Abstract
Data regarding the clinical outcomes of men with metastatic hormone sensitive prostate cancer (mHSPC) who harbor homologous recombination repair (HRR) gene alterations have not been fully characterized. Here, we examine a cohort of mHSPC patients who underwent genomic sequencing to evaluate the impact of HRR gene alterations on time to castrate resistance and other outcomes. We found that the presence of an HRR gene alteration is associated with a shorter time to mCRPC.
Introduction:
The homologous recombination repair (HRR) pathway is a frequently mutated pathway in advanced prostate cancer. The clinical course of patients with HRR gene alterations who have metastatic hormone sensitive prostate cancer (mHSPC) has not been fully characterized. Here, we examine the outcomes of men with mHSPC with HRR alterations.
Methods:
We conducted a single-center retrospective analysis of men with mHSPC who underwent next generation sequencing. The primary objective was to assess the time from diagnosis of mHSPC to metastatic castrate resistance prostate cancer (mCRPC) in patients with pathogenic HRR alterations compared to individuals lacking these alterations. Key secondary objectives included time to mCRPC in prespecified cohorts, PSA response, and overall survival.
Results:
151 men with mHSPC were identified for the study. 24% (N = 37) had pathogenic HRR gene alterations detected with the most common alterations found in BRCA2 (n = 15), ATM (n = 10), and CDK12 (n = 7). Time to mCRPC was significantly decreased in patients with HRR gene alterations versus those without such alterations (12.7 vs. 16.1 months, HR 1.95, P = .02). In multivariate analysis, the effect of HRR gene alterations on time to CRPC remained significant when adjusting for age, mHSPC therapy, the volume of disease, the presence of visceral metastases, and PSA (adjusted HR 1.69, P = .02). Stratified by specific HRR gene alteration, patients with BRCA2 or CDK12 had significantly decreased time to mCRPC compared to other HRR alterations.
Conclusion:
HRR gene alterations are associated with the worse outcomes in mHSPC with significantly shorter time to mCRPC. Given the established role of Poly (ADP-ribose) Polymerase (PARP) inhibitors in mCRPC, these data highlight an opportunity to examine PARP inhibitors earlier in the clinical course for prostate cancer patients. Ongoing prospective studies will further validate the role of PARP inhibitors in mHSPC patients.
Keywords: Biomarker, BRCA2, CDK12, CRPC, HRR, PARP inhibitors, PSA
Introduction
The management of metastatic hormone sensitive prostate cancer (mHSPC) has significantly evolved in the last 5 years with multiple studies showing overall survival benefit with intensification of therapy with the addition of either taxane chemotherapy1 or an androgen receptor signaling inhibitor (ARSI)2-4 (Table 1). Additionally, the recent studies have demonstrated that docetaxel combined with an ARSI improves survival for patients with mHSPC.5,6 While these therapies have improved survival and quality of life for patients with advanced prostate cancer, long term durable responses are limited and of the majority of patients will progress to metastatic castrate resistance prostate cancer (mCRPC).1-3,7-9 Despite a growing understanding of the genomic landscape of advanced prostate cancer,10 at the present time there are no biomarker selected therapies for patients with mHSPC.
Table 1.
Clinical Trials Demonstrating Benefit of Early Intensification in mHSPC Patients.
| Trial | Progression Free Survival Definition | Treatment Arm (PFS, in months) |
Control Arm (PFS, in months) |
HR | P-value | ||
|---|---|---|---|---|---|---|---|
| Clinical | Biochemical | Radiographic | |||||
| CHAARTED7 | x | x | X | Docetaxel ± ADT Time to CRPC*: 20.2 Time to clinical progression**: 33.0 |
ADT Time to CRPC*: 11.7 Time to clinical progression**: 19.8 |
0.61 | < .0001 |
| STAMPEDE1 | x | x | x | Abiraterone ± prednisolone ± ADT 43.9 |
ADT 30.0 |
0.40 | < .001 |
| LATITUDE8 | x | Abiraterone ± prednisone ± ADT 33.0 |
ADT 14.8 |
0.47 | < .001 | ||
| ENZAMET2 | x | x | x | Enzalutamide ± ADT ±/− docetaxel PSA PFS: 67% at 3 yrs (HR 0.39, P <.001) Clinical PFS***: 68% at 3 yrs (HR 0.40, P < .001) |
ADT ± NSAA ±/− docetaxel PSA PFS: 37% at 3 yrs (HR 0.39, P <.001) Clinical PFS: 41% at 3 yrs (HR 0.40, P < .001) |
||
| ARCHES9 | x | Enzalutamide ± ADT Not reached |
ADT 19.0 |
0.39 | < .001 | ||
| TITAN3 | x | Apalutamide ± ADT Could not be estimated |
ADT 22.1 |
0.48 | < .001 | ||
| PEACE 16 | x | Abiraterone ± prednisone ± ADT ± Docetaxel ±/− local radiotherapy 4.5 yrs |
ADT ± Docetaxel ±/− local radiotherapy 2.0 years |
0.50 | < .0001 | ||
| ARASENS5 | x | Darolutamide ± ADT ± Docetaxel Time to CRPC*: Not reached |
Placebo ± ADT ± Docetaxel Time to CRPC*: 19.1 |
0.36 | < .001 | ||
Time to CRPC defined as time to clinical deterioration, radiographic progression, serologic progression, or death due to any cause.
Clinical progression defined as time to clinical deterioration, radiographic progression, or death due to any cause.
Based on clinical symptoms, radiographic progression, or change in therapy.Abbreviations: ADT = androgen deprivation therapy, CRPC = Castrate Resistant Prostate Cancer, NSAA = Nonsteroidal antiandrogen, PFS= progression free survival.
The homologous recombination pathway is frequently altered in advanced prostate cancer. Among patients with mCRPC, the rate of HRR gene alterations derived from several large sequencing analyses is between 20-40%.10,11 The PROFOUND trial paved the way for the use of Poly (ADP-ribose) Polymerase (PARP) inhibitors in the treatment of mCRPC patients with HRR gene alterations, in which men with alterations in BRCA1/2 or ATM who received olaparib were found to have longer overall survival compared to enzalutamide or abiraterone.11 This trial led to the FDA approval of olaparib for the treatment of mCRPC harboring HRR gene alterations, the first approval of a biomarker-driven therapy specific to advanced prostate cancer. Additionally, the TRITON2 study investigated the role of rucaparib in patients with mCRPC with HRR gene alterations and demonstrated improved objectives responses with this agent, leading to the FDA approval of rucaparib in mCRPC in men with BRCA1/2 alterations.12
There is an increasing evidence of the prognostic significance of HRR gene alterations in patients with advanced prostate cancer. Several retrospective studies have demonstrated that patients with germline HRR gene alterations have a shorter time to castration resistance and worse overall survival.13,14 With regards to patients with mCRPC, the data are mixed regarding the prognostic significance of HRR gene alterations.15,16 Additionally, several studies have demonstrated differential responses to ARSIs and taxane chemotherapy in patients harboring HRR gene alterations.16,17
As therapies move earlier into the treatment landscape for metastatic prostate cancer, a key question regarding the role of PARP inhibition in earlier disease states is understanding the outcomes of patients with HRR alterations in the metastatic hormone sensitive setting. To help provide insights into this question, we investigated the clinical outcomes of men with mHSPC with and without HRR gene alterations.
Materials and Methods
Patients
We analyzed patients seen at the University of California San Diego Moores Cancer Center (La Jolla, CA), with mHSPC who underwent either tissue-based or circulating tumor DNA (ctDNA) next generation sequencing (NGS) from 2014 to 2020. Key eligibility criteria included a diagnosis of stage IVB prostate cancer and the availability of follow-up data after diagnosis of mHSPC. This cohort included only patients with adenocarcinoma histology. Clinical, disease, and genomic characteristics were collected consecutively via electronic medical record into a secure HIPAA compliant database. This study was approved by the University of California San Diego Institutional Review Board.
Genomic Analysis
Genomic testing was done using standard of care Clinical Laboratory Improvement Amendments (CLIA)-based NGS assays. All patients underwent somatic profiling with tissue-based and/or ctDNA NGS testing. A subset of patients also underwent germline testing (Table 2). Patients were stratified into 2 cohorts: those with the presence of HRR gene alterations versus the absence of HRR gene alterations (biomarker negative). HRR gene altered patients were defined as having a pathogenic or suspected pathogenic alteration in the following 15 genes as previously identified in the PROFOUND trial: ATM, BRCA1, BRCA2, BRIP1, BARD1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L.10,11 Supplemental Table 1 notes the details of the tissue, ctDNA, and germline genomic assays utilized for determination of HRR gene alteration status (Table S1).
Table 2.
Baseline Characteristics.
| Characteristic | HRR altered (N = 37) |
Wild Type (N = 114) |
Overall Cohort (N = 151) |
P-value |
|---|---|---|---|---|
| Age at diagnosis, median (range) | 63 (46-79) | 64 (44-93) | 64 (44-93) | .89 |
| Ethnicity | .88 | |||
| Hispanic | 3 (8%) | 11 (10%) | 14 (9%) | |
| Non-Hispanic | 34 (92%) | 103 (90%) | 137 (91%) | |
| Race | .97 | |||
| American Indian | 0 (0%) | 2 (2%) | 2 (1%) | |
| Asian | 1 (3%) | 7 (6%) | 8 (5%) | |
| Black/African American | 2 (5%) | 8 (7%) | 10 (7%) | |
| Native Hawaiian | 0 (0%) | 1 (1%) | 1 (1%) | |
| White | 29 (78%) | 82 (72%) | 111 (74%) | |
| Other race/Mixed | 5 (14%) | 14 (12%) | 19 (13%) | |
| Disease status at diagnosis | .99 | |||
| Localized | 14 (38%) | 44 (39%) | 58 (38%) | |
| De novo metastatic | 23 (62%) | 70 (61%) | 93 (62%) | |
| Gleason Score at Prostate Cancer Diagnosis | ||||
| 6 | 0 (0%) | 1 (1%) | 1 (1%) | 1.0 |
| 7 | 6 (16%) | 23 (20%) | 29 (19%) | .81 |
| > = 8 | 25 (68%) | 68 (60%) | 93 (62%) | .44 |
| Unknown | 6 (16%) | 22 (19%) | 28 (19%) | .81 |
| Prostate Directed Therapy | ||||
| Radical prostatectomy | 7 (19%) | 33 (29%) | 40 (26%) | .35 |
| Radiation therapy | 15 (41%) | 43 (38%) | 58 (38%) | .80 |
| PSA at mHSPC diagnosis, median (range) | 30.3 (0.5-5000) | 39.6 (1.1-4249) | 38.3 (0.5-5000) | .34 |
| Sites of metastatic disease at mHSPC diagnosis | ||||
| Bone | 31 (84%) | 94 (82%) | 125 (83%) | 1.0 |
| Lymph Node | 19 (51%) | 62 (54%) | 81 (54%) | .85 |
| Liver | 0 (0%) | 3 (3%) | 3 (2%) | 1.0 |
| Lung | 2 (5%) | 17 (15%) | 19 (13%) | .16 |
| Bladder | 2 (5%) | 4 (4%) | 6 (4%) | .64 |
| Other | 0 (0%) | 3 (3%) | 3 (2%) | 1.0 |
| mHSPC Therapies | ||||
| ADT Alone | 18 (49%) | 51 (45%) | 69 (46%) | .71 |
| ARSI + ADT | 8 (22%) | 33 (29%) | 41 (27%) | .52 |
| Docetaxel + ADT | 11 (30%) | 30 (26%) | 41 (27%) | .68 |
Abbreviations: ADT = androgen deprivation therapy, ARSI = androgen receptor signaling inhibitor, mHSPC = metastatic hormone sensitive prostate cancer.
Endpoints
The primary endpoint of the study was the time from diagnosis of mHSPC to onset of mCRPC in patients with somatic and/or germline HRR gene alterations compared to patients who lacked HRR alterations. mCRPC was defined as receipt of castration therapy or bilateral orchiectomy, with either a successively rising PSA or radiographic progression as defined by Response Evaluation Criteria in Solid Tumors version 1.1 and Prostate Cancer Working Group 3 (PCWG3) principals.18 Key secondary endpoints included time to mCRPC in the predefined cohorts based on mHSPC type of therapy and individual HRR gene alteration. We additionally examined PSA kinetics and overall survival. PSA kinetics included PSA-50, defined as a decline in PSA of ≥ 50% from initiation of mHSPC therapy as per PCWG3 criteria,18,19 rates of PSA < 0.2 at 7 months, and PSA nadir. Overall survival was defined as the time from mHSPC diagnosis to the either death or last follow-up, whichever came first.
Statistical Analysis
Time to event analysis was performed using Kaplan Meier analysis and Cox hazard proportional hazards analysis. A Cox proportional hazards model was used to assess time to mCRPC, adjusting for age, mHSPC therapy, metastatic disease burden,7 the presence of visceral metastases, and PSA at therapy initiation. mHSPC therapies were categorized as ADT alone, docetaxel with ADT, or ARSI with ADT. High volume of metastatic disease was defined as the presence of visceral metastases or ≥ 4 bone lesion with ≥ 1 lesion beyond vertebral bodies and pelvis detected on conventional imaging with CT and/or bone scan.7 The proportion of patients achieving a 50% decline in PSA (PSA-50) was calculated in each cohort and compared using Fisher’s Exact Test for significance. All tests were 2-sided and considered statistically significant if P < .05. All statistical analysis was performed using SPSS Statistics (version 26).
Results
Patient Characteristics
From 2014 to 2020, we identified 151 men with metastatic prostate cancer who underwent somatic NGS profiling, with a subset who also underwent additional germline testing (N = 91, 60%). The median age of the cohort was 64 years (range 44-93). 62% (N = 93) were found to have de novo metastatic disease at diagnosis (Table 2). 26% of patients were non-white (N = 40). With regards to mHSPC therapy, 46% (n = 69) were treated with ADT alone, 28% (N = 41) with the addition of an ARSI, and 27% (N = 41) with the addition of docetaxel (Table 2). Overall, the baseline characteristics were similar among the patients with and without HRR gene alterations (Table 2).
Genomic Alterations
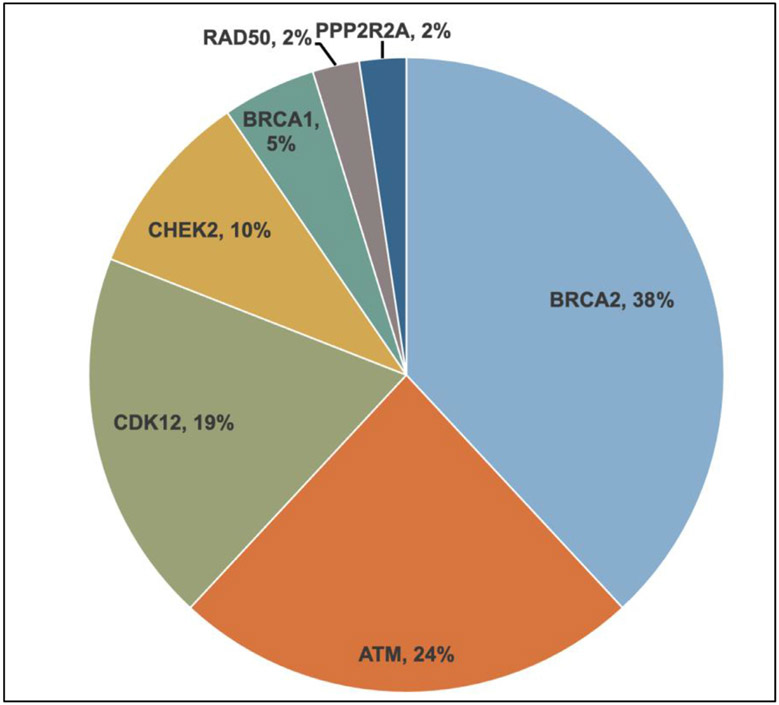
The majority of patients underwent tissue profiling (n = 90, 71.9%) of which the prostate was the most common specimen source (n = 78) (Table S1). Among the 151 patients analyzed, 25% (N = 37) of patients had HRR gene alterations detected. 32 (86%) patients had monoallelic mutations, while 5 (13%) had biallelic mutations. The most common somatic alterations were in BRCA2 (N = 12), ATM (N = 8), and CDK12 (N = 8). Of the 91 patients having undergone germline testing, 10 patients (11%) were found to have germline HRR gene alterations, of whom all 10 also had somatic tumor profiling performed. Of patients with concurrent germline and somatic tumor profiling, 6 had the same gene alteration identified on both germline and somatic testing platforms. The most common germline alterations were in CHEK2 (n = 4) and BRCA2 (n = 4). Figure 1 shows a breakdown of the detected alterations among patients with HRR gene alterations.
Figure 1.
Landscape of Detected HRR Alterations.
Clinical Outcomes
Time to MCRPC.
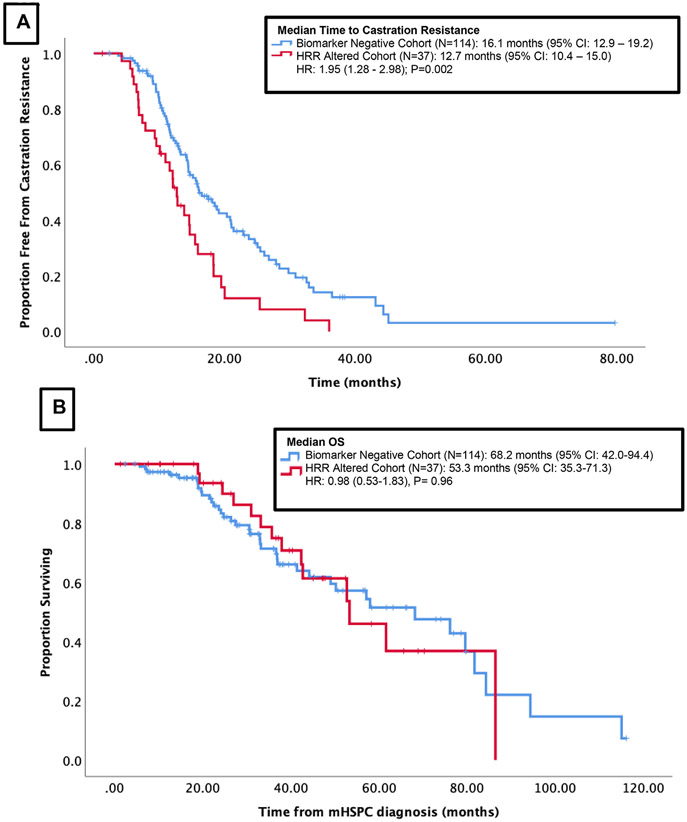
Median follow up was 31.7 months (3.2-319.7 months) for the entire cohort. Median time from mHSPC to castration resistance was significantly shorter in patients with HRR gene alterations when compared to patients lacking HRR gene alterations (12.7 months vs. 16.1 months, HR 1.95, P = .02) (Figure 2A). Using a multivariate model, the effect of HRR gene alteration on time to mCRPC remained significant when controlling for age, mHSPC therapy, volume of disease, presence of visceral metastases, and PSA (adjusted HR 1.69, P = .02) (Table 4).
Figure 2.
Outcomes of the cohort stratified by the presence of HRR alteration (A): Time to Castration Resistance stratified by cohort with HRR alteration versus biomarker negative (B): Overall survival from time of mHSPC diagnosis, stratified by cohort with HRR alteration versus biomarker negative.
Table 4.
Multivariable Analysis of Time to Castration Resistance.
| Characteristics | aHR (95% CI) | P-value |
|---|---|---|
| HRR alteration present Yes (n = 37) vs. No (n = 114) | 1.68 (1.06-2.65) | .027* |
| Age < = 65 (n=68) vs. > 65 yo (n = 83) | 1.10 (0.73-1.66) | .65 |
| PSA at mHSPC diagnosis | 1.00 (0.99-1.01) | .12 |
| Volume of Disease High (n = 72) vs. Low (n = 75) | 1.67 (1.02-2.74) | .044* |
| Visceral metastases Present (n = 27) vs. Not (n = 124) | 0.89 (0.48-1.63) | .70 |
| De novo metastatic (N = 93) vs. Not (n = 58) | 1.01 (0.65-1.57) | .97 |
| mHSPC therapy | ||
| ADT Alone [n = 69] | Reference | - |
| ADT + ARSI [n = 41] | 0.42 (0.23-0.77) | .005* |
| ADT + Docetaxel [n = 41] | 0.70 (0.40-1.23) | .21 |
P < .05 considered statistically significant.Abbreviations: aHR (Adjusted Hazard Ratio)
When stratified by individual HRR genes, the presence of an alteration in either BRCA2 (HR 2.18, P = .02) or CDK12 (HR 2.52, P = .03) was associated with inferior time to castration resistance compared to other HRR alterations (Table 5). When stratified by individual types of therapy for mHSPC, patients with HRR alterations who received only ADT had worsened time to mCRPC (HR 1.90, P = .02). There were no significant differences in time to mCRPC among HRR altered compared to biomarker negative patients treated with either the addition of an ARSI or docetaxel (Table 5).
Table 5.
Time to mCRPC in HRR Altered vs. Biomarker Negative Cohorts by Clinical and Genomic Characteristics.
| Clinical/Genomic Characteristic |
Univariate HR (95% CI) |
P value |
|---|---|---|
| Any HRR alteration (n = 37) | 1.95 (1.28-2.98) | .020 |
| ATM (n = 9) | 1.61 (0.73-3.52) | .24 |
| BRCA2 (n = 12) | 2.18 (1.12-4.24) | .021 |
| CDK12 (n = 6) | 2.52 (1.09-5.82) | .031* |
| Co-occurring alterations (n = 5)** | 5.37 (2.11-13.68) | < .001 |
| Other HRR alterations (n = 5)*** | 0.80 (0.27-2.74) | .86 |
| Therapy Type | ||
| ADT Alone (n = 69) | 1.90 (1.09 - 3.33) | .024* |
| ADT + ARSI (n = 41) | 1.77 (0.55 -5.69) | .34 |
| ADT + Docetaxel (n = 41) | 2.06 (0.89 -4.77) | .090 |
P < .05 considered statistically significant.
Co-alterations: ATM/CDK12, BRCA1/BRCA2, BRCA2/CDK12, BRCA2/CHEK2
Other HRR alterations: CHEK2, PPP2R2A, RAD51C
PSA Response.
Median PSA at time of cancer diagnosis in HRR altered patients was 28.4 ng/mL ( < 0.1-2867.0) compared to 20.5 ng/mL (< 0.1-40862.0) in biomarker negative individuals (P = .85). Median PSA at metastatic disease diagnosis in HRR altered patients was 49.2 ng/mL (0.5-5,000.0) compared to 34.5 ng/mL (1.1-4249.0) in biomarker negative patients (P = .30). Fewer than 10% of patients in both the HRR altered group and biomarker negative did not have a PSA-50 response following treatment. Patients with HRR alterations had a significantly higher mean PSA nadir compared to biomarker negative individuals (32.9 vs. 5.4 ng/mL; P = .02). Rates of PSA response <0.2 ng/mL at 7 months were similar between HRR altered patients compared to biomarker negative patients (41.7% vs. 31.3%, P = .34).
Overall Survival.
Of the 151 patients included in this analysis, 41 have died. There was no a significant difference in OS among patients with HRR gene alterations compared to patients lacking HRR gene alterations (median 68.2 months vs. 53.3 months respectively, HR 0.98, P = .96) (Figure 2B).
Discussion
While there is a growing data on the predictive role of select HRR gene alterations in patients with mCRPC, the prognostic significance of these alterations in mHSPC remains poorly understood. This study represents the real world data of patients with advanced prostate cancer having undergone clinical-grade NGS. Our results demonstrate that patients with mHSPC with HRR gene alterations were found to have significantly worse clinical outcomes compared to patients lacking such alterations. While there was no difference in overall survival between cohorts, likely driven by the sample size and duration of follow-up, patients with HRR gene alterations had a significantly shorter time to mCRPC. We observe the most dramatic affects in patients with BRCA2 or CDK12 alterations. Given the therapeutic role of PARP inhibitors for patients with HRR altered tumors,11,12 these data highlight the importance of somatic and germline profiling in men with advanced prostate cancer. Additionally, the results provide rationale for testing of PARP inhibitors earlier in the prostate cancer disease natural history.
Over the past 5 years, data have emerged regarding the increased the prevalence of HRR gene alterations in patients with metastatic prostate cancer.4 In the landmark study by Robinson and colleagues, DNA damage repair gene alterations were identified in 23% of 150 mCRPC biopsy samples.4 A larger series including data from 1013 prostate cancer tumors identified DNA repair gene alterations in 10% of primary tumor samples and 27% of metastases.20 Furthermore, screening for 15 HRR gene alterations in the context of the phase 3 PROFOUND study testing olaparib in men with mCRPC identified alterations in 28% of samples analyzed (n = 2792), with similar prevalence between primary (27%) and metastasis (32%) sites, suggesting that HRR gene alterations are likely early events in the prostate cancer disease evolution.11
A significant finding from these early reports was the identification that approximately 8% of mCRPC patients harbored germline mutations in HRR genes.4 These data were confirmed in a pooled multicenter retrospective analysis of 692 patients with metastatic prostate cancer, in which 11.8% of patients were identified to carry a pathogenic mutation in at least one of 20 DNA damage repair genes21.
These revolutionary discoveries have dramatically impacted diagnostic testing and targeted treatments for men with prostate cancer. The PROFOUND trial is the first positive phase 3, biomarker-driven clinical trial in men with mCRPC.11 This study enrolled men with mCRPC having progressed on a prior ARSI and randomized patients to olaparib versus the alterative ARSI. Patients were stratified into one of two cohorts based on the HRR gene alteration present (cohort A: BRCA1/2 or ATM; cohort B: other). The trial met its primary endpoint demonstrating improved radiographic progression-free survival in patients receiving olaparib enrolled onto cohort A. Additionally, treatment was associated with improved radiographic progression-free survival in the intent-to-treatment population (both cohorts A and B) and improved overall survival in patients with BRCA1/2 or ATM altered tumors (cohort A). Data from the TOPARP-A and TOPARP-B studies also support the role of olaparib in mCRPC.22,23 Furthermore, the TRITON2 and GALAHAD studies demonstrated respectable radiographic responses with the alternative PARP inhibitors rucaparib and niraparib, respectively.12,24 To date, there are 2 FDA approved PARP inhibitors for use clinically: olaparib (mCPRC post ARSI for patients with one of 14 HRR gene alterations) and rucaparib (mCRPC post ARSI and docetaxel for patients with BRCA1/2 mutated tumors). In addition to PARP inhibitors, platinum-based chemotherapy has also demonstrated efficacy in patients with HRR gene alterations. As a result of these data, the National Comprehensive Cancer Network (NCCN) and other guidelines panels have now recommended somatic and germline tumor profiling for all men with metastatic prostate cancer given implications for treatment, cancer screening, and cascade testing for family members.
Studies have also investigated the role of PARP inhibitor in combination with ARSIs in patients with mCRPC independent of HRR gene alterations. The PROPEL and MAGNITUDE studies tested olaparib and niraparib, respectively, in combination with abiraterone in patients with treatment-naïve mCRPC unselected for HRR gene alteration. The PROPEL study demonstrated an improved in radiographic progression-free survival of olaparib plus abiraterone compared to placebo plus abiraterone in all patients, while the Magnitude trial demonstrated an improved radiographic progression-free survival of niraparib plus abiraterone compared to placebo plus abiraterone in HRR-positive patients only.25,26 To date, mature data on the impact on overall survival and granular data in biomarker subgroups are lacking and the clinical application of this data are still to be determined. Additional studies are investigating the combination of PARP inhibitors with enzalutamide in patients with treatment-naïve mCRPC independent of HRR gene status and will shed further light on this approach: TALAPRO-2 testing talazoparib plus enzalutamide (NCT03395197) and CASPER testing rucaparib plus enzalutamide (NCT04455750).
In parallel to these advances in genomics and targeted treatments for patients with mCRPC, a series of phase 3 studies were reported over the past 6 years that tested the hypothesis of early treatment intensification for men with mHSPC. Studies evaluating docetaxel, abiraterone, enzalutamide, and apalutamide have uniformly demonstrated efficacy of early use of taxane chemotherapy or ARSI treatment in the setting of hormone sensitive disease.1-3,7-9 Additionally, the more recent data from the PEACE-1 trial and also the ARASENS trial demonstrated that the combination of docetaxel with abiraterone or darolutamide, respectively, also improves overall survival for patients with advanced prostate cancer.5
Taken together with the emerging role of PARP inhibitors for mCRPC, these data provide rationale for investigation of early PARP inhibition or platinum chemotherapy for patients with mHSPC with somatic or germline HRR gene alterations. To successfully test this approach in selected patients with mHSPC, a clear understanding of the prognostic significance of HRR gene alterations in the hormone sensitive setting is warranted. Our study aims to achieve this goal and defines the prognostic significance of HRR gene alterations in men with mHSPC. While longer follow up is warranted, we demonstrate that patients with HRR gene alterations have a shorter time to development of mCRPC. Furthermore, our data highlight differential responses to therapy in the hormone sensitive setting. Patients with tumors with HRR gene alterations had an inferior response to ADT alone compared to patients lacking such alterations, but had no significant difference in time to mCRPC when receiving therapy escalation with ADT plus ARSI or ADT plus docetaxel. Prior studies have demonstrated that patients with HRR gene alterations appear to have similar response to standard therapies compared to patients lacking HRR gene alterations, though validation studies are warranted.16,17,27
There are several ongoing studies evaluating the use of PARP inhibitors in patients with hormone sensitive prostate cancer with HRR gene alterations. These studies include TAPAPRO-3: talazoparib combined with enzalutamide in patients with tumors with HRR gene alterations (NCT04821622) and Amplitude: niraparib combined with abiraterone in patients with HRR gene altered tumors (NCT04497844). Additionally, several studies are investing PARP inhibitors in the BCR, non-metastatic setting including olaparib in combination with durvalumab (NCT03810105) and rucaparib in patients with BRCAness genotype (NCT03533946).
Key limitations of this study include the retrospective nature of the analysis. Additionally, germline testing was only performed on 60.3% of patients. Genetic profiling was not obtained uniformly at diagnosis for all patients. Additionally, a variety of clinical grade sequencing platforms were used in this cohort, reflective of the real-world patient population, which could potentially add variability into analysis. We also acknowledge that this cohort is comprised primarily of white men, and recognize that it may not accurately represent a diverse patient population.
HRR alterations carry the clinical significance in the mHSPC patient, and with the increased use of PARP inhibitors in patients with mCRPC, it is a rational to test the role of PARP inhibitors in patients with mHSPC. Our data further support the standard use of early testing for somatic and germline HRR alterations for all patients with metastatic disease to provide insight into both prognostication and treatment options for patients with advanced disease.
Clinical Practice Points
Alterations in the homologous recombination repair (HRR) pathway have been observed in roughly one quarter of patients with advanced prostate cancer. Despite this, little is known about the clinical course of patients with metastatic hormone sensitive prostate cancer (mHSPC) who harbor alterations in the HRR pathway. Here, we examined outcomes of 151 men with mHSPC who underwent genomic sequencing. The primary objective was to examine time to mCRPC in patients with HRR alterations compared to individuals lacking such alterations. Among this cohort, 24% (N = 37) had pathogenic HRR gene alterations. Time to mCRPC was significantly decreased in patients with HRR gene alterations compared to patients lacking such alterations (12.7 vs. 16.1 months, HR 1.95, P = .02). In multivariate analysis, the effect of HRR gene alterations on time to mCRPC remained significant (adjusted HR 1.69, P = .02). When stratified by specific HRR gene alteration, patients with BRCA2 or CDK12 alterations had significantly decreased time to mCRPC compared to individuals with other HRR gene alterations. These data show that patients with HRR gene alterations appear to have more aggressive disease and shorter time to castration resistance. Additionally, given that Poly (ADP-ribose) Polymerase (PARP) inhibitors have been approved in the management of mCRPC, ongoing studies which are exploring the use of PARP inhibitors in mHSPC will be important in attempting to optimize therapy for group of patients.
Supplementary Material
Table 3.
Genomic Characteristics of Cohort.
| Genomic Characteristic | N (%N), N = 151 total |
|---|---|
| Underwent somatic profiling | 151 (100%) |
| Tissue NGS only | 68 (45%) |
| Circulating tumor DNA (ctDNA) only | 38 (25%) |
| Both Tissue NGS/ctDNA | 45 (30%) |
| Underwent germline profiling | 91 (60.3%) |
| HRR alterations detected | 37 (24.5%) |
| Somatic Alteration Only | 27 (73%) |
| Germline Alteration Only | 8 (22%) |
| Somatic/Germline Co-Alteration | 2 (5%) |
| Single HRR alteration detected | 32 (86.5%) |
| Compound HRR alterations detected | 5 (13.5%) |
Footnotes
Conflicts of Interest
RRM received research funding from Bayer, Pfizer, Tempus; serves on Advisory Board/consultant for AstraZeneca, Aveo, Bayer, Bristol Myers Squib, Calithera, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, Tempus, Telix.
The remaining authors have no conflicts of interest to disclose.
Disclosure
RRM received research funding from Bayer, Pfizer, Tempus; serves on Advisory Board/consultant for AstraZeneca, Aveo, Bayer, Bristol Myers Squib, Calithera, Caris, Dendreon, Exelixis, Janssen, Merck, Myovant, Novartis, Pfizer, Sanofi, Sorrento Therapeutics, Tempus, Telix.
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clgc.2022.06.016.
References
- 1.James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med. 2019;381(2):121–131. [DOI] [PubMed] [Google Scholar]
- 3.Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2019;381(1):13–24. [DOI] [PubMed] [Google Scholar]
- 4.Robinson D, Van Allen EM, Wu YM, et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell. 2015;162(2):454. [DOI] [PubMed] [Google Scholar]
- 5.Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022;386(12):1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fizazi K LBA5_PR - A phase III trial with a 2x2 factorial design in men with de novo metastatic castration-sensitive prostate cancer: Overall survival with abiraterone acetate plus prednisone in PEACE-1. Annals of Oncology. 2021;162(2):S1283–S1346. [Google Scholar]
- 7.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. N Engl J Med. 2015;373(8):737–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Engl J Med. 2017;377(4):352–360. [DOI] [PubMed] [Google Scholar]
- 9.Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974–2986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383(24):2345–2357. [DOI] [PubMed] [Google Scholar]
- 11.de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091–2102. [DOI] [PubMed] [Google Scholar]
- 12.Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a. J Clin Oncol. 2020;38(32):3763–3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro E, Goh C, Olmos D, et al. Germline BRCA mutations are associated with higher risk of nodal involvement, distant metastasis, and poor survival outcomes in prostate cancer. J Clin Oncol. 2013;31(14):1748–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Annala M, Struss WJ, Warner EW, et al. Treatment Outcomes and Tumor Loss of Heterozygosity in Germline DNA Repair-deficient Prostate Cancer. Eur Urol. 2017;72(1):34–42. [DOI] [PubMed] [Google Scholar]
- 15.Annala M, Vandekerkhove G, Khalaf D, et al. Circulating Tumor DNA Genomics Correlate with Resistance to Abiraterone and Enzalutamide in Prostate Cancer. Cancer Discov. 2018;8(4):444–457. [DOI] [PubMed] [Google Scholar]
- 16.Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2019;37(6):490–503. [DOI] [PubMed] [Google Scholar]
- 17.Antonarakis ES, Lu C, Luber B, et al. Germline DNA-repair Gene Mutations and Outcomes in Men with Metastatic Castration-resistant Prostate Cancer Receiving First-line Abiraterone and Enzalutamide. Eur Urol. 2018;74(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scher HI, Morris MJ, Stadler WM, et al. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J Clin Oncol. 2016;34(12):1402–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harshman LC, Chen YH, Liu G, et al. Seven-Month Prostate-Specific Antigen Is Prognostic in Metastatic Hormone-Sensitive Prostate Cancer Treated With Androgen Deprivation With or Without Docetaxel. J Clin Oncol. 2018;36(4):376–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Armenia J, Wankowicz SAM, Liu D, et al. The long tail of oncogenic drivers in prostate cancer. Nat Genet. 2018;50(5):645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375(5):443–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med. 2015;373(18):1697–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith M Pre-specified interim analysis of GALAHAD: A phase 2 study of niraparib in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD). Annals of Oncology. 2019;30. [Google Scholar]
- 25.Chi KN, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first–line therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. Journal of Clinical Oncology. 2022;40(6_suppl):12.34752147 [Google Scholar]
- 26.Saad F, Armstrong AJ, Thiery-Vuillemin A, et al. PROpel: Phase III trial of olaparib (ola) and abiraterone (abi) versus placebo (pbo) and abi as first-line (1L) therapy for patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Journal of Clinical Oncology. 2022;40(6_suppl):11. [Google Scholar]
- 27.Mateo J, Carreira S, de Bono JS. PARP Inhibitors for Advanced Prostate Cancer: Validating Predictive Biomarkers. Eur Urol. 2019;76(4):459–460 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.