Abstract
Contribution of platelets in tissue regeneration and their possible application in regenerative medicine, which is primarily mediated via secretion of granular components following platelet activation, has been well established in the recent decades. Therefore, platelet rich plasma (PRP), as a portion of plasma with higher concentrations of platelets than the baseline level, is now an attractive therapeutic option in various medical fields mainly for tissue repair and regeneration following injuries. Burn injuries are devastating trauma with high rate of morbidities affecting several aspects of the patient’s life. They require a long-time medical care and high costs. However, even following the best treatment procedures, post-burn scars are inevitable consequence of burn healing process. Therefore, development of new treatment modalities for both burn healing and prevention of post-burn scar establishment seems to be necessary. Regarding the well-known role of PRP in wound healing, here we aimed to provide a comprehensive insight in the possible application of PRP as an adjuvant therapy for the management of burn injuries and subsequent scars. In terms of the following keywords (individually or in combination), original/review articles were searched in PubMed, Scopus, and Google Scholar databases from 2009 to 2021: platelet rich plasma, PRP therapy, platelet biology, platelet function, burn healing, burn scar, scar formation, burn management, wound healing, regenerative medicine. All type of articles or book chapters in English language and relevant data were included in this review. This review initially focused on PRP, its mechanisms of action, preparation methods, and available sources. Then, pathophysiology of burns and subsequent scars were discussed. Finally, their current conventional therapeutic modalities and implication of PRP in their healing process were highlighted.
Keywords: Burns, Platelet Rich Plasma, Wound Healing
Introduction
Platelet rich plasma (PRP) is a portion of plasma with 3-5 times higher concentration of platelets than the baseline level obtained from whole blood (WB) after centrifugation (1). The PRP term was initially created in the 1970s by hematology experts for describing a blood product with high platelet concentrations utilized for its hemostatic characteristics in patients with thrombocytopenia. Later, in the 1980s, application of platelet rich fibrin (PRF) was introduced in maxillofacial surgeries. Subsequently, with increased evidences on the beneficial effects of PRP, interests of PRP application in the both clinical and research settings were grown up.
Contribution of platelets in regenerative medicine and tissue repair makes PRP therapy an attractive option in various medical fields predominantly including sport medicine, orthopedics, dermatology, cosmetology, plastic surgery, ophthalmology, etc. (2-4). In the field of dermatology, PRP is now being successfully used for skin rejuvenation and treatment of several pathological conditions including acne scar, melasma, skin ulcers, periorbital hyperpigmentation, hair loss and alopecia (1). Burn wounds and post-burn scars, which result in significant morbidities and mortalities, may also take advantages of platelet products (5). According to the world health organization (WHO) 11,000,000 burn injuries take globally place every year, while 90% of them involve populations with poor economic conditions (6). Burn injuries are responsible for 180,000 annual deaths worldwide, which is decreasing regarding novel therapeutic strategies particularly in high-income areas, whereby burn induced morbidities has become the focus of treatment in the last decades (7-9). Burn care is a long-time and resource demanding process which often should be performed in specialized centers (6). The application of PRP in the management of burn wounds and post-burn scars has been evaluated in several studies. Here, we tried to have a comprehensive review on the contribution of PRP in the treatment of burns and subsequent scars.
Platelet biology and mechanism of action in regenerative medicine
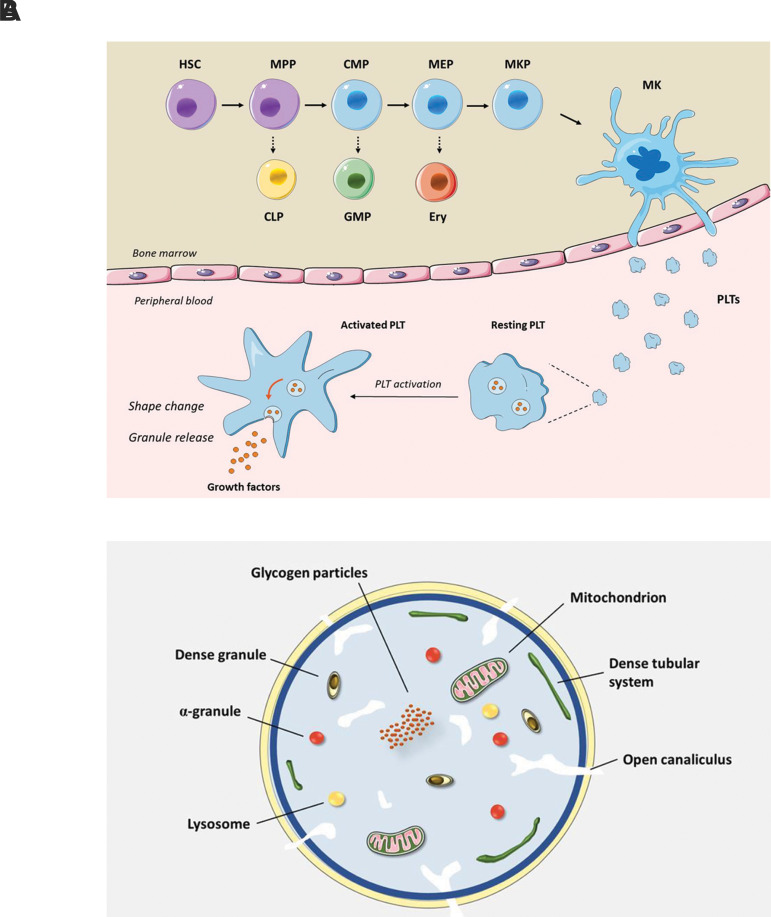
Platelets are small discoid-shaped anucleate cells which circulate in blood with a short lifespan of about 7-10 days. The normal count of platelets ranges from 150,000 to 400,000 per µl. Platelet production, referred to thrombopoiesis, is a precisely-regulated process occurring in the bone marrow, where hematopoietic stem cells differentiate into multiple blood cell lineages including megakaryocytes (Fig .1A). Each mature megakaryocyte, subsequently, produces a vast number of proplatelets which then develop into platelets as small pieces of cytoplasm (10). Platelets contain intracellular organelles and structures such as mitochondria, fragments of endoplasmic reticulum and three main types of granules including α-granules, dense bodies, and lysosomes (Fig .1B).
Fig.1.
Platelet biogenesis, activation and content. A. Megakariopoiesis, platelet production and platelet activation. MKs are developed from HSC in bone marrow. Following MK maturation, a vast number of platelets are produced from each MK, extruded into the peripheral circulation. In normal conditions, platelets are present in a resting state. Upon platelet activation, several events will occur, including but not limited to platelet shape change owing to reorganization of platelet cytoskeleton and granule release. Platelet α-granules contain a variety of growth factors and inflammatory mediators which contribute in tissue regeneration. B. Platelet ultrastructure. Platelets are anucleate cells, containing intracellular structures such as mitochondria and dense tubular system as well as three types of granules including α-granules, dense bodies (also known as δ-granules) and lysosomes. HSC; Hematopoietic stem cell, MPP; Multipotent progenitor, CMP; Common myeloid progenitor, MEP; Megakaryocyte erythroid progenitor, MKP; Megakaryocyte progenitor, MK; Megakaryocyte, CLP; Common lymphoid progenitor, GMP; Granulocyte monocyte progenitor, EryP; Erythroid progenitor, and PLT; Platelet.
Platelet function in regenerative medicine
Beside the pivotal and well-known role of platelets in maintaining hemostasis, their contribution in regenerative medicine and tissue repair have been elucidated in the last decades (11, 12). Therefore, there is a growing interest regarding platelet administration in a wide range of conditions with tissue injuries. The rationale for such applications was mainly ascribed to the vast amount of growth factors and inflammatory mediators, stored in the platelet α-granules (11). Platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), fibroblast growth factor (FGF), epidermal growth factor (EGF), and vascular endothelial growth factor (VEGF) are among the most important proteins secreted by activated platelets, promoting cellular proliferation and differentiation (13). Such factors also contribute in angiogenesis and epithelialization which accelerate tissue repair (9). It has been indicated that PRP contains much more concentrations of growth factors compared to WB; as measured by enzyme-linked immunosorbent assay (ELISA), levels of TGF-β, PDGF and EGF were 7-fold, 30-fold and 10-fold higher than WB, respectively (14).
Another possible mechanism of PRP action ascribed to the bone morphogenic proteins (BMPs), a group of growth factors from TGF-β superfamily which are also present in the platelet α-granules. BMPs are not only involved in development of bones and cartilages, but also contribute in development and differentiation of adipocytes. It has been suggested that specific BMPs can lead to myofibroblast dedifferentiation into adipocytes and therefore they may play a role in scar modulation following PRP administration (15).
Platelet α-granules also contain several inflammatory mediators, including chemokines and interleukins, which in turn modulate recruitment of immune and inflammatory cells to the site of injury. It has been revealed that control and modulation of the local inflammation is one of the main mechanisms of platelet-related tissue repair (16). Platelets also act as key elements in the immune system and play different roles in host defense through several mechanisms (17).
Platelet rich plasma preparation
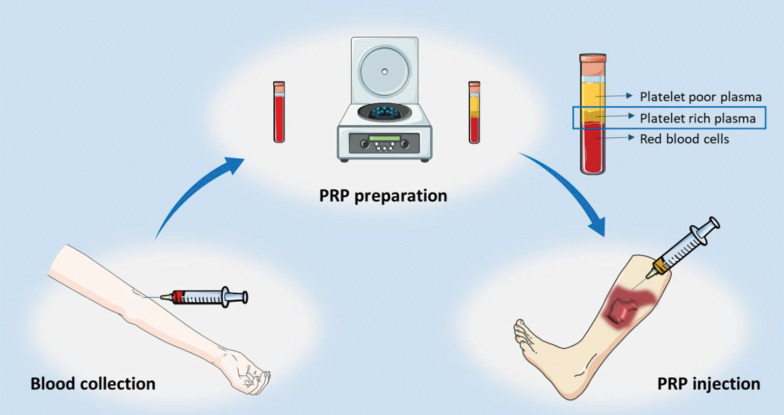
PRP preparation can be performed manually, through automated devices, or commercially available kits which results in different products with diverse components, biological properties and platelet yields (Fig .2) (3). For manual PRP preparation, the venous WB is obtained from the patient and collected into standard bags containing anticoagulant citrate dextrose-A solution (ACD-A). Two main procedures, including PRP or buffy coat method, may be applied for PRP preparation. In PRP method, which is the widely used procedure, WB is firstly encountered a soft spin and then the supernatant containing platelets underwent a hard spin in order to isolate platelet concentrate. The final step includes removing the upper platelet poor plasma (PPP) (18). In contrast, the buffy coat method initiates with high centrifugation of WB which results in three layers, from the lowest to the uppermost, including red blood cells (RBCs), buffy coat and the supernatant PPP. The middle buffy coat layer, containing platelets and leukocytes, is then isolated and undergo a low spin to separate platelets from leukocytes (18). PRP is stable for about 8 hours following preparation (19).
Fig.2.
Process of the platelet rich plasma (PRP) therapy for burn scar. The patient’s blood is collected in a tube and following the preparation of PRP, it is injected near the scar.
Prior to PRP administration, it requires to be activated in order to release the granular contents. Several methods are available for PRP activation among which thrombin and calcium chloride are commonly used. Thrombin, which can be obtained from autologous or bovine source, has also hemostatic features and potential to induce fibroblast proliferation (20).
Platelet rich plasma sources
The majority of studies took advantages of autologous platelets, mainly due to the safety of autologous sources regarding both transmissible infections and antigenic components. Furthermore, they are more affordable and less time taking. Despite the all superiorities of autologous products, they are usually a source of variability resulting in diverse platelet counts and qualities with different levels of growth factors. On the other hand, such sources impose an extra burden to the patients particularly in critically ill patients, which make the application of allogenous products inevitable. Furthermore, allogenic sources should be considered when the use of autologous platelets is a case of contraindication, like the cases with thrombocytopenia or platelet defects, long-term consumption of anti-platelet medications, hematologic disorders, inflammatory or autoimmune conditions, anemia and severe edema, in addition to the cases having trouble with venous access or when there is a patient refusal or discomfort.
Several studies have been denoted to survey the efficacy and safety of allogenic platelets for intentions of regenerative medicine. He et al. (21) investigated safety and effectiveness of allogenic PRP in the healing diabetic ulcers compared to autologous PRP and conventional wound treatment. They indicated that allogenic PRP was also a safe and effective alternative method, resulting in no antibody formation against platelet or HLA class I antigens. Another study, performed by Liao et al. (22), revealed effectiveness of allogenic PRP on chronic refractory wounds. Similar promising findings were obtained in the field of osteoarthritis (23, 24).
The main concern regarding allogenic PRP is risk of contamination or disease transmission. Hence, such studies typically applied leuko-depleted and gammairradiated platelet concentrate from healthy donors which were tested for blood product-transmissible infections including HIV, hepatitis B, hepatitis C, and sometimes treponema pallidum. Furthermore, to avoid immunogenic reactions, ABO, and Rh-D matched unites were usually considered.
It has been indicated that umbilical cord blood (UCB) could be a potential source of platelets, as an effective and available alternative to autologous counterparts. In addition, platelet products can be derived from donated UCB units with low counts of nucleated cells which therefore are not useful for hematopoietic stem cell transplantation and are potentially available as a source of platelets (25). In a multicenter attempt with contribution of 13 public cord blood banks, a standardized procedure for preparation of cryopreserved platelet concentrates from cord blood units has been proposed, facilitating development of clinical trials using cord blood platelets (26).
According to the studies, UCB-derived platelets exert similar or even more extent of functionality as adult products. Murphy et al. (27) revealed that UCB-derived PRP encompassed higher concentrations of some mitogenic and angiogenic factors including PDGF (PDGF-AB and-BB), FGF-2, VEGF and the chemokine RANTES in comparison with adult PRP. Furthermore, UCB-derived PRP resulted in a considerably higher proliferation of human/rat mesenchymal stem cell (MSCs) and represented greater potency for MSC migration. Another study proposed that platelet gel obtained from UCB platelets with high concentrations of VEGF and PDGF-BB could be an appropriate product in regenerative medicine (28). Interestingly, cryopreservation did not impose a negative impact on the level of growth factors. In contrast, it was indicated that amount of growth factors in cryopreserved UCB-derived PRP, compared to the baseline levels, were considerably increased (29).
Another possible source of PRP is lyophilized platelets, although its application in clinical setting is still in its infancy. Lyophilized or freeze-dried platelets are off the shelf products with long-term stability at room temperature (typically several months), which can overcome the limitation of platelet storage and preservation. Freeze-drying process offers a product with standard amount of growth factors and platelet count. In addition, it facilitates availability, storage, handling, and shipping of PRP products, while preserving the platelets bioactivity. Nonetheless, lack of a standardized and optimized freeze-drying procedure, costs of production and subtle risk of contamination, in addition to transmission of infections remains the main challenges for the wide application of lyophilized platelets, implying the necessity of future studies.
Currently, efficacy and safety of lyophilized platelets in clinical practice has been investigated in some studies. In a study conducted by Yeung et al. (30), effectiveness of lyophilized PRP powder produced from allogenous blood units for burn wound healing was surveyed. They enrolled 27 patients with deep second-degree burns including 15 cases and 12 controls who received lyophilized PRP solution or placebo solution. The results were indicative of the benefits of lyophilized PRP for wound closure and healing rate.
Burn caused wounds
The prevalence of burn injuries is higher in lower socioeconomic populations, partly due to the lack of appropriate safety education and preventive measures, such as application of smoke detectors. Generally, an overall decline in the incidence of burn injuries, its severity and related mortality has been indicated in several countries, specifically extremely developed ones. Men have an approximately double prevalence compared to women with a 1.92:1 mean ratio of men/women incidence, which mainly attributed to the different work settings and different free time activities, however the gender distribution is reported almost equal in children (31).
Based on the depth of trauma, burn lesions are classified into four degrees with diverse characteristics requiring different therapeutic interventions. First degree burns, also known as superficial thickness, are benign injuries only involving the epidermis. No blisters and scars are developed in these burns, but are usually very painful. Second degree burns, also named partial thickness, affects dermis and vary from superficial partial thickness to deep partial thickness, the latter usually required surgery. With increasing depth of injury, probability of infection and scar formation raises, but the affected area is less painful. Third degree or full thickness burn injuries are usually painless, but generally need skin grafting. Finally, forth degree burns affect muscles or bones and may results in loss of the injured area (6). Severe burns are devastating and disabling injuries leading to significant morbidities. Annoying pain, risk of infections, sluggish healing process and scarring are considered as the principal challenges in the treatment of burn injuries. Infection is the most frequent reason of death in these patients and therapy-resistant microorganisms and fungi are the most challenging (5). Management of severe burns conventionally includes fluid resuscitation, measures to prevent or treatment of infections, wound excision, skin grafting or applying skin substitute and finally rehabilitation (6).
Implication of platelet rich plasma in the burn wound healing
The effectiveness of PRP application in improving burn injuries have been primarily examined in animal models. By studding the rat models, Venter et al. (32) revealed that treatment with PRP resulted in a faster wound closure in deep second-degree (but not in third-degree) burns, and Lee et al. (33) indicated the efficacy of PRP administration in saving the zone of stasis. From another point of view, Ozcelik et al. (8) evaluated histopathological alterations of the injured tissues following topical PRP application in the rat models with partial thickness burn injuries. Findings were indicative of a significant reduction of infiltration of inflammatory cells in the PRP treated group compared to the controls. But, regarding epithelialization, vascularization, collagen production and fibroblast development, no significant difference was found. However, contribution of PRP in promoting angiogenesis and re-epithelialization of full-thickness surgical wounds has been reported (34). Furthermore, the beneficial point of PRP injection in ameliorating post-burn pain was displayed in the rat models (35, 36).
Beside the animal studies, several clinical investigations (Table 1) were also dedicated to appraising efficacy of PRP therapy in human burn wounds (30, 37, 38). Another study surveyed the effect of allogenic platelet dressing versus silver sulfadiazine in 50 patients with burn wounds, suggesting a better wound healing with improved epithelialization and formation of granulation tissue. Furthermore, a pain reduction was reported by the all patients, following platelet dressing (37).
Table 1.
Some human studies evaluating the role of platelet rich plasma (PRP) in improving burn injuries
|
| |||||
|---|---|---|---|---|---|
| Study (Reference) | Year | Country | Number of patients | Intervention in case group/areas | Intervention in control group/areas |
|
| |||||
| Maghsoudi et al. (37) | 2013 | Iran | 50 | Topical allogenic platelet concentrate | Silver sulfadiazine |
| Aggarwal et al. (38) | 2018 | India | 12 | Subcutaneous injections of autologous PRP + tangential excision and skin graft | Tangential excision and skin graft |
| Yeung et al. (30) | 2018 | China | 27 | Topical spray lyophilized PRP | Topical spray of DW |
|
| |||||
DW; Distilled water.
Post-burn scarring
Scarring is an inevitable consequence of the burn healing process even following the best treatment procedures; however modern treatment modalities can result in less scarring and the improved aesthetic aspects. After a burn injury, particularly depending on depth and severity of damage, normal or pathological scars may occur. Normal scars show higher collagenase activity, while pathologic scars are associated with excess collagen deposition. Furthermore, normal scars are accompanied by lower expression of TGF-β. Pathological scars, following heat injuries, are usually composed of two subtypes, including hypertrophic and keloids. Hypertrophic scarring is almost a common finding post-burns, occurred in 30-90% of injured patients (6). It is considered as a complication of wound healing in dermal injuries (39). The risk of such scarring is augmented in cases with more than three weeks delay in wound healing (14) or persistence inflammation. Hypertrophic scars are red, nodular and stiff scars with increased height resulting from uncontrolled fibroblast proliferation and inordinate collagen production by fibroblasts.
Interestingly, other animals do not develop hypertrophic scars and they are restricted to humans, but the reason is not known (39).
Despite great advances in the field of burn management, pathologic post-burn scarring remains a major challenge. Depending on the severity of burn and the subsequent scarring, it may lead to functional insufficiency, especially if it involves upper extremities (40). Furthermore, disfiguration and change of appearance can have a psychological impact, while they negatively influence body image and the patient’s self-confidence which in turn affect the patient’s quality of life, mental health and reintegration into society (41).
One of the significant and common complications related to post-burn scars is contractures due to decreased elasticity of the periarticular tissues, therefore limiting the range of motion of neighboring joints. Depending on the pattern of formed bands, contractures may be categorized as linear or diffuse (42). The reported incidence of contractures is highly variable among the studies. In a systematic review surveying 10 studies with a total of 6337 patients, prevalence of contractures was 38-54% at discharge, but it was lower after a longer time following burn insult. The study also revealed that severe burn injuries, flame burns, female gender and children were more prone to develop contractures. Furthermore, contractures were more common in upper extremities and cervical spine (43).
Another irritating complication of burn scars is pruritus that may disturb patient’s activities. This has been reported in about 87% of injured patients. Inflammatory mediators and increased number of axons with positive substance P were suggested to be responsible for pruritus (42).
Therefore, many efforts have been advocated in order to ameliorate burn scars through developing new treatment strategies.
Wound healing process and pathophysiology of scar formation
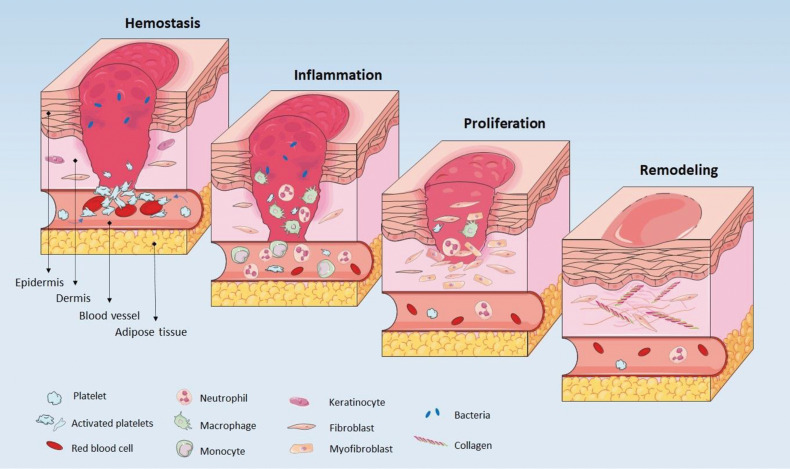
Wound healing is a physiological phenomenon in response to tissue injury which involves a diversity of cells, growth factors, cytokines and mediators (44). It consists of four phases including hemostasis, inflammation, proliferation, and remodeling, which eventually may lead to scar formation (Fig .3). The process is primarily initiated by vasoconstriction, platelet activation and secretion of growth factors, finally leading to fibrin clot formation. In addition to stop bleeding, the constructed fibrin clot provided a transient scaffold for subsequent repair stages (6, 44, 45). Recruitment of neutrophiles, monocytes and macrophages, releasing plenty growth factors, cytokines and chemokines, results in initiation of inflammation. The inflammation stage may last from several days to weeks or even months. Inflammatory cells also remove pathogens and cellular debris (6, 44). The proliferation phase is then initiated by activation of keratinocytes and fibroblasts and it is known by granulation tissue formation, neovascularization and epithelialization. In this stage, transient scaffold is replaced by connective tissue. Ultimately, remodeling or maturation phase occurs, resulted in remodeling of extracellular matrix and enhancement of tensile strength (6, 44). It may last for up to two years (46).
Fig.3.
Four stages of wound healing. Hemostasis is the first step usually followed by inflammation. After fibroblasts proliferation the final step is remodeling.
Impairment of the balance between deposition and destruction of extracellular matrix components results in the formation of excessive scars (47, 48).
Degradation of extra cellular matrix (ECM) and modification of type III collagen to type I collagen occurs in optimal healing conditions, however, in hypertrophic scars, collagen production and/or degradation is disrupted leading to increased type III and decreased type I collagens (48). Increased production of fibronectin and hyaluronic acid, reduced production of decorin and absence of elastin were also observed in post-burn hypertrophic scars. Furthermore, balance of T helper1 (Th1) and T helper 2 (Th2) cells tipped in favor of Th2 response which resulted in a fibrotic environment with increased expression of IL4, IL5, IL10 and thereby TGF-β, increased fibrocyte differentiation to myofibroblast and decreased collagenase activity of fibroblasts (46).
Scar assessment
Evaluation of burn scars necessitates measurement modalities in order to compare the scare quality pre- and post-intervention and determine treatment response. Given this, different evaluation scar scales have been defined which provide quantitative or qualitative measurements (49). The latter type, referred to subjective methods, requires specific devices to assess the physical parameters of scar. Such devices are non-invasive and allows for accurate, reproducible and reliable scar assessment (46).
In contrast, qualitative measurements are observer dependent, who could be a clinician or patient. Different scar scales have been developed for subjective measurements which defined a range of scores for each parameter and thereby provided semiquantitative measurements for subjective methods. There are currently about five designed scare scales, two of which -including vancouver scar scale (VSS) and patient and observer scar assessment scale (POSAS)- are more frequently used for burn scars (49).
Treatment modalities for post-burn scars
Prevention of excessive scar formation appears to be more efficacious than scar treatment (47). Therefore, goal of the recent therapeutic interventions is impeding the profibrotic responses prior to abnormal scar formation, while historical approaches were based on reducing the on-going fibrotic response (50). Immature scars with intact overlaying epithelium are the most successfully treated among hypertrophic scars and keloids. Management of skin scars are primarily based on the experience of clinicians instead of evidence-based studies and clinical trials (47). Various therapeutic procedures have been employed for improving quality of post-burn scars, including surgical and non-surgical treatments (like compression garments, silicone-based products, cryotherapy, radiotherapy, laser and administration of interferon, 5-fluorouracil and intra-lesional corticosteroids). Considering the fact that none of the strategies are individually full effective, they are usually preferred to be applied as part of a combined treatment protocol (42, 46). An illustrative comparison of different nonsurgical methods for treatment of hypertrophic scars is provided in Table 2. Surgical interventions may be helpful in cases with refractory scars which remains unimproved with non-surgical measures (46).
Table 2.
Characteristics of the main non-surgical methods for treatment of hypertrophic burn scars
|
| ||||
|---|---|---|---|---|
| Treatment modality | Mechanisms of action (reference) | Effects (reference) | Possible adverse effects/ Disadvantages (reference) | Other considerations (reference) |
|
| ||||
| Silicone gel sheets | Collagen remodeling by local increasing of oxygen and temperature, hydration, chemical effects, increasing number of mast cells, and polarizing scar tissue (7) | Increased elasticity, decreased pruritus and erythema, decreased scar thickness and height (42), especially effective in fresh scars (47), | Folliculitis, lack of efficacy on mature scars (47) | Effectiveness in preventing burn scars is conflicting (46) |
| Compression garments | Inhibition of TGF-β1 secretion and thereby attenuating fibroblast activity (42), Reduction of collagen production through reducing blood flow at the site of injury and thereby reducing oxygen and nutrients transfer (47) | Decreased scar thickness and hardness, decreased erythema (42) | Low patient compliance and adherence (<40%) because of discomfort, appearance, and movement difficulties (42) | Efficacy is highly depending on the anatomic position of the injured are (47) |
| Skin rash, erosion, pruritus, swelling, skeletal deformities (47) | ||||
| Intralesional corticosteroid injections | Inhibition of inflammation and immunosuppression, vasoconstriction, inhibition of fibroblast and keratinocyte proliferation (47) | Increased pliability, decreased height and volume of scars, decreased pain and pruritus (46, 47) | High rate of adverse effects (up to 60%) including hypopigmentation, skin atrophy, telangiectasias, rebound effects, ineffectiveness and injection pain. | Improved efficacy if combined with other therapies, decreased adverse effects in combination with 5-FU (47) |
| Variable response rate (50-100%), possibility of recurrence (9-50%) (47) | ||||
| Laser and light therapy | Based on the type of laser: collagen remodeling, induction of necrosis in the target capillaries and therefore reduction of vascularity (46) | Increased pliability, decreased erythema, scar height and volume, pain, pruritus, color, and abnormal texture (46) | ||
| Fat grafting | Efficacy is mainly attributed to adipose-derived stem cells that may change fibroblast ECM production (46) | Enhancement of wound closure, increased pliability, decreased fibrosis, decreased scar height and skin hardness (46) | ||
| Interferons (α, β, γ) | Enhancement of collagen breakdown, inhibition of TGF-β and fibrosis by IFN-γ, inhibition of proliferation by IFN-α2b (47) | Improved appearance of scars, decreased recurrence of keloid scars (47) | Painful injections, Flu-like adverse effects (47) | An expensive therapy, but a promising method to treat extreme scarring (47) |
|
| ||||
Implication of platelet rich plasma in scar improvement
Hypertrophic scar pathogenesis, at least in some part, is attributed to high levels of TGF-β, while blockade of TGF-β signaling pathway seems to be a therapeutic strategy for skin scar reduction (51). Efficacy of PRP in scar improvement has been indicated by several studies, although the underlying mechanism of PRP action in such conditions remains to be fully understood. In a pioneer study conducted by Nam and Kim (39), it has been proposed that induction of a negative feedback mechanism of TGF-β1 signaling may be responsible for the PRP function in hypertrophic scars. This hypothesis was originated from in vitro investigations of TGF-β1 and connective tissue growth factor (CTGF) in cultures of primary dermal fibroblasts, while the culture medium was supplemented with PRP or PPP. Accordingly, by adding excessive amounts of TGF-β1, the negative feedback mechanism of TGF-β1 signaling is triggered. This, in turn, results in down-regulation of CTGF gene transcription and subsequently its protein level, finally leading to hypertrophic scar improvement. Impact of CTGF suppression on reducing hypertrophic scars has been demonstrated previously (52).
There are few studies evaluating efficacy of PRP administration in improving burn scars (Table 3). A case of post second-degree burn scar was successfully managed by autologous PRP injections and resulted in appropriate cicatrization and significant decrease in Vancouver and POSAS scores after 10 months followup (53).
Table 3.
Some studies evaluating the role of PRP in improving burn scars
|
| ||||||
|---|---|---|---|---|---|---|
| Study (Reference) | Year | Country | Number of patients | Intervention in case group/area | Intervention in control group/areas | Evaluation method |
|
| ||||||
| Mark et al. (55) | 2016 | Netherlands | 52 | Autologous buffy-coat PRP | SSG alone | POSAS, dermospectrometer, cutometer |
| Elsayed et al. (54) | 2017 | Egypt | 38 | Autologous PRP | Silicone-based products | POSAS |
| Ruiz et al. (53) | 2018 | Colombia | Case report | Autologous PRP | Vancouver and POSAS | |
| Karakol and Bozkurt (40) | 2021 | Turkey | 29 | ROM, POSAS, histopathological | ||
|
| ||||||
PRP; Platelet rich plasma, POSAS; Patient and observer scar assessment scale, and ROM; Range of motion.
In a study conducted on 38 patients with post-burn scars, effectiveness of autologous PRP injections were investigated in comparison with controls receiving silicone-based products in a period of six months follow-up. POSAS scores were suggestive of the better function of PRP in improving scar quality in most of the scar features, particularly itching, pigmentation and pliability parameters. However, thickness of scar tissue showed a better improvement by silicon-based products (54).
However, Marck et al. (55) found no difference in the scar quality of PRP-treated versus control comparable areas in 52 patients with deep burns receiving split skin graft after 12 months follow-up.
It has been indicated that conventional methods, including incision and skin graft, following application of acellular dermal matrix, in combination with PRP and stem cell rich fat injection (as recently developed therapeutic strategies), could be an effective approach in enhancing functional features of burn contractures. The results suggested a substantial improve in the ROM score and reduction of POSAS scores. Furthermore, enhancement of vascularization, collagen deposition and subcutaneous tissue thickness were found in histopathological investigations (40).
Conclusion
According to the literature, there are conflicting consequences on the benefits of PRP application which can be ascribed to a plenty of variables affecting each stage of investigation from study design to report the results. Different number of the enrolled participants with various types and degrees of burn or post-burn scars are main variables in the studies. On the other hand, variation in PRP preparation and administration are the other important factors. Different protocols and techniques, in addition to the lack of standardization give rise to different platelet concentrations with different qualities. Activation of platelets with conserved cellular integrity may be affected by different types of platelet activators, such as thrombin or calcium gluconate. Rout of PRP administration (i.e. subcutaneous/intradermal injections, topical application, and times of administration) may potentially affect efficacy of the intervention. Another crucial source of variation is the combination therapies which also take advantages of the other treatment modalities coincide with PRP application. Ultimately, all of these variations gave rise to conflict findings and made the results less comparable. It highlights the necessity of more standardized and well-defined studies which could provide more reliable insight into the usefulness of PRP in managing burns and the subsequent scars.
Acknowledgements
This work was financially supported by the Royan Stem Cell Technology Company (Tehran, Iran) and Ministry of Science and Higher Education of the Russian Federation within the framework of state support for the creation and development of World-Class Research Centers "Digital biodesign and personalized healthcare" (N. 075-15-2022- 304/5). Maryam Sadat Hosseini, Masoume Nouri, and Morteza Zarrabi are employed by the Royan Stem Cell Technology Company. All the authors declare that they have no conflict of interest.
Authors’ Contributions
M.S.H.; Wrote the manuscript and drew the figures. M.N., M.Z.; Conceived the presented idea and reviewed the manuscript. A.Sh., P.T., M.H., M.J.F.; Developed the concept and edited the manuscript. M.V.; Developed the concept, performed the professional editing, and reviewing, and approved the final version. All authors read and approved the final manuscript.
References
- 1.Merchán WH, Gómez LA, Chasoy ME, Alfonso-Rodríguez CA, Muñoz AL. Platelet-rich plasma, a powerful tool in dermatology. J Tissue Eng Regen Med. 2019;13(5):892–901. doi: 10.1002/term.2832. [DOI] [PubMed] [Google Scholar]
- 2.Alves R, Grimalt R. A review of platelet-rich plasma: history, biology, mechanism of action, and classification. Skin Appendage Disord. 2018;4(1):18–24. doi: 10.1159/000477353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alser OH, Goutos I. The evidence behind the use of platelet-rich plasma (PRP) in scar management: a literature review. Scars Burn Heal. 2018;4:2059513118808773–2059513118808773. doi: 10.1177/2059513118808773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baklaushev VP, Bogush VG, Kalsin VA, Sovetnikov NN, Samoilova EM, Revkova VA, et al. Tissue engineered neural constructs composed of neural precursor cells, recombinant spidroin and PRP for neural tissue regeneration. Sci Rep. 2019;9(1):3161–3161. doi: 10.1038/s41598-019-39341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Beekman J, Hew J, Jackson S, Issler-Fisher AC, Parungao R, et al. Burn injury: challenges and advances in burn wound healing, infection, pain and scarring. Adv Drug Deliv Rev. 2018;123:3–17. doi: 10.1016/j.addr.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 6.Jeschke MG, van Baar ME, Choudhry MA, Chung KK, Gibran NS, Logsetty S. Burn injury. Nat Rev Dis Primers. 2020;6(1):11–11. doi: 10.1038/s41572-020-0145-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Baar ME. In: Textbook on scar management.Cham.Springer. Téot L, Mustoe TA, Middelkoop E, Gauglitz GG, editors. Springer; 2020. Epidemiology of scars and their consequences: burn scars; pp. 37–43. [PubMed] [Google Scholar]
- 8.Ozcelik U, Ekici Y, Bircan HY, Aydogan C, Turkoglu S, Ozen O, et al. Effect of topical platelet-rich plasma on burn healing after partial-thickness burn injury. Med Sci Monit. 2016;22:1903–1909. doi: 10.12659/MSM.895395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shpichka A, Butnaru D, Bezrukov EA, Sukhanov RB, Atala A, Burdukovskii V, et al. Skin tissue regeneration for burn injury. Stem Cell Res Ther. 2019;10(1):94–94. doi: 10.1186/s13287-019-1203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Meijden PEJ, Heemskerk JWM. Platelet biology and functions: new concepts and clinical perspectives. Nat Rev Cardiol. 2019;16(3):166–179. doi: 10.1038/s41569-018-0110-0. [DOI] [PubMed] [Google Scholar]
- 11.De Pascale MR, Sommese L, Casamassimi A, Napoli C. Platelet derivatives in regenerative medicine: an update. Transfus Med Rev. 2015;29(1):52–61. doi: 10.1016/j.tmrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Etulain J. Platelets in wound healing and regenerative medicine. Platelets. 2018;29(6):556–568. doi: 10.1080/09537104.2018.1430357. [DOI] [PubMed] [Google Scholar]
- 13.Chicharro-Alcántara D, Rubio-Zaragoza M, Damiá-Giménez E, Carrillo-Poveda JM, Cuervo-Serrato B, Peláez-Gorrea P, et al. Platelet rich plasma: new insights for cutaneous wound healing management. J Funct Biomater. 2018;9(1):10–10. doi: 10.3390/jfb9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arora NS, Ramanayake T, Ren YF, Romanos GE. Platelet-rich plasma: a literature review. Implant Dent. 2009;18(4):303–310. doi: 10.1097/ID.0b013e31819e8ec6. [DOI] [PubMed] [Google Scholar]
- 15.Sayadi LR, Obagi Z, Banyard DA, Ziegler ME, Prussak J, Tomlinson L, et al. Platelet-rich plasma, adipose tissue, and scar modulation. Aesthet Surg J. 2018;38(12):1351–1362. doi: 10.1093/asj/sjy083. [DOI] [PubMed] [Google Scholar]
- 16.Marques LF, Stessuk T, Camargo IC, Sabeh Junior N, dos Santos L, Ribeiro-Paes JT. Platelet-rich plasma (PRP): methodological aspects and clinical applications. Platelets. 2015;26(2):101–113. doi: 10.3109/09537104.2014.881991. [DOI] [PubMed] [Google Scholar]
- 17.Nicolai L, Gaertner F, Massberg S. Platelets in Host defense: experimental and clinical insights. Trends Immunol. 2019;40(10):922–938. doi: 10.1016/j.it.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Dhurat R, Sukesh M. Principles and methods of preparation of platelet-rich plasma: a review and author’s perspective. J Cutan Aesthet Surg. 2014;7(4):189–197. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arora G, Arora S. Platelet-rich plasma-where do we stand today?. A critical narrative review and analysis. Dermatol Ther. 2021;34(1):e14343–e14343. doi: 10.1111/dth.14343. [DOI] [PubMed] [Google Scholar]
- 20.Marck RE, Middelkoop E, Breederveld RS. Considerations on the use of platelet-rich plasma, specifically for burn treatment. J Burn Care Res. 2014;35(3):219–227. doi: 10.1097/BCR.0b013e31829b334e. [DOI] [PubMed] [Google Scholar]
- 21.He M, Guo X, Li T, Jiang X, Chen Y, Yuan Y, et al. Comparison of allogeneic platelet-rich plasma with autologous platelet-rich plasma for the treatment of diabetic lower extremity ulcers. Cell Transplant. 2020;29:963689720931428–963689720931428. doi: 10.1177/0963689720931428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liao X, Liang JX, Li SH, Huang S, Yan JX, Xiao LL, et al. Allogeneic platelet-rich plasma therapy as an effective and safe adjuvant method for chronic wounds. J Surg Res. 2020;246:284–291. doi: 10.1016/j.jss.2019.09.019. [DOI] [PubMed] [Google Scholar]
- 23.Gato-Calvo L, Magalhaes J, Ruiz-Romero C, Blanco FJ, Burguera EF. Platelet-rich plasma in osteoarthritis treatment: review of current evidence. Ther Adv Chronic Dis. 2019;10:2040622319825567–2040622319825567. doi: 10.1177/2040622319825567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caiaffa V, Ippolito F, Abate A, Nappi V, Santodirocco M, Visceglie D. Allogenic platelet concentrates from umbilical cord blood for knee osteoarthritis: preliminary results. Med Glas (Zenica) 2021;18(1):260–266. doi: 10.17392/1330-21. [DOI] [PubMed] [Google Scholar]
- 25.Orlando N, Pellegrino C, Valentini CG, Bianchi M, Barbagallo O, Sparnacci S, et al. Umbilical cord blood: current uses for transfusion and regenerative medicine. Transfus Apher Sci. 2020;59(5):102952–102952. doi: 10.1016/j.transci.2020.102952. [DOI] [PubMed] [Google Scholar]
- 26.Rebulla P, Pupella S, Santodirocco M, Greppi N, Villanova I, Buzzi M, et al. Multicentre standardisation of a clinical grade procedure for the preparation of allogeneic platelet concentrates from umbilical cord blood. Blood Transfus. 2016;14(1):73–79. doi: 10.2450/2015.0122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy MB, Blashki D, Buchanan RM, Yazdi IK, Ferrari M, Simmons PJ, et al. Adult and umbilical cord blood-derived platelet-rich plasma for mesenchymal stem cell proliferation, chemotaxis, and cryo-preservation. Biomaterials. 2012;33(21):5308–5316. doi: 10.1016/j.biomaterials.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 28.Parazzi V, Lazzari L, Rebulla P. Platelet gel from cord blood: a novel tool for tissue engineering. Platelets. 2010;21(7):549–554. doi: 10.3109/09537104.2010.514626. [DOI] [PubMed] [Google Scholar]
- 29.Baba K, Yamazaki Y, Sone Y, Sugimoto Y, Moriyama K, Sugimoto T, et al. An in vitro long-term study of cryopreserved umbilical cord blood-derived platelet-rich plasma containing growth factors- PDGF-BB, TGF-β, and VEGF. J Craniomaxillofac Surg. 2019;47(4):668–675. doi: 10.1016/j.jcms.2019.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Yeung CY, Hsieh PS, Wei LG, Hsia LC, Dai LG, Fu KY, et al. Efficacy of lyophilised platelet-rich plasma powder on healing rate in patients with deep second degree burn injury: a prospective double-blind randomized clinical trial. Ann Plast Surg. 2018;80(2S Suppl 1):S66–S69. doi: 10.1097/SAP.0000000000001328. [DOI] [PubMed] [Google Scholar]
- 31.Smolle C, Cambiaso-Daniel J, Forbes AA, Wurzer P, Hundeshagen G, Branski LK, et al. Recent trends in burn epidemiology worldwide: a systematic review. Burns. 2017;43(2):249–257. doi: 10.1016/j.burns.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Venter NG, Marques RG, Santos JS, Monte-Alto-Costa A. Use of platelet-rich plasma in deep second- and third-degree burns. Burns. 2016;42(4):807–814. doi: 10.1016/j.burns.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Choi TH, Kim SW. Effect of platelet-rich plasma on burn wounds according to time of application: an experimental study on rats. J Korean Burn Soc. 2011;14(1):1–5. [Google Scholar]
- 34.Xu P, Wu Y, Zhou L, Yang Z, Zhang X, Hu X, et al. Platelet-rich plasma accelerates skin wound healing by promoting re-epithelialization. Burns Trauma. 2020;8:tkaa028–tkaa028. doi: 10.1093/burnst/tkaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang SH, Wu SH, Lee SS, Lin YN, Chai CY, Lai CS, et al. Platelet- rich plasma injection in burn scar areas alleviates neuropathic scar pain. Int J Med Sci. 2018;15(3):238–247. doi: 10.7150/ijms.22563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ren ZQ, Du B, Dong HJ, Duan GH, Du AC, Wang Y, et al. Autologous platelet-rich plasma repairs burn wound and reduces burn pain in rats. J Burn Care Res. 2022;43(1):263–268. doi: 10.1093/jbcr/irab079. [DOI] [PubMed] [Google Scholar]
- 37.Maghsoudi H, Nezami N, Mirzajanzadeh M. Enhancement of burn wounds healing by platelet dressing. Int J Burns Trauma. 2013;3(2):96–101. [PMC free article] [PubMed] [Google Scholar]
- 38.Aggarwal A, Chittoria RK, Dutta S, Reddy KS, Chavan V, Gupta S, et al. Autologous platelet rich plasma-an adjunct to early tangential excision and grafting in burns. Plast Aesthet Res. 2018;5:47–47. [Google Scholar]
- 39.Nam SM, Kim YB. The effects of platelet-rich plasma on hypertrophic scars fibroblasts. Int Wound J. 2018;15(4):547–554. doi: 10.1111/iwj.12896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karakol P, Bozkurt M. Recent strategic approach in postburn extremity scars and contractures. J Plast Surg Hand Surg. 2021;55(3):153–161. doi: 10.1080/2000656X.2020.1856670. [DOI] [PubMed] [Google Scholar]
- 41.Lawrence JW, Mason ST, Schomer K, Klein MB. Epidemiology and impact of scarring after burn injury: a systematic review of the literature. J Burn Care Res. 2012;33(1):136–146. doi: 10.1097/BCR.0b013e3182374452. [DOI] [PubMed] [Google Scholar]
- 42.Willows BM, Ilyas M, Sharma A. Laser in the management of burn scars. Burns. 2017;43(7):1379–1389. doi: 10.1016/j.burns.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 43.Oosterwijk AM, Mouton LJ, Schouten H, Disseldorp LM, van der Schans CP, Nieuwenhuis MK. Prevalence of scar contractures after burn: a systematic review. Burns. 2017;43(1):41–49. doi: 10.1016/j.burns.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 44.Wallace HA, Basehore BM, Zito PM. Wound healing phases. Wound healing phases; 2023. 2023 [Google Scholar]
- 45.Semenov AN, Lugovtsov AE, Shirshin EA, Yakimov BP, Ermolinskiy PB, Bikmulina PY, et al. Assessment of fibrinogen macromolecules interaction with red blood cells membrane by means of laser aggregometry, flow cytometry, and optical tweezers combined with microfluidics. Biomolecules. 2020;10(10):1448–1448. doi: 10.3390/biom10101448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Finnerty CC, Jeschke MG, Branski LK, Barret JP, Dziewulski P, Herndon DN. Hypertrophic scarring: the greatest unmet challenge after burn injury. Lancet. 2016;388(10052):1427–1436. doi: 10.1016/S0140-6736(16)31406-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Arno AI, Gauglitz GG, Barret JP, Jeschke MG. Up-to-date approach to manage keloids and hypertrophic scars: a useful guide. Burns. 2014;40(7):1255–1266. doi: 10.1016/j.burns.2014.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fayzullin A, Ignatieva N, Zakharkina O, Tokarev M, Mudryak D, Khristidis Y, et al. Modeling of old scars: histopathological, biochemical and thermal analysis of the scar tissue maturation. Biology (Basel) 2021;10(2):136–136. doi: 10.3390/biology10020136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fearmonti R, Bond J, Erdmann D, Levinson H. A review of scar scales and scar measuring devices. Eplasty. 2010;10:e43–e43. [PMC free article] [PubMed] [Google Scholar]
- 50.Ladak A, Tredget EE. Pathophysiology and management of the burn scar. Clin Plast Surg. 2009;36(4):661–674. doi: 10.1016/j.cps.2009.05.014. [DOI] [PubMed] [Google Scholar]
- 51.Zhang T, Wang XF, Wang ZC, Lou D, Fang QQ, Hu YY, et al. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed Pharmacother. 2020;129:110287–110287. doi: 10.1016/j.biopha.2020.110287. [DOI] [PubMed] [Google Scholar]
- 52.Jensen J, Gentzkow G, Berman G, Senne L, Jewell M, Connall TP, et al. Anti-CTGF oligonucleotide reduces severity of postsurgical hypertrophic scars in a randomized, double-blind, within-subject, placebo-controlled study. Plast Reconstr Surg. 2018;142(2):192e–201e. doi: 10.1097/PRS.0000000000004590. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz A, Cuestas D, Garcıa P, Quintero J, Forero Y, Galvis I, et al. Early intervention in scar management and cutaneous burns with autologous platelet-rich plasma. J Cosmet Dermatol. 2018;17(6):1194–1199. doi: 10.1111/jocd.12554. [DOI] [PubMed] [Google Scholar]
- 54.Elsayed M, Moaty MA, Moghazy A, Eldeen OS. Evaluation of the effect of platelet-rich plasma on post-burn scars. J Surg. 2017;5(2):6–10. [Google Scholar]
- 55.Marck RE, Gardien KL, Stekelenburg CM, Vehmeijer M, Baas D, Tuinebreijer WE, et al. The application of platelet-rich plasma in the treatment of deep dermal burns: a randomized, double-blind, intra-patient controlled study. Wound Repair Regen. 2016;24(4):712–720. doi: 10.1111/wrr.12443. [DOI] [PubMed] [Google Scholar]