Abstract
Objective:
Parkinson’s disease (PD) is a neurodegenerative disorder described by the dynamic decline of dopaminergic neurons in the substantia nigra pars compacta (SNpc). Stem cell transplantation is a new therapeutic strategy in the treatment of PD. The objective of the study was to assess the impact of intravenous infusion of adipose-derived mesenchymal stem cells (AD-MSCs) on memory disorder in Parkinsonian rats.
Materials and Methods:
In this experimental study, male Wistar rats were randomly divided to four groups containing sham, cell treatment, control, and lesion. The cell treatment group received intravenous injection of AD-MSCs 12 days after PD induction by bilateral injection of 6-hydroxydopamine. Four weeks after lesion formation, spatial memory was examined using the Morris water maze (MWM) assessment. The rats’ brains were removed and assessed by bromodeoxyuridine (BrdU), tyrosine hydroxylase (TH), and glial fibrillary acidic protein (Gfap) immunostaining.
Results:
Statistical analyses revealed a significant addition and reduction in time spent and escape latency in the target quadrant, respectively, in the cell group as compared to the lesion group. Also, BrdU-labeled cells were present in the substantia nigra (SN). The density of TH-positive cells was significantly increased in the AD-MSCs transplantation group as compared to the lesion group, and the density of astrocytes significantly diminished in the AD-MSCs transplantation group as compared to the lesion group.
Conclusion:
It appears that AD-MSCs treatment for Parkinson’s could decrease the density of astrocytes and promote the density of TH-positive neurons. It appears that AD-MSCs could improve spatial memory impairment in PD.
Keywords: Dopaminergic Neurons, Mesenchymal Stem Cells, Parkinson’s Disease, 6-Hydroxydopamine
Introduction
Parkinson’s disease (PD) which causes motor disorders is a neurodegenerative condition described by dynamic and extensive damage of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc) and their terminals in the striatum (1). This disturbing disease has afflicted more than 1 million people in the United States and its prevalence is expanding worldwide. Many studies have indicated that DA plays a certain role in the modulation of neuronal activities and contributes to various types of learning and memory processes occurring in specific regions of the brain (2). Furthermore, other studies have demonstrated that interactions are crucial between the hippocampus, the dopaminergic systems, adaptive memory, and motivated behavior (2, 3). Intracerebral administration of 6-OHDA in rats has been widely used to induce nigrostriatal DA depletion (4). Today, 6-OHDA represents one of the most common neurotoxins used to induce degeneration of central catecholaminergic projections including the nigrostriatal system in vivo (5). Experimental and clinical studies have confirmed cognitive dysfunctions in the hippocampus, a temporal lobe structure essential for physiological learning and memory, and this finding has been observed in some PD patients (3, 6).
Currently, available pharmacological and surgical treatments have improved clinical indications in the initial stages of the disease, but they cannot stop or reverse the degeneration of DA neurons. Therefore, inhibition of disease progression with efficacious and safe neuroprotective drugs is essential to prevent patients from reaching the late stages of PD. Other strategies have been established to reconstruct the damaged neural tissue, and most of them, especially cell-based therapies, have been tested preclinically in animal models (7). Therefore, cell substitution methods have come to the forefront as promising regenerative methodologies in PD research (8). Adult stem cell-based therapies, such as mesenchymal stem cells (MSCs), are considered a good choice in terms of regenerative medicine. MSCs have two particular therapeutic properties; i. The capacity to produce different lineages and ii. Self-renewal capacity. MSCs through the interaction with other cells increase their growth and development in vitro (9). MSCs have been used in the treatment of diseases such as ischemia, and they have been also been deployed as a therapeutic strategy against several neurodegenerative diseases in animal models, clinical trials, and preclinical studies (10, 11). Increasing evidence shows that transplanted MSCs significantly improve performance in patients with PD (12). AD-MSCs are a reliable cell source and can be ideal for application among other kinds of MSCs. Their ease of access from the patient’s fat tissue, besides not having ethical issues, is the major benefit of AD-MSCs therapy which has caused the use of AD-MSCs to be suitable for both auto- and allo-transplantation. Also, high and rapid proliferation makes these cells suitable for clinical application (13). Therefore, it appears that there is a high therapeutic potential in AD-MSCs for the treatment of various diseases, including PD (2, 13). The objective of this study was to determine the ability of AD-MSCs to improve cognitive deficits in Parkinsonian rat models constructed by 6-OHDA.
Materials and Methods
Animals and license
In this experimental study, adult male Wistar rats (age: 10 weeks old; weight: 220 to 280 g) were obtained from Shahid Beheshti University Laboratory Animal Center and maintained (5 per cage) in 12 hours: 12 hours light: dark cycle with free access to food and water. All the experimental protocols were approved by the Damghan University Committee of Animal Use for Research and Education (1817-28/7/1393).
Experimental protocol
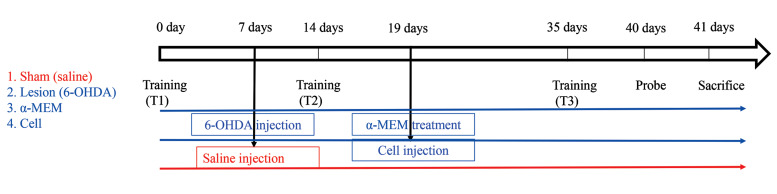
The animals were randomly divided into four groups (n=10). Before the experiments, animals in the four groups could learn how to perform the Morris water maze (MWM) task, and the learning potential of all the animals was investigated using the MWM. Animal grouping was as follows (Fig .1):
Fig.1.
The experimental design of the study.
Group I: the sham group that only received normal saline via a bilateral injection into the SN and was not treated with AD-MSCs. Group II: cell group that was treated with AD-MSCs (106 cells in 500 µl medium) 12 days after bilateral injection of 6-hydroxydopamine (6- OHDA) into the SN. Group III: rats that only received α-minimal essential medium (α-MEM, 500 µl) 12 days after the induction of SN lesion by 6-OHDA. Group IV: lesion group that was only subjected to 6-OHDA injection. In each group, except the sham, each rat was subjected to 2 µl of 6-OHDA injection using the stereotaxic apparatus. 6-OHDA infused by a Hamilton syringe and the rats were allowed to recover after lesion induction. Subsequently the rats were experimented for spatial memory using the MWM assay at 1 and 4 weeks after 6-OHDA injection. Finally, the rats were killed and their brains were removed. Next, the survival of dopaminergic neurons in the SN and astrocytes was determined.
The administration of 6-OHDA
6-OHDA was prepared following Ferro et al. (14). The rats were anesthetized by 10 mg/kg xylazine and 100 mg/ kg ketamine. Afterward, each rat was located on a stereotaxic stand, to expose the cranium the skin overlying the skull was cut, and for the SNpc the coordinates were exactly measured (mediolateral −2.1 mm from the midline, anteroposterior −0.5 mm from bregma, and dorsoventral −7.7 mm from the skull). Then, 6 μg of 6-OHDA, dissolved in 2 μL of 0.9% physiological saline solution containing 0.02% ascorbic acid (Sigma, USA), was perfused into the SNpc using 10 μl Hamilton syringes at a rate of 0.5 μl/minutes (the flow rate was controlled by a microsyringe using an EICOM EP-60 pump).
Morris water maze test
We performed Morris water maze (MWM) test according to previously published protocols (15). The MWM used in our research was a black circular pool (140 cm in diameter and 45 cm in depth) filled with water (at 24 ± 2°C with 30 cm depth). The pool was divided into four quadrants of the same size. An invisible escape platform (10 cm in diameter) was located in the middle of one of the quadrants (2 cm below the water surface) equidistant from the side walls and in the middle of the pool. The behaviors of the animals (i.e., latency, distance, etc.) were monitored by way of a video camera, fixed on the ceiling above the center of the pool, and a computerized tracking system (Ethovision; Noldus IT, The Netherlands). Four different starting positions had been similarly placed around the pool. For each rat, the training session comprised of four trials per day for four sequential days starting from one of the four start locations, used in a random sequence. Each trial was begun with putting the rat in the water facing the pool wall at one of the starting points. It was guided to the platform through the experimenter if a rat failed to escape within 60 seconds. When the rat reached the platform, it was allowed to remain there for 30 seconds, and it became located in a holding cage for an inter-trial interval of 30 seconds. The time that the animals climbed the hidden platform was recorded as escape latency and the distance to reach the platform was recorded as distance moved. Retention of the spatial education (probe trial) was evaluated 24 hours after the ultimate training session with a 60 seconds free-swim probe trial by a new starting position. The parameters gauged on the probe trial were time spent within the quadrant including the platform during training (time in the target quadrant), initial latency to cross the platform location (Escape location latency), and total swim distance (total distance moved) (16). All the groups were trained three times a day for four days, once before lesion induction and twice after lesion induction (one, and four weeks following lesion induction). The first, second and third training are called T1, T2, and T3, respectively. The experiment timeline is shown in Figure 1.
Adipose-derived mesenchymal stem cells culture and injection
AD-MSCs were collected under sterile conditions from intracapsular subcutaneous adipose tissue of rats. The sample tissue was washed in cold sterile phosphatebuffered saline (PBS, Ca/Mg Free, Invitrogen, USA). Fat pads were mechanically triturated and then enzymatically digested in 0.2% collagenase (Gibco, USA) for 30-40 minutes at 37°C accompanied by mechanical dissociation to yield a cell suspension which was gathered by centrifugation at 1756 rpm for 5 minutes at 37°C. The cell pellet was resuspended in α-MEM supplemented with 10% fetal bovine serum (FBS), 1% penicillinstreptomycin and incubated in 25-cm2 culture flasks at a density of 1-5×106 cells/cm2 . After 72 hours, non-adherent cells were eliminated by replacing the medium. Adherent cells shaped a confluent layer (80%), which was then passaged. Before transplantation, AD-MSCs were labeled with 10 μM BrdU 48 hours before injection (17). A total of 1×106 BrdU-labeled AD-MSCs (the fourth passage) in a 500 µl medium was injected into the tail vein 12 days after lesion induction (18, 19).
Histology assessments
Four weeks after lesion induction, following anesthesia, the brains were fixed by transcardial perfusion of a fixative solution containing 4% paraformaldehyde in 0.1 M phosphate buffer pH=7.3. The fixed brains were dehydrated, embedded in paraffin, sectioned into 5 μm. The sections were derived from the SN and hippocampus. A series of sections were used for BrdU and some for TH and Gfap immunostaining. According to the Paxinos atlas, SN is located at 4.68 to 6.24 mm of bregma. From each animal, 5 out of 62 sections (310 μm) were used for TH immunostaining, while for Gfap immunostaining, 10 out of 30 (150 μm) dentate gyrus (DG) sections (2.8 to 4.52 mm of bregma) were selected.
Immunohistochemistry and histological study
BrdU and TH staining protocols were used to investigate AD-MSCs migration into the SNpc and hippocampus, respectively, and to evaluate the influence of AD-MSCs treatment on the density of dopaminergic neurons in a rat model of PD. Gfap immunohistochemistry was also performed to investigate the density of astrocytes in all the groups. After deparaffinization and rehydration the sections were retrieved in the microwave using sodium citrate buffer for 10 minutes at 95-100°C and kept for 10 minutes at room temperature. The slides were washed two times and sections were incubated in blocking serum containing 10% Triton for 1 hour at room temperature. For BrdU and TH, slides were incubated with mouse monoclonal anti-BrdU antibody (1:500; B 2531, Sigma, USA) and anti-TH (Millipore-AB152) at 4°C overnight, respectively. Then, they were washed two times with PBS and labeled with horseradish peroxidase (HRP) secondary antibody for 1 hour at 37°C. For Gfap, slides were incubated with monoclonal anti-Gfap (Sigma, Aldrich, Anti Rabit IgG) at 4°C overnight. Afterwards, they were washed twice with PBS and labeled with secondary antibody (goat antimouse conjugate with rhodamin-AP124 R) for 1 hour at 37°C and nuclei stained with DAPI. Consequently, all the slides were washed twice each time for 5 minutes in PBS and the tissue slides for BrdU and TH immunohistochemistry were incubated with DAB for 5 minutes at room temperature. Then, the sections were mildly washed with distilled water and incubated with 70, 80, and 100% ethanol. Finally, coverslips were mounted on slides using the mounting solution. Photos of slides were captured by a digital camera (Nikon, DXM 120, USA) and analyzed by the Image J software (National Institutes of Health, USA).
Immunostaining for BrdU, nestin, and Oct4 markers
Anti-nestin and oct4 immunostaining were employed to identify the stemness of AD-MSCs. Briefly, the cells were fixed by 4% paraformaldehyde solution for 20 minutes at room temperature and washed three times in PBS. [For the BrdU-labeling assay, the cells were fixed using 70% ethanol for 10 minutes, and they were exposed to 2 M of HCl for 1 hour at 37°C. Subsequently, the cells were incubated in 0.1 M of borate buffer (pH=8.5) for 10 minutes]. Next, the cells were permeabilized with 0.3% Triton X-100 in PBS for 15 minutes and 10% goat serum for 15 minutes at room temperature and were allowed to incubate overnight at 4°C with primary antibodies such as anti-BrdU primary antibody (1:500; B 2531; Sigma, USA), anti-nestin (1:500; N5413; Sigma, USA) and anti-Oct4 [(1:100); ab18976; rabbit anti Oct4 antibody, Abcam, Cambridge, UK]. Next, the cells were washed twice in PBS and stained with anti-mouse secondary antibody Rhodamine-conjugated (1:100; Millipore, USA, AP124R), fluorescein isothiocyanate (FITC)- conjugated secondary anti-mouse (1:100) for nestin and FITC-conjugated secondary anti-rabbit (1:100) for Oct4 at 37°C for 1 hour. Cells were examined by fluorescent microscope (E600 Eclipse; Nikon, Netherlands) equipped with a digital camera (DXM 1200; Nikon Digital).
Statistical analysis
Data are displayed as mean ± SEM and were statistically analyzed by one-way and two way ANOVA and followed by Tukey’s test. All the statistical analyses were performed using SPSS version 26 (IBM, USA). P<0.05 were significantly considered.
Results
Morphological characterizations of the AD-MSCs
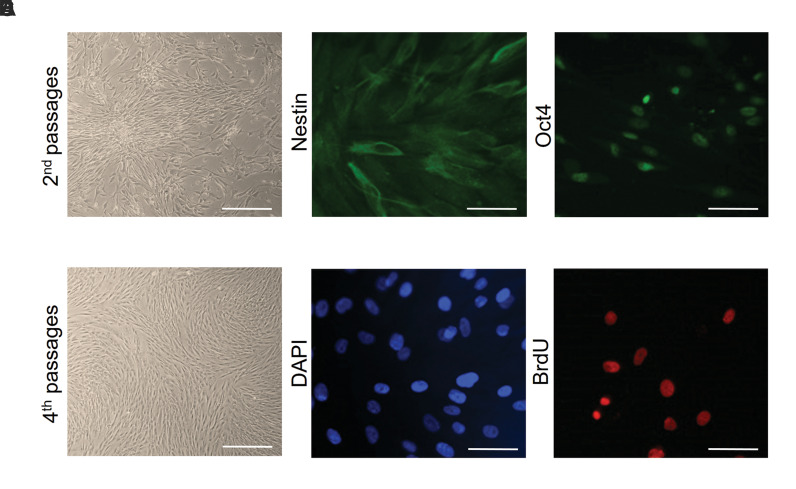
MSCs derived from adipose tissue had spindle-shaped morphology (Fig .2A, B). Previously, the isolation and characterization of the AD-MSCs and their neural differentiation have been reported (17-19).
Fig.2.
Characterization and labeling adipose-derived mesenchymal stem cells (AD-MSCs). Thin, elongated, and spindle-shaped cell bodies were observed. A. Second passages (scale bar: 200 µm) and B. Fourth passages (scale bar: 200 µm). C. Immunostaining indicated that MSCs expressed nestin which appears as green in fluorescence (scale bar: 50 µm) and D. Nuclei with DAPI in blue color (scale bar: 50 µm). E. Immunofluorescence staining indicated that AD-MSCs expressed Oct4 which appears as green in fluorescence (scale bar: 50 µm) and F. BrdU-labeled cells in the cell culture before transplantation (scale bar: 50 µm).
Immunocytochemical testing was performed to characterize mesenchymal cells using specific markers CD71 and CD90 (20). Also, adipogenic and osteogenic differentiation was authenticated by the formation of lipid droplets and the production of calcium phosphate after 21 days (21).
Immunofluorescence for the expression level of Nestin, Oct4, and BrdU
The expression levels of Nestin, Oct4, and BrdU were assessed to verify the AD-MSC phenotype of these cells. The result showed that 87.50 ± 0.565% of AD-MSCs expressed Nestin (Fig .2C, D). Oct4 immunostaining showed AD-MSCs to be positive for this marker (Fig .2E). BrdU labeling was used as a marker in cell transplantation. Cultured AD-MSCs were labeled in culture by uptake of BrdU, before grafting and more than 95% of the cells were BrdU positive (Fig .2F).
Morris water maze test
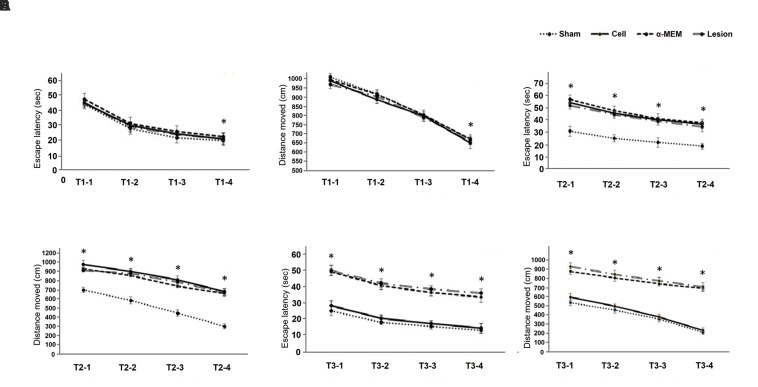
At first, the average time spent to find the platform on each training day for all the animals was measured. Paired-samples t test analysis between the results obtained on the first and fourth days of training presented significant differences in all the groups (P<0.05). Difference in time spent was compared among the groups by two-way ANOVA: 1st (F[3,28]=112, P=0.935), 2nd (F[3,28]=0.142, P=0.934), 3rd (F[3,28]=0.180, P=0.909), and 4th (F[3,28]=0.030, P=0.993) days, showing no significant differences in time spent to find the platform among the groups (Fig .3A). Another index that was measured was the distance traveled to find the platform on each of the training days for all the animals. Pairedsamples t test analysis between the first and fourth days of training showed significant differences (P<0.05). The distance traveled to find the platform decreased from the first to the fourth day. The traveled distance was compared among the groups using two-way ANOVA: 1st (F[3,28]=0.385, P=0.765), 2nd (F[3,28]=1.415, P=0.259), 3rd(F[3,28]=0.173, P=0.914), and 4th (F[3,28]=0.983, P=0.415) days, indicating no significant differences in the distance traveled to find the platform among the groups (Fig .3B). Training days’ data exhibited no significant difference between different groups and all the animals could learn the MWM task.
Fig.3.
Training after initiation of the experiment. A. Comparing the average time spent to find the platform in the studied groups (before the experiment). B. Comparing the average distance traveled to find the platform in the studied groups (before the experiment). C. Comparing the average time spent to find the platform in the studied groups (one week after the lesion). D. Comparing the average distance traveled to find the platform in the studied groups (one week after the lesion). E. Comparing the average time spent to find the platform in the studied groups (four weeks after the lesion). F. Comparing the average distance traveled to find the platform in the studied groups (four weeks after the lesion). Data were expressed as mean ± SEM. *; P0.05> compared to the first day in all the groups, n=5.
Training days one week after initiation of the experiment
On the fifth day of the study, animals of the lesion, cell, and α-MEM groups were injured as mentioned in the materials and methods section. One week after lesion induction, the intended indices were measured. The average time spent to find the platform on each training day for all the animals was measured one week following lesion induction. Paired-samples t test analysis between the first and fourth days of training showed significant differences in all the groups (P<0.05). The spent time was compared among the groups using two-way ANOVA: 1st (F[3,28]=101.243, P=0.000), 2nd (F[3,28]=114.176, P=0.000), 3rd (F[3,28]=101.028, P=0.000) and 4th (F[3,28]=91.479, P=0.000) days. Tukey’s test revealed that escape latency was significantly increased in the lesion, cell, and α-MEM groups as compared to the sham group (P<0.05, Fig .3C). Also, the average distance traveled to find the platform was measured on each training day for all the animals. Paired-samples t test analysis between the first and fourth days of training indicated significant differences (P<0.05). The distance traveled to find the platform decreased from the first to the fourth day. The traveled distance was compared among the groups using two-way ANOVA: 1st (F[3,28]=30218.06, P=0.000), 2nd (F[3,28]=28749.24, P=0.000), 3rd (F[3,28]=42028.57, P=0.000) and 4th (F[3,28]=36672.76, P=0.000) days. Tukey’s test showed that the traveled distance was significantly increased in the lesion, cell, and α-MEM groups as compared to the sham group (P<0.05, Fig .3D).
Training days four weeks after the initiation of the experiment
Four weeks following lesion induction, the MWM task was performed again. The time spent to find the platform was measured. Paired-samples t test analysis between the first and fourth days of training showed significant differences (P<0.05). In other words, the learning ability of the animals was increased. The spent time was compared among the groups using two-way ANOVA: 1st (F[3,28]=197.396, P=0.000), 2nd (F[3,28]=168.526, P=0.000), 3rd (F[3,28]=160. 286, P=0.000) and 4th (F[3,28]=172.677, P=0.000) days. Tukey’s test exposed that escape latency was significantly increased in the lesion and α-MEM groups as compared to the sham group (P<0.05). In this way, the time spent to find the platform in the lesion and α-MEM groups was significantly increased as compared to the sham group, but between the cell and sham groups, no significant differences were observed. Indeed, spatial memory in the lesion and α-MEM groups significantly declined as compared to the sham group. α-MEM injection could not improve the damage, and no significant difference was observed among the lesion and α-MEM groups. However, cell injection could improve the damage; therefore, the time spent to find the platform in the cell group was significantly decreased compared to the lesion group (Fig .3E).
Regarding the distance traveled to find the platform, paired-samples t test analysis between the first and fourth days of training showed significant differences in all the groups (P<0.05). The traveled distance among the groups was compared using two-way ANOVA: 1st (F[3,28]=35861.05, P=0.000), 2nd (F[3,28]=33239, P=0.000), 3rd (F[3,28]=44945.53, P=0.000) and 4th (F[3,28]=74495.47, P=0.000) days. Tukey’s test showed that the traveled distance was significantly increased in the lesion and α-MEM groups compared to the sham group (P<0.05). As previously mentioned, the α-MEM injection could not improve the damage, hence, no significant difference was observed between the lesion and α-MEM groups. Cell injection may have restored the damaged tissue resulting in a decreased travel time compared to the lesion group (Fig .3F).
Probe trial assay
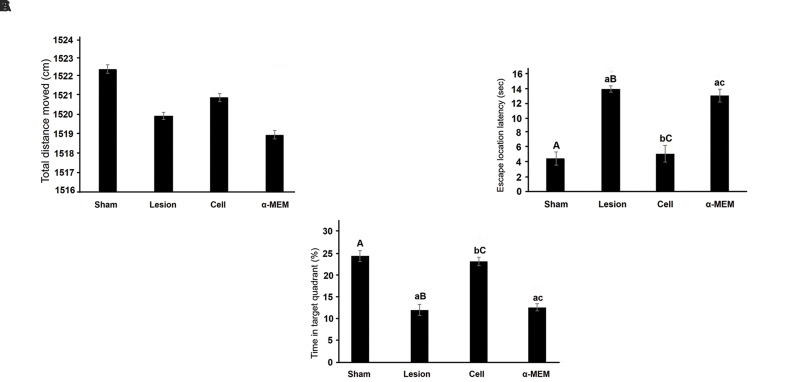
To study the animals’ movement ability and motivation, the total distance they moved was measured. One-way ANOVA displayed no significant differences between the studied groups (F [3,28] =0.251, P=0.086). In other words, movement ability and motivation did not differ significantly among the groups (Fig .4A). Also, the time spent to find the platform was measured. Animals that reached the platform in less time had a better spatial memory. The comparison of spent time between the groups using one-way ANOVA showed significant differences: (F[3,28]=74.251, P=0.000). Tukey’s test showed that escape latency was significantly increased in the lesion and α-MEM groups compared to the sham group (P<0.05), but there were no significant differences between the cell and sham groups in this regard. Indeed, cell injection could improve spatial memory (Fig .4B). Moreover, the percentage of time spent in the target quadrant (the quadrant where the platform was located on the training days) was measured. The comparison of spent time among the groups using one-way ANOVA showed significant differences: (F [3, 28] =83.521, P=0.000). Tukey’s test showed a significant decrease in time spent in the target quadrant in the lesion and α-MEM groups compared to the sham group (P<0.05). Cell injection could significantly increase the percentage of time spent in the target quadrant compared to the lesion and α-MEM groups. This increase reflected an improvement in memory (Fig .4C).
Fig.4.
Probe trial assay. A. The total distance moved in four weeks after creating the model in the studied groups. B. Comparing the average time spent to find the platform in the studied groups. C. Comparing the percentage of time spent in the target quadrant in the studied groups. All data are presented as mean ± ESM. Small letters (a, b, c) indicate the significance (P<0.05) compared to groups labeled by similar capital letters (A, B, C), n=3.
Immunohistochemistry for TH
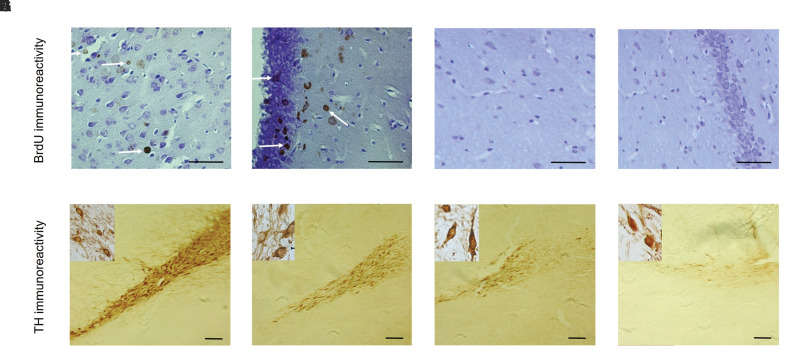
BrdU immunohistochemistry showed the presence of labeled AD-MSCs in the SN and hippocampus of the cell group animals, while labeled cells were not seen in this area of the sham group (Fig .5A-D) (Statistical results are not shown).
Fig.5.
The Immunohistochemical staining for BrdU and TH. A-D. The results showed that the BrdU-labeled cells were observed in the substantia nigra and Hippocampal granular layer after cell transplantation [A, B. Cell group (scale bar: 50 µm) and C, D. Sham group (scale bar: 50 µm)]. E-H. The results of immunohistochemical staining for TH indicated numerous TH-positive neurons were found within the cell group in comparison other groups. E. Sham group (scale bar: 100 µm); F. Cell group (scale bar: 100 µm), G. α-MEM group (scale bar: 100 µm), and H. Lesion group (scale bar: 100 µm).
Also, neural differentiation of the transplanted ADMSCs was evaluated using TH marker staining. The TH-positive neurons (density of TH fibers) in the SN were evaluated by the Image J software. One-way ANOVA demonstrated a significant difference among the groups (F [3, 36] = 4991.939, P=0.000). The results showed a significant decrease in the density of dopaminergic neurons in the SN in the α-MEM and lesion groups compared to the sham group (P<0.05), while a significant increase in this parameter was observed in the cell group compared to the lesion and α-MEM groups (Fig .5E-H).
Immunohistochemistry for GFAP
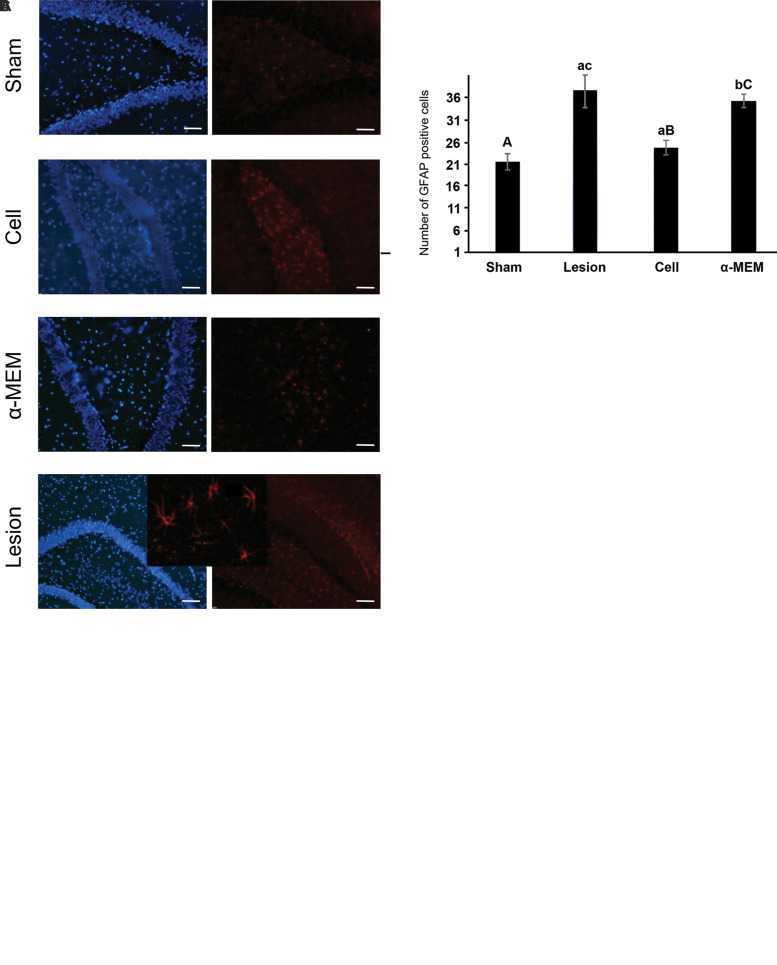
The GFAP-positive cells (density of astrocytes) in the DG were evaluated by Gfap immunohistochemistry using the Image J software. One-way ANOVA demonstrated significant differences among the groups (F [3, 76] = 96.154, P=0.000). The results showed a significant increase in the density of astrocytes in the DG in the α-MEM and lesion groups compared to the sham group (P<0.05), and this density was significantly decreased in the cell group compared to the lesion and α-MEM groups (P<0.05, Fig .6).
Fig.6.
The immunofluorescence staining for GFAP and TH markers. A. The number of TH-positive cells were significantly higher in the AD-MSCstreatment group than in the group treated with α-MEM and the lesion group. B. The number of Gfap-positive cells were significantly lower in the sham and AD-MSCs-treatment groups than in the group treated with α-MEM and the lesion group. C. The results showed that the GFAP-positive cells (red) in the sham and ADSC-treatment groups were decreased compared to the lesion and α-MEM groups. All data are presented as mean ± SEM. Small letters (a, b, c) indicate the significance (P<0.05) compared to groups labeled by similar capital letters (A, B, C), n=5. TH; Tyrosine hydroxylase, AD-MSCs; Adipose-derived mesenchymal stem cells, and α-MEM; α-minimal essential medium.
Discussion
Due to the disadvantages of drug therapy and surgicalbased treatments of PD, considerable efforts have been devoted to finding more efficient therapeutic strategies. Cell therapy has special importance and AD-MSCs may be an appropriate treatment. The current results demonstrate that transplantation of AD-MSCs improved learning and spatial memory in a Parkinson’s rat model.
Cognitive impairment in patients with AD is attributed to the loss of dopaminergic neurons in the SNpc. This study showed that the density of TH-positive neurons was significantly increased in the cell transplantation group as compared to the lesion group. The cognitive performance of rats was investigated by the MWM assay, which is one of the best tools for the evaluation of spatial learning and memory in rodents (4, 22).
Results of training days one week before the initiation of the experiment demonstrated that all the groups followed the MWM task, and they showed an equal potential for learning. But one week after the initiation of the study, the learning potential of all the groups, except the sham group, was significantly diminished. It appears that 6-OHDA injection induces neurodegeneration, and consequently, memory impairment. Moreover, 12 days after the commencement of the experiment, treatment with AD-MSCs in the cell group was started. Results of training days four weeks after the commencement of the experiment demonstrated that all the groups could learn the MWM task, such that the spent time and the traveled distance on the fourth day as compared to the first day were reduced in all the groups.
Comparison among the groups showed that the learning potential of the lesion and α-MEM groups was lower than that of the sham group; therefore, escape latency and traveled distance were significantly increased compared to the sham group on all days. In contrast, cell injection significantly improved the cognitive impairment induced by 6-OHDA. For this reason, the two abovementioned indices were significantly reduced in the cell group compared to the lesion group, but they were not significantly different from the sham group, suggesting that learning potential in the cell group was improved.
Results of the probe trial indicated no significant difference in the traveled distance among the groups, reflecting that the animals’ ability and incentive for moving were similar. Measuring escape latency in the probe trial revealed a significant increase in the lesion and α-MEM groups compared to the sham group. 6-OHDA treatmentincreased GFAP levels indicate astrocyte hypertrophy, which occurs when inflammation and degeneration occur in the nerve tissue. The results of the MWM test confirm that there is a disturbance in the nervous system because the PD model rats have a disturbance in their memory, which indicates a decrease in memory in this group.
Spatial memory of the rats must be impaired by 6-OHDA. Also, as escape latency showed a significant decrease in the cell group compared to the lesion and α-MEM groups, it can be stated that treatment with ADMSCs can improve spatial memory impairment, resulting in a reduction of time spent to find the platform. Time spent in the target quadrant is also another index for evaluating spatial memory (4).
The lesion and α-MEM groups compared to the sham group spent significantly less time in the target quadrant, but there was no significant difference among the cell group and the sham group that indicated treatment with cells can improve the spatial memory impairment induced by 6-OHDA. Although all animals could learn to perform the MWM task at the beginning of the experiment, 6-OHDA injection impaired learning and spatial memory, and AD-MSCs improved learning and spatial memory impairment.
In this regard, previous studies reported that intravenous transplantation of stem cells into brains in rat models of PD may provide trophic support to endogenous cells and improve spatial memory impairment (1, 23). Chen et al. (22) showed that transplantation of neural stem cells improved spatial memory in an Alzheimer’s Disease rat model.
Other studies established that bone marrow mesenchymal stem cells (BM-MSCs) transplantation improved spatial learning and memory in an Alzheimer’s Disease mouse model (24-26). These results were consistent with our findings as well. In our experiment, to check whether cells reached the brain parenchyma and engrafted in injured sites, the cells were labeled with BrdU.
MSCs characteristically migrate toward injured brain areas in animal models of PD induced by 6-OHDA, possibly in response to signals that are up-regulated under injury conditions (27). Additionally, 6-OHDA injection into the nigrostriatal tract moderately increases bloodbrain barrier permeability at the sites of neuronal damage (4, 28, 29). Also, the investigation has demonstrated that MSCs can pass through the blood-brain-barrier to reach the damaged area and can differentiate into TH cells (30). This is in line with an earlier study that showed MSCs were able to move through the bloodstream and migrate to damaged regions.
A previous study indicated that after bone marrow transplantation, donor-derived cells could migrate to damaged organs and differentiate into tissue cells. This indicates that stem cells can reside in damaged sites and participate in tissue regeneration (28). BrdU immunohistochemistry showed the presence of labeled AD-MSCs in the SN and hippocampus of the cell group animals, it seems that AD-MSCs can reside in damaged sites and participate in host tissue. Taken together, these results are consistent with the findings of previous works.
Dopaminergic neurons play an important role in PD, and it appears that dopaminergic neuron density in the SN of a rat model of 6-OHDA-induced PD was ameliorated by the cell treatment. For this proposal, TH immunohistochemistry was performed and images were evaluated.Results showed that the density of TH-positive neurons was significantly increased in the cell group as compared to the lesion and α-MEM groups.
According to several studies, it can be stated that MSCs have three mechanisms of action, including differentiation into dopaminergic neurons and replacement of degenerated cells with functional cells, neuroprotection where transplanted stem cells provide environmental aid to the affected brain cells by secreting cytokines and neurotrophic factors and increment of intrinsic neurogenesic capability (31).
Intravenous administration of AD-MSCs can restore impaired learning and spatial memory and recover dopaminergic neurons. Previously, the expression of neurotrophic factors such as Gdnf and Bdnf genes by AD-MSCs in vitro have been reported (32). Paracrine secretion of soluble factors by MSCs was implicated in their intrinsic capacity and anti-apoptotic and immunomodulatory properties. All these activities can contribute to neural regeneration, but the determination of the exact mechanisms leading to increased TH-positive cell density requires further investigations.
However, the results demonstrate that AD-MSCs differentiated into TH-positive cells and replaced the damaged dopaminergic neurons in a rat model of PD. Pro-inflammatory mediators may unexpectedly induce the degeneration of SNpc dopaminergic neurons (33). In addition, astrocytes appear as pivotal regulators of CNS inflammatory responses. Moreover, pro-inflammatory mediators released upon microglial activation can activate astrocytes, which in turn, play a major role in the neuroinflammatory processes involved in PD (34-37).
Several studies have shown that MSCs possess immunomodulatory and anti-inflammatory properties (10, 37) and MSCs can suppress microglial activation (10, 37, 38). Overall, these studies advocate that astrocyte-mediated inflammatory responses contribute to the pathophysiology of PD by degeneration of SNpc dopaminergic neurons. Our results demonstrated that the density of astrocytes was significantly decreased in the cell group compared to the lesion and α-MEM groups.
Conclusion
Transplantation of AD-MSCs improved learning and spatial memory in a PD rat model. Another important finding was that AD-MSCs secrete a variety of bioactive molecules, such as neurotrophic factors able to modulate immunoreactivity response with a reduction in Gfap markers or astrocyte number. AD-MSCs may be a useful therapeutic agent for the treatment of the neurodegenerative disorders, including PD.
Acknowledgements
The authors thank the Biological School of Damghan University for financiall supporting this research. The authors have no actual or potential declarations of interest.
Authors’ Contributions
H.H.; Investigation, Software. Sh.Gh.; Writing- Original draft preparation, Software, Reviewing and Editing. L.M.; Software, Reviewing and Editing, Methodology. K.A.; Methodology, Software. P.M.; Reviewing and Editing, Software, Methodology. M.T.Gh.; Supervision, Conceptualization, Methodology. All authors read and approved the final manuscript.
References
- 1.Reddy AP, Ravichandran J, Carkaci-Salli N. Neural regeneration therapies for Alzheimer’s and Parkinson’s disease-related disorders. Biochim Biophys Acta Mol Basis Dis. 2020;1866(4):16550–16550. doi: 10.1016/j.bbadis.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 2.Jiang W, Liang G, Li X, Li Z, Gao X, Feng S, et al. Intracarotid transplantation of autologous adipose-derived mesenchymal stem cells significantly improves neurological deficits in rats after MCAo. J Mater Sci Mater Med. 2014;25(5):1357–1366. doi: 10.1007/s10856-014-5157-9. [DOI] [PubMed] [Google Scholar]
- 3.Liu XL, Zhang W, Tang SJ. Intracranial transplantation of human adipose-derived stem cells promotes the expression of neurotrophic factors and nerve repair in rats of cerebral ischemia-reperfusion injury. Int J Clin Exp Pathol. 2013;7(1):174–183. [PMC free article] [PubMed] [Google Scholar]
- 4.Yuan Z, Zhou H, Zhou N, Dong D, Chu Y, Shen J, et al. Dynamic evaluation indices in spatial learning and memory of rat vascular dementia in the morris water maze. Sci Rep. 2019;9(1):7224–7224. doi: 10.1038/s41598-019-43738-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weintraub D, Moberg PJ, Duda JE, Katz IR, Stern MB. Effect of psychiatric and other nonmotor symptoms on disability in Parkinson’s disease. J Am Geriatr Soc. 2004;52(5):784–788. doi: 10.1111/j.1532-5415.2004.52219.x. [DOI] [PubMed] [Google Scholar]
- 6.Darbinyan LV, Hambardzumyan LE, Simonyan KV, Chavushyan VA, Manukyan LP, Sarkisian VH. Rotenone impairs hippocampal neuronal activity in a rat model of Parkinson’s disease. Pathophysiology. 2017;24(1):23–30. doi: 10.1016/j.pathophys.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Tieu K. A guide to neurotoxic animal models of Parkinson’s disease. Cold Spring Harb Perspect Med. 2011;1(1):a009316–a009316. doi: 10.1101/cshperspect.a009316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, et al. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16(6):653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- 9.Sarabadani M, Tavana S, Mirzaeian L, Fathi R. Co-culture with peritoneum mesothelial stem cells supports the in vitro growth of mouse ovarian follicles. J Biomed Mater Res A. 2021;109(12):2685–2694. doi: 10.1002/jbm.a.37260. [DOI] [PubMed] [Google Scholar]
- 10.Shinozuka K, Dailey T, Tajiri N, Ishikawa H, Kim DW, Pabon M, et al. Stem cells for neurovascular repair in stroke. J Stem Cell Res Ther. 2013;4(4):12912–12912. doi: 10.4172/2157-7633.S4-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C, Wang Y, Yang GY. Stem cell-mediated gene delivering for the treatment of cerebral ischemia: progress and prospectives. Curr Drug Targets. 2013;14(1):81–89. doi: 10.2174/138945013804806497. [DOI] [PubMed] [Google Scholar]
- 12.Ding X, Li Y, Liu Z, Zhang J, Cui Y, Chen X, et al. The sonic hedgehog pathway mediates brain plasticity and subsequent functional recovery after bone marrow stromal cell treatment of stroke in mice. J Cereb Blood Flow Metab. 2013;33(7):1015–1024. doi: 10.1038/jcbfm.2013.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du S, Guan J, Mao G, Liu Y, Ma S, Bao X, et al. Intra-arterial delivery of human bone marrow mesenchymal stem cells is a safe and effective way to treat cerebral ischemia in rats. Cell Transplant. 2014;23(Suppl 1):S73–S82. doi: 10.3727/096368914X685023. [DOI] [PubMed] [Google Scholar]
- 14.Ferro MM, Bellissimo MI, Anselmo-Franci JA, Angellucci ME, Canteras NS, Da Cunha C. Comparison of bilaterally 6-OHDA- and MPTP-lesioned rats as models of the early phase of Parkinson’s disease: histological, neurochemical, motor and memory alterations. J Neurosci Methods. 2005;148(1):78–87. doi: 10.1016/j.jneumeth.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 15.Nazari Z, Bahrehbar K, Golalipour MJ. Effect of MDMA exposure during pregnancy on cell apoptosis, astroglia, and microglia activity in rat offspring striatum. Iran J Basic Med Sci. 2022;25(9):1091–1096. doi: 10.22038/IJBMS.2022.64980.14308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mohammadi HS, Goudarzi I, Lashkarbolouki T, Abrari K, Elahdadi Salmani M. Chronic administration of quercetin prevent spatial learning and memory deficits provoked by chronic stress in rats. Behav Brain Res. 2014;270:196–205. doi: 10.1016/j.bbr.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 17.Taghi GM, Ghasem Kashani Maryam H, Taghi L, Leili H, Leyla M. Characterization of in vitro cultured bone marrow and adipose tissue- derived mesenchymal stem cells and their ability to express neurotrophic factors. Cell Biol Int. 2012;36(12):1239–1249. doi: 10.1042/CBI20110618. [DOI] [PubMed] [Google Scholar]
- 18.Ning H, Liu G, Lin G, Yang R, Lue TF, Lin CS. Fibroblast growth factor 2 promotes endothelial differentiation of adipose tissue-derived stem cells. J Sex Med. 2009;6(4):967–979. doi: 10.1111/j.1743-6109.2008.01172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chi H, Guan Y, Li F, Chen Z. The effect of human umbilical cord mesenchymal stromal cells in protection of dopaminergic neurons from apoptosis by reducing oxidative stress in the early stage of a 6-ohda-induced parkinson’s disease model. Cell Transplant. 2019;28(Suppl 1):87S–99S. doi: 10.1177/0963689719891134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramezani M, Mirzaeian L, Ghezelayagh Z, Ghezelayagh Z, Ghorbanian MT. Comparing the mesenchymal stem cells proliferation rate with different labeling assessments. The Nucleus. 2023;66(2):1–7. [Google Scholar]
- 21.Ghorbanian MT, Haji-Ghasem-Kashani M, Hossein-Pour L, Mirzaiyan L. Expression of nestin and nerve growth factors in adiposederived mesenchymal stem cells. Feyz J Kashan Univ Med Sci. 2012;15(4):322–330. [Google Scholar]
- 22.Chen SQ, Cai Q, Shen YY, Wang PY, Li MH, Teng GY. Neural stem cell transplantation improves spatial learning and memory via neuronal regeneration in amyloid-β precursor protein/presenilin 1/tau triple transgenic mice. Am J Alzheimers Dis Other Demen. 2014;29(2):142–149. doi: 10.1177/1533317513506776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rad SNH, Kashani MHG, Abrari K. Pre-treatment by rosemary extract or cell transplantation improves memory deficits of parkinson’s disease: when tradition meets the future.Braz Arch Biol Technol. Braz Arch Biol Technol; 2021. pp. 64–64. [Google Scholar]
- 24.Lee JK, Jin HK, Endo S, Schuchman EH, Carter JE, Bae JS. Intracerebral transplantation of bone marrow-derived mesenchymal stem cells reduces amyloid-beta deposition and rescues memory deficits in Alzheimer’s disease mice by modulation of immune responses. Stem Cells. 2010;28(2):329–343. doi: 10.1002/stem.277. [DOI] [PubMed] [Google Scholar]
- 25.Na Kim H, Yeol Kim D, Hee Oh S, Sook Kim H, Suk Kim K, Hyu Lee P. Feasibility and efficacy of intra-arterial administration of mesenchymal stem cells in an animal model of double toxin-induced multiple system atrophy. Stem Cells Transl Med. 2017;6(5):1424–1433. doi: 10.1002/sctm.16-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qin C, Lu Y, Wang K, Bai L, Shi G, Huang Y, et al. Transplantation of bone marrow mesenchymal stem cells improves cognitive deficits and alleviates neuropathology in animal models of Alzheimer’s disease: a meta-analytic review on potential mechanisms. Transl Neurodegener. 2020;9(1):20–20. doi: 10.1186/s40035-020-00199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang YL, Liu XS, Wang SS, Xue P, Zeng ZL, Yang XP, et al. Curcumin- activated mesenchymal stem cells derived from human umbilical cord and their effects on MPTP-mouse model of parkinson’s disease: a new biological therapy for parkinson’s disease. Stem Cells Int. 2020;2020:4636397–4636397. doi: 10.1155/2020/4636397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponte AL, Marais E, Gallay N, Langonné A, Delorme B, Hérault O, et al. The in vitro migration capacity of human bone marrow mesenchymal stem cells: comparison of chemokine and growth factor chemotactic activities. Stem Cells. 2007;25(7):1737–1745. doi: 10.1634/stemcells.2007-0054. [DOI] [PubMed] [Google Scholar]
- 29.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: the devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Kuroda Y, Kitada M, Wakao S, Dezawa M. Bone marrow mesenchymal cells: how do they contribute to tissue repair and are they really stem cells? Arch Immunol Ther Exp (Warsz) 2011;59(5):369–378. doi: 10.1007/s00005-011-0139-9. [DOI] [PubMed] [Google Scholar]
- 31.Zappa Villar MF, Lehmann M, García MG, Mazzolini G, Morel GR, Cónsole GM, et al. Mesenchymal stem cell therapy improves spatial memory and hippocampal structure in aging rats. Behav Brain Res. 2019;374:111887–111887. doi: 10.1016/j.bbr.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Ghorbanian MT, Tiraihi T, Mesbah-Namin SA, Fathollahi Y. Selegiline is an efficient and potent inducer for bone marrow stromal cell differentiation into neuronal phenotype. Neurol Res. 2010;32(2):185–193. doi: 10.1179/174313209X409016. [DOI] [PubMed] [Google Scholar]
- 33.Zhang BP, Wu L, Wu XW, Wang F, Zhao X. Dexmedetomidine protects against degeneration of dopaminergic neurons and improves motor activity in Parkinson’s disease mice model. Saudi J Biol Sci. 2021;28(6):3198–3203. doi: 10.1016/j.sjbs.2021.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norden DM, Muccigrosso MM, Godbout JP. Microglial priming and enhanced reactivity to secondary insult in aging, and traumatic CNS injury, and neurodegenerative disease. Neuropharmacology. 2015;96(Pt A):29–41. doi: 10.1016/j.neuropharm.2014.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dabrowska S, Andrzejewska A, Janowski M, Lukomska B. Immunomodulatory and regenerative effects of mesenchymal stem cells and extracellular vesicles: therapeutic outlook for inflammatory and degenerative diseases. Front Immunol. 2021;11:591065–591065. doi: 10.3389/fimmu.2020.591065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nat Rev Neurosci. 2015;16(5):249–263. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson’s disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19–19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rappold PM, Tieu K. Astrocytes and therapeutics for Parkinson’s disease. Neurotherapeutics. 2010;7(4):413–423. doi: 10.1016/j.nurt.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]