Abstract
Immune checkpoint inhibitors are a well-established treatment option for advanced urothelial carcinoma, and biomarkers of response are needed for better patient selection. We show that metastatic disease confined to lymph nodes is associated with better outcomes, while metastases to liver, bone or both are associated with poor outcomes with immune checkpoint inhibitor therapy. Results are hypothesis-generating but relevant to practice.
Background:
Sites of metastasis have prognostic significance in advanced urothelial carcinoma (aUC), but more information is needed regarding outcomes based on metastatic sites in patients treated with immune checkpoint inhibitors (ICI). We hypothesized that presence of liver/bone metastases would be associated with worse outcomes with ICI.
Methods:
We identified a retrospective cohort of patients with aUC across 26 institutions, collecting demographics, clinicopathological, treatment, and outcomes information. Outcomes were compared with logistic (observed response rate; ORR) and Cox (progression-free survival; PFS, overall survival; OS) regression between patients with/without metastasis beyond lymph nodes (LN) and those with/without bone/liver/lung metastasis. Analysis was stratified by 1st or 2nd+ line.
Results:
We identified 917 ICI-treated patients: in the 1st line, bone/liver metastases were associated with shorter PFS (Hazard ratio; HR: 1.65 and 2.54), OS (HR: 1.60 and 2.35, respectively) and lower ORR (OR: 0.48 and 0.31). In the 2nd+ line, bone/liver metastases were associated with shorter PFS (HR: 1.71 and 1.62), OS (HR: 1.76 and 1.56) and, for bone-only metastases, lower ORR (OR: 0.29). In the 1st line, LN-confined metastasis was associated with longer PFS (HR: 0.53), OS (HR:0.49) and higher ORR (OR: 2.97). In the 2nd+ line, LN-confined metastasis was associated with longer PFS (HR: 0.47), OS (HR: 0.54), and higher ORR (OR: 2.79); all associations were significant.
Conclusion:
Bone and/or liver metastases were associated with worse, while LN-confined metastases were associated with better outcomes in patients with aUC receiving ICI. These findings in a large population treated outside clinical trials corroborate data from trial subset analyses.
Keywords: Bladder cancer, Immune checkpoint inhibitors, Advanced urothelial carcinoma, Outcomes, Metastatic cancer
Introduction
Locally advanced/unresectable or metastatic (herein described as ‘advanced’) urothelial carcinoma (aUC) can cause significant morbidity and mortality. Recently there have been major advances in the management of aUC with the use of immune checkpoint inhibitors (ICI) in the frontline, switch maintenance and salvage settings,1,2 as well as antibody-drug conjugates (enfortumab-vedotin, sacituzumab-govitecan) and the Fibroblast Growth Factor Receptor (FGFR) inhibitor erdafitinib in the salvage setting.3–5 However, the observed response rates (ORR) and progression-free survival (PFS) achieved with ICI remain modest across treatment settings, while there is a considerable risk of immune related adverse events.1 This status emphasizes the need for the discovery and validation of accurate both prognostic and predictive biomarkers to help select patients more likely to benefit and reduce (or delay) ICI exposure to those who are unlikely to benefit. While predictive biomarkers have higher value in patient selection for particular therapies and optimal sequencing of agents in aUC, prognostic biomarkers can still help inform discussions about prognostic estimates, as well as in clinical trial design, stratification, and interpretation of results.
There have been several proposed clinical and molecular putative biomarkers associated with survival with ICI in aUC.6–11 Several clinical factors, such as performance status, sites of metastasis (eg, liver) albumin and hemoglobin levels have been shown to hold prognostic value for patients receiving ICI in the first (1st) line setting.10 Moreover, data from clinical trials and retrospective studies suggest that patients with liver and bone metastasis who receive ICI have overall poor outcomes.12–14 A retrospective study also has suggested that bone metastasis at time of aUC diagnosis is associated with poor outcomes with ICI.15
However, questions remain regarding the response and outcomes with ICI based on specific sites of metastasis and line of therapy, when adjusting, as possible, for other known prognostic factors. Additionally, data on the association between lung metastasis and response or outcomes with ICIs in aUC remains scarce at this point. Presence of liver, bone or lung metastases could potentially be associated with immunosuppression and poor response and outcomes with ICI. To further investigate the association between metastatic sites and outcomes with ICI, we evaluated response and survival with ICI in patients with aUC according to metastatic site. We hypothesized that response and survival with ICI would be inferior in patients with metastases not confined to lymph nodes (LNs), especially those with liver and/or bone metastases, as opposed to patients with LN-confined metastatic disease. We also hypothesized that there would be a significant interaction between the co-presence of liver and bone metastases regarding outcomes with ICI.
Patients & Methods
Study Design, Patients and Data Collection
In concordance with the Declaration of Helsinki and after obtaining institutional review board approval, we performed a retrospective cohort study, using a cohort of patients from 25 institutions across the United States and Europe.10,16–18 Consecutive patients at each institution were identified using a combination of provider-driven and electronic health record search algorithms.
Patients were included for analysis if they were treated with ICI monotherapy for aUC. We excluded patients who received ICI for a different cancer type or in a combination regimen or on a clinical trial, had pure non-urothelial histology, received more than one line of ICI therapy, or the sites of metastasis were unknown (Figure 1-Consort diagram). In addition, patients were excluded from the efficacy analysis, eg, ORR, PFS, overall survival (OS) if the information regarding the endpoint was missing.
Figure 1.

Consort diagram.
For data collection and storage, we used web-based, secure and standardized REDCap capture tools hosted at the Institute of Translational Sciences at the University of Washington.19,20 Data collected included demographics, stage, histology type, prior therapies for earlier UC settings, laboratory values, sites of metastatic disease, laboratory parameters and clinical endpoints (eg, response, progression, death). For metastatic sites, we collected information about metastasis to the following sites: lymph node, soft tissue, lung, liver, bone, adrenal gland, CNS, peritoneum, intestine. Information on metastases on other miscellaneous sites outside of the categories above, was also collected.
Pathology and radiology data were assessed from notes in the electronic health record based on comprehensive investigator review; no centralized review of images or pathology was feasible. All patients underwent imaging at the discretion of treating provider. Evaluation of response and progression were determined by the investigator review based on the best available information in clinic notes and radiographic study reports without formal central radiology review based on strict RECIST 1.1 criteria.
Statistical Analysis
Baseline characteristics were summarized using descriptive statistics and compared via chi-square and Student’s t-tests, for categorical and continuous variables, respectively. ORR was calculated as the number of patients with complete or partial response divided by the total number of patients. OS was defined as time from ICI initiation to date of death from any cause, and PFS as time from ICI initiation until date of radiographic or clinical progression or death from any cause. Patients who did not experience death or progression were censored at the date of last follow-up.
All outcomes (ORR, PFS, OS) were analyzed separately for patients treated with ICI in the first line (1st) and the subsequent setting (second line and beyond; 2nd+). No patient with switch maintenance ICI was included. For our analysis, we created 3 separate models: in model A we compared patients with versus without LN confined metastasis; those with soft tissue recurrent/metastatic disease were classified as non-LN confined; in model B, we compared patients with no bone or liver metastasis with those having bone-only, liver-only, or both. We also tested the interaction between bone and liver metastases in this model, by modeling both bone and liver metastases as separate binary variables and including a multiplicative interaction term in the model. Finally, in model C, we compared patients with no bone, liver or lung metastasis with those having any combination of those 3 sites, such as liver-only, bone-only or lung-only, bone and liver without lung, liver and lung without bone, bone and lung without liver, or all 3 sites together. We also tested for any interaction between these sites (bone-liver, bone-lung, liver-lung, and bone-liver-lung).
We used univariable and multivariable logistic regression to estimate the odds ratios (OR) and confidence intervals (CI) for ORR dependent on exposure. For OS and PFS, we estimated median survival time using the Kaplan-Meier method. Hazard ratios (HR) dependent on exposure were calculated using univariable and multivariable Cox Regression. For multivariable modeling, we a priori identified factors hypothesized to possibly confound the relationship between metastatic site and efficacy endpoints (ORR, PFS, OS). We tested each factor independently in a model, retaining any variable for the final model that changed the outcome of interest (OR or HR) by > 10%. The factors that were tested were age (as a continuous variable), sex (male, female), white race (yes, no), smoking history (yes, no), history of cystectomy (or [nephro]ureterectomy for upper tract UC) (yes, no), histology (pure urothelial carcinoma vs. mixed), presence of upper tract urothelial carcinoma (yes, no) and ECOG PS (0–4, modeled as discrete categories) along with hemoglobin, albumin, and Neutrophil to Lymphocyte Ratio (NLR). Hemoglobin, albumin and NLR were all modeled as continuous variables. The alpha level was set at 0.05 for all analyses, which were performed with Stata IC 16.0 (Stata LLC, College Station, Texas).
Results
Patient Characteristics
We analyzed data from 1283 patients with aUC treated with ICIs across 26 institutions between 2013 and 2021. After exclusions, we identified 917 patients that were treated with ICI monotherapy for aUC. Ultimately, 886 were included in the ORR, 898 patients were included in the PFS and 876 in the OS analysis, respectively. Baseline characteristics of patients are shown on Table 1, stratified by line of therapy (1st and 2nd+). Among 504 patients treated with 1st line and 413 patients with 2nd+ line ICIs, pure urothelial histology was present in 69% and 74% of cases, respectively. At ICI initiation, LN-metastatic disease was present in 72% and 79% for 1st and 2nd+ line, respectively, while the prevalence of liver metastasis was 17% and 25% for 1st and 2nd+ line, of bone metastasis was 22% and 32%, and of lung metastasis was 32% and 39% respectively. (Table 1) Patient characteristics by site of metastasis (LN and bone, lung or liver) and line of therapy (1st or 2nd+) can be found in supplementary tables. (Table S1–S4).
Table 1.
Patient Population Demographics.
| First Line ICI N = 504 | Subsequent Line ICI N = 413 | ||
|---|---|---|---|
| Age at ICI initiation | 71 (11) | 68 (10) | |
| Sex | Male | 360 (71%) | 313 (76%) |
| Female | 144 (29%) | 100 (24%) | |
| Smoking History | No | 162 (32%) | 136 (33%) |
| Yes | 339 (68%) | 273 (67%) | |
| White race | no | 109 (22%) | 89 (22%) |
| yes | 395 (78%) | 324 (79%) | |
| History of cystectomy or (nephro)ureterectomy | No | 210 (44%) | 192 (50%) |
| Yes | 268 (56%) | 191 (50%) | |
| Histology | Pure urothelial | 344 (69%) | 302 (74%) |
| Mixed urothelial | 158 (32%) | 108 (26%) | |
| Hemoglobin < 10mg/dl at ICI initiation | No | 371 (75%) | 291 (72%) |
| Yes | 124 (25%) | 113 (28%) | |
| Prior platinum chemotherapy | No | 303 (60%) | 29 (7%) |
| Yes | 201 (40%) | 384 (93%) | |
| ECOG PS at ICI initiation | 0 | 121 (26%) | 93 (25%) |
| 1 | 211 (46%) | 210 (57%) | |
| 2 | 112 (24%) | 53 (14%) | |
| 3 | 14 (3%) | 12 (3%) | |
| 4 | 2 (< 1%%) | 0 (0%) | |
| Bellmunt score at ICI initiation | 0 | 90 (20%) | 63 (17%) |
| 1 | 228 (50%) | 166 (46%) | |
| 2 | 117 (26%) | 110 (30%) | |
| 3 | 17 (4%) | 25 (7%) | |
| Site of Primary Tumor | Bladder | 410 (83%) | 316 (79%) |
| Upper GU | 25 (5%) | 25 (6%) | |
| Urethra | 5 (1%) | 4 (1%) | |
| Renal Pelvis | 39 (8%) | 33 (8%) | |
| Ureter | 14 (3%) | 24 (6%) | |
| Lymph node metastasis at ICI initiation | no | 143 (28%) | 88 (21%) |
| yes | 361 (72%) | 325 (79%) | |
| Lung metastasis at ICI initiation | no | 342 (68%) | 251 (61%) |
| yes | 162 (32%) | 162 (39%) | |
| Liver metastasis at ICI initiation | no | 420 (83%) | 312 (76%) |
| yes | 84 (17%) | 101 (25%) | |
| Bone metastasis at ICI initiation | no | 394 (78%) | 282 (68%) |
| yes | 110 (22%) | 131 (32%) |
Observed Response Rate
A total of 886 patients were included in the ORR analysis; of those, 479 received ICI in the 1st line and 407 in the 2nd+ line. In the model A (“LN model”) analysis of both the 1st and 2nd+ lines, patients with LN-only metastasis had a significantly higher ORR compared to those with disease spread beyond LNs. (Table 2).
Table 2.
Lymph Node Metastatic Disease Model.
| Metastatic Sites | ORR | OR | PFS | HR | OS | HR | |
|---|---|---|---|---|---|---|---|
| 1st line ICI | Non LN - confined disease | 22 (18–26) | reference | 3 (3–4) | reference | 9 (7–12) | reference |
| LN - confined disease | 45 (37–54) | 2.97 (1.92–4.59) a | 11 (7–16) | 0.53 (0.41–0.69) a | 22 (14-NR) | 0.49 (0.37–0.65) a | |
| 2nd line ICI or greater | |||||||
| Non LN - confined disease | 19 (15–23) | reference | 4 (3–4) | reference | 8 (6–9) | reference | |
| LN - confined disease | 38 (28–49) | 2.79 (1.50–5.16) a b | 13 (6–21) | 0.47 (0.34–0.65) a | 18 (11-NR) | 0.54 (0.38–0.77) a c |
P < .05.
adjusted for cystectomy and albumin at ICI initiation.
adjusted for albumin.
In the model B (“bone-liver model”) analysis, the presence of bone metastases, liver metastases or both were each significantly associated with lower ORR versus those without bone or liver metastases in the 1st line setting; among patients treated with ICI as 2nd+ line, only bone metastases were associated with a significantly lower ORR (Table 3). However, in our third model (Model C) only liver metastases retained a significant association with lower ORR in the 1st line. Similarly, in the 2nd+ line, only bone and bone & lung metastases showed a significant association with lower ORR. Lung-only metastases were not significantly associated with lower ORR (Table 4). We did not identify a significant interaction between the presence of bone, liver or lung metastases in either 1st or 2nd+ line setting.
Table 3.
Liver and Bone Metastatic Disease Model.
| Metastatic Sites | ORR | aOR | PFS | aHR | OS | aHR | |
|---|---|---|---|---|---|---|---|
| 1st line ICI | |||||||
| No liver-bone | 33 (28–39) | Reference | 9 (6–13) | Reference | 17 (13–21) | Reference | |
| Bone - only | 21 (14–32) | 0.48 (0.25–0.91) a b | 3 (2–6) | 1.65 (1.19–2.30) a c | 7 (4–11) | 1.60 (1.13–2.25) a d | |
| Liver - only | 15 (7–28) | 0.31 (0.13–0.75) a b | 2 (2–3) | 2.54 (1.77–3.66) a c | 5 (3–7) | 2.35 (1.66–3.34) a d | |
| Liver & bone | 4 (1–22) | 0.08 (0.01–0.06) a b | 2 (1–3) | 3.23 (1.96–5.32) a c | 2 (1–5) | 3.66 (2.31–5.79) a d | |
| 2nd line ICI or greater | |||||||
| No liver-bone | 28 (23–35) | Reference | 6 (5–8) | Reference | 12 (9–15) | Reference | |
| Bone - only | 12 (7–20) | 0.29 (0.13–0.67) a e | 4 (3–5) | 1.71 (1.28–2.28) a f | 7 (5–9) | 1.76 (1.32–2.35) a f | |
| Liver - only | 25 (16–37) | 0.70 (0.34–1.45) e | 3 (2–6) | 1.62 (1.09–2.41) a f | 7 (4–11) | 1.56 (1.04–2.34) a f | |
| Liver & Bone | 11 (4–27) | 0.45 (0.15–1.39) e | 2 (2–3) | 2.32 (1.34–4.03) a f | 3 (2–5) | 2.53 (1.47–4.38) a f |
P < .05.
adjusted for cystectomy, site of primary (upper vs lower) and albumin at ICI initiation.
adjusted for albumin at ICI initiation.
adjusted for ECOG PS at ICI initiation.
adjusted for cystectomy, hemoglobin, albumin and NLR at ICI initiation.
adjusted for cystectomy and NLR at ICI initiation.
Table 4.
Liver, Bone and Lung Metastasic Disease Model.
| Metastatic Sites | ORR | aOR | PFS | aHR | OS | aHR | |
|---|---|---|---|---|---|---|---|
| 1st line ICI | |||||||
| No liver-bone-lung | 37 (31–43) | Reference | 7 (5–11) | Reference | 17 (12–22) | Reference | |
| Bone - only | 20 (12–33) | 0.48 (0.23–1.01) b | 3 (2–5) | 1.42 (0.93–2.16) c | 7 (3–13) | 1.55 (0.96–2.51) d | |
| Liver - only | 13 (5–29) | 0.21 (0.06–0.72) a b | 2 (1–3) | 2.43 (1.55–3.83) a c | 5 (3–14) | 2.46 (1.54–3.94) a d | |
| Lung-only | 26 (18–35) | 0.69 (0.40–1.21) b | 4 (3–6) | 1.32 (0.96–1.83) c | 18 (11–23) | 1.25 (0.87–1.79) d | |
| Liver & bone | 6 (1–32) | 0.14 (0.02–1.04) b | 1 (1–4) | 2.09 (1.08–4.05) a c | 2 (1–5) | 2.81 (1.46–5.37) a d | |
| Lung & bone | 24 (10–46) | 0.31 (0.09–1.11) b | 4 (1–12) | 1.39 (0.71–2.74) c | 11 (3-NR) | 1.59 (0.80–3.14) d | |
| Lung & liver | 19 (6–45) | 0.33 (0.07–1.65) b | 2 (2–3) | 2.52 (1.40–4.55) a c | 4 (2–7) | 3.74 (2.11–6.62) a d | |
| Lung, liver & bone | 0 | - | 2 (0.2–2) | 3.92 (1.77–8.70) a c | 2 (0.2–13) | 4.41 (2.19–8.88) a d | |
| 2nd line ICI or greater | |||||||
| No liver-bone | 32 (25–41) | Reference | 7 (5–10) | Reference | 14 (11–19) | Reference | |
| Bone - only | 14 (7–25) | 0.25 (0.09–0.72) a e | 4 (3–7) | 1.75 (1.17–2.63) a f | 8 (5–10) | 1.87 (1.24–2.82) a g | |
| Liver - only | 24 (13–39) | 0.49 (0.20–1.23) e | 3 (2–4) | 2.20 (1.34–3.59) a f | 9 (4–14) | 1.61 (0.93–2.80) g | |
| Lung-only | 22 (15–32) | 0.67 (0.33–1.36) e | 4 (3–6) | 1.58 (1.10–2.28) a f | 8 (6–12) | 1.33 (0.90–1.97) g | |
| Liver & Bone | 9 (2–30) | 0.28 (0.05–1.52) e | 2 (1–3) | 2.91 (1.30–6.52) a f | 2 (2–5) | 2.80 (1.21–6.44) a g | |
| Lung & bone | 8 (3–23) | 0.25 (0.07–0.87) a e | 3 (2–5) | 2.49 (1.72–3.61) a f | 6 (4–9) | 1.96 (1.31–2.95) a g | |
| Lung & liver | 26 (12–47) | 1.13 (0.34–3.83) e | 3 (2–3) | 2.34 (1.27–4.33) a f | 4 (3–9) | 2.14 (1.14–4.02) a g | |
| Lung, liver & bone | 15 (4–45) | 0.60 (0.13–2.80) e | 2 (1–3) | 2.64 (1.24–5.60) a f | 3 (1–9) | 2.78 (1.26–6.15) a g |
P < .05.
adjusted for cystectomy, site of primary (upper vs. lower), albumin at ICI initiation and NLR at ICI initiation.
adjusted for cystectomy, site of primary (upper vs. lower), and albumin at ICI initiation.
adjusted for cystectomy, site of primary (upper vs. lower), ECOG at ICI initiation, albumin, hemoglobin and NLR at ICI initiation.
adjusted for cystectomy, site of primary (upper vs. lower), albumin, hemoglobin and NLR at ICI initiation.
adjusted for cystectomy, albumin, hemoglobin and NLR at ICI initiation.
adjusted for cystectomy, ECOG at ICI initiation, albumin, hemoglobin and NLR at ICI initiation.
Progression-Free Survival
A total of 898 patients were included in the PFS analysis; of those, 495 were treated with ICI in the 1st line and 403 in the 2nd+ line. In both treatment settings, the presence of LN-confined metastasis was significantly associated with longer PFS compared to non-LN confined metastasis (Table 2, Figure 2).
Figure 2.

Progression-free survival. 2a. Lymph node (LN) model (model A). 2b. Liver & bone model (model B).
In the model B (bone-liver) analysis, the presence of bone metastasis, liver metastasis or both were associated with shorter PFS compared to neither bone or liver, in both 1st line and 2nd+ line settings (Table 3). In Model C, liver-only metastasis, as well as co-presence of liver and bone, liver and lung, as well as liver, lung & bone metastases were associated with significantly shorter PFS in the 1st line setting. In the 2nd+ line, all those single sites (liver, lung, bone) of metastases as well as their combinations (liver-bone, liver-lung, bone-lung, and liver-bone-lung) were significantly associated with shorter PFS (Table 4). No significant interaction was detected between bone, liver or lung metastases for either 1st or 2nd+ line.
Overall Survival
A total of 876 patients were included in the OS analysis: 485 received 1st line ICI and 391 2nd+ line ICI. In the model A (LN model) analysis, patients treated with 1st line or 2nd+ ICIs with LN-confined metastasis had significantly longer OS compared to those without LN-confined metastasis (Table 2, Figure 3).
Figure 3.
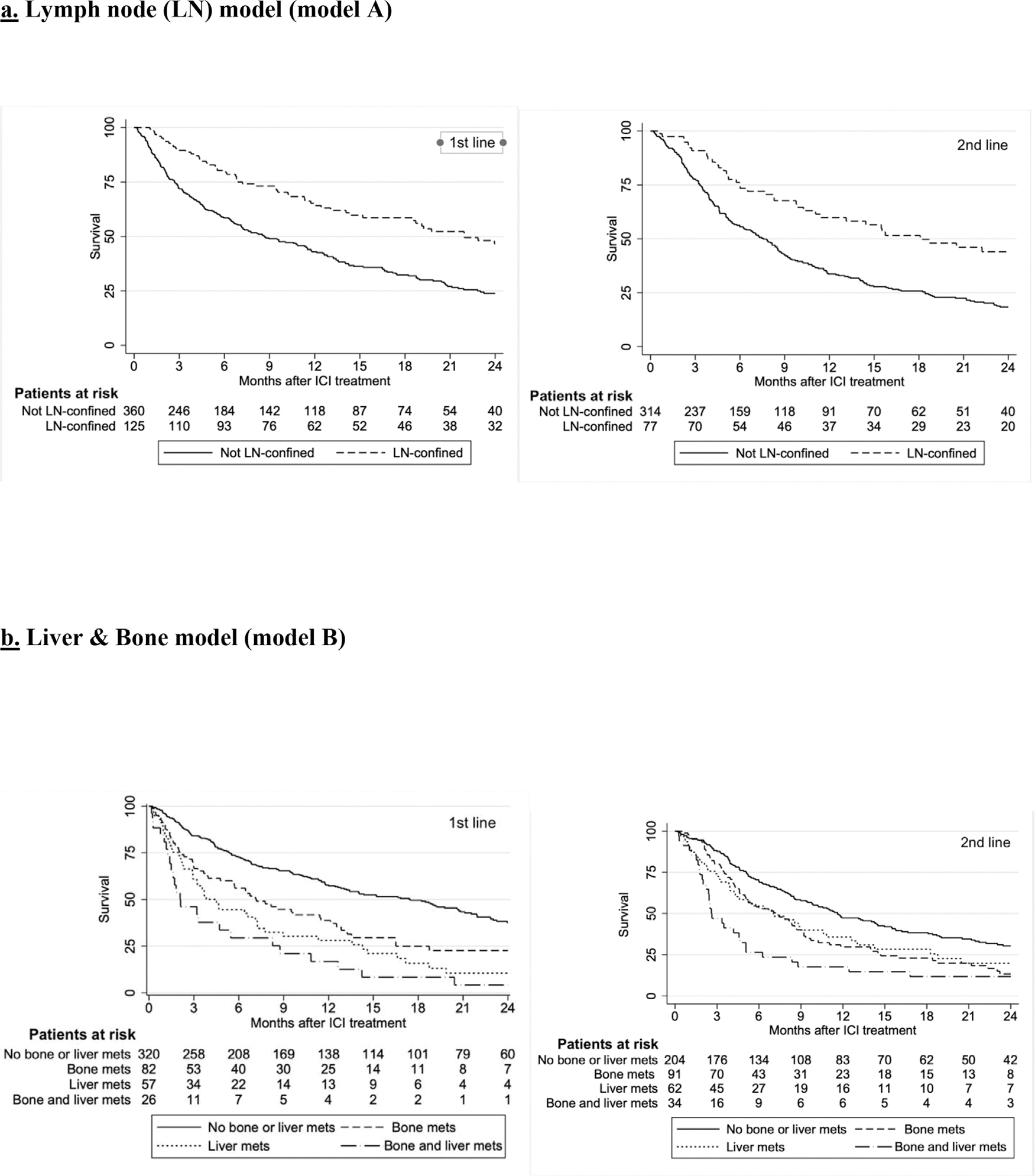
Overall survival 3a. Lymph node (LN) model (model A). 3b. Liver & bone model (model B).
In the model B (bone-liver) analysis, patients with bone metastases, liver metastases or both had shorter OS compared to patients without either bone or liver metastases in both 1st line and 2nd+ line settings (Table 3, Figure 3). In the model C, liver-only metastases, along with bone and liver, lung and liver, as well as co-occurrence of bone, liver and lung metastases were significantly associated with shorter OS in the 1st line setting. In the 2nd+ line, bone-only metastases along with all combinations (bone and lung, bone and liver, liver and lung, as well as bone-liver-lung) were significantly associated with shorter OS (Table 4). Like ORR and PFS analyses, no significant interaction between liver and bone lesions was detected in either the 1st or the 2nd+ line.
Discussion
In this retrospective cohort of 917 patients with aUC treated with ICI outside clinical trials, we demonstrated that sites of metastases were associated with different outcomes on ICI therapy. Specifically, patients with aUC confined to LNs had significantly higher ORR, longer PFS and OS compared to patients without LN-confined disease, when treated with ICI as either 1st or 2nd+ line of therapy. Moreover, bone and liver metastases were associated with significantly lower ORR, shorter PFS and OS in both therapy settings. We did not identify a significant interaction between metastatic sites regarding outcomes with ICI therapy.
Previous studies have reported the association between specific sites of metastases and treatment outcomes in aUC. In a widely used prognostic model, Bajorin et al. identified the presence of visceral metastasis and poor ECOG PS to be associated with shorter OS in patients treated with 1st line platinum-based chemotherapy.8 Similarly, the prognostic model by Bellmunt et al. in patients with platinum-refractory aUC treated with vinflunine chemotherapy identified liver metastasis, along with ECOG PS greater than 0 and Hgb < 10 g/dL, as clinical factors associated with shorter OS. More recently, prognostic models developed in patients treated with ICI have also identified metastatic sites to have prognostic significance. A risk score developed by Khaki et al.10 using an earlier data-lock of this cohort and assessing prognostic factors for 1st line ICI treatment in aUC, showed that liver metastases were associated with worse prognosis. For those treated with ICI in the 2nd+ line, a 5-factor prognostic model for response to ICI also identified liver metastasis as associated with significantly shorter OS,9 while another prognostic model by Nassar et al.11 identified visceral metastasis as a poor prognostic feature.
While multiple prior prognostic models have specifically identified liver metastasis as a poor prognostic factor, an important additional finding in our study is the similarly poor prognostic outcomes with bone metastasis. Bone metastasis has been recently identified as a risk factor for worse outcomes in aUC. Nelson et al. reported a significant association between the presence of early bone metastases (defined as present at the time of metastatic diagnosis) and shorter OS for patients receiving either chemotherapy or ICI for aUC. In a separate analysis comparing the effect of bone metastasis to treatment with ICI versus chemotherapy, no significant difference in survival was found, suggesting that presence of bone metastasis is a negative prognostic factor independent of treatment type. Of note, liver metastasis was also identified as a negative prognostic factor in that study.21 In another study of patients with aUC treated with 2nd+ line ICI, Raggi et al. showed that bone metastasis was associated with significantly shorter OS and PFS.22
Similarly, another study by de Liano Lista et al. showed that concurrent presence of bone and liver metastases, along with multi-metastatic disease (defined as ≥3 sites) was associated with shorter OS in patients with aUC treated with ICIs in the 1st line setting. The association persisted in the 2nd line, while the presence of liver-only and bone-only metastasis was significantly associated with shorter OS, while LN-confined metastasis was associated with longer OS.23 Moreover, Ma et al. assessed 160 patients with urothelial or renal cell carcinoma treated with ICI and showed that the presence of liver metastasis was associated with significantly worse ORR, PFS and OS. Of interest, comparing liver to LN-confined metastasis, CNS or bone metastasis showed that patients with liver metastasis had worse outcomes.24
In our study, we also investigated the putative association between lung metastasis and outcomes with ICI; interestingly, presence of lung, without liver or bone metastases, was significantly associated with shorter PFS only in the 2nd+ line setting. Lung metastasis was associated with worse outcomes when co-present with liver and/or bone metastases. Results may suggest that lung-confined metastasis may not have the same magnitude as a negative prognostic factor in patients with aUC treated with ICI, but more studies are needed to evaluate this hypothesis.
Subgroup analyses from clinical trials evaluating ICI in aUC are aligned with our findings. In the IMvigor 210 trial, ORR with atezolizumab was 23% (95% CI:16–31) for the entire cohort 1 (consisting of cisplatin-ineligible patients without prior treatment for aUC), but numerically higher in patients with LN-confined metastasis (n = 31; 32% [95% CI:17–51]) and lower for those with liver metastases (n = 25, 8% [95%CI: 1–26]). Median OS in patients with liver metastasis was also shorter (5.5 vs. 15.9 months in all patients).12 In the Keynote 052 trial, patients with LN-confined metastasis (n = 51) had higher ORR on pembrolizumab than those with visceral metastases (n = 315): 47% (95% CI:31–62) versus 23% (95% CI:18–29).13 In the Keynote 045 trial, while the HR for OS was <1 (favoring pembrolizumab vs. salvage chemotherapy) for both patients with and without liver metastasis, HR for those with liver metastasis was higher (0.85 vs. 0.67), which might suggest lower degree of benefit in this population, though the trial was not powered for these subgroup analyses.14
Although the above subset analyses were purely exploratory and should not be over-interpreted due to the small sample size and number of events, they raise the hypothesis of worse outcomes in patients with non-LN metastases treated with ICI. These results in the context of our findings suggest that the spread of tumors beyond LNs may possibly herald a shift in cancer biology and/or suggest that the LN-confined metastasis could possibly be more sensitive to ICI due to a strong presence of immune cells in LNs. However, it is important to note that visceral (liver, lung or bone) metastasis appear as a negative prognostic factor since patients seem to have worse outcomes regardless of therapy, eg, chemotherapy or ICI. Moreover, there are not adequate datasets to clarify the putative predictive role of metastatic site with regards to response and survival to individual therapies. The latter ‘predictive’ question requires further analysis in randomized trials and cannot be answered in single arm trials and retrospective studies.
It is unclear whether the above findings could be attributed to underlying cancer biology/organotropism, such as increased clonal evolution, tumor heterogeneity, cancer burden, tumor mutation burden, quality of neoantigens, PD-L1 or other factors in the tumor microenvironment, and/or host-related factors, such as immunosuppression, T cell clonality and diversity, and other components of immune response.25,26 Emerging evidence also suggests that differences exist on immune-related factors, such as tumor-infiltrating lymphocytes and PDL1 expression on immune cells between primary tumors and metastatic sites.27 Such immune heterogeneity across metastatic sites could pose significant treatment challenges. Disease heterogeneity seems to extend to a molecular level as well; recent work showed that MTAP-deficient tumors seem significantly more likely to present with visceral metastasis, such as liver or lung, and are associated with worse prognosis with ICI.28 Another study on heterogeneity following chemotherapy showed that chemotherapy-treated aUC had increased mutational variation between different clones, while clonal expansion of primary tumors happened early in the disease course.29 Similarly, a recently developed model/score of clinical (NLR ≥5, visceral metastasis) and molecular factors (Single-Nucleotide Variant < 10) correlated with no benefit with ICI in patients with aUC.11 Such findings require further validation in larger cohorts; they, however, may suggest a distinct molecular background associated with early visceral metastasis and poor outcomes with ICI, and could be useful in deciding optimal sequencing of ICI therapy in the future.
Notably, in addition to ICI, other agents, such as enfortumab-vedotin, erdafitinib and sacituzumab-govitecan have been approved by the FDA for treatment-refractory aUC. For patients with FGFR2 or FGFR3 activating mutation or fusion, the BLC 2001 single arm trial with erdafitinib showed an ORR 40% in patients with progression on at least one prior course of chemotherapy. In this study, outcomes were similar among patients with liver (ORR 35% [95% CI: 14–56]) and bone metastasis (ORR 48% (95% CI: 26–69) compared to the overall population.4 Similarly, in a subgroup analysis of the EV201 trial with 2nd line enfortumab-vedotin in cisplatin-ineligible patients previously treated with ICI, outcomes were similar between patients with or without liver metastasis; ORR in patients with liver lesions was 48% (95% CI: 26–70) and 53% (95% CI: 40–65) for those without.30 In the EV301 trial of EV for patients previously treated with platinum-based chemotherapy and ICI, HR for OS in patients with liver metastasis (HR: 0.66, 95% CI: 0.46–0.96) was similar to the HR for those without liver metastasis (HR: 0.73 95% CI:0.55–0.98). Similarly, in a real-world study of patients with aUC treated with enfortumab-vedotin (UNITE), patients with liver metastases demonstrated a higher ORR (64% vs. 47%, P < .05), but shorter OS (8.3 months, vs. 15.7 months, P= .05) compared to those without liver metastasis; the presence of bone metastases was not associated with a significant difference in ORR or OS in the UNITE study.21
In the context of the above separate datasets, a potential application of our findings could be to help inform treatment decisions among patients with aUC based on metastatic sites.31 For patients with bone and/or liver metastases, our data suggests poor response and survival with ICI. Recently, enfortumab-vedotin received FDA approval after one or more prior lines of therapy in cisplatin-ineligible patients. Moreover, the THOR (NCT03390504) phase III trial is comparing erdafitinib to pembrolizumab in patients with platinum-refractory aUC harboring a susceptible FGFR alteration (results are pending). So far, there has been no head-to-head comparison between either antibody drug conjugate or erdafitinib versus ICI in the platinum-refractory setting. Based on all the above considerations, therapeutic agents with higher ORR in patients with liver and/or bone metastasis could be possibly considered prior to use of ICI in this setting.
Treatment with ICI may depend more on the immune system robustness compared to cytotoxic regimens; patients with advanced cancer may have less robust immune system, which might dampen response to ICI.32 Recent trials with neoadjuvant ICI monotherapy (eg, ABACUS, PURE-01) have showed promising results with high pathologic complete response rates.33 Moreover, there have been practice-changing data in the adjuvant and switch maintenance settings based on the Checkmate 274 and Javelin Bladder 100 phase III trials.34,35 These findings, along with data on the limited efficacy of single-agent ICI in patients with visceral metastases and higher tumor burden might suggest that ICI used in earlier disease settings could possibly have greater benefit compared to the salvage setting. Several clinical trials have also investigated the role of ICI alone or combined with other systemic therapies and/or radiation in earlier disease settings, with promising results.36–39 However, this hypothesis is not confirmed, while the role of ICI is evolving in UC.
Our work has limitations inherent to its retrospective design, such as lack of randomization, possibility for selection bias, and residual confounding that cannot be measured or fully accounted for. Clinical practices, surveillance schedules and follow-up times may not be perfectly consistent across 26 different institutions. Additionally, no centralized review of pathology and imaging was feasible, but all participating sites were academic institutions with expert genitourinary medical oncologists, radiologists and pathologists. Although we tried to capture information on the extent of metastatic burden, detailed data on the number of distinct lesions per organ system, as well as their size, were not available. Sample size in many of the metastatic subgroups was also limited by low power (~10–30 patients). Response and progression to treatment were determined by systematic comprehensive chart review based upon clinical and radiological notes, while prespecified interval assessments using strictly the RECIST 1.1 criteria were not mandated. We did not capture data on switch maintenance ICI or on molecular biomarkers. However, our study has several strengths, including the utilization of real-world data outside clinical trials, a relatively large sample size, and participation of multiple institutions across 2 continents with a diverse patient population.
In conclusion, we showed that in patients receiving ICI for aUC outside clinical trials, the presence of bone and/or liver metastasis was significantly associated with lower ORR, as well as shorter PFS and OS. Despite inherent limitations, these results are complementary to findings from relatively limited trial subset analyses and could possibly help inform therapeutic discussions, prognostic estimates, as well as the design, stratification, and interpretation of clinical trials with ICI in aUC. Future research is needed to further identify and validate not only prognostic, but also predictive, biomarkers that can help inform optimal use of ICI in patients with aUC.
Supplementary Material
Clinical Practice Points.
Immune checkpoint inhibitors (ICI) are a well-established treatment option for patients with advanced urothelial carcinoma (aUC), but response rates remain modest.
There is an urgent need for more accurate biomarkers of response to ICIs.
In this retrospective cohort study, we show that patients with metastatic disease confined to lymph nodes have better outcomes with ICIs both in the 1st line and salvage (2nd line and beyond) settings compared to those with cancer spread beyond lymph nodes.
We also demonstrate that patients with bone or liver metastasis have worse outcomes with ICIs in both the 1st and salvage settings, while co-existence of bone and liver metastasis was associated with the worst outcomes.
Findings are hypothesis-generating but can provide valuable and clinically relevant information regarding optimal therapy selection and sequence in clinical practice, especially in the context of other FDA-approved agents, such as antibody drug conjugates and FGFR inhibitor.
Results can help in the development of prognostic tools at the era of ICI use in aUC, as well as with clinical trial design, stratification, and interpretation.
Acknowledgments
D Makrakis and LN Diamantopoulos acknowledge the support of Kure It Cancer Research. P Grivas, EY Yu, RB Montgomery acknowledge the support of the Seattle Translational Tumor Research Program at Fred Hutchinson Cancer Center. DJ. Pinato acknowledges the infrastructure support provided by Imperial Experimental Cancer Medicine Centre, Cancer Research UK Imperial Centre, the Imperial College Healthcare NHS Trust Tissue Bank and the Imperial College BRC. AR Khaki and R Talukder were supported by the National Cancer Institute under training grant T32CA009515. Research Electronic Data Capture at the Institute of Translational Health Sciences is supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award UL1 TR002319. David J. Pinato is supported by grant funding from the Wellcome Trust Strategic Fund (PS3416) and acknowledges grant support from the Cancer Treatment and Research Trust (CTRT) and infrastructural support by the Cancer Research UK Imperial Centre and the NIHR Imperial Biomedical Research Centre.
Abbreviations:
- aUC
advanced urothelial carcinoma
- ICI
immune checkpoint inhibitors
- IRAEs
immune related adverse effects
- LN
lymph node
- MIBC
muscle-invasive bladder cancer
- PDL1
programmed death ligand 1
- TME
tumor microenvironment
Footnotes
Disclosure
Dimitrios Makrakis has no COI to declare Rafee Talukder has no COI to declare Genevieve Ihsiu Lin has no COI to declare Leonidas N. Diamantopoulos has no COI to declare Scott Dawsey has received honoraria from MJH Life Sciences Shilpa Gupta has received personal fees from Bristol Myers Squibb, Merck, Janssen, Seattle Genetis, EMD Sorono and Pfizer, and has received grants from Astellas, BMS and Bristol Myers Squibb. Lucia Carril-Ajuria has no COI to declare. Daniel Castellano has served as advisor/consultant for Lilly, Pierre-Fabre, Boehringer Ingelheim, Roche/Genentech, AstraZeneca, Ipsen, Bayer, Sanofi, Janssen Oncology, Pfizer, Bristol-Myers Squibb, Astellas Pharma, Novartis, MSD Oncology and Sanofi. His institution has received research funding from Janssen Oncology and has received travel accomodations/expenses from Roche, Pfizer, Astra Zeneca Spain and Bristol-Myers Squibb. Ivan de Kouchkovsky has no COI to declare Vadim S. Koshkin has no COI to declare Joseph J. Park has no COI to declare Ajjai Alva has received grants/contracts from Arcus Biosciences, AstraZeneca Pharmaceuticals, LP Bristol-Myers Squibb Company, Eisai Inc., Esanik, Ionnis, Merck & Co., Inc. and Prometheus, consulting fees from Bristol-Myers Squibb Company, EMD Serono, Merck & Co., Inc., and Pfizer Inc., and had a leadership/fiduciary role in ASCO TAPUR/CRC Mehmet A. Bilen has acted as a paid consultant for and/or as a member of the advisory boards of Exelixis, Bayer, BMS, Eisai, Pfizer, AstraZeneca, Janssen, Calithera Biosciences, Genomic Health, Nektar, EMD Serono, SeaGen, and Sanofi, and has received grants to his institution from Merck, Xencor, Bayer, Bristol-Myers Squibb, Genentech/Roche, SeaGen, Incyte, Nektar, AstraZeneca, Tricon Pharmaceuticals, Genome & Company, AAA, Peloton Therapeutics, and Pfizer for work performed as outside of the current study. Tyler F. Stewart has no COI to declare Rana R. McKay has received compensation as advisor/consultant for AstraZeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Tempus. Financing of Scientific Research: Pfizer, Tempus, Bayer Nishita Tripathi has no COI to declare Neeraj Agarwal has served as consultant to Astellas, Astra Zeneca, Aveo, Bayer, Bristol Myers Squibb, Calithera, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Foundation Medicine, Genentech, Gilead, Janssen, Merck, MEI Pharma, Nektar, Novartis, Pfizer, Pharmacyclics, and Seattle Genetics, and has received research funding for his institution from Astellas, Astra Zeneca, Bavarian Nordic, Bayer, Bristol Myers Squibb, Calithera, Celldex, Clovis, Eisai, Eli Lilly, EMD Serono, Exelixis, Genentech, Gilead, Glaxo Smith Kline, Immunomedics, Janssen, Medivation, Merck, Nektar, New Link Genetics, Novartis, Pfizer, Prometheus, Rexahn, Roche, Sanofi, Seattle Genetics, Takeda, and Tracon. Naomi Vather-Wu has no COI to declare Yousef Zakharia has been in the advisory board for Bristol Myers Squibb, Amgen, Roche Diagnostics, Novartis, Janssen, Eisai, Exelixis, Castle Bioscience, Array, Bayer, Pfizer, Clovis and EMD Serono, has received grants for his institution from NewLink Genetics, Pfizer, Exelixis, Eisai, has received honoraria from Pfizer and Novartis, Rafael Morales-Barrera has served as consultant/advisor for Sanofi, AstraZeneca, Astellas Pharma and MSD, has been in the speaker’s Bureau for Astellas Pharma, Merck/Pfizer and MSD Oncology, and has received travel accomodations from Sanofi, Pfizer, MSD, Astellas Pharma, Astra Zeneca, Bayer, Roche/Genentech. Michael E. Devitt has no COI to declare. Alessio Cortellini has served as consultant for Astrazeneca, MSD, BMS and Roche, and has received speaker’s compensation from Astrazeneca, Novartis and Eisai. Fulgenzi Claudia Angela Maria has no COI to declare David J. Pinato received lecture fees from ViiV Healthcare and Bayer Healthcare and travel expenses from BMS and Bayer Healthcare; consulting fees for Mina Therapeutics, EISAI, Roche, and Astra Zeneca, and received research funding to his institution from MSD and BMS. Ariel Nelson has no COI to declare. Christopher J. Hoimes has received consulting fees from Merck, Seagen, Astellas, BMS and speaker’s compensation for Eisai, Seagen, Astellas, BMS. Kavita Gupta has no COI to declare. Benjamin A. Gartrell has no COI to declare Alex Sankin has no COI to declare. Abhishek Tripathi has received Honoraria from Urology times, grants/contracts from Clovis Oncology, Corvus Pharamceuticals, Bayer, EMD Serono, Aravive, WindMIL, Exact Sciences, and Pfizer and served as an advisor for Foundation Medicine and Pfizer, Genzyme, EMD Serono, Exelixis, Deka Biosciences and Seattle Genetics. Roubini Zakopoulou has no COI to declare. Aristotelis Bamias has received grants/contracts from Pfizer, BMS, Astra Zeneca, Ipsen, and served as advisor and received payment/honoraria for lectures from BMS, Ipsen, MSD. Jure Murgic has received honoraria from Roche and Astellas Pharma, has served as consultant/advisor for Bristol-Myers Squibb and Sandoz, has received research funding from Astellas Pharma and has received travel expenses from BMS, Roche and Janssen. Ana Fröbe has served as consultant/advisor for Bayer Germany, Astellas, BMS, Pfizer, Sandoz and Janssen Oncology. Alejo Rodriguez-Vida has served as advisor for MSD, Pfizer, BMS, Astellas, Janssen, Bayer, Clovis, Ipsen and Roche has received honoraria or travel expenses from Pfizer, MSD, Astellas, BMS, Janssen, Astra Zeneca, Roche, Bayer, Ipsen and Sanofi Aventis, and has received research funding from Takeda, Pfizer, and Merck. Alexandra Drakaki has served as consultant for Bristol-Myers Squibb, AstraZeneca, RADMETRIX, Seattle Genetics, Janssen, PACT Pharma, Merck, Roche/Genentech, Exelixis, Dyania Health, has received research funding from Kite/Gilead, AstraZeneca, Genentech/Roche, BMS, Merck Sharp & Dohme, Jounce Therapeutics, Infinity Pharmaceuticals, Seattle Genetics/Astellas, and has received travel expenses from Lilly, AstraZeneca and Seattle Genetics. Sandy Liu has received honoraria from Esai, Exelixis and EMD-Serono. Eric Lu has no COI to declare. Vivek Kumar has no COI to declare. Giuseppe Di Lorenzo has no COI to declare.Monika Joshi has received research funding to her institution from Astra Zeneca, Eisai and Pfizer, has served as an advisor for Seagen and Sanofi Pedro Isaacsson-Velho had received grants from ASCO Conquer Cancer Foundation, consulting fees from Bayer, Astellas and AstraZeneca, payment for lectures and travel expenses from Astellas, Pfizer, AstraZeneca, Merck, MSD, Janssen and BMS, and has served as an advisor for Astellas, Pfizer and AstraZeneca Lucia Alonso Buznego has no COI to declare. Ignacio Duran has received honoraria for speaker engagements, advisory roles or funding of continuous medical education from Astellas, Bristol Myers Squibb, EUSA Pharma, Immunomedics Inc., IPSEN, Jansen, Merck, MSD, Novartis, Pfizer, Roche Genentech, Astra Zeneca and Seattle Genetics, and has received research grants from AstraZeneca, Astellas, and Roche-Genentech. Marcus Moses has no COI to declare. Albert Jang has no COI to declare. Pedro Barata has served as consultant for Astellas; Eisai; Janssen, EMD Serono; Dendreon; Pfizer, Seattle Genetics, BMS, Bayer, Guardant Health, has received research grants from BlueEarth Diagnostics, has been in the speaker’s bureau for Bayer, Caris and Myovant, and has contracted institutional research for AstraZeneca and Merck. Vivek Kumar has no COI to declare Guru Sonpavde has been in the advisory board for BMS, Genentech, EMD Serono, Merck, Sanofi, Seattle Genetics/Astellas, Astrazeneca, Exelixis, Janssen, Bicycle Therapeutics, Pfizer, Gilead, Scholar Rock, G1 Therapeutics, Eli Lilly/Loxo Oncology, Infinity Pharmaceuticals, has received research support from Sanofi, Astrazeneca, Gilead, QED, Lucence, Predicine, BMS, has served in a steering committee of studies for BMS, Bavarian Nordic, Seattle Genetics, QED, G1 Therapeutics (all unpaid), and Astrazeneca, EMD Serono, Debiopharm, has served in the data safety monitoring committee for Mereo, has received travel compensation from BMS, AstraZeneca, has received writing/editor fees from Uptodate, Editor of Elsevier Practice Update Bladder Cancer Center of Excellence and received speaking fees from Physicians Education Resource (PER), Onclive, Research to Practice, Medscape, Cancer Network, Masters Lecture Series (MLS). Evan Y. Yu has received research funding to his institution Bayer, Blue Earth, Daiichi Sankyo, Dendreon, Lantheus, Merck, Seagen, Taiho and received consulting fees from Abbvie, Advanced Accelerator Applications, Bayer, Clovis, Exelixis, Janssen, Merck, Sanofi. Robert Bruce Montgomery has received research funding to his institution from Janssen Oncology, AstraZeneca, Clovis, Astellas Pharma and Beigene. Petros Grivas has done paid consulting with AstraZeneca, Astellas Pharma, Bristol Myers Squibb, Dyania Health, EMD Serono, Exelixis, Foundation Medicine, Genentech/Roche, Genzyme, GlaxoSmithKline, Guardant Health, Gilead Sciences, Infinity Pharmaceuticals, Janssen, Lucence, Merck, Mirati Therapeutics, Pfizer, QED Therapeutics, Regeneron Pharmaceuticals, Seattle Genetics, Silverback Therapeutics, UroGen, 4D Pharma PLC. His institution has received grants from Bavarian Nordic, Bristol Myers Squibb, Clovis Oncology, Debiopharm, EMD Serono, G1 Therapeutics, Gilead Sciences, GlaxoSmithKline, Merck, Mirati Therapeutics, Pfizer, QED Therapeutics. Ali Raza Khaki has received honoraria from OncLive/MJH Life Sciences, has owned stocks of Merck and Sanofi, has ongoing research collaborations with Tempus Labs and Natera and has had uncompensated relationships with Seagen/Astellas.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clgc.2022.06.001.
References
- 1.Gopalakrishnan D, Koshkin VS, Ornstein MC, Papatsoris A, Grivas P. Immune checkpoint inhibitors in urothelial cancer: recent updates and future outlook. Ther Clin Risk Manag. 2018;14:1019–1040 Published 2018 Jun 5. doi: 10.2147/TCRM.S158753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 3.Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med. 2021;384:1125–1135. doi: 10.1056/NEJMoa2035807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loriot Y, Necchi A, Park SH, et al. Erdafitinib in locally advanced or metastatic urothelial carcinoma. N Engl J Med. 2019;381:338–348. doi: 10.1056/NEJMoa1817323. [DOI] [PubMed] [Google Scholar]
- 5.Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: A phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol. 2021;39:2474–2485. doi: 10.1200/JCO.20.03489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apolo AB, Ostrovnaya I, Halabi S, et al. Prognostic model for predicting survival of patients with metastatic urothelial cancer treated with cisplatin-based chemotherapy. J Natl Cancer Inst. 2013;105:499–503. doi: 10.1093/jnci/djt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28:1850–1855. doi: 10.1200/JCO.2009.25.4599. [DOI] [PubMed] [Google Scholar]
- 8.Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17:3173–3181. doi: 10.1200/JCO.1999.17.10.3173. [DOI] [PubMed] [Google Scholar]
- 9.Sonpavde G, Manitz J, Gao C, et al. Five-factor prognostic model for survival of post-platinum patients with metastatic urothelial carcinoma receiving PD-L1 inhibitors [published correction appears in J Urol. 2021 Mar;205(3):942]. J Urol. 2020;204:1173–1179. doi: 10.1097/JU.0000000000001199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khaki AR, Li A, Diamantopoulos LN, et al. A new prognostic model in patients with advanced urothelial carcinoma treated with first-line immune checkpoint inhibitors. Eur Urol Oncol. 2021;4:464–472. doi: 10.1016/j.euo.2020.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nassar AH, Mouw KW, Jegede O, et al. A model combining clinical and genomic factors to predict response to PD-1/PD-L1 blockade in advanced urothelial carcinoma. Br J Cancer. 2020;122:555–563. doi: 10.1038/s41416-019-0686-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. The Lancet. 2017;389:67–76. doi: 10.1016/S0140-6736(16)32455-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017;18:1483–1492. doi: 10.1016/S1470-2045(17)30616-2. [DOI] [PubMed] [Google Scholar]
- 14.Fradet Y, Bellmunt J, Vaughn DJ, et al. Randomized phase III KEYNOTE-045 trial of pembrolizumab versus paclitaxel, docetaxel, or vinflunine in recurrent advanced urothelial cancer: results of >2 years of follow-up. Ann Oncol. 2019;30:970–976. doi: 10.1093/annonc/mdz127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nelson AA, Cronk RJ, Lemke EA, et al. Early bone metastases are associated with worse outcomes in metastatic urothelial carcinoma. Bladder Cancer. 2021;7:33–42. doi: 10.3233/BLC-200377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaki AR, Li A, Diamantopoulos LN, et al. Impact of performance status on treatment outcomes: a real-world study of advanced urothelial cancer treated with immune checkpoint inhibitors. Cancer. 2020;126:1208–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller NJ, Khaki AR, Diamantopoulos LN, et al. Histological subtypes and response to PD-1/PD-L1 blockade in advanced urothelial cancer: a retrospective study. J Urol. 2020;204:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esagian SM, Khaki AR, Diamantopoulos LN, et al. Immune checkpoint inhibitors in advanced upper and lower tract urothelial carcinoma: a comparison of outcomes. BJU Int. 2021;128:196–205. [DOI] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95. doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koshkin VS, Henderson N, James M, et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: analysis from the urothelial cancer network to investigate therapeutic experiences (UNITE) study [published online ahead of print, 2021 Dec 9]. Cancer. 2021. 10.1002/cncr.34057. doi: 10.1002/cncr.34057. [DOI] [PubMed] [Google Scholar]
- 22.Raggi D, Giannatempo P, Marandino L, et al. Role of bone metastases in patients receiving immunotherapy for pre-treated urothelial carcinoma: the multicentre, retrospective meet-URO-1 bone study [published online ahead of print, 2021 Dec 16]. Clin Genitourin Cancer. 2021:S1558–S7673 00238-X. doi: 10.1016/j.clgc.2021.12.008. [DOI] [PubMed] [Google Scholar]
- 23.Gómez de Liaño Lista A, van Dijk N, de Velasco Oria de Rueda G, et al. Clinical outcome after progressing to frontline and second-line Anti–PD-1/PD-L1 in advanced urothelial cancer. Eur Urol. 2020;77:269–276. doi: 10.1016/j.eururo.2019.10.004. [DOI] [PubMed] [Google Scholar]
- 24.Ma VT, Su CT, Hu M, et al. Characterization of outcomes in patients with advanced genitourinary malignancies treated with immune checkpoint inhibitors. Urol Oncol Semin Orig Investig. 2021;39:437 e1–437.e9. doi: 10.1016/j.urolonc.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Dijk N, Funt SA, Blank CU, Powles T, Rosenberg JE, van der Heijden MS. The cancer immunogram as a framework for personalized immunotherapy in urothelial cancer. Eur Urol. 2019;75:435–444. doi: 10.1016/j.eururo.2018.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahoor H, Grivas P. The cancer immunogram: a pledge for a comprehensive biomarker approach for personalized immunotherapy in urothelial cancer. Eur Urol. 2019;75:445–447. doi: 10.1016/j.eururo.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Eckstein M, Sikic D, Strissel PL, Erlmeier F. Evolution of PD-1 and PD-L1 gene and protein expression in primary tumors and corresponding liver metastases of metastatic bladder cancer. Eur Urol. 2018;74:527–529. doi: 10.1016/j.eururo.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 28.Alhalabi O, Zhu Y, Hamza A, et al. Integrative clinical and genomic characterization of mtap-deficient metastatic urothelial cancer. Eur Urol Oncol. 2021;14 Published online November. doi: 10.1016/j.euo.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faltas BM, Prandi D, Tagawa ST, et al. Clonal evolution of chemotherapy-resistant urothelial carcinoma. Nat Genet. 2016;48:1490–1499. doi: 10.1038/ng.3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu EY, Petrylak DP, O’Donnell PH, et al. Enfortumab vedotin after PD-1 or PD-L1 inhibitors in cisplatin-ineligible patients with advanced urothelial carcinoma (EV201): a multicentre, single-arm, phase 2 trial [published correction appears in Lancet Oncol. 2021 Jun;22(6):e239]. Lancet Oncol. 2021;22:872–882. doi: 10.1016/S1470-2045(21)00094-2. [DOI] [PubMed] [Google Scholar]
- 31.Msaouel P, Lee J, Thall PF. Making patient-specific treatment decisions using prognostic variables and utilities of clinical outcomes. Cancers. 2021;13. doi: 10.3390/cancers13112741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zucali PA, Cordua N, D’Antonio F, et al. Current perspectives on immunotherapy in the perioperative setting of muscle-infiltrating bladder cancer. Front Oncol. 2020;10 Published 2020 Oct 21. doi: 10.3389/fonc.2020.568279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma. N Engl J Med. 2021;384:2102–2114. doi: 10.1056/NEJMoa2034442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med. 2020;383:1218–1230. doi: 10.1056/NEJMoa2002788. [DOI] [PubMed] [Google Scholar]
- 36.Balar AV, Milowsky MI, O’Donnell PH, et al. Pembrolizumab (pembro) in combination with gemcitabine (Gem) and concurrent hypofractionated radiation therapy (RT) as bladder sparing treatment for muscle-invasive urothelial cancer of the bladder (MIBC): a multicenter phase 2 trial. J Clin Oncol. 2021;39(15_suppl):4504 4504. doi: 10.1200/JCO.2021.39.15_suppl.4504. [DOI] [Google Scholar]
- 37.Rodriguez-Moreno JF, de Velasco G, Bravo Fernandez I, et al. Impact of the combination of durvalumab (MEDI4736) plus olaparib (AZD2281) administered prior to surgery in the molecular profile of resectable urothelial bladder cancer: NEODURVARIB trial. J Clin Oncol. 2020;38(6_suppl):542 542. doi: 10.1200/JCO.2020.38.6_suppl.542. [DOI] [Google Scholar]
- 38.van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nat Med. 2020;26:1839–1844. doi: 10.1038/s41591-020-1085-z. [DOI] [PubMed] [Google Scholar]
- 39.Hoimes CJ, Albany C, Hoffman-Censits J, et al. A phase Ib/II study of neoadjuvant pembrolizumab (pembro) and chemotherapy for locally advanced urothelial cancer (UC). Ann Oncol. 2018;29:viii726. doi: 10.1093/annonc/mdy424.039. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


