Abstract
Purpose
To compare results after ILM peeling and ILM inverted flap technique utilized the repair of full thickness macular holes, irrespective of their size.
Patients and Methods
Pre- and postoperative data of 109 patients who suffered from a full thickness macular hole were retrospectively analyzed. Forty-eight patients were treated with an inverted ILM flap technique, 61 patients were treated with ILM peeling. All patients received a gas tamponade. The primary endpoint was macular hole closure as demonstrated by OCT scanning. Secondary endpoints were best corrected visual acuity and clinical complication rates.
Results
For small and medium-sized macular holes the closure rates in the ILM flap technique group were 100% and 94%, respectively. For ILM peeling, the closure rate was identical (95%). For large macular holes, the closure rate was 100% in the flap versus 50% in the ILM peeling group, but visual acuity improved in both groups (ILM flap p=0.001, ILM peeling p=0.002). In both treatment groups, larger holes were associated with a reduced final visual outcome. For medium-sized macular holes, visual acuity significantly improved only in the ILM peeling group. Both techniques were associated with minimal and comparable side effects.
Conclusion
In our limited series, the inverted ILM flap technique for repair of macular holes demonstrated a high closure rate. For large MHs, we saw a trend towards a better closure rate in the flap technique compared to ILM peel only. However, final visual acuity showed no significant difference between the groups. Clinical results and complications appeared to be comparable in both groups.
Keywords: ILM peeling, flap technique, pars plana vitrectomy
Introduction
After the landmark publication of Kelly and Wendel1 in 1991, which described results of a pilot study for vitreous surgery for idiopathic macular holes, many modifications have been investigated in trials, studies, and case series. Examples of major steps for improving the outcome in macular hole surgery2 are the use of staining agents,3,4 the application of platelet concentrate,5,6 the removal of the inner limiting membrane (ILM),7–9 and more recently, the development of an ILM flap10,11 which can be used to seal the macular hole (MH). The ILM flap technique seems to be especially helpful in the repair of larger macular holes.12,13
In many vitreoretinal centers, the standard procedure for idiopathic macular holes consists mainly of a more or less extended core vitrectomy, staining and peeling of any epiretinal tissue including the ILM. Usually, the eye is then filled with a non-expanding gas, eg, SF 6 20% and the patient is advised for prone positioning for one week. With this technique, success rates for macular hole closure of up to 100% with a considerable improvement of visual acuity were reported.14,15
Prospective clinical trials to investigate the best technique for macular hole repair are difficult to perform, because of the high success rate of the standard of care technique. In order to demonstrate that a new technique is better than the standard of care, a prospective clinical trial would require inclusion of several hundred patients assuming that standard of care has a success rate of 90% and a new method has a success rate of 95% (further assuming a power of approximately 80% with alpha = 0.05). Prospective clinical trials of such size are difficult to perform. However, some studies suggest that large macular holes close better with the inverted flap technique than with simple ILM peeling.12–17 If this is the case, one may wonder, if the inverted flap technique may also be better for any size of macular holes.
In the current single-center case series, we report outcomes of surgery for idiopathic and secondary macular holes from three experienced vitreoretinal (VR) surgeons with two surgeons still using the ILM peeling technique as the primary procedure for all macular holes and a third surgeon using the inverted flap technique for all cases.
We retrospectively compared the data with respect to safety, macular hole closure, and visual acuity.
Materials and Methods
Ethical Approval
The study adhered to the tenets of the Helsinki Declaration. The study protocol was reviewed and approved by the Medical Ethics Committee of the University Hospital RWTH Aachen (ethical approval no. 20-157). The requirement for informed consent was waived by the Ethics Committee due to the retrospective nature of the study.
Inclusion Criteria
We included all adult patients undergoing surgery for a full thickness macular hole between August 30, 2013, and May 20, 2020. Patients were excluded, if the documentation was incomplete or no follow-up data was available or if they had already macular surgery before for any indication.
Definitions
Macular hole sizes were defined as small (≤250µm), medium (>250 ≤400µm) and large (>400µm) according to the definition described by the IVTS Group.18
Patient Groups
Patients were grouped according to the surgical intervention: Group ILM-peeling (ILM-P): The patients received a more or less extended core vitrectomy, staining of the preretinal tissue, removal of any preretinal tissue and the ILM followed by gas endotamponade. Group ILM-flap (ILM-F): Here, after extended core vitrectomy and staining and removal of preretinal tissue, if present, the ILM was incised, lifted up from the retina with the base temporal to the edge of the MH.10,11
Technique of ILM Flap
The technique of Michalewska12 was applied. Briefly, the flap was flipped over the macular hole by gentle suction on the opposite side during air exchange at the end of the surgery. We did not place the ILM into the hole. Surgeries in the ILM P group were performed by two surgeons A and B, whereas all procedures in group ILM F were performed by a third surgeon C. All surgeons were experienced VR surgeons.
Preoperative Assessment
Preoperatively, all patients underwent a standardized work-up as follows:
Automated refractometry, best corrected visual acuity (BCVA) testing with Snellen charts, Goldmann applanation tonometry, slit-lamp examination, lens status evaluation, IOL Master® biometry (Zeiss, Oberkochen, Germany) and spectral domain ocular coherence tomography (OCT) (Spectralis, Heidelberg Engineering, Heidelberg, Germany). The following OCT parameters were evaluated: macular hole diameter at base, thinnest macular hole diameter, retinal thickness measured nasally and temporally at an edge of a macular hole, tractional components, and foveal thickness after closure of the macular hole.
Postoperative Follow-Up
Postoperatively, the patients were examined at day 1 postoperatively and after 6-weeks (range, 2–12 weeks). A final visit was analyzed (up to 6.4 years). At follow-up the same examinations were performed as preoperatively.
Primary Endpoint
The primary endpoint measure of this study was macular hole closure on OCT scans after one single procedure at all follow-up examinations.
Secondary Endpoints
Secondary endpoints were side effects, the change in BCVA, and duration of surgery.
Statistics
All analyses were conducted in statistical software R, version 4.1.1 (R Core Team (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org) with a confidence level of α = 0.05. Visualizations of plots were made using the Plotly and ggplot2 libraries (with R language). Qualitative variables were summarized by giving n and percentage, and quantitative variables were summarized by giving mean and standard deviation or median with quartiles 1 and 3 (depending on distribution normality, which was analyzed by the Shapiro–Wilk test). Fisher’s exact and chi-square tests were used to analyze the dependency between groups and other qualitative variables. Both parametric and nonparametric tests were conducted to examine differences in the level of selected quantitative variables. Furthermore, Student’s t-test or Mann–Whitney U-test was used when analyzing two independent samples. The analysis of variance (ANOVA) or Kruskal–Wallis test was used when analyzing three independent samples. The ANOVA and Friedman tests were used for repeated measures and analyzing three dependent samples, respectively. In addition, ANOVA post hoc tests were conducted with Bonferroni correction. The Kendall tau-b correlation coefficient was used to examine linear relationships between two quantitative variables.19
Results
This study included 109 patients who had surgery between August 30, 2013, and May 20, 2020. Among these, 48 and 61 patients were included in the ILM-F and ILM-P groups, respectively.
The characteristics of the study groups are presented in Table 1 and Table 2. The treatment groups were comparable in terms of their demographic data, gender distribution, and percentage of an intraoperative phacoemulsification. Combined surgery was performed in the ILM-F group in 37.5% and in the ILM-P in 36.1% (Table 1 and Table 2).
Table 1.
Group Characteristics (Demographic and Preoperative Parameters) for Patients from ILM Flap and ILM Peeling Groups
| Characteristic | ILM Flap | ILM Peeling | p |
|---|---|---|---|
| n = 48 | n = 61 | ||
| Age, Me (Q1; Q3) | 70.43 (67.42; 76.15) | 63.84 (70.54; 74.16) | 0.557a |
| Eye (left), n (%) | 23 (47.9) | 28 (45.9) | 0.987c |
| Sex (female), n (%) | 31 (64.6) | 34 (55.7) | 0.461c |
| High myopia, n (%) | 7 (14.6) | 9 (14.8) | > 0.999c |
| Intraoperative gas tamponade, n (%) | |||
| C3F8 | 1 (2.1) | 10 (16.4) | 0.021 |
| SF6 | 47 (97.9) | 51 (83.6) | |
| Phacoemulsification intraoperative, n (%) | 18 (37.50) | 22 (36.1) | > 0.999c |
| Preoperative macular hole diameter at its base, M ± SD | 885.61 ± 239.58 | 830.36 ± 351.93 | 0.337b |
| Macular hole size (µm), M ± SD | 421.90 ± 158.53 | 322.61 ± 159.62 | 0.002b |
| Preoperative retinal thickness temporal, M ± SD | 401.69 ± 69.48 | 383.93 ± 72.54 | 0.197b |
| Preoperative retinal thickness nasal, M ± SD | 418.60 ± 58.99 | 410.07 ± 71.53 | 0.496b |
| VMT, n (%) | 11 (22.9) | 19 (31.1) | 0.460c |
| Macular hole (MH) grade, n (%) | |||
| Large | 24 (50.0) | 20 (32.8) | 0.017c |
| Medium | 18 (37.5) | 19 (31.1) | |
| Small | 6 (12.5) | 22 (36.1) | |
| Idiopathic vs secondary macular hole, n (%) | |||
| Idiopathic macular hole | 42 (87.5) | 45 (73.8) | 0.125c |
| Secondary macular hole | 6 (12.5) | 16 (26.2) | |
| Lens status pre-op, n (%) | |||
| Phakic | 29 (60.4) | 47 (77.0) | 0.089 |
| Aphakic | 2 (4.2) | 0 (0.0) | |
| Pseudophakic | 17 (35.4) | 14 (23.0) | |
| VA at baseline (logmar), Me (Q1; Q3) | 0.75 (0.50; 1.30) | 0.70 (0.60; 1.00) | 0.822a |
| Surgery duration (min), Me (Q1; Q3) | 34.50 (30.00; 40.00) | 34.00 (29.00; 45.00) | 0.985a |
| Dye, n (%) | |||
| BB | 9 (18.8) | 60 (98.4) | < 0.001c |
| DB | 39 (81.3) | 1 (1.6) |
Notes: High myopia: if it exceeded 6 dioptres or if an axial eye length was > 26 mm; p < 0.05 was considered statistically significant; n (%) was given for qualitative variables, M ± SD or Median with quartile 1 and 3 for quantitative variables; Analyses were made with Mann-Whitey’s U-testa or Student’s t-testb (quantitative variables), Fisher’s exact test or chi-square testc (qualitative variables).
Abbreviations: M, mean; SD, standard deviation; Me, median; Q1, Q3, quartile 1 and 3; n, number of subjects in a group. ILM, membrana limitans interna; VMT, vitreomacular traction; RT, retinal thickness; VA, visual acuity; BB, brilliant blue; DB, dual blue.
Table 2.
Demographic and Preoperative Parameters in Both Groups in Regard to Macular Hole Grade
| Small MH Grade | |||
| Characteristics | ILM Flap | ILM Peeling | p |
| n = 6 | n = 22 | ||
| Age, M ± SD | 65.99 ± 10.27 | 67.30 ± 8.12 | 0.780a |
| Eye (left), n (%) | 4 (66.7) | 11 (50.0) | 0.655 |
| Sex (female), n (%) | 3 (50.0) | 12 (54.5) | > 0.999 |
| High myopia, n (%) | 2 (33.3) | 4 (18.2) | 0.581 |
| Intraoperative gas tamponade, n (%) | |||
| C3F8 | 0 (0.0) | 4 (18.2) | 0.549 |
| SF6 | 6 (100.0) | 18 (81.8) | |
| Phacoemulsification intraoperatively, n (%) | 2 (33.3) | 4 (18.2) | 0.581 |
| Preoperative macular hole diameter at its base (µm), Me (Q1; Q3) | 640.00 (482.75; 721.50) | 516.00 (435.25; 722.5) | 0.764b |
| Macular hole size (µm), M ± SD | 192.17 ± 40.88 | 156.14 ± 57.90 | 0.110a |
| Preoperative retinal thickness temporal (µm), M ± SD | 354.50 ± 65.50 | 367.64 ± 73.80 | 0.682a |
| Preoperative retinal thickness nasal (µm), M ± SD | 373.17 ± 57.51 | 402.23 ± 73.90 | 0.267a |
| VMT, n (%) | 1 (16.7) | 8 (36.4) | 0.630 |
| Idiopathic vs secondary MH, n (%) | |||
| Idiopathic MH | 5 (83.3) | 16 (72.7) | > 0.999 |
| Secondary MH | 1 (16.7) | 6 (27.3) | |
| Preoperative lens status, n (%) | |||
| Phakic | 3 (50.0) | 18 (81.2) | 0.144 |
| Aphakic | 0 (0.0) | 0 (0.0) | |
| Pseudophakic | 3 (50.0) | 4 (18.8) | |
| Visual acuity at baseline (logMar) - overall, Me (Q1; Q3) | 0.45 (0.40; 0.58) | 0.60 (0.50; 0.68) | 0.256b |
| Medium MH Grade | |||
| Characteristics | ILM Flap | ILM Peeling | p |
| n = 18 | n = 19 | ||
| Age, M ± SD | 69.27 (65.52; 75.68) | 71.52 (68.15; 75.92) | 0.298b |
| Eye (left), n (%) | 8 (44.4) | 5 (26.3) | 0.418c |
| Sex (female), n (%) | 11 (61.1) | 10 (52.6) | 0.851c |
| High myopia, n (%) | 4 (77.8) | 2 (10.5) | 0.405 |
| Intraoperative gas tamponade, n (%) | |||
| C3F8 | 0 (0.0) | 0 (0.0) | - |
| SF6 | 18 (100.0) | 19 (100.0) | |
| Phacoemulsification intraoperatively, n (%) | 7 (38.9) | 11 (57.9) | 0.408c |
| Preoperative macular hole diameter at its base (µm), Me (Q1; Q3) | 795.82 ± 181.57 | 881.95 ± 292.52 | 0.291a |
| Macular hole size (µm), M ± SD | 331.72 ± 42.63 | 335.21 ± 44.51 | 0.809a |
| Preoperative retinal thickness temporal (µm), M ± SD | 389.56 ± 61.34 | 387.00 ± 62.28 | 0.903a |
| Preoperative retinal thickness nasal (µm), M ± SD | 411.67 ± 49.98 | 405.84 ± 67.60 | 0.767a |
| VMT, n (%) | 5 (27.8) | 7 (36.8) | 0.812c |
| Idiopathic vs secondary MH, n (%) | |||
| Idiopathic MH | 14 (77.8) | 15 (78.9) | > 0.999 |
| Secondary MH | 4 (22.2) | 4 (21.1) | |
| Preoperative lens status, n (%) | |||
| Phakic | 14 (77.8) | 16 (84.2) | 0.825 |
| Aphakic | 1 (5.6) | 0 (0.0) | |
| Pseudophakic | 3 (16.6) | 3 (15.8) | |
| Visual acuity at baseline (logMAR) - overall, Me (Q1; Q3) | 0.60 (0.43; 0.78) | 0.70 (0.60; 1.00) | 0.153b |
| Large MH Grade | |||
| Characteristics | ILM Flap | ILM Peeling | p |
| n = 24 | n = 20 | ||
| Age, M ± SD | 70.54 (67.50; 75.24) | 67.76 (63.76; 75.01) | 0.476b |
| Eye (left), n (%) | 11 (45.8) | 12 (60.0) | 0.526c |
| Sex (female), n (%) | 17 (70.8) | 12 (60.0) | 0.663c |
| High myopia, n (%) | 1 (4.2) | 3 (15.0) | 0.316 |
| Intraoperative gas tamponade, n (%) | |||
| C3F8 | 1 (4.2) | 6 (30.0) | 0.035 |
| SF6 | 23 (95.8) | 14 (70.0) | |
| Phacoemulsification intraoperatively, n (%) | 9 (37.5) | 7 (35.0) | > 0.999c |
| Preoperative macular hole diameter at its base (µm), Me (Q1; Q3) | 1029.30 ± 183.66 | 1008.95 ± 244.17 | 0.762a |
| Macular hole size (µm), M ± SD | 534.00 (435.75; 607.00) | 466.00 (424.50; 500.75) | 0.075b |
| Preoperative retinal thickness temporal (µm), M ± SD | 422.58 ± 70.71 | 398.95 ± 77.49 | 0.301a |
| Preoperative retinal thickness nasal (µm), M ± SD | 436.50 (404.00; 478.50) | 406.00 (367.25; 448.00) | 0.252b |
| VMT, n (%) | 5 (20.8) | 4 (20.0) | > 0.999 |
| Idiopathic vs secondary MH, n (%) | |||
| Idiopathic MH | 23 (95.8) | 14 (70.0) | 0.035 |
| Secondary MH | 1 (4.2) | 6 (30.0) | |
| Preoperative lens status, n (%) | |||
| Phakic | 12 (50.0) | 13 (65.0) | 0.637 |
| Aphakic | 1 (4.2) | 0 (0.0) | |
| Pseudophakic | 11 (45.8) | 7 (35.0) | |
| Visual acuity at baseline (logMar) - overall, Me (Q1; Q3) | 1.00 (0.80; 1.30) | 1.15 (0.95; 1.30) | 0.745a |
| High Myopia | |||
| Characteristics | ILM Flap | ILM Peeling | p |
| n = 7 | n = 9 | ||
| Age, M ± SD | 63.59 (52.08; 65.76) | 61.98 (59.34; 68.05) | 0.758b |
| Eye (left), n (%) | 3 (42.9) | 3 (33.3) | > 0.999 |
| Sex (female), n (%) | 4 (57.1) | 4 (44.4) | > 0.999 |
| High myopia, n (%) | |||
| Intraoperative gas tamponade, n (%) | 0 (0.0) | 4 (44.4) | 0.088 |
| C3F8 | 7 (100.0) | 5 (55.6) | |
| SF6 | 1 (14.3) | 1 (11.1) | > 0.999 |
| Phacoemulsification intraoperatively, n (%) | 660.29 ± 255.93 | 735.89 ± 209.85 | 0.539a |
| Preoperative macular hole diameter at its base (µm), Me (Q1; Q3) | 325.00 ± 167.35 | 310.33 ± 157.54 | 0.861a |
| Macular hole size (µm), M ± SD | 372.71 ± 71.42 | 367.44 ± 63.98 | 0.881a |
| Preoperative retinal thickness temporal (µm), M ± SD | 397.86 ± 55.42 | 403.11 ± 59.15 | 0.858a |
| Preoperative retinal thickness nasal (µm), M ± SD | 1 (14.3) | 0 (0.0) | 0.438 |
| VMT, n (%) | |||
| Idiopathic vs secondary MH, n (%) | 6 (85.7) | 4 (44.4) | 0.145 |
| Idiopathic MH | 1 (14.3) | 5 (55.6) | |
| Secondary MH | |||
| Preoperative lens status, n (%) | 3 (42.9) | 5 (55.6) | 0.786 |
| Phakic | 1 (14.3) | 0 (0.0) | |
| Aphakic | 3 (42.9) | 4 (44.4) | |
| Pseudophakic | 0.50 (0.45; 0.65) | 1.30 (0.60; 1.30) | 0.145b |
| Visual acuity at baseline (logMAR) - overall, Me (Q1; Q3) | 0.50 (0.40; 0.60) | 0.50 (0.50; 0.65) | 0.556b |
Notes: Small MH grade, ≤250 µm; Medium MH grade, >250 – < 400 µm; Large MH grade, ≥400 µm; High myopia: if it exceeded 6 dioptres or if an axial eye length was > 26 mm; p < 0.05 was considered statistically significant; n (%) was given for qualitative variables, M ± SD or Median with quartile 1 and 3 for quantitative variables; Analyses were made with Student’s t-testa or Mann-Whitey’s U-testb (quantitative variables), Fisher’s exact test or chi-square testc (qualitative variables).
Abbreviations: M, mean; SD, standard deviation; Me, median; Q1, Q3, quartile 1 and 3; n, number of subjects in a group; IL, membrana limitans interna; MH, macular hole; VMT, vitreomacular traction; VA, visual acuity.
The groups differed significantly in terms of macular hole size. The MH size was 421.9 ± 158 µm in the ILM flap group and 322 ± 159 µm in the ILM peeling group. In the ILM flap group there were had 6 small, 18 medium sized, and 24 large MHs, whereas in the ILM peeling group there were 22 small, 19 medium-sized MHs, and 20 large macular holes.
In the ILM-F group, 87.5% (42/48) of MHs were idiopathic, and 12.5% (6/48) were considered as secondary MHs (eg after retinal detachment, posttraumatic, after vitrectomy for other causes, and other conditions). In the ILM-P group 73.8% (45/61) cases were idiopathic MHs and 26.2% secondary cases, respectively. Two patients were included with a bilateral macular hole. Both patients had the same subsequent surgery in both eyes, one by ILM flap technique, the other by ILM peeling technique. Both eyes of both patients were analyzed in this data set.
Primary Endpoint
The closure rate was significantly higher in the ILM-F group both in the overall evaluation and in patients with large MHs (p = 0.006 and p < 0.001, respectively). For the small and medium-sized macular holes the closure rates were high: 6/6 small MHs in the ILM flap group and 21/22 small MHs in the ILM peeling group could be closed and 17/18 medium-sized MHs in the ILM flap technique and 18/19 in the ILM peeling technique were closed. However, for large macular holes with ILM peeling alone only 50% of the macular hole could be closed with one procedure, whereas with the flap technique all holes could be closed (Table 3, Figure 1).
Table 3.
Correlation Between Main Outcome (Open or Closed Macular Hole) and Operative Technique (ILM Flap or ILM Peeling)
| Variable | Intervention n (%) | p | |
|---|---|---|---|
| ILM Flap | ILM Peeling | ||
| Overall | n = 48 | n = 61 | |
| Open | 1 (2.1) | 12 (19.7) | 0.006 |
| Closed | 47 (97.9) | 49 (80.3) | |
| Small MH | n = 6 | n = 22 | |
| Open | 0 (0.0) | 1 (4.5) | > 0.999 |
| Closed | 6 (100.0) | 21 (95.5) | |
| Medium MH | n = 18 | n = 19 | |
| Open | 1 (5.6) | 1 (5.3) | > 0.999 |
| Closed | 17 (94.4) | 18 (94.7) | |
| Large MH | n = 24 | n = 20 | |
| Open | 0 (0.0) | 10 (50.0) | < 0.001 |
| Closed | 24 (100.0) | 10 (50.0) | |
| High myopia | n = 7 | n = 9 | |
| Open | 0 (0.0) | 3 (33.3) | 0.213 |
| Closed | 7 (100.0) | 6 (66.7) | |
Notes: Small MH grade, ≤250 µm; Medium MH grade, >250 – < 400 µm; Large MH grade, ≥400 µm; High myopia: if it exceeded 6 dioptres or if an axial eye length was > 26 mm; n (%) was given for each variable; p < 0.05 was considered statistically significant; Analyses were made with Fisher’s exact test.
Abbreviations: n, number of subjects in a group; MH, macular hole.
Figure 1.
Success and failures of macular hole surgery with regard to size of the macular hole and procedure.
Secondary Endpoints
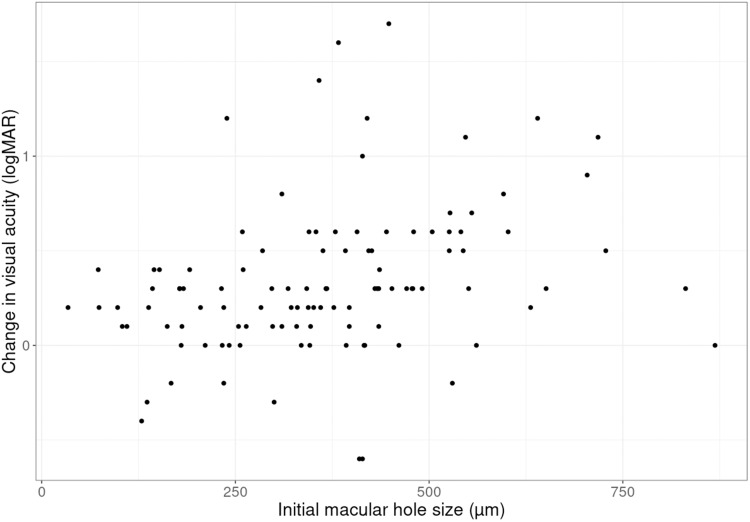
The initial preoperative visual acuity correlated with the size of the macular hole (Figure 2). The final visual acuity was better when the macular hole before surgery was small. With larger holes, the improvement in visual acuity was not as good. The improvement in visual acuity was smaller for small and large size macular holes and higher for medium size macular holes (Figures 3 and 4).
Figure 2.
Preoperative visual acuity and macular size hole determined by OCT scanning. Scatter plot for preoperative Visual Acuity (logMAR) and initial macular hole size (µm) with regression line and 95% confidence intervals.
Figure 3.
Final best corrected visual acuity (BCVA) and initial macular hole size (µm). Scatter plot for final BCVA (logMAR) and initial macular hole size (µm) with regression line and 95% confidence intervals.
Figure 4.
Change in best corrected visual acuity and initial macular hole size. Scatter plot for change in visual acuity (logMAR) and initial macular hole size (µm).
BCVA significantly improved in both treatment groups. There was no statistically significant difference in the postoperative improvement of visual acuity between both groups. In the subgroup analysis (comparison between ILM-P and ILM-F groups), a statistically significant BCVA improvement in the ILM-F group was found only in phacovitrectomy cases of large MHs, but not in patients with small- and medium-size MHs, isolated vitrectomy, and high myopia (Table 4).
Table 4.
Pre- and Postoperative Visual Acuity Comparison
| Overall | ILM Flap | ILM Peeling | p |
| n = 48 | n = 61 | ||
| BCVA preoperative | 0.75 (0.50; 1.30) | 0.70 (0.60; 1.00) | 0.822 |
| BCVA postoperative | 0.45 (0.38; 0.63) | 0.50 (0.40; 0.70) | 0.469 |
| p | < 0.001 | < 0.001 | |
| Isolated ppv | Small + medium MH | ||
| n = 15 | n = 26 | ||
| BCVA preoperative | 0.50 (0.40; 0.70) | 0.60 (0.50; 0.95) | 0.256 |
| BCVA postoperative | 0.40 (0.30; 0.55) | 0.40 (0.33; 0.50) | 0.782 |
| p | 0.082 | 0.002 | |
| Isolated ppv | Large MH | ||
| n = 15 | n = 13 | ||
| BCVA preoperative | 1.00 (0.80; 1.60) | 1.00 (1.00; 1.30)/ 1.12 ± 0.34 | 0.906 |
| BCVA postoperative | 0.60 (0.35; 0.90)/ 0.61 ± 0.35 | 0.64 ± 0.38 | 0.857a |
| p | 0.001 | 0.002a | |
| Isolated ppv | High myopia | ||
| n = 6 | n = 8 | ||
| BCVA preoperative | 0.50 (0.43; 0.58) | 1.30 (0.75; 1.30) | 0.029 |
| BCVA postoperative | 0.45 (0.40; 0.65) | 0.60 (0.35; 0.85) | 0.896 |
| p | > 0.999 | 0.090 | |
| Phacovitrectomy | Small + medium MH | ||
| n = 9 | n = 15 | ||
| BCVA preoperative | 0.60 (0.40; 0.70) | 0.60 (0.55; 0.80) | 0.608 |
| BCVA postoperative | 0.40 (0.20; 0.50) | 0.20 (0.15; 0.55) | 0.410 |
| p | 0.015 | 0.008 | |
| Phacovitrectomy | Large MH | ||
| n = 9 | n = 7 | ||
| BCVA preoperative | 1.00 (0.80; 1.30)/ 1.03 ± 0.42 | 1.30 (0.75; 1.60)/ 1.23 ± 0.52 | 0.437a |
| BCVA postoperative | 0.40 (0.30; 0.40) | 0.70 (0.70; 0.95) | 0.002 |
| p | 0.004 | 0.136 | |
Notes: Small + medium MH, <400 µm; Large MH, ≥400 µm; High myopia: if it exceeded 6 dioptres or if an axial eye length was > 26 mm; M ± SD or Median with quartile 1 and 3 was given for each variable; Analyses were made with Wilcox’s test or with Student’s t-testa (both for dependent or independent samples). p < 0.05 was considered statistically significant.
Abbreviations: MH, macular hole; BCVA, best-corrected visual acuity.
The duration of surgery (incision–suture time) in the ILM-P and ILM-F groups was similar with 38.3 ± 14.0 and 37.5 ± 11.8 min, respectively.
The most common intraoperative issue was the identification of a retinal break which needed to be treated with cryo- or laser-retinopexy (31% in both groups). No severe complication (eg endophthalmitis, severe bleedings) was noted in either group. In both groups, a few cases of temporarily elevated intraocular pressure were observed but resolved with medical treatment. In the ILM-P group, three postoperative retinal detachments were noted. In the ILM-F group, four cases of Irvine–Gass syndrome in the phacovitrectomy subgroup were observed. In the long-term follow-up, cataracts and posterior capsular opacifications were observed in both groups (ILM-F, 25%; ILM-P, 32%).
In 18% of the patients in the ILM-P group revision surgery for a persisting MH was necessary (heavy silicone oil or another vitrectomy with long-lasting gas tamponade). In the ILM-F group, only one closure failure was observed. The patient refused any further procedures. Late re-openings of the macular hole were not seen in these cohorts.
For the ILM-P group, a regression analysis was performed to better understand the possible factors that lead to failure. The regression analysis for the ILM-F group was not performed because only one failure case was noted.
The univariate logistic regression model showed a statistically significant effect on the closure rate for the following five preoperative parameters: basal hole size (OR = 0.998, p = 0.045), macular hole size (OR = 0.992, p = 0.006), retinal thickness temporally of the fovea (OR 0.988, p = 0.018), retinal thickness nasally of the fovea (OR 0.99, p = 0.043), and large macular hole grade (OR = 0.05, p = 0.006, Table 5).
Table 5.
Logistic Regression Analysis
| Univariate Logistic Regression Models for Main Outcome | |||
|---|---|---|---|
| Variable | OR | 95% CI for OR | p |
| ILM Peeling | |||
| High myopia (no vs yes) | 0.42 | 0.09; 2.27 | 0.274 |
| Intraoperative gas tamponade (C3F8 vs SF6) | 3.58 | 0.77; 15.74 | 0.086 |
| Phacoemulsification intraoperatively (no vs yes) | 0.74 | 0.21; 2.84 | 0.653 |
| Preoperative macular hole diameter (µm) | 0.998 | 0.995; 0.999 | 0.045 |
| Macular hole size (µm) | 0.992 | 0.985; 0.997 | 0.006 |
| Preoperative retinal thickness temporal (µm) | 0.988 | 0.977; 0.997 | 0.018 |
| Preoperative retinal thickness nasal (µm) | 0.99 | 0.98; 0.99 | 0.043 |
| Macular hole grade (small = baseline) | |||
| Medium | 0.86 | 0.03; 22.72 | 0.915 |
| Large | 0.05 | < 0.01; 0.30 | 0.006 |
| Macular hole idiopathic vs secondary | 0.41 | 0.11; 1.60 | 0.183 |
| Preoperative lens status (phakic vs pseudophakic) | 0.56 | 0.28; 1.13 | 0.095 |
Notes: High myopia: if it exceeded 6 dioptres or if an axial eye length was > 26 mm; Dependent variable was coded as follows: 0 – open (unsuccessful outcome), 1 – close (successful outcome); OR with 95% CI, odds ratio with 95% confidence intervals; p < 0.05 was considered statistically significant; p value for each predictor in a regression analysis.
Abbreviation: ILM, membrana limitans interna.
Discussion
This study represents a single-center case series that compares patients after introduction of a new surgical technique with a historical control group. We are aware of several drawbacks, such as the limited patient number, and the fact that it is not a single surgeon experience. Moreover, we were unable to demonstrate statistically significant differences in the visual acuity despite a better closure rate in large macular holes.
Nevertheless, it appears that the introduction of the new technique was not associated with an increase in complications. Moreover, the closure rate in ILM flap patients seemed to be better, which may be viewed as an encouraging result. A prospective randomized trial to provide statistical evidence for a possible benefit of this technique could be difficult to perform because of the necessary large sample size. Other data analysis approaches such as the analysis of data from larger multicenter registers with a high degree of standardization would be helpful to find considerable and statistically significant benefits of different surgical techniques. In our series, we may carefully draw the conclusion that the ILM inverted flap technique appeared to be also feasible for small and medium-sized holes, and also for secondary macular holes.
In our series, both techniques are also comparable in terms of procedure time and side effects. We did not change the diameter of the peeling area with respect to the size of the macular hole.
Both groups differ in the incidence of secondary macular holes (26.2% vs 12.5%) with a larger proportion of secondary MHs in the ILM peeling group. There is no specific reason why the ILM peeling was more frequently used in the historic control ILM-P group for secondary MH. We feel that it is a coincident finding.
Secondary macular holes do have a worse prognosis than idiopathic macular holes, which contributes to this difference in outcome. In our series in the ILM peeling group 31% of the secondary MHs did not close after one procedure, whereas 16.7% of the idiopathic MHs failed. In the flap group only one secondary macular hole failed. On the other hand, the macular holes in the flap group were significantly larger than in the peeling group (421.9 µm vs 322.61 µm). The reason for that is, when we started with the flap technique, we usually assigned the larger macular holes for the ILM flap procedure.
An important issue in the evaluation of procedures for macular hole repair is visual acuity. Long-term observations of patients who had vitrectomy for MH repair showed after a short faster visual acuity increase a slow but long-lasting recovery and improvement effect on visual acuity.14 In contrast to the improvement in retinal architecture and remodeling, cataract formation is known to interfere with the visual acuity.20–22 To avoid problems with the interpretation of pre- and postoperative visual acuity data, patients in clinical trials for MH should be pseudophakic. To reduce the impact of cataract formation, postoperative BCVA at the 6-week visit was used for calculation for all patients (overall) as well as for patients with a phakic preoperative lens status without any signs of the opaque lens and who received isolated vitrectomy. For all the other patients, the last postoperative BCVA was used for calculations.
The results of the current study confirmed the statistically significant BCVA improvement in both groups for all conditions but also in patients with large-size MHs. Only in patients presenting with a macular hole associated with high myopia, the improvement in visual acuity was not statistically significant.
An interesting issue is that the increase in visual acuity was very good for medium size holes and not so convincing for small holes and large holes. Small holes were usually associated with a better visual acuity. Therefore, the improvement may not have been as sustained. Also, it has to be considered that in large holes the retinal damage prevents a large improvement in visual acuity. However, there is a large variation in the changes of visual acuity in all three groups and the sample size is too small to draw stronger conclusions.
Where available, we included all follow-up data of the operated patients in our series. The maximum follow-up was 6.4 years. During the follow-up, we did not observe any re-opening of the macular hole. Because of the retrospective nature of this series, we cannot exclude such incidents. The percentage of late re-openings in the recent literature is 8%.23 Furthermore, the postoperative formation of an epiretinal membrane after ILM-flap technique as reported by Kanda24 could not be seen in our series.
Conclusion
We conclude that the ILM flap technique appears to be safe in our series and further clinical studies should include larger patient numbers to evaluate if this new technique is applicable for all sizes of MHs.
Acknowledgments
No funding or other support was received to assist with the preparation of this manuscript.
All authors certified no affiliations with or involvement in any organization or entity with any financial or nonfinancial interest in the subject matter or materials discussed in this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results of a pilot study. Arch Ophthalmol. 1991;109(5):654–659. doi: 10.1001/archopht.1991.01080050068031 [DOI] [PubMed] [Google Scholar]
- 2.Robles-Holmes HK, Staropoli PC, Yannuzzi N, Sridhar J. Management of large or recurrent macular holes. Curr Ophthalmol Rep. 2020;8(2):62–68. doi: 10.1007/s40135-020-00231-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumagai K, Furukawa M, Ogino N, Uemura A, Larson E. Long-term outcomes of internal limiting membrane peeling with and without indocyanine green in macular hole surgery. Retina. 2006;26(6):613–617. doi: 10.1097/01.iae.0000236471.79066.fe [DOI] [PubMed] [Google Scholar]
- 4.Sheidow TG, Blinder KJ, Holekamp N, et al. Outcome results in macular hole surgery: an evaluation of internal limiting membrane peeling with and without indocyanine green. Ophthalmology. 2003;110(9):1697–1701. doi: 10.1016/S0161-6420(03)00562-1 [DOI] [PubMed] [Google Scholar]
- 5.Korobelnik JF, Hannouche D, Belayachi N, Branger M, Guez JE, Hoang-Xuan T. Autologous platelet concentrate as an adjunct in macular hole healing: a pilot study. Ophthalmology. 1996;103(4):590–594. doi: 10.1016/S0161-6420(96)30648-9 [DOI] [PubMed] [Google Scholar]
- 6.Paques M, Chastang C, Mathis A, et al. Effect of autologous platelet concentrate in surgery for idiopathic macular hole: results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery Group. Ophthalmology. 1999;106(5):932–938. doi: 10.1016/S0161-6420(99)00512-6 [DOI] [PubMed] [Google Scholar]
- 7.Christensen UC. Value of internal limiting membrane peeling in surgery for idiopathic macular hole and the correlation between function and retinal morphology. Acta Ophthalmol. 2009;87(2):1–23. doi: 10.1111/j.1755-3768.2009.01777.x [DOI] [PubMed] [Google Scholar]
- 8.Spiteri Cornish K, Lois N, Scott N, et al. Vitrectomy with internal limiting membrane (ILM) peeling versus vitrectomy with no peeling for idiopathic full-thickness macular hole (FTMH). Cochrane Database Syst Rev. 2013;6:CD009306. [DOI] [PubMed] [Google Scholar]
- 9.Ben Simon GJ, Desatnik H, Alhalel A, Treister G, Moisseiev J. Retrospective analysis of vitrectomy with and without internal limiting membrane peeling for stage 3 and 4 macular hole. Ophthalmic Surg Lasers Imaging. 2004;35(2):109–115. doi: 10.3928/1542-8877-20040301-05 [DOI] [PubMed] [Google Scholar]
- 10.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Nawrocki J. Inverted internal limiting membrane flap technique for surgical repair of myopic macular holes. Retina. 2014;34(4):664–669. doi: 10.1097/IAE.0000000000000042 [DOI] [PubMed] [Google Scholar]
- 11.Michalewska Z, Michalewski J, Dulczewska-Cichecka K, Adelman RA, Nawrocki J. Temporal inverted internal limiting membrane flap technique versus classic inverted internal limiting membrane flap technique: a comparative study. Retina. 2015;35(9):1844–1850. doi: 10.1097/IAE.0000000000000555 [DOI] [PubMed] [Google Scholar]
- 12.Michalewska Z, Michalewski J, Adelman RA, Nawrocki J. Inverted internal limiting membrane flap technique for large macular holes. Ophthalmology. 2010;117(10):2018–2025. doi: 10.1016/j.ophtha.2010.02.011 [DOI] [PubMed] [Google Scholar]
- 13.Velez-Montoya R, Ramirez-Estudillo JA, Sjoholm-Gomez de Liano C, et al. Inverted ILM flap, free ILM flap and conventional ILM peeling for large macular holes. Int J Retina Vitreous. 2018;4:8. doi: 10.1186/s40942-018-0111-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim SH, Kim HK, Yang JY, Lee SC, Kim SS, Korean J. Visual recovery after macular hole surgery and related prognostic factors. Ophthalmol. 2018;32(2):140–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gu C, Qiu Q. Inverted internal limiting membrane flap technique for large macular holes: a systematic review and single-arm meta-analysis. Graefes Arch Clin Exp Ophthalmol. 2018;256(6):1041–1049. doi: 10.1007/s00417-018-3956-2 [DOI] [PubMed] [Google Scholar]
- 16.Marques RE, Sousa DC, Leal I, Faria MY, Marques-Neves C. Complete ILM peeling versus inverted flap technique for macular hole surgery: a meta-analysis. Ophthalmic Surg Lasers Imaging Retina. 2020;51(3):187–A2. doi: 10.3928/23258160-20200228-08 [DOI] [PubMed] [Google Scholar]
- 17.Silva N, Ferreira A, Nawrocka Vel Michalewska ZA, Meireles A. Inverted internal limiting membrane flap technique: is it the best option for macular holes? Clin Ophthalmol. 2021;15:3295–3303. doi: 10.2147/OPTH.S284614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duker JS, Kaiser PK, Binder S, et al. The international vitreomacular traction study group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120(12):2611–2619. doi: 10.1016/j.ophtha.2013.07.042 [DOI] [PubMed] [Google Scholar]
- 19.Hosmer DW, Lemeshow S, Sturdivant RX. Applied Logistic Regression. John Wiley & Sons; 2013. [Google Scholar]
- 20.Muselier A, Dugas B, Burelle X, et al. Macular hole surgery and cataract extraction: combined vs consecutive surgery. Am J Ophthalmol. 2010;150(3):387–391. doi: 10.1016/j.ajo.2010.04.008 [DOI] [PubMed] [Google Scholar]
- 21.Theocharis IP, Alexandridou A, Gili NJ, Tomic Z. Combined phacoemulsification and pars plana vitrectomy for macular hole treatment. Acta Ophthalmol Scand. 2005;83(2):172–175. doi: 10.1111/j.1600-0420.2005.00417.x [DOI] [PubMed] [Google Scholar]
- 22.Thompson JT, Glaser BM, Sjaarda RN, Murphy RP. Progression of nuclear sclerosis and long-term visual results of vitrectomy with transforming growth factor beta-2 for macular holes. Am J Ophthalmol. 1995;119(1):48–54. doi: 10.1016/S0002-9394(14)73812-7 [DOI] [PubMed] [Google Scholar]
- 23.Elhusseiny AM, Schwartz SG, Flynn HW, Smiddy WE. Long-term outcomes after macular hole surgery. Ophthalmol Retina. 2020;4(4):369–376. doi: 10.1016/j.oret.2019.09.015 [DOI] [PubMed] [Google Scholar]
- 24.Kanda K, Nakashima H, Emi K. Macular pucker formation after inverted internal limiting membrane flap technique: two case reports. Am J Ophthalmol Case Rep. 2022;25:101282. doi: 10.1016/j.ajoc.2022.101282 [DOI] [PMC free article] [PubMed] [Google Scholar]