Abstract
Background
We tested whether neuroaffective responses to motivationally salient stimuli are associated with vulnerability to cue-induced e-cigarette use in e-cigarette naïve adults who smoke daily. We hypothesized that individuals with stronger neuroaffective responses to nicotine-related cues than to pleasant stimuli (the C>P reactivity profile) would be more vulnerable to cue-induced nicotine self-administration than individuals with stronger neuroaffective responses to pleasant stimuli than to nicotine-related cues (the P>C reactivity profile).
Methods
We used event-related potentials (ERPs, a direct measure of cortical activity) to measure neuroaffective reactivity to pleasant, unpleasant, neutral, and nicotine-related cues indicating the opportunity to use an e-cigarette in 36 participants. For each picture category, we computed the amplitude of the late positive potential (LPP), a robust index of motivational salience. To identify each individual’s neuroaffective reactivity profile we applied k-means cluster analysis on the LPP responses. We compared the e-cigarette use frequency across profiles using quantile regression for counts.
Results
K-means cluster analysis assigned 18 participants to the C>P profile and 18 participants to the P>C profile. Individuals with the C>P neuroaffective profile used the e-cigarette significantly more often than those with the P>C profile. Significant differences in the number of puffs persisted across different quantiles.
Conclusions
These results support the hypothesis that individual differences in the tendency to attribute motivational salience to drug-related cues underlie vulnerability to cue-induced drug self-administration. Targeting the neuroaffective profiles that we identified with tailored treatments could improve clinical outcomes.
Keywords: Drug-related cues, Nicotine self-administration, Event-Related Potentials, Late Positive Potential, Motivational Salience, Quantile Regression
1. Introduction
Neurobiological models of addiction posit that individuals with substance use disorders are prone to compulsive drug use because they attribute high levels of incentive salience to drug-related cues (i.e., stimuli that are associated with a drug and its effects) (Robinson et al., 2013; Volkow et al., 2019). Incentive salience refers to the motivational properties that make rewards (e.g., water, food, sex, or drugs) and reward-related cues attractive (Berridge, 2012). Results from animal models show that individuals that attribute high incentive salience to reward-related cues are more vulnerable to cue-induced compulsive reward seeking behaviors, including drug self-administration (Flagel et al., 2009). We proposed that humans also vary in their tendency to attribute incentive salience to reward-related cues and that those attributing high levels of incentive salience to drug-related cues would be more prone to cue-induced drug seeking (Versace et al., 2017). Identifying a biomarker of the tendency to attribute high incentive salience to drug-related cues could have significant clinical implications because it will give clinicians a new treatment target and could foster the development of personalized treatments aimed at reducing vulnerability to cue-induced compulsive substance use.
The amplitude of the late positive potential (LPP) has been proposed as an index of incentive salience attributed to drug-related and other motivationally salient stimuli (Webber et al., 2022). The LPP is a robust component of the event-related potentials (ERPs, a direct measure of cortical activity) that peaks over central scalp sites approximately 600 ms after the presentation of a stimulus and its amplitude increases as a function of a stimulus’ motivational relevance, irrespective of its hedonic value (Lang and Bradley, 2010; Minnix et al., 2013; Schupp et al., 2000; Weinberg and Hajcak, 2010). Even though drug-related cues tend to evoke larger LPP responses in individuals with substance use disorders than in controls (Littel et al., 2012; Robinson et al., 2015), there are large individual differences in the neurophysiological responses evoked by drug-related cues. Applying multivariate classification algorithms to the LPP responses evoked by drug-related and non-drug-related motivationally relevant stimuli, we identified two replicable neuroaffective reactivity profiles: one characterized by larger LPPs to drug-related cues than to pleasant stimuli (C>P), the other by larger LPPs to pleasant stimuli than drug-related cues (P>C) (Kypriotakis et al., 2020; Versace et al., 2023, 2017; Webber et al., 2021). We also showed that these two neuroaffective reactivity profiles are associated with different drug-related behaviors: among people with cocaine use disorder, individuals characterized by the C>P profile have a stronger attentional bias towards drug-related cues than individuals characterized by the P>C profiles (Webber et al., 2021), and among adults who smoke, those characterized by the C>P profile are more likely to resume smoking than those with the P>C profile (Frank et al., 2020; Versace et al., 2012).
While these findings support the hypothesis that individual differences in the tendency to attribute incentive salience to drug-related cues affect drug-related behaviors, they do not directly link the two neuroaffective profiles to differences in drug self-administration. Accordingly, we designed this study to test the hypothesis that individuals characterized by the C>P profile are more prone to cue-induced nicotine self-administration than those characterized by the P>C profile.
To test our hypothesis, we developed a new neurobehavioral assessment: the “cued nicotine availability task”. During the cued nicotine availability task, we recorded ERPs while e-cigarette–naïve adults who smoked cigarettes looked at non-drug-related motivationally relevant images and at images of people using e-cigarettes. These images signaled that an electronic nicotine delivery system (ENDS) was available for use. Hence, unlike previous passive picture viewing tasks, the cued nicotine availability task allowed us to measure both the motivational relevance of drug-related cues (relative to non-drug-related motivationally relevant stimuli), and actual nicotine self-administration. We hypothesized that a) applying k-means cluster analysis to the LPP responses recorded during the cued nicotine delivery task would yield the C>P and P>C profiles and b) individuals characterized by the C>P profile would use the ENDS to self-administer nicotine significantly more often than those characterized by the P>C profile.
2. Methods
2.1. Participants
We enrolled adults who reported daily cigarette smoking and no prior use of e-cigarettes. Participants were older than 18 and did not report psychiatric disorders. We reimbursed participants with a $50 gift card at the end of the session. The original recruitment plan included 60 participants. However, owing to the COVID-19 pandemic, our laboratory shut down in March 2020. After one year of inactivity, the protocol associated with this study was closed and only 36 participants were available for the analyses. Table 1 shows the characteristics of the sample.
Table 1.
Demographic characteristics
| All (N=36) | C>P (N=18) | P>C (N=18) | |
|---|---|---|---|
| Mean age (SD) | 46 (11) | 42 (12) | 49 (10) |
| Females | 42% | 39% | 44% |
| Race | |||
| White | 17% | 22% | 11% |
| Black | 83% | 78% | 89% |
| Questionnaire scores | |||
| FTCD | 4.7 (4.7) | 5.2 (1.6) | 4.2 (2.1) |
| QSU | 47.1 (29.4) | 53.2 (31.9) | 50.1 (30.4) |
| BIS attention | 14.9 (3.2) | 14.4 (3.4) | 15.4 (3) |
| BIS motor | 22.2 (4.7) | 21.4 (4.6) | 23.1 (4.8) |
| BIS nonplanning | 24.5 (4.3) | 23.9 (3.3) | 25.1 (5.1) |
| PANAS negative | 17.6 (5.4) | 17.7 (5.2) | 17.6 (5.7) |
| PANAS positive | 34 (8.7) | 33.9 (9.2) | 34 (8.5) |
| SHAPS | 9.9 (5.5) | 10.6 (5.4) | 9.2 (5.6) |
NOTE: FTCD = Fagerström Test for Cigarette Dependence, QSU=Questionnaire for Smoking Urges, BIS = Barratt Impulsiveness Scale, PANAS=Positive and Negative Affect Scale, SHAPS=Snaith-Hamilton Pleasure Scale.
2.2. Procedures
The University of Texas MD Anderson Cancer Center Institutional Review Board approved all procedures. We asked participants to continue smoking at their regular rate but to refrain from using other drugs (including marijuana) in the 24 hours and caffeine in the 2 hours preceding the visit. At the visit, we explained the procedures and collected informed consent. Then, participants completed a battery of self-report questionnaires (see Supplementary Materials for details) about nicotine dependence (Fagerström Test for Cigarette Dependence, FTCD; Fagerstrom, 2012) smoking urges (Questionnaire of Smoking Urges, QSU-brief; Cox et al., 2001), impulsivity (Barratt Impulsiveness Scale, BIS; Patton et al., 1995), hedonic capacity (Snaith-Hamilton Pleasure Scale, SHAPS; Snaith et al., 1995), and mood (Positive and Negative Affect Scale, PANAS; Watson et al., 1988). We used the responses to these questionnaires to test for differences between groups. After the questionnaire data collection, we prepared the participant for the EEG session and completed the cued nicotine availability task. At the end of the session, participants were debriefed, encouraged to quit smoking, and compensated.
2.3. Materials
2.3.1. Cued nicotine self-administration task
The task included 300 images divided into 6 equivalent blocks. The images (selected from the International Affective Picture System (Lang et al., 2008) and other picture collections; see Supplementary Table 1 for the IAPS picture numbers) belonged to 8 categories: pleasant high salience (PH, erotic scenes of naked couples), pleasant low salience (PL, romantic scenes of couples hugging or kissing), food (FD, sweet palatable foods), neutral (NE, ordinary objects and people engaged in mundane activities), unpleasant objects (UO, accidents and pollution), unpleasant low salience (UL, sadness and violence), unpleasant high salience (UH, mutilations), and ENDSs (EC, images of people vaping or images of ENDSs). Figure 1 outlines the trial structure. The images (except EC) were presented for 2 seconds, followed by a 1.5- to 3-second variable inter-trial interval. EC images were presented for 1 second, and then a banner appeared at the top of the screen to indicate that it was possible to take 1 puff from the ENDS (Model: THERION-BF-DNA75C, loaded with tobacco-flavored e-liquid with 1.2% nicotine). The ENDS rested inside a receptacle within arm’s reach of the participant (See Figure 1 inset and Supplementary Figure S1). A photocell in the receptacle detected when the participant picked up the ENDS and paused the task until the ENDS was placed back in the receptacle. If the participant decided not to use the ENDS, they pressed a button to advance to the next trial. Each block included 10 vaping opportunities. To familiarize participants with the ENDS, we ran a practice block of 10 trials with 2 vaping opportunities. We asked participants to take 1 puff at both practice vaping opportunities.
Figure 1. Schematic sequence of events during the cued nicotine availability task.
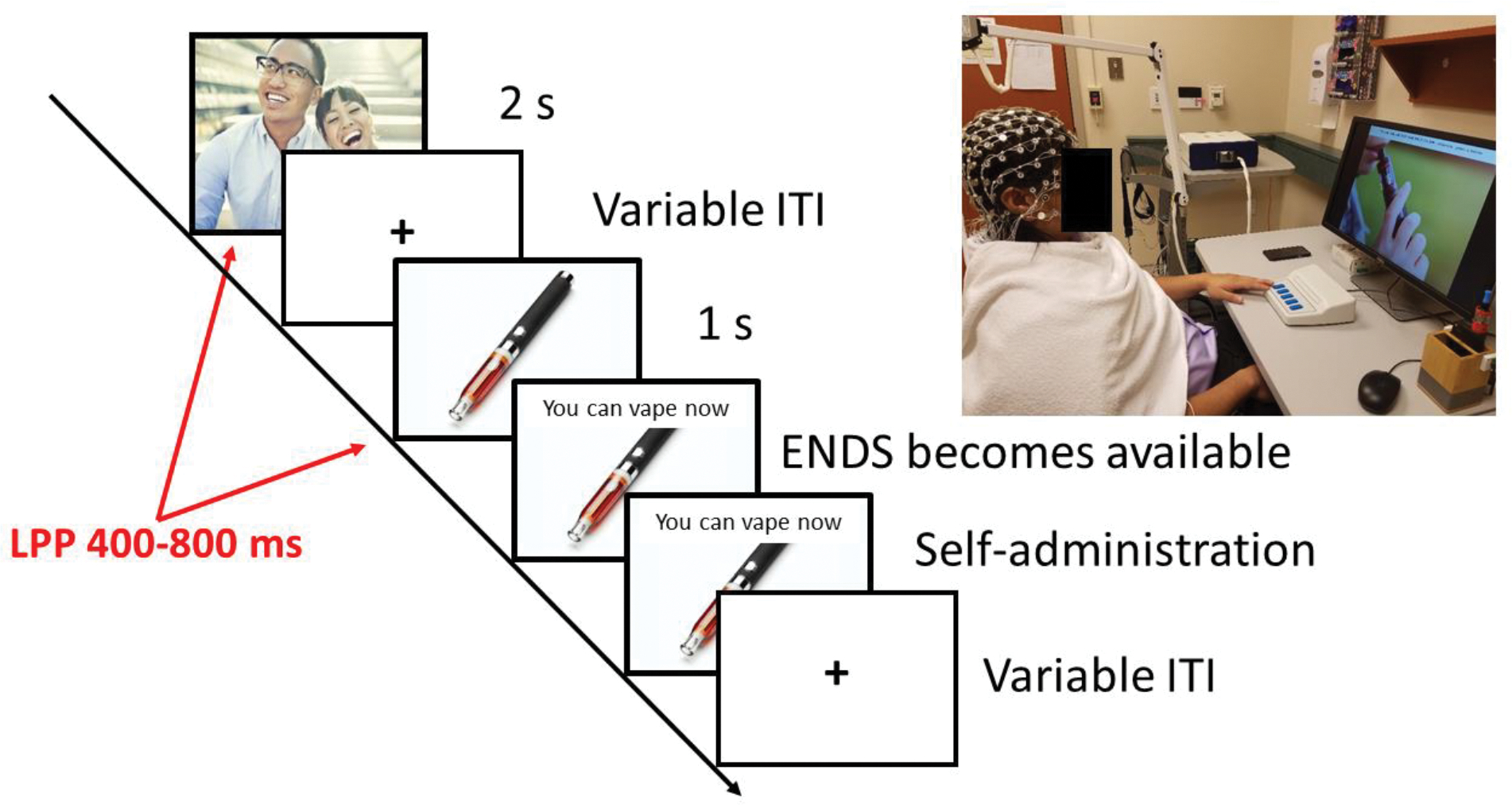
During the cued nicotine availability task, non-drug-related cues are presented for two seconds and are followed by a blank screen. Nicotine-related cues (images of people vaping or images of e-cigarettes) are presented for 1 second, then a banner appears on the top of the screen to let the participant know that the electronic nicotine delivery system (ENDS) is ready for use. The participant decides whether to take one puff from the ENDS or to push a button to move to the next trial (See inset and Supplementary Figure 1 for details about the nicotine self-administration apparatus). During the task, EEG is continuously recorded and the amplitude of the late positive potential (LPP) is computed offline for every picture category presented during the study. Supplementary Figure S1 details the elements of the apparatus depicted in the right panel.
2.3.2. EEG data acquisition
During the task, we collected EEG using a 129-channel Geodesic Sensor Net, amplified with a Geodesic EEG System 400 amplifier. The sampling rate was 250 Hz, and all electrodes were referenced to Cz.
2.3.3. EEG data reduction and LPP amplitude calculation
Offline EEG data reduction followed a standard pipeline (Versace et al., 2019, 2012). It included filtering (0.1–30 Hz), EEG visual inspection and interpolation of broken channel (using spherical splines), average reference calculation, and eye movements and blink correction (as implemented in BESA 5.3). Then, data were imported into Brain Vision Analyzer 2 and divided into 1000-ms segments starting 100 ms before picture onset. After baseline correction, channels contaminated by artifacts were identified using pre-defined criteria of relative and absolute voltage amplitude. Channels contaminated by artifacts in more than 40% of the segments were interpolated using spherical splines. Segments with more than 10% of contaminated channels were discarded, and average amplitudes for each picture category were computed at each scalp site. For each participant, we calculated the mean LPP amplitude of each category as the mean voltage recorded between 400 and 800 ms after picture onset across 10 central and parietal sensors (EGI HydroCel Geodesic Sensor Net sensors: 7, 31, 37, 54, 55, 79, 80, 87, 106, 129; see Figure 1 inset). The Supplementary Results section reports the number of channels interpolated per subject, the number of trials included in each condition, and the standardized measurement error for each condition at the end of the data reduction process (Luck et al., 2021).
2.4. Statistical Analyses
2.4.1. Event-related potentials
As a manipulation check, we analyzed the LPP responses to the 8 stimulus categories (EC, PH, PL, FD, NE, UO, UL, UH) in a repeated measures ANOVA. We expected to replicate previous findings showing that both pleasant and unpleasant images increase the LPP amplitude as a function of their motivational relevance and that nicotine cues prompt larger LPP responses than neutral stimuli.
2.4.1.1. Participant classification
To identify participants attributing high or low salience to cues, we applied k-means cluster analysis to the LPP responses evoked by the 8 image categories. K-means cluster analysis is a multivariate classification procedure that, by minimizing within-group variability and maximizing between-group variability, groups individuals according to common features. This is the same procedure used in our previous studies, and, based on those findings (Versace et al., 2019, 2012; Webber et al., 2021), we hypothesized that the 2 groups would show the following neuroaffective reactivity profiles: one group would show high reactivity to EC relative to pleasant stimuli (C>P); the other, low reactivity to EC relative to pleasant stimuli (P>C). We also expected that, irrespective of reactivity to EC, both groups would show larger LPP responses as a function of motivational relevance for both pleasant and unpleasant stimuli.
2.4.1.2. Cue-induced nicotine self-administration
We tested the relationship between group membership and number of puffs using quantile regression (QR, Koenker, 2005). Conceptually, QR is an extension of linear regression, but instead of estimating the mean of the dependent variable, it estimates the median. Hence, QR is most useful in the presence of outliers and when assumptions of the linear model are not met (including assumptions of homoscedasticity, linearity and normality). Furthermore, in addition to estimating the median (i.e., the 0.5 quantile), QR can be used to estimate any quantile (e.g., 0.25 or 0.75 quantile) of the outcome variable’s distribution. Hence, by deriving regression estimates at multiple quantiles, QR provides a more comprehensive and insightful analysis than linear regression. Because our dependent variable (the number of puffs) is not continuous, we used QR for counts (Machado and Santos Silva, 2005), and for estimation we used 5,000 bootstrap samples to calculate 95% confidence intervals (CIs) around the 0.25, 0.5, and 0.75 quantile.
3. Results
3.1. Late Positive Potential
Figure 1 shows the grand-averaged ERP waveforms for neutral, pleasant, unpleasant, and nicotine-related images and the mean LPP amplitude for each category. As expected, pleasant, unpleasant, and nicotine-related stimuli prompted larger LPPs than neutral images (Bonferroni-corrected P values <0.005), and the amplitude of the LPP increased as a function of motivational relevance for both pleasant and unpleasant stimuli (polynomial contrast for the quadratic trend including PH, PL, FD, NE, UO, UL, UH; F(1,35)=123.3; p<0.001).
K-means cluster analysis assigned 18 subjects to each group. As hypothesized (see Figure 2), both groups had larger LPP responses to motivationally salient images than to neutral images (the quadratic trend was significant [p<0.001] in both groups), but one group showed the C>P profile (i.e., larger LPP responses to EC than to pleasant images), while the other showed the P>C profile. The 2 groups did not differ according to demographic characteristics, nicotine dependence, mood, or impulsivity (see Table 1).
Figure 2.
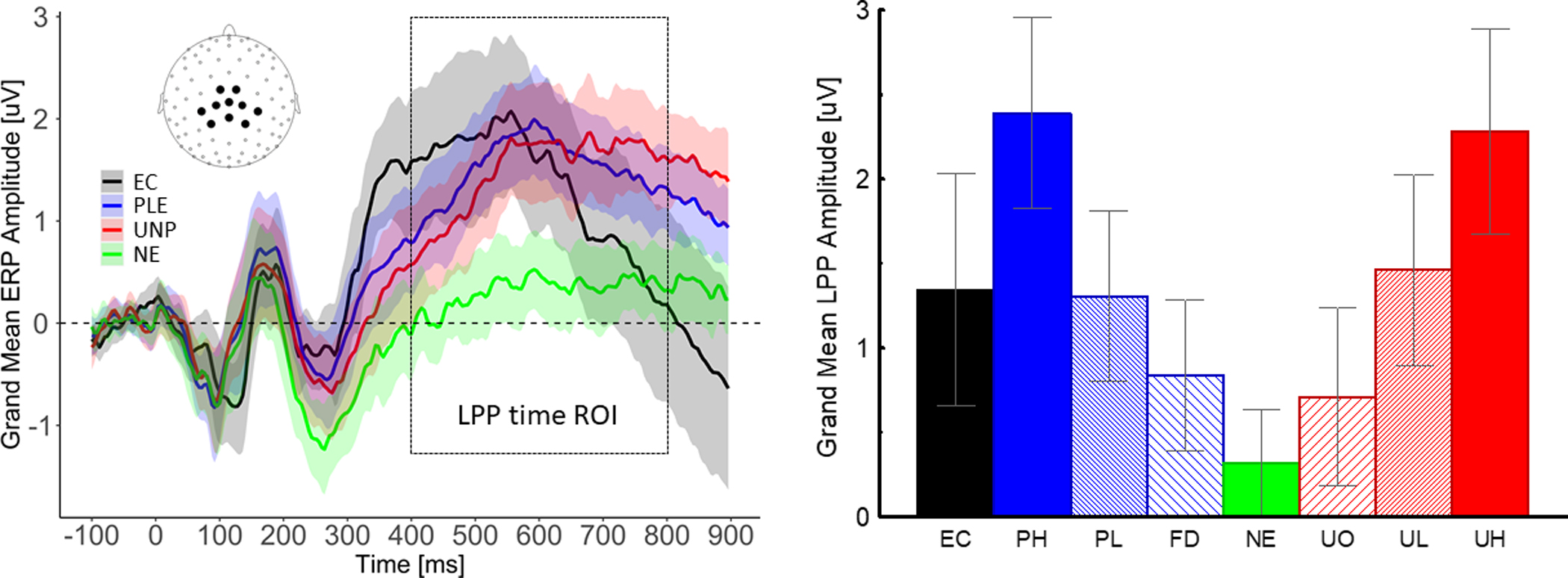
Left: Motivationally salient images (including EC) prompted larger LPPs than neutral images. Right: The LPP amplitude increased as a function of motivational salience for both pleasant and unpleasant contents. Note: LPP=late positive potential, ROI=region of interest, EC=E-cigarettes, PLE=pleasant (PH, PL, FD averaged), UNP=unpleasant (UH, UL, UO averaged), NE=neutral, PH=pleasant high motivational salience (erotica), PL=pleasant low motivational salience (romantic), FD=food (appetizing sweet food), UO=unpleasant objects (accidents, pollution), UL=unpleasant low motivational salience (violence), UH=unpleasant high motivational salience (mutilations). The values are calculated averaging the voltage across the 10 sensors shown in the inset on the left panel. The shaded areas in the left panel and the error in the right panel represent ±95% confidence intervals around the means.
3.2. Cue-induced nicotine self-administration
The results of the QR analysis for counts are presented in Figure 2 and Table 2. Because the unstandardized coefficients are derived from a log-linear model and do not have an intuitive interpretation, we estimated marginal effects that have a direct interpretation. Marginal effects are the differences in the number of puffs between the groups derived from each quantile regression model. For all 3 estimated quantiles, the C>P group took a significantly higher number of puffs than the P>C group. Specifically, the differences in the estimated number of puffs were 5 (C>P = 9 vs. P>C = 4), 5 (C>P = 15 vs. P>C =10), and 8 (C>P = 35 vs. P>C = 27) puffs at the 25th, 50th, and 75th percentiles, respectively.
Table 2.
Unstandardized and marginal effects of group membership on the number of puffs.
| Model | Effect | CI | p-value |
|---|---|---|---|
| Unstandardized Effects | |||
| Quantile 0.25 | −0.80 | (−1.29, −0.31) | 0.001 |
| Quantile 0.50 | −0.41 | (−0.76, −0.07) | 0.018 |
| Quantile 0.75 | −0.26 | (−0.43, −0.09) | 0.002 |
| Marginal effects of differences between groups | |||
| Quantile 0.25 | −5.00 | (−7.61, −2.39) | < 0.001 |
| Quantile 0.50 | −5.08 | (−9.66, −0.49) | 0.029 |
| Quantile 0.75 | −7.99 | (−12.95, −3.03) | 0.002 |
4. Discussion
By applying cluster analysis to the neuroaffective responses evoked by drug-related and non-drug-related motivationally salient stimuli, among individuals who smoke cigarettes we identified those who attribute high salience to cues indicating nicotine availability (C>P), and those who do not attribute high salience to such cues (P>C). We showed that individuals characterized by the C>P neuroaffective profile are more likely to self-administer nicotine than those characterized by the P>C neuroaffective reactivity profile when given the opportunity to do so.
These results align with preclinical (Flagel et al., 2009) and clinical findings (Versace et al., 2014, 2012; Webber et al., 2021) indicating that individual differences in the tendency to attribute incentive salience to cues predicting rewards underlie vulnerability to the maladaptive cue-induced behaviors that characterize substance use disorders. The C>P profile could become a clinical target to match patient-to-treatment (Cinciripini et al., 2017; Frank et al., 2020) and to develop new personalized treatments for substance use disorders and other behavioral disorders characterized by cue-induced compulsive behaviors (Houston and Schlienz, 2018).
Notwithstanding their potential theoretical and clinical significance, these results should still be considered preliminary. The main limitation of this study is its small sample size: it has been shown that low statistical power reduces the likelihood that a nominally statistically significant finding reflects a true effect (Button et al., 2013) and the findings that we report should be replicated in a larger sample. Nevertheless, we think that the features of the cued nicotine availability task and the analytic approach that we chose allowed us to produce valid and reliable results that will likely replicate. First, because the cued nicotine availability task includes several non-drug-related motivationally relevant conditions, it allowed us to evaluate the reliability of the results across multiple active control conditions. In line with the existing literature (Lang and Bradley, 2010; Weinberg and Hajcak, 2010), we showed that the amplitude of the LPP increases as a function of motivational salience for both pleasant and unpleasant images within both the C>P and the P>C group (Figure 3). Replicating in both groups the canonical affective modulation of the LPP for non-drug-related motivationally relevant stimuli indicates that the LPP modulation observed for drug-related cues is unlikely to be spurious. A second feature that, in our opinion, supports the claim that our findings are reliable stems from our decision to use k-means clustering to identify participants’ neuroaffective profiles. K-means clustering is a data-driven multivariate classification procedure that groups individuals by minimizing the Euclidian distance of each participant to the centroid of the cluster (Pollard, 1981). While cluster analysis must yield two groups, the neuroaffective profiles of the groups identified by cluster analysis are not predetermined and are driven by the data. Yet, the profiles identified in this new sample replicate those that emerged in our previous studies (Versace et al., 2019, 2016, 2014, 2012; Webber et al., 2021), an outcome that supports our claim that the results reported are reliable, despite the small sample size. It is important to note that while the largest reactivity differences involve drug-related cues and pleasant stimuli (hence the labels that we assigned to the groups), reactivity to unpleasant and neutral stimuli somewhat contributes to the classification outcomes: the voltage differences between pleasant and drug-related cues do not predict outcomes as accurately as the results from multivariate clustering. Finally, using nicotine self-administration as the outcome measure, rather than self-reports, strengthens the validity of our conclusions. During most brain imaging studies that assess cue reactivity, participants do not have the option to self-administer nicotine, hence researchers often opt to investigate the relationship between neurophysiological responses evoked by drug-related cues and self-reported nicotine craving (Engelmann et al., 2012). While craving is considered a symptom with clinical significance (Hasin et al., 2013), empirical findings show that it is not consistently associated with smoking cessation outcomes (Wray et al., 2013). These inconsistencies suggest that self-reported craving may not be a reliable surrogate measure for validating neurophysiological markers aimed at predicting cue-induced drug use. By measuring drug self-administration rather than self-reported craving, the cued nicotine availability task allows to directly investigate the psychophysiological underpinnings of cue-induced drug use.
Figure 3.
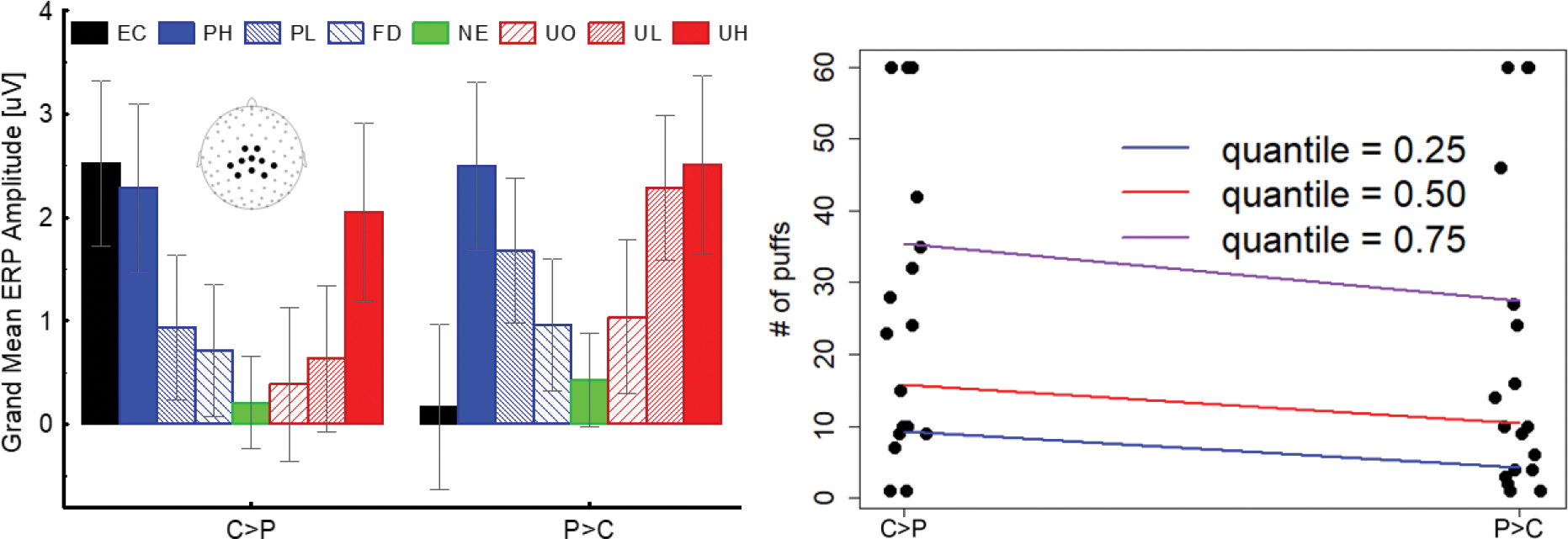
Left: The cluster analysis identified two groups of individuals with the hypothesized characteristics: one group (C>P) had larger late positive potential (LPP) responses to EC than to pleasant images and the other (P>C) had larger LPP responses to pleasant than to EC images. Right: Individuals categorized as C>P took significantly more puffs from the electronic nicotine delivery system (ENDS) than individuals categorized as P>C. The between-groups difference in number of puffs was significant (p<.05) at every predicted quantile (.25, .50, and .75). Note: The error bars represent the ±95% confidence intervals around the means. EC=images of people vaping, PH=pleasant high motivational salience, PL=pleasant low motivational salience, FD=food, NE=neutral, UO=unpleasant objects, UL=unpleasant low motivational salience, UH= unpleasant high motivational salience. The LPP values are calculated averaging the voltage across the 10 sensors shown in the inset. LPP responses from each subject for EC, PLE, NE, and UNP contents are shown in Supplement Figure S4
We encourage addiction neuroscientists to adapt the cued nicotine availability task to other drugs and other environments: following our recommendations of measuring reactivity to non-drug-related motivationally relevant stimuli and making rewards immediately available during the task (Versace et al., 2023) is likely to foster the discovery of new biomarkers and treatment targets for substance use disorders.
5. Conclusions
Our results show that neuroaffective responses to motivationally salient stimuli are associated with vulnerability to cue-induced nicotine self-administration: when individuals who smoke combustible cigarettes are given the opportunity to use an e-cigarette, those with stronger neuroaffective responses to nicotine-related cues than to pleasant stimuli (C>P) used the device more often than those with stronger neuroaffective responses to pleasant stimuli than to nicotine-related cues (P>C). Determining the extent to which the neurobehavioral outcomes that we obtained using this laboratory task predict real-world vulnerability to cue-induced compulsive smoking following an attempt to quit, the next step in our research agenda, will contribute to the development and optimization of new targeted clinical interventions for substance use disorders.
Supplementary Material
Highlights.
We used ERPs to measure neuroaffective reactivity to emotional and drug-related cues
Drug-related cues signaled the opportunity to puff from an e-cigarette
We identified neuroaffective reactivity profiles associated with e-cigarette use
Smokers reacting more to drug-related than pleasant cues vaped more
Targeting this neuroaffective biomarker could foster personalized treatments
Acknowledgements:
We thank Liz Lee, Kendra Lumbi, and Menton Deweese for help with data collection.
Role of Funding Source:
This work was supported by the National Institute on Drug Abuse of the National Institutes of Health (R01DA032581 to FV) and by MD Anderson’s Cancer Center Support Grant P30CA016672. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interests: The authors declare no competing financial interests.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data availability statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- Berridge KC, 2012. From prediction error to incentive salience: Mesolimbic computation of reward motivation. Eur. J. Neurosci. 35, 1124–1143. 10.1111/j.1460-9568.2012.07990.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPA, Mokrysz C, Nosek BA, Flint J, Robinson ESJ, Munafò MR, 2013. Power failure: why small sample size undermines the reliability of neuroscience. Nat. Rev. Neurosci. 14, 365–376. 10.1038/nrn3475 [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Green CE, Robinson JD, Karam-Hage MA, Engelmann JM, Minnix JA, Wetter DW, Versace F, 2017. Benefits of varenicline vs. bupropion for smoking cessation: A Bayesian Analysis of the interaction of reward sensitivity and treatment. Psychopharmacology (Berl). 234. 10.1007/s00213-017-4580-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG, 2001. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine Tob. Res. 3, 7–16. 10.1080/14622200020032051 [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Versace F, Robinson JD, Minnix JA, Lam CY, Cui Y, Brown VL, Cinciripini PM, 2012. Neural substrates of smoking cue reactivity: A meta-analysis of fMRI studies. Neuroimage 60, 252–262. 10.1016/j.neuroimage.2011.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerstrom KO, 2012. Determinants of Tobacco Use and Renaming the FTND to the Fagerstrom Test for Cigarette Dependence. Nicotine Tob. Res. 14, 75–78. 10.1093/ntr/ntr137 [DOI] [PubMed] [Google Scholar]
- Flagel SB, Akil H, Robinson TE, 2009. Individual differences in the attribution of incentive salience to reward-related cues: Implications for addiction. Neuropharmacology 56, 139–148. 10.1016/j.neuropharm.2008.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank DW, Cinciripini PM, Deweese MM, Karam-Hage MA, Kypriotakis G, Lerman C, Robinson JD, Tyndale RF, Vidrine DJ, Versace F, 2020. Toward precision medicine for smoking cessation: Developing a neuroimaging-based classification algorithm to identify smokers at higher risk for relapse. Nicotine Tob. Res. 22, 1277–1284. 10.1093/ntr/ntz211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF, 2013. DSM-5 criteria for substance use disorders: Recommendations and rationale. Am. J. Psychiatry 170, 834–851. 10.1176/appi.ajp.2013.12060782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston RJ, Schlienz NJ, 2018. Event-related potentials as biomarkers of behavior change mechanisms in substance use disorder treatment. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 3, 30–40. 10.1016/J.BPSC.2017.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenker R, 2005. Quantile Regression, Econometri. ed. Cambridge University Press, Cambridge, MA. 10.1017/CBO9780511754098 [DOI] [Google Scholar]
- Kypriotakis G, Cinciripini PM, Versace F, 2020. Modeling neuroaffective biomarkers of drug addiction: A Bayesian nonparametric approach using Dirichlet process mixture. J. Neurosci. Methods 341, 108753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, 2010. Emotion and the motivational brain. Biol. Psychol. 84, 437–450. 10.1016/j.biopsycho.2009.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN, 2008. International affective picture system (IAPS): Affective ratings of pictures and instruction manual. Technical Report A-8., Technical Report A-8. University of Florida, Gainesville, FL., Gainesville, FL. 10.1016/j.epsr.2006.03.016 [DOI] [Google Scholar]
- Littel M, Euser AS, Munafò MR, Franken IHA, 2012. Electrophysiological indices of biased cognitive processing of substance-related cues: A meta-analysis. Neurosci. Biobehav. Rev. 36, 1803–1816. 10.1016/j.neubiorev.2012.05.001 [DOI] [PubMed] [Google Scholar]
- Luck SJ, Stewart AX, Simmons AM, Rhemtulla M, 2021. Standardized measurement error: A universal metric of data quality for averaged event-related potentials. Psychophysiology 58, 1–15. 10.1111/psyp.13793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado JAF, Santos Silva JMC, 2005. Quantiles for counts. J. Am. Stat. Assoc. 100, 1226–1237. 10.1198/016214505000000330 [DOI] [Google Scholar]
- Minnix JA, Versace F, Robinson JD, Lam CY, Engelmann JM, Cui Y, Brown VL, Cinciripini PM, 2013. The late positive potential (LPP) in response to varying types of emotional and cigarette stimuli in smokers: A content comparison. Int. J. Psychophysiol. 89, 18–25. 10.1016/j.ijpsycho.2013.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JH, Stanford MS, Barratt ES, 1995. Factor structure of the Barratt Impulsiveness Scale. J. Clin. Psychol. 51, 768–774. [DOI] [PubMed] [Google Scholar]
- Pollard D, 1981. Strong Consistency of K-Means Clustering. Ann. Stat. 9, 135–140. [Google Scholar]
- Robinson JD, Versace F, Engelmann JM, Cui Y, Slapin A, Oum R, Cinciripini PM, 2015. The Motivational Salience of Cigarette-Related Stimuli Among Former, Never, and Current Smokers. Exp. Clin. Psychopharmacol. 23, 37–48. 10.1037/a0038467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MJF, Robinson TE, Berridge KC, 2013. Incentive salience and the transition to addiction, in: Miller PM (Ed.), Biological Research on Addiction. Academic Press, San Diego, CA, pp. 391–399. 10.1016/B978-0-12-398335-0.00039-X [DOI] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley MM, Cacioppo JT, Ito T, Lang PJ, 2000. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37, 257–261. 10.1111/1469-8986.3720257 [DOI] [PubMed] [Google Scholar]
- Snaith RP, Hamilton M, Morley S, Humayan a., Hargreaves D, Trigwell P, 1995. A scale for the assessment of hedonic tone. The Snaith-Hamilton Pleasure Scale. Br. J. Psychiatry 167, 99–103. 10.1192/bjp.167.1.99 [DOI] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Deweese MM, Robinson JD, Green CE, Lam CY, Minnix JA, Karam-Hage M, Wetter DW, Schembre SM, Cinciripini PM, 2017. Beyond cue reactivity: Non-drug-related motivationally relevant stimuli are necessary to understand reactivity to drug-related cues. Nicotine Tob. Res. 19, 663–669. 10.1093/ntr/ntx002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Engelmann JM, Robinson JD, Jackson EF, Green CE, Lam CY, Minnix JA, Karam-hage M, Brown VL, Wetter DW, Cinciripini PM, 2014. Prequit fMRI responses to pleasant cues and cigarette-related cues predict smoking cessation outcome. Nicotine Tob. Res. 16, 697–708. 10.1093/ntr/ntt214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Frank DW, Stevens EMEM, Deweese MMMM, Guindani M, Schembre SMSM, 2019. The reality of “food porn”: Larger brain responses to food-related cues than to erotic images predict cue-induced eating. Psychophysiology 56, e13309. 10.1111/psyp.13309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Kypriotakis G, Basen-Engquist K, Schembre SM, 2016. Heterogeneity in brain reactivity to pleasant and food cues: evidence of sign-tracking in humans. Soc. Cogn. Affect. Neurosci. 11, 604–611. 10.1093/scan/nsv143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Lam CY, Engelmann JM, Robinson JD, Minnix JA, Brown VL, Cinciripini PM, 2012. Beyond cue reactivity: Blunted brain responses to pleasant stimuli predict long-term smoking abstinence. Addict. Biol. 17, 991–1000. 10.1111/j.1369-1600.2011.00372.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace F, Robinson JD, Cinciripini PM, 2023. Towards neuromarkers for tailored smoking cessation treatments. Addict. Neurosci 10.1016/j.addicn.2023.100075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, Baler R, 2019. The neuroscience of drug reward and addiction. Physiol. Rev. 99, 2115–2140. 10.1152/physrev.00014.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A, 1988. Development and validation of brief measures of positive and negative affect: The PANAS Scales. J. Pers. Soc. Psychol. 54, 1063–1070. 10.1037/0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Webber HE, de Dios C, Kessler DA, Schmitz JM, Lane SD, Suchting R, 2022. Late positive potential as a candidate biomarker of motivational relevance in substance use: Evidence from a meta-analysis. Neurosci. Biobehav. Rev. 141, 104835. 10.1016/j.neubiorev.2022.104835 [DOI] [PubMed] [Google Scholar]
- Webber HE, De Dios C, Wardle MC, Suchting R, Green CE, Schmitz JM, Lane SD, Versace F, 2021. Electrophysiological responses to emotional and cocaine cues reveal individual neuroaffective profiles in cocaine users. Exp. Clin. Psychopharmacol. 30, 514–524. 10.1037/pha0000450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg A, Hajcak G, 2010. Beyond good and evil: the time-course of neural activity elicited by specific picture content. Emotion 10, 767–782. 10.1037/a0020242 [DOI] [PubMed] [Google Scholar]
- Wray JM, Gass JC, Tiffany ST, 2013. A systematic review of the relationships between craving and smoking cessation. Nicotine Tob. Res. 15, 1167–1182. 10.1093/ntr/nts268 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


