Abstract
Objective:
Osteosarcoma (OS) is an uncommon sarcoma with osteoid formation in conjunction with malignant mesenchymal cells on histological examination. SP-8356 has been reported to exhibit anti-cancer properties in human cancers. However the impact of SP-8356 on OS is largely unknown. The metabolic pathways are coordinated by AMPactivated protein kinase (AMPK), which maintains a balance between the supply and demand of nutrients and energy. This study aimed to investigate effect of SP-8356 on proliferation and apoptosis of OS cells and tumor growth in mice. Furthermore, involvement of PGC-1α/TFAM and AMPK-activation was studied.
Materials and Methods:
In the experimental study, Saos-2 and MG63 cells were cultured with SP-8356 for 24 hours and analysed for cellular proliferation using MTT assay. DNA fragmentation was studied using ELISA based kit. Furthermore, transwell chambers assay was used to determine cell migration and cell invasion. Targeted protein expression levels were assessed using western blotting. For in vivo studies, mice (5-6 weeks old) were implanted with either Saos-2 or MG63 cells on dorsal surface subcutaneously and they were administered with SP-8356 (10 mg/kg) for two weeks prior to bone tumor induction.
Results:
We found that SP-8356 exerted anti-proliferative effects on Saos-2 and MG63 cells. Furthermore, SP-8356 treatment significantly restricted migration and invasion of Saos-2 and MG63 cells. Compared to the control, SP-8356 significantly reduced apoptotic cell death, while it increased PGC-1α and TFAM expressions. Without affecting body weight, SP-8356 significantly reduced tumor development in mice, as compared to the control group.
Conclusion:
SP-8356 was found to inhibit proliferation, suppressed cells migration and invasion and decreased OS tumor growth. Furthermore, SP-8356 was found to act through PGC-1α/TFAM and AMPK activations. SP-8356 can be therefore used as therapeutic agent for OS treatment.
Keywords: Apoptosis, Mice, Osteosarcoma, SP-8356, Tumor Growth
Introduction
Osteosarcoma (OS) is a primary mesenchymal malignant bone tumor (1). Adjuvant chemotherapy was first used to treat OS in the 1970s, boosting survival rates to 50%. (2). OS is extremely rare in children ≤ 5 years of age (3). OS infrequently occurs in only a few cases linked to known hereditary disorders in cell cycle regulation, while chromosomal abnormalities are still seen in over 70% of tumor specimens (4, 5).
The essential energy, needed for cellular processes, is produced by mitochondria. Furthermore, mitochondria cause removal of unwanted cells in the programmed manner (6). Dysfunction of mitochondria acts as a potent target for anti-cancer therapies (7). In different human cancers, alterations of mitochondria quantity have been reported. A decreased mitochondrial number has been reported in gastric, breast, renal and liver cancers, while an increased mitochondrial quantity has been found in neck, ovarian and esophageal carcinomas. Increased cancer progression with poor prognosis has been associated with reduced mitochondrial count in breast and hepatocellular carcinoma (8). A lower mitochondrial count has been reported in OS tissues compared to normal tissues (9).
Peroxisome proliferator-activated receptor-gamma coactivator 1ɑ (PGC-1ɑ) has become a crucial component in the mitochondrial biogenesis (10, 11). PGC-1ɑ affected transcriptional activity of numerous crucial mitochondrial genes, like mitochondrial transcription factor A (TFAM), and therefore increased amount of mitochondrial DNA (12). Expression level of TFAM was responsible for maintaining mitochondrial DNA levels (13). PGC-1ɑ up-regulation increased mitochondrial count, and thereby led to apoptosis of sarcoma cells through mitochondrial pathway (14). Thus, it provided strong evidence that regulation of mitochondrial biogenesis could act as therapeutic target for treating sarcoma. Although many chemotherapy techniques are used to treat human sarcomas, these treatments have not been successful for high-grade sarcomas (15). High-grade sarcomas can be treated in a variety of ways, according to the previous reports. Numerous studies have linked decreased mitochondrial numbers to neoplastic transformation and/or tumor growth (16). Several nuclear receptors and transcriptional factors involved in mitochondrial biogenesis are under the control of PGC-1ɑ (17). There have been reports of fewer mitochondria in osteosarcoma. It has been noted that forced mitochondrial augmentation in human sarcoma cell lines results in mitochondrial apoptosis and consequently cell death (18). In human malignancies, alterations in the copy number of mitochondrial DNA (mtDNA) have been thoroughly documented (19, 20). Additionally, TFAM silencing has been linked to mitochondrial dysfunction and cancer development (21).
Previously, it was reported that essential oils containing (1S)-()-verbenone possessed anti-inflammatory effects (22). Furthermore, a wide variety of modified (1S)-()-verbenone have been synthesized to increase their cytoprotective potential with enhanced anti-oxidant and anti-inflammatory properties (23). Cytoprotective effects of a variety of (1S)-()-verbenone derivatives were strengthened by stronger anti-inflammatory and antioxidative activities, and it was discovered that some (1S)-()-verbenone derivatives hindered cell motility via inhibiting NF-B signalling pathways. Certain modified (1S)-()-verbenone derivatives were reported. SP-8356 is a (1S)-()-verbenone derivative that posses anti-cancer properties in human cancers. The present study, therefore aimed to investigate role of SP8356 on proliferation, apoptosis and tumor growth in osteosarcoma.
Materials and Methods
In this experimental, cell death assay kit, DNA extraction kit and MTT reagent were obtained from Sigma Aldrich Co. (USA). All of the antibodies used in the present study were procured from thermo scientific (USA). SP-8356 (Cat No.: 462434, purity: >98%) was obtained from MedKoo Biosciences Inc. (USA). All of the other chemicals and reagents, unless those mentioned, were purchased from Sigma Aldrich Co. (USA).
The Ethics Committee of Hanzhong Hospital of Traditional Chinese Medicine (Hanzhong, China) granted approval to the current experimental investigation under the following number: XZC/20200611.
Cell culture and treatment
Saos-2 and MG63 cell lines were acquired from ATCC. The cells were cultured for 24 hours in DMEM with 10% FBS and antibiotic solution penicillin (100 U/ml) and streptomycin (100 mg/ml), as supplements. In 12-well plates, the cells were either untreated or treated.
MTT assay
MG63 and Saos-2 cells were cultured in 96-well plates (1×103 cells/well). Initially, the cells were cultured for 24 hours and they were later treated with different amount of SP-8356 (2-16 µM) for 24 hours. After 24-hour treatment period, the medium was decanted, and each well received 200 μl of MTT stock solution (5 mg/ml) and further incubated for 4 hours at 37°C. Next, the culture medium was aspirated out and the insoluble emerging formazan crystals were dissolved in 50 μl Dimethylsulfoxide (DMSO). It was subsequently incubated for 30 minutes in an ELISA plate shaker. A microplate reader was used for measuring the absorbance at 570 nm and 650 nm. Calculation of live and dead cell percentages was done as the following formula:
(treated cell absorbance/control cell absorbance)×100.
Cell migration and invasion analyses
Transwell chambers assay was used to monitor rate of cell migration and invasion in Saos-2 and MG63 cells, post SP-8356 treatment. In brief, MG63 and Saos-2 cells were plated with 1×104 cells/well concentration of the upper transwell chambers maintaining Dulbecco’s Modified Eagle medium (DMEM). The cells were treated with variant concentration of SP-8356 viz 4 and 8 µM, and later, pre-treated cells were added to the upper transwell chambers. The lower chambers were supplied with 600 µl of DMEM, bearing 10% fetal bovine serum. Afterwards, the cells were let migrate till 24 hours post-treatment under incubation at 37o C. The un-migrated cells were then swept with a cotton swab. The migrated cells were analyzed and their numbers were counted under a light microscope. Likewise, the invasive capability of MG63 and Saos-2 cells post SP-8356 treatment was determined.
Apoptosis assay
The cells were grown for 24 hours in 96-well plates to perform apoptotic assay. The cells were treated with SP8356 (4 µM or 8 µM). To evaluate apoptosis, a cell death test kit (ELISA based) was utilized based on the proper manufacturer’s protocol.
Protein extraction
Using lysis buffer, Saos-2 and MG63cells cell lysate were prepared (NP-40). Halt Protease Inhibitor Cocktail was employed to stop proteolysis of the cell lysate. To extract supernatant, the cell lysate was centrifuged at 3000 rpm for 10 minutes. Protein concentration was calculated using the Bradford assay.
Western blotting
Preparation of protein samples was performed as described by Ahmad Waza et al. (24). Bradford’s assay was performed to examine concentration of the isolated proteins. Exactly 35 µg of protein samples were isolated using Sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS-PAGE). The resolved proteins were transferred to PVDF membrane (Millipore, USA). To block the membranes, 5% skimmed milk powder was used for one hour. Next, the PVDF membranes were incubated with primary antibodies, like anti-PGC-1α, anti-p-AMPKα (Thr172), anti-TFAM, anti-AMPKα, anti-PARP, anticaspase-3/9 and anti-GAPDH, overnight at 4°C. This was followed by the incubation of the membranes with HRP-conjugated secondary antibody for one hour at room temperature. Finally, the protein bands were detected through chemiluminescence and analyzed with FR-200 system (Shanghai FURI Technology, China).
Quantitative polymerase chain reaction
In cultured cells, examination of amount of mtDNA relative to nuclear DNA was determined to evaluate the mitochondrial number. Genomic DNA was extracted from the cells using DNA extraction kit obtained from Sigma-Aldrich. For amplification of mitochondrial DNA (mtDNA), specific primers were used:
sense: 5ˊ-GCAGATTTGGGTACCACCCAAGTATTGA CTCACCC-3ˊ and
anti-sense: 5ˊ-GCATGGAGAGCTCCCGTGAGTGGT TAATAGGGTGATAG-3ˊ.
The amplification conditions included: initial cycle (94°C for 14 minutes), and 30 cycles of (94°C for 25 seconds, 57°C for 25 seconds, and 70°C for 85 seconds). The ΔΔCt method was used for analysis of mtDNA, relative to nuclear DNA.
Animal model of bone cancer
Thirty BALB/C nude mice were employed in the current investigation. According to the previous description, the mice were used to establish experimental bone tumor model (25). In the current study, three groups of nude mice were used and they were hospitalized for 52 days. Group 1 served as the control group, receiving a vehicle (Sham). Group 2 was induced by bone tumors (Model). Group 3 received SP-8356 (10 mg/kg) until the end of the experiment. After anesthesia with pentobarbital (35 mg/kg), the mice (groups 2 and 3) were subcutaneously implanted with either Saos-2 or MG63 cells at a concentration of 2.0×106 in 400 μl of PBS for the purpose of inducing bone tumors. SP-8356 was administered intraperitoneally to the mice at a dose of 10 mg/kg for 40 days (treated group). The groups of sham mice and model mice received standard saline dosages every day until the end of experiment; a routine body weight checkup was done (25).
Statistical analysis
Statistical analysis was done using SPSS software (IBM SPSS Statistics for Windows, Version 27.0. Armonk, NY: IBM Corp). Mean and standard error of the mean were used to present experimental results. ANOVA was used to determine statistical significance, and a post-hoc test was utilized to account for multiple comparisons (statistical significance was considered P<0.05).
Results
SP-8356 decreased cell proliferation and induced apoptosis
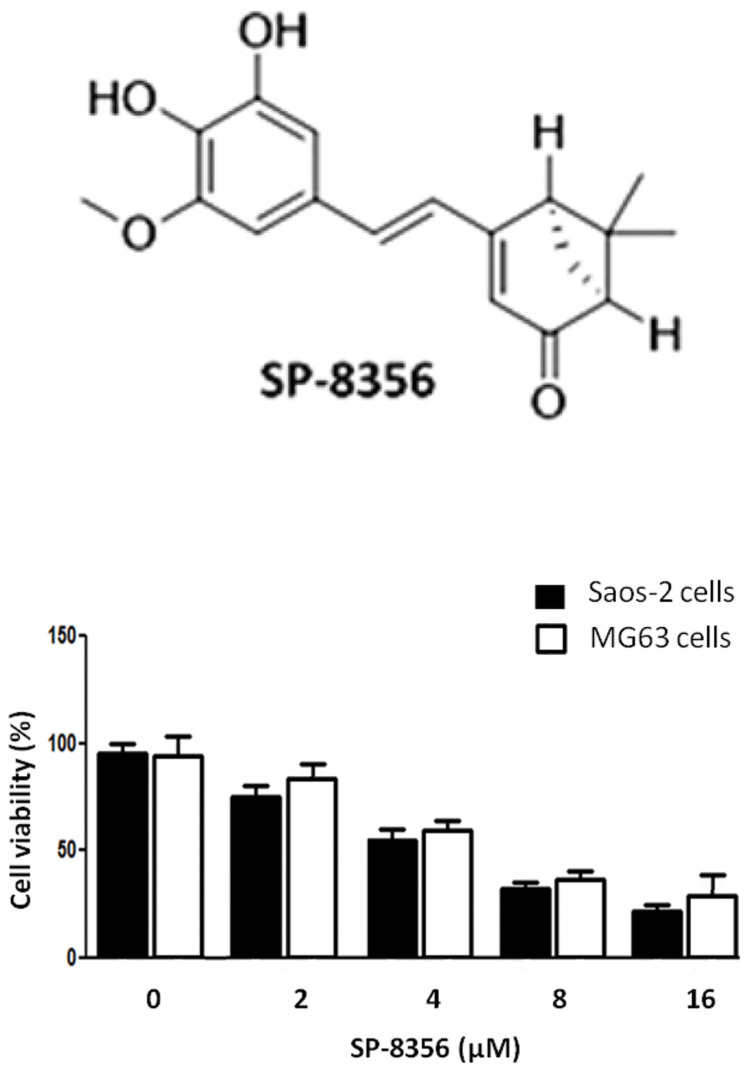
Saos-2 and MG63 cell viability was determined against different doses (2, 4, 8 and 16 μM) of SP-8356 (Fig .1). Increasing concentration of SP-8356 significantly decreased cell viability at 24 hours. SP-8356 dosages of 2, 4, 8 and 16 μM decreased cell viability to 78, 61, 48, and 27 respectively.
Fig.1.
Effect of SP-8356 on Saos-2 and MG63 cell viability. The Saos-2 and MG63 cells with SP-8356 dosages of 2, 4, 8 and 16 μM were incubated for 24 hours, followed by MTT assay. The experiments for each drug concentration were repeated thrice and data were presented as mean ± standard error mean (SEM).
Using the Cell Death Detection ELISA technique, apoptotic cell death was performed to check SP-8356- induced death in Saos-2 and MG63 cells. During apoptosis, the cells released histone and fragmented DNA from the nucleus. Histones and fragmented DNA were measured in the cytoplasm using an ELISA-based assay. Compared to the control, we discovered that SP-8356 treatment (4 and 8 μM) significantly (P<0.05) enhanced apoptosis (Fig .2A).
Fig.2.
Effects of SP-8356 on Saos-2 and MG63 cell apoptotic death and pro-apoptotic proteins. Saos-2 and MG63 cells with SP-8356 dosages of 2, 4, 8 and 16 μM were incubated for 24 hours. A. SP-8356-induced apoptosis was measured using the ELISA-based apoptotic assay. B. Using immunoblotting assay, pro-apoptotic protein expression was analyzed. The internal control was GAPDH. C. Figure shows densitometry analysis of pro-apoptotic proteins. The experiments were repeated thrice and data were presented as mean ± SEM.
To look for the underlying mechanism of SP8356-induced death in Saos-2 and MG63 cells, immunoblotting assay was performed. Following treatment with SP-8356, caspase-3/9 levels and cleaved PARP were increased (Fig .2B). Densitometry analysis (Fig .2C) showed that SP-8356 treatment (4 and 8 μM) increased expression levels of caspase-3 by 2 and 2.8 fold, respectively, compared to the control. Expression level of caspase-9 was increased by 1.7 and 2.4 fold with SP-8356 treatment (4 and 8 μM), compared to the control. Similarly, it was observed that SP-8356 (8 μM) increased expression levels of cleaved PARP by 4 folds, compared to SP-8356 (4 μM).
We discovered that treatment with SP-8356 greatly reduced growth of Saos-2 cells (Fig .1). Furthermore, SP8356 treatment significantly increased cell death in Saos2 cells in dose dependent manner (Fig .2A).
We find that, following treatment with SP-8356 in a dose-dependent manner, the caspase-3 and -9 proteins were significantly up-regulated (Fig .2B). In the current investigation, SP-8356 treatment induced significant PARP cleavage.
SP-8356 inhibited migration and invasion
Tendency of Saos-2 cells to migrate and invade was examined through transwell chambers assay. The results showed that SP-8356 restricted migratory movement of the Saos-2 cells in a concentration dependent manner (Fig .3A). The migrated cells counted at controls were recorded as almost 280 cells which were remarkably reduced to almost 45 cells, post SP-8356 treatment. Similarly, SP-8356 restricted invasive movement of Saos2 cells (Fig .3B). The counted invasive cells at controls were almost 240 cells, while the number was dramatically changed to about 35 cells post SP-8356 treatment.
Fig.3.
Transwell chambers assay. A. Photographs show reduced migration of Saos-2 cells post SP-8356 treatment at the indicated doses. B. Photographs show reduced invasion of Saos-2 cells post SP-8356 treatment at the indicated doses. Graphical representation indicates reduced number of the migrated and invaded cells at individual drug concentration. The experiments were repeated thrice and data were presented as mean ± SEM.
SP-8356 increased p-AMPK expression
To look for expression levels of p-AMPK in SP-8356 treatment cells, SP-8356 dosages of 4 and 8 μM was used for 24 hours. It was observed that SP-8356 treatment (4 and 8 μM) significantly increased expression of p-AMPK (Fig .4A). Densitometry analysis (Fig .4B) showed that SP8356 treatment (4 and 8 μM) increased expression levels of p-AMPK by 1.5 and 3.7 fold, respectively, compared to the control.
Fig.4.
Effect of SP-8356 treatment on p-AMPK expression. The Saos-2 cells with SP-8356 dosages of 4 and 8 μM were incubated for 24 hour. A. With SP-8356 treatment, p-AMPK expression levels were significantly increased. B. Graph shows densitometry analysis of the blot. The experiments were repeated thrice and data were presented as mean ± SEM.
SP-8356 induced mitochondrial proliferation and PGC-1α/TFAM expression
It was found that SP-8356 treatment (4 and 8 μM) significantly increased mtDNA copies in dose dependent manner, at 24 hours (Fig .5A). To look for expression levels of PGC-1ɑ and TFAM in SP-8356 treated cells, SP-8356 dosages of 4 and 8 μM was used for 24 hours. It was observed that SP-8356 treatment (4 and 8 μM) significantly increased expression of PGC-1ɑ (Fig .5B). Densitometry analysis showed that SP-8356 treatment (4 and 8 μM) increased expression levels of PGC-1ɑ by 2.5 and 5.5 fold, respectively, compared to the control (Fig .5C). It was further observed that SP-8356 treatment (4 and 8 μM) significantly increased expression of PGC1ɑ TFAM (Fig .5D). Similarly, SP-8356 treatment (4 and 8 μM) increased expression levels of TFAM by 3 and 6.5 fold, respectively, compared to the control (Fig .5E).
Fig.5.
SP-8356 treatment impact on PGC-1/TFAM expression and mitochondrial proliferation. The Saos-2 cells with SP-8356 dosages of 4 and 8 μM were incubated for 24 hours. A. Treatment with SP-8356 led to an increase in mtDNA copies. B., D. With SP-8356 treatment, a significant rise in PGC-1 and TFAM expression levels was observed. C., E. Images show densitometry analysis of the immunoblots for respectively PGC-1ɑ and TFAM. The experiments were repeated thrice and data were presented as mean ± SEM.
In the current investigation, we noticed a considerable, dose-dependent increase in the number of mtDNA copies, following SP-8356 treatment. It was discovered that PGC-1ɑ regulated mitochondrial biogenesis. According to the reports, TFAM silencing linked mitochondrial malfunction to the development of cancer. Following treatment with SP-8356, higher PGC-1ɑ and TFAM expression levels were seen.
SP-8356 decreased tumor growth in osteosarcoma mice model
In vivo antitumor ability of SP-8356 was determined in mice model of Saos-2 and MG63 cell xenograft (Fig .6A). It was observed that SP-8356 (5 mg/kg) treatment of Saos-2 and MG63 cell implanted models significantly inhibited tumor growth significantly compared to control group. However, it was noted that the mice’s body weight was unaffected by SP-8356 administration during the study period (Fig .6B).
Fig.6.
Effect of SP-8356 on osteosarcoma tumor growth in mice. Saos-2 and MG63 cells were implanted into mice followed by treatment with SP-8356 (5 mg/kg) for 40 days post implantation. Graph shows A. Tumor volume (mm3) and B. Bogy weight (g) post-implantations. The experiments were repeated thrice and data are presented as mean ± SEM.
Discussion
Despite the significant advancements in medicine, majority of the anti-osteosarcoma chemotherapeutics have a limited clinical application due to side-effects on normal cells, lack of sensitivity and selectivity to tumor cells, poor pharmacokinetics, multidrug resistance (MDR), etc (26, 27). Blood-bone marrow barrier also hinders effective delivery of the antitumor medicines to bone (28). Therefore, finding new anti-tumor targets is crucial for effectively treating OS (29). The goal of the current investigation was to identify potential anti-cancer agents in OS cells. We discovered that treatment with SP-8356 greatly reduced growth of Saos-2 cells. Furthermore, SP-8356 treatment significantly increased Saos-2 cell death in dose dependent manner. Our results are consistent with the earlier studies carried out on liver cancer cells (30).
The dynamics of pro- and anti-apoptotic protein expressions primarily demonstrated survival and death of cells. Expression of pro-apoptotic proteins, like caspases, was increased during cell death (31). Following treatment with SP-8356 in a dose-dependent manner, we found that proteins caspases 3 and 9 were significantly upregulated. During apoptosis, PARP proteins (113 kDa) were found to split into two smaller fragments, 89 and 24 kDa (32). In the current investigation, SP-8356 treatment induced significant PARP cleavage. Similar results were obtained earlier (33).
Cell migration and invasion are two cellular processes which aids cancer metastasis and angiogenesis (34). Herein, SP-8356 molecule substantially restricted migratory and invasive movement of the Saos-2 cells. The results showed that SP-8356 restricted migratory movement of Saos-2 cells in a concentration dependent manner. Similarly, SP-8356 restricted invasive movement of the Saos-2 cells. The counted invasive cells at controls were almost 240 cells, while the number was dramatically changed to about 35 cells post SP-8356 treatment.
By phosphorylating threonine 172, AMP-activated protein kinase (AMPK) was activated in response to a variety of metabolic stressors (35, 36). Cell growth was inhibited by AMPK activation via suppression of the mTOR pathway (37, 38). After SP-8356 treatment, we noticed a considerable rise in the expression of p-AMPK.
In human malignancies, alterations in the copy number of mtDNA have been thoroughly documented. In the current investigation, we noticed a considerable, dose-dependent increase in the number of mtDNA copies, following the SP-8356 treatment. It was discovered that PGC-1ɑ regulated mitochondrial biogenesis. According to reports, TFAM silencing linked mitochondrial malfunction to the development of cancer. Following treatment with SP-8356, higher PGC-1ɑ and TFAM expression levels were seen.
Conclusion
We come to the conclusion that SP-8356 inhibited OS cell proliferation by activating p-AMPK, while it caused apoptosis. Additionally, SP-8356 increased PGC-1α and TFAM expression levels as well as mitochondrial biogenesis. Finally, SP-8356 treatment of Saos-2 and MG63 cells implanted in mice model significantly reduced tumor growth. In the current study, SP-8356 emerged as an effective therapeutic agent in OS treatment.
Acknowledgements
There is no financial support and conflict of interest in this study.
Authors’ Contributions
W.C.; Carried out the experimental work. D.Y.; Experimental design, manuscript writing, reviewed and edited the manuscript. X.C., H.Y.; Helped in the experimental works, data analysis and paper writing. All authors read and approved the final manuscript.
References
- 1.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125(1):229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eilber F, Giuliano A, Eckardt J, Patterson K, Moseley S, Goodnight J. Adjuvant chemotherapy for osteosarcoma: a randomized prospective trial. J Clin Oncol. 1987;5(1):21–26. doi: 10.1200/JCO.1987.5.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Kager L, Zoubek A, Dominkus M, Lang S, Bodmer N, Jundt G, et al. Osteosarcoma in very young children: experience of the cooperative osteosarcoma study group. Cancer. 2010;116(22):5316–5324. doi: 10.1002/cncr.25287. [DOI] [PubMed] [Google Scholar]
- 4.Morrow JJ, Khanna C. Osteosarcoma genetics and epigenetics: emerging biology and candidate therapies. Crit Rev Oncog. 2015;20(3-4):173–197. doi: 10.1615/critrevoncog.2015013713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Misaghi A, Goldin A, Awad M, Kulidjian AA. Osteosarcoma: a comprehensive review. SICOT J. 2018;4:12–12. doi: 10.1051/sicotj/2017028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan DC. Mitochondria: dynamic organelles in disease, aging, and development. Cell. 2006;125(7):1241–1252. doi: 10.1016/j.cell.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Neuzil J, Dong LF, Rohlena J, Truksa J, Ralph SJ. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion. 2013;13(3):199–208. doi: 10.1016/j.mito.2012.07.112. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Nomoto S, Fujii T, Kaneko T, Takeda S, Inoue S, et al. Correlation between copy number of mitochondrial DNA and clinico-pathologic parameters of hepatocellular carcinoma. Eur J Surg Oncol. 2006;32(3):303–307. doi: 10.1016/j.ejso.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13(9):935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 10.Scarpulla RC. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim Biophys Acta. 2011;1813(7):1269–1278. doi: 10.1016/j.bbamcr.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeBleu VS, O’Connell JT, Gonzalez Herrera KN, Wikman H, Pantel K, Haigis MC, et al. PGC-1α mediates mitochondrial biogenesis and oxidative phosphorylation in cancer cells to promote metastasis. Nat Cell Biol. 2014;16(10):992-1003, 1-15. doi: 10.1038/ncb3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Puigserver P, Wu Z, Park CW, Graves R, Wright M, Spiegelman BM. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 13.Ekstrand MI, Falkenberg M, Rantanen A, Park CB, Gaspari M, Hultenby K, et al. Mitochondrial transcription factor A regulates mtDNA copy number in mammals. Hum Mol Genet. 2004;13(9):935–944. doi: 10.1093/hmg/ddh109. [DOI] [PubMed] [Google Scholar]
- 14.Polychronopoulos P, Magiatis P, Skaltsounis AL, Myrianthopoulos V, Mikros E, Tarricone A, et al. Structural basis for the synthesis of indirubins as potent and selective inhibitors of glycogen synthase kinase-3 and cyclin-dependent kinases. J Med Chem. 2004;47(4):935–946. doi: 10.1021/jm031016d. [DOI] [PubMed] [Google Scholar]
- 15.Bajpai J, Susan D. Adjuvant chemotherapy in soft tissue sarcomas… Conflicts, consensus, and controversies. South Asian J Cancer. 2016;5(1):15–19. doi: 10.4103/2278-330X.179687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masuike Y, Tanaka K, Makino T, Yamasaki M, Miyazaki Y, Takahashi T, et al. Esophageal squamous cell carcinoma with low mitochondrial copy number has mesenchymal and stem-like characteristics, and contributes to poor prognosis. PLoS One. 2018;13(2):e0193159–e0193159. doi: 10.1371/journal.pone.0193159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cantó C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20(2):98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xie H, Lev D, Gong Y, Wang S, Pollock RE, Wu X, et al. Reduced mitochondrial DNA copy number in peripheral blood leukocytes increases the risk of soft tissue sarcoma. Carcinogenesis. 2013;34(5):1039–1043. doi: 10.1093/carcin/bgt023. [DOI] [PubMed] [Google Scholar]
- 19.O’Hara R, Tedone E, Ludlow A, Huang E, Arosio B, Mari D, et al. Quantitative mitochondrial DNA copy number determination using droplet digital PCR with single-cell resolution. Genome Res. 2019;29(11):1878–1888. doi: 10.1101/gr.250480.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reznik E, Miller ML, Şenbabaoğlu Y, Riaz N, Sarungbam J, Tickoo SK, et al. Mitochondrial DNA copy number variation across human cancers. Elife. 2016;5:e10769–e10769. doi: 10.7554/eLife.10769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Araujo LF, Siena ADD, Plaça JR, Brotto DB, Barros II, Muys BR, et al. Mitochondrial transcription factor A (TFAM) shapes metabolic and invasion gene signatures in melanoma. Sci Rep. 2018;8(1):14190–14190. doi: 10.1038/s41598-018-31170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mander S, Kim DH, Thi Nguyen H, Yong HJ, Pahk K, Kim EY, et al. SP-8356, a (1S)-(-)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-κB signaling. Sci Rep. 2019;9(1):6595–6595. doi: 10.1038/s41598-019-41224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ju C, Song S, Hwang S, Kim C, Kim M, Gu J, et al. Discovery of novel (1S)-(-)-verbenone derivatives with anti-oxidant and antiischemic effects. Bioorg Med Chem Lett. 2013;23(19):5421–5425. doi: 10.1016/j.bmcl.2013.07.038. [DOI] [PubMed] [Google Scholar]
- 24.Ahmad Waza A, Andrabi K, Ul Hussain M. Adenosine-triphosphate- sensitive K+ channel (Kir6.1): a novel phosphospecific interaction partner of connexin 43 (Cx43) Exp Cell Res. 2012;318(20):2559–2566. doi: 10.1016/j.yexcr.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 25.Fu B, Yin G, Song K, Mu X, Xu B, Zhang X. Indirubin-3’-Oxime (IDR3O) inhibits proliferation of osteosarcoma cells in vitro and tumor growth in vivo through AMPK-activation and PGC- 1α/TFAM up-regulation. Dokl Biochem Biophys. 2020;495(1):354–360. doi: 10.1134/S1607672920060022. [DOI] [PubMed] [Google Scholar]
- 26.Wang L, Xue GB. Catalpol suppresses osteosarcoma cell proliferation through blocking epithelial-mesenchymal transition (EMT) and inducing apoptosis. Biochem Biophys Res Commun. 2018;495(1):27–34. doi: 10.1016/j.bbrc.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 27.Xie L, Ji T, Guo W. Anti-angiogenesis target therapy for advanced osteosarcoma (Review) Oncol Rep. 2017;38(2):625–636. doi: 10.3892/or.2017.5735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valkenburg KC, de Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–381. doi: 10.1038/s41571-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu R, Fu C, Sun J, Wang X, Geng S, Wang X, et al. A new perspective for osteosarcoma therapy: proteasome inhibition by MLN9708/2238 successfully induces apoptosis and cell cycle arrest and attenuates the invasion ability of osteosarcoma cells in vitro. Cell Physiol Biochem. 2017;41(2):451–465. doi: 10.1159/000456598. [DOI] [PubMed] [Google Scholar]
- 30.Kim DH, Yong HJ, Mander S, Nguyen HT, Nguyen LP, Park HK, et al. SP-8356, a (1S)-(-)-verbenone derivative, inhibits the growth and motility of liver cancer cells by regulating NF-κB and ERK signaling. Biomol Ther (Seoul) 2021;29(3):331–341. doi: 10.4062/biomolther.2020.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bartke T, Siegmund D, Peters N, Reichwein M, Henkler F, Scheurich P, et al. p53 upregulates cFLIP, inhibits transcription of NF-kappaB-regulated genes and induces caspase-8-independent cell death in DLD-1 cells. Oncogene. 2001;20(5):571–580. doi: 10.1038/sj.onc.1204124. [DOI] [PubMed] [Google Scholar]
- 32.Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31–31. doi: 10.1186/1478-811X-8-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mander S, Kim DH, Thi Nguyen H, Yong HJ, Pahk K, Kim EY, et al. SP-8356, a (1S)-(-)-verbenone derivative, exerts in vitro and in vivo anti-breast cancer effects by inhibiting NF-κB signaling. Sci Rep. 2019;9(1):6595–6595. doi: 10.1038/s41598-019-41224-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fares J, Fares MY, Khachfe HH, Salhab HA, Fares Y. Molecular principles of metastasis: a hallmark of cancer revisited. Signal Transduct Target Ther. 2020;5(1):28–28. doi: 10.1038/s41392-020-0134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat Cell Biol. 2011;13(9):1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.He G, Zhang YW, Lee JH, Zeng SX, Wang YV, Luo Z, et al. AMP-activated protein kinase induces p53 by phosphorylating MDMX and inhibiting its activity. Mol Cell Biol. 2014;34(2):148–157. doi: 10.1128/MCB.00670-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowling RJ, Zakikhani M, Fantus IG, Pollak M, Sonenberg N. Metformin inhibits mammalian target of rapamycin-dependent translation initiation in breast cancer cells. Cancer Res. 2007;67(22):10804–10812. doi: 10.1158/0008-5472.CAN-07-2310. [DOI] [PubMed] [Google Scholar]
- 38.Kimura N, Tokunaga C, Dalal S, Richardson C, Yoshino K, Hara K, et al. A possible linkage between AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR) signalling pathway. Genes Cells. 2003;8(1):65–79. doi: 10.1046/j.1365-2443.2003.00615.x. [DOI] [PubMed] [Google Scholar]