Abstract
Rationale
Clear definition of optimal positive airway pressure therapy usage in patients with obstructive sleep apnea is not possible because of scarce data on the relationship between usage hours and major clinical outcomes.
Objective
To investigate the dose–response relationship between positive airway pressure usage and healthcare resource utilization and determine the minimum device usage required for benefit.
Methods
A linked data set combined deidentified payer-sourced administrative medical/pharmacy claims data from more than 100 U.S. health plans and individual patient positive airway pressure usage data. Eligible adults (age ⩾18 yr) had a new obstructive sleep apnea diagnosis between June 2014 and April 2018. All received positive airway pressure therapy (AirSense 10; ResMed) with claims data for ⩾1 year before, and 2 years after, device setup. Healthcare resource utilization was determined on the basis of the number of all-cause hospitalizations and emergency room visits over 3, 12, and 24 months after positive airway pressure initiation.
Results
Data from 179,188 patients showed a clear dose–response relationship between daily positive airway pressure usage and healthcare utilization. Minimum device usage required for benefit was 1–3 hours per night. There was a statistically significant decrease in hospitalizations and emergency room visits at all time points (all Ps < 0.0001) with increasing positive airway pressure usage. Each additional hour of usage per night decreased hospitalizations and emergency room visits by 5–10% and 5–7%, respectively.
Conclusions
These data provide compelling evidence for a dose–response relationship between positive airway pressure usage and healthcare utilization, with benefits seen even when usage was as low as 1–2 hours per night.
Keywords: positive airway pressure, dose–response, OSA, healthcare resource utilization, hospitalization, emergency room visits
Obstructive sleep apnea (OSA) is thought to affect up to 1 billion people worldwide and has major neurocognitive and cardiometabolic sequelae (1–3). Continuous positive airway pressure (CPAP) is the first-line treatment for OSA (4), but its effectiveness is limited by variability in adherence to treatment. Alternative therapies for OSA exist but are also affected by variable adherence and incomplete efficacy. Several strategies have been utilized to try and improve CPAP adherence, including heated humidification, patient engagement tools, mask resupply programs, expiratory pressure relief strategies, intensive support, and others (5–7). However, optimizing CPAP usage in clinical practice is challenging. Moreover, optimal CPAP usage remains unclear, because the dose–response relationship between usage hours and major clinical outcomes has not been adequately studied (8).
The U.S. Centers for Medicare and Medicaid Services (CMS) defines CPAP compliance as device usage for 4 hours per night for more than four nights per week during a 30-day period in the first 90 days of therapy. However, although these thresholds are relatively arbitrary, they have been adopted by many parties around the world as metrics of adequate adherence to CPAP therapy. At present, there is insufficient evidence to guide the optimal amount of CPAP usage, particularly when one considers that the optimal duration of device usage may vary depending on the outcome of interest. For example, the hours of CPAP usage needed to lower blood pressure might differ from the hours of CPAP usage needed to improve clinical symptoms. Furthermore, there is debate about whether improvements during CPAP therapy show a threshold effect (e.g., occurring when usage is more than 4 hours per night) or a dose–response effect (i.e., “more is better”). Although some data suggest that patient-reported outcomes may improve with increased CPAP usage (8–12), studies have generally been small and have not assessed important objective outcomes (e.g., risk of hospitalization and/or emergency room [ER] visits).
Technological improvements have facilitated the assessment of large-scale positive airway pressure (PAP) usage on the basis of big data or real-world data-analytic approaches. Using recently developed techniques, we can now assess how PAP usage predicts clinical outcomes on a large scale in a manner compliant with the Health Insurance Portability and Accountability Act. Therefore, this study investigated the dose–response relationship between PAP usage and healthcare resource utilization and determined the minimum PAP usage required to see a benefit. In addition, we sought to test the hypothesis that a dose–response curve would be present whereby increasing usage of CPAP would yield improved health outcomes rather than having a threshold above which hours of CPAP usage become irrelevant.
Methods
Study Design and Data Sources
This study was conducted using a linked data set that combined de-identified payer-sourced administrative medical and pharmacy claims data from more than 100 U.S. health plans (Inovalon Insights LLC) and individual patient PAP usage data from cloud-connected devices (via AirView; ResMed Corporation). Objective PAP data collected in AirView devices include treatment usage, clinical therapy metrics, and residual respiratory events (13–15). This allowed the assessment of the effect of increased PAP usage on healthcare resource utilization. The databases were linked through a tokenized process, and the resulting matched database underwent expert determination to ensure patient privacy and compliance with the Health Insurance Portability and Accountability Act. The study design was reviewed by an institutional review board (Advarra, ref. Pro0004005) and deemed exempt from board oversight.
Study Participants
Patients ⩾18 years old with a new OSA diagnosis (diagnosis code 327.23 in the International Classification of Diseases, Ninth Revision, Clinical Modification; and diagnosis code G47.33 in the International Classification of Diseases, Tenth Revision, Clinical Modification) within 60 days of a sleep test between June 2014 and April 2018 were eligible for inclusion in the study. All included patients received PAP therapy using an AirSense 10 device (ResMed Corporation) with claims data for ⩾1 year before the first sleep test and 2 years after device setup. Those who did not have insurance enrollment for the full 3-year study period were excluded. Patients with evidence of PAP resupply, use of ventilation modes, pregnancy, end-stage renal disease, dialysis use, central sleep apnea, or nocturnal hypoventilation in the year before device setup were excluded.
Outcomes and Predictors
Primary outcomes were the number of all-cause hospitalizations and ER visits within 3, 12, and 24 months after PAP initiation. The primary predictor of interest was the average hours of PAP use per night over 3, 12, and 24 months (defined as the total hours used per number of days in each study time period). Average PAP usage was categorized into ten 1-hour increments (from <1 to ⩾9 h per night).
Covariates of interest included patient demographics (age at setup, payer, sex, and obesity), comorbidities (including hypertension, hyperlipidemia, gastroesophageal reflux disease, type 2 diabetes, anxiety, depression, psychotic disorders, other mood disorders, asthma, chronic obstructive pulmonary disease, pneumonia, cancer, coronary artery disease, cerebrovascular disease, atrial fibrillation and other arrhythmias, and heart failure; defined on the basis of diagnoses present in the claims in the year before PAP setup), and prior year healthcare resource use (all-cause inpatient hospitalization and ER visits). Among the subset of patients undergoing statin (16), antihypertensive, or long-acting β2 agonist (LABA) bronchodilator therapy (one or more claims for a given medication within 180–360 days before PAP setup), adherence to medication was defined as a proxy for healthy behaviors. Patients with a proportion of days covered that was ⩾80% for a given therapy were classified as adherent. All covariates were selected on the basis of their potential to confound the relationship between PAP usage and healthcare resource use outcomes. Patients with greater comorbidity burden are likely to have a harder time using their devices consistently and to have greater resource use over time, as are those with greater healthcare resource use in the year before PAP setup. Those with other healthy behaviors are likely to have both more consistent PAP use and better outcomes.
Statistical Analysis
Outcomes at each time point were modeled separately to assess the effects of increased PAP usage over different time frames. The numbers of all-cause hospitalizations and ER visits were capped at the larger of two or the 99.5 percentile for each outcome and modeled using negative binomial regression. A single risk score for each patient was defined on the basis of the coefficients of all potential confounders and was used as a covariate in subsequent risk-adjusted models. Crude and risk-standardized rates (17), based on the uncapped values (per 1,000 people) of annualized healthcare resource utilization values, with 95% confidence intervals, were calculated using the ‘epitools’ package in R (18) and were plotted against nightly hours of PAP usage.
On the basis of previously used methodology (19), both the minimum threshold to confer a significant benefit and the incremental benefit with increased use were assessed using risk-adjusted negative binomial regression models, based on the capped values. The minimum threshold was determined by identifying the first usage category that differed significantly (P < 0.05) from the reference of <1 hour per night. Incremental benefit was modeled with PAP usage as a continuous variable. The predicted event rate was derived from the model (total predicted events/number of patients) and reported per 1,000 people. The exponentiated coefficient for hours of PAP usage gave the incidence rate ratio with 95% confidence interval, which was expressed as the percent reduction (1 − IRR) in the number of outcomes with each additional hour of PAP use. The percent reduction was applied to the overall predicted event rate to report an absolute reduction in the predicted number of events per 1,000 people.
Sensitivity analyses for 12-month hospitalization and ER visits were conducted among those undergoing statin, antihypertensive, or LABA therapy. Adherence to these maintenance medications was included as an additional covariate to assess the potential for healthy user confounding. All analyses were conducted using Python (version 3.6.10) and R (version 3.6.1).
Results
Study Population
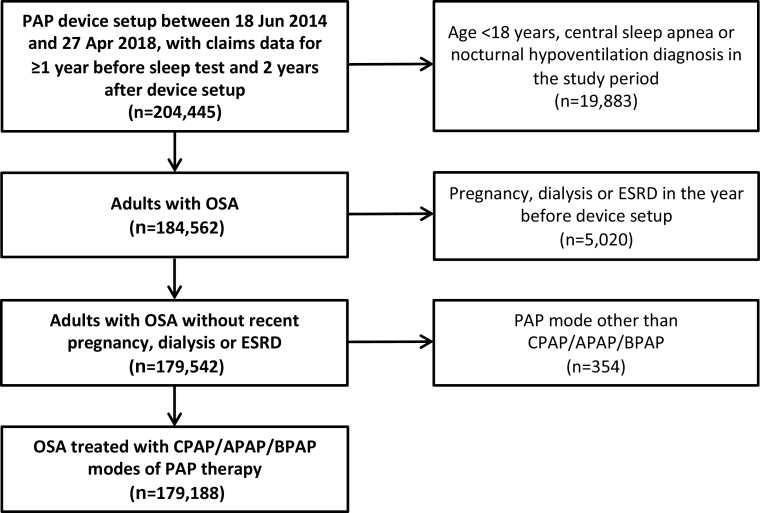
There were 204,445 patients who had PAP device setup during the study inclusion period, with claims data covering the period from at least 1 year before the sleep test to 2 years after the device setup claims date; of these, 179,188 met all other study inclusion criteria and were included in the analysis (Figure 1). The average age was 52.5 years, there were more males than females in the sample, more than half of all patients were obese, comorbidities (especially hypertension) were common, and most patients had commercial insurance (Table 1).
Figure 1.
Cohort flow chart. APAP = automatically titrating continuous positive airway pressure; BPAP = bilevel positive airway pressure; CPAP = continuous positive airway pressure; ESRD = end-stage renal disease; OSA = obstructive sleep apnea; PAP = positive airway pressure.
Table 1.
Baseline characteristics of the study population
| Characteristic | Study Population (N = 179,188) |
|---|---|
| Female, n (%) | 72,571 (40.5) |
| Age, yr, mean (median) | 52.5 (53) |
| Obesity, n (%) | 92,282 (51.5) |
| Comorbidities, n (%) | |
| Hypertension | 102,496 (57.2) |
| Coronary artery disease | 21,503 (12.0) |
| Atrial fibrillation | 11,110 (6.2) |
| Heart failure | 10,035 (5.6) |
| Type 2 diabetes | 40,496 (22.6) |
| Chronic obstructive pulmonary disease | 15,948 (8.9) |
| Depression | 28,133 (15.7) |
| None | 53,219 (29.7) |
| Payer, n (%) | |
| Commercial/other | 139,408 (77.8) |
| Medicaid | 21,861 (12.2) |
| Medicare advantage | 18,098 (10.1) |
| Residual AHI, events/h, mean (median) | |
| At 3 mo | 3.0 (1.8) |
| At 12 mo | 2.7 (1.7) |
| At 24 mo | 2.7 (1.6) |
| Healthcare utilization in the year before PAP | |
| Mean number of hospitalizations per patient | 0.11 |
| Mean number of emergency room visits per patient | 0.53 |
Definition of abbreviations: AHI = apnea-hypopnea index; PAP = positive airway pressure.
Device Usage
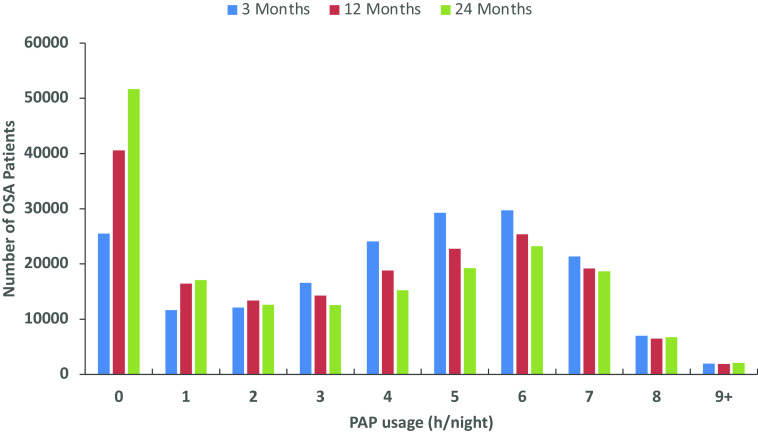
The proportion of patients with <1 hour of PAP usage per night increased as follow-up time increased (Figure 2). Across all three follow-up visits, usage of 3–7 hours per night was most common, and no more than 5% of patients had usage ⩾8 hours per night in any time period (Figure 2).
Figure 2.
Number of OSA patients by hours of PAP usage per night at 3, 12, and 24 months of follow-up. OSA = obstructive sleep apnea; PAP = positive airway pressure.
Risk Factors for Hospitalizations and ER visits
For hospitalizations and ER visits across all time points, resource utilization in the year before PAP setup was a major significant predictor of increased events after PAP initiation. Other significant predictors of hospitalizations and ER visits included older age, Medicaid insurance, female gender, and presence of comorbidities.
Minimum Usage and Incremental Benefit between PAP Usage and Healthcare Resource Utilization
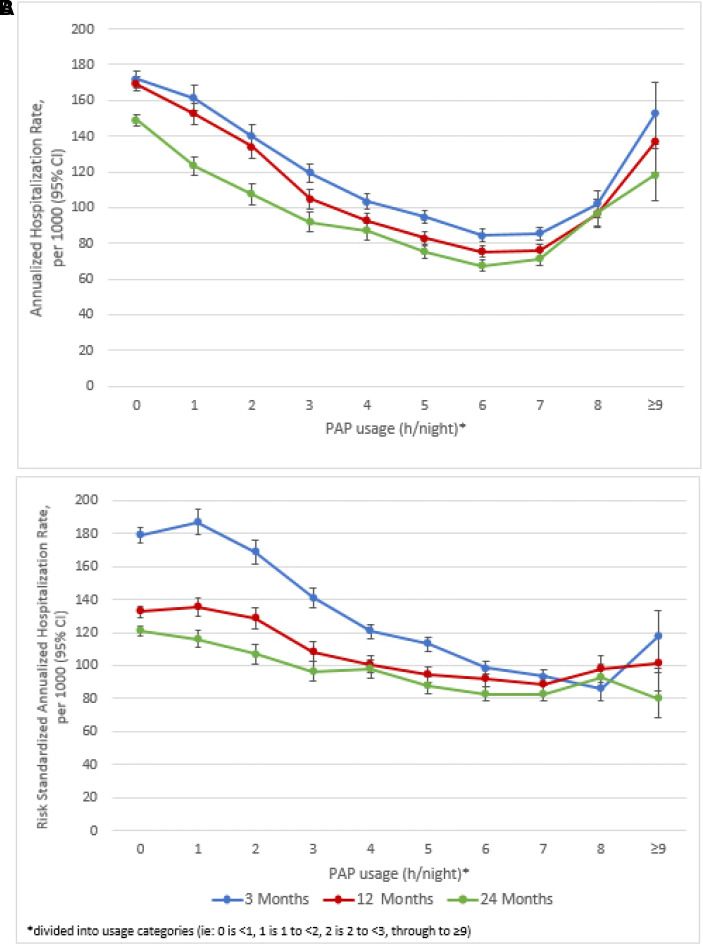
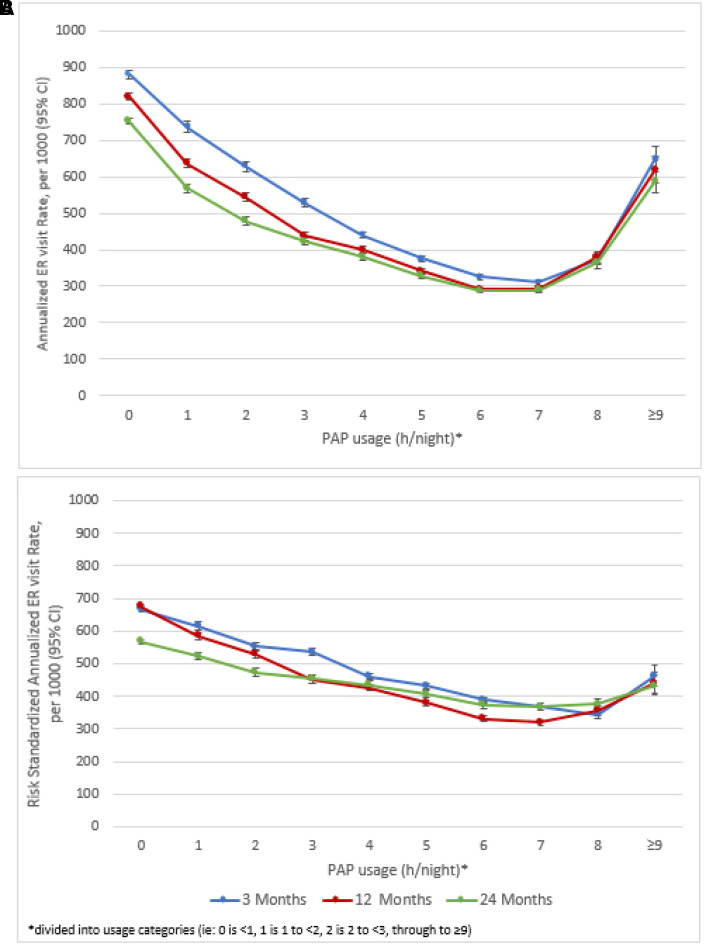
At 3, 12, and 24 months after PAP device setup, healthcare utilization decreased with increasing nightly PAP usage up until 8 hours per night (Figures 3A and 4A). There was a slight increase in event rates when PAP usage was ⩾8 hours per night, but event rates remained below those with <1 hour of PAP usage per night. When standardized to the risk score distribution of the overall sample, this increase was mitigated for both hospitalizations (Figure 3B) and ER visits (Figure 4B).
Figure 3.
(A and B) Annualized crude rate, per 1,000 of all-cause hospitalizations (A) and annualized risk-standardized rate, per 1,000 of all-cause hospitalizations (B) at 3, 12, and 24 months after PAP device setup. 95% CI = 95% confidence interval; PAP = positive airway pressure.
Figure 4.
(A and B) Annualized crude rate per 1,000 of all-cause emergency room (ER) visits (A) and annualized risk-standardized rate per 1,000 of all-cause ER visits (B) at 3, 12, and 24 months after PAP device setup. PAP = positive airway pressure.
Capped values for hospitalizations were 2, 3, and 5 and for ER visits were 3, 8, and 14 for 3, 12, and 24 months, respectively. After risk adjustment, the threshold for minimum hours of PAP usage to derive a significant benefit was at most between 3 and 4 hours per night for all outcomes and time points. The lowest threshold was between 1 and 2 hours per night to prevent ER visits at all three time points (Table 2). There was a statistically significant decrease in hospitalizations and ER visits at all time points (all Ps < 0.0001) with increasing PAP usage. There was a 5.1–9.7% reduction in predicted event rates with each additional hour per night of PAP usage. This corresponded to a range of predicted absolute reductions of 3 to 11 hospitalizations and 9 to 48 ER visits per 1,000 patients with each additional hour of PAP usage (Table 2).
Table 2.
Risk-adjusted minimum positive airway pressure usage threshold and incremental benefit
| 3 months |
12 months |
24 months |
||||
|---|---|---|---|---|---|---|
| Hospitalization | ER visits | Hospitalization | ER visits | Hospitalization | ER visits | |
| Predicted event rate per 1,000 patients | 32 | 119 | 109 | 471 | 202 | 930 |
| Reduction per additional hour of PAP usage, % (95% CI)* | 9.7 (8.7–10.6) | 7.4 (6.9–8.0) | 6.8 (6.2–7.4) | 5.8 (5.5–6.1) | 5.4 (4.9–5.8) | 5.1 (4.9–5.4) |
| Absolute reduction in events per additional hour of PAP usage, per 1,000 patients (95% CI)† | 3.1 (2.8–3.4) | 8.9 (8.2–9.5) | 7.4 (6.7–8.0) | 27 (25.9–29.0) | 10.9 (9.9–11.8) | 48 (45.5–50.3) |
| Minimum usage for benefit, h/night‡ | 3 | 1 | 3 | 1 | 2 | 1 |
Definition of abbreviations: CI = confidence interval; ER = emergency room; IRR = incidence rate ratio; PAP = positive airway pressure.
Calculated as 1 − IRR.
Based on the percent reduction and lower/upper confidence limits applied to the predicted event rate.
Divided into usage categories (i.e., 0 = <1, 1 = 1 to <2, 2 = 2 to <3, through to ⩾9).
Healthy User Effect
Statins, antihypertensives, and LABAs were prescribed to 21%, 33%, and 3% of the cohort, respectively; and 67%, 78%, and 28% were adherent to these therapies, respectively. Results were similar for all sensitivity analyses including adherence to maintenance medication as a covariate. After adjusting for medication adherence, there remained a statistically significant relationship between increased PAP usage and decreased 12-month healthcare resource utilization.
Discussion
Our new findings add to the literature in several important ways. The results showed a consistent dose–response relationship between PAP usage hours and all-cause hospitalizations and ER visits over 2 years of follow-up. The magnitude of the effect of PAP therapy was clinically important (Table 2). For every additional hour of PAP usage up to 9 hours per night, there was a 5.4–9.7% decrease in hospitalizations and a 5.1–7.4% decrease in ER visits. These benefits were observed even when PAP usage was 1–3 hours per night, which is below the traditionally defined minimum usage threshold of 4 hours per night. On the basis of current CMS compliance criteria, such patients would have therapy withdrawn despite the potential clinical benefits of low-level PAP usage in the first 90 days of treatment that was documented in this study.
Our data showed an apparent attenuation of the positive effects of PAP therapy on healthcare resource utilization when device usage was ⩾9 hours per night. The underlying reasons are unclear, but the finding is consistent with data and recommendations highlighting the risk of excess sleep (20, 21), which is likely confounded by factors such as coexisting depression and cardiovascular disease, social isolation, and inflammatory conditions (22, 23). Thus, our findings most likely represent a known relationship between excess sleep and adverse outcomes rather than any “toxicity” of extended PAP usage. Our clinical experience suggests that extremes of CPAP usage (e.g., >9 h per night) are sometimes seen in moribund patients who are bedridden or preterminal patients who use CPAP for extended time periods. Of note, risks of ER visits and hospitalizations with PAP usage of ⩾9 hours per night were still lower than with <1 hour of usage per night, despite the potential confounding effect of excessive sleep duration and other factors.
PAP usage may also be linked with health behaviors such that the benefits observed could be a function of education, diet, exercise, or other behaviors common in patients with good adherence. Platt and colleagues (16) reported that PAP usage tracks with statin use and suggested that PAP usage might be confounded by medication adherence. We, therefore, investigated the impact of this “healthy user” effect. This analysis showed no difference in PAP benefits between statin-adherent and nonadherent patients, indicating that the healthy user effect is unlikely to be a key mechanism underlying our observations and that, therefore, reductions in healthcare resource use were likely attributable to PAP use itself rather than a function of some confounding variable. Additional sensitivity analyses were conducted using adherence to antihypertensives and bronchodilators, in which we saw similar results. Although we attempted to control for potential healthy user confounding, it is possible that some residual confounding exists. However, on the basis of the clear relationship with increased PAP usage, it seems unlikely that it could be entirely explained by healthy user effect.
Strengths of this study are the large sample size, rigorous analyses, and evaluation of objective and important clinical outcomes. However, we acknowledge several limitations. First, the administrative data used lack some information, including underlying disease severity. Thus, we are supportive of smaller scale mechanistic studies for gathering more granular data than is feasible with our study design. Second, this was not a randomized controlled trial; therefore, the results may reflect correlation rather than causation. However, we view these real-world data as informative and relevant for clinical practice. Randomized controlled trials are not feasible on the large scale of the present study. Moreover, one could not ethically randomize patients to receive adequate versus inadequate therapy. Nonetheless, we hope that our new findings help to guide/encourage subsequent studies. Third, the administrative database did not include Medicare fee-for-service patients. Thus, the findings may not be applicable to these patients or those without access to health care, and conclusions are therefore limited to the population studied.
In conclusion, these real-world data show a clear dose–response relationship between PAP usage and healthcare resource utilization, with benefits seen even when device usage was 2–3 hours per night. These data could help inform evidence-based guidelines for PAP usage and reimbursement until more definitive data are available. On the basis of CMS adherence criteria alone, the current data have the potential to impact on medical decision-making for OSA patients worldwide, possibly allowing a greater number of individuals to benefit from even low-level usage of CPAP therapy.
Acknowledgments
Acknowledgment
The authors thank Nicola Ryan, an independent medical writer, for assistance with editing and formatting after preparation of the first draft of the manuscript; and Walter Linde-Zwirble, an independent statistician, for consultation on healthy user effect analysis.
Footnotes
This work was supported by ResMed. The medXcloud Group is an academic-industry collaboration involving employees and consultants of ResMed and global academic thought leaders in the fields of sleep and respiratory medicine. Representatives of the study sponsors were involved in the study design, collection, analysis and interpretation of data, writing of the report, and the decision to submit the paper for publication. A.M. had final responsibility for the decision to submit for publication.
Author Contributions: Conception and design: A.M., K.L.S., P.A.C., J.-L.P., C.M.N., and A.V.B. Analysis: J.C., C.W., N.A., and S.M. Interpretation: all authors. Drafting the first version of the manuscript: A.M. and A.V.B. Review, editing, and approval of the manuscript: all authors.
Data sharing statement: Because of the data sources used in this study, the authors do not have appropriate permissions to share the data publicly. A.M. had full access to all the data in the study and had final responsibility for the decision to submit for publication. J.C., C.W., and N.A. have personally reviewed the data, understand the statistical methods used for all analyses, and confirm an understanding of these analyses, that the methods are clearly described, and that they are a fair way to report the results.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Benjafield AV, Ayas NT, Eastwood PR, Heinzer R, Ip MSM, Morrell MJ, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med . 2019;7:687–698. doi: 10.1016/S2213-2600(19)30198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Punjabi NM, Caffo BS, Goodwin JL, Gottlieb DJ, Newman AB, O’Connor GT, et al. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med . 2009;6:e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kendzerska T, Gershon AS, Hawker G, Leung RS, Tomlinson G. Obstructive sleep apnea and risk of cardiovascular events and all-cause mortality: a decade-long historical cohort study. PLoS Med . 2014;11:e1001599. doi: 10.1371/journal.pmed.1001599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Patil SP, Ayappa IA, Caples SM, Kimoff RJ, Patel SR, Harrod CG. Treatment of adult obstructive sleep apnea with positive airway pressure: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med . 2019;15:335–343. doi: 10.5664/jcsm.7640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Malhotra A, Crocker ME, Willes L, Kelly C, Lynch S, Benjafield AV. Patient engagement using new technology to improve adherence to positive airway pressure therapy: a retrospective analysis. Chest . 2018;153:843–850. doi: 10.1016/j.chest.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoy CJ, Vennelle M, Kingshott RN, Engleman HM, Douglas NJ. Can intensive support improve continuous positive airway pressure use in patients with the sleep apnea/hypopnea syndrome? Am J Respir Crit Care Med . 1999;159:1096–1100. doi: 10.1164/ajrccm.159.4.9808008. [DOI] [PubMed] [Google Scholar]
- 7. Hwang D, Chang JW, Benjafield AV, Crocker ME, Kelly C, Becker KA, et al. Effect of telemedicine education and telemonitoring on continuous positive airway pressure adherence. The Tele-OSA randomized trial. Am J Respir Crit Care Med . 2018;197:117–126. doi: 10.1164/rccm.201703-0582OC. [DOI] [PubMed] [Google Scholar]
- 8. Weaver TE, Maislin G, Dinges DF, Bloxham T, George CF, Greenberg H, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep . 2007;30:711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weaver TE. Novel aspects of CPAP treatment and interventions to improve CPAP adherence. J Clin Med . 2019;8:2220. doi: 10.3390/jcm8122220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bakker JP, Weaver TE, Parthasarathy S, Aloia MS. Adherence to CPAP: what should we be aiming for, and how can we get there? Chest . 2019;155:1272–1287. doi: 10.1016/j.chest.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 11. Ye L, Malhotra A, Kayser K, Willis DG, Horowitz JA, Aloia MS, et al. Spousal involvement and CPAP adherence: a dyadic perspective. Sleep Med Rev . 2015;19:67–74. doi: 10.1016/j.smrv.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev . 2011;15:343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cistulli PA, Armitstead J, Pepin JL, Woehrle H, Nunez CM, Benjafield A, et al. Short-term CPAP adherence in obstructive sleep apnea: a big data analysis using real world data. Sleep Med . 2019;59:114–116. doi: 10.1016/j.sleep.2019.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Drager LF, Malhotra A, Yan Y, Pépin JL, Armitstead JP, Woehrle H, et al. medXcloud group Adherence with positive airway pressure therapy for obstructive sleep apnea in developing vs. developed countries: a big data study. J Clin Sleep Med . 2021;17:703–709. doi: 10.5664/jcsm.9008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu D, Armitstead J, Benjafield A, Shao S, Malhotra A, Cistulli PA, et al. Trajectories of emergent central sleep apnea during CPAP therapy. Chest . 2017;152:751–760. doi: 10.1016/j.chest.2017.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Platt AB, Kuna ST, Field SH, Chen Z, Gupta R, Roche DF, et al. Adherence to sleep apnea therapy and use of lipid-lowering drugs: a study of the healthy-user effect. Chest . 2010;137:102–108. doi: 10.1378/chest.09-0842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Naing NN. Easy way to learn standardization: direct and indirect methods. Malays J Med Sci . 2000;7:10–15. [PMC free article] [PubMed] [Google Scholar]
- 18.Aragon TJ, Fay MP, Wollschlaeger D, Omidpanah A.2020. https://cran.r-project.org/web/packages/epitools/epitools.pdf
- 19. Kirsch DB, Yang H, Maslow AL, Stolzenbach M, McCall A. Association of positive airway pressure use with acute care utilization and costs. J Clin Sleep Med . 2019;15:1243–1250. doi: 10.5664/jcsm.7912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep . 2010;33:585–592. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. St-Onge MP, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. American Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; and Stroke Council Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a scientific statement from the American Heart Association. Circulation . 2016;134:e367–e386. doi: 10.1161/CIR.0000000000000444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Patel SR, Malhotra A, Gottlieb DJ, White DP, Hu FB. Correlates of long sleep duration. Sleep . 2006;29:881–889. doi: 10.1093/sleep/29.7.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kwok CS, Kontopantelis E, Kuligowski G, Gray M, Muhyaldeen A, Gale CP, et al. Self-reported sleep duration and quality and cardiovascular disease and mortality: a dose–response meta-analysis. J Am Heart Assoc . 2018;7:e008552. doi: 10.1161/JAHA.118.008552. [DOI] [PMC free article] [PubMed] [Google Scholar]