ABSTRACT
Background:
The studies which investigated the relationship between anti-Mullerian hormone (AMH) level and abortion rate have conflicting results.
Aims:
This retrospective study aimed to evaluate the relationship between AMH levels and abortion in women who achieved pregnancy with in vitro fertilisation (IVF) treatment.
Settings and Design:
This retrospective study was conducted in the Department of Gynecology and Obstetrics, Etlik Zubeyde Hanim Women's Health Training and Research Hospital, between January 2014 and January 2020.
Materials and Methods:
Patients below 40 years of age who conceived after IVF-embryo transfer treatment during a 6-year period and had a serum AMH level measurement were included. The patients were divided into three groups according to the serum AMH levels as low AMH (L-AMH, ≤1.6 ng/mL), intermediate AMH (I-AMH, 1.61–5.6 ng/mL) and high AMH (H-AMH, >5.6 ng/mL). The groups were compared in terms of obstetric, treatment cycle characteristics and abortion rates.
Statistical Analysis Used:
The Mann–Whitney U-test was used in comparison of non-parametric data of two groups; the Kruskal–Wallis test was used to compare the data of more than two groups. When a statistically significant difference was found in the Kruskal–Wallis test result, the groups were compared in pairs using the Mann–Whitney U-test, and the groups that made a statistical difference were determined. The Pearson's Chi-square and Fisher's exact tests were used to compare the independent categorical variables.
Results:
L-AMH (n = 164), I-AMH (n = 153) and H-AMH (n = 59) groups were similar in terms of obstetric histories and number of cycles applied, with an abortion rate of 23.8%, 19.6% and 16.9%, respectively (P = 0.466). The same analyses were repeated in two subgroups under 34 years of age and above, and no difference was found in terms of miscarriage rates. The number of oocytes retrieved and the number of mature oocytes were higher in H-AMH group compared to intermediate and low groups.
Conclusion:
No relationship was found between serum AMH level and abortion rate in women who achieved clinical pregnancy with IVF treatment.
KEYWORDS: Abortion, anti-Mullerian hormone, in vitro fertilisation, miscarriage, pregnancy
INTRODUCTION
Assisted reproductive technology (ART) is used in infertile patients when other treatment modalities fail; however, in some cases such as bilateral tubal occlusion or severe male factor, in vitro fertilisation and embryo transfer (IVF-ET) is the first choice. Although a significant progress has been made in ART over the years, the success rates have not still reached to the expected level.[1] Abortion is the most common complication of the first-trimester pregnancy, and 26% of all pregnancies and 10% of clinically proven pregnancies result in abortion, whereas this rate is approximately 15%–25% in IVF-ET pregnancies.[2]
Anti-Mullerian hormone (AMH) plays a crucial role in folliculogenesis.[3] AMH, used as one of the biomarkers of ovarian reserve, progressively declines over the years and becomes undetectable near menopause.[3] Serum AMH level is measured for evaluation of the ovarian reserve besides other ovarian reserve tests.[4,5] Many studies reported serum AMH level as superior to other commonly measured markers in predicting ovarian stimulation response in ART cycles.[4] Previous studies have shown that women with normal AMH levels respond better to ovarian stimulation and have lower cycle cancellation rate, higher retrieved oocyte count and higher pregnancy rate per cycle in IVF treatment.[6,7] However, the results from a meta-analysis are controversial as a weak association was reported between AMH level and clinical pregnancy rates.[8] In a recent study, Sun et al. reported that AMH to be a predictor of clinical pregnancy besides age and the number of retrieved oocytes.[9] The impact of AMH levels on oocyte and, subsequently, embryo quality have been investigated.[10] The number of studies evaluating the relationship between the pregnancy outcome and early pregnancy loss in women who achieved pregnancy with IVF treatment and serum AMH level is limited. The results of the two studies evaluating the relationship between AMH level and abortion rates are conflicting.[11,12] One study showed that the abortion risk increased, especially in pregnant women aged 34 and above in patients with low AMH (L-AMH) (<1.6 ng/mL) level.[11] On the other hand, in Peuranpaa's study, investigating the relationship between L-AMH levels and IVF results, no relationship was found between L-AMH and abortion rates.[12]
We aimed to evaluate the relationship between serum AMH levels and abortion rate in women who conceived by IVF-ET treatment.
MATERIALS AND METHODS
The study participants were women who achieved pregnancy with IVF-fresh ET treatment between January 2014 and January 2020 at our hospital. The Ethics Committee approval was obtained from the Ethics Comittee of …… Hospital (Approval number: 2020/76, Date: 17 June 2020).
Of the 748 evaluated patients, 376 women who met the study criteria were included in the study [Figure 1]. Inclusion criteria were: being aged between 18 and 40 years, having clinical pregnancy detected after the IVF-ET cycle, having no history of polycystic ovary syndrome (PCOS), reccurent pregnancy loss, genetic disorder, or giving birth to an offspring with congenital anomaly and having serum AMH level measured within the past 12 months before the IVF treatment at our institution. Women aged <18 and >40 years of age, who had a family history of a genetic disorder or a congenital malformation, with detected uterine anomaly or malformation, PCOS and had a history of recurrent pregnancy loss were excluded [Figure 1]. As a part of the policy of the institution, all the patients who received treatment at our institution are required to give consent to the utilization of their medical data anonymously.
Figure 1.
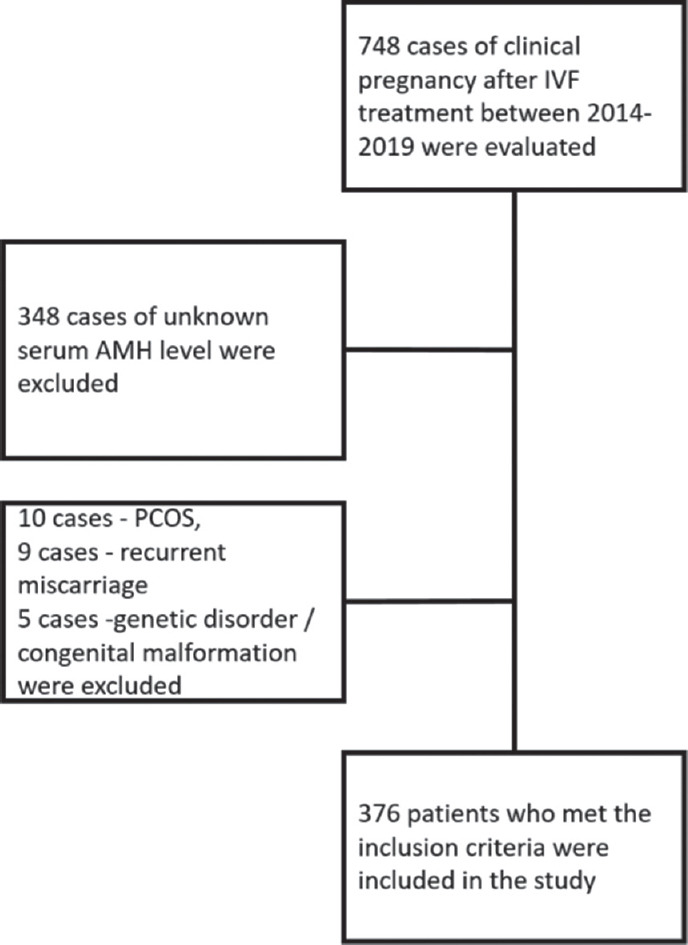
Flow chart of the study. IVF = In vitro fertilisation, AMH = Anti-Mullerian hormone, PCOS = Polycystic ovary syndrome
Patients’ age, aetiology of infertility, body mass index (BMI), serum AMH levels, number of oocytes retrieved, number of mature oocytes, number of fertilised oocytes, number of embryos transferred and abortion rates were obtained from the patient's files and hospital's electronical records. The presence of an embryo with a positive heartbeat on transvaginal ultrasonography performed 6 weeks after ET was defined as clinical pregnancy. The gestational age was calculated according to the last menstrual date, and it was confirmed by the measurement of crown-rump length with transvaginal ultrasonography. Abortion was defined as ’the termination of a pregnancy within 20 weeks of pregnancy after accompanied by, resulting in or closely followed by the death of the embryo or foetus’ as defined by Merriam-Webster's Medical Dictionary.[13]
Based on a study by Tarasconi et al.,[11] the patients were classified into three groups according to the serum AMH levels as (L-AMH; ≤1.6 ng/mL), intermediate AMH (I-AMH, 1.61–5.6 ng/mL) and high AMH (H-AMH >5.6 ng/mL). The groups were compared in terms of demographic, clinical, obstetric, cycle characteristics and abortion. In addition, the same analysis was performed in two subgroups, defined by the age of the women (a) under 34 years and (b) 34 and above.
The study of Tarasconi et al.[11] was taken as a reference, and the G*Power software determined that the sample number should consist of a minimum of 75 abortion cases.
Statistical analysis
The independent samples t-test was used to compare the data of two groups showing normal distribution between independent groups; ANOVA test was used to compare the data of more than two groups. When a statistically significant difference was detected as a result of the ANOVA test, Post hoc Tukey analysis determined which groups made this difference. The Mann–Whitney U-test was used in comparison of non-parametric data of two groups; the Kruskal–Wallis test was used to compare the data of more than two groups. When a statistically significant difference was found in the Kruskal–Wallis test result, the groups were compared in pairs using the Mann–Whitney U-test, and the groups that made a statistical difference were determined. The Pearson's Chi-square and Fisher's exact tests were used to compare the independent categorical variables. Receiver operator characteristic (ROC) analysis was performed to determine the optimum cut-off value of serum AMH level that could predict abortion. Statistical analysis of the study was done in two ways, and P < 0.05 was considered statistically significant.
RESULTS
The mean age of 376 patients included in the study was 31 ± 4.3 years (median: 30 years, range: 19–39 years). Unexplained infertility was the most common cause of infertility among the patients (36.5%), followed by male factor (31.6%) and diminished ovarian reserve (20.5%) [Table 1]. Abortion occured in 21% of women who had a clinical pregnancy after IVF-ET cycle. The patients were evaluated in three groups according to their serum AMH levels, and a comparison of age, BMI, number of stimulation cycle and IVF-ET data of the three groups is shown in Table 1. The L-AMH group's median age was higher than the I-AMH and H-AMH groups (P < 0.001 and P = 0.002, respectively). The most common cause of infertility in the L-AMH group was diminished ovarian reserve (40.9%), whereas it was male factor (44.4%) in the I-AMH group and unexplained infertility in the H-AMH group (69.5%). There was no significant difference between the groups in terms of BMI and the number of stimulation cycles (P = 0.768 and P = 0.391). The number of retrieved, mature and fertilised oocytes in the L-AMH group was significantly lower than in the I-AMH and H-AMH groups (P = 0.001). In addition, the number of retrieved oocytes, mature oocytes and fertilised oocytes in the H-AMH group was higher than the I-AMH group (P = 0.001, P = 0.002 and P = 0.003).
Table 1.
Comparison of the groups determined according to serum anti-Mullerian hormone levels in terms of age, in vitro fertilisation indications, body mass index, number of cycles and oocyte counts
| All patients | Groups according to serum AMH levels | P | |||
|---|---|---|---|---|---|
|
| |||||
| L-AMH ≤1.6 ng/mL (n=164) | I-AMH 1.61-5.6 ng/mL (n=153) | H-AMH >5.6 ng/mL (n=59) | |||
| Age (year), median (IQR) | 30 (19–39) | 32 (28–36) | 29 (26–33) | 30 (27–32) | <0.001” |
| Indications for IVF-ET, n (%) | |||||
| Male factor | 119 (31.6) | 42 (25.6) | 68 (44.4) | 9 (15.2) | NA |
| Tubal factor | 28 (7.4) | 9 (5.5) | 16 (10.5) | 3 (5.1) | |
| Diminished ovarian reserve | 77 (20.5) | 67 (40.9) | 7 (4.6) | 3 (5.1) | |
| Endometriosis | 15 (4.0) | 8 (4.9) | 4 (2.6) | 3 (5.1) | |
| Unexplained infertility | 137 (36.5) | 38 (23.1) | 58 (37.9) | 41 (69.5) | |
| BMI (kg/m2), mean±SD | 26.8±5.3 | 26.7±5.7 | 26.7±4.9 | 27.2±5.1 | 0.768 |
| Treatment cycle, n (%) | |||||
| 1st | 207 (55.1) | 89 (54.3) | 87 (56.8) | 31 (52.5) | 0.391 |
| 2nd | 99 (26.3) | 42 (25.6) | 44 (28.8) | 13 (22.0) | |
| ≥3rd | 70 (18.6) | 33 (20.1) | 22 (14.4) | 15 (25.5) | |
| Stimulation protocol, n (%) | |||||
| Long agonist | 124 (33.0) | 60 (36.6) | 52 (34.0) | 12 (20.3) | 0.071 |
| Antagonist | 252 (67.0) | 104 (63.4) | 101 (66.0) | 47 (79.7) | |
| Number of oocytes retrieved (IQR) | 10 (6–14) | 7 (5–9) | 11 (8–15) | 15 (12–21) | <0.001” |
| Number of mature oocytes (IQR) | 7 (5–11) | 6 (4–8) | 9 (5–12) | 12 (9–15) | <0.001” |
| Number of fertilised oocytes (IQR) | 4 (3–7) | 3 (2–5) | 5 (3–7) | 6 (5–10) | <0.001” |
| Oocyte quality index (IQR) | 5.2 (2.0–6.1) | 5.3 (4.9–5.7) | 5.1 (4.5–5.6) | 5.1 (4.7–5.5) | 0.107 |
| Number of embryo transfer cycles, mean±SD | 1.3±0.5 | 1.3±0.5 | 1.2±0.4 | 1.3±0.5 | 0.126 |
AMH=Anti-Mullerian hormone, IVF=In vitro fertilisation, BMI=Body mass index, IQR=Interquartile range, SD=Standard deviation, NA=Not applicable, ET=Embryo transfer, L-AMH=Low AMH, I-AMH=Intermediate AMH, H-AMH=High-AMH
The data are compared in patients aged <34 and ≥34 years as shown in Table 2. The abortion rates in the low, I-AMH and H-AMH groups were 23.8%, 19.6% and 16.9% for all patients, 24.7%, 18.2% and 18.0% for patients <34 years of age and it was 22.4%, 25.0% and 11.1% for patients ≥34 years of age (P = 0.466, P = 0.436 and P = 0.675, respectively) [Figure 2]. These numerically different abortion rates of the subgroups were not statistically significant.
Table 2.
Comparison of the age, in vitro fertilisation indications, body mass index, number of cycles and oocyte counts of the patients under and above 34 years of age
| <34 years of age | ≥34 years of age | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Low ≤1.6 (n=164) | Intermediate 1.61–5.6 (n=153) | High >5.6 (n=59) | P | Low ≤1.6 (n=164) | Intermediate 1.61–5.6 (n=153) | High >5.6 (n=59) | P | |
| Age (year), median (IQR) | 29 (27–31) | 28 (26–30) | 29 (27–30) | 0.110 | 36 (35–37) | 36 (34–37) | 35 (34–37) | 0.300 |
| IVF indications, n (%) | ||||||||
| Male factor | 27 (27.8) | 60 (49.6) | 7 (14.0) | NA | 15 (22.4) | 8 (25.0) | 2 (22.2) | NA |
| Tubal factor | 6 (6.2) | 10 (8.3) | 2 (4.0) | 3 (4.5) | 6 (18.8) | 1 (11.1) | ||
| Diminished ovarian reserve | 43 (44.4) | 5 (4.1) | 1 (2.0) | 24 (35.7) | 2 (6.3) | 2 (22.2) | ||
| Endometriosis | 5 (5.2) | 3 (2.5) | 3 (6.0) | 3 (4.5) | 1 (3.1) | 0 (0) | ||
| Unexplained | 16 (16.4) | 43 (45.5) | 37 (74.0) | 22 (32.9) | 15 (46.8) | 4 (44.4) | ||
| BMI (kg/m2), mean±SD | 26.6±5.8 | 26.5±4.5 | 27.0±5.0 | 0.829 | 26.9±5.5 | 27.4±6.0 | 28.7±5.6 | 0.674 |
| Cycles, n (%) | ||||||||
| 1st | 52 (53.6) | 67 (55.4) | 25 (50.0) | 0.279 | 37 (55.2) | 20 (62.5) | 6 (66.7) | 0.882 |
| 2nd | 27 (27.8) | 39 (32.2) | 12 (24.0) | 15 (22.4) | 5 (15.6) | 1 (11.1) | ||
| ≥3rd | 18 (18.6) | 15 (12.4) | 13 (26.0) | 15 (22.4) | 7 (21.9) | 2 (22.2) | ||
| Stimulation protocol, n (%) | ||||||||
| Long agonist | 37 (38.1) | 38 (31.4) | 9 (18.0) | 0.045 | 23 (34.3) | 14 (43.8) | 3 (33.3) | 0.643 |
| Antagonist | 60 (61.9) | 83 (68.6) | 41 (82.0) | 44 (65.7) | 18 (56.3) | 6 (66.7) | ||
| Number of oocytes retrieved (IQR) | 7 (5–10) | 12 (8–16) | 15 (12–23) | <0.001 | 6 (5–8) | 10 (7–12) | 14 (12–20) | <0.001” |
| Number of mature oocytes (IQR) | 6 (4–8) | 9 (5–13) | 12 (9–15) | <0.001 | 5 (4–7) | 8 (5–11) | 9 (8–12) | <0.001” |
| Number of fertilised oocytes (IQR) | 3 (2–5) | 5 (3–7) | 6 (5–10) | <0.001 | 3 (2–5) | 4 (3–6) | 5 (5–9) | 0.002” |
| Oocyte quality index (IQR) | 5.2 (4.8–5.7) | 5.1 (4.5–5.5) | 5.2 (4.7–5.5) | 0.320 | 5.3 (5.0–5.7) | 5.2 (4.8–5.7) | 4.9 (4.6–5.2) | 0.215 |
| Number of embryo transfer cycles, mean±SD | 1.1±0.3 | 1.1±0.3 | 1.2±0.4 | 0.167 | 1.7±0.4 | 1.7±0.4 | 1.7±0.5 | 0.986 |
AMH=Anti-Mullerian hormone, IVF=In vitro fertilisation, BMI=Body mass index, IQR=Interquartile range, SD=Standard deviation, NA=Not applicable
Figure 2.
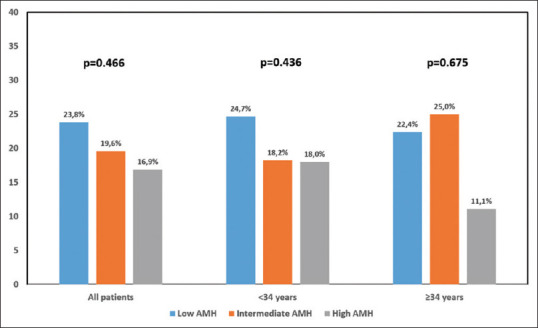
Abortion rates according to AMH groups. AMH = Anti-Mullerian hormone
The patients were evaluated in two groups according to the pregnancy outcome: Group A included the patients whose pregnancy resulted with abortion, and Group B included those resulted with live birth. Age, IVF indications, BMI and the number of stimulation cycles of the groups were compared. The number of oocytes retrieved, the number of mature and fertilised oocytes and the oocyte quality index and AMH levels were similar in both groups [P = 0.839, P = 0.581, P = 0.573 and P = 0.283 and P = 0.450, respectively, Table 3]. Whether the serum AMH level predicted abortion rate was evaluated by ROC analysis [Figure 3].
Table 3.
Comparison of the patients who experienced abortion with the patients with ongoing pregnancy after in vivo exposure therapy treatment
| State of pregnancy | P | ||
|---|---|---|---|
|
| |||
| Group A (abortion group) (n=79) | Group B (ongoing pregnancy) (n=297) | ||
| Age (year), median (IQR) | 31 (29–34) | 30 (27–34) | 0.062 |
| Age (year), n (%) | |||
| <34 | 55 (69.6) | 213 (71.7) | 0.714 |
| ≥34 | 24 (30.4) | 84 (28.3) | |
| IVF indications, n (%) | |||
| Male factor | 31 (39.2) | 88 (29.6) | NA |
| Tubal factor | 4 (5.1) | 24 (8.1) | |
| Diminished ovarian reserve | 4 (5.1) | 73 (24.6) | |
| Endometriosis | 3 (3.8) | 12 (4.0) | |
| Unexplained infertility | 37 (46.8) | 100 (33.7) | |
| BMI (kg/m2), mean±SD | 27.3±5.8 | 26.6±5.1 | 0.294 |
| Cycles, n (%) | |||
| 1st | 38 (48.1) | 169 (56.9) | 0.351 |
| 2nd | 25 (31.6) | 74 (24.9) | |
| ≥3rd | 16 (20.3) | 54 (18.2) | |
| Stimulation protocol, n (%) | |||
| Long agonist | 25 (31.6) | 99 (33.3) | 0.777 |
| Antagonist | 54 (68.4) | 198 (66.7) | |
| Number of oocytes retrieved (IQR) | 9 (7–14) | 10 (6–14) | 0.839 |
| Number of mature oocytes (IQR) | 7 (5–12) | 7 (5–11) | 0.581 |
| Number of fertilised oocytes (IQR) | 4 (3–7) | 4 (3–6) | 0.573 |
| Oocyte quality index (IQR) | 5.3 (4.8–5.7) | 5.2 (4.7–5.6) | 0.283 |
| Number of embryo transfer cycles, mean±SD | 1.3±0.5 | 1.3±0.4 | 0.266 |
| AMH level (ng/mL), mean±SD | 2.74±3.43 | 3.04±2.94 | 0.450 |
AMH=Anti-Mullerian hormone, IVF=In vitro fertilisation, BMI=Body mass index, IQR=Interquartile range, SD=Standard deviation, NA=Not applicable
Figure 3.
ROC analysis to determine the optimum AMH value that can predict abortion. AMH = Anti-Mullerian hormone, ROC = Receiver operator characteristic, AUC = Area under curve
DISCUSSION
The live birth rate in fresh and frozen-thawed ET cycles still needs improvement as even the most recent studies present live birth rate values below 50%.[14] There has been ongoing research to identfy the risk factors for pregnancy loss in pregnancies achieved after intracytoplasmic sperm injection-ET (IVF/ICSI-ET)treatment cycles to obtain higher live birth rates. In a large series presented by Yang et al., the rate of pregnancy loss was 19.7%, and age and BMI were shown as the risk factors.[15] In the presented study, 376 women achieved pregnancy with fresh IVF/ET cycles, and the abortion rate was 21%. Peuranpää et al. analysed 1323 pregnancies conceived by IVF/ICSI cycles with fresh and frozen-thawed ET and reported miscarriage in 12.7% and non-visualised pregnancy loss in 25.4%.[12] Peuranpää reported a lower cumulative live birth rate in women with L-AMH (<2 μg/L) compared to the ones with an AMH level ≥2.0 μg/L during the first treatment cycle; however, the risk of early pregnancy loss was not found to be related with AMH levels. In the presented study, we evaluated the clinical pregnancies that ended in abortion, and non-visualised pregnancy loss was not investigated. Cornille et al. investigated the miscarriage rate in women aged under 37 years with L-AMH and normal AMH levels and reported a miscarriage rate of 9.5% and 6.8%, respectively, in fresh cycles.[16] L-AMH level was defined as <10th percentile by Cornille et al., and these low levels did not alter the miscarriage rate in young women.
L-AMH levels were proposed to be related with pre-term birth, pre-eclampsia and recurrent miscarriage.[17,18,19] The relationship between serum AMH level and abortion in women getting pregnant through natural conception was also evaluated in studies, and conflicting results were obtained.[20,21,22] Lyttle Schumacher et al. reported that low serum AMH level (0.4 ng/mL) could predict abortion in their prospective cohort study, which included 533 women aged 30–44 years.[20] Atasever et al. showed that women with recurrent pregnancy loss were three times more likely to have L-AMH levels (<1 ng/mL) than the control group.[21] However, Zarek et al. did not find any relationship between serum AMH level and abortion.[22] Similarly, Cornille et al. failed to find any relationship between L-AMH levels and miscarriage rate in young women in fresh IVF-ET cycles.[16] Peuranpää et al. did not detect a higher miscarriage rate in fresh and frozen-thawed ET cycles in women with moderately low and L-AMH levels. In the other hand, they reported higher cumulative live birth rate among women with normal AMH levels than women with L-AMH, related to the higher number of oocytes and embryos obtained.[12] However, Szafarowska et al. found a negative correlation between H-AMH levels and abortion rates when AMH rate was >2.5 ng/mL.[23] In the same study, the clinical pregnancy rates in the three groups with AMH levels <1 ng/mL, 1–2.5 ng/mL and >2.5 ng/ml were 42.3%, 41.1% and 38.9%, respectively (P > 0.05). In the presented study, the relationship between serum AMH level and abortion was evaluated in women who achieved clinical pregnancy with fresh IVF-ET treatment, and the abortion rates were 23.8%, 19.6% and 16.9%, respectively. The abortion rate was slightly higher, but not statistically significant in women with L-AMH.
The predictive value of various clinical and biochemical markers in IVF/ICSI treatment cycle success has been investigated widely; however, using these makers in prediction of abortion rate is a new field of research. Increasing maternal age has been related with a lower pregnancy and higher abortion rate in IVF-ET cycles.[24] Tan et al. reported a miscarriage rate of 15.1% in women aged below 30 years, while the rates increased to 30% and 47.7% at 38 and 39 years of age, respectively.[24] AMH together with age is investigated for prediction of IVF treatment cycles,[25] and pregnancies and live births were achieved in young patients with lower AMH levels.[26] Tarasconi et al. evaluated the relationship between AMH and abortion rates in 1060 patients who achieved clinical pregnancy after IVF-ET, and showed that the risk of abortion was doubled in women aged 34 years and above who had low serum AMH levels (≤1.60 ng/mL). In the young patient group (<34 years of age), the risk of abortion was not increased in patients with L-AMH.[11] The researchers speculated that this result was due to the lower incidence of abortion in the younger patient group compared to the older patient group.[11] However, when the study details were examined, there were 467 patients in the young age patient group, and the abortion rate was 15%.
There are several limitations arising from the retrospective nature of the study. However, the abortion rate of 21% in the patient population included in the study, and its compatibility with the literature suggested that our sample size could represent the study population. The study groups based on AMH levels are not homogenous in the published studies as different researchers used different values for defining low, I-AMH and H-AMH levels. For this reason, the studies failed to report a standard cut-off value for serum AMH levels in terms of predicting abortion and live birth rate. This limitation is valid for almost all cited studies. Therefore, the cut-off values used in the study of Tarasconi et al. were taken as a reference while creating AMH groups in our study. On the other hand, the exclusion of patients with PCOS from the study provided an objective comparison of mean AMH values, which was one of the strengths of our study. Many factors affecting abortion may have affected the results. Another study limitation was that these factors could not be matched between groups.
CONCLUSION
In women who achieved pregnancy with IVF treatment, there was no relationship between serum AMH levels and abortion rates. The conflicting results from previous studies evaluating the relationship between serum AMH level and abortion suggest that comprehensive prospective randomised studies are needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Data availability
Our data are available for the journal if requested by the editor(s). Data can be shared as per requirement.
REFERENCES
- 1.Paul RC, Fitzgerald O, Lieberman D, Venetis C, Chambers GM. Cumulative live birth rates for women returning to ART treatment for a second ART-conceived child. Hum Reprod. 2020;35:1432–40. doi: 10.1093/humrep/deaa030. [DOI] [PubMed] [Google Scholar]
- 2.Dugas C, Slane VH. In: StatPearls. Treasure Island (FL): StatPearls Publishing Copyright© 2020, StatPearls Publishing LLC; 2020. Miscarriage. [Google Scholar]
- 3.Bedenk J, Vrtačnik-Bokal E, Virant-Klun I. The role of anti-müllerian hormone (AMH) in ovarian disease and infertility. J Assist Reprod Genet. 2020;37:89–100. doi: 10.1007/s10815-019-01622-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fanchin R, Schonäuer LM, Righini C, Guibourdenche J, Frydman R, Taieb J. Serum anti-müllerian hormone is more strongly related to ovarian follicular status than serum inhibin B, estradiol, FSH and LH on day 3. Hum Reprod. 2003;18:323–7. doi: 10.1093/humrep/deg042. [DOI] [PubMed] [Google Scholar]
- 5.Barad DH, Weghofer A, Gleicher N. Comparing anti-müllerian hormone (AMH) and follicle-stimulating hormone (FSH) as predictors of ovarian function. Fertil Steril. 2009;91:1553–5. doi: 10.1016/j.fertnstert.2008.09.069. [DOI] [PubMed] [Google Scholar]
- 6.Daney de Marcillac F, Pinton A, Guillaume A, Sagot P, Pirrello O, Rongieres C. What are the likely IVF/ICSI outcomes if there is a discrepancy between serum AMH and FSH levels. A multicenter retrospective study? J Gynecol Obstet Hum Reprod. 2017;46:629–35. doi: 10.1016/j.jogoh.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 7.Wang S, Zhang Y, Mensah V, Huber WJ, 3rd, Huang YT, Alvero R. Discordant anti-müllerian hormone (AMH) and follicle stimulating hormone (FSH) among women undergoing in vitro fertilization (IVF): Which one is the better predictor for live birth? J Ovarian Res. 2018;11:60. doi: 10.1186/s13048-018-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tal R, Tal O, Seifer BJ, Seifer DB. Antimüllerian hormone as predictor of implantation and clinical pregnancy after assisted conception: A systematic review and meta-analysis. Fertil Steril. 2015;103:119–30.e3. doi: 10.1016/j.fertnstert.2014.09.041. [DOI] [PubMed] [Google Scholar]
- 9.Sun XY, Lan YZ, Liu S, Long XP, Mao XG, Liu L. Relationship between anti-müllerian hormone and ın vitro fertilization-embryo transfer in clinical pregnancy. Front Endocrinol (Lausanne) 2020;11:595448. doi: 10.3389/fendo.2020.595448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotlyar A, Seifer DB. Anti-müllerian hormone as a qualitative marker – Or just quantity? Curr Opin Obstet Gynecol. 2020;32:219–26. doi: 10.1097/GCO.0000000000000623. [DOI] [PubMed] [Google Scholar]
- 11.Tarasconi B, Tadros T, Ayoubi JM, Belloc S, de Ziegler D, Fanchin R. Serum antimüllerian hormone levels are independently related to miscarriage rates after in vitro fertilization-embryo transfer. Fertil Steril. 2017;108:518–24. doi: 10.1016/j.fertnstert.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 12.Peuranpää P, Hautamäki H, Halttunen-Nieminen M, Hydén-Granskog C, Tiitinen A. Low anti-müllerian hormone level is not a risk factor for early pregnancy loss in IVF/ICSI treatment. Hum Reprod. 2020;35:504–15. doi: 10.1093/humrep/deaa008. [DOI] [PubMed] [Google Scholar]
- 13.Definition of Abortus. 2021. [Last accessed on 2021 Oct 10]. Available from: https://www.merriam-webster.com/dictionary/abortion .
- 14.Sunderam S, Kissin DM, Crawford SB, Folger SG, Jamieson DJ, Warner L, et al. Assisted reproductive technology surveillance — United States, 2012. MMWR Surveill Summ. 2015;64:1–29. [PubMed] [Google Scholar]
- 15.Yang AM, Xu X, Han Y, Wei JJ, Hao GM, Cui N, et al. Risk factors for different types of pregnancy losses: Analysis of 15,210 pregnancies after embryo transfer. Front Endocrinol (Lausanne) 2021;12:683236. doi: 10.3389/fendo.2021.683236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornille AS, Sapet C, Reignier A, Leperlier F, Barrière P, Caillet P, et al. Is low anti-mullerian hormone (AMH) level a risk factor of miscarriage in women<37 years old undergoing in vitro fertilization (IVF)? Hum Fertil (Camb) 2022;25:600–6. doi: 10.1080/14647273.2021.1873431. [DOI] [PubMed] [Google Scholar]
- 17.Kerkhof GF, Leunissen RW, Willemsen RH, de Jong FH, Visser JA, Laven JS, et al. Influence of preterm birth and small birth size on serum anti-müllerian hormone levels in young adult women. Eur J Endocrinol. 2010;163:937–44. doi: 10.1530/EJE-10-0528. [DOI] [PubMed] [Google Scholar]
- 18.Stegmann BJ, Santillan M, Leader B, Smith E, Santillan D. Changes in antimüllerian hormone levels in early pregnancy are associated with preterm birth. Fertil Steril. 2015;104:347–55.e3. doi: 10.1016/j.fertnstert.2015.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McCormack CD, Leemaqz SY, Furness DL, Dekker GA, Roberts CT. Anti-müllerian hormone levels in recurrent embryonic miscarriage patients are frequently abnormal, and may affect pregnancy outcomes. J Obstet Gynaecol. 2019;39:623–7. doi: 10.1080/01443615.2018.1552669. [DOI] [PubMed] [Google Scholar]
- 20.Lyttle Schumacher BM, Jukic AM, Steiner AZ. Antimüllerian hormone as a risk factor for miscarriage in naturally conceived pregnancies. Fertil Steril. 2018:1065–71.e1. doi: 10.1016/j.fertnstert.2018.01.039. 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atasever M, Soyman Z, Demirel E, Gencdal S, Kelekci S. Diminished ovarian reserve: İs it a neglected cause in the assessment of recurrent miscarriage.A cohort study? Fertil Steril. 2016;105:1236–40. doi: 10.1016/j.fertnstert.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 22.Zarek SM, Mitchell EM, Sjaarda LA, Mumford SL, Silver RM, Stanford JB, et al. Antimüllerian hormone and pregnancy loss from the effects of aspirin in gestation and reproduction trial. Fertil Steril. 2016;105:946–52.e2. doi: 10.1016/j.fertnstert.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Szafarowska M, Molinska-Glura M, Jerzak MM. Anti-müllerian hormone concentration as a biomarker of pregnancy success or failure. Neuro Endocrinol Lett. 2014;35:322–6. [PubMed] [Google Scholar]
- 24.Tan TY, Lau SK, Loh SF, Tan HH. Female ageing and reproductive outcome in assisted reproduction cycles. Singapore Med J. 2014;55:305–9. doi: 10.11622/smedj.2014081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brodin T, Hadziosmanovic N, Berglund L, Olovsson M, Holte J. Comparing four ovarian reserve markers – Associations with ovarian response and live births after assisted reproduction. Acta Obstet Gynecol Scand. 2015;94:1056–63. doi: 10.1111/aogs.12710. [DOI] [PubMed] [Google Scholar]
- 26.Revelli A, Biasoni V, Gennarelli G, Canosa S, Dalmasso P, Benedetto C. IVF results in patients with very low serum AMH are significantly affected by chronological age. J Assist Reprod Genet. 2016;33:603–9. doi: 10.1007/s10815-016-0675-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Our data are available for the journal if requested by the editor(s). Data can be shared as per requirement.


