chloroform extraction. RNA was then precipitated and quantified using a spectrophotometer by reading the 260/280 nm absorbance ratio. 1 µg of total RNA was reverse-transcribed into the first-strand cDNA. Quantitative PCR (qPCR) was performed using the iCycler (Bio-Rad Laboratories, Hercules, CA, USA) with SYBR Green (Qiagen, USA). Specific primers (Tib-Molbiol, Berlin, Germany) were used to assess the expression of each of the following inflammatory mediators in lung tissues from different mice groups;
IL-1β F: CACCTCTCAAGCAGAGCACAG, IL-1β R: GGGTTCCATGGTGAAGTCAAC
IL-6 F: TCCTACCCCAACTTCCAATGCTC, IL-6 R: TTGGATGGTCTTGGTCCTTAGCC
TNF-α F: AATGGGCTCCCTCTCATCAGTTC, TNF-α R: TCTGCTTGGTGGTTTGCTACGAC.
PCR products and their corresponding melting temperatures were analyzed using the iQ5 Optical System Software (Bio-Rad Laboratories). The expressions of the different inflammatory mediators were normalized against the cDNA levels of the GAPDH used as the housekeeping gene (GAPDH F: GTATTGGGCGCCTGGTCACC, GAPDH R: CGCTCCTGGAAGATGGTGATGG).
Lung Histopathology
The upper lobes of the right lung were fixed in 10% buffered formalin, embedded in paraffin, sectioned (4 μm), and stained with hematoxylin-eosin (H&E) and Masson trichrome stains. Sections from the different experimental groups were blindly evaluated by light microscopy for the degree of lung injury based on the degree of inflammatory cell infiltration, alveolar edema, as well as collagen deposit.
Assessment of Apoptosis – TUNEL Assay
The terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay was used to monitor the extent of DNA fragmentation as a measure of apoptosis in paraffin-embedded sections (Sigma-Aldrich, USA). Paraffin lung sections were placed on slides. Fluorescein-conjugated dUTP incorporated in nucleotide polymers were detected and quantified using fluorescence microscopy (Zeiss LSM 410). Positive and negative controls were used to verify the specificity of the TUNEL assay. Positive controls were treated with DNase I (Sigma Chemical Co., Saint-Louis, Mo) to enzymatically induce DNA fragmentation. TUNEL-positive nuclei were distinguished from the TUNEL-negative nuclei by counterstaining with Hoechst 33258.
Both ROS and TUNEL mean fluorescence intensity analyses were normalized to the mean fluorescence intensity of the DAPI nuclear staining. Thus, mean values were compared between conditions per an equal number of cells.
Assessment of ROS Production
Dihydroethidium (DHE) (Invitrogen, Molecular Probes, USA) at the concentration of 10 µmol/l dissolved in DMSO was applied to lung sections and then incubated in a light-protected humidified chamber at 37°C for 20 min. Fluorescent images of ethidium-stained tissues were captured using a laser scanning confocal microscope (LSM 710, Zeiss, Germany). Ethidium bromide was excited at 488 nm, and fluorescence was detected at 560 nm. 50 images of each experimental group were analyzed using ImageJ software for ROS scoring.18
Statistical Analysis
Results were expressed as the mean ± standard error of the mean (SEM). Statistical comparisons were performed using the analysis of variance (ANOVA) test, followed by Tukey’s multiple comparisons test. Differences were considered statistically significant for a p-value < 0.05.
Results
W/D Ratio and Albumin Leak
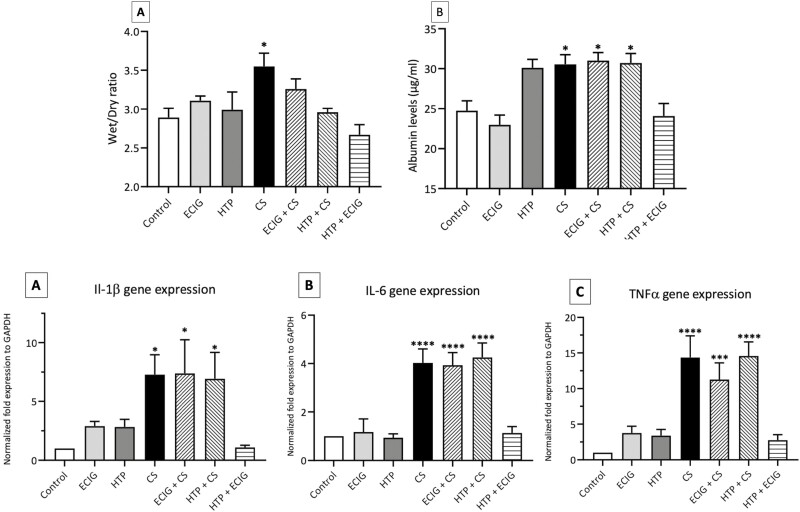
A significant (p < 0.05) increase in the W/D ratio for animals exposed to CS was detected when compared to the control. All other groups demonstrated no significant increase in W/D ratio when compared to the Control (Fig. 1A).
Figure 1.
A) Mean Wet to dry ratio (W/D); Albumin levels in the bronchoalveolar lavage fluid (BALF); and pro-inflammatory mediators’ expression of IL-1β (C), IL-6 (D), and TNF-α (E) in lung tissues of Control, ECIG, HTP, CS, ECIG + CS, HTP + CS, and HTP + ECIG. Each experimental group consisted of eight, Two-month-old male C57BL/6, mice. A: Bar graphs displayed Wet/Dry ratio analysis; B: BALF was collected, and albumin levels were measured for the different groups. C, D, and E: Histograms show normalized gene expression of IL-1β (C), IL-6 (D), and TNF-α (E) in lung tissues as detected by quantitative polymerase chain reaction (qPCR). W/D ratio was significantly increased in CS and the expression of the three different genes was significantly amplified in CS, ECIG + CS, and HTP + CS groups as compared to the control. Results are presented as means ± SEM of eight independent mice. One-way ANOVA, *p < 0.05, ***p < 0.005, ****p < 0.0001.
As for albumin leak into the BALF, only CS, ECIG + CS, and HTP + CS groups demonstrated a significant increase in albumin leak (p < 0.05) when compared to the Control (Fig. 1B).
Transcriptional Expression Profile of Inflammatory Mediators
A significant increase in the expression of IL-1β was shown in CS, ECIG + CS, and HTP + ECIG groups (p < 0.05) when compared to Control where IL-1β expression increased about six folds in these groups (Fig. 1C).
IL-6
CS, ECIG + CS, and HTP + CS exposure triggered a significant increase in IL-6 expression levels in CS, ECIG + CS, and HTP + CS exposed mice, as compared to Control (approximately a 4-fold increase; p < 0.0001). However, ECIG, HTP, and HTP + ECIG groups didn’t demonstrate any increase in IL-6 expression when compared to the Control group (Fig. 1D).
TNF-α
ECIG + CS exposure resulted in a significant increase in TNF-α expression as compared to Control (10-fold increase; p < 0.005). Similarly, CS and HTP + CS demonstrated a significant increase in TNF- α expression with an approximately 15-fold increase as compared to Control (p < 0.0001). No increase in the expression of TNF-α was observed in ECIG, HTP, and HTP + ECIG groups (Fig. 1E).
These findings indicate that ECIG + CS and HTP + CS dual exposed mice are associated with an increase in pro-inflammatory mediators’ expression when compared to non-CS exposed mice.
Lung Histopathology
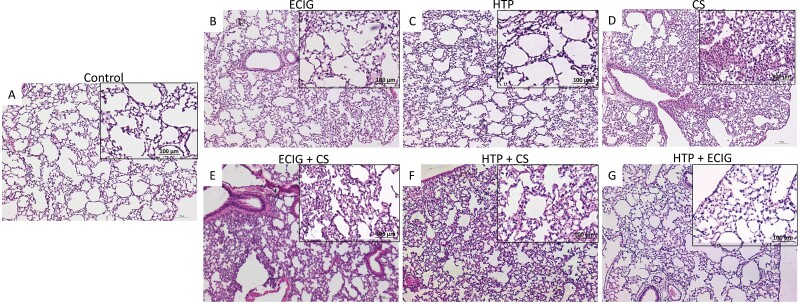
H&E sections from CS, ECIG + CS, and HTP + CS dual users groups showed significant lung injury characteristics as compared to controls. Increased infiltration of inflammatory cells around the bronchioles, a decrease in the alveolar spaces, and thickened alveolar walls are noted in these three groups (Fig. 2D, E, F). These findings are abnormal when compared to Control but are less conspicuous when compared to CS-exposed mice (Fig. 2D). ECIG, HTP, and HTP + ECIG groups demonstrated limited lung injury. CS-associated lung injury was the worst among all experimental groups (Table 1).
Figure 2.
H&E examination under light microscopy of lung tissues from (A) Control, (B) ECIG, (C) HTP, (D) CS, (E) ECIG + CS, (F) HTP + CS, and (G) HTP + ECIG. Original magnification: ×40. Lung issues of two-month-old male C57BL/6 mice were examined. A: Normal mouse lung showing thin interstitial alveolar wall and fine capillary vessels. B & C: After one week of days of exposure to (ECIG) or HTP, rare inflammatory cells, minimal injury, damage to the lung parenchyma, and preservation of pulmonary alveoli are observed. D: CS exposure is associated with a significant influx of inflammatory cells with thickened alveolar walls and destruction of alveolar spaces. E & F: ECIG +CS and HTP + CS demonstrated milder forms of lung injury when compared to CS exposure. HTP + ECIG No evidence of lung injury was observed. Insets represent magnified images; Scale bar 100 μm.
Table 1.
Qualitative Assessments in Histopathological Examination of Lung Tissues From ECIG, HTP, CS, ECIG + CS, HTP + CS, and HTP + ECIG, as Compared to Control Lung Tissues
| CS > ECIG + CS, and HTP + CS | ECIG, HTP, and HTP + ECIG | |
|---|---|---|
| H&E staining | 1. Increased infiltration of inflammatory cells around the bronchioles 2. Decreased alveolar spaces 3. Thickened alveolar walls |
Limited lung injury |
| Masson trichrome staining (MS) (Collagen deposits) | Abundant MS around bronchioles | Limited MS around bronchioles |
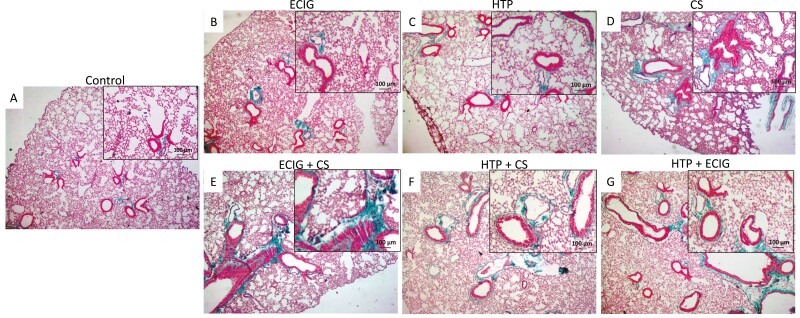
As for Masson trichrome staining, collagen deposits (stained in blue) in dual users’ groups (ECIG + CS and HTP + CS) were, more importantly, visible around bronchioles as compared to other groups and to Control (Fig. 3E, F). This observation is secondary to the recruitment of fibroblasts by immune cells at the site of injury. Settled fibroblasts produce more extracellular matrix proteins, notably collagen, and participate in the remodeling of the lung resulting in a fibrotic phenotype.
Figure 3.
Masson staining of lung tissues from (A) Control, (B) ECIG, (C) HTP, (D) CS, (E) ECIG + CS, (F) HTP + CS, and (G) HTP + ECIG. Collagen deposits are stained in light blue. Original magnification: ×20. Significant collagen deposits were more abundant around bronchioles in CS, ECIG + CS, and HTP + CS when compared to ECIG, HTP, and HTP + ECIG and control. Insets represent magnified images; Scale bar 100 μm.
Assessment of Apoptosis and Oxidative Stress
The data obtained for the oxidative stress analysis demonstrated a significant increase in cellular death as reflected by an increase in the number of TUNEL-positive apoptotic nuclei, in all groups exposed to CS (CS, ECIG + CS, and HTP + CS groups). In addition, the intensity of oxidative stress was statically more intense in CS when compared to ECIG + CS, and HTP + CS.
As for apoptosis secondary to exposure, Groups exposed to ECIG, HTP, or HTP + ECIG demonstrated a percentage of apoptotic cells similar to the Control group (Fig. 4, Fig. 1S). A significant increase in ROS production was noted in CS, as well as in ECIG + CS and HTP + CS as compared to Control. (Figs. 2S and 3S). The intensity of apoptosis, however, was more intense in CS when compared to ECIG + CS, and HTP + CS.
Discussion
In this study, we have shown that mice groups, dually exposed to 50% CS and 50% HTP or ECIG, developed similar characteristics and features of lung injury after one week of exposure similar to those exclusively exposed to 100% CS. These characteristics included an increase in the expression of pro-inflammatory mediators (IL-1β, IL-6, and TNF-α), a significant elevation in the albumin leakage into the BALF, an aggravation in ROS production, and an augmented percentage of cellular death. Furthermore, histopathological examination of these groups revealed infiltration of inflammatory cells, decrease of air space, and thickened alveolar wall, or alveolar edema. Masson trichrome staining demonstrated collagen fibers around bronchioles and among smooth muscles after exposure to CS, ECIG + CS, and HTP + CS when compared to the Control. This observation may indicate the initiation of lung remodeling and future fibrosis19
We also demonstrated that swapping up to 50% of CS exposure with ECIG or HTP, overall, did not result in significant attenuation of lung injury secondary to CS. The apoptosis, oxidative stress data of ECIG + CS and HTP + CS may suggest a reduction in lung injury when compared to CS only exposure. All other elements, however, were not reduced. In a clinical study, similar adverse effects were described by Wang et.al who noted that ECIG + CS exposure was associated with higher cardiopulmonary risks when compared to CS only. In addition, the study noted that dual users smoked more conventional cigarettes and scored lower health rates when compared to exclusive conventional cigarette smokers.20 This places dual users at a higher risk compared to CS and the main associating factor appears to be nicotine levels, which were higher in dual users when compared to CS.21,22 These observations clearly indicate that dual users, in real life, are not equally substituting CS with alternatives on a one-to-one ratio, and hence the current model is a conservative representation of current dual users’ practices. It may be the case that conventional cigarette smokers who are also ECIG or HTP consumers end up with higher nicotine dependency due to the greater availability of nicotine by the combination of CS, ECIG, or HTP consumption, and simply augment rather than substitute.9 Even with our conservative estimate and with a reduction of 50% of CS, our study did not demonstrate a significant attenuation of lung injury in our animal model.
In previous work, exposure to only ECIG attenuated the cardiovascular risk as compared to only CS.23 As for HTP, the manufacturer published various research papers showing a less harmful effect of HTP compared to CS exposure that was accredited to the elimination of combustion products.24–26 At the cellular level, HTP aerosols displayed less cytotoxicity than CS but higher than ECIG.27 The findings of limited lung injury by ECIG- or HTP-only exposure have led to recommendations without adequate substantiation from organizations, groups, and a few medical societies urging traditional cigarette smokers to quit smoking or adopt a “dual user” approach.28 Intriguingly, in the United Kingdom, authorities have banned traditional smoking in indoor public areas and permitted people to switch and smoke electronic cigarettes in these designated areas29 In this context, the findings of our study signal against dual use and the associated benefit or risk reduction. The notion that dual use is a reduced-risk alternative to quitting combustible tobacco smoking is not supported by the findings of this study.
Most studies on dual-use concentrated on the behavioral and psychosocial aspects of dual smoking.30,31 Pokhrel et.al reported that dual users are influenced by many social and psychological characteristics, including eating, stress, as well as permission of smoking outdoors, and dual smoking at younger ages was associated with different sociable factors when compared to ECIG users only or non-smokers.32 In this study, we examined the physiologic effects of combined product use in an animal model that may be utilized in future studies aimed at understanding the pathophysiology of different combinations of tobacco products. It is estimated that 63% of ECIG users in Canada and up to 70% of ECIG users in the United States are also CS users. The high percentage of dual smokers,10,28 necessitates the creation of consistent “dual users” animal models for future studies that incorporate the multifaceted physiological and psychological aspects of the use of these products.
Our findings of lung injury, secondary to HTP exposure, are different from those observed by Bhat et al who did illicit lung injury after exposing animals to a five hour per day of HTP exposure in a closed chamber for two weeks.33 We believe that the design of Bhat et al included confounding parameters that created a more intense exposure to animals and is more likely to result in lung injury when compared to our study.
Finally, our study demonstrated no significant lung injury by combining exposure to ECIG and HTP in an animal model. There was no significant increase in inflammatory markers expression, no infiltration of inflammatory cells, and no apoptosis or ROS production. While additional studies are needed to document and analyze this interesting observation, one possible explanation may be the limited time exposure of one week as per the design of the study. Another explanation is the different mechanisms of lung injury that are associated with ECIG when compared to pathways of lung injury secondary to HTP exposure. It appears, from this study, that an equal mix of HTP + ECIG exposure for one week did not reach the threshold needed to illicit significant lung injury even though our study design ensured that ECIG and HTP exposure was more intense when compared to CS exposure.
A limitation of this study includes the acute duration of animal exposure. It is possible that chronic studies may reveal different results as animals may adapt and adjust to the chronicity of exposure resulting in different observations. Another limitation of this study is the assigned amount of exposure to ECIG, HTP, and CS. Different combinations and different percentages of exposure to each element may lead to different conclusions. Also, the study did not rely on nicotine blood levels to determine the intensity of ECIG exposure. Finally, this study used a single ECIG product operating at a fixed power and with a fixed liquid composition; varying combinations of device, power, and composition have been shown to result in widely varying toxicant emissions across and within products3
Conclusion
This study examined the effects on the lung of partially substituting CS with ECIG or HTP aerosol that mimics dual users in humans. We found that substituting fifty percent of CS exposure with ECIG or HTP did not reduce lung injury. These results suggest a potentially grave misunderstanding by cigarette users that partially substituting cigarette use with ECIG or HTP use can reduce health risks.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Acknowledgments
The authors acknowledge the assistance in performing the animal studies of Hashem Nassereddine, Farah Allouch, Mazin Sabeh, Omar Sleiman, Elias Bechara, and Andrea Haddad.
Contributor Information
Ahmad Husari, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine; American University of Beirut, Beirut, Lebanon.
Mohammad El-Harakeh, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine; American University of Beirut, Beirut, Lebanon.
Alan Shihadeh, Department of Mechanical Engineering, American University of Beirut, Beirut, Lebanon; Center for the Study of Tobacco Products, Virginia Commonwealth University, Richmond, VA, USA.
Michella Abi Zeid Daou, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine; American University of Beirut, Beirut, Lebanon.
Hala Bitar, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine; American University of Beirut, Beirut, Lebanon.
Nareg Karaoghlanian, Department of Mechanical Engineering, American University of Beirut, Beirut, Lebanon; Center for the Study of Tobacco Products, Department of Psychology, Virginia Commonwealth University, Richmond, VA, USA.
Ghazi Zaatari, Department of Pathology & Laboratory Medicine, American University of Beirut, Beirut, Lebanon.
Marwan El-Sabban, Department of Anatomy, Cell Biology, and Physiological Sciences, Faculty of Medicine, American University of Beirut, Beirut, Lebanon.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.
Funding
This work was supported by Hamdi el Zaim Interstitial lung diseases center at the American University of Beirut. The National Institute on Drug Abuse of the National Institutes of Health (grant number P50DA036105) and the Center for Tobacco Products of the U.S. Food and Drug Administration. The content is solely the responsibility of the authors and does not necessarily represent the official views of the American University of Beirut, the National Institutes of Health, or the Food and Drug Administration. The funders had no role in the study design, data collection, analysis, decision to publish, or preparation of the manuscript.
Declaration of Interests
None declared.
References
- 1. Davis B, Williams M, Talbot P.. iQOS: evidence of pyrolysis and release of a toxicant from plastic. Tob Control. 2019;28(1):34–41. [DOI] [PubMed] [Google Scholar]
- 2. Onor IO, Stirling DL, Williams SR, et al. Clinical effects of cigarette smoking: epidemiologic impact and review of pharmacotherapy options. Int J Environ Res Public Health. 2017;14(10):1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res. 2015;17(2):150–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Werner AK, Koumans EH, Chatham-Stephens K, et al. ; Lung Injury Response Mortality Working Group. Hospitalizations and deaths associated with EVALI. N Engl J Med. 2020;382(17):1589–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Daou MAZ, Shihadeh A, Hashem Y, et al. Role of diabetes in lung injury from acute exposure to electronic cigarette, heated tobacco product, and combustible cigarette aerosols in an animal model. PLoS One. 2021;16(8):e0255876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Philip Morris International. Our Smoke-Free Products . Available from: https://www.pmi.com/smoke-free-products.
- 7. Live Science. Are ‘Heat-Not-Burn’ Tobacco Products Safer Than Cigarettes? 2018. Available from: https://www.livescience.com/61538-heat-not-burn-tobacco-iqos.html.
- 8. Pisinger C, Dagli E, Filippidis FT, et al. ; ERS Tobacco Control Committee, on behalf of the ERS. ERS and tobacco harm reduction. Eur Respir J. 2019;54(6):1902009. [DOI] [PubMed] [Google Scholar]
- 9. Gravely S, Fong GT, Sutanto E, et al. Perceptions of harmfulness of heated tobacco products compared to combustible cigarettes among adult smokers in Japan: findings from the 2018 ITC Japan Survey. Int J Environ Res Public Health. 2020;17(7):2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Coleman BN, Rostron B, Johnson SE, et al. Electronic cigarette use among US adults in the population assessment of tobacco and health (PATH) Study, 2013-2014. Tob Control. 2017;26(e2):e117–e126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jackson SE, Shahab L, West R, Brown J.. Associations between dual use of e-cigarettes and smoking cessation: A prospective study of smokers in England. Addict Behav. 2020;103(Apr):106230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Piper ME, Baker TB, Benowitz NL, Kobinsky KH, Jorenby DE.. Dual users compared to smokers: demographics, dependence, and biomarkers. Nicotine Tob Res. 2019;21(9):1279–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smith DM, Christensen C, van Bemmel D, et al. Exposure to nicotine and toxicants among dual users of tobacco cigarettes and E-Cigarettes: population assessment of tobacco and health (PATH) Study, 2013-2014. Nicotine Tob Res. 2021;23(5):790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shihadeh A, Eissenberg T.. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine Tob Res. 2015;17(2):158–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Husari A, Shihadeh A, Talih S, Yasmine H, Marwan ES, Ghazi Z.. Acute exposure to electronic and combustible cigarette aerosols: effects in an animal model and in human alveolar cells. Nicotine Tob Res. 2016;18(5):613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Salman R, Talih S, El-Hage R, et al. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob Res. 2019;21(9):1285–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goel R, Trushin N, Reilly SM, et al. A survey of nicotine yields in small cigar smoke: influence of cigar design and smoking regimens. Nicotine Tob Res. 2018;20(10):1250–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rueden CT, Schindelin J, Hiner MC, et al. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinf. 2017;18(1):529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Avila G, Bazan-Perkins B, Sandoval C, et al. Interstitial collagen turnover during airway remodeling in acute and chronic experimental asthma. Exp Ther Med. 2016;12(3):1419–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang JB, Olgin JE, Nah G, et al. Cigarette and e-cigarette dual use and risk of cardiopulmonary symptoms in the Health eHeart Study. PLoS One. 2018;13(7):e0198681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Flower M, Nandakumar L, Singh M, David W, Morgan W, David F.. Respiratory bronchiolitis-associated interstitial lung disease secondary to electronic nicotine delivery system use confirmed with open lung biopsy. Respirol Case Rep. 2017;5(3):e00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Heydari G, Ahmady AE, Chamyani F, Masjedi M, Fadaizadeh L.. Electronic cigarette, effective or harmful for quitting smoking and respiratory health: A quantitative review papers. Lung India. 2017;34(1):25–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Benowitz NL, Fraiman JB.. Cardiovascular effects of electronic cigarettes. Nat Rev Cardiol. 2017;14(8):447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Malinska D, Szymanski J, Patalas-Krawczyk P, et al. Assessment of mitochondrial function following short- and long-term exposure of human bronchial epithelial cells to total particulate matter from a candidate modified-risk tobacco product and reference cigarettes. Food Chem Toxicol. 2018;115:1–12. [DOI] [PubMed] [Google Scholar]
- 25. Schaller JP, Keller D, Poget L, et al. Evaluation of the Tobacco Heating System 2.2. Part 2: Chemical composition, genotoxicity, cytotoxicity, and physical properties of the aerosol. Regul Toxicol Pharmacol. 2016;81(Suppl 2):S27–S47. [DOI] [PubMed] [Google Scholar]
- 26. Smith MR, Clark B, Ludicke F, et al. Evaluation of the Tobacco Heating System 2.2. Part 1: Description of the system and the scientific assessment program. Regul Toxicol Pharmacol. 2016;81(Suppl 2):S17–S26. [DOI] [PubMed] [Google Scholar]
- 27. Dusautoir R, Zarcone G, Verriele M, et al. Comparison of the chemical composition of aerosols from heated tobacco products, electronic cigarettes and tobacco cigarettes and their toxic impacts on the human bronchial epithelial BEAS-2B cells. J Hazard Mater. 2021;401:123417. [DOI] [PubMed] [Google Scholar]
- 28. Lee AS, Hart JL, Walker KL, Keith RJ, Ridner SL.. Dual users and electronic cigarette only users: consumption and characteristics. Int J Health Med Sci. 2018;4(6):111–116. [PMC free article] [PubMed] [Google Scholar]
- 29. Cooper M, Case KR, Loukas A, Creamer MR, Perry CL.. E-cigarette dual users, exclusive users and perceptions of tobacco products. Am J Health Behav. 2016;40(1):108–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Staff J, Maggs JL, Seto C, Dillavou J, Vuolo M.. Electronic and combustible cigarette use in adolescence: links with adjustment, delinquency, and other substance use. J Adolesc Health. 2020 Jan;66(1):39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Reid JL HD, Tariq U, Burkhalter R, Rynard VL, Douglas O.. Tobacco Use in Canada: Patterns and Trends: Waterloo, ON: Propel Center for Population Health Impact, University of Waterloo; 2019. Edition.
- 32. Pokhrel P, Herzog TA, Muranaka N, Regmi S, Fagan P.. Contexts of cigarette and e-cigarette use among dual users: a qualitative study. BMC Public Health. 2015;4(15):859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhat TA, Kalathil SG, Leigh N, et al. Acute Effects of Heated Tobacco Product (IQOS) aerosol inhalation on lung tissue damage and inflammatory changes in the lungs. Nicotine Tob Res. 2021;23(7):1160–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.