Abstract
In idiopathic pulmonary fibrosis (IPF), the normal delicate lung architecture is replaced with rigid extracellular matrix (ECM) as a result of the accumulation of activated myofibroblasts and excessive deposition of ECM. Lamins have a role in fostering mechanosignaling from the ECM to the nucleus. Although there is a growing number of studies on lamins and associated diseases, there are no prior reports linking aberrations in lamins with pulmonary fibrosis. Here, we discovered, through analysis of RNA sequencing data, a novel isoform of lamin A/C that is more highly expressed in IPF compared with control lung. This novel LMNA (lamin A/C) splice variant includes retained introns 10 and 11 and exons 11 and 12 as documented by rapid amplification of cDNA ends. We found that this novel isoform is induced by stiff ECM. To better clarify the specific effects of this novel isoform of lamin A/C and how it may contribute to the pathogenesis of IPF, we transduced the lamin transcript into primary lung fibroblasts and alveolar epithelial cells and found that it impacts several biological effects, including cell proliferation, senescence, cell contraction, and the transition of fibroblasts to myofibroblasts. We also observed that type II epithelial cells and myofibroblasts in the IPF lung exhibited wrinkled nuclei, and this is notable because this has not been previously described and is consistent with laminopathy-mediated cellular effects.
Keywords: IPF, lamin A/C, RNA splicing, lung
Clinical Relevance
This is the first study to discover a novel splice isoform of lamin in the idiopathic pulmonary fibrosis (IPF) lung and discusses a potential role of this novel splice isoform in the pathogenesis of IPF. This novel splice variant of lamin is induced by stiff matrix and promotes cell senescence and fibroblast–myofibroblast transition. Our study reveals that fibroblasts and alveolar epithelial cells exhibit wrinkled nuclei in the IPF lung, which is a feature of laminopathies. We anticipate that our studies will garner significant interest from the pulmonary community, and we expect that this study will open a new area of research.
Idiopathic pulmonary fibrosis (IPF) is a progressive and fatal lung disease characterized by accumulation of activated myofibroblasts and excessive deposition of extracellular matrix (ECM) in fibroblastic foci that harbor senescent cells. The IPF lung exhibits destruction of normal lung architecture and increased ECM rigidity (1). The ECM is composed of more than 300 proteins and is an essential regulator for progressive pulmonary fibrosis, in part by modulating the mechanical stiffness of the lung (2). Epithelial injury, genetic/epigenetic alteration, and aging are considered to contribute to the pathobiology of IPF (3).
The elasticity of ECM determines the mechanical properties of tissue and also leads to nuclear rigidity through accumulation of lamin (4). The mechanical stimuli of ECM to/from the nucleus occurs via signaling molecules and direct cell–cell contacts and/or tension forces, specifically through integrins and focal adhesion complexes (5). The linker of nucleoskeleton and cytoskeleton, named the LINC, involves several proteins (e.g., Nesprin and SUN) that play a critical role in force transmission among the ECM, cell membrane, and nucleus by connecting the actin cytoskeleton to the lamina on the inside of the nuclear envelope.
The nuclear lamina consists of lamins and associated proteins. As type V intermediate filament proteins, lamin proteins contain three structures, including an α-helical coiled-coil rod domain, a nuclear localization signal, and an immunoglobulin-fold motif within the C-terminal tail (6, 7). Lamins are classified into A and B types encoded by LMNA and LMNB, respectively (8), which yield alternative splicing transcripts of lamins A, C, AΔ10, and C2 and lamins B1, B2, and B3, respectively (9). LMNA has 12 exons encoding 664 amino acids. The first 10 exons, encoding 566 amino acids of LMNA, are identical in lamin C and prelamin A (the precursor to mature lamin A), but their carboxyl-terminal sequences differ (9). The 572 amino acids of lamin C contain six unique carboxyl terminal sequences (farnesylated tail domain) plus the sequence of 566 consensus amino acids, without posttranslational processing, whereas prelamin A contains 646 amino acids including the 98 unique amino acid sequences encoded by exons 11–12. Prelamin A undergoes several posttranslational modifications at its carboxyl-terminal region that convert the prelamin A to mature lamin A and allows for incorporation of prelamin A into the nuclear envelope through modifications such as farnesylation, methylation, and endoproteolytic processing (10, 11).
Lamin interacts with inner nuclear proteins directly or indirectly and is thereby involved in regulation of gene expression and signaling, DNA replication and repair, chromatin organization, cell proliferation, apoptosis, senescence, and differentiation (12–15). Therefore, dysregulation or mutation of lamin has been closely associated with aging or aging-related hereditary disorders in muscle, adipose tissue, and nerves. These mutations lead to disorders known as laminopathies, such as Hutchinson-Gilford progeria syndrome (HGPS), LMNA-related cardiomyopathies, lipodystrophy, and muscular dystrophy (16–18). For example, an alternative A>G mutation at a 3′ splice site (ss) in exon 4 adds three amino acids and leads to dilated cardiomyopathy (19); a single-nucleotide mutation G>C at a 5′ ss causes intron 8 retention, resulting in familial partial lipodystrophy type 2 (20); a G>C SNP at a 5′ ss leads to intron 9 retention and causes limb-girdle muscular dystrophy 1B (21); and an alternative SNP at a 5′ ss on exon 11 leads to a 150-bp deletion that translates to the protein progerin that causes HGPS (18, 22).
Despite the growing number of studies on lamins and their associated diseases, there are no prior reports linking aberrations in lamins with pulmonary fibrosis. In the present study, we report a novel isoform of lamin A/C. We discovered through analysis of RNA sequencing (RNA-seq) data and immunoblots that this isoform is more highly expressed in IPF lung tissue compared with control lung. To better clarify the specific function of this isoform of lamin A/C and how it may contribute to the pathogenesis of IPF, we transduced this novel lamin transcript into human lung fibroblasts and alveolar epithelial cell lines.
Methods
Ethical Approval Statement
The use of specimens in the present study was approved by the Tulane University Biomedical Institutional Review Board (approval 12-334398E) and by the University of Alabama at Birmingham (approval N120410001).
RNA Extraction and RT-PCR
TRIzol Reagent, RNeasy plus, and QIAshredder were used for total RNA isolation from lung tissue. One microgram of RNA was reverse-transcribed into cDNA using the iScript cDNA Synthesis Kit. mRNA levels were quantified using iQ SYBR Green Supermix and the CFX96 system. Relative gene expression was normalized to the housekeeping gene 36B4 and calculated using the Δ-Δ Cq method as described previously (23).
Cell Culture
A549 and HEK 293 cells were grown in Dulbecco’s modified Eagle medium. HPL1D cells were maintained in Ham’s F-12 medium (24). Normal human lung fibroblasts (i.e., NHLF1) and human pulmonary fibroblasts (HPFs) were cultured per the suppliers’ instructions (Lonza and ScienCell, respectively).
Preparation of Lysates and Western Blotting
Frozen lung tissue was resuspended in 300 μl of 1.5× cold radioimmunoprecipitation assay buffer containing PMSF and protease inhibitor cocktail. Protein was quantified using the Pierce BCA Protein Assay Kit. Thirty micrograms of protein were subjected to SDS-PAGE separation and transferred onto polyvinylidene difluoridemembrane for Western blotting.
Gene Synthesis and Transduction
Lamin genes were synthesized and inserted into the pLVX-puro lentiviral expression vector from Synbio Technologies. Lamin viruses were generated by transfecting these plasmids into 293T cells using Lenti-XTM Packaging Single Shots. Transduction was performed in a 6-well format in the presence of 6 μg/ml of polybrene.
Rapid Amplification of cDNA Ends Assay
Rapid amplification of cDNA ends (RACE) PCR was performed using the SMARTer RACE kit. Briefly, first-strand 5′-RACE/3′-RACE cDNA was synthesized with 1 μg of total RNA. The cDNA ends were amplified using primers spanning LMNA intron 10 at 68°C for 30 seconds for 25 cycles.
RNA in situ Hybridization and RNA–Protein Codetection Assay
An LMNAin (lamin A/C splice variant with retained introns 10 and 11) probe was designed by Advanced Cell Diagnostics to target LMNAin specifically. Fluorescence in situ hybridization was performed in lung tissue and cultured lung fibroblasts using the BaseScope 2.5 HD assay and RNA–protein codetection kit.
Senescence Assay
Senescence-associated β-galactosidase (SA-β-gal) activity was detected at the indicated time periods after infection using the Senescence β-Galactosidase Staining Kit per the manufacturer’s protocol. Cells were then observed and enumerated using an inverted microscope.
Proliferation Assay and Immunofluorescence Staining
Cell-proliferation assays were performed at the indicated times after stable expression of LMNAin using the Click-iT EdU Cell Proliferation Kit. To combine with immunofluorescence staining, slides were incubated with antibodies against lamins after 5-Ethynyl-2′-deoxyuridine (EdU) reaction. Images were acquired using an Olympus BX43 microscope and analyzed with Olympus cellSens.
Three-Dimensional Culture and Stiffness Assay
Cells were seeded in hydrogel collagen–coated 6-well plates containing 2 ml of culture media at stiffnesses of 1 kPa, 8 kPa, and 25 kPa as described by the manufacturer (Matrigen).
Fluorescent Immunohistochemistry and Image Acquisition
Formalin-fixed, paraffin-embedded slides of control and IPF lungs were obtained from the LTRC (Lung Tissue Research Consortium) and University of Alabama at Birmingham. Staining by IHC was performed by the Morphology and Imaging Core at Louisiana State University (LSU). Imaging was captured with a confocal microscope (Nikon Eclipse Ti2).
Telomere Length Quantification
A total of 5 ng of DNA was used for telomere length quantification. Telomere length was quantified per the manufacturer’s instructions (ScenCell). The total telomere length of the sample per diploid cell was calculated as reference sample telomere length × 2−ΔΔCq, in which ΔΔCq is ΔCq(telomere) − ΔCq(single copy reference).
Contraction Assay
Collagen-based contraction assays were performed in 24-well plates as described by the manufacturer (Cell Biolabs). The contraction index was measured at the indicated times with a ruler, and images were acquired with the ChemiDoc imaging system.
Statistical Analysis
GraphPad Prism was used to graph the data and analyze the statistics. The results are expressed as the mean ± standard deviation. Differences between groups were analyzed using one-way analysis of variance with the Student’s t test or two-way ANOVA multiple-comparison tests. A P value lower than 0.05 was considered statistically significant.
Results
A Novel LMNA Splice Variant Is Expressed in IPF Lungs
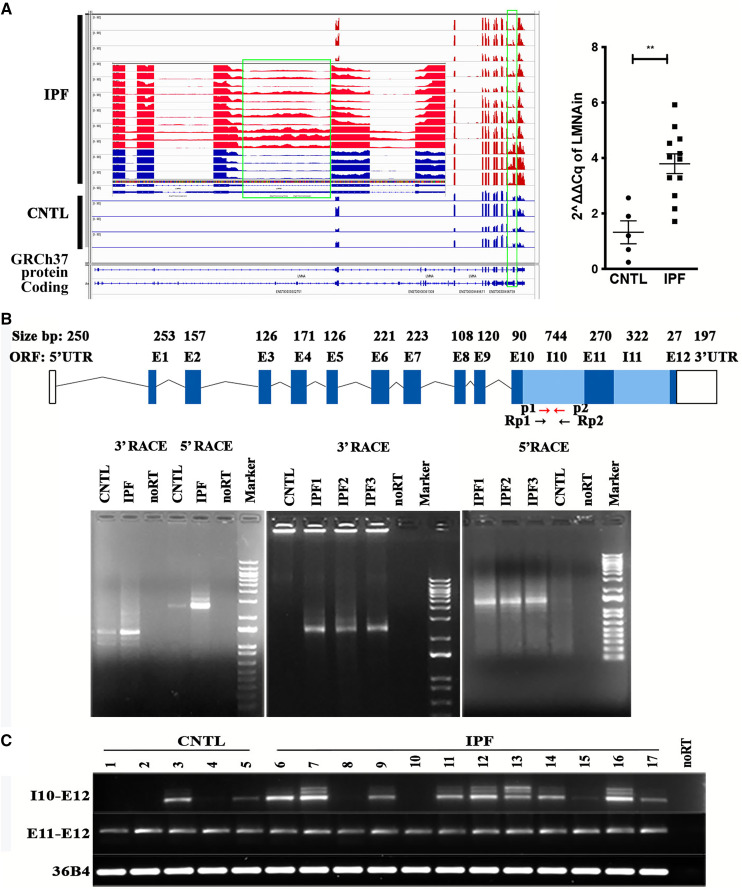
To assess regulation of mRNA alternative splicing in IPF lungs, we conducted a splicing analysis within RNA-seq data from control and IPF lungs using the dSpliceType methodology (http://dsplicetype.sourceforge.net) (25, 26). Detailed subject demographic information was described in our prior published paper (23). As shown in Figure 1A, the type V intermediate filament gene nuclear lamin A/C displayed alternative splicing. The matrix gene LMNA displayed two primary splice variants, including one that was missing exon 10 within the lamin tail domain and another with retained introns 10 and 11, at ratios of 1.37 and 2.67, respectively. These splicing events of LMNA were not listed on Ensembl or the University of California, Santa Cruz genome browser database, indicating that our work uncovered novel LMNA splice variants. Visualization with Integrative Genomics Viewer confirmed the findings observed using RNA-seq. The lamin A/C alternative splicing event demonstrates retention of introns 10 and 11 (LMNAin) in RNA from IPF lungs (Figure 1A). RT-PCR validated the RNA-seq findings. Retained intron 10 of LMNA (Figure 1A, right) was confirmed with primers spanning a region within intron 10.
Figure 1.
RNA sequencing (RNA-seq) and rapid amplification of cDNA ends (RACE) assays showed that a novel lamin A/C mRNA alternative splice isoform was more highly expressed in idiopathic pulmonary fibrosis (IPF) compared with control lung. (A) A novel transcript of LMNA (lamin A/C) with enhanced expression in IPF compared with control lung was discovered using analyses of RNA-seq data. Left: Visualization of LMNA RNA splicing events through the Integrative Genomics Viewer. The green box indicates the retention of introns 10 and 11. An enlarged image in the middle shows the retained intron 10 (controls in blue, IPFs in red). Right: Detection of the LMNAin (lamin A/C splice variant with retained introns 10 and 11) splicing event in IPF and control lungs through real-time RT-PCR with primers spanning intron 10. (B) RACE revealed a unique transcript using primers spanning LMNA intron 10. Top panel: Schematic diagram of the LMNAin isoform. Arrows indicate the primers used for RT-PCR (in red, labeled p1 and p2) and RACE (in black, labeled Rp1 and Rp2). Left lower panel: RACE assays using one control (CNTL) and one IPF sample. Amplification product without reverse transcription of the RNA from the IPF sample is labeled “noRT.” Left lower middle: 3′ RACE assays of RNA from three independent IPF samples (IPF1, IPF2, and IPF3) with RNA from normal lung (CNTL) and no reverse transcription of RNA from IPF1 (noRT). Left lower right: 5′ RACE assay of RNA from three independent IPF samples (IPF1, IPF2, and IPF3) with RNA from normal lung (CNTL) and no reverse transcription of IPF1 (noRT). On all three gels, the DNA marker is the 1 kb plus DNA ladder. (C) Selective detection by RT-PCR product containing intron 10 to exon 12 (I10–E12) and exon 11 to exon 12 (E11–E12) in RNA from patients with IPF (lanes 6–17) compared with RNA from control lungs (lanes 1–5). UTR = untranslated region.
Identification of a Unique Longer 3′UTR of LMNA mRNA in IPF Lung Tissue
Although RNA-seq and Integrative Genomics Viewer visualization showed the lamin A/C isoforms with retained introns 10 and 11, they cannot interpret the RNA isoform information. For LMNA, there are more than 20 isoforms (splice variants) based on the National Center for Biotechnology Information and the Ensembl database. One of them is long noncoding RNA LMNA-216 that contains intron 10 (ENST00000496738.5). To determine the full-length transcripts of LMNAin, 5′ and 3′ RACE was conducted using control and IPF lung tissue and primers Rp1 forward for 3′ RACE and Rp2 reverse for 5′ RACE. There is a 149-bp overlap between these two primers located in LMNA intron 10 (Figure 1B). No PCR product was detected in IPF samples in the absence of reverse transcription. Similar assays of RNA from normal lung revealed little to no PCR product. The 5′ RACE identified exon 1 to exon 10 and retained intron 10 of LMNAin, and 3′ RACE recognized the retained intron 10 to 3′UTR of the cDNA LMNAin. RACE products were cloned into the plasmid for cloning of RACE products, pRACE. Ten clones of each sample were selected from each RACE for enzyme digestion to identify the correct clones, and three clones of each sample were sequenced by Sanger sequencing. After aligning the sequence, the RACE sequence of LMNAin was Basic Local Alignment Search Tool–searched on the University of California, Santa Cruz genome browser; MegAlign Pro; and SeqMan Pro. All three programs showed that the lamin RACE product was consistent with the RNA-seq findings, and it included the sequence of exon 1–10, intron 10, exon 11, intron 11, exon 12, and 3′UTR (Figure 1B, top) of LMNA. Concordantly, the RACE assay also revealed that expression of LMNAin was enriched in IPF compared with control lung (Figure 1B, bottom). The amplicon intensity of cDNA from IPF lung is more prominent than that of controls with primers spanning intron 10 to exon 12, but not the RT-PCR fragments from primers spanning exon 11 to exon 12 (Figure 1C). As a control, RNA from all samples displayed a comparable RT-PCR product corresponding to the region spanning exon 11 to exon 12. These results demonstrate that IPF lung expresses higher levels of the intron 10 splice variant specifically as a manifestation of LMNAin expression.
The Ratio of Lamin A to Lamin C Isoforms is Greater in IPF Lung Compared to Control Lung
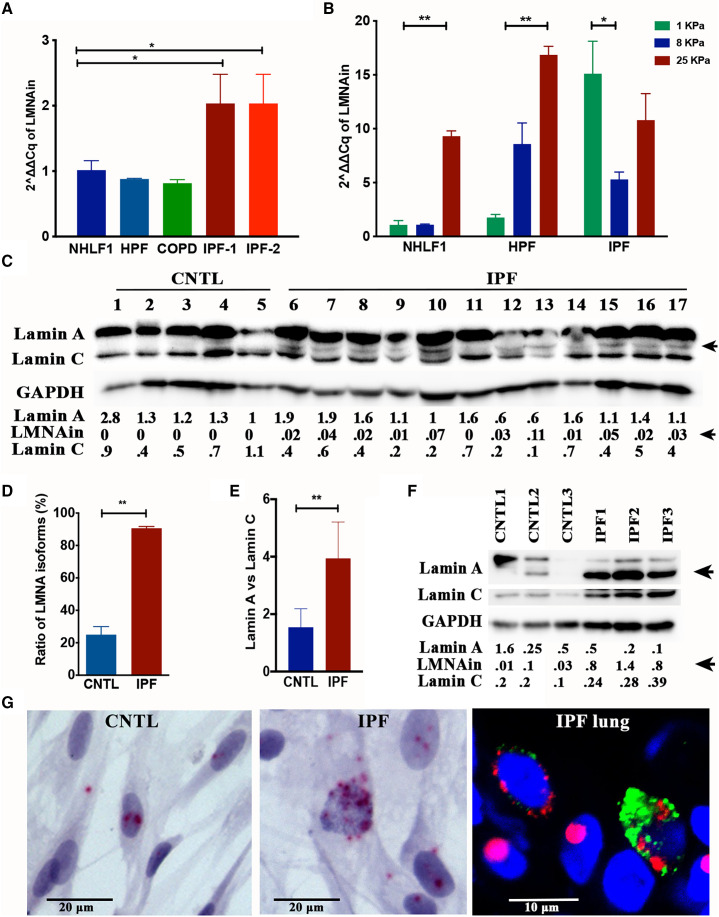
To determine whether LMNAin is expressed in various lung cell types, RT-PCR was conducted in fibroblasts from various origins. As shown in Figure 2A, compared with control lung fibroblasts (i.e., NHLF1), two independent IPF fibroblast isolates displayed higher levels of LMNAin. To test the hypothesis that matrix stiffness may influence LMNAin expression, primary lung fibroblasts were cultured on matrices that are similar in stiffness to control and fibrotic lung (Figure 2B). These experiments indicate that matrix stiffness is capable of inducing expression of the LMNAin splice variant in normal fibroblasts but did not enhance further expression in IPF fibroblasts. Next, we examined whether a more rigid matrix could induce changes in the lamin A/C isoform ratio in IPF lungs. RNA-seq–based isoform analysis allowed us to calibrate the lamin A/C stoichiometries and thereby determine which isoform dominates in IPF. Twenty-five lamin isoforms were detected by RNA-seq, including seven protein-coding A-type lamin isoforms and four protein-coding C-type lamin isoforms (Table E2 in the data supplement). We observed that the expression levels of A-type lamin isoforms were higher than those of C-type lamin isoforms in IPF compared with control lungs (Figure 2D). This result was confirmed by RT-PCR (Figure 2E). Further, we detected more A-type lamin protein isoforms using Western blotting in IPF using an antibody against the LMNA N-terminus (4 of 15 controls and 27 of 30 IPF lung specimens; Figures 2C and 2D), thus demonstrating higher expression of lamin A/C isoforms in fibrotic lungs at the RNA and protein levels. Concordantly, higher lamin A isoform expression in relation to lamin C was detected in the fibroblasts isolated from IPF lung tissue (Figure 2F). To evaluate intracellular distribution of individual LMNAin transcripts in single cells, a probe was designed to target the LMNAin specifically for the fluorescence in situ hybridization assay. The LMNAin RNAs were visualized in the cytoplasm and nucleus, suggesting that the LMNAin RNAs were exported to the cytoplasm for translation (Figure 2G). Moreover, the RNA–protein codetection results showed LMNAin mRNAs located in the alveolar type II (AT2) cells expressing surfactant protein C (SP-C) and other cell types in the IPF lung (Figure 2G, right).
Figure 2.
Detection of LMNA isoforms in IPF lung. (A) The transcript of LMNAin was expressed highly in fibroblasts isolated from the IPF lung, but not in normal primary fibroblasts (normal human lung fibroblasts [NHLF1 cells] and human pulmonary fibroblasts [HPFs]) or fibroblasts derived from a chronic obstructive pulmonary disease (COPD) lung based on a qRT-PCR assay. The graph shows the fold change and standard deviation of LMNAin RNA levels (2ΔΔCq method using 36B4 mRNA as internal control) from the indicated fibroblasts (NHLF1, HPF, COPD, IPF-1, and IPF-2). The results were normalized to those in NHLF1. (B) The transcript of LMNAin increased with increasing stiffness in primary fibroblasts (NHLF1 and HPF), but not in fibroblasts isolated from the IPF lung. The graph shows the relative level of LMNAin RNA (2ΔΔCq method using 36B4 mRNA as internal control) detected by qRT-PCR in the indicated fibroblasts (NHLF1, HPF, and IPF) grown on matrices with the indicated stiffness. (C) Western blotting reveals an extra lamin band in IPF lungs. Immunoblot of whole lung extracts prepared from normal (CNTL, lanes 1–5) and IPF (lanes 6–17) lung tissue samples. The blot was probed with an antibody that detects isoforms of lamin A and C. The arrow indicates a lamin A isoform selectively detected in IPF samples. The blot was probed with an antibody to GAPDH to confirm equal loading. The band densitometry analyses were performed with ImageJ. The relative optical density of lamins normalized to GAPDH is shown numerically. (D) Quantification of lamin A isoform (percentage of samples) in 15 control (CNTL) and 30 IPF lungs based on Western blotting results. (E) The ratio of lamin A to lamin C in IPF and control lung based on RT-PCR. (F) The lamin protein isoform was highly expressed in fibroblasts isolated from IPF lung compared with control lung fibroblasts. The numbers indicate the relative optical density of lamins normalized to GAPDH. (G) Analysis of LMNAin transcript subcellular localization by fluorescence in situ hybridization. Detection of LMNAin RNA in fibroblasts using the LMNAin probe (red) and nuclear counterstain (blue). Normal lung fibroblasts are on the left, and IPF lung–derived fibroblasts are in the middle. Scale bars, 20 μm. Simultaneous coimaging of surfactant protein C (SP-C; green), LMNAin RNAs (red), and nuclei (blue) in human IPF lung are shown on the right. The images were taken using an Olympus BX43 microscope at 40× original magnification (left and middle) and Nikon A1R confocal microscope (right) at 40× original magnification. Scale bar, 10 μm.
LMNAin Expression Influences Cellular Biological Processes
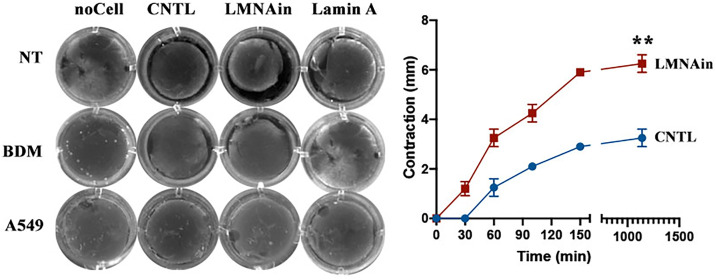
To evaluate the biological effect of overexpressing LMNAin, we cloned the LMNAin sequence into a lentiviral expression vector and stably expressed LMNAin in lung epithelial cell lines (A549 and HPL1D) and lung fibroblast cell lines (NHLF1, HPF, and 3T3) (Figure E1 in the data supplement). We performed cell-senescence, telomere-length, cell-cycle, and cell-proliferation assays in these cells. Assessment of cell proliferation in situ using EdU demonstrated lower cell proliferation in LMNAin-transduced fibroblasts (i.e., NHLF1) (Figure 3A). In contrast, we did not observe that LMNAin promoted senescence or inhibited proliferation in LMNAin-expressing alveolar epithelial cancer cells (i.e., A549) or transformed peripheral lung epithelial cells (i.e., HPL1D). However, we did observe telomere length shortening in fibroblasts and epithelial cells that stably expressed LMNAin, including NHLF1 and A549 cells (Figure 3B). To explore potential consequences of this telomere erosion, stably transfected cells of LMNAin were subjected to a cellular senescence assay by quantification of SA-β-gal activity (hydrolysis of X-gal) in NHLF1 cells from passage 4 to passage 8 (Figure 4A). As shown in Figure 4A (right panel), approximately 50% of LMNAin-expressing fibroblasts exhibited X-gal blue staining (i.e., increased SA-β-gal activity), whereas only 5% of the control vector cells did so at passage 4. LMNAin-transduced cells continued to increase the percentage of senescent cells with subculture over ensuing passages (99% of cells were blue at passage 8). Moreover, LMNAin induced more SA-β-gal activity compared with the lamin A or lamin C vectors (Figure 4B), and the lamin A and lamin C vectors’ SA-β-gal activities were like that observed in control vector cells. Using the ability of fibroblast cells to contract collagen gels, we determine the effect of LMNAin polymerization on mechanical tension generation. As shown in cell-contraction assays (Figure 5), LMNAin but not lamin A mediated the increased intracellular tension in fibroblasts, enabling these cells to exert increased force on the ECM and exerting more contractive force on this ECM gel (Figure 5, top left and right). Cell contractions were reversed by the contraction inhibitor 2,3-butanedion monoxime (Figure 5, left middle). As a negative control, there was no contraction observed in A549 cells (Figure 5, left bottom).
Figure 3.

Overexpression of LMNAin diminished cell proliferation in normal human lung fibroblasts. (A) Effect of LMNAin on 5-Ethynyl-2′-deoxyuridine (EdU) incorporation. Left panels: The indicated control vector (CNTL) and LMNAin (transduced with a lentivirus expressing LMNAin) cells were grown in culture overnight with EdU. The cells were fixed and stained with DAPI (blue), and EdU incorporation (green) was detected. The scale bar represents 50 μm. Right panel: Quantification of proliferating cells based on EdU expression. The ratio of green (EdU incorporation) to blue (DAPI) was calculated (left panel). (B) Expression of LMNAin induced telomere length shortening in A549 and NHLF1 cells. Average telomere length and SEM are shown. Student’s t test was used to analyze the statistical significance.
Figure 4.
Expression of LMNAin-induced cell senescence in fibroblasts (A, NHLF1; B, HPF) based on the cell senescence assay using β-galactosidase staining. (A) Left panel: Quantification of senescent cells at passage 6. Middle panel: Senescent cells appear enlarged and express β-galactosidase activity (blue-green). Scale bars, 100 μm. Right panel: Quantification of senescent cells over several passages in culture following stable expression of LMNAin. Two-way ANOVA and multiple unpaired t tests were used for statistical analysis. (B) There are more senescence cells in LMNAin-expressing HPF cells than in lamin A– and lamin C–expressing HPF cells after 21 days in culture. SA-β-gal = senescence-associated β-galactosidase. Scale bars, 20 μm.
Figure 5.
Evaluation of the effect of LMNAin on cell contraction using three-dimensional collagen gels: 0.5 × 106 fibroblasts or A549 cells were cultured for 2 days within a collagen gel lattice before releasing collagen gels. Fibroblasts (left panel, top two rows) were untreated or treated with 10 mM 2,3-butanedione monoxime for 1 hour before initiating contraction. The left panel is images, and the right panel is the measurement of collagen gel diameter at the indicated times in fibroblasts. Statistical analysis was performed using two-way ANOVA with multiple-comparisons tests. BDM = 2,3-butanedione monoxime; NT = not treated.
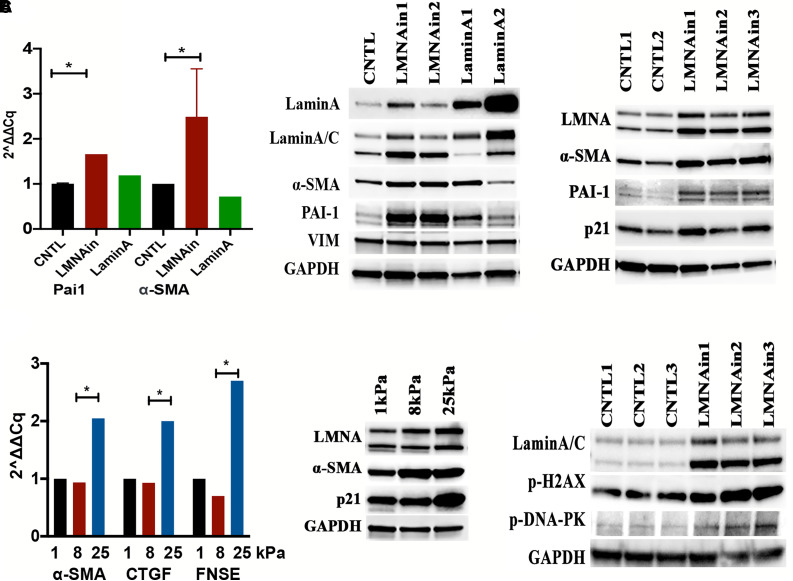
Given the importance of myofibroblasts in fibrotic regions, we sought to further elucidate the role of LMNAin on the transition of fibroblasts to myofibroblasts through measuring expression of α-smooth muscle actin (SMA), a molecular marker for myofibroblasts, and plasminogen activator inhibitor-1 (PAI-1), a marker of senescence. RT-PCR and Western blotting showed that stable expression of LMNAin induced α-SMA and PAI-1 expression in primary fibroblasts (Figure 6A), indicating that LMNAin promotes the transition of fibroblasts to myofibroblasts. Expression of LMNAin also induced p21 expression. Furthermore, increasing matrix stiffness from 1 kPa to 25 kPa stimulated the expression of LMNAin and the profibrotic markers α-SMA, CTGF, and FNSE (fibronectin EDA) (Figure 6B). Finally, expression of LMNAin induced the expression of phosphorylation of histone H2AX at Ser 139, (p-H2AX) and phosphorylation of DNA-dependent protein kinase (p-DNA-PK) (Figure 6C), suggesting that LMNAin may promote DNA double-strand break damage. These experiments, taken together, indicate that overexpression of LMNAin leads to expression of several indicators of cell senescence.
Figure 6.
Fibroblast–myofibroblast transition and DNA damage were associated with the expression of LMNAin and matrix stiffness. (A) LMNAin induced expression of myofibroblasts markers in normal human lung fibroblast cells. RT-PCR (left panel) and Western blotting (middle and right panels) showed that α-smooth muscle actin (α-SMA) and plasminogen activator inhibitor-1 were increased in normal human lung fibroblasts (HPF in middle panel and NHLF1 in right panel) transduced with LMNAin after 14 days of selection. (B) Mechanical stiffness of extracellular matrix stimulated fibroblast differentiation. RT-PCR (left) and Western blotting (right) showed that profibrotic markers (α-SMA, CTGF, EDA-FN) were increased with increasing matrix stiffness. (C) Transduction of LMNAin promoted DNA damage markers (p-H2AX and p-DNA-PK), as shown by Western blotting. CNTL indicates control or empty vector. Student’s t test was used to analyze the statistical significance. CTGF = connective tissue growth factor. PAI-1 = plasminogen activator inhibitor-1.
The Function of A-Type Lamin in Nuclear Morphology and Function Regarding the Pathobiology of IPF
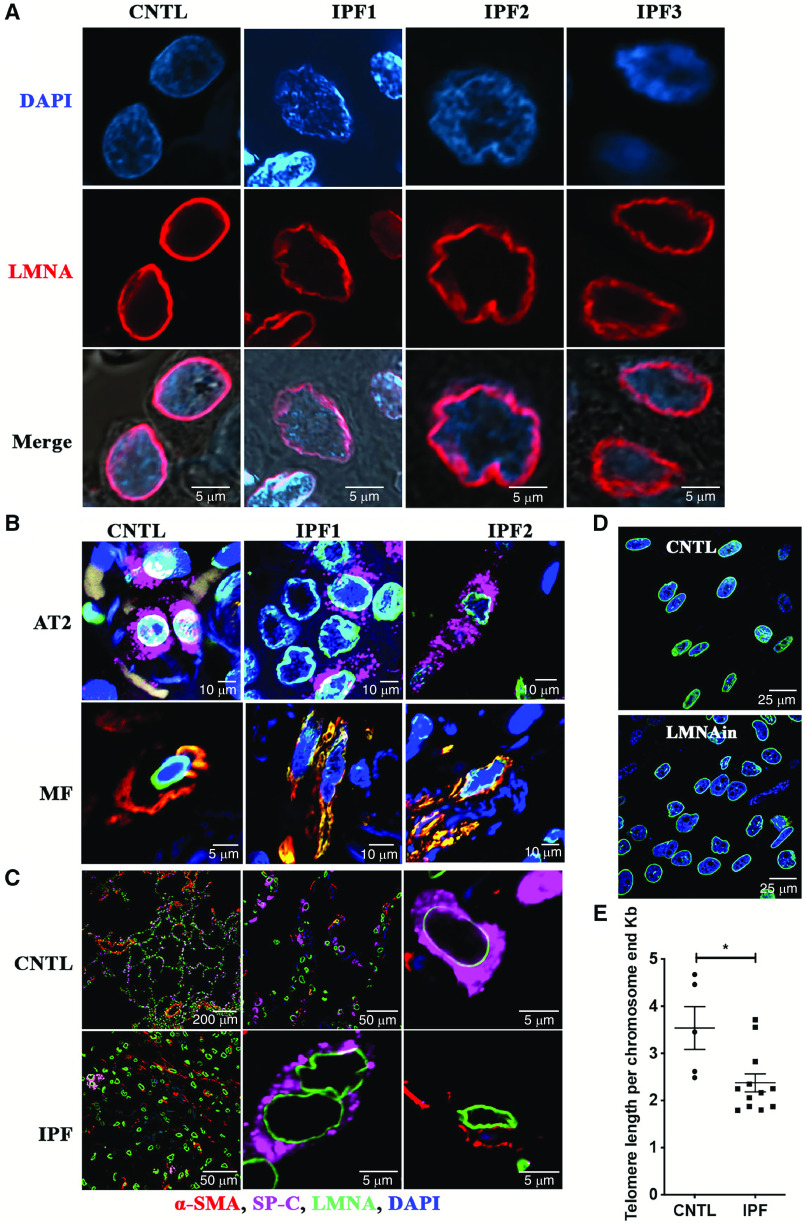
Nuclear lamins line the nucleoplasmic side of the inner nuclear membrane to support nuclear structure; therefore, we examined whether a change in the ratio of lamin A to lamin C is sufficient to induce nuclear morphology changes. Hence, fluorescent immunohistochemistry using an antibody against lamin A/C was conducted in three control and four IPF lung tissue specimens. Cells in IPF lung tissue exhibited wrinkled nuclei, similar to that observed in HGPS, but this was not observed in control lung (Figure 7A). Next, we sought to determine what cell type exhibited wrinkled nuclei in the IPF lung. Type II cell–associated SP-C and α-SMA staining revealed that SP-C/α-SMA–positive and –negative cells showed wrinkled nuclei and distorted nuclear shape, suggesting that cell types other than AT2 cells and myofibroblasts exhibit changes in their nuclear shape and size in the IPF lung (Figure 7B). Furthermore, the nuclear shapes were different between normal areas and fibrotic areas in the same IPF lung tissue specimen. The nuclear shape of most cells was smooth and round in normal areas (Figure 7C, top), whereas the nuclear shape of the majority of cells was wrinkled in fibrotic areas (Figure 7C, bottom). It has been previously reported that IPF lungs exhibit diminished telomere length comparison with control lungs, even though only a minority of patients with IPF show expression of mutations to account for this (27). Furthermore, we observed the changes in nuclear shape in cells cultured in a three-dimensional matrix as shown by immunofluorescence with antibody against lamin B (Figure 7D) in LMNAin-transduced fibroblasts. There were abnormal nuclear shapes and sizes that included invagination, enlargement, and lobulation (Figure 7D). Telomere-length assays were performed using DNA isolated from control and IPF lungs. As expected, based on prior reports (28), significantly shorter telomere length was observed in IPF lungs compared with controls (Figure 7E). These findings are consistent with our observation that introduction of LMNAin into lung fibroblasts and AT2 cell lines resulted in telomere shortening (Figure 3B).
Figure 7.
Wrinkled nuclei and telomere length shortening were detected in IPF lung tissue (original magnification, 60×). (A) Confocal microscopy showing wrinkled nuclei in IPF lungs on fluorescent immunohistochemistry using a lamin A/C antibody. Lamin A/C is identified by the red color and located at the nuclear periphery. Scale bar, 5 μm. (B) Nuclear morphology changes were observed in alveolar type II (AT2) cells, myofibroblasts, and other types of lung cells. Fluorescent immunohistochemistry staining with AT2 cell marker, SP-C antibody (magenta), fibroblast marker, α-SMA (red), nuclear membrane protein, and lamin A/C antibody (green) in control and IPF lungs. (C) The nuclear shapes were compared between normal area and fibrotic area in the same IPF lung tissue. The nuclear morphological shape characteristics were examined by fluorescent immunohistochemistry and stained with lamin A/C as green, SP-C as magenta, and α-SMA as red. Original magnifications of 10× (top left panel) and 60× were used to acquire the images. (D) Nuclear morphology changes in LMNAin-transduced fibroblasts. Confocal immunofluorescent analysis was conducted in cells cultured in three dimensions using antibody against lamin B (green). Images were acquired at 60× original magnification by Nikon A1R confocal microscopy. Scale bars in B–D are as labeled. (E) Telomere length is shorter in IPF lung compared with control lung. Average telomere length and SE are shown. Student’s t test was used to analyze the statistical significance.
Discussion
We identified a previously unknown LMNA isoform by comparing IPF and control lung. Although there are abundant changes in RNA splicing in IPF lung compared with control, the LMNA isoform drew our attention for two reasons. First, the isoform switch was among the most prominent of isoform splice variations observed. Second, we recognized that LMNA mutations are associated with premature senescence. This latter point is notable because there is burgeoning evidence that the IPF lung exhibits features of senescence (29).
Our initial finding was the identification of a previously undescribed LMNA splice variant with retained introns 10 and 11 that was more highly expressed in IPF versus control lungs. Further investigation revealed that this splice variant was a slightly longer lamin C with a longer 3′UTR. Expression of the transcript of LMNAin was detected in A549, HPL1D, 3T3, and NHLF1 cells (Figure E1). Inasmuch as laminopathies lead to nuclear wrinkling, we performed fluorescent immunohistochemistry on IPF and control lungs. We found that cells, including AT2 cells and myofibroblasts, in the IPF lung exhibited wrinkled nuclei, and this is notable because this has not been previously described and is consistent with laminopathy-mediated cellular effects.
Further experiments were directed at understanding what was driving the expression of the LMNAin splice variant and what the effects of expressing the splice variant construct were in regard to cell senescence. It has been previously reported that stiff matrices induce enhanced nuclear expression of lamin. Thus, we explored the effect of culturing normal and IPF fibroblasts on matrices that approximate the stiffness of normal and IPF lung and found that culturing fibroblasts on stiff matrix leads to enhanced expression of the LMNAin splice variant. We do not presently know what signaling pathways are involved in this event.
Our findings that fibrotic lung and stiff matrices are associated with expression of LMNAin are consistent with the possibility that LMNAin isoform expression contributes to the pathobiology of IPF; however, only an association was demonstrated, and the study did not address the effects of LMNAin expression in lung cells. Subsequently, we conducted experiments in which LMNAin was introduced into primary human lung fibroblasts and epithelial cell lines. The focus of these experiments was directed toward examining whether there are findings consistent with the LMNAin construct’s capacity to induce senescence. Figures 3–5 show that enhancing expression of LMNAin in normal fibroblast cells resulted in several cellular changes indicative of senescence, including a broader shape and β-gal expression, diminished proliferative capacity, and telomere length shortening. We did not observe diminished proliferation in the epithelial cells and speculate that this may be related to the fact that these were cancer cells or a transformed cell line. We confirmed the work of others showing that telomeres are shorter in IPF compared with control lung. It is known that some cases of familial pulmonary fibrosis are associated with point mutations in Tert and Terc that lead to telomere shortening. However, only approximately 10% of patients with familial IPF present with such a mutation (30). We speculate that expression of LMNAin may also help explain telomere length shortening in the IPF lung.
Short telomere length in the IPF lung has been associated with the pathogenesis and progression of IPF (28). In our study, the telomere length shortening in IPF lung tissue correlates with higher expression of endogenous LMNAin. Importantly, telomere shortening is seen on overexpression of LMNAin. These results suggest that LMNAin may interact with the chromosomes and cause telomere length shortening. Our findings are compatible with what has been previously reported, specifically, that lamins affect chromosomal organization and genomic instability through affecting telomere length (31). Other research shows that loss of A-type lamin or overexpression of mutant lamin A is associated with telomere shortening (31). This may support the hypothesis that LMNAin-induced telomere shortening is due to LMNAin rather than expression of other A-type lamins.
It has been demonstrated that telomere length shortening triggers DNA damage and repair, senescence, and apoptosis (32). Particularly, Chen and colleagues detailed enrichment of transcripts of genes harboring longer 3′UTR in senescent cells, such as RRas2 (33). On the contrary, we showed that overexpression of LMNAin promotes cellular senescence in primary lung fibroblasts and inhibits cell proliferation through interaction with several genes, including p21 and PAI-1. In contrast, we did not detect senescence in cells overexpressing lamin A constructs, indicating that the LMNAin-induced senescence is not due to overexpression of lamin A/C in general. Our data show that expression of LMNAin induced Η2ΑΧ, and future experiments will be performed to investigate the DNA damage caused by expression of LMNAin, including measuring DNA double-strand breaks and accumulation of DNA repair foci.
Normal lung compliance at ranges of 0.5–5 kPa is much more supple than that of fibrotic lung at ranges of 20–100 kPa (34, 35). We, therefore, cultured fibroblasts on matrices of various stiffness to imitate normal and fibrotic tensile conditions. LMNAin increased with increasing stiffness in primary fibroblasts but not in fibroblasts isolated from IPF lung; however, IPF fibroblast expression of LMNAin on compliant matrix is higher than that of normal lung fibroblast on compliant matrix and similar to that in normal lung fibroblasts cultured on stiff matrix. Furthermore, transduction of fibroblasts with LMNAin promotes the expression of α-SMA, indicating that LMNAin promotes fibroblast–myofibroblast transition. This observation is consistent with previous findings of others concluding that stiffness of the matrix is not only a consequence of fibrosis but also can induce the activation of myofibroblasts. We speculate that our results may reflect that LMNAin could be a regulator that is activated by matrix stiffness to stimulate fibroblast-to-myofibroblast transition and further promote fibrosis. In addition, the fact that lamins A and C, but not lamin B, are involved in cellular mechanical properties (36, 37) suggests that LMNAin may play a role in mechanotransduction in IPF. Our other results showing that overexpression of LMNAin in IPF lungs, and especially in fibroblast cells isolated from IPF, strengthens this hypothesis. Our results are also consistent with the previous studies that found that A-type lamins, but not B-type lamins, are upregulated upon cell culture on increasingly stiff substrates (36), and that expression of mutations in LMNA can cause defective mechanotransduction (38). Further research is needed to fully clarify the role of LMNAin in lung fibrogenesis.
Ratiometric expression of lamin isoforms has shown that their ratio changes with varying matrix stiffness and that the lamin A/C ratio is very important for cell differentiation and disease pathogenesis (39). Expression of mutant lamin affects the ratio of lamin A to lamin C and the synthesis of mature lamins (22, 40). The well-known aging-associated lamin peptide progerin, which harbors a point mutation in exon 11, prevents mature lamin A production and causes HGPS. Because IPF is considered an aging-associated disease, we considered that a mutant progeria-like lamin may be involved in the pathobiology of IPF. We did not detect higher expression of progerin in IPF compared with control lung, but we did detect a unique isoform of lamin A/C using antibodies specific for lamin A/C. Mass spectrometry analysis confirmed that the extra band was a lamin family member (Table E1). Nevertheless, we have not yet acquired the full-length protein sequence of this isoform. We did discover an RNA splice variant (LMNAin) that is highly expressed in IPF lung tissue using a RACE assay. Further, LMNAin modulates cellular biological function, including promoting senescence and inhibiting proliferation in fibroblasts. This is not altogether unexpected because changes in lamin ratio might regulate cell senescence and proliferation, leading to disease onset and progression (41, 42). Loss of lamin A/C enhances cell proliferation (43), lamin A can decrease vascular smooth muscle cell proliferation in rats (44), and other studies showed that expression of mutant lamin A promotes proliferation in embryonic cells (45). There is increasing evidence that cellular senescence is linked to lung fibrosis (46), and our experimental work supports the notion that LMNAin enhances senescence in fibroblasts.
As a major nuclear envelope protein, mutations in lamin A affect nuclear and cellular deformability, mechanotransduction, and cell polarization, which in turn leads to mechanically weaker nuclei, nuclear deformation, and irregular nuclear shape and size (47–49). Accumulation of LAΔ50, a lamin A mutant, causes nuclear shape abnormalities in patients with HGPS. Moreover, this nuclear shape cannot be restored by wild-type lamin A (49). Inhibition of this aberrant splicing event (Δ50 isoform) reverses nuclear structure defects associated with aging (42). Forcing loss of lamin A/C causes irregular nuclear shape, and reintroduction of prelamin A or lamin C does not rescue the abnormal nuclear shape in mouse embryonic fibroblasts (37). Similar research shows that mouse embryonic fibroblasts lacking all nuclear lamins have abnormal nuclear shapes, and that expression of lamin A partially rescues the nuclear abnormalities (48). We observed wrinkled nuclei in IPF lungs and in fibroblasts overexpressing LMNAin. It is intriguing to speculate that this novel isoform of LMNA may contribute to the irregular nuclear shape in IPF, but this requires further study.
Acknowledgments
Acknowledgment
The authors thank Melody C. Baddoo with the Next Generation Sequence Analysis Core supported by the National Cancer Institute (P01CA214091) for RNA splicing analysis, Jessie Guidry from the LSU proteomics core facility for providing the proteomics service, and the Morphology and Imaging Core at LSU for performing the immunohistochemical staining. HPL1D cells are gifts from Dr. Takashi Takahashi of Nagoya University Graduate School of Medicine.
Footnotes
Supported by the COBRE in aging and Regenerative Medicine at Tulane (J.A.L.), the John Deming Endowed Chair for Research (J.A.L.), the Carol Lavin Bernick Faculty Grant Program (Q.Y.), the Tulane School of Medicine Pilot Fund (Q.Y.), the Howard B. Kenyon Research Fund (J.A.L.), a U.S. Department of Veterans Affairs Merit Award (I01BX003056, V.J.T.), and National Institutes of Health grants P20 GM103629 (S.M.J. and J.A.L.) and P01 HL114470 (V.J.T.).
Author Contributions: Q.Y. designed and performed RNA sequencing data analysis and wrote the manuscript. G.F.M. contributed data interpretation and technical support. S.S. performed HPL1D, NHLF1, and A549 cell culture. Y.Z. performed fibroblast and lung tissue protein extraction. V.J.T. provided formalin-fixed, paraffin-embedded slides and IPF fibroblasts and assisted with data interpretation. S.M.J. edited the manuscript and provided idea development. J.A.L. contributed via conception and supervision of the project and cowrote the manuscript.
Data availability statement: Raw and processed RNA sequencing data are available at Gene Expression Omnibus with accession code GSE138283. The full sequence of LMNAin has been submitted to GenBank with accession code MZ054260.
This article has a data supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1165/rcmb.2022-0222OC on February 27, 2023
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Selman M, Pardo A. Alveolar epithelial cell disintegrity and subsequent activation: a key process in pulmonary fibrosis. Am J Respir Crit Care Med . 2012;186:119–121. doi: 10.1164/rccm.201206-0997ED. [DOI] [PubMed] [Google Scholar]
- 2. Upagupta C, Shimbori C, Alsilmi R, Kolb M. Matrix abnormalities in pulmonary fibrosis. Eur Respir Rev . 2018;27:180033. doi: 10.1183/16000617.0033-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wolters PJ, Collard HR, Jones KD. Pathogenesis of idiopathic pulmonary fibrosis. Annu Rev Pathol . 2014;9:157–179. doi: 10.1146/annurev-pathol-012513-104706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Watt FM, Huck WT. Role of the extracellular matrix in regulating stem cell fate. Nat Rev Mol Cell Biol . 2013;14:467–473. doi: 10.1038/nrm3620. [DOI] [PubMed] [Google Scholar]
- 5. Osmanagic-Myers S, Dechat T, Foisner R. Lamins at the crossroads of mechanosignaling. Genes Dev . 2015;29:225–237. doi: 10.1101/gad.255968.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fisher DZ, Chaudhary N, Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc Natl Acad Sci USA . 1986;83:6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKeon FD, Kirschner MW, Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature . 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 8. Prokocimer M, Davidovich M, Nissim-Rafinia M, Wiesel-Motiuk N, Bar DZ, Barkan R, et al. Nuclear lamins: key regulators of nuclear structure and activities. J Cell Mol Med . 2009;13:1059–1085. doi: 10.1111/j.1582-4934.2008.00676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lin F, Worman HJ. Structural organization of the human gene encoding nuclear lamin A and nuclear lamin C. J Biol Chem . 1993;268:16321–16326. [PubMed] [Google Scholar]
- 10. Vorburger K, Kitten GT, Nigg EA. Modification of nuclear lamin proteins by a mevalonic acid derivative occurs in reticulocyte lysates and requires the cysteine residue of the C-terminal CXXM motif. EMBO J . 1989;8:4007–4013. doi: 10.1002/j.1460-2075.1989.tb08583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M. The processing pathway of prelamin A. J Cell Sci . 1994;107:61–67. doi: 10.1242/jcs.107.1.61. [DOI] [PubMed] [Google Scholar]
- 12. Wilson KL, Berk JM. The nuclear envelope at a glance. J Cell Sci . 2010;123:1973–1978. doi: 10.1242/jcs.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lochs SJA, Kefalopoulou S, Kind J. Lamina associated domains and gene regulation in development and cancer. Cells . 2019;8:271. doi: 10.3390/cells8030271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho S, Vashisth M, Abbas A, Majkut S, Vogel K, Xia Y, et al. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev Cell . 2019;49:920–935.e5. doi: 10.1016/j.devcel.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ragnauth CD, Warren DT, Liu Y, McNair R, Tajsic T, Figg N, et al. Prelamin A acts to accelerate smooth muscle cell senescence and is a novel biomarker of human vascular aging. Circulation . 2010;121:2200–2210. doi: 10.1161/CIRCULATIONAHA.109.902056. [DOI] [PubMed] [Google Scholar]
- 16. Bonne G, Di Barletta MR, Varnous S, Bécane HM, Hammouda EH, Merlini L, et al. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet . 1999;21:285–288. doi: 10.1038/6799. [DOI] [PubMed] [Google Scholar]
- 17. Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, et al. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med . 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 18. Eriksson M, Brown WT, Gordon LB, Glynn MW, Singer J, Scott L, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature . 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Otomo J, Kure S, Shiba T, Karibe A, Shinozaki T, Yagi T, et al. Electrophysiological and histopathological characteristics of progressive atrioventricular block accompanied by familial dilated cardiomyopathy caused by a novel mutation of lamin A/C gene. J Cardiovasc Electrophysiol . 2005;16:137–145. doi: 10.1046/j.1540-8167.2004.40096.x. [DOI] [PubMed] [Google Scholar]
- 20. Morel CF, Thomas MA, Cao H, O’Neil CH, Pickering JG, Foulkes WD, et al. A LMNA splicing mutation in two sisters with severe Dunnigan-type familial partial lipodystrophy type 2. J Clin Endocrinol Metab . 2006;91:2689–2695. doi: 10.1210/jc.2005-2746. [DOI] [PubMed] [Google Scholar]
- 21. Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, et al. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet . 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 22. De Sandre-Giovannoli A, Bernard R, Cau P, Navarro C, Amiel J, Boccaccio I, et al. Lamin A truncation in Hutchinson-Gilford progeria. Science . 2003;300:2055. doi: 10.1126/science.1084125. [DOI] [PubMed] [Google Scholar]
- 23. Yin Q, Strong MJ, Zhuang Y, Flemington EK, Kaminski N, de Andrade JA, et al. Assessment of viral RNA in idiopathic pulmonary fibrosis using RNA-seq. BMC Pulm Med . 2020;20:81. doi: 10.1186/s12890-020-1114-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Masuda A, Kondo M, Saito T, Yatabe Y, Kobayashi T, Okamoto M, et al. Establishment of human peripheral lung epithelial cell lines (HPL1) retaining differentiated characteristics and responsiveness to epidermal growth factor, hepatocyte growth factor, and transforming growth factor beta1. Cancer Res . 1997;57:4898–4904. [PubMed] [Google Scholar]
- 25. Deng N, Sanchez CG, Lasky JA, Zhu D. Detecting splicing variants in idiopathic pulmonary fibrosis from non-differentially expressed genes. PLoS One . 2013;8:e68352. doi: 10.1371/journal.pone.0068352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhu D, Deng N, Bai C. A generalized dSpliceType framework to detect differential splicing and differential expression events using RNA-Seq. IEEE Trans Nanobioscience . 2015;14:192–202. doi: 10.1109/TNB.2015.2388593. [DOI] [PubMed] [Google Scholar]
- 27. Garcia CK. Running short on time: lung transplant evaluation for telomere-related pulmonary fibrosis. Chest . 2015;147:1450–1452. doi: 10.1378/chest.15-0077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alder JK, Chen JJ, Lancaster L, Danoff S, Su SC, Cogan JD, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci USA . 2008;105:13051–13056. doi: 10.1073/pnas.0804280105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kuwano K, Araya J, Hara H, Minagawa S, Takasaka N, Ito S, et al. Cellular senescence and autophagy in the pathogenesis of chronic obstructive pulmonary disease (COPD) and idiopathic pulmonary fibrosis (IPF) Respir Investig . 2016;54:397–406. doi: 10.1016/j.resinv.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 30. Armanios MY, Chen JJ, Cogan JD, Alder JK, Ingersoll RG, Markin C, et al. Telomerase mutations in families with idiopathic pulmonary fibrosis. N Engl J Med . 2007;356:1317–1326. doi: 10.1056/NEJMoa066157. [DOI] [PubMed] [Google Scholar]
- 31. Gonzalez-Suarez I, Redwood AB, Perkins SM, Vermolen B, Lichtensztejin D, Grotsky DA, et al. Novel roles for A-type lamins in telomere biology and the DNA damage response pathway. EMBO J . 2009;28:2414–2427. doi: 10.1038/emboj.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kruk PA, Rampino NJ, Bohr VA. DNA damage and repair in telomeres: relation to aging. Proc Natl Acad Sci USA . 1995;92:258–262. doi: 10.1073/pnas.92.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen M, Lyu G, Han M, Nie H, Shen T, Chen W, et al. 3′ UTR lengthening as a novel mechanism in regulating cellular senescence. Genome Res . 2018;28:285–294. doi: 10.1101/gr.224451.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Liu F, Mih JD, Tschumperlin D. Increasing matrix stiffness shifts normal human lung fibroblast secretions in favor of fibrosis. Am J Respir Crit Care Med . 2011;183:A3564. [Google Scholar]
- 35. Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, et al. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med . 2012;186:866–876. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. González-Cruz RD, Dahl KN, Darling EM. The emerging role of lamin C as an important LMNA isoform in mechanophenotype. Front Cell Dev Biol . 2018;6:151. doi: 10.3389/fcell.2018.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lammerding J, Fong LG, Ji JY, Reue K, Stewart CL, Young SG, et al. Lamins A and C but not lamin B1 regulate nuclear mechanics. J Biol Chem . 2006;281:25768–25780. doi: 10.1074/jbc.M513511200. [DOI] [PubMed] [Google Scholar]
- 38. Lammerding J, Schulze PC, Takahashi T, Kozlov S, Sullivan T, Kamm RD, et al. Lamin A/C deficiency causes defective nuclear mechanics and mechanotransduction. J Clin Invest . 2004;113:370–378. doi: 10.1172/JCI19670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bermeo S, Vidal C, Zhou H, Duque G. Lamin A/C acts as an essential factor in mesenchymal stem cell differentiation through the regulation of the dynamics of the Wnt/β-catenin pathway. J Cell Biochem . 2015;116:2344–2353. doi: 10.1002/jcb.25185. [DOI] [PubMed] [Google Scholar]
- 40. Lee JM, Nobumori C, Tu Y, Choi C, Yang SH, Jung HJ, et al. Modulation of LMNA splicing as a strategy to treat prelamin A diseases. J Clin Invest . 2016;126:1592–1602. doi: 10.1172/JCI85908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang Y, Wang J, Huang W, Cai J, Ba J, Wang Y, et al. Nuclear Nestin deficiency drives tumor senescence via lamin A/C-dependent nuclear deformation. Nat Commun . 2018;9:3613. doi: 10.1038/s41467-018-05808-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science . 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Van Berlo JH, Voncken JW, Kubben N, Broers JL, Duisters R, van Leeuwen RE, et al. A-type lamins are essential for TGF-beta1 induced PP2A to dephosphorylate transcription factors. Hum Mol Genet . 2005;14:2839–2849. doi: 10.1093/hmg/ddi316. [DOI] [PubMed] [Google Scholar]
- 44. Qi YX, Yao QP, Huang K, Shi Q, Zhang P, Wang GL, et al. Nuclear envelope proteins modulate proliferation of vascular smooth muscle cells during cyclic stretch application. Proc Natl Acad Sci USA . 2016;113:5293–5298. doi: 10.1073/pnas.1604569113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Piekarowicz K, Machowska M, Dratkiewicz E, Lorek D, Madej-Pilarczyk A, Rzepecki R. The effect of the lamin A and its mutants on nuclear structure, cell proliferation, protein stability, and mobility in embryonic cells. Chromosoma . 2017;126:501–517. doi: 10.1007/s00412-016-0610-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, et al. Cellular senescence mediates fibrotic pulmonary disease. Nat Commun . 2017;8:14532. doi: 10.1038/ncomms14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dahl KN, Ribeiro AJS, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res . 2008;102:1307–1318. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen NY, Kim P, Weston TA, Edillo L, Tu Y, Fong LG, et al. Fibroblasts lacking nuclear lamins do not have nuclear blebs or protrusions but nevertheless have frequent nuclear membrane ruptures. Proc Natl Acad Sci USA . 2018;115:10100–10105. doi: 10.1073/pnas.1812622115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Goldman RD, Shumaker DK, Erdos MR, Eriksson M, Goldman AE, Gordon LB, et al. Accumulation of mutant lamin A causes progressive changes in nuclear architecture in Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA . 2004;101:8963–8968. doi: 10.1073/pnas.0402943101. [DOI] [PMC free article] [PubMed] [Google Scholar]