Abstract
Objective:
Psoriasis is a common, auto-immune skin disease characterized by abnormal proliferation and differentiation of keratinocytes. Studies revealed the role of stress stimulators in the pathogenesis of psoriasis. Oxidative stress and heat shock are two important stress factors tuning differentiation and proliferation of keratinocytes, regarding to psoriasis disease. BCL11B is a transcription factor with critical role in embryonic keratinocyte differentiation and proliferation. Given this, in keratinocytes we have investigated potential role of BCL11B in stress-induced differentiation. Furthermore, we searched for a potential intercommunication between BCL11B expression and psoriasis-related keratinocyte stress factors.
Materials and Methods:
In this experimental study, data sets of psoriatic and healthy skin samples were downloaded in silico and BCL11B was chosen as a potential transcription factor to analyze. Next, a synchronized in vitro model was designed for keratinocyte proliferation and differentiation. Oxidative stress and heat shock treatments were employed on HaCaT keratinocytes in culture, and BCL11B expression level was measured. Cell proliferation rate and differentiation were analyzed by synchronized procedure test. Flow cytometry was done to analyze cell cycle alterations due to the oxidative stress.
Results:
Quantitative reverse transcription polymerase chain reaction (qRT-PCR) data revealed a significant upregulation of BCL11B expression in keratinocytes, by 24 hours after initiating differentiation. However, it was followed by a significant down-regulation in almost all the experiments, including the synchronized model. Flow cytometer data demonstrated a G1 cell cycle arrest in the treated cells.
Conclusion:
Results indicated a remarkable role of BCL11B in differentiation and proliferation of HaCaT keratinocytes. This data along with the results of flow cytometer suggested a probable role for BCL11B in stress-induced differentiation, which is similar to what is happening during initiation and progression of normal differentiation.
Keywords: BCL11B, Keratinocyte, Psoriasis, Stress-Induced Differentiation, Transcription Factor
Introduction
Psoriasis is a chronic immune mediated skin disease significantly affecting quality of life, both physically and psychologically (1, 2). Psoriasis frequency affects approximately 2 to 3 percent of general population. It is characterized by epidermal hyper proliferation, abnormal differentiation of keratinocytes and inflammation along with infiltrated immune cells in the skin (3). There existed different factors with crucial roles in pathogenesis of psoriasis, including several subtypes of T cells, environmental factors and complex genetic background of patients. A combination of these factors, most probably, results in abnormal proliferation and differentiation of epidermal keratinocytes in psoriasis patients (4, 5).
Stress is one of the environmental factors that interfere with homeostasis. It can also change molecular balance of different tissues. Skin is one the organs that responds to various stressors vigorously. Several different signals are received by the skin and responded based on their importance (6). It has been reported that some stressors trigger skin responses eventually leading to psoriasis.
Reactive oxygen species (ROS) is one of the skin stressors which involve in oxidative stress (7). ROS is produced from both endogenous sources and external pro-oxidant stimuli which expose the skin to oxidative stress. ROS is involved in several cellular processes including cell proliferation, apoptosis, immune responses and cell differentiation. Moreover, it is also generated within dysregulated signal transduction events, and bimolecular (lipids, proteins, DNA) damage processes. Amount of ROS determines its positive or destructive role in cellular mechanisms (8). It has been shown that the imbalance between ROS and antioxidant cause oxidative stress with a role in the pathogenesis of psoriasis (7).
Heat shock response in the skin is another stressor in the pathogenesis of psoriasis. Heat shock proteins are the most important components of heat shock response involved in different biological processes including maintenance of intracellular redox potential balance, stabilization of the cytoskeleton, as well as regulation of complicated processes of cellular proliferation, differentiation and apoptosis (9).
Gene expression alterations could ignite all of the aforementioned molecular and cellular mechanisms. Transcription factors are the most important mediators of gene expression regulation. Transcription factors may be constitutively expressed or could be activated during special conditions. BCL11B, a zinc finger transcription factor, is highly expressed in skin and immune system. This gene was identified as a bifunctional transcriptional regulator and acts as a repressor or a trans-activator (10, 11). A critical role for BCL11B in epidermal proliferation and keratinocytes terminal differentiation of adult skin cells has already been reported. These findings make the gene a novel candidate for potential treatment of psoriasis (12). Given these information, we investigated here a probable role of BCL11B in keratinocytes differentiation, under different stress conditions related to psoriasis.
Materials and Methods
Ethical statements
This experimental study did not involve human participants, use human samples or tissues. It neither did include experimenting on animals. The in vitro experiments on commercial cell lines and standard techniques of molecular genetics was approved for a Ph.D. thesis proposal, by Tarbiat Modares Research Ethics Committee (IR.MODARES.REC.1398.119).
Bioinformatics analysis
All data sets were downloaded from the NCBI gene expression omnibus (GEO). A total of 170 microarray series, regarding the psoriasis research, were retrieved from GEO. After a careful review, three gene expression profiles (GSE166388, GSE68939, and GSE68937) were selected. GEO2R online analysis tool (https:// www.ncbi.nlm.nih.gov/geo/GEO2R/) was used to predict potential transcription factors of screened differentially expressed mRNAs of lesional psoriatic and healthy control skin biopsies. Transcription factors that met the cutoff criteria, adjusted P<0.05 and |logfc|≥ ± 2.0, were considered as potential transcription factors. Statistical analysis was carried out for each dataset and common data was identified using the Venn diagram webtool (bioinformatics.psb. ugent.be/webtools/venn/).
Each transcription factor was then checked for tissue specificity through the human protein atlas portal (website: http://www.proteinatlas.org/), GTEX portal (website: https://gtexportal.org). BCL11B was then selected according to the cutoff criteria and tissues in which it was expressed.
Cell culture
The spontaneously immortalized human keratinocyte (HaCaT) cells were kindly given by Dr. Majid Mojarrad, Mashhad University of Medical Sciences (Mashhad, Iran). HaCaT cells were cultured in dulbecco’s modified eagle’s medium, high glucose (DMEM, Thermo Fisher Scientific, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, USA), penicillin (100 u/ ml) and streptomycin (100 mg/ml, Bio basic, Canada), and maintained in a humidified atmosphere at 37°C with 5 % CO2 . The cells were sub-cultured every 2-3 days or when they reached 80-90% confluency.
Cell viability
The HaCaT cells at logarithmic growth phase were seeded at 1×105 cells/ml into each wells of a 96-well cell culture plate. Effect of H2 O2 on cell viability was measured using the 3-(4 5-dimethylthiazole-y)-2, 5-diphenyltetrazolium bromide assay (MTT, Sigma, USA). 100 μl medium containing H2 O2 (0 to 1000 μmol/l) was added to each well for 24 hours. 20 hours later incubation period, the medium was aspirated and 20 μl 5 mg/ml MTT solution was added. After 4 hours the supernatant was discarded, 150 μl dimethyl sulphoxide (DMSO) was added to dissolve the precipitate. Survival rate of the cells was then measured.
Synchronization procedure
Synchronization procedure of HaCaT cells done by cultivation at high density in the absence of serum. Cells were grown at complete DMEM medium containing 10% FBS at 100% confluence for 2 days. The culture medium was then replaced with serum free DMEM and incubated for more 2 days. The synchronized cells were trypsinized and seeded into new 24 wells plate in 10% FBS high-glucose DMEM. The cells were counted and their viability determined by trypan blue staining. Total RNA was collected at 0, 16, 24, 36 and 48 hours, when the cells reached 100% confluence in complete medium and/or in serum free medium.
Oxidative stress
The HaCaT cells were cultured and then for the next passage were seeded in a 48 wells plate (7×104 cells/well). Later, when cultures reached 70-80% confluence, the medium was changed with complete DMEM containing 300 μmol/l H2 O2 . The cells were incubated at a humidified atmosphere at 37°C with 5% CO2 for 2 days. Total RNA was collected 0, 1, 2, 4, 6, 8, 12, 24, 36 and 48 hours later, after oxidative stress.
Heat shock stress
Heat shock assay was conducted culturing HaCaT cells in 6-well plate (3×105 cells/well) incubating at 42°C for 1 hour. The plate was then incubated in normal condition at 37°C with 5% CO2 . Total RNA was then collected in different time points including 0, 1, 2, 4, 8, 12 and 24 hours after heat shock treatment.
Quantitative reverse transcription polymerase chain reaction
Total RNA was isolated from HaCaT keratinocytes using trizol (Thermo Fisher Scientific, USA) in different time points following the manufacturer’s instructions. RNA concentration was determined by measuring the 260 nm absorbance. In order to eliminate DNA contamination, 1 µg of RNA was first treated with DNase I (Thermo Fisher Scientific, USA), then first-strand cDNA was synthetized using the cDNA synthesis kit (Takara Bio, Japan). Quantitative reverse transcription polymerase chain reaction (qRT-PCR) experiments were performed to quantify the relative abundance of each interested mRNAs using biofact™ 2x real-time PCR master mix (Biofact, South Korea) through ABI StepOne real time PCR system (Thermo Fisher Scientific, USA). Specific primers were used for quantifying BCL11B-F: 5ˊCAAACAGGGCAACCCGCAGCA3ˊ R: 5ˊACAGGTGAGCAGGTCAGGGTC3ˊ
Abundance of the gene of interest was normalized to the expression of beta 2-microglubolin (b2m) of each examined sample.
Cell cycle characterization
Flow cytometry was employed to observe possible alterations in different phases of cell cycles, induced in oxidative stress. The keratinocyte cell line, HaCaT, was stimulated with 300 μmol/l H2 O2 for 24 hours, washed with phosphate buffered saline (PBS, Aminsan, Iran) and then fixed with 4% paraformaldehyde solution for 15 minutes. Subsequently, the cells were stained with propidium iodide (pi), and distribution of the cells in cell cycle phases was assessed by fluorescenceactivated cell sorting (FACS) and flow cytometer (Becton Dickinson, USA).
Statistical analysis
The 2-(∆ct) method was used for qRT-PCR data analysis and gene expression profiling. All experiments were analyzed using Graphpad prism (version 8.4; Graphpad software inc, La Jolla, CA, USA). P values were analyzed using the student t test and one-way ANOVA. Statistical significance was considered at a P<0.05.
Results
BCL11B transcription factor was down-regulated in skin tissues of psoriasis patients
Three gene expression profile (GSE166388, GSE68939, and GSE68937) were analyzed in this study. A total of 19 samples were acquired from 10 skin biopsies of lesional psoriatic skin and 9 healthy skin controls. Detailed information about each databases is presented in Table 1.
Table 1.
Gene expression profiles analyzed in this study
Based on the criteria of P<0.05 and |logfc|≥ ± 2, a number of transcription factors were short-listed. As we expected, data from the human protein atlas and GTEX portal indicated that among all nominated transcription factors, BCL11B mRNA expression level was relatively higher in skin and immune cells, which made this gene a potential candidate for the research. Expression changes of BCL11B was then plotted in the analyzed GEO dataset between psoriasis patients and healthy controls (Fig.1).
BCL11B was up-regulated during proliferation and differentiation of HaCaT keratinocytes in synchronized procedure
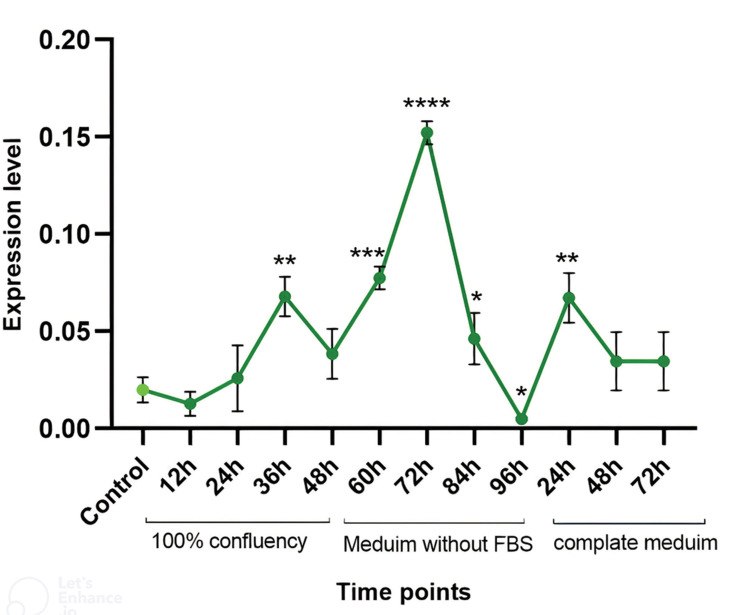
Role of BCL11B was studied under different conditions linking to stress in keratinocytes. For this purpose, the in vitro model of keratinocyte proliferation and differentiation was developed using the chemicalfree synchronized HaCaT keratinocytes. HaCaT cells became quiescent and differentiated in this model, due to the contact inhibition at high proliferation stage, followed by starvation at serum free medium. Passaging the cells release them from quiescent state and synchronous proliferation start by adding serum to the medium. In this study, we investigated kinetic of BCL11B in different aforementioned stages, using qRTPCR test. Analysis of qRT-PCR results indicated that expression level of BCL11B was significantly elevated 24 hours after 2 days of contact inhibition and inducing starvation. This was followed by a sharp decline in BCL11B expression, afterward. However, expression level of BCL11B was significantly increased again in proliferation stage by passaging and addition of FBS to the medium (Fig.2).
Fig.1.
BCL11B expression level in the online databases. A. Single cell sequencing of different skin cell types in the human protein atlas database shows expression level of BCL11B mostly in keratinocytes (website: http://www.proteinatlas.org/). B. Single cell sequencing of different immune cell types in the human protein atlas database shows high expression level of BCL11B in almost all cells of this tissue (website: http://www.proteinatlas.org/). C. GTEX portal online database indicate a high expression level of BCL11B in the skin tissue (website: https://gtexportal.org). D. Differentially expressed analysis of three different microarray dataset using GEO2R indicates a down-regulation of BCL11B among psoriasis skin biopsies and healthy controls.
Fig.2.
Kinetic analysis of BCL11B expression levels in synchronized HaCaT cells. HaCaT cells were grown in DMEM medium containing 10% FBS and 1% PS for 48 hours at 100% confluence. Confluent cells were serum starved for 48 hours by adding DMEM containing 1% PS without FBS. Cells then sub-cultured with complete fresh DMEM and followed for the next 72 hours. All experiments were repeated three times, each in triplicate statistical t test were used and *; P<0.05, **; P<0.01, ***; P<0.001, ****; P<0.0001, h; Hour, DMEM; Dulbecco’s Modified Eagle Medium, PS; Penicillin streptomycin, and FBS; Fetal bovine serum.
Cell cycle and BCL11B expression alteration in stressinduced differentiation
Cytotoxicity of H2 O2 on HaCaT cells was assayed by the MTT method. Results showed that LD50 value was 522 μmol/l H2 O2 for HaCaT cells. Concentration of 150 μmol/l H2 O2 was also for LD10 range (Fig.3).
Fig.3.
Cytotoxicity assessments by MTT in HaCaT cells. HaCaT cells were cultured with different concentrations of H2 O2 (0 to 1000 μmol/l) for 24 hours. Four hours prior to the end of the incubation period, MTT assays was done to quantify metabolic activity.
Expression level of BCL11B was increased significantly 24 hours after using H2 O2 , as a component to increase oxidative stress in HaCaT cells. Then, there was a decrease in the expression level of BCL11B (Fig.4).
To get more detail of potential contribution of BCL11B in cell cycle during oxidative stress, flow cytometry was done in different time points following oxidative stress procedure. The results of flow cytometry analysis indicated a meaningful G1 arrest in oxidative stress especially at 24 hours after treatment by H2 O2 (Fig.4).
Fig.4.
Cell cycle and BCL11B expression alteration in oxidative stress. A. HaCaT cells were cultured and 300 µM/ml H2 O2 were added to the meduim of the cells at the conflueny of 70%. Total RNA was extacted from the cells in different time points. Expression level of BCL11B was measured using Quantitative reverse transcription polymerase chain reaction (qRT-PCR). All experiments were repeated three times, each in triplicate statistical t test were used and ***; P<0.001, ****; P<0.0001. B. Normal control HaCaT cells with equal percentage of G1 and G2 (G1= 46%, G2=48%). C. H2 O2 treated HaCaT cells after 4 hours, percentages of G1 was increased compared to G2 (G1=85%, G2=5.05%). D. H2 O2 treated HaCaT cells after 12 hours, cells arrested more in G1 (G1=89%, G2=5%). E. H2 O2 treated HaCaT cells after 24 hours, highest level of g1 arrest (G1=91%, G2=0.16).
Expression level of BCL11B was increased significantly under heat shock treatment
Briefly, HaCaT cells were seeded at 6 wells plate and incubated at 42°C for 1 hour. One hour after heat shock, the cells were placed in the normal incubation condition of 37°C with 5% CO2 . total RNA was collected in different time points after heat shock treatment between 0-24 hour(s). Results of heat shock treatment on HaCaT cells indicated that expression level of BCL11B was increased significantly 12 hours after treatment, followed by a decrease until 24 hours post-treatment (Fig.5).
Fig.5.
Expression level of BCL11B in different time points of heat shock treatment. HaCaT cells were cultured at the conflueny of 70%, the cells were incubated at 42°C for 60 minutes. The cells were then incubated at normal condition of 37°C and 5% CO2 . Total RNA were then extacted from the cells in different time points. expression level of BCL11B were measured using quantitative reverse transcription polymerase chain reaction (qRT-PCR). All experiments were repeated three times, each in triplicate statistical t-test were used and *; P<0.05 and ***; P<0.001.
Discussion
BCL11B is a transcription factor which plays critical role in development and differentiation of the investigated organs, such as skin (13). Studies demonstrated that BCL11B acts as a regulator of keratinocytes proliferation and differentiation (11). Hyperproliferation and aberrant differentiation of keratinocytes are two most important characters of psoriasis (14). We recruited an in vitro model of keratinocyte proliferation and differentiation, using synchronized keratinocytes HaCaT cell line. In this model, both proliferation and differentiation stages were designed in order to explore expression pattern and probable role of the gene in each stages.
Our results demonstrated a notable up-regulation of BCL11B in differentiation stage in contact inhibition condition with serum free medium which indicated a potential role of BCL11B in keratinocytes differentiation. In addition, there was a remarkable increase of BCL11B expression in proliferation stage of the model system. The results of both differentiation and proliferation stages were well matched with those of previous studies. It has been demonstrated that BCL11B could regulate proliferation and differentiation of epidermal cells, via EGFR and notch signaling pathways (15, 16).
Given that serum starvation makes a stressful condition for cells and psoriasis is well known to connect to stressful conditions for different kinds of cells, especially keratinocytes. For these reasons, we aimed to investigate expression pattern of BCL11B under different conditions related to the stress in psoriasis.
Oxidative stress, which arises due to the imbalance of ROS, is an important factor to induce psoriasis (17). There are different stressful stimuli which can produce ROS to cause oxidative stress and affect skin, including ultraviolet (UV) radiation of the sun and oxygen from the air. Oxidative stress can promote inflammation and ROS can attract and activate neutrophils in the skin which results in reduced antioxidative capacity and leads to psoriasis formation (18). Studies showed that cell cycle arrest at H2 O2 induced oxidative stress condition resulting in DNA damage and cell death (19, 20). Moreover, it was demonstrated that some kind of cells can arrest cycle in G0/G1 and differentiate them (21). Our data demonstrated an induction of G1 cell cycle arrest in H2 O2 treatment, 24 hours after treatment, which was accompanied with an increased level of BCL11B expression at the same time under this treatment. In addition, this results was well matched to the results of keratinocytes synchronization experiment which showed a significant increase in the expression level of BCL11B at 24 hours after serum starvation indicating keratinocyte differentiation.
Studies showed that there was a relationship between BCL11B and STIR1, in which BCL11B- mediated repression in the transcription involved recruitment of STIR1 (22). It has been investigated that expression level of STIR1 was down-regulated in psoriatic skin (17). Considering the role of STIR1 in differentiation (23) and different cellular pathways, including NF-kB pathway, a possible role of BCL11B in stress-induced differentiation conditions, such as psoriasis, is suggested.
For further confirmation on the role of BCL11B in stress-related differentiation of the skin, we also analyzed the expression pattern of the genes under heat-shock stress condition. Several studies reported that expression level of heat-shock proteins was changed in some cells, during differentiation and injury with inflammatory mediators or any other stressful stimuli that affect the skin (24, 25). Our data revealed a significant increase followed by a significant decrease of BCL11B expression level in the keratinocytes, following the heat shock treatment, which indicated its role under heat stressful conditions of the cells.
Conclusion
Given that BCL11B is a bi-functional transcription factor, highly expressed in the skin, it is suggested that any change in the expression pattern of BCL11B could alter expression level of several other genes. We made a cellular monolayer in vitro model of differentiation and proliferation similar to psoriasis, using contact inhibition in the keratinocytes. Confirming the role of BCL11B in this in vitro model, we looked into expression alteration of BCL11B in different stressful conditions related to psoriasis including oxidative stress and heat shock treatment. During different stressful stimulation related to differentiation, similar pattern of BCL11B expression alteration (a significant increase, followed by a significant decrease), along with FACS results, suggested a potential role for BCL11B in the stress induced differentiation skin. It is suggested that BCL11B is needed for differentiation, and when the cells are going through complete or incomplete differentiation process, expression level of the gene will be decreased again. To better understand exact role of BCL11B in the skin stressful condition, more experiments are needed.
Acknowledgements
The authors respectfully thank Dr. Fatemeh Mirzadeh Azad for the help in the process of MTT assay. This manuscript was extracted from the Ph.D. thesis of Sara Parsa and did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors have no conflict of interest to disclose.
Authors’ Contributions
S.P.; Contributed to the study conception and design, performed all of the experiments, data and statistical analysis, and interpreted the data. Z.-S.S., S.J.M.; Supervised the research and help with the study plan. H.A.O., E.B., P.M., E.L.; Advised the research and help with the research progression. All authors read and approved the final manuscript.
References
- 1.Boehncke WH, Schön MP. Psoriasis. Lancet. 2015;386(9997):983–994. doi: 10.1016/S0140-6736(14)61909-7. [DOI] [PubMed] [Google Scholar]
- 2.Billi AC, Gudjonsson JE, Voorhees JJ. Psoriasis: past, present, and future. J Invest Dermatol. 2019;139(11):e133–e142. doi: 10.1016/j.jid.2019.08.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Price BA, Jackson JB. In: Psoriasis. Enna SJ, Bylund DB, editors. New York: Elsevier; 2007. Xpharm the comprehensive pharmacology reference; pp. 1–6. [Google Scholar]
- 4.Benhadou F, Mintoff D, Del Marmol V. Psoriasis: keratinocytes or immune cells - which is the trigger? Dermatology. 2019;235(2):91–100. doi: 10.1159/000495291. [DOI] [PubMed] [Google Scholar]
- 5.Zhou F, Zhu Z, Gao J, Yang C, Wen L, Liu L, et al. NFKB1 mediates Th1/Th17 activation in the pathogenesis of psoriasis. Cell Immunol. 2018;331:16–21. doi: 10.1016/j.cellimm.2018.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Pondeljak N, Lugović-Mihić L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin Ther. 2020;42(5):757–770. doi: 10.1016/j.clinthera.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Lin X, Huang T. Oxidative stress in psoriasis and potential therapeutic use of antioxidants. Free Radic Res. 2016;50(6):585–595. doi: 10.3109/10715762.2016.1162301. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Q, Mrowietz U, Rostami-Yazdi M. Oxidative stress in the pathogenesis of psoriasis. Free Radic Biol Med. 2009;47(7):891–905. doi: 10.1016/j.freeradbiomed.2009.06.033. [DOI] [PubMed] [Google Scholar]
- 9.Damasiewicz-Bodzek A, Szumska M, Tyrpień-Golder K. Antibodies to heat shock proteins 90α and 90β in psoriasis. Arch Immunol Ther Exp (Warsz) 2020;68(2):9–9. doi: 10.1007/s00005-020-00573-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen S, Huang X, Chen S, Yang L, Shen Q, Zheng H, et al. The role of BCL11B in regulating the proliferation of human naive T cells. Hum Immunol. 2012;73(5):456–464. doi: 10.1016/j.humimm.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Daher MT, Bausero P, Agbulut O, Li Z, Parlakian A. Bcl11b/Ctip2 in skin, tooth, and craniofacial system. Front Cell Dev Biol. 2020;8:581674–581674. doi: 10.3389/fcell.2020.581674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golonzhka O, Leid M, Indra G, Indra AK. Expression of COUP-TFinteracting protein 2 (CTIP2) in mouse skin during development and in adulthood. Gene Expr Patterns. 2007;7(7):754–760. doi: 10.1016/j.modgep.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enomoto T, Ohmoto M, Iwata T, Uno A, Saitou M, Yamaguchi T, et al. Bcl11b/Ctip2 controls the differentiation of vomeronasal sensory neurons in mice. J Neurosci. 2011;31(28):10159–10173. doi: 10.1523/JNEUROSCI.1245-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou X, Chen Y, Cui L, Shi Y, Guo C. Advances in the pathogenesis of psoriasis: from keratinocyte perspective. Cell Death Dis. 2022;13(1):81–81. doi: 10.1038/s41419-022-04523-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang LJ, Bhattacharya S, Leid M, Ganguli-Indra G, Indra AK. Ctip2 is a dynamic regulator of epidermal proliferation and differentiation by integrating EGFR and Notch signaling. J Cell Sci. 2012;125(Pt 23):5733–5744. doi: 10.1242/jcs.108969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang X, Bhattacharya S, Bajaj G, Guha G, Wang Z, Jang HS, et al. Delayed cutaneous wound healing and aberrant expression of hair follicle stem cell markers in mice selectively lacking Ctip2 in epidermis. PLoS One. 2012;7(2):e29999–e29999. doi: 10.1371/journal.pone.0029999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu F, Xu J, Xiong X, Deng Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. 2019;24(1):70–74. doi: 10.1080/13510002.2019.1658377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medovic MV, Jakovljevic VL, Zivkovic VI, Jeremic NS, Jeremic JN, Bolevich SB, et al. Psoriasis between autoimmunity and oxidative stress: changes induced by different therapeutic approaches. Oxid Med Cell Longev. 2022;2022:2249834–2249834. doi: 10.1155/2022/2249834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Emmert H, Culley J, Brunton VG. Inhibition of cyclin-dependent kinase activity exacerbates H2 O2 -induced DNA damage in Kindler syndrome keratinocytes. Exp Dermatol. 2019;28(9):1074–1078. doi: 10.1111/exd.14000. [DOI] [PubMed] [Google Scholar]
- 20.Thorn T, Gniadecki R, Petersen AB, Vicanova J, Wulf HC. Differences in activation of G2/M checkpoint in keratinocytes after genotoxic stress induced by hydrogen peroxide and ultraviolet A radiation. Free Radic Res. 2001;35(4):405–416. doi: 10.1080/10715760100300921. [DOI] [PubMed] [Google Scholar]
- 21.Wirt SE, Adler AS, Gebala V, Weimann JM, Schaffer BE, Saddic LA, et al. G1 arrest and differentiation can occur independently of Rb family function. J Cell Biol. 2010;191(4):809–825. doi: 10.1083/jcb.201003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Senawong T, Peterson VJ, Avram D, Shepherd DM, Frye RA, Minucci S, et al. Involvement of the histone deacetylase SIRT1 in chicken ovalbumin upstream promoter transcription factor (COUPTF)- interacting protein 2-mediated transcriptional repression. J Biol Chem. 2003;278(44):43041–43050. doi: 10.1074/jbc.M307477200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heo J, Lim J, Lee S, Jeong J, Kang H, Kim Y, et al. Sirt1 regulates DNA methylation and differentiation potential of embryonic stem cells by antagonizing Dnmt3l. Cell Rep. 2017;18(8):1930–1945. doi: 10.1016/j.celrep.2017.01.074. [DOI] [PubMed] [Google Scholar]
- 24.Jonak C, Klosner G, Trautinger F. Significance of heat shock proteins in the skin upon UV exposure. Front Biosci (Landmark Ed) 2009;14(12):4758–4768. doi: 10.2741/3565. [DOI] [PubMed] [Google Scholar]
- 25.Nixon B, Bromfield EG, Cui J, De Iuliis GN. Heat shock protein A2 (HSPA2): regulatory roles in germ cell development and sperm function. Adv Anat Embryol Cell Biol. 2017;222:67–93. doi: 10.1007/978-3-319-51409-3_4. [DOI] [PubMed] [Google Scholar]