Abstract
Following infection with SARS-CoV-2, a substantial minority of people develop lingering after-effects known as ‘long COVID’. Fatigue is a common complaint with a substantial impact on daily life, but the neural mechanisms behind post-COVID fatigue remain unclear. We recruited 37 volunteers with self-reported fatigue after a mild COVID infection and carried out a battery of behavioural and neurophysiological tests assessing the central, peripheral and autonomic nervous systems. In comparison with age- and sex-matched volunteers without fatigue (n = 52), we show underactivity in specific cortical circuits, dysregulation of autonomic function and myopathic change in skeletal muscle. Cluster analysis revealed no subgroupings, suggesting post-COVID fatigue is a single entity with individual variation, rather than a small number of distinct syndromes. Based on our analysis, we were also able to exclude dysregulation in sensory feedback circuits and descending neuromodulatory control. These abnormalities on objective tests may aid in the development of novel approaches for disease monitoring.
Keywords: fatigue, COVID, transcranial magnetic brain stimulation, myopathy, dysautonomia
Baker et al. report evidence for changes in the state of multiple neural systems in a group of participants suffering from persistent fatigue, several weeks after a non-severe COVID infection. This suggests neural dysregulation could either be predictive of or contribute to post-COVID fatigue.
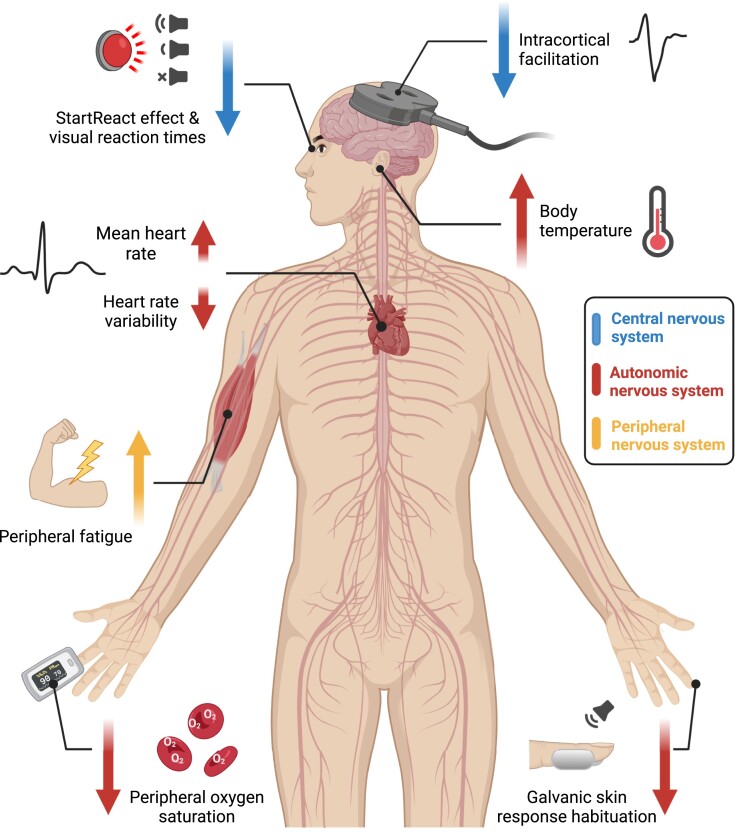
Graphical Abstract
Graphical abstract.
Introduction
Most people infected with SARS-CoV-2 do not require hospitalization. However, even after a mild infection, a minority develop symptoms that linger for weeks or months (long COVID). Persistent fatigue, where everyday actions become laborious, is one of the more commonly reported after-effects1 and can have a substantial impact on the quality of life and productivity of sufferers.2-4 At the time of publication, ∼2% of the UK population are experiencing long COVID; >50% report fatigue as their primary symptom.5
Fatigue appears to be a multisystem pathology associated with immunological, metabolic and hormonal anomalies. There are strong links between the immune and nervous systems with multiple pathways for possible interactions.6 These presumably generate changes in neurological function, which in turn lead to feelings of weakness, with physical and cognitive actions being more effortful. Such effects could result from changes at many levels of the nervous system; here we focused on five potential neural substrates of fatigue, that might not only result in increased performance fatigue but also increased perception of fatigue7:
Hypothesis 1. Motoneurons (and the muscles they innervate) are activated by multiple inputs from motor cortical areas, the brainstem and spinal cord. If any of these systems have reduced excitability or increased inhibition, as demonstrated in other chronic conditions associated with fatigue,8 this could contribute to a perception of fatigue.
Hypothesis 2. During normal self-generated movements, sensory feedback is attenuated.9 Incomplete sensory attenuation during movement could lead to heightened feedback and an increased sense of effort.10
Hypothesis 3. At the level of the periphery, other post-viral syndromes (such as Guillain–Barré and Miller Fisher syndrome) often lead to ineffective signal transmission at the neuromuscular junction, whereas myopathic changes within the muscle fibres themselves will cause weakness,11-13 requiring stronger voluntary drive to generate force, which could give rise to an increased perception of effort.14
Hypothesis 4. Monoaminergic neuromodulators are released in the spinal cord and regulate the gain of motoneuron responses to inputs through the activation of specific membrane conductances.15 If neuromodulatory inputs to motoneurons are affected in post-COVID fatigue (pCF),16 a stronger synaptic drive would be required for a given level of force. This could contribute to movements being perceived as more effortful.
Hypothesis 5. Autonomic dysregulation is often a predictor for fatigue in other chronic illnesses,17 and treating dysautonomia has shown promising results in improving the symptoms of fatigue.18 Autonomic dysregulation could also contribute to pCF.
In this study, we used an extensive battery of non-invasive tests to compare pCF sufferers with a matched control group, testing these varied hypotheses. Our results provide evidence for some of the hypothesized mechanisms, while suggesting that others are unlikely to contribute. pCF seems to result from dysregulation in specific components of the central, peripheral and autonomic nervous systems (ANSs).
Materials and methods
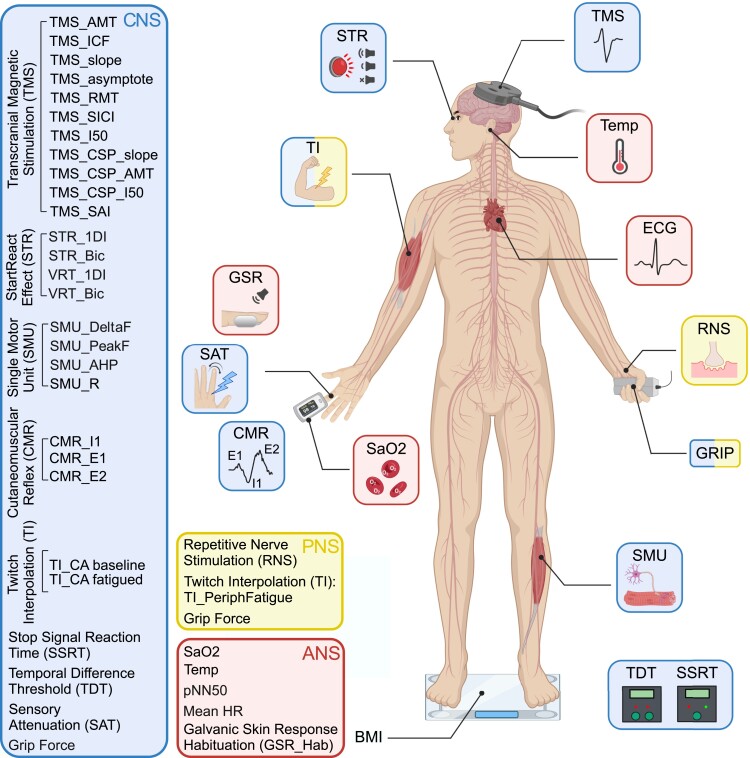
To understand the neural mechanisms behind pCF, we utilized a wide range of well-characterized non-invasive behavioural and neurological tests (summarized in Fig. 1 and described in detail in the Supplementary material). Through these tests, we were able to probe specific components within the CNS, PNS and ANSs. Transcranial magnetic stimulation (TMS) probed the state of intracortical motor circuits. Sensory nerve stimulation assessed the impact of sensory feedback on the CNS. Electrical stimulation of muscles assessed both central and peripheral levels of fatigue, while recordings of heart rate and galvanic skin responses assessed the state of the ANS. High-density surface electromyography extracted the activity of muscle motor units, from which we derived metrics of the state of neuromodulatory systems. Collectively, these tests yielded 35 measures [33 relating directly to the state of the nervous system, plus blood oxygen saturation (SaO2) and tympanic temperature]. These measures are referred to using consistent abbreviations in this report, as defined in the Supplementary material; in the text, these abbreviations are in italics. Please see Supplementary Fig. 1 for exemplar responses to some of these tests. Participants also completed a fatigue impact scale (FIS)19 questionnaire via a web-based survey tool.
Figure 1.
Neurophysiological tests performed. Schematic representation of the different tests performed, separated according to which components of the CNS, PNS and ANS they assessed. BMI, body mass index; CMR, cutaneomuscular reflex; GSR, habituation of the galvanic skin response to loud sound; ECG, electrocardiogram; RNS, repetitive nerve stimulation; SAT, sensory attenuation with movement; SMU, single motor unit recording; SSRT, stop signal reaction time; STR, StartReact effect; TDT, temporal difference threshold; TI, twitch interpolation; TMS, transcranial magnetic stimulation. Created with biorender.com.
Tests were carried out on two groups of volunteers—one who self-reporting as suffering from pCF, and a second cohort of control subjects with no fatigue. Inclusion criteria were age 18–65 years with no history of neurological disease and 6–26 weeks after infection (for the pCF cohort). In the control cohort, six subjects had knowingly had a mild COVID infection, but this had recovered without leading to pCF. The study was approved by the Ethics Committee of the Newcastle University Faculty of Medical Sciences; participants provided written informed consent to take part.
Statistical methods
Descriptive statistics are given as mean ± standard deviation (SD). Each of the measures we collected had different units and scales. To allow easy comparison of differences between measures and to avoid a metric with large values dominating the classification algorithm (see below), data were normalized as a Z-score for each feature. This was computed by finding the difference between the means of a measure between the pCF and control cohorts, and dividing by the SD of the control cohort. This is a measure of effect size and similar to Hedge’s g measure. To correct for multiple comparisons, we used the Benjamini–Hochberg procedure.20 Raw (uncorrected) P-values are given throughout this report, together with a statement of whether these values passed the Benjamini–Hochberg procedure.
Results
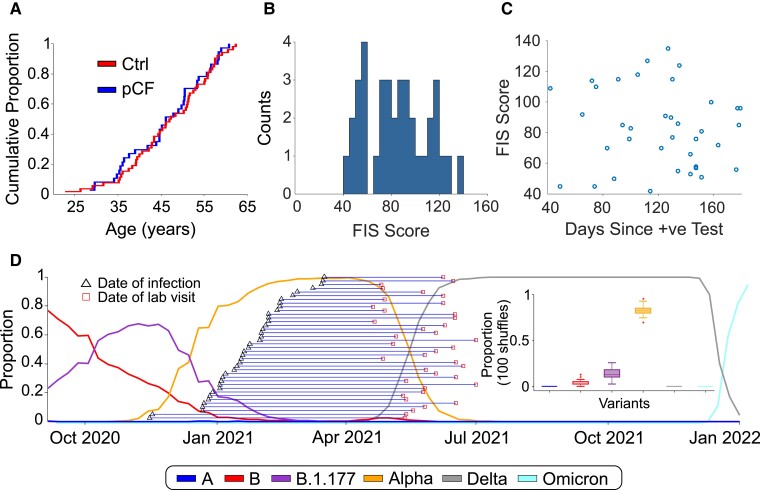
A total of 39 people with pCF and 53 controls who were not suffering from pCF were initially recruited to the study. Prior to attending the laboratory, volunteers with pCF underwent a structured telephone interview, which checked details of their medical history and possible exclusion criteria. Further measurements were then made during a single laboratory visit lasting around 4 h. Two participants with pCF were discovered during the course of the study to be under clinical investigation for neurological symptoms and signs not part of the typical long COVID syndrome. One additional participant from the control group was found to have an exaggerated startle response even to weak stimuli, which precluded gathering meaningful data on many of the protocols. These three individuals were excluded from the database, leaving 37 pCF (27 females, 73%) and 52 controls (37 females, 71%). The two cohorts were well matched for age, as illustrated by the cumulative distribution plots in Fig. 2A (and were not significantly different, P > 0.5, unpaired t-test). Full demographic information about the two cohorts are available in Supplementary Table 1.
Figure 2.
Cohort demographics. (A) Cumulative age distribution plots for pCF and control subjects. (B) Distribution histogram of FIS scores reported by pCF subjects. (C) Lack of correlation of FIS score with time since SARS-CoV-2 infection (Pearson r2 = 0.009, P = 0.59, t-test). (D) Proportions of the most common SARS-CoV-2 variants in circulation in England since October 2020 and the estimated expected proportion of each variant across our cohort (based on 100 shuffles).
The FIS score reflects functional limitation due to fatigue experienced within the last 4 weeks, rather than a measure of the level of fatigue, and for the pCF cohort the mean score was 83 ± 26 (range 42–135; Fig. 2B), out of a maximum value of 160, suggesting, on average, a moderate impact on daily life. The interval between diagnosis with SARS-CoV-2 and attending the laboratory was 121 ± 37 days (range 42–179 days). There was no correlation between the severity of fatigue measured by FIS score and time since infection (Fig. 2C; r2 = 0.009, P = 0.59). We did not routinely measure FIS for the control subjects, but some completed the questionnaire inadvertently as part of the web form used for initial recruitment; these 14 control individuals all scored below 6/160.
Although we do not have any way of definitively knowing the virus variant that our fatigue participants were infected with, we can estimate the likely proportions based on the known distribution of variants at the time. The weekly proportion of the six main variants circulating in England since November 2020 (A, Alpha, B, B.1.177, Delta & Omicron) was downloaded from the Sanger Institute COVID 19 Genomic Surveillance website (https://covid19.sanger.ac.uk/lineages/raw). For each subject in the pCF cohort, we randomly assigned a variant 100 times, with a probability based on the relative proportions of variants at the time of their week of infection. By collating all the data across all pCF subjects, we could then estimate the expected proportions of each variant across our fatigue cohort (shown in Fig. 2D). Based on the published relative incidence of SARS-CoV-2 variants in the UK, we thus estimate 83 ± 5% of our pCF cohort had the Alpha variant.
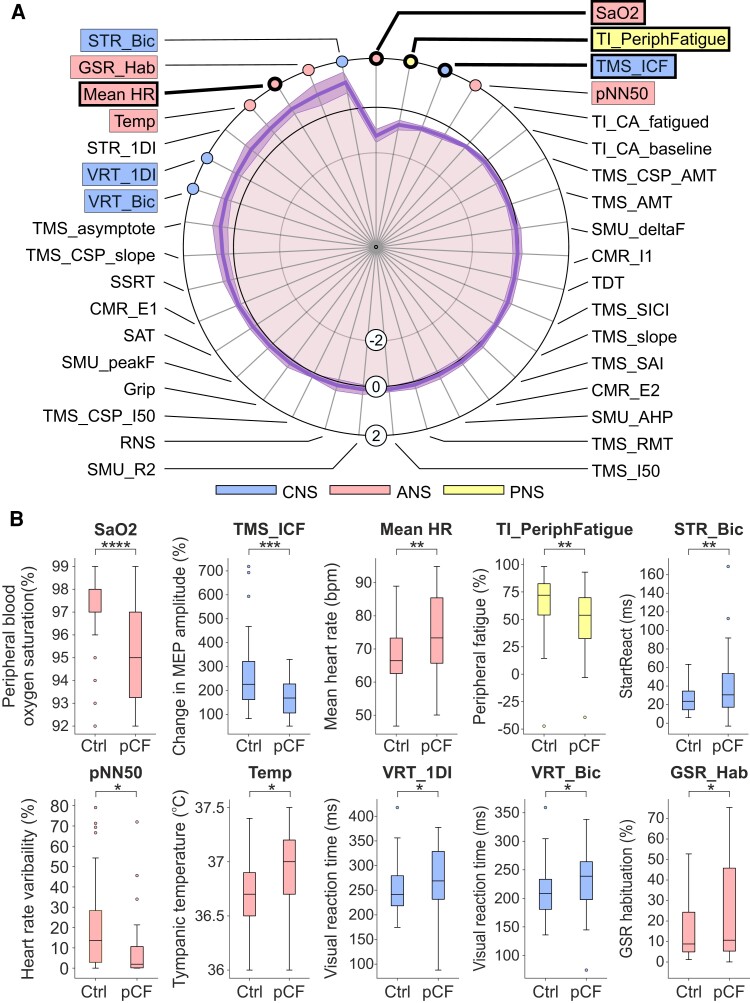
Figure 3A presents the normalized data for each metric as a spider plot,21 ordered so the greatest difference is located at the top of the figure; the shading indicates the standard error of the mean difference (calculated by dividing the SD of each metric by the square root of the number of data points available). The significance of differences between the pCF and control cohorts was assessed using unpaired t-tests. We highlight the 10 measures which had uncorrected P < 0.05 with coloured boxes on Fig. 3A. Figure 3B compares the distribution of these measures between the cohorts as box-and-whisker plots. Four of the measures had differences so great that they were assessed as significantly different even after adjustment for multiple comparisons; these are indicated with thicker lines in Fig. 3A. Full descriptive statistics for all measures in both cohorts are given in Supplementary Table 1.
Figure 3.
Differences between pCF and control cohorts. (A) Results from the tests outlined in Fig. 1, normalized as Z-scores (difference between pCF and control subjects, scaled by SD). Measures highlighted within boxes were individually significantly different between pCF and controls (P < 0.05); for those with thicker lines, significance passed the Benjamini–Hochberg correction for multiple comparisons. (B) Distribution of the 10 measures which had uncorrected P < 0.05 as box-and-whisker plots across the two cohorts. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001.
Voluntary activation of muscles relies on command signals from motor areas of the cortex; the state of cortical circuits has been linked to perception of effort and force output during fatiguing contractions.22,23 By using TMS to assess the function of primary motor cortex, we found that intracortical facilitation (TMS_ICF24) was significantly lower in pCF than controls (conditioned motor evoked potential relative to unconditioned 171 ± 79% versus 258 ± 140%, P < 0.001), suggesting reduced cortical excitability (Hypothesis 1). Other TMS measures also likely to be related to cortical excitability were no different between controls and pCF (the asymptote of the TMS recruitment curve, TMS_asymptote; the recruitment curve slope, TMS_slope; the intensity yielding 50% of the asymptote response amplitude, TMS_I50; active motor threshold, TMS_AMT; resting motor threshold, TMS_RMT). Multiple measures of cortical inhibition showed no significant differences between pCF and controls (short-interval intracortical inhibition, TMS_SICI; metrics of cortical silent period TMS_CSP_AMT, TMS_CSP_slope, TMS_CSP_I50). Possibly consistent with reduced cortical excitability, we also found a trend towards longer visual reaction times in pCF (in biceps muscle, VRT_Bic, 232 ± 52 versus 210 ± 41 ms; P = 0.026; in first dorsal interosseous muscle, VRT_1DI, 277 ± 61 versus 251 ± 46 ms, P = 0.024 for pCF versus controls, respectively; neither P-value crossed the significance threshold after adjustment for multiple comparisons).
Disturbances in sensory feedback processing have been previously hypothesized to contribute to an increased perception of effort25 (Hypothesis 2). However, the attenuation of sensory input during movement (SAT), short-latency afferent inhibition (TMS_SAI) and the different components of the cutaneomuscular reflex (CMR_E1, CMR_I1, CMR_E2) all showed no significant differences. This suggests that sensory abnormalities are unlikely to be a contributing factor to pCF in our cohort.
Fatigue could arise from a reduced ability of the neuromuscular apparatus to generate force; a given movement would then require stronger voluntary drive and perceived effort would rise. Changes could arise in the muscles themselves,26 due to a weakened connection from motoneurons to muscle fibres,27 or because motoneurons are less excitable. We found that maximal grip strength (Grip) was not significantly reduced in pCF, suggesting no deficit in force production for brief contractions. The efficacy of transmission at the neuromuscular junction (assessed using repetitive nerve stimulation, RNS), and intrinsic motoneuron excitability (assessed by estimating the peak firing rate of single motor units, SMU_peakF and the after-hyperpolarization of motoneurons, SMU_AHP) were also not significantly different between our two cohorts. However, when we tested changes during a prolonged maximal contraction, we found pCF subjects had an increased level of peripheral fatigue (size of maximum twitch evoked by direct electrical stimulation of the muscle after a sustained contraction compared with baseline, TI_PeriphFatigue, 48.5 ± 30.8% in pCF versus 67.1 ± 25.2% in controls, P = 0.003). This suggests that people with pCF develop metabolic changes in muscle fibres after prolonged activity, leading to reduced force output (Hypothesis 3).
We assessed the state of descending neuromodulatory pathways by looking at differences in the recruitment and de-recruitment of motoneurons (SMU_deltaF); the persistent inward currents that mediate this phenomenon are highly sensitive to serotonergic and noradrenergic inputs. We did not find any difference between our cohorts, suggesting that pCF is not associated with significant changes in descending neuromodulatory drive (Hypothesis 4).
Autonomic dysregulation is often associated with fatigue in other conditions17,28 and recent studies reported autonomic dysregulation after SARS-CoV-2 infection29-32 (although not universally33). We found a significantly increased resting heart rate in pCF (Mean_HR, 74.8 ± 11.1 versus 67.7 ± 8.8 beats/min, P = 0.0016). Other measures of autonomic function (tympanic temperature, Temp, 36.9 ± 0.4 versus 36.7 ± 0.3°C, P = 0.018; heart rate variability, pNN50, 8.8 ± 15.7 versus 20.2 ± 21.1%, P = 0.011; galvanic skin response habituation, GSR_Hab, 25.2 ± 24.5 versus 14.3 ± 12.2%, P = 0.026) also differed between cohorts, but did not pass correction for multiple comparisons. In our cohorts, only a small number of subjects had any medication that could potentially affect heart rate measurements [propranolol (n = 1, Control), atenolol (n = 1, Control) and amlodipine (n = 1, pCF)], and therefore, medications are unlikely to have had a significant impact on our results. These results all point towards a reduced vagal (relative to sympathetic) tone, suggesting at least some of our pCF subjects suffer from a degree of dysautonomia (Hypothesis 5).
Various behavioural measures did not show differences between pCF and control subjects. These included temporal difference threshold (TDT34) and stop signal reaction time (SSRT35); both are likely to be partly sensitive to inhibition in subcortical circuits. Central activation, which assesses the ability of the CNS to activate muscle maximally voluntarily, was also not different in pCF, either assessed at baseline (TI_CA_baseline) or after a fatiguing contraction (TI_CA_fatigue). The StartReact effect, which measures the acceleration of a visual reaction time by a loud (startling) sound and has been proposed to assess reticulospinal pathways,36 showed a trend to increase in the biceps muscle in pCF subjects (STR_Bic, 38.7 ± 34.1 versus 25.1 ± 12.3 ms, P = 0.010) but did not survive correction for multiple comparisons and was not significantly different in the first dorsal interosseous (STR_1DI). This is likely to be driven by the increased visual reaction time in pCF described above; because the startle reaction times were similar to controls, this led to an elevated difference.
The level of common input to a motoneuron pool can assess cortical control of muscles; this was not different between pCF and controls (SMU_R2). Finally, we found a significant reduction in SaO2 in the pCF subjects (SaO2, 95.3 ± 1.9 versus 97.2 ± 1.5%, P = 0.00002).
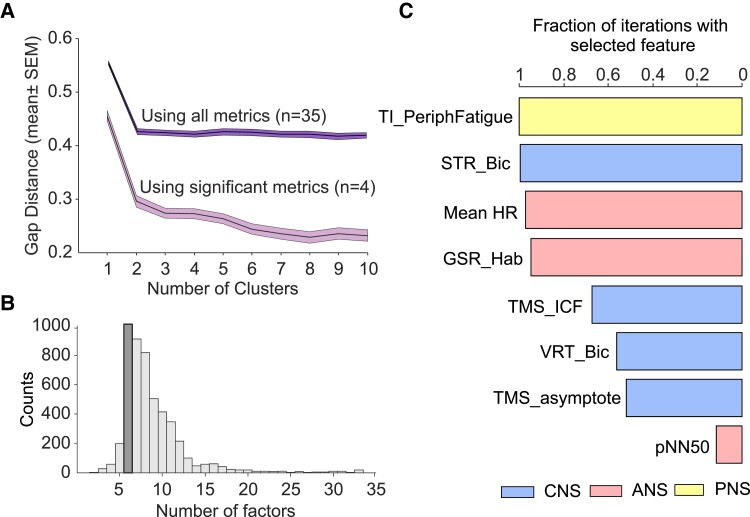
Overall, we found 10 measures that were different between pCF and control subjects (uncorrected P < 0.05), of which four passed significance correction for multiple comparisons. Our control cohort included six people who had knowingly had a mild COVID infection, but which had not led to pCF. When these individuals were excluded, the qualitative results as reported above were unchanged. We investigated whether these dysregulations occurred in the same people, or whether pCF could be subdivided into two or more different syndromes. K-means clustering on the pCF measurements followed by gap analysis revealed that the optimal cluster number was 1 (Fig. 4A), regardless of whether we included all metrics, or only the four that were significantly different after correction for multiple comparisons. Dysregulations thus appear to vary independently across the pCF population, rather than being clustered in particular subsets.
Figure 4.
Clustering and machine-learning analysis. (A) Gap analysis of number of clusters in the multivariate data set from pCF subjects. This is the result of 100 iterations. (B) Number of factors chosen by a machine-learning algorithm to maximize classification of pCF versus control subjects during 5000 iterations. (C) Fraction of iterations (n = 5000) of classification algorithm, with feature number fixed to 6, which included different features. Plot has been truncated to show the most common eight features.
For the metrics that failed to reach significance individually, it was important to determine if they could still distinguish between pCF and controls in combination. We used a machine-learning approach to classify participants as pCF or control, based on the multivariate data. Out of 33 available neurophysiological or behavioural metrics, repeated runs of the classifier determined the optimal feature number to maximize classifier accuracy. This had a mode of six (Fig. 4B). We then ran repeated classifications locked to six features and counted how often a given measure was used (Fig. 4C). With this approach, the mean classification accuracy was 70% (SD 3.6%). In addition to the three neurophysiological features that were individually significantly different as described above (TMS_ICF, Mean_HR and TI_PeriphFatigue), additional frequently selected metrics were the habituation of the galvanic skin response to a startling stimulus and heart rate variability (GSR_Hab, PNN50, autonomic measures), visual reaction times and StartReact effect (STR_Bic, VRT_Bic, multimodal measures of sensorimotor function) and another measure of cortical excitability (TMS_asymptote). All bar one of these additional measures had individual significance levels P < 0.05, but had failed the correction for multiple comparisons. The consistency between this analysis and that t-tests between control and pCF cohorts gives confidence in the robustness of the findings This analysis also suggests that there is little redundancy between measures—the cross correlation across all possible pairwise comparisons revealed 11% as significant (P < 0.05) with a median R2 of 13% for significant comparisons (interquartile range of 10–23%). Collectively, this suggests that each measure captures a separate dimension of dysregulation in pCF.
Discussion
The rapid development of successful vaccines against SARS-CoV-2 means that despite the evolution of variants, the majority of people in the UK are now largely protected from adverse short-term effects. Immunity also offers significant, but incomplete, protection from lingering sequelae37 and thus the incidence of pCF is likely to grow less rapidly than it has recently; nevertheless, the number of people still suffering remains staggering. Current estimates suggest ∼1% of the population have lasting fatigue, with enormous economic and social cost.
Much of our current research and understanding on the acute and chronic impacts of SARS-CoV-2 is centred on the inflammatory and immunological effects following an infection,38 which in turn can affect many other systems in the body. Indeed, there is mounting evidence that inflammatory markers remain elevated several months after an infection for patients with the longer term sequelae,39 but the relationship between inflammation and pCF remains unclear. Research on other chronic inflammatory conditions40 can be informative and shows that although fatigue is often associated with inflammation, a direct link between the two has proved elusive. Fatigue levels do not correlate well with circulating levels of inflammatory markers.41 Many rheumatoid arthritis patients undergoing anti-inflammatory treatment still report high levels of fatigue, even though their disease itself is in remission,42 suggesting that the relationship between fatigue and inflammation is not simple.
Although inflammation is likely to be important in the pathogenesis of pCF, a neural component is inevitable—the most common symptoms of pCF (as for fatigue in other conditions) are exhaustion after minimal physical or cognitive activity, both of which rely on neural circuits. There are multiple physiological pathways for the immune system to influence the nervous system and vice versa6 but of particular interest is the fact that pro-inflammatory cytokines in the brain, that are elevated following an infection, can have profound effects on neuroplasticity.43,44 Before being able to address whether such a mechanism operates in pCF, we first need to know which neural systems are affected.
In this study, we deployed an extensive battery of well-characterized non-invasive tests which are sensitive to different components of the nervous system. Although several measures were affected in pCF, it is important to emphasize that the majority of tests showed no difference between pCF sufferers and controls. Fatigue after SARS-CoV-2 infection does not result from a generalized deficit, but from specific changes in defined neural circuits. Our data not only support some of the hypotheses outlined in the Introduction, but also enable us to exclude some possible mechanisms.
Hypothesis 1 proposes that circuits providing inputs to motoneurons are less active in pCF; this could lead to weaker contractions, and an increased sense of effort. In support of this proposed mechanism, intra-cortical facilitation, a measure of intracortical glutamatergic function,45 was reduced in pCF.45 Other metrics of cortical state, which included measures of intra-cortical inhibition were not different—for example reduced facilitation was not countered by a concomitant reduction in intra-cortical GABAergic or cholinergic inhibition, suggesting a rebalancing of cortical activity and excitability to a lower level. As a result, corticospinal neurons could fire less vigorously for the same input from other upstream cortical areas, and hence plausibly lead to an increased sense of effort and fatigue. In agreement with these results, visual reaction times tended to be slower in pCF. This result also suggests that fatigue can affect cortical circuits differently in different cohorts as in a previous study,8 we instead found evidence for increased intra-cortical inhibition and normal intra-cortical facilitation.
Hypothesis 2 suggests that fatigue results from an impairment of sensory attenuation during movement. If sense of effort is judged from the level of feedback, this could make a movement feel more effortful than it actually was, and hence lead to fatigue.7 Importantly, a direct measure of sensory attenuation was unaffected in pCF; indeed, all measures related to sensory processing appeared normal. While this mechanism may contribute to fatigue in other pathologies (e.g. after stroke, see Kuppuswamy10), it does not appear important in pCF.
Hypothesis 3 is that pCF leads to myopathy, producing muscle weakness that requires an increased neural drive to generate a given contraction strength. Our results provide partial support for this idea. Individuals with pCF had normal grip strength, and there was no evidence of fatiguing transmission at the neuromuscular junction. As far as we could assess, the intrinsic excitability of motoneurons was also normal (measurements of persistent inward currents, SMU_deltaF and after-hyperpolarisation, SMU_AHP). However, myopathic changes became apparent after a sustained contraction, when the ability of muscle to produce force in response to electrical stimulation was significantly reduced in pCF subjects. This may reflect abnormalities in energy metabolism, leading to a more rapid depletion of muscle energy stores46 but this would need verification with further studies that directly measure muscle metabolic function. Clearly, such deficits could lead to a feeling of fatigue,47,48 although whether muscles are regularly pushed to the regime where such effects become noticeable in everyday life is perhaps debatable.
Hypothesis 4 relates to the extensive role played by neuromodulators in motoneuron function.15 Recent work has emphasized how active channels in the motoneuron dendrites amplify synaptic currents, and even generate sustained firing and thereby contractions in the absence of synaptic drive. The magnitude of these persistent inward currents is regulated by neuromodulators.49 There is evidence for changes in neuromodulatory centres following other inflammatory50 or autoimmune disorders16; thus even a small reduction in tonic levels of neuromodulators could leave motoneurons relatively unresponsive to descending drive,51 and hence generate feelings of weakness and fatigue. However, assessment of persistent inward currents showed no evidence for a difference in pCF, suggesting that this mechanism does not contribute to fatigue after SARS-CoV-2 infection.
Hypothesis 5 posits a role for the ANS in fatigue,17,52,53 and supporting this, we found multiple abnormalities in autonomic function. Resting heart rate was elevated, and heart rate variability reduced; this suggests a rebalancing of parasympathetic versus sympathetic drive in favour of the latter. Habituation of the galvanic skin response to a loud (startling) sound was also reduced in pCF subjects, again supporting excessive sympathetic output. The core body temperature was elevated, and SaO2 reduced. These metrics may reflect the continued long-lasting impacts of the original infection on lung function and immune activation, but they may also result from a generalized heightened sympathetic tone, in at least some of the pCF cohort.
A further hypothesis that we must consider is that the lower SaO2 values in our pCF cohort were the result of persistent pulmonary injury or vasculopathy. If so, this could potentially contribute to the findings we report here. However, it should be noted that although they were significant, the differences in SaO2 were small. Many clinical conditions lead to reductions in SaO2 larger than the 2% change we saw here, without producing symptoms of fatigue. It is thus unlikely that SaO2 is the sole driver for the differences in neural measures that we observed.
An ongoing challenge with fatigue is to determine the extent to which it is caused by disordered physiology versus psychological and social factors. Blindly accepting all reported symptoms as having an organic origin, versus uniformly rejecting the lived experience of fatigue sufferers, are equally unsatisfactory clinical approaches. In this study, we were able to identify a small number of metrics with abnormalities in pCF. Using these alongside immunological biomarkers39 may allow a more objective diagnosis on the basis of signs rather than symptoms alone. Interestingly, there was no evidence for more than one cluster within the pCF cohort, as we might expect if pCF originated from multiple causes (which could include a psychogenic origin). This finding should be treated as preliminary, given the relatively small size of our cohort, but it does suggest that treatment of pCF may not require extensive stratification to be successful.
An important and unavoidable limitation of our work is its cross-sectional nature and this applies both to the pCF and the control cohorts. Although we collected as much medical information as possible from our participants, their complete medical history was not available to us. It seems natural to assume that changes in metrics were caused by pCF, but it is equally possible that these were present prior to the SAR-CoV-2 infection, and that these perhaps conferred an increased risk for developing fatigue. We also do not know whether changes occurred early in the disease process prior to fatigue onset, or whether they developed alongside fatigue. These possibilities should be examined by a longitudinal study of individuals earlier after infection; objective metrics could help to identify individuals at risk of developing pCF, for whom more proactive management of an otherwise mild acute infection might then be warranted.
Conclusion
Our results provide evidence of dysregulation in all three main divisions of the nervous system, using tests that are straightforward to administer and could easily be incorporated into future trials to assess and treat pCF. Knowledge of which neural circuits are affected in pCF, whether as predictors of fatigue or due to the infection, may aid in the development of novel approaches for disease monitoring. Whether these results are applicable to other post-viral fatigue syndromes as well as chronic fatigue remains to be determined.
Supplementary Material
Acknowledgement
The authors thank Norman Charlton for mechanical engineering support.
Abbreviations
Abbreviations in italics refer to specific measurements, which are outlined in Fig. 1 and fully detailed in Supplementary material.
- ANS =
autonomic nervous system
- CMR_E1 =
the early excitatory phase of the cutaneomuscular reflex
- CMR_E2 =
the late excitatory phase of the cutaneomuscular reflex
- CMR_I1 =
the early inhibitory phase of the cutaneomuscular reflex
- FIS =
fatigue impact scale
- Grip =
the maximum grip force
- GSR_Hab =
habituation of the galvanic skin response following a loud sound
- Mean HR =
mean heart rate
- pCF =
post-COVID fatigue
- pNN50 =
proportion of successive heartbeat intervals which differ by >50 ms
- RNS =
repetitive nerve stimulation
- SaO2 =
blood oxygen saturation
- SAT =
sensory attenuation with movement
- SMU_AHP =
estimate of the duration of motoneuron after hyperpolarization made from single motor unit discharge
- SMU_deltaF =
difference in firing rate between recruitment and de-recruitment of single motor units
- SMU_peakF =
peak firing rate in single motor units
- SMU_R2 =
correlation coefficient between smoothed discharge of single motor units
- SSRT =
stop signal reaction time
- STR_1DI =
the StartReact effect (speeding up of reaction time by a loud sound) measured in the first dorsal interosseous muscle
- STR_Bic =
the StartReact effect (speeding up of reaction time by a loud sound) measured in the biceps muscle
- TDT =
temporal difference threshold
- Temp =
tympanic temperature
- TI_CA_baseline =
central activation measured by twitch interpolation at baseline
- TI_CA_fatigued =
central activation measured by twitch interpolation after a fatiguing contraction
- TI_PeriphFatigue =
peripheral fatigue measured during twitch interpolation experiment
- TMS =
transcranial magnetic stimulation
- TMS_AMT =
active motor threshold of TMS
- TMS_asymptote =
the asymptote of the sigmoid fit to the curve of TMS response versus stimulus intensity
- TMS_CSP_AMT =
the cortical silent period duration after TMS at an intensity equal to the active motor threshold
- TMS_CSP_I50 =
the cortical silent period duration after TMS at the intensity which generates a half-maximal response
- TMS_CSP_slope =
the slope of the relation between cortical silent period duration after TMS and stimulus intensity
- TMS_I50 =
intensity of TMS which generates a half-maximal response
- TMS_ICF =
intracortical facilitation measured with TMS
- TMS_RMT =
resting motor threshold of TMS
- TMS_SAI =
short latency afferent inhibition measured with TMS
- TMS_SICI =
intracortical inhibition measured with TMS
- TMS_slope =
measure related to the slope of the sigmoid fit to the curve of TMS response versus stimulus intensity
- VRT_1DI =
visual reaction time measured in the first dorsal interosseous muscle
- VRT_Bic =
visual reaction time measured in the biceps muscle
Contributor Information
Anne M E Baker, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, NE2 4HH, UK.
Natalie J Maffitt, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, NE2 4HH, UK.
Alessandro Del Vecchio, Department Artificial Intelligence in Biomedical Engineering, Friedrich–Alexander University Erlangen–Nürnberg, 91052 Erlangen, Germany.
Katherine M McKeating, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, NE2 4HH, UK.
Mark R Baker, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, NE2 4HH, UK.
Stuart N Baker, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, NE2 4HH, UK.
Demetris S Soteropoulos, Faculty of Medical Sciences, Newcastle University, Newcastle Upon Tyne, NE2 4HH, UK.
Supplementary material
Supplementary material is available at Brain Communications online.
Funding
This work was supported by a grant (MR/W004798/1) from the Medical Research Council UK Research and Innovation.
Competing interests
The authors report no competing interests.
Data availability
A spreadsheet containing Z-transformed values for measurements in all subjects and averages across cohorts is available in the Supplementary Material (see Supplementary Tables 1 and 2).
References
- 1. Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021;27(9):1607–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCrone P, Darbishire L, Ridsdale L, Seed P. The economic cost of chronic fatigue and chronic fatigue syndrome in UK primary care. Psychol Med. 2003;33(2):253–261. [DOI] [PubMed] [Google Scholar]
- 3. Collin SM, Crawley E, May MT, Sterne JA, Hollingworth W, UK CFS/ME National Outcomes Database . The impact of CFS/ME on employment and productivity in the UK: A cross-sectional study based on the CFS/ME national outcomes database. BMC Health Serv Res. 2011;11:217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cullinan J, Ni Chomhrai O, Kindlon T, Black L, Casey B. Understanding the economic impact of myalgic encephalomyelitis/chronic fatigue syndrome in Ireland: A qualitative study. HRB Open Res. 2020;3:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Office for National Statistics . Prevalence of ongoing symptoms following coronavirus (COVID-19) infection in the UK. 2022.https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/prevalenceofongoingsymptomsfollowingcoronaviruscovid19infectionintheuk/6january2022 [Accessed 6.1.2022]
- 6. Dantzer R. Neuroimmune interactions: From the brain to the immune system and vice versa. Physiol Rev. 2018;98(1):477–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kluger BM, Krupp LB, Enoka RM. Fatigue and fatigability in neurologic illnesses: Proposal for a unified taxonomy. Neurology. 2013;80(4):409–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McDonald C, Newton J, Lai HM, Baker SN, Jones DE. Central nervous system dysfunction in primary biliary cirrhosis and its relationship to symptoms. J Hepatol. 2010;53(6):1095–1100. [DOI] [PubMed] [Google Scholar]
- 9. Blakemore SJ, Wolpert DM, Frith CD. Central cancellation of self-produced tickle sensation. Nat Neurosci. 1998;1(7):635–640. [DOI] [PubMed] [Google Scholar]
- 10. Kuppuswamy A. The fatigue conundrum. Brain. 2017;140(8):2240–2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Milone M, Wong LJ. Diagnosis of mitochondrial myopathies. Mol Genet Metab. 2013;110(1–2):35–41. [DOI] [PubMed] [Google Scholar]
- 12. Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: Cellular mechanisms. Physiol Rev. 2008;88(1):287–332. [DOI] [PubMed] [Google Scholar]
- 13. Owen AM, Patel SP, Smith JD, et al. Chronic muscle weakness and mitochondrial dysfunction in the absence of sustained atrophy in a preclinical sepsis model. eLife. 2019;8:e49920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Vries JM, Hagemans ML, Bussmann JB, van der Ploeg AT, van Doorn PA. Fatigue in neuromuscular disorders: Focus on Guillain-Barre syndrome and Pompe disease. Cell Mol Life Sci. 2010;67(5):701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Johnson MD, Heckman CJ. Gain control mechanisms in spinal motoneurons. Front Neural Circuits. 2014;8:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rink C, Gortzen A, Veh RW, Pruss H. Serum antibodies targeting neurons of the monoaminergic systems in Guillain-Barre syndrome. J Neurol Sci. 2017;372:318–323. [DOI] [PubMed] [Google Scholar]
- 17. Davies K, Ng WF. Autonomic nervous system dysfunction in primary Sjogren’s syndrome. Front Immunol. 2021;12:702505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tarn J, Legg S, Mitchell S, Simon B, Ng WF. The effects of noninvasive vagus nerve stimulation on fatigue and immune responses in patients with primary Sjogren’s syndrome. Neuromodulation. 2019;22(5):580–585. [DOI] [PubMed] [Google Scholar]
- 19. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF. Measuring the functional impact of fatigue: Initial validation of the fatigue impact scale. Clin Infect Dis. 1994;18(Suppl 1):S79–S83. [DOI] [PubMed] [Google Scholar]
- 20. Benjamini Y, Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57:289–300. [Google Scholar]
- 21. Spider_plot. 2022. https://uk.mathworks.com/matlabcentral/fileexchange/59561-spider_plot [Accessed 6.1.2022]
- 22. Hunter SK, McNeil CJ, Butler JE, Gandevia SC, Taylor JL. Short-interval cortical inhibition and intracortical facilitation during submaximal voluntary contractions changes with fatigue. Exp Brain Res. 2016;234(9):2541–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thickbroom GW, Sacco P, Kermode AG, et al. Central motor drive and perception of effort during fatigue in multiple sclerosis. J Neurol. 2006;253(8):1048–1053. [DOI] [PubMed] [Google Scholar]
- 24. Kujirai T, Caramia MD, Rothwell JC, et al. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. De Doncker W, Dantzer R, Ormstad H, Kuppuswamy A. Mechanisms of poststroke fatigue. J Neurol Neurosurg Psychiatry. 2018;89(3):287–293. [DOI] [PubMed] [Google Scholar]
- 26. Agergaard J, Leth S, Pedersen TH, et al. Myopathic changes in patients with long-term fatigue after COVID-19. Clin Neurophysiol. 2021;132(8):1974–1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alekseeva TM, Gavrilov YV, Kreis OA, Valko PO, Weber KP, Valko Y. Fatigue in patients with myasthenia gravis. J Neurol. 2018;265(10):2312–2321. [DOI] [PubMed] [Google Scholar]
- 28. Pagani M, Lucini D. Chronic fatigue syndrome: A hypothesis focusing on the autonomic nervous system. Clin Sci (Lond). 1999;96(1):117–125. [PubMed] [Google Scholar]
- 29. Leitzke M, Stefanovic D, Meyer JJ, Schimpf S, Schonknecht P. Autonomic balance determines the severity of COVID-19 courses. Bioelectron Med. 2020;6(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pan Y, Yu Z, Yuan Y, et al. Alteration of autonomic nervous system is associated with severity and outcomes in patients with COVID-19. Front Physiol. 2021;12:630038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kurtoglu E, Afsin A, Aktas I, Akturk E, Kutlusoy E, Cagasar O. Altered cardiac autonomic function after recovery from COVID-19. Ann Noninvasive Electrocardiol. 2022;27(1):e12916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mol MBA, Strous MTA, van Osch FHM, et al. Heart-rate-variability (HRV), predicts outcomes in COVID-19. PLoS One. 2021;16(10):e0258841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Townsend L, Moloney D, Finucane C, et al. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS One. 2021;16(2):e0247280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lacruz F, Artieda J, Pastor MA, Obeso JA. The anatomical basis of somaesthetic temporal discrimination in humans. J Neurol Neurosurg Psychiatry. 1991;54(12):1077–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Logan GD, Cowan WB, Davis KA. On the ability to inhibit simple and choice reaction time responses: A model and a method. J Exp Psychol Hum Percept Perform. 1984;10(2):276–291. [DOI] [PubMed] [Google Scholar]
- 36. Tapia JA, Tohyama T, Poll A, Baker SN. The existence of the StartReact effect implies reticulospinal, not corticospinal, inputs dominate drive to motoneurons during voluntary movement. J Neurosci. 2022;42(40):7634-7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kuodi P, Gorelik Y, Zayyad H, et al. Association between vaccination status and reported incidence of post-acute COVID-19 symptoms in Israel: A cross-sectional study of patients tested between March 2020 and November 2021. medRxiv. 2022:2022.01.05.22268800. [Google Scholar]
- 38. Merad M, Blish CA, Sallusto F, Iwasaki A. The immunology and immunopathology of COVID-19. Science. 2022;375(6585):1122–1127. [DOI] [PubMed] [Google Scholar]
- 39. Phetsouphanh C, Darley DR, Wilson DB, et al. Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol. 2022;23(2):210-216. [DOI] [PubMed] [Google Scholar]
- 40. Davies K, Dures E, Ng WF. Fatigue in inflammatory rheumatic diseases: Current knowledge and areas for future research. Nat Rev Rheumatol. 2021;17(11):651–664. [DOI] [PubMed] [Google Scholar]
- 41. Howard Tripp N, Tarn J, Natasari A, et al. Fatigue in primary Sjogren’s syndrome is associated with lower levels of proinflammatory cytokines. RMD Open. 2016;2(2):e000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Druce KL, Bhattacharya Y, Jones GT, Macfarlane GJ, Basu N. Most patients who reach disease remission following anti-TNF therapy continue to report fatigue: Results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford). 2016;55(10):1786–1790. [DOI] [PubMed] [Google Scholar]
- 43. Stellwagen D, Beattie EC, Seo JY, Malenka RC. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J Neurosci. 2005;25(12):3219–3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lewitus GM, Pribiag H, Duseja R, St-Hilaire M, Stellwagen D. An adaptive role of TNFalpha in the regulation of striatal synapses. J Neurosci. 2014;34(18):6146–6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115(8):1717–1729. [DOI] [PubMed] [Google Scholar]
- 46. Filipe A, Chernorudskiy A, Arbogast S, et al. Defective endoplasmic reticulum-mitochondria contacts and bioenergetics in SEPN1-related myopathy. Cell Death Differ. 2021;28(1):123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gorman GS, Elson JL, Newman J, et al. Perceived fatigue is highly prevalent and debilitating in patients with mitochondrial disease. Neuromuscul Disord. 2015;25(7):563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Park JH, Niermann KJ, Olsen N. Evidence for metabolic abnormalities in the muscles of patients with fibromyalgia. Curr Rheumatol Rep. 2000;2(2):131–140. [DOI] [PubMed] [Google Scholar]
- 49. Heckman CJ, Lee RH, Brownstone RM. Hyperexcitable dendrites in motoneurons and their neuromodulatory control during motor behavior. Trends Neurosci. 2003;26(12):688–695. [DOI] [PubMed] [Google Scholar]
- 50. Katafuchi T, Kondo T, Take S, Yoshimura M. Brain cytokines and the 5-HT system during poly I:C-induced fatigue. Ann N Y Acad Sci. 2006;1088:230–237. [DOI] [PubMed] [Google Scholar]
- 51. Cushing S, Bui T, Rose PK. Effect of nonlinear summation of synaptic currents on the input-output properties of spinal motoneurons. J Neurophysiol. 2005;94(5):3465–3478. [DOI] [PubMed] [Google Scholar]
- 52. Escorihuela RM, Capdevila L, Castro JR, et al. Reduced heart rate variability predicts fatigue severity in individuals with chronic fatigue syndrome/myalgic encephalomyelitis. J Transl Med. 2020;18(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Newton JL, Okonkwo O, Sutcliffe K, Seth A, Shin J, Jones DE. Symptoms of autonomic dysfunction in chronic fatigue syndrome. QJM. 2007;100(8):519–526. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A spreadsheet containing Z-transformed values for measurements in all subjects and averages across cohorts is available in the Supplementary Material (see Supplementary Tables 1 and 2).