Abstract
Purpose
The incidence of triple-negative breast cancer (TNBC) is higher in Black women compared to White women which is not explained by racial differences in body mass index (BMI). As BMI has limitations as an anthropometric measure, we used different anthropometric measures to examine associations with TNBC by race.
Method
Of 161,808 postmenopausal participants in Women’s Health Initiative, eligible were a subsample of 121,744 White and Black postmenopausal women enrolled from 1993-1998, 50-79 years of age with anthropometric measures who were followed for breast cancer incidence until March 2019. At entry, BMI, waist circumference (WC) and waist-hip ratio (WHR) were measured using standardized methods. Breast cancers were verified by central medical record review. Associations between anthropometric measures and triple-negative breast cancer risk were examined using Cox proportional hazards regression models.
Results
After 17.6 years (median) follow-up, there were 87 Black women and 529 White women with incident triple-negative breast cancer. Overall, there was no significant associations between anthropometric measures and risk of triple-negative breast cancer. However, compared to White women with normal BMI, White women with obesity (BMI≥30) (HR=0.76, 95% CI: 0.60, 0.96) were significantly associated with a lower risk of triple-negative breast cancer. And larger waist circumference (HR=0.99, 95% CI: 0.99, 1.00) were significantly associated with a lower risk of triple-negative breast cancer among White women.
Conclusion
Overall, among postmenopausal women, anthropometric measures were not associated with risk of TNBC. The association among White women with larger waist circumference and women with obesity with a lower risk of triple-negative breast cancer needs further confirmation.
Keywords: Triple-negative breast cancer, BMI, waist, waist to hip ratio, racial disparity
Introduction
Triple-negative breast cancer (TNBC) is a subtype of breast cancer defined by negative status of the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2), which has a poor prognosis and accounts for 15% of US breast cancers [1]. These cancers are more common in younger women with genetic mutations (such as BRCA1, BRCA2 and others) and in Black women [1, 2].
While genetic factors play a role in the higher incidence of triple-negative breast cancer in Black women compared to White women, the contribution of other factors are unclear [3, 4]. While the prevalence of obesity in Black women is high compared to other racial groups, studies of obesity and triple-negative breast cancer in postmenopausal women are mixed. In some studies, obesity is associated with higher risk [5, 6], while in other studies, no association with obesity is seen [6, 7] and others have found obesity associated with lower triple-negative breast cancer risk [8, 9].
Although BMI is correlated with body fat measures (like skinfold measures and x-ray absorptiometry), BMI could disproportionately correlate to body fat in Black women, as compared to White women. Specifically, Black women have less visceral fat compared to White women with similar BMI. [10] Although there is no clear explanation for TNBC, studies demonstrated that anthropometric differences between Black women and White women can lead to differences in insulin sensitivity, glucose intolerance, hormone concentration, and inflammation, which are potential risks factors that can trigger breast cancer development. [11–13] While there is ongoing interest in the potential association of obesity, metabolic syndrome components [14–17] and triple negative breast cancer, clinical evidence has been limited [14, 18, 19]. Therefore, as different anthropometric measures reflect different aspects of body fat, we examined associations between three anthropometric measurements and risk of triple-negative breast cancer to assess whether the associations between different anthropometric measurements and risk of triple-negative breast cancer differed in White and Black women. For example, waist circumference is reported as a better measure of abdominal fat compared to other anthropometric measures, which is a key factor of metabolic syndrome that associated with insulin resistance and inflammation [16]. We hypothesized that associations between anthropometric measurements and risk of triple-negative breast cancer were different in White and Black women, and that waist circumference would have a stronger association with the risk of triple-negative breast cancer compared to other anthropometric measurements.
Method
Details of the Women’s Health Initiative have been previously described [17]. Briefly, in the study,161,808 postmenopausal women, aged 50 to 79 years, enrolled at 40 clinical centers throughout the United States from 1993 through 1998. Women were included in an observational cohort (n = 93,676) or one of four randomized clinical trials (n = 68,132).
Study Population
The original dataset had 161,808 participants. After exclusions, our analysis included 121,774 postmenopausal women (11,832 Black and 109,942 White), with 616 participants with incident triple-negative breast cancer during follow-up (median, 17.6 years) including 87 Black women and 529 White women. Excluded were 13,649 participants who were not White or Black, 3,042 with BMI <18.5, 11,474 with history of cancer at baseline, and 11,434 with missing covariates including education, smoking, history of hormone use, age at menarche, diet, physical activity, alcohol servings, breastfeeding duration, and parity (Figure 1).
Figure 1.
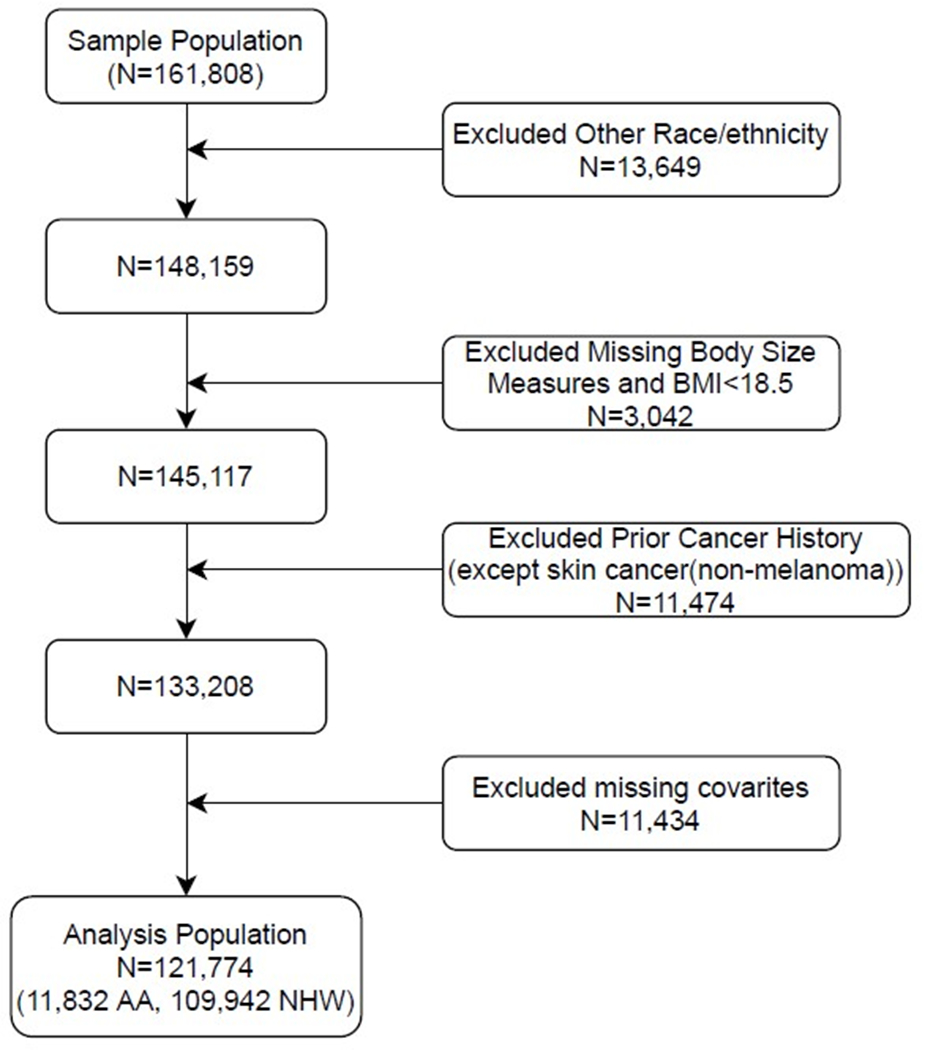
Study Population
Exposure
Anthropometric measurements were performed during the baseline clinic visit following standard protocols. Weight was measured and recorded in kilograms and height was measured and recorded as centimeters. BMI was computed from weight and height as body weight divided by the square of body height, recorded as kg/m2. Waist circumference was measured at the smallest circumference of the natural waist to the nearest 0.5 cm, hip circumference was measured at the widest part of the hips to the nearest 0.5 cm, at baseline. The Waist to Hip Ratio (WHR) was calculated as waist circumference(cm) divided by hip circumference(cm).
Outcomes
The primary outcome was triple-negative breast cancer incidence during follow-up through April 1, 2019. Breast cancers were confirmed after medical record review by clinical center physician adjudicators with final adjudication at the Clinical Coordinating Center. Receptor assays were based on review of local laboratory reports recorded as positive, negative, borderline, not available or unknown. Triple-negative breast cancers were defined as negative estrogen receptor (ER), negative progesterone receptor (PR), and negative human epidermal growth factor receptor 2 (HER2) breast cancers.
Covariates
Potential covariates included age, region, education, income, smoking, alcohol consumption, family history of breast cancer, diet, physical activity, different sub-cohorts (participating in OS/CTs and different arms), hormone therapy use, parity, age at menarche, breastfeeding duration [1, 17, 20]. Family history was defined as whether mother, full-blooded sisters, daughters, or grandmothers ever had breast cancer (Y/N). Diet quality was calculated using healthy eating index (HEI)-2015 score at baseline. Physical activity was defined as metabolic equivalent task (MET-hours/week) from recreational physical activity. Parity was collected in medical history questionnaire as “how many live births?” Age at menarche was self-reported in years. Breastfeeding duration was self-reported as total months breastfeeding (detailed information in Table 1).
Table 1:
Participant Baseline Characteristics between Black Women and Non-Hispanic White Women
| Black (N=11,832) | White (N=109,942) | P-value | ||
|---|---|---|---|---|
| Age at baseline (year) ** | 61.40 (7.09) | 63.51 (7.14) | <0.0001 | |
| Education * | <0.0001 | |||
| High school diploma or GED | 2988 (25.25) | 23197 (21.17) | ||
| Training School, Some college or Associate degree | 5642 (47.68) | 54057 (49.17) | ||
| College degree or higher | 3202 (27.06) | 32688 (29.73) | ||
| Income * | <0.0001 | |||
| Less than $35,000 | 5862 (53.44) | 39304 (38.14) | ||
| $35,000 to $74,999 | 3913 (35.67) | 42874 (41.61) | ||
| >$75,000 | 1194 (10.89) | 20868 (20.25) | ||
| Region * | <0.0001 | |||
| Northeast | 2077 (17.55) | 27455 (24.97) | ||
| South | 5537 (46.80) | 25233 (22.95) | ||
| Midwest | 2857 (24.15) | 25843 (23.51) | ||
| West | 1361 (11.50) | 31411 (28.57) | ||
| Smoking * | <0.0001 | |||
| Never smoked | 5870 (49.61) | 55053 (50.07) | ||
| Past smoker | 4641 (39.22) | 47871 (43.54) | ||
| Current smoker | 1321 (11.16) | 7018 (6.38) | ||
| Alcohol serving (Number of serving per week) ** | 1.08 (3.93) | 2.62 (5.02) | <0.0001 | |
| Diet ** | 62.71 (10.84) | 65.57 (10.31) | <0.0001 | |
| Physical activity ** | 9.65 (12.87) | 12.81 (13.72) | <0.0001 | |
| Family history of breast cancer * | <0.0001 | |||
| Yes | 1637 (13.84) | 19990 (18.18) | ||
| No | 10195 (86.16) | 89952 (81.82) | ||
| Use of female hormones * | <0.0001 | |||
| Yes | 4850 (40.99) | 64838 (58.97) | ||
| No | 6982 (59.01) | 45104 (41.03) | ||
| Parity * | <0.0001 | |||
| Never full-term pregnancy | 1580 (13.35) | 12568 (11.43) | ||
| 1-2 | 4522 (38.22) | 36825 (33.49) | ||
| 3-4 | 3643 (30.79) | 44974 (40.91) | ||
| 5 or more | 2087 (17.64) | 15575 (14.17) | ||
| Breastfeeding duration * | <0.0001 | |||
| Never breastfeeding | 6184 (52.27) | 53658 (48.81) | ||
| 1-6 months | 3162 (26.72) | 28052 (25.52) | ||
| 7-12 months | 1273 (10.76) | 12206 (11.10) | ||
| 13-23 months | 665 (5.62) | 9949 (9.05) | ||
| 24+ months | 548 (4.63) | 6077 (5.53) | ||
| Age to reach menarche (year) ** | 12.62 (1.64) | 12.59 (1.45) | 0.0230 | |
| BMI (kg/m2) ** | 31.27 (6.64) | 27.71 (5.70) | <0.0001 | |
| Waist circumference (cm) ** | 91.97(13.90) | 86.16 (13.55) | <0.0001 | |
| Waist to hip ratio ** | 0.82 (0.08) | 0.81 (0.08) | <0.0001 | |
Tested by chi-square test
Tested by one-way ANOVA
Statistical analyses
Descriptive analyses were conducted to describe participants’ characteristics at baseline for White and Black women in Table 1. All demographic and potential confounding variables are presented as the mean ± standard deviation for continuous variables, and frequencies and percentages for categorical variables. Chi-square tests were conducted for categorical covariates and ANOVA tests for continuous covariates. The survival time was defined from the date of enrollment until the date of diagnosis of breast cancer, the date of death, date of withdrawal from the study, or the end of follow-up (March 1st, 2019), whichever came first.
To examine the association between different anthropometric measurements and risk of triple-negative breast cancer, we constructed the crude and adjusted Cox proportional hazards regression models using competing risk developed by Fine and Gray [21], treating all other types of breast cancer cases as competing events. Before performing Cox proportional hazard models, the proportional hazards assumptions were evaluated based on the Schoenfeld Residuals. For covariates that did not meet the assumption, their interactions with time were included in the models. Because participating in clinical trials might disproportionally affect the association, different sub-cohorts (participating in OS/CTs and different arms) were treated as strata in the model to consider possible different baseline hazards. We performed Cox proportional hazard models used both continuous and categorical anthropometric measurements. BMI was categorized into three groups according to established cut points (normal: 18.5 to <25 kg/m2; overweight: 25.0 to <30 kg/m2; obese: 30.0 or higher kg/m2) [22]. Waist circumference was categorized as ≤88cm or >88cm based on established cut points [22]. Waist to hip ratio later was categorized as <0.85 and ≥0.85 [23]. Both continuous and categorical variables of different anthropometric measurements were assessed as main exposures, respectively. Covariables were selected a priori based on prior literature and all were retained in the final adjusted model. Potential interactions of race-anthropometric measurements were evaluated in Cox proportional hazard models at a significance level of 0.1. As weight change might have significant effect on the risk of triple-negative breast cancer, a sensitivity analysis was conducted with further adjusting for weight changes from baseline to year 3 [20, 24].
All statistical tests were two-tailed with a significance level of 0.05 (except interaction terms with a significance alpha=0.1). All analyses were performed in SAS 9.4.
Results
Participants’ characteristics at baseline between Black women and White women
Compared to White women, Black women were younger, had less education and lower household income, be current smokers, consume less alcohol, have lower diet quality, be less physically active, be less likely to have a breast cancer family history, be less likely to use hormone therapy, have lower parity, have shorter breastfeeding duration, be older at menarche, and have higher BMI, larger waist circumference, and larger waist-to-hip ratio (Table 1).
Associations between different anthropometric measurements and risk of triple-negative breast cancer between Black women and White women
There was no significant association between anthropometric measures (including BMI, waist circumference, or WHR when defined as categorical or continuous) and risk of triple-negative breast cancer among Black women, after adjusting for age, region, education, income, smoking status, alcohol consumption, family history, diet, exercise, hormone uses, age at menarche, parity and study arms (Table 2). However, obesity (HR=0.76, 95%CI: 0.60, 0.96) and waist circumference (HR=0.99, 95%CI: 0.99, 1.00) were significantly associated with lower triple-negative breast cancer risk among White women after adjusting for covariates. In White women, the association between waist circumference>88cm and lower triple-negative breast cancer risk had borderline significance (HR=0.83, 95%CI: 0.68, 1.00). Overall, there was no significant interaction between anthropometric measurements and race. The interaction between waist category (WC>88 cm) and race had borderline significance (p=0.1).
Table 2.
The Association Between Anthropometric Measurements and Risk of Triple-Negative Breast Cancer
| Overall (N=121,774, NBC=616) | Black (N=11,832, TNBC=87) | White (N=109,942, TNBC=529) | |||||
|---|---|---|---|---|---|---|---|
| Adjusted HR a (95% CI) | Test for interaction (P-value) | TNBC cases | Adjusted HR b (95% CI) | TNBC cases | Adjusted HR b (95% CI) | ||
| BMI | 0.99 (0.97, 1.00) | 0.93 | 87 | 1.00 (0.97, 1.03) | 529 | 0.99 (0.97, 1.00) | |
| BMI category | 0.55 | ||||||
| Normal (18.5-24.9) | (reference) | 12 | (reference) | 208 | (reference) | ||
| Overweight (25.0-29.9) | 0.92 (0.75, 1.13) | 31 | 1.34 (0.66, 2.73) | 186 | 0.92 (0.75, 1.12) | ||
| Obesity (≥30) | 0.77 (0.61, 0.97) | 44 | 1.20 (0.60, 2.43) | 135 | 0.76 (0.60, 0.96) ** | ||
| WC | 0.99 (0.99, 1.00) | 0.34 | 87 | 1.01 (0.99, 1.02) | 529 | 0.99 (0.99, 1.00) ** | |
| Waist category | 0.10 * | ||||||
| WC≤88 cm | (reference) | 32 | (reference) | 341 | (reference) | ||
| WC>88 cm | 0.84 (0.70, 1.02) | 55 | 1.42 (0.90, 2.22) | 188 | 0.83 (0.68, 1.00) * | ||
| WHR | 0.89 (0.76, 1.04) | 0.23 | 87 | 1.09 (0.86, 1.37) | 529 | 0.88 (0.75, 1.04) | |
| WHR category | 0.28 | ||||||
| WHR<0.85 | (reference) | 55 | (reference) | 403 | (reference) | ||
| WHR≥0.85 | 0.85 (0.69, 1.04) | 32 | 1.15 (0.74, 1.79) | 126 | 0.84 (0.69, 1.04) | ||
Age, race/ethnicity, region, education, income, smoking, alcohol consumption, weight changes, family history of breast cancer, diet, physical activity, different sub-cohorts (participating in OS/CTs and different arms), use of hormone, parity, age at menarche, and breast-feeding, interaction between race/ethnicity and anthropometric measures were adjusted in the COX model.
Age, region, education, income, smoking, alcohol consumption, weight changes, family history of breast cancer, diet, physical activity, different sub-cohorts (participating in OS/CTs and different arms), use of hormone, parity, age at menarche, and breast-feeding were adjusted in the COX model.
borderline significance. P=0.05 or p=0.1 (for interaction).
p<0.05
Sensitivity analysis of intentional weight changes
After additionally adjusting for weight changes in year 3, obesity and high waist circumference were significantly and associated with lower risk of triple-negative breast cancer in the total sample (Obesity: HR=0.73, 95%CI: 0.57, 0.95; waist circumference: HR=0.99, 95%CI: 0.98, 1.00) and in White women (Obesity: HR=0.73, 95%CI: 0.56, 0.94; waist circumference: HR=0.99, 95%CI: 0.98, 1.00).
Discussion
In this large, prospective cohort of postmenopausal women, there was no significant association between anthropometric measures and risk of triple-negative breast cancer overall. However, White women with larger waist circumference and those who were obese had significantly lower risk of triple-negative breast cancer. In Black women, there were no significant associations between any anthropometric measures and triple-negative breast cancer risk. Further studies are needed to confirm these associations.
The finding of significantly lower triple-negative breast cancer risk in White women who were obese or had larger waist circumference was surprising and did not support our study hypothesis, especially given the adverse metabolic effects associated with obesity [25]. This paradoxical finding highlights the complexity of the breast cancer and obesity relationship, further illustrated by findings in premenopausal versus postmenopausal women. In cohort studies, obesity has consistently been associated with significantly higher breast cancer risk, while in premenopausal women, obesity is associated with significantly lower breast cancer risk [26]. However, in the randomized Breast Cancer Prevention Trial, obese premenopausal women (BMI < 30.0 kg/m2) were found to be at increased breast cancer risk (HR 1.70 95% CI 1.10–2.63) compared with normal weight premenopausal women (BMI < 25.0 kg/m2) [27]. More recently, The Premenopausal Breast Cancer Collaborative Group investigators, with 758, 592 premenopausal women and 13,082 incident breast cancers, reported increased BMI associated with significantly lower breast cancer risk, with the reduction greater than in prior reports. However, for triple-negative cancer, there was no association of BMI for women 25 years or older [28].
There is difference in body fat composition between White and Black women. Black women have less visceral fat and more skeletal muscle compared to White women with similar BMI, while White women have significantly lower subcutaneous adipose tissue [10, 13, 29, 30] which is positively associated with breast cancer risk [31, 32]. However, these differences in anthropometrics by race do not explain our current study findings. Considering the small number of Black women with triple-negative breast cancer, our findings should be considered exploratory.
As several studies reported differential associations of BMI with triple-negative breast cancer based on time from menopause (≥10 years) [8, 33], we did a sensitivity analysis adjusting for this variable with no change in results. This study considered BMI at baseline. In the WHI Observational Study cohort, women with weight loss in a 3-year period had significantly lower breast cancer risk [20]. In a sensitivity analysis adjusting for weight change at year 3, the results were unchanged. Our findings of no significant association in Black women were similar to a prospective Black Women’s Health Study that found no significant association between obesity (measured with BMI) and receptor-negative breast cancers [34]. While obesity is a well-established risk factor for hormone receptor positive breast cancer, the association with triple-negative breast cancer remains unsettled [19].
Strength and limitations
Study strengths include use of a large cohort with ethnic diversity, breast cancers verified by central medical record review, and long-term follow-up. In addition, several anthropometric measurements were used that reflect different aspect of body fat with analyses which incorporated potential confounders and used competing risk models including other breast cancer subtypes as competing events. Study limitations include the lower number of Black women with triple-negative breast cancer compared to the number in White women, reliance on self-report for most covariates, and findings limited to postmenopausal women.
Conclusion
In this large prospective cohort study of postmenopausal women, anthropometric measurements were not associated with triple-negative breast cancer risk in Black women. However, in White women, larger waist circumference and obesity were associated with lower triple-negative breast cancer risk. Further studies are needed to examine these associations.
Acknowledgments
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts N01WH22110, 24152, 32100-2, 32105-6, 32108-9, 32111-13, 32115, 32118-32119, 32122, 42107-26, 42129-32, and 44221; The WHI Life and Longevity after Cancer (LILAC) study is funded by UM1 CA173642.
Footnotes
Rowan Chlebowski is a consultant for Novartis, AstraZeneca, and Genentech. No other authors declared conflicts.
The first draft of the manuscript was written by Fengge Wang and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
References
- 1.Howard FM and Olopade OI, Epidemiology of Triple-Negative Breast Cancer: A Review. The Cancer Journal, 2021. 27(1): p. 8–16.DOI: 10.1097/PPO.0000000000000500. [DOI] [PubMed] [Google Scholar]
- 2.Cho B, Han Y, Lian M, Colditz GA, Weber JD, Ma C, Liu Y. Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer. JAMA oncology. 2021. Jul 1;7(7):1016–23. DOI: 10.1001/jamaoncol.2021.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stead LA, et al. , Triple-negative breast cancers are increased in black women regardless of age or body mass index. Breast Cancer Research, 2009. 11(2): p. R18.DOI: 10.1186/bcr2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddharth S and Sharma D, Racial disparity and triple-negative breast cancer in African-American women: a multifaceted affair between obesity, biology, and socioeconomic determinants. Cancers, 2018. 10(12): p. 514.DOI: 10.3390/cancers10120514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle P, Triple-negative breast cancer: epidemiological considerations and recommendations. Annals of Oncology, 2012. 23(suppl_6): p. vi7–vi12.DOI: 10.1093/annonc/mds187. [DOI] [PubMed] [Google Scholar]
- 6.Pierobon M and Frankenfeld CL, Obesity as a risk factor for triple-negative breast cancers: a systematic review and meta-analysis. Breast cancer research and treatment, 2013. 137(1): p. 307–314. [DOI] [PubMed] [Google Scholar]
- 7.Munsell MF, et al. , Body mass index and breast cancer risk according to postmenopausal estrogen-progestin use and hormone receptor status. Epidemiologic reviews, 2014. 36(1): p. 114–136.DOI: 10.1093/epirev/mxt010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagrani R, et al. , Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. European Journal of Cancer, 2016. 66: p. 153–161.DOI: 10.1016/j.ejca.2016.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Cook LS, Tang MTC, et al. Body mass index and risk of luminal, HER2-overexpressing, and triple negative breast cancer. Breast Cancer Research and Treatment. 2016;157(3):545–554. DOI: 10.1007/s10549-016-3825-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Heymsfield SB, et al. , Why are there race/ethnic differences in adult body mass index–adiposity relationships? A quantitative critical review. Obesity reviews, 2016. 17(3): p. 262–275.DOI: 10.1111/obr.12358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camhi SM, et al. , The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity, 2011. 19(2): p. 402–408.DOI: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carroll JF, et al. , Visceral fat, waist circumference, and BMI: impact of race/ethnicity. Obesity, 2008. 16(3): p. 600–607.DOI: 10.1038/oby.2007.92. [DOI] [PubMed] [Google Scholar]
- 13.Lovejoy JC, et al. , Abdominal fat distribution and metabolic risk factors: effects of race. Metabolism, 1996. 45(9): p. 1119–1124.DOI: 10.1016/S0026-0495(96)90011-6. [DOI] [PubMed] [Google Scholar]
- 14.Davis AA and Kaklamani VG, Metabolic syndrome and triple-negative breast cancer: a new paradigm. International journal of breast cancer, 2012. 2012.DOI: 10.1155/2012/809291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phipps AI, Malone KE, Porter PL, Daling JR, Li CI. Body Size and Risk of Luminal, HER2-Overexpressing, and Triple-Negative Breast Cancer in Postmenopausal Women. Cancer Epidemiology, Biomarkers & Prevention. 2008;17(8):2078–2086. DOI: 10.1158/1055-9965.EPI-08-0206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. DOI: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 17.Luo J, et al. , Mediation analysis of racial disparities in triple-negative breast cancer incidence among postmenopausal women. Breast Cancer Research and Treatment, 2021: p. 1–11.DOI: 10.1007/s10549-021-06158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chlebowski RT, Chen Z, Anderson GL, Rohan T, Aragaki A, Lane D, Dolan NC, Paskett ED, McTiernan A, Hubbell FA, Adams-Campbell LL. Ethnicity and breast cancer: factors influencing differences in incidence and outcome. Journal of the National Cancer Institute. 2005. Mar 16;97(6):439–48. DOI: 10.1093/jnci/dji064. [DOI] [PubMed] [Google Scholar]
- 19.Berger ER, Iyengar NM. Obesity and Energy Balance Considerations in Triple-Negative Breast Cancer. The Cancer Journal. 2021;27(1):17–24. DOI: 10.1097/PPO.0000000000000502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan K, et al. , Weight loss, diet composition and breast cancer incidence and outcome in postmenopausal women. Oncotarget, 2019. 10(33): p. 3088.DOI: 10.18632/oncotarget.26864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. Journal of the American statistical association. 1999; 94:496–509. [Google Scholar]
- 22.Heart N, et al. , Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. 1998: National Heart, Lung, and Blood Institute. [Google Scholar]
- 23.Organization, W.H., Waist circumference and waist-hip ratio: report of a WHO expert consultation, Geneva, 8–11 December 2008. 2011. [Google Scholar]
- 24.Chlebowski RT, et al. , Weight loss and breast cancer incidence in postmenopausal women. Cancer, 2019. 125(2): p. 205–212.DOI: 10.1002/cncr.31687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brown JC, Carson TL, Thompson HJ, Agurs-Collins T. The Triple Health Threat of Diabetes, Obesity, and Cancer—Epidemiology, Disparities, Mechanisms, and Interventions. Obesity. 2021;29(6):954–959. DOI: 10.1002/oby.23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Anderson GL, Neuhouser ML. Obesity and the Risk for Premenopausal and Postmenopausal Breast Cancer. Cancer prevention research. 2012. Apr 1;5(4):515–21. DOI: [DOI] [PubMed] [Google Scholar]
- 27.Cecchini RS, Costantino JP, Cauley JA, et al. Body Mass Index and the Risk for Developing Invasive Breast Cancer among High-Risk Women in NSABP P-1 and STAR Breast Cancer Prevention Trials. Cancer prevention research. 2012. Apr 1;5(4):583–92. DOI: 10.1158/1940-6207.CAPR-11-0482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The Premenopausal Breast Cancer Collaborative Group. Association of Body Mass Index and Age with Subsequent Breast Cancer Risk in Premenopausal Women. JAMA Oncol. 2018;4(11): e181771. DOI: 10.1001/jamaoncol.2018.1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silva AM, et al. , Ethnicity-related skeletal muscle differences across the lifespan. American Journal of Human Biology: The Official Journal of the Human Biology Association, 2010. 22(1): p. 76–82.DOI: 10.1002/ajhb.20956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katzmarzyk PT, et al. , Racial differences in abdominal depot–specific adiposity in white and African American adults. The American journal of clinical nutrition, 2010. 91(1): p. 7–15.DOI: 10.3945/ajcn.2009.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schapira DV, et al. , Abdominal obesity and breast cancer risk. Annals of internal medicine, 1990. 112(3): p. 182–186.DOI: 10.7326/0003-4819-112-3-182. [DOI] [PubMed] [Google Scholar]
- 32.He Y, et al. , Adipose tissue levels of polybrominated diphenyl ethers and breast cancer risk in Chinese women: A case–control study. Environmental research, 2018. 167: p. 160–168. DOI: 10.1016/j.envres.2018.07.009. [DOI] [PubMed] [Google Scholar]
- 33.Gaudet MM, et al. , Risk factors by molecular subtypes of breast cancer across a population-based study of women 56 years or younger. Breast cancer research and treatment, 2011. 130(2): p. 587–597.DOI: 10.1007/s10549-011-1616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palmer JR, et al. , A prospective study of body size and breast cancer in black women. Cancer Epidemiology and Prevention Biomarkers, 2007. 16(9): p. 1795–1802.DOI: 10.1158/1055-9965.EPI-07-0336. [DOI] [PubMed] [Google Scholar]


