Abstract
Objective:
In spite of the advances in therapeutic modalities, morbidity, due to multiple sclerosis (MS), still remains high. Therefore, a large body of research is endeavouring to discover or develop novel therapies with improved efficacy for treating MS patients. In the present study, we examined the immunomodulatory effects of apigenin (Api) on peripheral blood mononuclear cells (PBMCs) isolated from MS patients. We also developed an acetylated form of Api (apigenin- 3-acetate) to improve In its blood-brain barrier (BBB) permeability. Additionally, we compared its anti-inflammatory properties to original Api and methyl-prednisolone-acetate (a standard therapy), as a potential option in treating MS patients.
Materials and Methods:
The current study was an experimental-interventional research. The half maximal inhibitory concentration (IC50) values for apigenin-3-acetate, apigenin, and methyl-prednisolone-acetate were determined in healthy volunteers’ PBMCs (n=3). Gene expressions of T-box transcription factor (TBX21 or T-bet) and IFN-γ, as well as proliferation of T cells isolated from MS patients’ PBMCs (n=5), were examined in co-cultures of apigenin-3-acetate, Api and methyl-prednisolone-acetate after 48 hours of treatment, using quantitative reverse transcription polymerase chain reaction (qRT-PCR).
Results:
Our findings showed that apigenin-3-acetate, apigenin, and methyl-prednisolone-acetate at concentrations of 80, 80, and 2.5 M could inhibit Th1 cell proliferation after 48 hours (P=0.001, P=0.036, and P=0.047, respectively); they also inhibited T-bet (P=0.015, P=0.019, and P=0.022) and interferon-γ (IFN-γ) gene expressions (P=0.0001).
Conclusion:
Our findings suggested that Api may have anti-inflammatory properties, possibly by inhibiting proliferation of IFN-producing Th1 cells. Moreover, comparative immunomodulatory effects were found for the acetylated version of apigenin-3-acetate versus Api and methyl-prednisolone-acetate.
Keywords: Apigenin, Apoptosis, Multiple Sclerosis, Proliferation, Th1
Introduction
Multiple sclerosis (MS), as the most common source of neurological disability among adults, is an autoimmunemediated inflammatory disease that affects central nervous system (CNS) and results in serious physical or cognitive disabilities (1). The disease can develop at any age and even may affect children or elderly people, but it most often occurs in adults in their 20 and 30 seconds, where women are twice as likely to suffer from MS compared to men (2-4). Neurologists have suggested four classes or subtypes for MS patients, comprising relapsing-remitting MS (RRMS), primary progressive MS (PPMS), secondary progressive MS (SPMS), and progressive relapsing MS (PRMS) (5). The first subtype accounts for almost 85% of MS cases. It is characterized by episodes of reversible acute attacks, followed by progressive neurological remission periods (6).
MS is mediated mainly by pathogenic T cells that target myelin antigens (self-antigens), thereby inducing a broad spectrum of neurodegenerative processes that subsequently result in development of the disease. A dysregulated activity of the several subtypes of T cells, including T-helper 1, T-helper 17, and regulatory T cells [interferon-γ (IFN-γ), interleukin-17 (IL-17), and transforming growth factor-beta (TGF-β)-producing cells, respectively], has been revealed to show a role in MS pathogenesis. Notably, IFN-γ-producing CD4+ T cells (primarily Th1 cells) have been identified in the brain tissue of individuals suffering from the disease early stages. Hence, they are assumed to play crucial roles in orchestrating inflammatory responses, leading to the recruitment and stimulation of immune cells, which negatively impact physiological activities of the oligodendrocytes (7, 8).
With advances in our knowledge of the pathobiology of MS, several therapies have been introduced for the management of affected patients. However, considering the relapsing nature of MS and less responsiveness to or serious side-effects of the currently available anti-inflammatory and immunosuppressive drugs, especially in long-term use (such as heart rate change, flu-like symptoms, rare brain infections, chest pain, hair loss, bladder infection, leukemia occurrence, etc.), unsolved challenges related to the treatment of MS patients still exist and need to be solved (9, 10). Therefore, this is an active research area to devise novel therapies or change existing options to improve their safety and therapeutic potential (11, 12).
During the last decades, herbal compounds or plant extracts with anti-inflammatory effects have widely been studied for potential use in clinical conditions, like MS disease (13-15). Among these compounds, apigenin (4ˊ, 5, 7‑trihydroxyflavone), is an easily extractable plant-derived flavonoid with strong anti-inflammatory characteristics, demonstrated in vitro and in vivo studies (13, 16). Previous studies have reported multiple biological functions for apigenin, such as anti-inflammatory, antioxidative, free radical-scavenging, and anti-carcinogenic effects (13, 17). The hydroxyl groups in flavonoids can form complexes with oxidizing species, allowing these compounds to scavenge and stabilize free radicals and reducing oxidative damage, as a hallmark of many chronic diseases (18, 19). Strikingly, apigenin (Api) has also been proven to counteract the neurodegenerative effects of nitric oxide (NO), inducible nitric oxide synthase (iNOS), and cyclooxygenase-2 (COX-2) (20). Moreover, Api was shown to reduce production of inflammatory cytokines, especially tumor necrosis factor (TNF), IL-6, and IL-1, by inhibiting expression of NF-κB and AP-1 transcription factors (21, 22). The therapeutic potential has also been investigated in several inflammatory conditions comprising alzheimer’s disease (23-25), parkinson’s disease (26), Arthritis (27, 28), lupus (29), etc.
Based on the mentioned notes, in this study, we tested immunomodulatory effects of Api on peripheral blood mononuclear cells (PBMCs) isolated from MS patients. We also developed an acetylated form of Api (apigenin-3-acetate) to improve its blood-brain barrier (BBB) permeability and compared its anti-inflammatory properties to Api and methylprednisolone-acetate (as a standard therapy), as a potential option in treating MS patients.
Materials and Methods
Patients and peripheral blood mononuclear cells isolation
The current study was an experimental-interventional research and it was conducted in the Department of Immunology at the Isfahan University of Medical Sciences, Isfahan, Iran. Patients recruited into this study were those diagnosed according to the revised McDonald criteria (30) and enrolled in the MS center at Kashani Hospital, Isfahan, Iran. The blood samples were only taken from patients with RRMS before their first corticosteroid dose. Patients who had received anti-inflammatory and immunosuppressive drugs before blood sampling, were diagnosed with other inflammatory diseases, or pregnant were excluded.
Ten millilitres of peripheral blood samples were collected from the all subjects (five patients and three healthy donors) in heparinized tubes to isolate PBMCs. According to the manufacturer’s guideline (inno-train Diagnostik GmbH, Germany), PBMCs were isolated using Ficoll-Paque centrifugation and washed with phosphate-buffered saline (PBS, pH=7.3) several times. Afterwards, PBMC cells were centrifuged at 2800 rpm for 25 minutes. In order to perform cell count, a hemocytometer was used and trypan blue dye exclusion (0.4% trypan blue in PBS) was used to assess cell viability. Cells with 98% viability were used for further experiments.
Carboxyfluorescein succinimidyl ester dye labeling
To determine proliferation capacity of PBMCs, carboxyfluorescein diacetate succinimidyl ester (CFSE) staining was performed. For this purpose, cells were suspended in 1 ml of Roswell Park Memorial Institute (RPMI1640, Sigma-Aldrich Co., Ireland) at a concentration of 5×-1× cells/ml. Then, CFSE staining solution at a concentration of 5 mM was added to the cell mixture to make a solution in 5 µM concentration for cell suspension. The cells were incubated in aluminium foil at 37°C, 5% CO2 for 15 minutes, and after that, 10 ml of the whole RPMI medium was added to quench the staining. Then, after twice washing the stained cells with RPMI medium, the cells were used in the following experiments.
Preparation of the pharmaceutical compounds
To prepare a 10000 µM stock solution, powders of apigenin-3-acetate (Api-3A, 5 mg powder, Isfahan Pharmaceutical Sciences Research Center, Iran), Api (5 mg powder, Aktin Chemicals, China), and methylprednisolone-acetate (M-Pre-A, 40 mg ampoule, Kaspean Taemin, Iran) were dissolved in 1.851, 1.262 and 9.615 ml of dimethyl sulphoxide (DMSO), respectively. All subsequent dilutions were prepared in RPMI medium to run Api-3A and Api concentrations of 100, 10, and 1 µM, as well as M-Pre-A concentrations of 5, 0.5, and 0.05 µM.
Dose response and time coursing
Flow cytometry was used to determine amount of inhibition in CD4+ lymphocyte proliferation (IC50) with Api3A concentrations of 1, 10, and 100 µM over 24 hours, 48 hours, and 72 hours. For this purpose, carboxyfluorescein succinimidyl ester dye (CFSE) labelled PBMCs were cultured (/well) in complete RPMI 1640 medium (BIO-IDEA, Iran), containing 10% heat-inactivated fetal bovine serum (FBS, Gibco, USA) and 1% penicillin/ streptomycin (SigmaAldrich, USA), while it was activated with soluble anti-CD3 and anti-CD28 monoclonal antibodies (mAb) OKT-3 (0.1 μg/ml, Mabtech, Sweden). After 24 hours, the cells were restimulated by soluble IL-2 (100 U/ml, Pepro Tech, UK) and simultaneously treated with various concentrations of Api-3A (100, 10, and 1 μM) for different incubation times (24,48, and 72 hours) under normal culture conditions (37°C, 95% humidified atmosphere with 5% CO2). Negative control cells were treated with DMSO and RPMI, instead of a drug. Then, DMSO was added to the negative control with equivalent volumes of the maximum doses of Api-3A.
In the following, after collecting cultured cells from the wells, they were stained with anti-human CD4-Percp. Cy5.5 (Biolegend, USA) at 4°C for 20 minutes. Using flow cytometry, a determinate dose capable of inhibiting 50% of proliferation in the best time course was calculated and nominated by Excel and graphs were provided by GraphPad Prism 6.0 (GraphPad Software, San Diego, CA). IC50 determination for M-Pre-A has also been accomplished using 0.05, 0.5, and 5 µM concentrations in the elected hour mediated by Api-3A with Excel. The time course for realtime polymerase chain reaction (PCR) experiments was also determined by three times pilot testing of the chosen dose at 24, 48, and 72 hours intervals.
FITC Annexin V-Propidium Iodide staining for apoptosis assay and cytotoxicity effects
The cytotoxicity of Api-3A, Api, and M-Pre-A at the optimum dose was evaluated using FITC annexin V-PI (Abcam, USA) double staining. Briefly, PBMCs (cells/ ml) were stimulated with soluble CD3, CD28, and IL-2, treated with their IC50 concentrations, and incubated in 24-well culture plates for the elected time (37°C, 5% CO2 humidified atmosphere). The cells were then harvested, centrifuged, re-suspended in 200 µl of binding buffer, and stained with FITC Annexin V (5 μl/195 µl). The cells were incubated (10 minutes, in darkroom) and were washed with binding buffer. Once more, the cells were re-suspended in 200 µl of binding buffer and stained with PI (10 µl/190 µl). Cell apoptosis and cytotoxicity effects of Api-3A, Api, and M-Pre-A in comparison with DMSO were then analyzed by a FACS Calibur flow cytometry (Becton Dickenson, Bioscience, USA); using FITC signal detector; Ex=488 nm; Em=530 nm for Annexin V (usually FL1) and PI signal detector for PI (FL3), and analyzed by Cell Quest software (Becton Dickenson, Bioscience, USA).
Flow cytometry
After culturing, stimulating, and treating the CFSElabeled PBMCs from MS patients with the elected dose of each drug, they were stained with anti-CD4 and antiCXCR3 surface markers and matched isotype controls as negative controls according to the Biolegend (USA) recommended method, and finally, proliferation of CD4+ , CXCR3+ (Th1) cells were evaluated by flow cytometry using a FACS Calibur (BD, USA) and analyzed by Cell Quest software (BD, USA) in the following.
RNA extraction and cDNA synthesis
Total RNA was extracted from the PBMCs using the Yekta tajhiz azma kit (Yekta tajhiz azma Co., Iran) in accordance with the manufacturer’s instructions. Concentration and purity of the total extracted RNAs were measured by Nano Drop (Biochrom WPA, UK).
Subsequent to the RNA extractions, 9 μl of total RNA was directly reverse transcribed (RT) in a 20 μl final volume, using BioFact MicroRNA reverse transcription kit (Biofact Co., South Korea) in accordance with the manufacturer’s instructions. The 20 μl reactions were incubated in an Applied Biosystems 2720 Thermal Cycler (in a 96-well plate) for 5 minutes at RT , 60 minutes at 50°C, 5 minutes at 95°C and, then held at 4°C. The products were kept at -20°C, for quantitative reverse transcription PCR (qRT-PCR) amplification.
Quantification of TBX21 and IFN-γ gene expressions by quantitative reverse transcription polymerase chain reaction
qRT-PCR analysis of TBX21 and IFN-γ was done using the SYBR Green Master Mix protocol (Biofact Co., South Korea) to detect gene expressions under the following cycling conditions: primary denaturation at 95°C for 15 minutes, 40 cycles of amplification consisting of denaturation at 95°C for 20 seconds and extension at 60°C for 60 seconds, followed by melting curve analysis to verify the qRT-PCR product identity. The 10 µl reaction system, contained 1 μl cDNA, 0.25 μl of each pair of oligonucleotide primers, 3.5 μl deoxyribonuclease (DNase)-free and ribonuclease (RNase)-free water, and 5 μl SYBR Green Master Mix. Each run included a non-template control consisting of 1 µl nuclease-free water instead of cDNA template. The primers were designed by Primer Express 2.0 Software (Perkin-Elmer, USA) and used to quantify T-bet and IFN-γ mRNA:
T-bet:
F: 5ˊ-CAGATGATTGTGCTCCAGTCC-3ˊ
R: 3ˊ-CTGAGTAATCTCGGCATTCTGGTA-5ˊ
IFN-γ:
F: 5ˊ-TGTATTGCTTTGCGTTGGAC-3ˊ
R: 3ˊ-TGACCAGAGCATCCAAAAGA-5ˊ
β-act:
F: 5ˊ-ATAGCACAGCCTGGATAGCAACGTAC-3ˊ
R: 5ˊ-CACCTTCTACAATGAGCTGCGTGTG-3ˊ
Normalization of the gene levels was performed using those of β-act, as an internal control, and relative expression levels were assessed using the method.
Statistics
All results were as mean ± standard error of the mean (SEM). They were analyzed by one-way analysis of variance (ANOVA) and unpaired t tests, followed by KolmogorovSmirnov test to determine normal distribution of data. To compare the groups with non-normal distributions, MannWhitney and Kruskal-Wallis tests were used. All statistical calculations were achieved using GraphPad Prism version 6.0 (GraphPad Software Inc., USA) and SPSS (Version. 22, IBM, USA). The standard level of significance was P<0.05.
Ethics approval
The study was approved by the Ethical Committees of Isfahan University Medical of Science, Isfahan, Iran (IR. MUI.MED.REC.1398.407). Informed consent was obtained from all persons before participating in this study.
Results
Calculation of IC50 for apigenin, apigenin-3-acetate, and methyl-prednisolone-acetate
We determined the half maximal inhibitory concentration (IC50) of newly synthesized apigenin-3-acetate with the PBMCs obtained from healthy donors, who were then followed by testing its anti-inflammatory effects on PBMCs isolated from MS samples (Table 1).
Table 1.
Characteristics of the study participants
|
| ||
|---|---|---|
| Characteristics | MS patients | Healthy volunteers |
|
| ||
| Number | 5 | 3 |
| Age (mean) Y | 22-46 (34.6) | 26-39 (33) |
| Gender (female/male) | 3/2 | 2/1 |
| EDSS | <3 | -- |
|
| ||
MS; Multiple sclerosis and EDSS; The Expanded Disability Status Scale.
IC50 is a measurement method indicating the required amount of a particular drug or other substance to inhibit a given biological process by half. For this purpose, we investigated the IC50 of Api-3A and M-Pre-A by evaluating the effects on the proliferation inhibition of CD4+ lymphocytes isolated from PBMCs of healthy donors. In this regard, the cells were treated with logarithmic escalating concentrations of Api-3A (1, 10, and 100 µM) for 24, 48, and 72 hours, respectively. A drastic reduction in the CD4 cell proliferation was observed after 48 hours of treatment, and the IC50 was calculated at a concentration of 80 µM Api-3A (Fig .1A). The elected dose was adapted for Api investigation, in order to further comparing Api-3A with Api, as its basic compound. Consequently, IC50 determination for M-Pre-A was made after 48 hours of culturing cells in doses of 0.05, 0.5, and 5 µM and it was calculated at a 2.5 µM concentration (Fig .1B). IC50 was calculated using Graphpad prism 6. (GraphPad Software Inc., USA).
Fig.1.
IC50 calculation. CFSE-stained PBMCs of the heathy donors were co-cultured with the escalating logarithmic doses of A. Api-3A (1, 10, and 100) for 24, 48, and 72 hours and B. MPA (0.05, 0.5, and 5) for 48 hours in order to determine the capable dose of inhibiting the CD4 cells proliferation by half using flow cytometry. Data were analyzed by excel to calculate IC50. Graphs were depicted by Prism software. Overlay of graphs were painted by FlowJo 7.6.1 software. 80 µM and 2.5 µM concentrations were calculated IC50 doses of Api-3A and MPA following 48 hours of culture, respectively. Data were pooled from three independent healthy people and expressed as means ± SEM. IC50; Half maximal inhibitory concentration, CFSE; Carboxyfluorescein succinimidyl ester, PBMCs; Peripheral blood mononuclear cells, Api-3A; Apigenin-3 Acetate, and MPA; Methyle Prednisolone acetate.
Apigenin 3-acetate showed apoptotic effects on PBMCs-isolated from MS patients compared to apigenin, and methyl-prednisolone-acetate
FITC annexin V-PI double staining was accomplished in order to identify cytotoxicity of Api-3A, Api, and M-Pre-A on PBMCs using flow cytometry. The cells were incubated for 48 hours after treating with their IC50 concentrations. We did not observe any significant difference in the early and late apoptosis as well as necrosis of PBMCs, when treating with Api-3A (18.79%), Api (17.72%), and M-Pre-A (16.25%) in comparison with DMSO (17.15%, Fig .2).
Fig.2.
Cytotoxicity effects of Apigenin-3 Acetate (Api-3A), Apigenin (Api), and Methyl-Prednisolone-Acetate (M-Pre-A) on PBMCs. A. After 48 hours of treating PBMCs with Api-3A (80 µM), Api (80 µM), and M-Pre-A (2.5 µM), no significant cytotoxicity effects were observed from these three compounds in their IC50 doses on viability of PBMCs, comparing to DMSO (18.79, 17.72, 16.25, and 17.25% of late apoptosis and necrosis for Api-3A, Api, M-Pre-A and DMSO, respectively). B. The upper right, upper left, lower right and lower left quadrants respectively represent late apoptosis, necrosis, early apoptosis and live cells. PBMCs; Peripheral blood mononuclear cells, DMSO; Dimethyl Sulfoxide, and IC50; Half maximal inhibitory concentration.
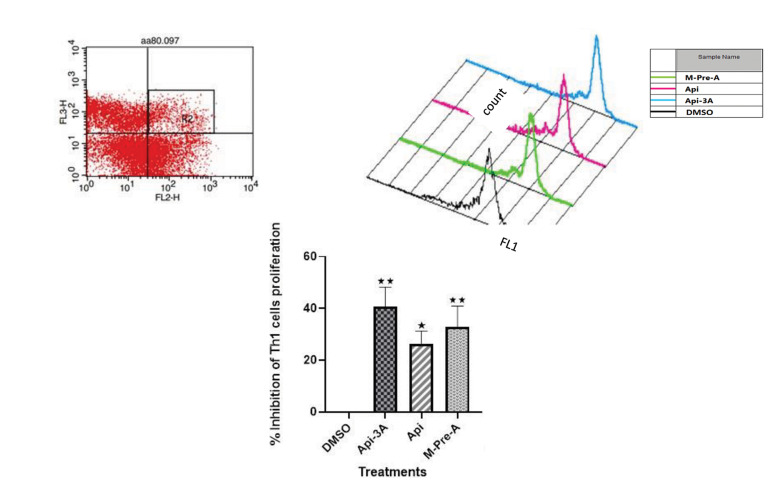
Effects of apigenin-3-acetate, apigenin and methylprednisolone-acetate on the proliferation of Th1 cells
CFSE-stained PBMCs of newly diagnosed MS patients were treated with Api-3A (80 µM), Api (80 µM) and M-Pre-A (2.5 µM) for 48 hours, followed by staining with anti-CD4 and anti-CXCR3 antibodies (for indicating Th1 cells) and assessement by CFSE flow cytometric analysis. Accordingly, Api-3A diminished Th1 proliferation to approximately 41% (P=0.001), while the proliferation reductions by Api and M-Pre-A were approximately 26% and 33% (P=0.036, and P=0.047) respectively, compared to the proliferation inhibition by DMSO as the control. In this experiment inhibition rate of DMSO was normalized to zero, Fig.3).
Fig.3.
Effects of Apigenin-3 Acetate (Api-3A), Apigenin (Api) and Methyl-Prednisolone-Acetate (M-Pre-A) on the proliferation of Th1 cells in MS patients. After 48 hours of incubating the CFSE-stained PBMCs with or without the selected doses of Api-3A, Api, and M-Pre-A (80 µM, 80 µM and 2.5 µM, respectively), the cells were collected, stained with anti-CD4 and anti-CXCR3 antibodies to detect Th1 cells. They were then analyzed by flow cytometer. In this regard, double positive cells were gated and then proliferation percentage of these gated cells were assessed using Cell Quest software comparing to DMSO. Data, represented from five independent experiments for MS patients and expressed as means ± SEM respectively, indicate statistically significant differences respectively between Api-3, Api and M-Pre-A with DMSO (40.61, 26.21, and 32.82% of Th1 cells proliferation inhibition by Api-3A, Api and M-Pre-A, respectively; with the highest rate for Api-3A; P=0.001, P=0.036, and P=0.047). The FL2-H and FL3-H channels respectively represent the cells labeled with anti-CD4 and anti-CXCR3 markers and the FL1-H channel represents CFSE stained cells. MS; Multiple sclerosis, CFSE; Carboxyfluorescein succinimidyl ester, PBMCs; Peripheral blood mononuclear cells, and DMSO; Dimethyl sulfoxide.
Quantitative reverse trascription polymerase chai reaction time coursing
qRT-PCR time coursing of the elected dose in 24, 48 and 72 hours showed that effectiveness and reduction of T-bet (Fig .4) and IFN-γ (Fig .5) genes were much more significant 48 hours post-treatment.
Fig.4.
Effects of Apigenin-3 Acetate (Api-3A), Apigenin (Api) and Methyl-PrednisoloneAcetate (M-Pre-A) on T-bet gene expressions in PBMCs of MS patients. After 48 hours of incubating PBMCs of MS patients with or without the selected doses of Api-3A, Api, and M-Pre-A, relative gene expression of T-bet was measured using qRT-PCR. All components were capable to significantly inhibit expression of T-bet gene expression (0.69, 0.67 and 0.65 reduction of T-bet gene expression by Api-3A, Api and M-Pre-A, respectively; P=0.015, P=0.019, and P=0.022). X-axis represents the relative T-bet gene expression and Y-axis represents the components used for treating cells including DMSO, Api-3A, Api and M-Pre-A. Depicted results are representative of five independent newly diagnosed MS patients and expressed as means ± SEM. PBMCs; Peripheral blood mononuclear cells, MS; Multiple sclerosis, and qRT-PCR; Quantitiative reverse transcriptase polymerase chain reaction.
Fig.5.
Effects of Apigenin-3 Acetate (Api-3A), Apigenin (Api) and MethylPrednisolone-Acetate (M-Pre-A) on IFN-γ gene expressions in PBMCs of MS patients. After 48 hours of incubating the PBMCs of MS patients with or without the selected doses of Api-3A, Api, and M-Pre-A, relative gene expression of IFN-γ gene was measured using qRT-PCR. All components were strongly capable to inhibit expression of IFN-γ gene expression (0.99, 0.97, and 0.98 reduction of IFN-γ gene expression by Api-3A, Api and M-Pre-A, respectively; P<0.0001). X-axis represents relative IFN-γ gene expression and Y-axis represents the components used for treating cells including DMSO, Api-3A, Api, and M-Pre-A. Depicted results are representative of five independent newly diagnosed MS patients and expressed as means ± SEM. IFN-γ; Interferon-γ, PBMCs; Peripheral blood mononuclear cells, MS; Multiple sclerosis, qRT-PCR; Quantitiative reverse transcriptase polymerase chain reaction, and DMSO; Dimethyl sulfoxide.
Apigenin-3 acetate exhibited similar effects on downregulating T-bet in Th1 cells-isolated from MS patients versus apigenin and methyl-prednisolone-acetate
After co-cultureing Th1 cells isolated from MS patients with 80 μM of Api-3A for 48 hours, we examined expression levels of T-bet by qRT-PCR, in order to investigate whether or not Api-3A would affect gene expression in Th1 cells (Fig .4). The same experiments were done with the same doses of Api and M-Pre-A. As it is obvious (Fig .4), Api-3A was able to significantly down-regulate expression of T-bet in Th1 cells isolated from MS patients compared to DMSO (P≤0.001). Down-regulation of T-bet was also significant for 2.5 μM of M-pre-A and 80 μM of Api for 48 hours of treatment, respectively (P=0.015, and P=0.022). However, no significant difference was observed for the expression of T-bet in treating Th1 cells with Api-3A, Api or M-Pre-A. This showed similar potential for Api-3A in reducing T-bet expression compared to its Api counterpart and M-Pre-A as standard therapy
Apigenin-3 acetate significantly down-regulated expression of IFN-γ in Th1 cells-isolated from MS Patients
We also assessed effects of Api-3A on the expression of IFN-γ as the main pro-inflammatory cytokine produced by Th1 cells using qRT-PCR. As shown in Figure 5, treating Th1 cells-isolated from MS patients with Api-3A for 48 hours resulted in a remarkable reduction of IFN-γ expression. Api and M-pre-A were also capable to reduce IFN-γ expression into a significant level (P≤0.0001). However, these findings were more outstanding for Api3A compared to Api and M-Pre-A.
Discussion
Although several studies showed that Api had antiinflammatory and immunomodulatory effects (28, 29), its clinical application in autoimmune diseases such as MS received little attention, most likely due to its low permeability across the BBB. Thus, a strategy to improve Api permeation through the BBB would be of interest and it may introduce a novel candidate for treatment of MS patients. In this regard, in the present study, we developed an acetylated form of Api (Api-3A) and examined its modulatory effects on Th1 cells isolated from MS patients compared to parental Api and methyl prednisolone acetate (M-Pre-A, as a standard option).
The data presented here clearly demonstrated that Api-3A, Api, and M-Pre-A have the potential to inhibit proliferation of Th1 cells in PBMCs of MS patients in a dose-dependent manner. This inhibition in Th1 cell proliferation was much higher when treating them with Api-3A and M-Pre-A rather than Api (40.61, 32.82, and 26.20%, respectively), which points to the same-level or even much higher efficacy of Api-3A compared to the commonly used drug for MS patients (M-Pre-A).
The inhibitory effects of M-pre and Api on PBMC proliferation was proven in the previous study (20). For instance, Leussink et al. (31) concluded that both cell apoptosis and anergy after exposing cells to glucocorticoids (GC), such as Methyl-Prednisolone (M-Pre), could result in inhibition of peripheral blood lymphocyte (PBL) proliferation (especially CD4 T cells), and development of anti-inflammatory conditions. This document is contrary to our results obtained by flow cytometry assessment, which showed inhibition of Th1 proliferation without a significant difference in the apoptosis percentage of PBMCs after treatment with M-Pre-A, compared to the DMSO group. Furthermore, investigating anti-proliferative effect of M-Pre in another autoimmune disease (32, 33) showed that using different doses of M-Pre, at least 48% inhibition of PBMC proliferation was prohibited in Rheumatoid Arthritis (RA) patients. Additiotnally, by compring healthy people and RA patients, it was indicated that size of this prohibitory effect on PBMCs was not significant. This was consistent with our findings indicating that the chosen dose of M-Pre-A resulted in 50 and 33% inhibition of PBMC proliferation, respectively in healthy donors when calculating IC50 and in MS patients.
According to the results reported by Namgoong et al. (34) on 34 different structures, Api was one of the most active flavonoids that could greatly inhibit lymphocyte proliferation. According to their investigations, unsaturation at positions 2 and 3, along with the absence of the hydroxyl group at position 3 in these molecules and other flavonoids, could possibly be very important in their inhibitory effect (35). In our newly synthesized compound, these properties were still retained in proportion to the basic Api. According to these results, Lee et al. (16) concluded that methylation and addition of a lipophilic compound at position 4’ of the Api compound, as well as the presence of this translocation at positions 7 and 4, increased Api inhibitory activity on cell proliferation. As mentioned earlier, the Api-acetate compound contains lipophilic acetate at positions 4ˊ, 5ˊ, and 7, which may be a reason for the better inhibitory effect of Api-acetate on Th1 cell proliferation compared to the basic Api.
On the other hand, in relation to the anti-proliferative efficacy of Api, Xu et al. (36) previously proved that Api caused apoptosis of recurrently activated T cells through regulation of the NF-κB signaling pathway, which has been well known for its importance in survival, proliferation, and T-cell effector functions. Its activation is the common denominator in the anti-apoptotic pathways (37). Eventhough the elected dose in our study was much higher than the under-investigated dose in their study (80 µM versus 12.5 µM), we did not observe any significant difference in the apoptosis percentage of the cells treated with Api compared to the cells treated with DMSO. This represented that the elected dose of Api in our study had appropriate efficacy and yet the lowest apoptosis and cell death rate.
A study by Verbeek et al. (38) showed that two components (Api and luteolin) were more effective in inhibiting proliferation of human and mouse T cells activated against myelin antigens than other flavonoids in the flavanone and flavonol subtypes. This study investigated inhibition of all T lymphocytes and found a significant inhibition of these lymphocytes in the exposure of Api, whereas, in our study, the Th1 subgroup was studied solely indicating lower inhibition of proliferation rate. In the Verbeek study (38), Api could have the inhibitory effect on T lymphocyte proliferation possibly by other members of the lymphocyte family, such as TCD8+ and other T helper cells, including Th17 cells.
Furthermore, the regulatory effect of Api in IFN-γ production has been proven earlier (35, 36). Reduction of the Th1 cells transcription factor (T-bet) and their indicator cytokine (IFN-γ) by Api-3A, Api, and M-Pre-A were also observed in our study. Additionally, it has been proven in previous studies that GCs and M-Pre were responsible for shifting the Th1-dominant cellular responses in autoimmune disease toward Th2-dominant responses (specific efficacy on Th1 cells cytokines and not Th2 cells cytokines). It has been hypothesized that selective effects of M-Pre on Th1 cells might be due to different numbers of GC receptors or different GC receptor affinities in these Th1 and Th2 cells (36-38).
Moreover, more potent inhibitory efficacy of Api-3A, Api and M-Pre-A on IFN-γ gene expression rather a T-bet gene expression as well as T-cell proliferation can be vindicated by the wide-range production of IFN-γ via different kinds of inflammatory cells and pathways in the inflammatory conditions. It can be proposed that multilateral efficacy of these drugs on different immunological cells (including monocytes, macrophages, Th1 cells, T-reg cells and Th17 cells) would result in the significant reduction of IFN-γ. In an interesting study performed by Momcilović et al. (39), it was found that severe decrease of IFN-γ in the presence of methylprednisolone, toward zero by antibodies against this cytokine, would increase IL-17 in the presence or absence of methyl Prednisolone. This means that IFN-γ acted as a negative regulator for IL-17, even in very small amounts, and complete exclusion of its inhibitory affected IL-17. Moreover, by noticing our investigations on IFN-γ gene expression in our study, no significant decrease in the expression of this gene was observed in any of the three-drug combinations; thus, none of them was able to bring it down to zero.
Besides the decreased expression of α4-chain observed in peripheral DCs and T cells (38, 39), and reduction of monocyte adherence to vascular endothelium due to the down-regulations of vascular cell adhesion molecule-1 (VCAM-1), intra-cellular adhesion molecule-1 (ICAM1), and E-selectin upon Api treatment as well as reducing the ability of cells for crossing the BBB, as a result (38, 39). Adding three acetate groups in positions 4, 5, and 7 to the recently produced combination (Api-3A) was investigated in our study, whose increased lipophilicity would be able to increasingly elevate its potential for passing the BBB and more specified effects.
But before any actions, it was necessary to investigate whether or not these recently added acetate groups led to more anti-inflammatory and anti-proliferative effects compared to the basic compound. The results were acceptable. Additionally, significant difference in the inhibitory effects of Api-3A and its parent compound was only observed by evaluating proliferation inhibition; in other evaluating processes, we also faced (although slightly and not significantly) this difference.
Depite realising that the observed efficacy of Api-3A was not solely due to its parent combination and these three acetate groups also played a part in inserting the efficacy, we had to make sure about their conversion in the body (whether they converted to their basic compound or the other unique derivatives) and subsequently insert their efficacies. Perceiving this fact required more in vivo studies in line with our study, which was pioneer for the in vitro phase in the long journey of investigating Api-3A, as a newly synthesized compound.
The limitation of this study was few number of underinvestigated MS patients; it will be better generalized and concluded, if it is more extensively investigated, in the future. On the other hand, in contrast to a 2004 published study demonstrating any beneficial effects on murine EAE by oral flavonoids, including Api and quercetin (20), an updated study by Ginwala indicated a prominent decrease in disease severity and some anti-inflammatory reactions in the treated animals by Api (40). This could be encouraged to continue studying this basic compound and its novel design, Api-3A, in animal models of EAE in vivo as well as the patients with MS, in the future, to ensure that its passage through the BBB is improved, compared to the basic compound without acetylation.
A notable point leading to the confirmation of our results was that we investigated the multilateral anti-immunological effects of Api-3A and its parent compound, Api, in the same dose. So that we could greatly understand their different rates of efficacy. Api-3A had a significant efficacy on Th1 cell proliferation, T-bet and IFN- γ gene expressions, while there was neither significant increase in apoptosis (either early or late apoptosis) nor necrosis compared to the control test. That would increase our reliance on our promising compound. This result would be confirmation of the fact that Api-3A exerted no toxicity or deleterious side effects.
Conclusion
The importance of controlling Th1 cells, as the initiator cells responsible for progression of many treatment steps in MS patients, is well understood. In addition, controlling activity of cells affecting the main site of inflammation, especially brain tissue, would make us able to particularly prevent the general suppression of the immune system and its destructive effects, such as increased susceptibility to a variety of diseases. Therefore, considering the modulatory and anti-inflammatory properties of Api-3A on Th1 cells and the higher possibility of this compound crossing the BBB than the basal compound can make this compound a useful choice in treating MS patients.
Acknowledgements
This work was supported by grants from Faculty of Medicine (396895), and Physiology Research Center of Isfahan University of medical science (298091, Isafahan, Iran), and also pharmaceutical compounds were kindly provided by Isfahan Pharmaceutical Sciences Research Center (Isfahan, Iran). We appreciate the collaboration of all individuals in the study. The authors declare no conflict of interest, financial or otherwise.
Authors’ Contributions
N.K., S.M.Gh., M.R., L.A., N.E.; Carried out the study concept, design and writing of the manuscript. N.K., M.R., L.A.; Carried out the experimental works. N.K., M.R., F.A., A.P., N.E., L.A.; Carried out data collection. N.K., S.M.G., M.R., L.A., R.H., N.E.; Carried out analysis of data and interpretation. All authors read and approved the final manuscript.
References
- 1.Filippi M, Bar-Or A, Piehl F, Preziosa P, Solari A, Vukusic S, et al. Multiple sclerosis. Nat Rev Dis Primers. 2018;4(1):43–43. doi: 10.1038/s41572-018-0041-4. [DOI] [PubMed] [Google Scholar]
- 2.Miclea A, Salmen A, Zoehner G, Diem L, Kamm CP, Chaloulos-Iakovidis P, et al. Age-dependent variation of female preponderance across different phenotypes of multiple sclerosis: a retrospective cross-sectional study. CNS Neurosci Ther. 2019;25(4):527–531. doi: 10.1111/cns.13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jia D, Zhang Y, Yang C. The incidence and prevalence, diagnosis, and treatment of multiple sclerosis in China: a narrative review. Neurol Sci. 2022;43(8):4695–4700. doi: 10.1007/s10072-022-06126-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simpson-Yap S, Atvars R, Blizzard L, van der Mei I, Taylor BV. Increasing incidence and prevalence of multiple sclerosis in the greater hobart cohort of tasmania, Australia. J Neurol Neurosurg Psychiatry. 2022 doi: 10.1136/jnnp-2022-328932. jnnp-2022-328932. [DOI] [PubMed] [Google Scholar]
- 5.Kalincik T. Multiple sclerosis relapses: epidemiology, outcomes and management.A systematic review. Neuroepidemiology. 2015;44(4):199–214. doi: 10.1159/000382130. [DOI] [PubMed] [Google Scholar]
- 6.Giovannoni G, Lang S, Wolff R, Duffy S, Hyde R, Kinter E, et al. A systematic review and mixed treatment comparison of pharmaceutical interventions for multiple sclerosis. Neurol Ther. 2020;9(2):359–374. doi: 10.1007/s40120-020-00212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Amedei A, Prisco D, D’Elios MM. Multiple sclerosis: the role of cytokines in pathogenesis and in therapies. Int J Mol Sci. 2012;13(10):13438–13460. doi: 10.3390/ijms131013438. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 8.Merrill JE, Kono DH, Clayton J, Ando DG, Hinton DR, Hofman FM. Inflammatory leukocytes and cytokines in the peptide-induced disease of experimental allergic encephalomyelitis in SJL and B10.PL mice. Proc Natl Acad Sci USA. 1992;89(2):574–578. doi: 10.1073/pnas.89.2.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasiri N, Rahmati M, Ahmadi L, Eskandari N. The significant impact of apigenin on different aspects of autoimmune disease. Inflammopharmacology. 2018;26(6):1359–1373. doi: 10.1007/s10787-018-0531-8. [DOI] [PubMed] [Google Scholar]
- 10.Landi D, Albanese M, Buttari F, Monteleone F, Boffa L, Rossi S, et al. Management of flu-like syndrome with cetirizine in patients with relapsing-remitting multiple sclerosis during therapy with interferon beta: results of a randomized, cross-over, placebo-controlled pilot study. PLoS One. 2017;12(7):e0165415–e0165415. doi: 10.1371/journal.pone.0165415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Zheng Y, Chen X. Drugs for autoimmune inflammatory diseases: from small molecule compounds to Anti-TNF biologics. Front Pharmacol. 2017;8:460–460. doi: 10.3389/fphar.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keyhanmehr N, Motedayyen H, Eskandari N. The effects of silymarin and cyclosporine A on the proliferation and cytokine production of regulatory Tcells. Immunol Invest. 2019;48(5):533–548. doi: 10.1080/08820139.2019.1571506. [DOI] [PubMed] [Google Scholar]
- 13.Rahmati M, Ghannadian SM, Kasiri N, Ahmadi L, Motedayyen H, Shaygannejad V, et al. Modulation of Th17 proliferation and IL-17A gene expression by acetylated form of apigenin in patients with multiple sclerosis. Immunol Invest. 2021;50(2-3):216–229. doi: 10.1080/08820139.2020.1726381. [DOI] [PubMed] [Google Scholar]
- 14.Afsharzadeh N, Lavi Arab F, Sankian M, Samiei L, Tabasi NS, Afsharzadeh D, et al. Comparative assessment of proliferation and immunomodulatory potential of Hypericum perforatum plant and callus extracts on mesenchymal stem cells derived adipose tissue from multiple sclerosis patients. Inflammopharmacology. 2021;29(5):1399–1412. doi: 10.1007/s10787-021-00838-3. [DOI] [PubMed] [Google Scholar]
- 15.Rasool R, Ullah I, Shahid S, Mubeen B, Imam SS, Alshehri S, et al. In vivo assessment of the ameliorative impact of some medicinal plant extracts on lipopolysaccharide-induced multiple sclerosis in wistar rats. Molecules. 2022;27(5):1608–1608. doi: 10.3390/molecules27051608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, Zhou HY, Cho SY, Kim YS, Lee YS, Jeong CS. Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules. Arch Pharm Res. 2007;30(10):1318–1327. doi: 10.1007/BF02980273. [DOI] [PubMed] [Google Scholar]
- 17.Kim HP, Mani I, Iversen L, Ziboh VA. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fatty Acids. 1998;58(1):17–24. doi: 10.1016/s0952-3278(98)90125-9. [DOI] [PubMed] [Google Scholar]
- 18.Almasi E, Gharagozloo M, Eskandari N, Almasi A, Sabzghabaee AM. Inhibition of apoptosis and proliferation in T Cells by immunosuppressive silymarine. Iran J Allergy Asthma Immunol. 2017;16(2):107–119. [PubMed] [Google Scholar]
- 19.Hougee S, Sanders A, Faber J, Graus YM, van den Berg WB, Garssen J, et al. Decreased pro-inflammatory cytokine production by LPS-stimulated PBMC upon in vitro incubation with the flavonoids apigenin, luteolin or chrysin, due to selective elimination of monocytes/macrophages. Biochem Pharmacol. 2005;69(2):241–248. doi: 10.1016/j.bcp.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 20.Eskandari N, Wickramasinghe T, Peachell PT. Effects of phosphodiesterase inhibitors on interleukin-4 and interleukin-13 generation from human basophils. Br J Pharmacol. 2004;142(8):1265–1272. doi: 10.1038/sj.bjp.0705892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patil RH, Babu RL, Naveen Kumar M, Kiran Kumar KM, Hegde SM, Nagesh R, et al. Anti-inflammatory effect of apigenin on LPSinduced pro-inflammatory mediators and AP-1 factors in human lung epithelial cells. Inflammation. 2016;39(1):138–147. doi: 10.1007/s10753-015-0232-z. [DOI] [PubMed] [Google Scholar]
- 22.Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, et al. Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NFkappaB through the suppression of p65 phosphorylation. J Immunol. 2007;179(10):7121–7127. doi: 10.4049/jimmunol.179.10.7121. [DOI] [PubMed] [Google Scholar]
- 23.Siddique YH, Rahul A, Ara G, Afzal M, Varshney H, Gaur K, et al. Beneficial effects of apigenin on the transgenic Drosophila model of Alzheimer’s disease. Chem Biol Interact. 2022;366:110120–110120. doi: 10.1016/j.cbi.2022.110120. [DOI] [PubMed] [Google Scholar]
- 24.Dourado NS, Souza CDS, de Almeida MMA, Bispo da Silva A, Dos Santos BL, Silva VDA, et al. Neuroimmunomodulatory and neuroprotective effects of the flavonoid apigenin in in vitro models of neuroinflammation associated with alzheimer›s disease. Front Aging Neurosci. 2020;12:119–119. doi: 10.3389/fnagi.2020.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alsadat AM, Nikbakht F, Hossein Nia H, Golab F, Khadem Y, Barati M, et al. GSK-3β as a target for apigenin-induced neuroprotection against Aβ 25-35 in a rat model of Alzheimer’s disease. Neuropeptides. 2021;90:102200–102200. doi: 10.1016/j.npep.2021.102200. [DOI] [PubMed] [Google Scholar]
- 26.Yarim GF, Kazak F, Yarim M, Sozmen M, Genc B, Ertekin A, et al. Apigenin alleviates neuroinflammation in a mouse model of Parkinson’s disease.Int J Neurosci. Int J Neurosci; 2022. pp. 1–10. [DOI] [PubMed] [Google Scholar]
- 27.Li X, Han Y, Zhou Q, Jie H, He Y, Han J, et al. Apigenin, a potent suppressor of dendritic cell maturation and migration, protects against collagen-induced arthritis. J Cell Mol Med. 2016;20(1):170–180. doi: 10.1111/jcmm.12717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun QW, Jiang SM, Yang K, Zheng JM, Zhang L, Xu WD. Apigenin enhances the cytotoxic effects of tumor necrosis factor-related apoptosis- inducing ligand in human rheumatoid arthritis fibroblast-like synoviocytes. Mol Biol Rep. 2012;39(5):5529–5535. doi: 10.1007/s11033-011-1356-3. [DOI] [PubMed] [Google Scholar]
- 29.Kang HK, Ecklund D, Liu M, Datta SK. Apigenin, a non-mutagenic dietary flavonoid, suppresses lupus by inhibiting autoantigen presentation for expansion of autoreactive Th1 and Th17 cells. Arthritis Res Ther. 2009;11(2):R59–R59. doi: 10.1186/ar2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69(2):292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leussink VI, Jung S, Merschdorf U, Toyka KV, Gold R. High-dose methylprednisolone therapy in multiple sclerosis induces apoptosis in peripheral blood leukocytes. Arch Neurol. 2001;58(1):91–97. doi: 10.1001/archneur.58.1.91. [DOI] [PubMed] [Google Scholar]
- 32.Weston MC, Anderson N, Peachell PT. Effects of phosphodiesterase inhibitors on human lung mast cell and basophil function. Br J Pharmacol. 1997;121(2):287–295. doi: 10.1038/sj.bjp.0701115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keyhanmehr N, Motedayyen H, Eskandari N. The effects of silymarin and cyclosporine A on the proliferation and cytokine production of regulatory T cells. Immunol Invest. 2019;48(5):533–548. doi: 10.1080/08820139.2019.1571506. [DOI] [PubMed] [Google Scholar]
- 34.Namgoong SY, Son KH, Chang HW, Kang SS, Kim HP. Effects of naturally occurring flavonoids on mitogen-induced lymphocyte proliferation and mixed lymphocyte culture. Life Sci. 1994;54(5):313–320. doi: 10.1016/0024-3205(94)00787-x. [DOI] [PubMed] [Google Scholar]
- 35.Almasi E, Gharagozloo M, Eskandari N, Almasi A, Sabzghabaee AM. Inhibition of apoptosis and proliferation in T cells by immunosuppressive silymarine. Iran J Allergy Asthma Immunol. 2017;16(2):107–119. [PubMed] [Google Scholar]
- 36.Xu L, Zhang L, Bertucci AM, Pope RM, Datta SK. Apigenin, a dietary flavonoid, sensitizes human T cells for activation-induced cell death by inhibiting PKB/Akt and NF-kappaB activation pathway. Immunol Lett. 2008;121(1):74–83. doi: 10.1016/j.imlet.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shi MD, Shiao CK, Lee YC, Shih YW. Apigenin, a dietary flavonoid, inhibits proliferation of human bladder cancer T-24 cells via blocking cell cycle progression and inducing apoptosis. Cancer Cell Int. 2015;15:33–33. doi: 10.1186/s12935-015-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Verbeek R, Plomp AC, van Tol EA, van Noort JM. The flavones luteolin and apigenin inhibit in vitro antigen-specific proliferation and interferon-gamma production by murine and human autoimmune T cells. Biochem Pharmacol. 2004;68(4):621–629. doi: 10.1016/j.bcp.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Momcilović M, Miljković Z, Popadić D, Marković M, Savić E, Ramić Z, et al. Methylprednisolone inhibits interleukin-17 and interferongamma expression by both naive and primed T cells. BMC Immunol. 2008;9:47–47. doi: 10.1186/1471-2172-9-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ginwala R, Bhavsar R, Chigbu DI, Jain P, Khan ZK. Potential role of flavonoids in treating chronic inflammatory diseases with a special focus on the anti-inflammatory activity of apigenin. Antioxidants (Basel) 2019;8(2):35–35. doi: 10.3390/antiox8020035. [DOI] [PMC free article] [PubMed] [Google Scholar]