The lining of the normal human tracheobronchial tree is classically described as a pseudostratified, ciliated columnar epithelium. The basic cell types giving rise to this epithelial layer were first described in the mid-1800s, and work during the past five decades has revealed the complexity of the underlying biology (1). Basal cells function as pluripotent stem cells, capable of recapitulating the ciliated epithelium when cultured at an air–liquid interface (ALI) (2). The other major cell types of the conducting airway epithelium include goblet cells, which produce mucus, ciliated cells, and club cells (3). One particularly interesting group of cells found in mouse tracheal epithelium are termed hillock cells. Hillock cells are cuboidal and exist in isolated clumps. Intriguingly, these cells also exhibit markers of squamous differentiation, such as KRT13 (keratin-13) (4). In 2018, a complete transcriptomic analysis of the entire human pulmonary tree revealed subsets of cells exhibiting markers of squamous cell differentiation (5, 6). The significance of these squamous cell markers on pulmonary epithelial function remains unknown.
In this issue of the Journal, Zhang and colleagues (pp. 664–678) provide valuable insight into the development of squamous epithelium in the tracheobronchial tree (7). The authors begin by identifying patches of squamous-appearing cells in samples taken from the posterior trachea of healthy subjects. These areas are confirmed to be squamous epithelium by positive staining for KRT13 and lack of staining for lineage markers of ciliated and goblet cells (Figure 1). Importantly, they examined tracheal sections from other species commonly used in translational studies (mouse, rabbit, and pig) to confirm that this phenomenon is conserved across species.
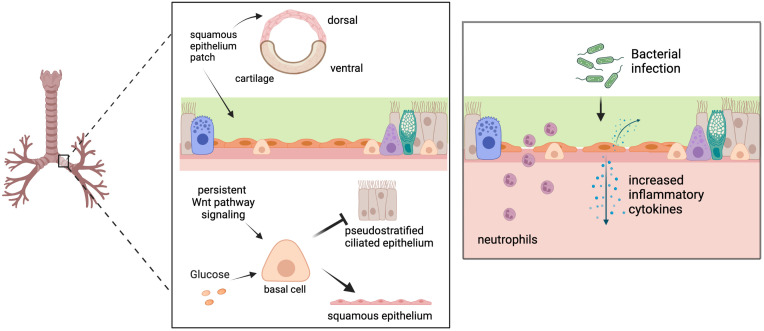
Figure 1.
(Left) In healthy conducting airways, patches of flat, nonmucociliary squamous epithelium exist in dorsal, noncartilaginous regions. Airway basal cells are directed away from differentiation to normal pseudostratified ciliated epithelium and toward squamous cell differentiation (keratin-13–positive) with exposure to prolonged canonical or noncanonical Wnt pathway signaling or high concentrations of glucose. (Right) Airway cells induced to exhibit squamous differentiation lack mucociliary clearance, have disrupted tight-junction protein expression, are unjammed, express and secrete increased inflammatory cytokines, and allow greater neutrophil transepithelial migration following bacterial infection compared with normal pseudostratified airway epithelium. Images created with Biorender.com.
Cells from these regions were expanded at an ALI. However, when grown ex vivo, the cells failed to exhibit squamous morphology. From this finding, the authors reasoned that some signaling process within the in vivo environment must be driving squamous differentiation. They subjected ALI cultures to a broad range of signaling molecules known to stimulate squamous differentiation. Two molecules, CHIR99021 and BIO, produced a recapitulation of the previously seen squamous morphology. Using whole-genome analysis and time-course experiments, the authors demonstrate the exposure to CHIR99021 can induce a complete squamous morphology and that persistent signaling is required (Figure 1).
The use of CHIR99021 to induce squamous differentiation represents a potential weakness of the subsequent experiments. The authors identify this molecule as a Wnt signaling agonist. However, it would be more precise to say that it is a Wnt pathway agonist because it specifically binds and activates GSK3β, which is a critical effector in the canonical Wnt pathway (8, 9). To clear up this potential confusion, Zhang and colleagues isolated the canonical and noncanonical Wnt pathways with constitutively active or knockdown β-catenin constructs. The constitutively active construct induced squamous differentiation in a time-dependent manner as expected. The β-catenin knockdown construct failed to ablate the effect of CHIR99021. This finding complicates interpretation of the prior results. If CHIR99021 were acting solely through the canonical Wnt pathway, β-catenin knockdown should eliminate the effect. Therefore, there exist at least two signaling pathways, β-catenin and CHIR99021-induced signaling, that can result in squamous differentiation.
To clarify the mechanism of squamous differentiation during CHIR99021 stimulation, the authors investigated a variety of antagonists downstream of the canonical and noncanonical Wnt pathway. They identify 12-O-tetracanoylphyorbol-13-acetate, a protein kinase C (PKC) agonist (10), for its ability to antagonize CHIR90021-induced squamous differentiation. Thus, this finding implicates the Wnt/Ca2 +-dependent pathway in squamous differentiation. However, given that PKC is an effector of the noncanonical Wnt pathway, this finding seems to raise more questions than it answers. The authors reason that squamous differentiation is the result of an imbalance between canonical and noncanonical Wnt signaling. This assertion requires further investigation in future studies.
Zhang and colleagues chose to investigate the metabolic effects of canonical and noncanonical Wnt signaling. Both arms of the Wnt signaling cascade regulate aspects of the central metabolic control system, including mTOR and insulin signaling cascades. By stimulating cells grown at an ALI in the presence of rapamycin and metformin, the authors demonstrate that squamous differentiation is primed by alteration in energy metabolism pathways. This observation carries clinical and translational significance.
Translationally, the authors note that several common media used in the culturing of airway epithelial cells at an ALI contain supranormal glucose concentrations. When otherwise healthy airway epithelial cells were cultured in media containing a physiologic glucose concentration, far less squamous differentiation was observed after CHIR99021 stimulation than when cells were cultured using standard ALI media. This finding should raise particular concern for anyone performing airway cell research using ALI models because minor perturbations of glucose concentrations in media may significantly alter cell-differentiation patterns and affect baseline cellular functions as well as responses to exogenous stimuli.
Clinically, Zhang and colleagues investigated the effect on airway barrier function and susceptibility to pulmonary infections. They demonstrate that squamous cell differentiation results in increased inflammation, decreased barrier function, and decreased mucociliary clearance (Figure 1). These observations shed light on the increased risk of hospitalization with pneumonia in patients with uncontrolled diabetes. Pneumonia risk correlates with glycemic control (11, 12), and the findings presented in this paper hint at a potential mechanism for this increased pulmonary infection susceptibility and severity associated with hyperglycemia.
In summary, Zhang and colleagues provide valuable insight on the role of metabolically deranged and ongoing PKC signaling in the development of abnormal airway epithelium. They also provide valuable translational insights by correlating the findings across several species commonly used as experimental models. They bring to light an important concern related to the effect of culture media on normal in vitro cell growth and differentiation. These findings are somewhat limited by the numerous pathways that converge on PKC, and further work is required to identify which in vivo cell signaling transducers are likely responsible for the prolonged signaling required to induce squamous cell differentiation in human airway health and disease.
Footnotes
Originally Published in Press as DOI: 10.1165/rcmb.2023-0065ED on March 15, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Breeze RG, Wheeldon EB. The cells of the pulmonary airways. Am Rev Respir Dis . 1977;116:705–777. doi: 10.1164/arrd.1977.116.4.705. [DOI] [PubMed] [Google Scholar]
- 2. Tata PR, Mou H, Pardo-Saganta A, Zhao R, Prabhu M, Law BM, et al. Dedifferentiation of committed epithelial cells into stem cells in vivo. Nature . 2013;503:218–223. doi: 10.1038/nature12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Davis JD, Wypych TP. Cellular and functional heterogeneity of the airway epithelium. Mucosal Immunol . 2021;14:978–990. doi: 10.1038/s41385-020-00370-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vieira Braga FA, Kar G, Berg M, Carpaij OA, Polanski K, Simon LM, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019;25:1153–1163. doi: 10.1038/s41591-019-0468-5. [DOI] [PubMed] [Google Scholar]
- 5. Plasschaert LW, Žilionis R, Choo-Wing R, Savova V, Knehr J, Roma G, et al. A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature . 2018;560:377–381. doi: 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruiz García S, Deprez M, Lebrigand K, Cavard A, Paquet A, Arguel MJ, et al. Novel dynamics of human mucociliary differentiation revealed by single-cell RNA sequencing of nasal epithelial cultures. Development . 2019;146:dev177428. doi: 10.1242/dev.177428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang Y, Black KE, Phung TN, Thundivalappil SR, Lin T, Wang W, et al. Human airway basal cells undergo reversible squamous differentiation and reshape innate immunity. Am J Respir Cell Mol Biol . 2023;68:664–678. doi: 10.1165/rcmb.2022-0299OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An WF, Germain AR, Bishop JA, Nag PP, Metkar S, Ketterman J, et al. Probe reports from the NIH Molecular Libraries Program. Bethesda, MD: National Center for Biotechnology Information; 2010. Discovery of potent and highly selective inhibitors of GSK3b. [Google Scholar]
- 9. Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang X, et al. Wnt/β-catenin signalling: function, biological mechanisms, and therapeutic opportunities. Signal Transduct Target Ther . 2022;7:3. doi: 10.1038/s41392-021-00762-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Radaszkiewicz KA, Beckerová D, Woloszczuková L, Radaszkiewicz TW, Lesáková P, Blanářová OV, et al. 12-O-Tetradecanoylphorbol-13-acetate increases cardiomyogenesis through PKC/ERK signaling. Sci Rep . 2020;10:15922. doi: 10.1038/s41598-020-73074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kornum JB, Thomsen RW, Riis A, Lervang H-H, Schønheyder HC, Sørensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care . 2008;31:1541–1545. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brunetti VC, Ayele HT, Yu OHY, Ernst P, Filion KB. Type 2 diabetes mellitus and risk of community-acquired pneumonia: a systematic review and meta-analysis of observational studies. CMAJ Open . 2021;9:E62–E70. doi: 10.9778/cmajo.20200013. [DOI] [PMC free article] [PubMed] [Google Scholar]