Abstract
The present investigation was envisaged for large scale in-silico genome wide identification and characterization of glutathione S-transferases (GSTs) in Chenopodium quinoa. In this study, a total of 120 GST genes (CqGSTs) were identified and divided into 11 classes of which tau and phi were highest in numbers. The average protein length of protein was found to be 279.06 with their corresponding average molecular weight of 31,819.4 kDa. The subcellular localization analysis results showed that proteins were centrally localized in the cytoplasm followed by chloroplast, mitochondria and plastids. Structural analysis revealed the presence of 2 -14 exons in CqGST genes. Most of the proteins possessed two exon one intron organization. MEME analysis identified 15 significantly conserved motifs with a width of 6–50 amino acids. Motifs 1, 3, 2, 5, 6, 8, 9 and 13 were found specifically in tau class family; motifs 3, 4, 5, 6, 7 and 9 were found in phi class gene family, while motifs 3, 4, 13 and 14 were found in metaxin class. Multiple sequence alignment revealed highly conserved N-terminus with active site serine (Ser; S) or cysteine (Cys; C) residue for the activation of GSH binding and GST catalytic activity. The gene loci were found to be unevenly distributed across 18 different chromosomes with a maximum of 17 genes located on chromosome number 7. Dominance of alpha helix was followed by coil, extended strand and beta turns. Gene duplication analysis revealed that segmental duplication and purifying type selection were highest in number and found to be main source of expansion of GST gene family. Cis acting regulatory elements analysis showed the presence of 21 different elements involved in stress, hormone and light response and cellular development. The evolutionary relationship of CqGST proteins carried out using maximum likelihood method revealed that all the tau and phi class GSTs were closely associated with those of G. max, O. sativa and A. thaliana. Molecular docking of GST molecules with the fungicide metalaxyl showed that the CqGSTF1 had the lowest binding energy. The comprehensive study of CqGST gene family in quinoa provides groundwork for further functional analysis of CqGST genes in the species at molecular level and has potential applications in plant breeding.
Keywords: Quinoa, GST, Characterization, Phylogenetic analysis, Chromosomal localization, Gene duplication
Introduction
Quinoa (Chenopodium quinoa Willd.) is an allotetraploid (2n = 4x = 36) pseudocereal of Amaranthaceae family that originated in the Andean region of South America (Hong et al. 2017). Quinoa produces gluten free nutritious grains that have exceptional balance between carbohydrates, vitamins, minerals, essential amino acids, dietary fibers and oils (Yasui et al. 2016; Dakhili et al. 2019). It is usually cultivated in clay loam or sandy loam soils, with approximate pH of 5.5–8.5 with good drainage and moderate slopes (Fuentes and Bhargava 2011). The optimum temperature for quinoa is around 8–15 °C, although it can withstand up to − 4 °C (Alvar-Beltrán et al. 2020). Quinoa is recognized as a crop of great value due to its nutritious grain and high tolerance towards abiotic stresses (Yasui et al. 2016). Quinoa is adapted to a wide range of marginal soils, including drought prone ones and those with high salinity, which help it to thrive under adverse agroclimatic conditions (Morales et al. 2017; Vita et al. 2021). Due to its potential health benefits and adaptability to adverse climatic conditions, the year 2013 was declared by the FAO as the International Year of Quinoa (Bhargava and Ohri 2015; Jarvis et al. 2017). Considering its economic and nutritional significance, it could be a fascinating challenge for plant breeders to use transgenic approaches to develop varieties resistant against diverse biotic and abiotic stresses. The availability of sequenced genome of quinoa provides an opportunity to search and characterize diverse gene families which are functionally important. Recently, several gene families like HSP 70 (Liu et al. 2018), WRKY (Yue et al. 2019), trihelix transcription factor (Li et al. 2022), PYL (Pizzio 2022) and CIPK CBL (Xiaolin et al. 2022) have been identified and well characterized in quinoa.
Environmental stresses such as high salt, drought, extreme temperatures are often very harmful to plants, and they produce reactive oxygen species (ROS) to cause oxidative stress and cell damage (Czarnocka and Karpiński 2018). In order to maintain the redox balance within cells, plants have a series of antioxidant enzymes such as superoxide dismutase (SOD), peroxidase (POD), catalase (CAT), glutathione peroxidase (GPx) and glutathione-S-transferase (GST) and non-enzymatic mechanisms like glutathione, alpha-tocopherol, carotenoids and flavonoids to maintain the balance between production and elimination of ROS (Mittler et al. 2004).
GSTs (EC 2.5.1.8) constitute ancient protein superfamily of multifunctional proteins and are an integral part of the antioxidant enzymatic defense system in plants that works downstream of Cyt P450 by catalyzing the nucleophilic conjugation of reduced tripeptide glutathione (GSH) (g-Glu–Cys–Gly) into wide variety of hydrophobic and electrophilic substrates to make a chemical compound more hydrophilic to be expelled from the cell (Marimo et al. 2016; Vaish et al. 2020; Song et al. 2021). GSTs are characterized by the presence of canonical thioredoxin fold in the highly conserved N-terminal domain which dominantly consists of α-helices and β-strands with a β1α1β2α2β3β4α3 topology. It possesses a G-site for glutathione binding. The variable C-terminal domains consisting of all the α-helices and possesses an H-site for secondary hydrophobic substrate binding (Vaish et al. 2020).
In plants, GSTs play vital roles in the detoxification of xenobiotics and toxic lipid peroxides (Chronopoulou et al. 2014; Fafián-Labora et al. 2020), glucosinolate biosynthesis (Czerniawski and Bednarek 2018) and in metabolism (Liu et al. 2014). The GST proteins of plants have been classified into 14 distinct classes, namely tau, phi, theta, zeta, lambda, γ-subunit of the eukaryotic translation elongation factor 1B (EF1B), dehydroascorbate reductase (DHAR), metaxin, tetrachlorohydroquinone dehalogenase (TCHQD), Ure2p, microsomal prostaglandin E synthase type 2 (mPGES2), hemerythrin, iota, and glutathionyl-hydroquinone reductases (GHR) (Nianiou-Obeidat et al. 2017). Given the important detoxification effects of GSTs during plant stress, a number of studies have been carried out on GSTs in many plants. With the advent of whole genome sequencing, a large number of GSTs have been reported and characterized through genome-wide analyses in a number of plants like Physcomitrella patens (Liu et al. 2013), Solanum Lycopersicum (Islam et al. 2017), Ipomoea batatas (Ding et al. 2017), Cucurbita maxima (Kayum et al. 2018), Vigna radiata (Vaish et al. 2018), Malus domestica (Fang et al. 2020), Raphanus sativus (Gao et al. 2020), Cicer arietinum (Ghangal et al. 2020), Medicago truncatula (Han et al. 2018; Hasan et al. 2021), Triticum aestivum (Hao et al. 2021) and Cucumis melo (Song et al. 2021).
Despite the availability of whole genome sequence of quinoa, large scale in-silico genome wide identification and characterization of GSTs have not been carried out in this potential crop. Till date, there is no report of GST gene family identification and characterization in quinoa which provided us with an opportunity to perform in-silico genome-wide analysis of the GST gene family in this underutilized crop.
Material and methods
The in-silico analysis was carried out using 1 Gbps LAN speed on 8 GB RAM personal computer having AMD Ryzen 5 5500 U processor with Radeon Graphics 2.10 Ghz and 64-Bit operating system.
Protein sequence retrieval and search in quinoa database available at Phytozome
C. quinoa genome database available at Phytozome (https://phytozome-next.jgi.doe.gov/) was used to conduct in-silico genome identification and characterization of GST genes. GST protein sequences of Arabidopsis thaliana were obtained from The Arabidopsis Information Resource (TAIR) (http://www.arabidopsis.org/). Glycine max, Physcomitrella patens and Medicago truncatula GST sequences were retrieved from NCBI by the locus ID or the accession number published by Liu et al. (2013), Liu et al. (2015) and Hasan et al. (2021), respectively, while sequences of Oryza sativa were obtained from Rice Genome Annotation Project (RGAP) by the accession number published by Jain et al. (2010). pBLAST searches of each GST class was performed separately using retrieved GST protein sequences of five different species as query with an e-value of 0.0001. The identified amino acid, genomic and coding sequences were downloaded and subjected to NCBI Batch CD (conserved domain) search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (Marchler-Bauer et al. 2017), SMART (Simple Modular Architecture Research Tool) database (http://smart.embl-heidelberg.de/) (Letunic et al. 2021) and Pfam search (http://pfam.xfam.org/search) for confirmation of conserved C-terminal domain and conserved N-terminal domain with the thioredoxin fold.
Subcellular localization and in-silico physicochemical characterization of identified GSTs
Subcellular localization of the identified GSTs were predicted by using three different tools namely CELLO online tool v.2.5 (http://cello.life.nctu.edu.tw/) (Yu et al. 2006), WoLF PSORT (www.genscript.com/wolf-psort.html) (Horton et al. 2007) and DeepLoc (Armenteros et al. 2017) (http://www.cbs.dtu.dk/services/DeepLoc/). The physicochemical parameters such as isoelectric point (pI), molecular weight, aliphatic index and Grand Average of Hydropathy (GRAVY) were analysed using default parameters of ProtParam tool at Expasy server (http://web.expasy.org/protparam/) (Gasteiger et al. 2005).
Gene structure visualization
The exon/intron organization of quinoa GSTs were analysed using Gene Structure Display Server 2.0 (GSDS, https://gsds.cbi.pku.edu.cn/) (Hu et al. 2015) by aligning CDS and genomic sequences that were retrieved from Phytozome (https://phytozome-next.jgi.doe.gov/).
Motif identification
Conserved motifs of quinoa GSTs were identified using the Mutiple Em for Motif Elicitation (MEME) analysis (http://meme-suite.org/) (Bailey et al. 2009). The parameters for analysis were 20 as motif number while the motif width was 6- 50. The results were visualized using TBtool software.
Protein sequence alignment and catalytic residue position prediction
The CqGSTs protein sequences were aligned with protein sequences of Arabidopsis thaliana, O. sativa, G. max, P. patens and M. truncatula using Clustal Omega (Sievers et al. 2011). The protein sequence alignments were visualized in ESPript 3.0 (http://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi) (Robert and Gouet 2014).
Chromosomal localization and gene duplication analysis of CqGST genes
The genomic locations of the CqGST genes were retrieved from the genomic data (Jarvis et al. 2017). The locations of these genes were diagrammatically depicted on their respective chromosomes using online TBtools software v0.667 (https://github.com/CJ-Chen/TBtools). Gene duplication events were analysed by pBLAST search of CqGSTs against each other on NCBI pBLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi?PAGE=Proteins). The CqGSTs having sequence similarity > 80% were assumed to be duplicated genes (Kong et al. 2013). The homologous pair genes present on same chromosome were considered as tandem duplicated (TD), while those located at different chromosomal locations were depicted as segmental duplicated (SD) genes (Holub et al. 2001). PAL2NAL online tool (https://bio.tools/pal2nal) (Suyama et al. 2006) was used for the estimation of synonymous rate (dS), non-synonymous rate (dN), and evolutionary constraint (dN/dS) between the duplicated CqGST gene pairs. This was carried out using sequences that were aligned using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/) along with their respective mRNA sequences. dN/dS ratio is the basis of mode of selection between duplicated genes. Positive, neutral and purifying selection was considered based on the values > 1, = 1 and < 1, respectively. The divergence time T (million years ago i.e., Mya) of each duplicated gene pair was calculated using the formula: (T = dS/2λ), where T is divergence time, dS is the number of synonymous substitutions per site, and λ is the fixed rate of 1.5 × 10–8 synonymous substitutions per site per year for dicotyledonous plants (Koch et al. 2000).
Protein secondary structure prediction
The components of protein secondary structure like random coil, beta turn, extended strand and alpha helix of CqGSTs were predicted using Self-Optimized Prediction Method with Alignment (SOPMA), a secondary structure prediction tool (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html) (Combet et al. 2000).
Evolutionary or phylogenetic analysis
The evolutionary relationship of CqGST proteins was carried out with P. patens (a bryophyte), A. thaliana (an angiosperm), O. sativa, G. max, M. truncatula GST proteins in MEGA 7 (Molecular Evolutionary Genetics Analysis) (Kumar et al. 2016) using maximum likelihood method. Bootstrap analysis was performed with 1000 replicates for the accuracy of the constructed tree.
Promoter analysis in CqGST genes
In order to analyze cis acting elements in promoter region, 2000 base pair upstream genomic sequences of initiation codon (ATG) of the identified CqGSTs were retrieved from Chenopodium database (https://www.cbrc.kaust.edu.sa/chenopodiumdb/). To identify various elements, the extracted genomic sequences were subjected to online software PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) (Lescot et al. 2002).
Homology modelling and molecular docking analysis
One member from each GST family was selected for docking against the acylalanine fungicide, metalaxyl (methyl N-(methoxyacetyl)-N-(2,6-xylyl)-dl-alaninate) which is used to control downy mildew infestation in a number of crops, including quinoa (Danielsen et al. 2003). Docking was not performed for metaxin and EF1B since the catalytic residue was not predicted for these two families. Homology modelling of protein was done by using I-TASSER (Zheng et al. 2021) (https://zhanggroup.org/I-TASSER/). It selects template having best identity from protein data bank hit and gives predicted model. The 3D structure of the ligand was downloaded from PubChem database (http://www.pubchem.ncbi.nlm.nih.gov) in SDF format. These structures were further converted into PDB file format using the PyMol tool and used for docking studies with identified protein members of quinoa. Molecular docking study was carried out using AutoDock v.4 tool (Morris et al. 2009).
Results
Quinoa GST genes
Comprehensive searches using Phytozome database led to the identification of total 120 GST genes in quinoa. To classify GSTs, all protein sequences were retrieved from Phytozome and analyzed through SMART, Pfam and NCBI Batch CD search database. A total of 120 full length GST genes containing both the domain were named as CqGSTs and classified into 11 different classes: tau (65 members), phi (19 members), lambda (2 members), theta (3 members), zeta (4 members), DHAR (2 members), hemerythrin (4 members), metaxin (2 members), mPGES (6 members), EF1B (7 members), GHR (6 members). Plant GSTs tau and phi were highest in numbers. All the classes of CqGSTs were named CqGSTU, CqGSTF, CqGSTL, CqGSTT, CqGSTZ, CqDHAR, CqGSTH, CqGSTM, CqGSTmi, CqGSTEF1B and CqGSTGHR. The numbering for each member of the class was done based on their chromosomal localization in the ascending order (Table 1).
Table 1.
List of identified GST members in Chenopodium quinoa along with their detailed genomic information and physicochemical features
| Sr no. | Locus ID | Gene name | Chr. no. | Start | End | Base pair | Strand | Protein (aa) | pI | Mol.Wt. (kDa) | GRAVY | Aliphatic\Index |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | AUR62037578 | CqGSTU1 | Chr05 | 22,258,067 | 22,265,792 | 7725 | Forward | 229 | 5.64 | 24,913.84 | − 0.4 | 65.76 |
| 2 | AUR62037579 | CqGSTU2 | Chr05 | 22,266,529 | 22,267,465 | 936 | Reverse | 222 | 5.92 | 25,784.89 | − 0.216 | 98.78 |
| 3 | AUR62037930 | CqGSTU3 | Chr05 | 31,850,247 | 31,851,615 | 1368 | Reverse | 172 | 5.21 | 20,184.12 | − 0.459 | 79.24 |
| 4 | AUR62037936 | CqGSTU4 | Chr05 | 32,278,507 | 32,279,315 | 808 | Forward | 155 | 6.21 | 18,104.97 | − 0.166 | 90.65 |
| 5 | AUR62037937 | CqGSTU5 | Chr05 | 32,279,645 | 32,281,429 | 1784 | Reverse | 225 | 5.18 | 26,005.1 | − 0.257 | 90.18 |
| 6 | AUR62037938 | CqGSTU6 | Chr05 | 32,288,793 | 32,295,251 | 6458 | Forward | 387 | 4.97 | 42,858.16 | − 0.055 | 97.26 |
| 7 | AUR62037941 | CqGSTU7 | Chr05 | 32,449,451 | 32,450,850 | 1399 | Forward | 222 | 4.89 | 26,250.07 | − 0.345 | 90.45 |
| 8 | AUR62037944 | CqGSTU8 | Chr05 | 32,654,240 | 32,654,593 | 353 | Forward | 117 | 4.58 | 13,507.64 | 0.144 | 121.62 |
| 9 | AUR62017444 | CqGSTU9 | Chr06 | 43,484,072 | 43,484,836 | 764 | Reverse | 220 | 6 | 24,952 | − 0.125 | 96.5 |
| 10 | AUR62044663 | CqGSTU10 | Chr00 | 45,020,735 | 45,021,025 | 290 | Reverse | 96 | 4.84 | 11,060.67 | − 0.051 | 94.38 |
| 11 | AUR62042823 | CqGSTU11 | Chr06 | 57,771,916 | 57,775,409 | 3493 | Reverse | 220 | 6.84 | 24,997.03 | − 0.138 | 97.45 |
| 12 | AUR62026482 | CqGSTU12 | Chr06 | 66,508,196 | 66,510,448 | 2252 | Reverse | 227 | 4.98 | 26,087.02 | − 0.171 | 85.07 |
| 13 | AUR62026484 | CqGSTU13 | Chr06 | 66,489,506 | 66,492,004 | 2498 | Reverse | 229 | 5.99 | 25,748.75 | − 0.18 | 89.08 |
| 14 | AUR62017445 | CqGSTU14 | Chr06 | 43,533,054 | 43,534,970 | 1916 | Reverse | 210 | 6.83 | 24,091.14 | − 0.039 | 100.24 |
| 15 | AUR62026483 | CqGSTU15 | Chr06 | 66,493,346 | 66,495,958 | 2612 | Reverse | 229 | 4.87 | 26,220.13 | − 0.187 | 92.05 |
| 16 | AUR62042824 | CqGSTU16 | Chr06 | 57,779,096 | 57,780,971 | 1875 | Forward | 222 | 5.54 | 25,364.37 | − 0.112 | 90.45 |
| 17 | AUR62035903 | CqGSTU17 | Chr07 | 24,669,985 | 24,671,288 | 1303 | Forward | 224 | 5.43 | 26,261.42 | − 0.176 | 96.56 |
| 18 | AUR62035904 | CqGSTU18 | Chr07 | 24,672,990 | 24,673,832 | 842 | Forward | 149 | 6.3 | 17,470.16 | − 0.317 | 79.87 |
| 19 | AUR62001302 | CqGSTU19 | Chr07 | 98,865,582 | 98,867,040 | 1458 | Reverse | 235 | 6.07 | 27,207.71 | − 0.319 | 92.64 |
| 20 | AUR62001701 | CqGSTU20 | Chr07 | 103,459,631 | 103,460,839 | 1208 | Reverse | 202 | 6.54 | 23,733.44 | − 0.39 | 89.26 |
| 21 | AUR62025397 | CqGSTU21 | Chr07 | 106,343,591 | 106,346,764 | 3173 | Forward | 161 | 5.02 | 18,404.18 | − 0.111 | 96.21 |
| 22 | AUR62025473 | CqGSTU22 | Chr07 | 107,763,570 | 107,765,588 | 2018 | Forward | 223 | 5.58 | 25,745.54 | − 0.473 | 87.44 |
| 23 | AUR62021697 | CqGSTU23 | Chr08 | 8,943,013 | 8,945,332 | 2319 | Forward | 347 | 7.63 | 38,973.37 | − 0.131 | 95.19 |
| 24 | AUR62021696 | CqGSTU24 | Chr08 | 8,946,584 | 8,947,569 | 985 | Forward | 226 | 6.42 | 26,211.54 | − 0.25 | 105.18 |
| 25 | AUR62021695 | CqGSTU25 | Chr08 | 8,966,413 | 8,970,065 | 3652 | Reverse | 228 | 8.28 | 26,524 | − 0.232 | 91.45 |
| 26 | AUR62021692 | CqGSTU26 | Chr08 | 9,050,946 | 9,051,821 | 875 | Forward | 200 | 5.9 | 23,015.75 | − 0.149 | 106.25 |
| 27 | AUR62021691 | CqGSTU27 | Chr08 | 9,068,011 | 9,069,374 | 1363 | Forward | 230 | 6.54 | 26,555.9 | − 0.24 | 91.52 |
| 28 | AUR62021690 | CqGSTU28 | Chr08 | 9,072,050 | 9,075,464 | 3414 | Reverse | 348 | 6.11 | 40,313.64 | − 0.261 | 93.56 |
| 29 | AUR62021689 | CqGSTU29 | Chr08 | 9,077,561 | 9,078,839 | 1278 | Forward | 232 | 5.74 | 26,702.89 | − 0.316 | 98.32 |
| 30 | AUR62021688 | CqGSTU30 | Chr08 | 9,080,067 | 9,081,578 | 1511 | Forward | 230 | 5.39 | 26,466.62 | − 0.287 | 92.87 |
| 31 | AUR62021687 | CqGSTU31 | Chr08 | 9,085,491 | 9,087,253 | 1762 | Forward | 231 | 5.61 | 26,657.75 | − 0.383 | 90.78 |
| 32 | AUR62018920 | CqGSTU32 | Chr10 | 11,529,705 | 11,531,271 | 1566 | Reverse | 224 | 5.28 | 25,766.81 | − 0.114 | 96.16 |
| 33 | AUR62018919 | CqGSTU33 | Chr10 | 11,531,703 | 11,533,254 | 1551 | Reverse | 214 | 4.96 | 24,449.53 | − 0.057 | 100.61 |
| 34 | AUR62033918 | CqGSTU34 | Chr10 | 47,430,348 | 47,431,888 | 1540 | Reverse | 224 | 5.5 | 25,805.84 | − 0.287 | 89.6 |
| 35 | AUR62035336 | CqGSTU35 | Chr11 | 11,262,390 | 11,263,905 | 1515 | Reverse | 224 | 5.42 | 26,026.11 | − 0.163 | 98.3 |
| 36 | AUR62035335 | CqGSTU36 | Chr11 | 11,336,767 | 11,337,934 | 1167 | Reverse | 149 | 6.52 | 17,588.21 | − 0.462 | 73.36 |
| 37 | AUR62041794 | CqGSTU37 | Chr12 | 34,308,452 | 34,310,039 | 1587 | Forward | 224 | 6.23 | 26,150.29 | − 0.261 | 98.35 |
| 38 | AUR62026287 | CqGSTU38 | Chr14 | 25,331,081 | 25,334,093 | 3012 | Reverse | 197 | 4.75 | 22,448.84 | − 0.072 | 88.17 |
| 39 | AUR62026289 | CqGSTU39 | Chr14 | 25,349,451 | 25,353,218 | 3767 | Reverse | 228 | 5.26 | 25,944.95 | − 0.131 | 96.36 |
| 40 | AUR62026290 | CqGSTU40 | Chr14 | 25,355,749 | 25,356,129 | 380 | Reverse | 126 | 5.01 | 13,945 | − 0.13 | 81.51 |
| 41 | AUR62026291 | CqGSTU41 | Chr14 | 25,369,752 | 25,370,216 | 464 | Reverse | 107 | 5.88 | 12,293.33 | − 0.172 | 101.12 |
| 42 | AUR62037811 | CqGSTU42 | Chr14 | 44,238,894 | 44,240,398 | 1504 | Forward | 219 | 5.3 | 25,413.43 | − 0.295 | 94.75 |
| 43 | AUR62037813 | CqGSTU43 | Chr14 | 44,344,324 | 44,348,405 | 4081 | Forward | 203 | 5.56 | 23,561.36 | − 0.114 | 99.9 |
| 44 | AUR62037814 | CqGSTU44 | Chr14 | 44,358,959 | 44,360,695 | 1736 | Forward | 227 | 8.19 | 25,675.99 | − 0.076 | 96.61 |
| 45 | AUR62025080 | CqGSTU45 | Chr15 | 50,947,192 | 50,947,825 | 633 | Reverse | 114 | 6.15 | 13,639.66 | − 0.455 | 75.26 |
| 46 | AUR62025085 | CqGSTU46 | Chr15 | 50,990,916 | 50,992,018 | 1102 | Forward | 202 | 7.7 | 23,848.52 | − 0.473 | 84.95 |
| 47 | AUR62025088 | CqGSTU47 | Chr15 | 51,029,526 | 51,035,545 | 6019 | Forward | 371 | 5.72 | 42,037.14 | − 0.231 | 88.54 |
| 48 | AUR62025095 | CqGSTU48 | Chr15 | 51,102,015 | 51,111,215 | 9200 | Reverse | 187 | 4.95 | 21,387.47 | − 0.212 | 84.97 |
| 49 | AUR62008398 | CqGSTU49 | Chr16 | 4,065,234 | 4,070,967 | 5733 | Reverse | 429 | 5.29 | 49,549.39 | − 0.236 | 101.72 |
| 50 | AUR62008404 | CqGSTU50 | Chr16 | 4,153,451 | 4,154,576 | 1125 | Forward | 221 | 5.34 | 25,390.38 | − 0.237 | 89.5 |
| 51 | AUR62008405 | CqGSTU51 | Chr16 | 4,162,275 | 4,163,834 | 1559 | Forward | 228 | 5.17 | 26,813.09 | − 0.289 | 96.62 |
| 52 | AUR62008406 | CqGSTU52 | Chr16 | 4,165,237 | 4,166,614 | 1377 | Forward | 232 | 5.22 | 26,717.93 | − 0.18 | 105.86 |
| 53 | AUR62008407 | CqGSTU53 | Chr16 | 4,169,081 | 4,169,886 | 805 | Reverse | 226 | 5.53 | 25,852.93 | − 0.208 | 97.57 |
| 54 | AUR62008408 | CqGSTU54 | Chr16 | 4,171,850 | 4,173,231 | 1381 | Reverse | 234 | 5.91 | 27,070.29 | − 0.344 | 92.52 |
| 55 | AUR62008409 | CqGSTU55 | Chr16 | 4,179,428 | 4,181,751 | 2323 | Reverse | 225 | 5.61 | 26,142.13 | − 0.322 | 92.27 |
| 56 | AUR62008410 | CqGSTU56 | Chr16 | 4,186,653 | 4,188,495 | 1842 | Reverse | 230 | 5.65 | 26,513.68 | − 0.289 | 89.04 |
| 57 | AUR62041790 | CqGSTU57 | Chr17 | 40,148,842 | 40,149,664 | 822 | Forward | 221 | 5.75 | 25,605.64 | − 0.209 | 95.7 |
| 58 | AUR62041791 | CqGSTU58 | Chr17 | 40,150,004 | 40,151,521 | 1517 | Reverse | 225 | 5.34 | 26,205.29 | − 0.274 | 88.44 |
| 59 | AUR62041792 | CqGSTU59 | Chr17 | 40,156,851 | 40,179,701 | 22,850 | Forward | 290 | 6.38 | 33,610.25 | − 0.592 | 75.31 |
| 60 | AUR62008765 | CqGSTU60 | Chr17 | 66,283,593 | 66,287,858 | 4265 | Forward | 236 | 6.27 | 26,596.27 | − 0.813 | 80.59 |
| 61 | AUR62008847 | CqGSTU61 | Chr17 | 67,490,100 | 67,491,129 | 1029 | Reverse | 222 | 5.39 | 25,660.54 | − 0.285 | 92.79 |
| 62 | AUR62008848 | CqGSTU62 | Chr17 | 67,491,834 | 67,492,999 | 1165 | Reverse | 223 | 5.36 | 26,018.82 | − 0.35 | 86.91 |
| 63 | AUR62025684 | CqGSTU63 | Chr18 | 23,464,056 | 23,477,054 | 12,998 | Reverse | 240 | 6.66 | 27,708.19 | − 0.363 | 92.75 |
| 64 | AUR62020080 | CqGSTU64 | Chr18 | 30,540,853 | 30,542,115 | 1262 | Forward | 220 | 6.85 | 25,895.92 | − 0.392 | 82.86 |
| 65 | AUR62020081 | CqGSTU65 | Chr18 | 30,542,691 | 30,544,606 | 1915 | Forward | 221 | 6.91 | 25,739.75 | − 0.309 | 89.1 |
| 1 | AUR62026020 | CqGSTF1 | Chr07 | 80,504,079 | 80,505,139 | 1060 | Forward | 216 | 7.74 | 24,561.57 | − 0.161 | 86.71 |
| 2 | AUR62033160 | CqGSTF2 | Chr07 | 94,403,967 | 94,405,978 | 2011 | Forward | 217 | 5.5 | 24,562.2 | − 0.19 | 93.5 |
| 3 | AUR62033161 | CqGSTF3 | Chr07 | 94,433,227 | 94,443,156 | 9929 | Forward | 240 | 6.67 | 27,399.39 | − 0.403 | 88.21 |
| 4 | AUR62033162 | CqGSTF4 | Chr07 | 94,485,894 | 94,489,106 | 3212 | Forward | 214 | 6.24 | 24,012.56 | − 0.265 | 87.52 |
| 5 | AUR62033175 | CqGSTF5 | Chr07 | 95,026,283 | 95,027,659 | 1376 | Reverse | 242 | 5.9 | 27,546.77 | − 0.126 | 91.57 |
| 6 | AUR62033176 | CqGSTF6 | Chr07 | 95,036,182 | 95,036,815 | 633 | Reverse | 184 | 5.56 | 21,203.59 | − 0.15 | 94.51 |
| 7 | AUR62033177 | CqGSTF7 | Chr07 | 95,037,534 | 95,039,279 | 1745 | Reverse | 214 | 5.75 | 24,289.96 | − 0.233 | 93.5 |
| 8 | AUR62033178 | CqGSTF8 | Chr07 | 95,040,874 | 95,041,248 | 374 | Reverse | 124 | 5.34 | 14,487.85 | − 0.091 | 103.95 |
| 9 | AUR62033179 | CqGSTF9 | Chr07 | 95,057,377 | 95,058,945 | 1568 | Reverse | 214 | 5.31 | 24,037.62 | − 0.08 | 83.46 |
| 10 | AUR62033180 | CqGSTF10 | Chr07 | 95,061,685 | 96,064,148 | 1E + 06 | Reverse | 218 | 5.04 | 25,000.56 | − 0.29 | 91.7 |
| 11 | AUR62013858 | CqGSTF11 | Chr10 | 15,176,795 | 15,179,961 | 3166 | Reverse | 223 | 6.12 | 25,280.79 | − 0.409 | 83.5 |
| 12 | AUR62008598 | CqGSTF12 | Chr17 | 62,528,187 | 62,530,381 | 2194 | Forward | 197 | 5.75 | 22,882.2 | − 0.434 | 89.14 |
| 13 | AUR62008599 | CqGSTF13 | Chr17 | 62,534,839 | 62,536,278 | 1439 | Forward | 214 | 5.3 | 24,008.56 | − 0.071 | 82.99 |
| 14 | AUR62008602 | CqGSTF14 | Chr17 | 62,632,018 | 62,638,824 | 6806 | Forward | 430 | 6.32 | 48,830.46 | − 0.209 | 92.14 |
| 15 | AUR62008604 | CqGSTF15 | Chr17 | 62,658,237 | 62,669,988 | 11,751 | Forward | 218 | 5.91 | 24,720.42 | − 0.248 | 83.21 |
| 16 | AUR62008607 | CqGSTF16 | Chr17 | 62,679,846 | 62,681,734 | 1888 | Forward | 214 | 5.91 | 24,108.69 | − 0.19 | 91.17 |
| 17 | AUR62008609 | CqGSTF17 | Chr17 | 62,850,817 | 62,853,467 | 2650 | Forward | 214 | 6.25 | 24,060.62 | − 0.286 | 85.28 |
| 18 | AUR62008610 | CqGSTF18 | Chr17 | 62,859,591 | 62,861,331 | 1740 | Forward | 218 | 5.8 | 24,992.13 | − 0.136 | 100.14 |
| 19 | AUR62008630 | CqGSTF19 | Chr17 | 63,533,471 | 63,533,854 | 383 | Forward | 127 | 8.52 | 15,012.66 | − 0.134 | 99.13 |
| 1 | AUR62040536 | CqGSTL1 | Chr00 | 13,469,432 | 13,473,006 | 3574 | Reverse | 260 | 5.21 | 29,085.43 | − 0.139 | 92.73 |
| 2 | AUR62041623 | CqGSTL2 | Chr01 | 100,372,908 | 100,385,913 | 13,005 | Reverse | 1028 | 6.82 | 113,884.86 | − 0.115 | 84.37 |
| 1 | AUR62010591 | CqGSTT1 | Chr13 | 10,768,543 | 10,777,898 | 9355 | Forward | 230 | 9.22 | 26,192.53 | − 0.188 | 100.52 |
| 2 | AUR62010590 | CqGSTT2 | Chr13 | 10,781,037 | 10,783,997 | 2960 | Forward | 233 | 6.5 | 26,741.66 | − 0.279 | 90.86 |
| 3 | AUR62017234 | CqGSTT3 | Chr16 | 68,183,290 | 68,195,926 | 12,636 | Forward | 292 | 9.14 | 33,000.1 | − 0.276 | 94.93 |
| 1 | AUR62024246 | CqGSTZ1 | Chr06 | 1,069,058 | 1,070,356 | 1298 | Reverse | 229 | 5.14 | 26,235.94 | − 0.335 | 93.28 |
| 2 | AUR62013634 | CqGSTZ2 | Chr10 | 12,347,463 | 12,349,907 | 2444 | Forward | 223 | 5.24 | 25,317.09 | − 0.246 | 85.25 |
| 3 | AUR62032505 | CqGSTZ3 | Chr14 | 42,313,607 | 42,321,587 | 7980 | Forward | 242 | 8.44 | 27,732.89 | − 0.404 | 92.81 |
| 4 | AUR62004214 | CqGSTZ4 | Chr01 | 116,540,900 | 116,543,258 | 2358 | Reverse | 224 | 5.36 | 25,201.91 | − 0.205 | 83.57 |
| 1 | AUR62035961 | CqGSTDHAR1 | Chr04 | 22,089,257 | 22,094,551 | 5294 | Reverse | 174 | 5.95 | 19,123.12 | − 0.03 | 97.47 |
| 2 | AUR62022530 | CqGSTDHAR2 | Chr01 | 93,473,248 | 93,477,932 | 4684 | Reverse | 212 | 6.09 | 23,533.29 | − 0.099 | 96.57 |
| 1 | AUR62008342 | CqGSTH1 | Chr16 | 3,358,572 | 3,371,304 | 12,732 | Forward | 1143 | 6.09 | 131,200.91 | − 0.364 | 77.02 |
| 2 | AUR62021756 | CqGSTH2 | Chr08 | 8,201,575 | 8,214,048 | 12,473 | Reverse | 1120 | 5.8 | 128,670.9 | − 0.376 | 76.5 |
| 3 | AUR62037305 | CqGSTH3 | Chr14 | 10,282,014 | 10,295,553 | 13,539 | Forward | 1246 | 5.7 | 139,397.54 | − 0.275 | 79.26 |
| 4 | AUR62002840 | CqGSTH4 | Chr06 | 19,255,868 | 19,269,917 | 14,049 | Forward | 1247 | 5.81 | 139,438.58 | − 0.288 | 78.97 |
| 1 | AUR62021331 | CqGSTM1 | Chr01 | 16,537,256 | 16,542,557 | 5301 | Reverse | 289 | 5.77 | 32,460.06 | − 0.192 | 87.4 |
| 2 | AUR62033246 | CqGSTM2 | Chr01 | 52,855,100 | 52,861,286 | 6186 | Reverse | 329 | 5.22 | 36,922.8 | − 0.257 | 86.53 |
| 1 | AUR62006187 | CqGSTMi1 | Chr15 | 3,433,426 | 3,442,275 | 8849 | Forward | 363 | 8.2 | 39,964 | − 0.146 | 86.47 |
| 2 | AUR62040567 | CqGSTMi2 | Chr00 | 14,528,356 | 14,539,835 | 11,479 | Forward | 328 | 8.89 | 36,427.4 | − 0.278 | 76.46 |
| 3 | AUR62013931 | CqGSTMi3 | Chr10 | 16,899,006 | 16,906,074 | 7068 | Reverse | 377 | 9.1 | 41,690.79 | − 0.183 | 80 |
| 4 | AUR62037507 | CqGSTMi4 | Chr12 | 26,058,521 | 26,062,960 | 4439 | Forward | 151 | 9.28 | 17,148.39 | 0.35 | 85.83 |
| 5 | AUR62028521 | CqGSTMi5 | Chr17 | 44,448,009 | 44,456,799 | 8790 | Forward | 315 | 8.55 | 34,578.84 | − 0.131 | 85.11 |
| 6 | AUR62004745 | CqGSTMi6 | Chr05 | 61,536,667 | 61,541,109 | 4442 | Reverse | 151 | 9.06 | 17,112.38 | 0.407 | 85.23 |
| 1 | AUR62035146 | CqGSTEF1B1 | Chr03 | 1,343,991 | 1,349,912 | 5921 | Reverse | 225 | 5.78 | 26,332.32 | − 0.794 | 64.49 |
| 2 | AUR62022367 | CqGSTEF1B2 | Chr10 | 27,104,863 | 27,109,452 | 4589 | Reverse | 415 | 5.74 | 47,454.59 | − 0.343 | 78.51 |
| 3 | AUR62022369 | CqGSTEF1B3 | Chr10 | 27,140,328 | 27,159,888 | 19,560 | Reverse | 491 | 6.6 | 55,025.98 | − 0.465 | 74.73 |
| 4 | AUR62034332 | CqGSTEF1B4 | Chr03 | 51,421,029 | 51,424,593 | 3564 | Forward | 416 | 5.79 | 47,231.4 | − 0.354 | 79.5 |
| 5 | AUR62034333 | CqGSTEF1B5 | Chr03 | 51,481,446 | 51,485,168 | 3722 | Forward | 416 | 5.45 | 47,374.58 | − 0.324 | 81.83 |
| 6 | AUR62042585 | CqGSTEF1B6 | Chr10 | 58,953,083 | 58,953,451 | 368 | Reverse | 122 | 4.59 | 14,489.55 | − 0.481 | 67.05 |
| 7 | AUR62042586 | CqGSTEF1B7 | Chr10 | 58,955,993 | 58,959,931 | 3938 | Reverse | 311 | 8.83 | 34,887.53 | − 0.221 | 92.15 |
| 1 | AUR62033029 | CqGSTGHR1 | Chr01 | 8,677,338 | 8,680,969 | 3631 | Forward | 329 | 6.56 | 37,869.89 | − 0.488 | 72.61 |
| 2 | AUR62033030 | CqGSTGHR2 | Chr01 | 8,731,951 | 8,736,630 | 4679 | Forward | 348 | 6.27 | 39,855.23 | − 0.437 | 78.48 |
| 3 | AUR62040763 | CqGSTGHR3 | Chr02 | 14,209,838 | 14,214,347 | 4509 | Reverse | 348 | 7.07 | 40,147.73 | − 0.435 | 82.1 |
| 4 | AUR62040764 | CqGSTGHR4 | Chr02 | 14,222,320 | 14,225,704 | 3384 | Reverse | 296 | 6.32 | 33,928.12 | − 0.611 | 69.19 |
| 5 | AUR62008787 | CqGSTGHR5 | Chr17 | 66,538,547 | 66,543,242 | 4695 | Forward | 408 | 8 | 46,176.97 | − 0.257 | 88.6 |
| 6 | AUR62025453 | CqGSTGHR6 | Chr07 | 107,323,805 | 107,335,636 | 11,831 | Forward | 408 | 7.09 | 46,048.76 | − 0.236 | 88.6 |
Subcellular localization analysis
Subcellular localization analysis revealed that most of the proteins were found majorly in cytoplasm. The average protein length of protein was found to be 279.06 with their corresponding average molecular weight of 31,819.4 kDa. The estimated mean isoelectric point (pI) was found to be 6.14. The highest pI value was 9.2 in theta and mPGES class. The grand average of hydropathy values of most of the proteins of all the classes were negative indicating that all the CqGST proteins were hydrophilic having good interaction with water. Mean aliphatic index of proteins was 89.33. The subcellular localization analysis results showed that proteins were centrally localized in the cytoplasm followed by chloroplast, mitochondria, plastid, plasma membrane, nucleus, extracellular, ER and cytoskeleton (Table 2).
Table 2.
Subcellular location of various CqGSTs
| Sr no. | Locus ID | Gene name | Subcellular localization by WoLFPSORT | Subcellular localization by DeepLoc | Subcellular localization by CELLO |
|---|---|---|---|---|---|
| 1 | AUR62037578 | CqGSTU1 | Cytoskeleton | Soluble cytoplasmic | Cytoplasmic |
| 2 | AUR62037579 | CqGSTU2 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 3 | AUR62037930 | CqGSTU3 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 4 | AUR62037936 | CqGSTU4 | Nucleus | Soluble cytoplasmic | Cytoplasmic |
| 5 | AUR62037937 | CqGSTU5 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 6 | AUR62037938 | CqGSTU6 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 7 | AUR62037941 | CqGSTU7 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 8 | AUR62037944 | CqGSTU8 | Cytoplasm | Soluble cytoplasmic | Extracellular |
| 9 | AUR62017444 | CqGSTU9 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 10 | AUR62044663 | CqGSTU10 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 11 | AUR62042823 | CqGSTU11 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 12 | AUR62026482 | CqGSTU12 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 13 | AUR62026484 | CqGSTU13 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 14 | AUR62017445 | CqGSTU14 | Nucleus | Soluble cytoplasmic | Cytoplasmic |
| 15 | AUR62026483 | CqGSTU15 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 16 | AUR62042824 | CqGSTU16 | Nucleus | Soluble cytoplasmic | Cytoplasmic |
| 17 | AUR62035903 | CqGSTU17 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 18 | AUR62035904 | CqGSTU18 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 19 | AUR62001302 | CqGSTU19 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 20 | AUR62001701 | CqGSTU20 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 21 | AUR62025397 | CqGSTU21 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 22 | AUR62025473 | CqGSTU22 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 23 | AUR62021697 | CqGSTU23 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 24 | AUR62021696 | CqGSTU24 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 25 | AUR62021695 | CqGSTU25 | Cytoplasm | Soluble cytoplasmic | Extracellular |
| 26 | AUR62021692 | CqGSTU26 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 27 | AUR62021691 | CqGSTU27 | Mitochondria | Soluble cytoplasmic | Cytoplasmic |
| 28 | AUR62021690 | CqGSTU28 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 29 | AUR62021689 | CqGSTU29 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 30 | AUR62021688 | CqGSTU30 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 31 | AUR62021687 | CqGSTU31 | Mitochondria | Soluble cytoplasmic | Cytoplasmic |
| 32 | AUR62018920 | CqGSTU32 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 33 | AUR62018919 | CqGSTU33 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 34 | AUR62033918 | CqGSTU34 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 35 | AUR62035336 | CqGSTU35 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 36 | AUR62035335 | CqGSTU36 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 37 | AUR62041794 | CqGSTU37 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 38 | AUR62026287 | CqGSTU38 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 39 | AUR62026289 | CqGSTU39 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 40 | AUR62026290 | CqGSTU40 | Chloroplast | Soluble mitochondrial | Cytoplasmic |
| 41 | AUR62026291 | CqGSTU41 | Chloroplast | Soluble cytoplasmic | Mitochondrial |
| 42 | AUR62037811 | CqGSTU42 | Nucleus | Soluble cytoplasmic | Cytoplasmic |
| 43 | AUR62037813 | CqGSTU43 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 44 | AUR62037814 | CqGSTU44 | Nucleus | Soluble cytoplasmic | Chloroplast |
| 45 | AUR62025080 | CqGSTU45 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 46 | AUR62025085 | CqGSTU46 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 47 | AUR62025088 | CqGSTU47 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 48 | AUR62025095 | CqGSTU48 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 49 | AUR62008398 | CqGSTU49 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 50 | AUR62008404 | CqGSTU50 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 51 | AUR62008405 | CqGSTU51 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 52 | AUR62008406 | CqGSTU52 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 53 | AUR62008407 | CqGSTU53 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 54 | AUR62008408 | CqGSTU54 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 55 | AUR62008409 | CqGSTU55 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 56 | AUR62008410 | CqGSTU56 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 57 | AUR62041790 | CqGSTU57 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 58 | AUR62041791 | CqGSTU58 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 59 | AUR62041792 | CqGSTU59 | Cytoplasm | Soluble plastid | Cytoplasmic |
| 60 | AUR62008765 | CqGSTU60 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 61 | AUR62008847 | CqGSTU61 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 62 | AUR62008848 | CqGSTU62 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 63 | AUR62025684 | CqGSTU63 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 64 | AUR62020080 | CqGSTU64 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 65 | AUR62020081 | CqGSTU65 | Cytoskeleton | Soluble cytoplasmic | Cytoplasmic |
| 1 | AUR62026020 | CqGSTF1 | Soluble cytoplasmic | Cytoplasmic | |
| 2 | AUR62033160 | CqGSTF2 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 3 | AUR62033161 | CqGSTF3 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 4 | AUR62033162 | CqGSTF4 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 5 | AUR62033175 | CqGSTF5 | Cytoplasm | Soluble mitochondrial | Cytoplasmic |
| 6 | AUR62033176 | CqGSTF6 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 7 | AUR62033177 | CqGSTF7 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 8 | AUR62033178 | CqGSTF8 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 9 | AUR62033179 | CqGSTF9 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 10 | AUR62033180 | CqGSTF10 | Extracellular | Soluble cytoplasmic | Cytoplasmic |
| 11 | AUR62013858 | CqGSTF11 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 12 | AUR62008598 | CqGSTF12 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 13 | AUR62008599 | CqGSTF13 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 14 | AUR62008602 | CqGSTF14 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 15 | AUR62008604 | CqGSTF15 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 16 | AUR62008607 | CqGSTF16 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 17 | AUR62008609 | CqGSTF17 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 18 | AUR62008610 | CqGSTF18 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 19 | AUR62008630 | CqGSTF19 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 1 | AUR62040536 | CqGSTL1 | Chloroplast | Soluble plastid | Cytoplasmic |
| 2 | AUR62041623 | CqGSTL2 | Plastid | Membrane plastid | Plasma membrane |
| 1 | AUR62010591 | CqGSTT1 | Mitochondria | Soluble nucleus | Cytoplasmic |
| 2 | AUR62010590 | CqGSTT2 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 3 | AUR62017234 | CqGSTT3 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 1 | AUR62024246 | CqGSTZ1 | Mitochondria | Soluble cytoplasmic | Cytoplasmic |
| 2 | AUR62013634 | CqGSTZ2 | Chloroplast | Soluble cytoplasmic | Chloroplast |
| 3 | AUR62032505 | CqGSTZ3 | Mitochondria | Soluble mitochondrial | Mitochondrial |
| 4 | AUR62004214 | CqGSTZ4 | Chloroplast | Soluble cytoplasmic | Chloroplast |
| 1 | AUR62035961 | CqGSTDHAR1 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 2 | AUR62022530 | CqGSTDHAR2 | Cytoplasm | Soluble cytoplasmic | Cytoplasmic |
| 1 | AUR62008342 | CqGSTH1 | Nucleus | Soluble nucleus | Nuclear |
| 2 | AUR62021756 | CqGSTH2 | Nucleus | Soluble nucleus | Nuclear |
| 3 | AUR62037305 | CqGSTH3 | cytoplasm | Soluble nucleus | Nuclear |
| 4 | AUR62002840 | CqGSTH4 | Nucleus | Soluble nucleus | Nuclear |
| 1 | AUR62021331 | CqGSTM1 | Nucleus | Membrane mitochondrial | Plasma membrane |
| 2 | AUR62033246 | CqGSTM2 | Cytoplasm | Membrane mitochondrial | Plasma membrane |
| 1 | AUR62006187 | CqGSTMi1 | Chloroplast | Membrane plastid | Chloroplast |
| 2 | AUR62040567 | CqGSTMi2 | Chloroplast | Membrane mitochondrial | Chloroplast |
| 3 | AUR62013931 | CqGSTMi3 | Chloroplast | Membrane mitochondrial | Chloroplast |
| 4 | AUR62037507 | CqGSTMi4 | Cytoplasm | Membrane ER | Plasma membrane |
| 5 | AUR62028521 | CqGSTMi5 | Chloroplast | Membrane plastid | Chloroplast |
| 6 | AUR62004745 | CqGSTMi6 | Cytoplasm | Membrane ER | Plasma membrane |
| 1 | AUR62035146 | CqGSTEF1B1 | Mitochondria | Soluble cytoplasmic | Cytoplasmic |
| 2 | AUR62022367 | CqGSTEF1B2 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 3 | AUR62022369 | CqGSTEF1B3 | Cytoplasm | Soluble nucleus | Chloroplast |
| 4 | AUR62034332 | CqGSTEF1B4 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 5 | AUR62034333 | CqGSTEF1B5 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 6 | AUR62042585 | CqGSTEF1B6 | Chloroplast | Soluble cytoplasmic | Cytoplasmic |
| 7 | AUR62042586 | CqGSTEF1B7 | Cytoplasm | Membrane peroxisome | Cytoplasmic |
| 1 | AUR62033029 | CqGSTGHR1 | Cytoplasm | Soluble cytoplasmic | Mitochondrial |
| 2 | AUR62033030 | CqGSTGHR2 | Mitochondria | Soluble mitochondrial | Mitochondrial |
| 3 | AUR62040763 | CqGSTGHR3 | Chloroplast | Soluble mitochondrial | Mitochondrial |
| 4 | AUR62040764 | CqGSTGHR4 | Cytoplasm | Soluble cytoplasmic | Mitochondrial |
| 5 | AUR62008787 | CqGSTGHR5 | Chloroplast | Soluble plastid | Mitochondrial |
| 6 | AUR62025453 | CqGSTGHR6 | Chloroplast | Soluble plastid | Extracellular |
Gene structure organization
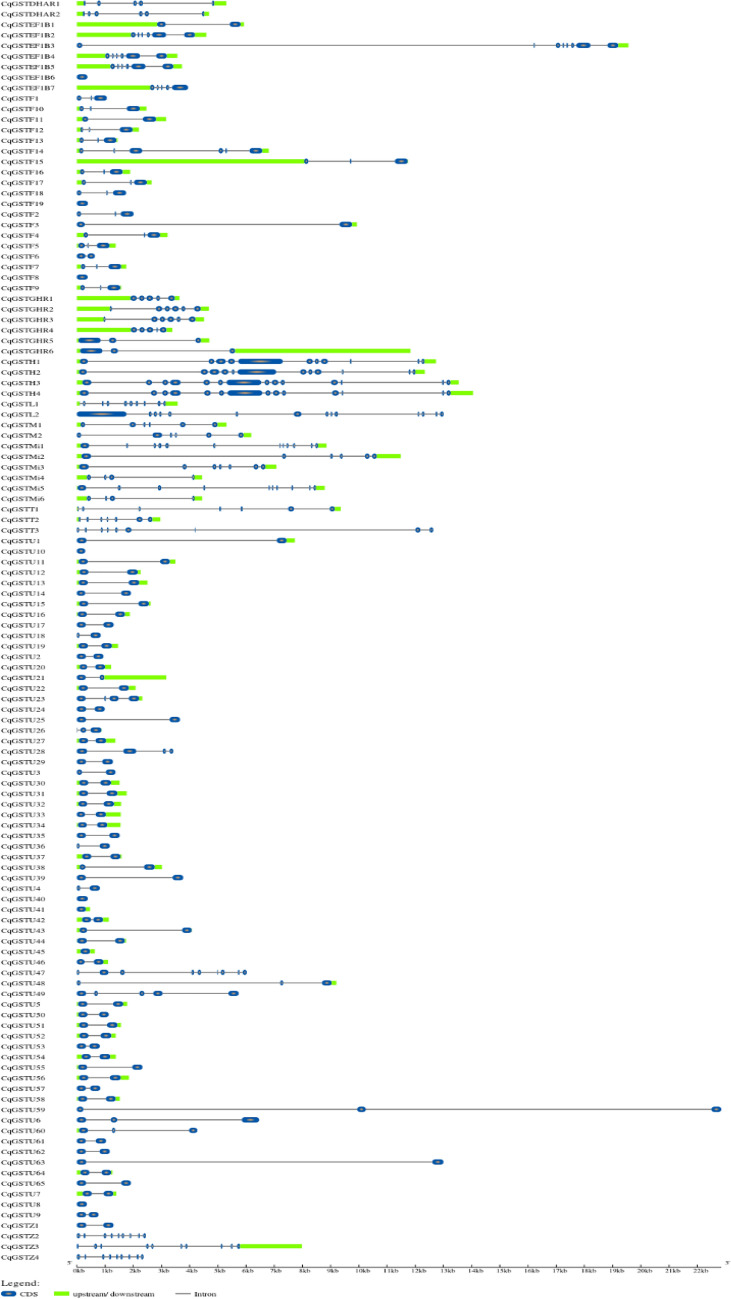
Gene structure organization having number of exons and introns were analyzed using coding and genomic sequences in Gene Structure Display server 2.0 to investigate possible structural evolution of GST gene family. Structural analysis revealed the presence of 2–14 exons in CqGST genes (Fig. 1). Most of the proteins possessed two exon one intron organization. The classes showed greater intron numbers with mixed conservation of splice site sequence.
Fig. 1.
Gene structure analyses of quinoa CqGST genes drawn using GSDS tool. Color description; CDS—blue color, intron—black thread, upstream and downstream—green
Motif analysis
Further, we analyzed quinoa GST proteins for motif discovery via MEME suite (v4.11.2) using parameters, motif discovery mode: Normal; site distribution: 0 or 1 occurrence per sequence; motif length 10–50; number of motifs: 15. Top fifteen motifs based on lowest e-value were identified and selected. Motifs 1, 3, 2, 5, 6, 8, 9 and 13 were found specifically in tau class family, motifs 3, 4, 5, 6, 7 and 9 were found in phi class gene family, while motif 3, 4, 13 and 14 were found in metaxin class. Motif 15 and motif 11 were highly conserved for GHR and EF1B class respectively, while motif 4 was present in almost all classes (Fig. 2).
Fig. 2.
Conserved motif analyses of CqGSTs were identified with MEME suite by inputting complete CqGST protein sequence with 15 motifs. Individual motifs were represented by different colored boxes
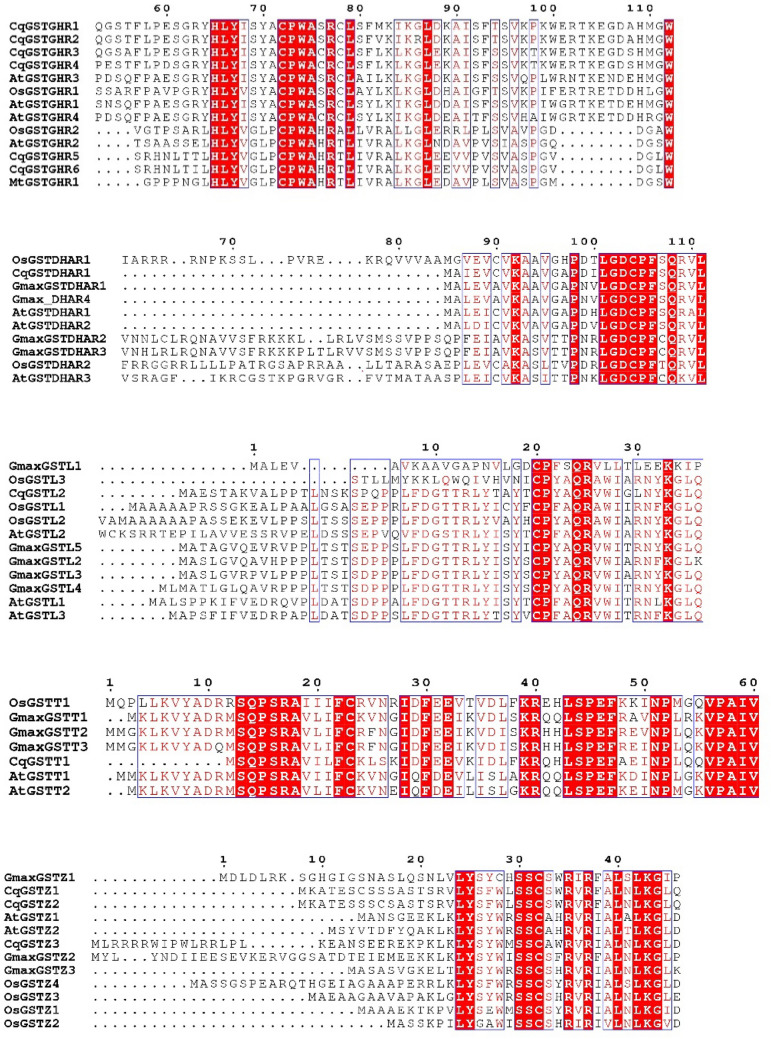
Multiple sequence alignment
The multiple sequence alignment was performed with GST protein sequences of A. thaliana, O. sativa, G. max, P. patens and M. truncatula to identify the conserved and catalytic residue among different GST classes (Fig. 3). It revealed highly conserved N-terminus with active site serine (Ser; S) or cysteine (Cys; C) residue for the activation of GSH binding and GST catalytic activity. Tau, phi, theta and zeta possessed serine active site residues, whereas lambda, GHR, DHAR mPGES had cysteine active site residues.
Fig. 3.
Catalytic residue depiction in CqGST proteins using ESpript. Multiple sequence alignment was performed with A. thaliana, G. max, P. patens, M. truncatula and O. sativa GST sequences. The red colour indicate the active site cysteine in GHR, DHAR and Lambda while serine in theta, zeta and tau CqGSTs
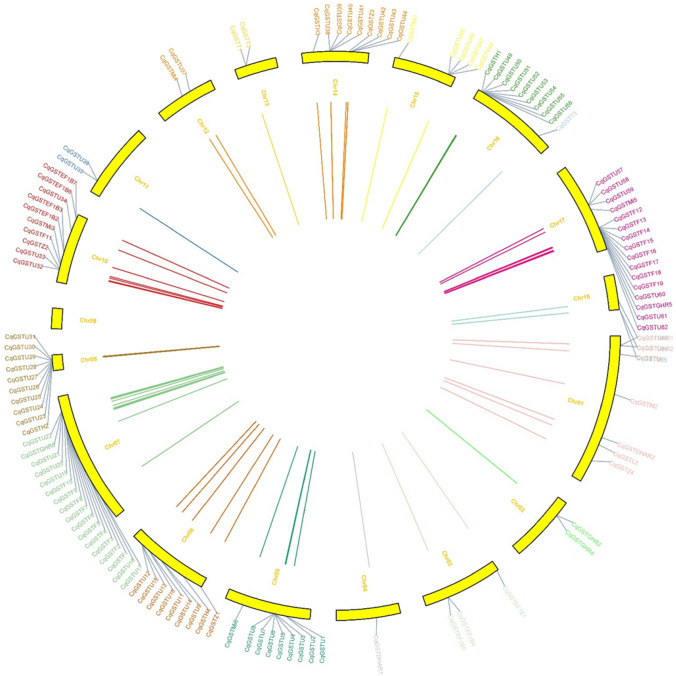
Chromosomal localization and gene duplication analysis of CqGST genes
All 120 CqGST gene loci were found to be unevenly distributed across 18 different chromosomes. Seventeen genes were located on chromosome 7, 16 on chromosome 17, 10 on chromosome 08, chromosome 10 and chromosome 16, 9 genes on chromosome 14, chromosome 5 and chromosome 6. Only two genes were found to be present on chromosomes 2, 11, 12 and 13; and a single gene on chromosome 4. No genes were found on chromosome 9 (Fig. 4). Tandem and segmental duplication play an important role in gene family expansion. Further analysis revealed that segmental duplication and purifying type selection were highest in number and found to be main source of expansion of GST gene family as compared to tandem duplication and positive type selection. We found 92 duplicated gene pairs (Fig. 5) with percent identity of more than 80% against each other using blast search and further calculated the ratio of non-synonymous substitutions and synonymous substitutions (Ka/Ks) for all duplicated CqGST genes to examine that genes were naturally favored or not. Twenty-one duplicated gene pairs were found to be tandem duplicated while 71 were duplicated segmentally. Ka/Ks ratio of gene pairs was less than 1 indicating that non-synonymous substitutions were not favored among duplicated genes and purifying selection was more prevalent. We also calculated the divergence time for these genes. The mean average divergence time for these genes was approximately 9.27 million years ago (MYA) (Table 3). CqGSTU genes were majorly involved in gene duplication event which played a major role in quinoa GST gene family expansion.
Fig. 4.
Chromosomal distribution of 120 CqGST genes on 18 different chromosomes using TB Tool software. CqGST Genes present on different chromosome were represented by different colors. Color description: genes present on chr1—rust orange, chr2—light green, chr3 and chr4—grey, chr5 and chr11—aqua, chr6—brown, chr7 and chr 16—green, chr8—dark brown, chr10—red, chr 12 and chr14—mustard yellow, chr 13 and chr 15—yellow, chr 17—pink and chr 18—sky blue
Fig. 5.
A circular plot depicting duplication of 92 gene pairs present in quinoa with different colors using TB tool software
Table 3.
Estimated Ka/Ks ratios and divergence times of the duplicated CqGST genes
| S no. | Gene name 1 | Chr no. | Gene name 2 | Chr no. | Percent identity | Ka | Ks | Ka/Ks | Duplication time (Mya) | Duplication type | Selection type |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | CqGSTU1 | Chr05 | CqGSTU5 | Chr05 | 89.43% | 0.4158 | 0.7940 | 0.5236 | 26.46 | Tandem | Purifying |
| 2 | CqGSTU1 | Chr05 | CqGSTU58 | Chr17 | 88.62% | 0.4201 | 0.7123 | 0.5898 | 23.74 | Segmental | Purifying |
| 3 | CqGSTU2 | Chr05 | CqGSTU4 | Chr05 | 93.59% | 0.0316 | 0.1078 | 0.2932 | 3.593 | Tandem | Purifying |
| 4 | CqGSTU2 | Chr05 | CqGSTU57 | Chr16 | 94.62% | 0.0321 | 0.0972 | 0.3306 | 3.24 | Segmental | Purifying |
| 5 | CqGSTU5 | Chr05 | CqGSTU58 | Chr17 | 92.04% | 0.0381 | 0.1432 | 0.2663 | 4.77 | Segmental | Purifying |
| 6 | CqGSTU6 | Chr05 | CqGSTU59 | Chr17 | 93% | 0.5209 | 0.9775 | 0.5328 | 32.58 | Segmental | Purifying |
| 7 | CqGSTU8 | Chr05 | CqGSTU37 | Chr11 | 90.48% | 0.1207 | 0.1955 | 0.6178 | 6.51 | Segmental | Purifying |
| 8 | CqGSTU9 | Chr06 | CqGSTU44 | Chr14 | 92.76% | 0.0324 | 0.1090 | 0.2976 | 3.63 | Segmental | Purifying |
| 9 | CqGSTU10 | Chr00 | CqGSTU17 | Chr07 | 100% | 0.0000 | 0.0000 | 0.2035 | 0 | Segmental | Purifying |
| 10 | CqGSTU10 | Chr00 | CqGSTU35 | Chr10 | 92.78% | 0.0303 | 0.1070 | 0.2829 | 3.56 | Segmental | Purifying |
| 11 | CqGSTU12 | Chr06 | CqGSTU38 | Chr12 | 93.79% | 0.0785 | 0.1124 | 0.6981 | 3.74 | Segmental | Purifying |
| 12 | CqGSTU12 | Chr06 | CqGSTU41 | Chr14 | 84.31% | 0.1181 | 0.5356 | 0.2205 | 17.85 | Segmental | Purifying |
| 13 | CqGSTU13 | Chr06 | CqGSTU40 | Chr14 | 92.80% | 0.0418 | 0.1305 | 0.3204 | 4.35 | Segmental | Purifying |
| 14 | CqGSTU13 | Chr06 | CqGSTU41 | Chr14 | 97.14% | 0.0269 | 0.0848 | 0.3170 | 2.82 | Segmental | Purifying |
| 15 | CqGSTU14 | Chr06 | CqGSTU43 | Chr14 | 91.67% | 0.0386 | 0.1567 | 0.2462 | 5.22 | Segmental | Purifying |
| 16 | CqGSTU15 | Chr06 | CqGSTU39 | Chr14 | 92.95% | 0.0363 | 0.1519 | 0.2387 | 5.06 | Segmental | Purifying |
| 17 | CqGSTU17 | Chr07 | CqGSTU35 | Chr10 | 95.96% | 0.0190 | 0.0717 | 0.2653 | 2.39 | Segmental | Purifying |
| 18 | CqGSTU18 | Chr07 | CqGSTU36 | Chr11 | 90.67% | 0.0470 | 0.0334 | 1.4071 | 1.11 | Segmental | Positive |
| 19 | CqGSTU19 | Chr07 | CqGSTU63 | Chr17 | 95.74% | 0.0222 | 0.1866 | 0.1189 | 6.22 | Segmental | Purifying |
| 20 | CqGSTU20 | Chr07 | CqGSTU45 | Chr14 | 92.98% | 0.0350 | 0.0281 | 1.2486 | 0.93 | Segmental | Positive |
| 21 | CqGSTU20 | Chr07 | CqGSTU46 | Chr15 | 94.58% | 0.0255 | 0.0511 | 0.4987 | 1.7 | Segmental | Purifying |
| 22 | CqGSTU20 | Chr07 | CqGSTU48 | Chr15 | 89.74% | 0.3383 | 0.3252 | 1.0404 | 10.84 | Segmental | Positive |
| 23 | CqGSTU20 | Chr07 | CqGSTU64 | Chr18 | 91.13% | 0.0398 | 0.1847 | 0.2155 | 6.15 | Segmental | Purifying |
| 24 | CqGSTU20 | Chr07 | CqGSTU65 | Chr18 | 87.19% | 0.0704 | 0.0754 | 0.9331 | 2.51 | Segmental | Purifying |
| 25 | CqGSTU21 | Chr07 | CqGSTU62 | Chr17 | 93.12% | 0.0374 | 0.0907 | 0.4128 | 3.02 | Segmental | Purifying |
| 26 | CqGSTU22 | Chr07 | CqGSTU60 | Chr17 | 92.91% | 0.3185 | 0.8690 | 0.3665 | 28.97 | Segmental | Purifying |
| 27 | CqGSTU23 | Chr07 | CqGSTU24 | Chr08 | 88.16% | 0.0639 | 0.1998 | 0.3197 | 6.66 | Segmental | Purifying |
| 28 | CqGSTU23 | Chr07 | CqGSTU49 | Chr15 | 87.50% | 0.0788 | 0.2402 | 0.3281 | 8.01 | Segmental | Purifying |
| 29 | CqGSTU24 | Chr08 | CqGSTU49 | Chr15 | 80.34% | 0.0925 | 0.2220 | 0.4166 | 7.40 | Segmental | Purifying |
| 30 | CqGSTU27 | Chr08 | CqGSTU50 | Chr16 | 88.74% | 0.0492 | 0.1251 | 0.3934 | 4.17 | Segmental | Purifying |
| 31 | CqGSTU29 | Chr08 | CqGSTU52 | Chr16 | 88.41% | 0.0642 | 0.1255 | 0.5120 | 4.18 | Segmental | Purifying |
| 32 | CqGSTU30 | Chr08 | CqGSTU31 | Chr08 | 84.48% | 0.0841 | 0.2455 | 0.3426 | 8.18 | Tandem | Purifying |
| 33 | CqGSTU30 | Chr08 | CqGSTU53 | Chr16 | 86.58% | 0.0604 | 0.1497 | 0.4035 | 4.99 | Segmental | Purifying |
| 34 | CqGSTU30 | Chr08 | CqGSTU54 | Chr16 | 82.13% | 0.0943 | 0.3304 | 0.2853 | 11.01 | Segmental | Purifying |
| 35 | CqGSTU31 | Chr08 | CqGSTU53 | Chr16 | 80.17% | 0.0994 | 0.2779 | 0.3576 | 9.26 | Segmental | Purifying |
| 36 | CqGSTU31 | Chr08 | CqGSTU54 | Chr16 | 92.34% | 0.0333 | 0.1984 | 0.1677 | 6.61 | Segmental | Purifying |
| 37 | CqGSTU33 | Chr10 | CqGSTU34 | Chr10 | 87.11% | 0.0654 | 0.0611 | 1.0700 | 2.04 | Tandem | Positive |
| 38 | CqGSTU38 | Chr12 | CqGSTU41 | Chr14 | 92.45% | 0.2519 | 1.2935 | 0.1948 | 43.12 | Segmental | Purifying |
| 39 | CqGSTU45 | Chr15 | CqGSTU46 | Chr15 | 96.49% | 0.0192 | 0.0574 | 0.3342 | 1.91 | Tandem | Purifying |
| 40 | CqGSTU45 | Chr15 | CqGSTU47 | Chr15 | 92.31% | 0.5645 | 0.9946 | 0.5675 | 33.15 | Tandem | Purifying |
| 41 | CqGSTU45 | Chr15 | CqGSTU48 | Chr15 | 93.86% | 0.0365 | 0.0245 | 1.4908 | 0.82 | Tandem | Positive |
| 42 | CqGSTU45 | Chr15 | CqGSTU64 | Chr18 | 91.23% | 0.0427 | 0.1953 | 0.2184 | 6.51 | Segmental | Purifying |
| 43 | CqGSTU45 | Chr15 | CqGSTU65 | Chr18 | 84.21% | 0.0989 | 0.1047 | 0.9445 | 3.49 | Segmental | Purifying |
| 44 | CqGSTU46 | Chr15 | CqGSTU64 | Chr18 | 90.64% | 0.0409 | 0.2245 | 0.1822 | 7.48 | Segmental | Purifying |
| 45 | CqGSTU46 | Chr15 | CqGSTU65 | Chr18 | 86.21% | 0.0719 | 0.1264 | 0.5691 | 4.21 | Segmental | Purifying |
| 46 | CqGSTU46 | Chr15 | CqGSTU48 | Chr15 | 91.45% | 0.3091 | 0.3581 | 0.8631 | 11.94 | Tandem | Purifying |
| 47 | CqGSTU47 | Chr15 | CqGSTU48 | Chr15 | 97.87% | 0.5602 | 0.7139 | 0.7847 | 23.80 | Tandem | Purifying |
| 48 | CqGSTU48 | Chr15 | CqGSTU64 | Chr18 | 88.03% | 0.3132 | 0.6329 | 0.4949 | 21.10 | Segmental | Purifying |
| 49 | CqGSTU48 | Chr15 | CqGSTU65 | Chr18 | 81.20% | 0.3798 | 0.4596 | 0.8264 | 15.32 | Segmental | Purifying |
| 50 | CqGSTU55 | Chr16 | CqGSTU56 | Chr16 | 89.04% | 0.0852 | 0.1898 | 0.4486 | 6.33 | Tandem | Purifying |
| 51 | CqGSTF2 | Chr07 | CqGSTF16 | Chr17 | 96.26% | 0.0175 | 0.0888 | 0.1973 | 2.96 | Segmental | Purifying |
| 52 | CqGSTF3 | Chr07 | CqGSTF4 | Chr07 | 91.39% | 0.2246 | 0.3272 | 0.6866 | 10.91 | Tandem | Purifying |
| 53 | CqGSTF3 | Chr07 | CqGSTF17 | Chr17 | 90.07% | 0.2242 | 0.3650 | 0.6142 | 12.17 | Segmental | Purifying |
| 54 | CqGSTF4 | Chr07 | CqGSTF17 | Chr17 | 97.67% | 0.0115 | 0.1161 | 0.0991 | 3.87 | Segmental | Purifying |
| 55 | CqGSTF5 | Chr07 | CqGSTF7 | Chr07 | 88.43% | 0.0780 | 0.1430 | 0.5455 | 4.77 | Tandem | Purifying |
| 56 | CqGSTF5 | Chr07 | CqGSTF14 | Chr17 | 93.64% | 0.1004 | 0.2293 | 0.4378 | 7.64 | Segmental | Purifying |
| 57 | CqGSTF6 | Chr07 | CqGSTF8 | Chr07 | 95.92% | 0.0314 | 0.0452 | 0.6948 | 1.51 | Tandem | Purifying |
| 58 | CqGSTF6 | Chr07 | CqGSTF19 | Chr17 | 85.71% | 0.0955 | 0.1053 | 0.9065 | 3.51 | Segmental | Purifying |
| 59 | CqGSTF7 | Chr07 | CqGSTF14 | Chr17 | 87.04% | 0.0618 | 0.1391 | 0.4444 | 4.64 | Segmental | Purifying |
| 60 | CqGSTF8 | Chr07 | CqGSTF19 | Chr17 | 83.87% | 0.0887 | 0.1014 | 0.8748 | 3.38 | Segmental | Purifying |
| 61 | CqGSTF9 | Chr07 | CqGSTF13 | Chr17 | 96.28% | 0.0170 | 0.1201 | 0.1411 | 4.00 | Segmental | Purifying |
| 62 | CqGSTF10 | Chr07 | CqGSTF12 | Chr17 | 91.37% | 0.0515 | 0.1630 | 0.3159 | 5.43 | Segmental | Purifying |
| 63 | CqGSTT2 | Chr13 | CqGSTT3 | Chr16 | 80.69% | 0.1201 | 0.3129 | 0.3837 | 10.43 | Segmental | Purifying |
| 64 | CqGSTZ2 | Chr10 | CqGSTZ4 | Chr01 | 95.09% | 0.0176 | 0.1252 | 0.1410 | 4.17 | Segmental | Purifying |
| 65 | CqGSTDHAR1 | Chr04 | CqGSTDHAR2 | Chr01 | 81.13% | 0.0075 | 0.1168 | 0.0643 | 3.89 | Segmental | Purifying |
| 66 | CqGSTH1 | Chr16 | CqGSTH2 | Chr08 | 92.42% | 0.0135 | 0.0597 | 0.2262 | 1.99 | Segmental | Purifying |
| 67 | CqGSTH3 | Chr14 | CqGSTH4 | Chr06 | 98.56% | 0.0071 | 0.0587 | 0.1216 | 1.96 | Segmental | Purifying |
| 68 | CqGSTM1 | Chr01 | CqGSTM2 | Chr01 | 85.20% | 0.0111 | 0.0750 | 0.1474 | 2.50 | Tandem | Purifying |
| 69 | CqGSTMi1 | Chr15 | CqGSTMi5 | Chr17 | 83.52% | 0.0301 | 0.1066 | 0.2829 | 3.55 | Segmental | Purifying |
| 70 | CqGSTMi2 | Chr00 | CqGSTMi3 | Chr10 | 84.13% | 0.0171 | 0.0695 | 0.2463 | 2.32 | Segmental | Purifying |
| 71 | CqGSTMi4 | Chr12 | CqGSTMi6 | Chr05 | 92.76% | 0.0324 | 0.0646 | 0.5008 | 2.15 | Segmental | Purifying |
| 72 | CqGSTEF1B1 | Chr03 | CqGSTEF1B2 | Chr10 | 82.74% | 0.0917 | 0.7587 | 0.1209 | 25.29 | Segmental | Purifying |
| 73 | CqGSTEF1B1 | Chr03 | CqGSTEF1B3 | Chr10 | 83.63% | 0.0851 | 0.8136 | 0.1046 | 27.12 | Segmental | Purifying |
| 74 | CqGSTEF1B1 | Chr03 | CqGSTEF1B4 | Chr03 | 84.07% | 0.0828 | 0.8725 | 0.0949 | 29.08 | Tandem | Purifying |
| 75 | CqGSTEF1B1 | Chr03 | CqGSTEF1B5 | Chr03 | 83.63% | 0.0847 | 0.8571 | 0.0988 | 28.57 | Tandem | Purifying |
| 76 | CqGSTEF1B1 | Chr03 | CqGSTEF1B6 | Chr10 | 99.19% | 0.0036 | 0.1574 | 0.0232 | 5.25 | Segmental | Purifying |
| 77 | CqGSTEF1B1 | Chr03 | CqGSTEF1B7 | Chr10 | 97.00% | 0.1271 | 0.2749 | 0.4622 | 9.16 | Segmental | Purifying |
| 78 | CqGSTEF1B2 | Chr10 | CqGSTEF1B3 | Chr10 | 90.41% | 0.0490 | 0.2030 | 0.2414 | 6.77 | Tandem | Purifying |
| 79 | CqGSTEF1B2 | Chr10 | CqGSTEF1B4 | Chr03 | 91.61% | 0.0437 | 0.2507 | 0.1743 | 8.36 | Segmental | Purifying |
| 80 | CqGSTEF1B2 | Chr10 | CqGSTEF1B5 | Chr03 | 90.41% | 0.0473 | 0.2175 | 0.2175 | 7.25 | Segmental | Purifying |
| 81 | CqGSTEF1B2 | Chr10 | CqGSTEF1B6 | Chr10 | 91.06% | 0.0407 | 0.6499 | 0.0626 | 21.66 | Tandem | Purifying |
| 82 | CqGSTEF1B3 | Chr10 | CqGSTEF1B4 | Chr03 | 98.56% | 0.0066 | 0.1303 | 0.0503 | 4.34 | Segmental | Purifying |
| 83 | CqGSTEF1B3 | Chr10 | CqGSTEF1B5 | Chr03 | 96.40% | 0.0188 | 0.1438 | 0.1306 | 4.79 | Segmental | Purifying |
| 84 | CqGSTEF1B3 | Chr10 | CqGSTEF1B6 | Chr10 | 92.68% | 0.0328 | 0.7165 | 0.0457 | 23.88 | Tandem | Purifying |
| 85 | CqGSTEF1B4 | Chr03 | CqGSTEF1B5 | Chr03 | 96.16% | 0.0186 | 0.1663 | 0.1120 | 5.54 | Tandem | Purifying |
| 86 | CqGSTEF1B4 | Chr03 | CqGSTEF1B6 | Chr10 | 92.62% | 0.0322 | 0.7986 | 0.0404 | 26.62 | Segmental | Purifying |
| 87 | CqGSTEF1B5 | Chr03 | CqGSTEF1B6 | Chr10 | 92.68% | 0.0328 | 0.6440 | 0.0510 | 21.47 | Segmental | Purifying |
| 88 | CqGSTGHR1 | Chr01 | CqGSTGHR2 | Chr01 | 84.66% | 0.0884 | 0.4827 | 0.1832 | 16.09 | Tandem | Purifying |
| 89 | CqGSTGHR1 | Chr01 | CqGSTGHR3 | Chr02 | 83.44% | 0.0998 | 0.4451 | 0.2241 | 14.84 | Segmental | Purifying |
| 90 | CqGSTGHR1 | Chr01 | CqGSTGHR4 | Chr02 | 84.05% | 0.0413 | 0.0960 | 0.4302 | 3.20 | Segmental | Purifying |
| 91 | CqGSTGHR2 | Chr01 | CqGSTGHR3 | Chr02 | 95.42% | 0.0232 | 0.0416 | 0.5579 | 1.39 | Segmental | Purifying |
| 92 | CqGSTGHR5 | Chr17 | CqGSTGHR6 | Chr07 | 97.07% | 0.0130 | 0.0556 | 0.2346 | 1.85 | Segmental | Purifying |
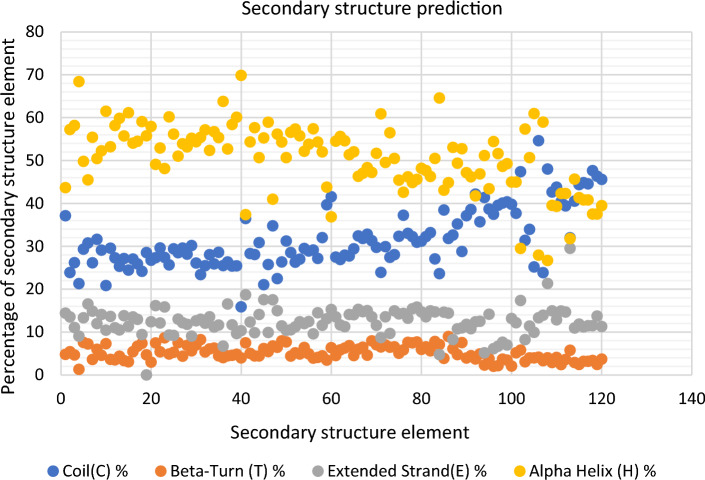
Protein secondary structure prediction
Dominance of alpha helix was followed by coil, extended strand and beta turns. Percentage of alpha helix was found to be higher in all CqGSTs except CqGSTU60, CqGSTZ3, CqGSTMi1, CqGSTMi5, CqGSTEF1B1, CqGSTEF1B2, CqGSTEF1B3, CqGSTEF1B6 and in all GHR genes in which high coil percentage was found. Highest alpha helix and coil percentage was present in CqGSTU40 and in CqGSTMi5 which is 69.84 and 54.6 respectively (Fig. 6) (Table 4).
Fig. 6.
Secondary structure prediction of all CqGSTs using SOPMA showed the dominance of alpha helices followed by coils
Table 4.
Quinoa GSTs secondary structure prediction using SOPMA
| Gene name | Coil (C) (%) | Beta-Turn (T) (%) | Extended strand (E) (%) | Alpha helix (H) (%) |
|---|---|---|---|---|
| CqGSTU1 | 37.12 | 4.8 | 14.41 | 43.67 |
| CqGSTU2 | 23.87 | 5.41 | 13.51 | 57.21 |
| CqGSTU3 | 26.16 | 4.65 | 11.05 | 58.14 |
| CqGSTU4 | 21.29 | 1.29 | 9.03 | 68.39 |
| CqGSTU5 | 29.33 | 7.56 | 13.33 | 49.78 |
| CqGSTU6 | 30.75 | 7.24 | 16.54 | 45.48 |
| CqGSTU7 | 26.13 | 3.6 | 14.86 | 55.41 |
| CqGSTU8 | 31.62 | 5.98 | 11.97 | 50.43 |
| CqGSTU9 | 29.09 | 4.55 | 14.09 | 52.27 |
| CqGSTU10 | 20.83 | 7.29 | 10.42 | 61.46 |
| CqGSTU11 | 29.55 | 3.64 | 13.64 | 53.18 |
| CqGSTU12 | 27.31 | 3.52 | 11.01 | 58.15 |
| CqGSTU13 | 25.33 | 4.37 | 10.48 | 59.83 |
| CqGSTU14 | 27.14 | 3.33 | 13.81 | 55.71 |
| CqGSTU15 | 24.45 | 3.06 | 11.35 | 61.14 |
| CqGSTU16 | 27.03 | 5.41 | 13.51 | 54.05 |
| CqGSTU17 | 25.89 | 6.7 | 12.95 | 54.46 |
| CqGSTU18 | 24.16 | 7.38 | 9.4 | 59.06 |
| CqGSTU19 | 28.51 | 4.68 | 11..06 | 55.74 |
| CqGSTU20 | 26.73 | 2.97 | 12.38 | 57.92 |
| CqGSTU21 | 27.33 | 7.45 | 16.15 | 49.07 |
| CqGSTU22 | 29.6 | 5.38 | 12.11 | 52.91 |
| CqGSTU23 | 27.38 | 8.65 | 15.85 | 48.13 |
| CqGSTU24 | 25.66 | 4.87 | 9.29 | 60.18 |
| CqGSTU25 | 29.39 | 5.26 | 9.21 | 56.14 |
| CqGSTU26 | 28.5 | 7.5 | 13 | 51 |
| CqGSTU27 | 29.57 | 4.35 | 12.17 | 53.91 |
| CqGSTU28 | 28.16 | 6.9 | 11.78 | 53.16 |
| CqGSTU29 | 30.17 | 5.6 | 9.05 | 55.17 |
| CqGSTU30 | 26.09 | 6.96 | 12.61 | 54.35 |
| CqGSTU31 | 23.38 | 8.23 | 12.99 | 55.41 |
| CqGSTU32 | 25.45 | 5.36 | 12.05 | 57.14 |
| CqGSTU33 | 28.04 | 6.07 | 13.55 | 52.34 |
| CqGSTU34 | 25.89 | 6.25 | 11.16 | 56.7 |
| CqGSTU35 | 28.57 | 4.46 | 11.61 | 55.36 |
| CqGSTU36 | 25.5 | 4.03 | 6.71 | 63.76 |
| CqGSTU37 | 26.34 | 4.46 | 16.52 | 52.68 |
| CqGSTU38 | 25.38 | 4.57 | 11.68 | 58.38 |
| CqGSTU39 | 25.44 | 4.82 | 9.65 | 60.09 |
| CqGSTU40 | 15.87 | 3.97 | 10.32 | 69.84 |
| CqGSTU41 | 36.45 | 7.48 | 18.69 | 37.38 |
| CqGSTU42 | 28.31 | 5.02 | 12.33 | 54.34 |
| CqGSTU43 | 28.08 | 4.43 | 9.85 | 57.64 |
| CqGSTU44 | 30.84 | 4.41 | 14.1 | 50.66 |
| CqGSTU45 | 21.05 | 6.14 | 17.54 | 55.26 |
| CqGSTU46 | 25.74 | 5.45 | 9.9 | 58.91 |
| CqGSTU47 | 34.77 | 6.74 | 17.52 | 40.97 |
| CqGSTU48 | 22.46 | 6.42 | 14.97 | 56.15 |
| CqGSTU49 | 26.34 | 7.93 | 11.42 | 54.31 |
| CqGSTU50 | 31.22 | 7.69 | 10.41 | 50.68 |
| CqGSTU51 | 28.51 | 4.39 | 10.53 | 56.58 |
| CqGSTU52 | 26.29 | 5.17 | 11.21 | 57.33 |
| CqGSTU53 | 26.99 | 4.87 | 12.39 | 55.75 |
| CqGSTU54 | 29.49 | 6.41 | 11.97 | 52.14 |
| CqGSTU55 | 28.89 | 4.89 | 12.44 | 53.78 |
| CqGSTU56 | 29.13 | 3.91 | 9.57 | 57.39 |
| CqGSTU57 | 27.15 | 4.07 | 14.48 | 54.3 |
| CqGSTU58 | 32 | 4.44 | 11.56 | 52 |
| CqGSTU59 | 39.66 | 3.45 | 13.1 | 43.79 |
| CqGSTU60 | 41.53 | 6.36 | 15.25 | 36.86 |
| CqGSTU61 | 27.48 | 4.5 | 13.51 | 54.5 |
| CqGSTU62 | 26.91 | 5.83 | 11.66 | 55.61 |
| CqGSTU63 | 27.92 | 6.25 | 11.25 | 54.58 |
| CqGSTU64 | 27.73 | 6.82 | 14.09 | 51.36 |
| CqGSTU65 | 29.41 | 4.52 | 14.03 | 52.04 |
| CqGSTF1 | 32.41 | 6.02 | 15.28 | 46.3 |
| CqGSTF2 | 31.8 | 6.45 | 14.75 | 47 |
| CqGSTF3 | 32.8 | 4.58 | 15 | 48.33 |
| CqGSTF4 | 31.31 | 7.94 | 13.55 | 47.2 |
| CqGSTF5 | 29.75 | 7.02 | 11.57 | 51.65 |
| CqGSTF6 | 23.91 | 6.52 | 8.7 | 60.87 |
| CqGSTF7 | 29.91 | 7.01 | 13.55 | 49.53 |
| CqGSTF8 | 27.42 | 6.45 | 9.68 | 56.45 |
| CqGSTF9 | 28.04 | 6.54 | 14.95 | 50.47 |
| CqGSTF10 | 32.32 | 5.05 | 14.22 | 45.41 |
| CqGSTF11 | 37.22 | 5.83 | 14.35 | 42.6 |
| CqGSTF12 | 32.99 | 7.61 | 13.2 | 46.19 |
| CqGSTF13 | 32.24 | 7.48 | 15.42 | 44.86 |
| CqGSTF14 | 30.93 | 7.67 | 15.81 | 45.58 |
| CqGSTF15 | 31.19 | 5.96 | 14.68 | 48.17 |
| CqGSTF16 | 32.24 | 6.54 | 13.55 | 47.66 |
| CqGSTF17 | 33.18 | 5.61 | 14.95 | 46.26 |
| CqGSTF18 | 27.06 | 7.8 | 14.68 | 50.46 |
| CqGSTF19 | 23.62 | 7.09 | 4.72 | 64.57 |
| CqGSTL1 | 38.46 | 3.85 | 14.62 | 43.08 |
| CqGSTL2 | 31.81 | 8.95 | 14.4 | 44.84 |
| CqGSTT1 | 32.61 | 6.09 | 8.26 | 53.04 |
| CqGSTT2 | 35.19 | 4.72 | 10.73 | 49.36 |
| CqGSTT3 | 28.77 | 7.53 | 10.96 | 52.74 |
| CqGSTZ1 | 37.12 | 3.93 | 11.79 | 47.16 |
| CqGSTZ2 | 38.57 | 4.48 | 10.76 | 46.19 |
| CqGSTZ3 | 42.15 | 3.72 | 12.4 | 41.74 |
| CqGSTZ4 | 35.71 | 4.91 | 12.5 | 46.88 |
| CqGSTDHAR1 | 41.38 | 2.3 | 5.17 | 51.15 |
| CqGSTDHAR2 | 38.68 | 3.77 | 14.15 | 43.4 |
| CqGSTH1 | 37.45 | 2.01 | 6.12 | 54.42 |
| CqGSTH2 | 39.55 | 2.14 | 6.7 | 51.61 |
| CqGSTH3 | 40.05 | 3.69 | 7.62 | 48.64 |
| CqGSTH4 | 40.34 | 3.45 | 6.98 | 49.24 |
| CqGSTM1 | 39.79 | 2.08 | 13.15 | 44.98 |
| CqGSTM2 | 37.69 | 5.17 | 12.16 | 44.98 |
| CqGSTMi1 | 47.38 | 5.79 | 17.36 | 29.48 |
| CqGSTMi2 | 31.4 | 3.05 | 8.23 | 57.32 |
| CqGSTMi3 | 33.95 | 3.98 | 11.41 | 50.66 |
| CqGSTMi4 | 25.17 | 3.97 | 9.93 | 60.93 |
| CqGSTMi5 | 54.6 | 4.13 | 13.33 | 27.94 |
| CqGSTMi6 | 23.84 | 3.31 | 13.91 | 58.94 |
| CqGSTEF1B1 | 48 | 4 | 21.33 | 26.67 |
| CqGSTEF1B2 | 42.65 | 2.89 | 14.94 | 39.52 |
| CqGSTEF1B3 | 43.79 | 4.07 | 12.83 | 39.31 |
| CqGSTEF1B4 | 40.38 | 2.4 | 14.9 | 42.31 |
| CqGSTEF1B5 | 39.42 | 3.61 | 14.66 | 42.31 |
| CqGSTEF1B6 | 31.97 | 5.74 | 29.51 | 31.79 |
| CqGSTEF1B7 | 40.51 | 2.89 | 10.93 | 45.66 |
| CqGSTGHR1 | 44.38 | 2.43 | 11.85 | 41.34 |
| CqGSTGHR2 | 44.83 | 3.16 | 11.21 | 40.8 |
| CqGSTGHR3 | 44.54 | 3.16 | 11.49 | 40.8 |
| CqGSTGHR4 | 47.64 | 3.38 | 11.49 | 37.5 |
| CqGSTGHR5 | 46.32 | 2.45 | 13.73 | 37.5 |
| CqGSTGHR6 | 45.59 | 3.68 | 11.27 | 39.46 |
Evolutionary or phylogenetic analysis
To understand evolutionary relationship between GST gene family members, an unrooted phylogenetic tree was generated between quinoa and other species including A. thaliana, O. sativa, G. max, P. patens and M. truncatula. The phylogenetic analysis showed that CqGSTs were grouped into 11 GST classes including tau, phi, theta, zeta, lambda, DHAR, mPGES, GHR, EF1B, metaxin and hemerythrin. In quinoa, tau and phi are the largest class of GSTs with 65 and 19 members respectively followed by EF1B (7 members), GHR and mPGES (6 member each), hemerythrin (4 members), theta (3 members), metaxin, DHAR and lambda (2 member each). The occurrence of tau and phi as major classes of GST gene family is in accordance with GSTs reported in G. max, O. sativa and A. thaliana. All the tau and phi class CqGSTs were found to be closely associated with G. max, O. sativa and A. thaliana. The analysis clearly represents that CqGSTs of specific class clustered with their respective class GSTs with the exception of lambda class which clustered with DHAR and metaxin that clustered with tau and mPGES class GSTs (Fig. 7).
Fig. 7.
Phylogenetic relationship among GST proteins of A. thaliana, G. max, O. sativa, M. truncatula, P. patens and C. quinoa using MEGA 7. A total of 11 different clades were depicted in different colors (Color description: Blue—Tau; Brown—Hemerythrin; Light green—Lambda; Purple—DHAR; Red—GHR; Light blue—Zeta; Yellow—Theta; Orange—EF1B; Dark green—Phi; Grey—Microsomal; Light orange—Metaxin)
Promoter analysis
Various cis- acting regulatory elements (CAREs) are found to be present in promoter region of the genes. This study identified 21 cis-elements which are responsible for various responses including light, hormone, stress, cellular development and other elements. After the core promoters (AT ~ TATA box, TATA box and CAAT box), MYB (395) was highest in number followed by STRE (280), ARE (279), ABRE (231), ERE (160), AAGAA (112), TCT (112), TGA (57), TC rich repeat (52), P box (44), Circadian (36), AE and CCAAT (35), TCCC (29), DRE (28), GARE (27), CTAG (15) and ACA (1). CqGSTL2 (275) and CqGSTU7 (243) possessed highest number of cis-acting elements while CqGSTU4 (49), CqGSTL1 and CqGSTT3 (50) have least (Fig. 8). Presence of highest number of stress responsive element (MYB) can be linked with their role in stress responses.
Fig. 8.
Cis-acting regulatory element in upstream promoter region of CqGSTs using PlantCARE. The scale represents the number of particular CARE elements in corresponding genes. Grey color is indicative of absence of cis acting regulatory elements
Molecular docking
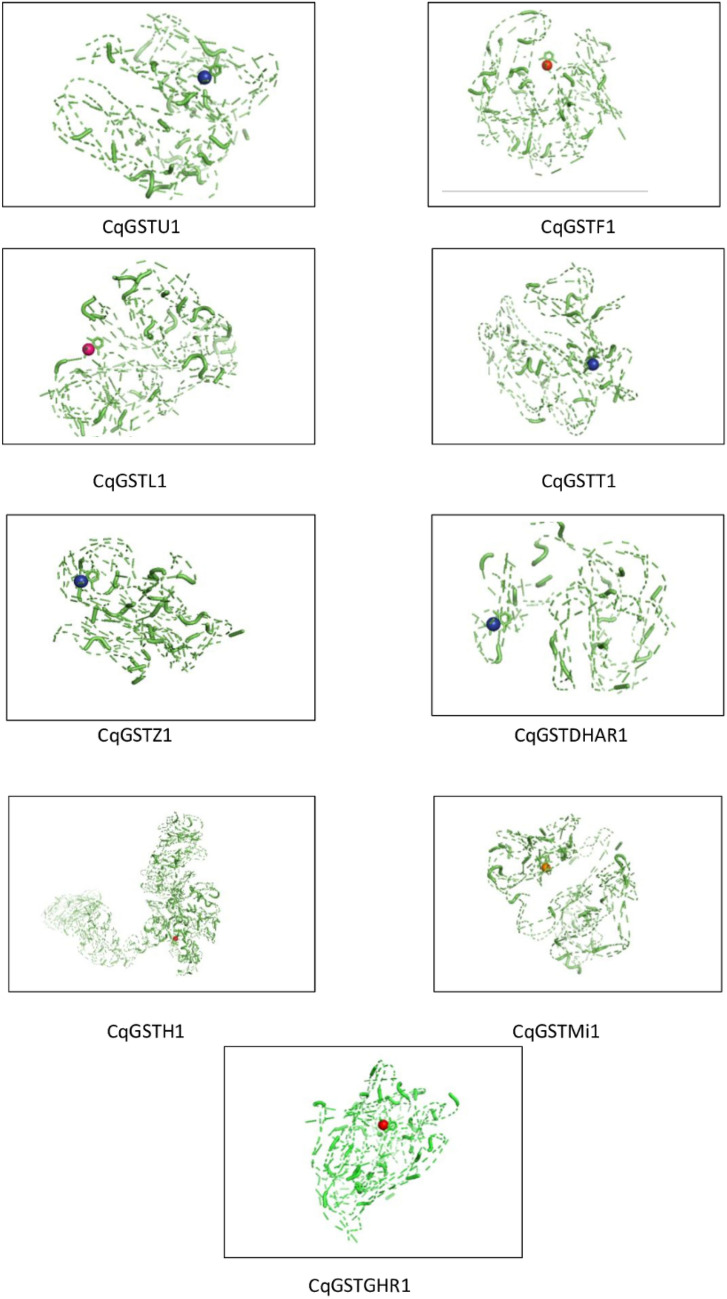
One candidate member from each identified family was selected for molecular docking study with metalaxyl. Docking study of molecules (CqGSTU1, CqGSTF1, CqGSTL1, CqGSTT1, CqGSTZ1, CqGSTDHAR1, CqGSTH1, CqGSTMi1, CqGSTGHR1) showed that metalaxyl binds with CqGSTF1 with lowest binding energy among all (Fig. 9) (Table 5).
Fig. 9.
Molecular docking study of one member of each quinoa GST gene family proteins with metalaxyl
Table 5.
Binding energies of CqGST molecules with metalaxyl
| Protein molecules | Run | Binding energy | Cluster RMSD | Reference RMSD |
|---|---|---|---|---|
| CqGSTU1 | 2 | − 3.63 | 0.00 | 99.10 |
| CqGSTF1 | 25 | − 5.21 | 0.00 | 97.63 |
| CqGSTL1 | 24 | − 4.77 | 0.00 | 90.22 |
| CqGSTT1 | 18 | − 3.25 | 0.00 | 98.47 |
| CqGSTZ1 | 22 | − 2.66 | 0.00 | 93.71 |
| CqGSTDHAR1 | 13 | − 2.52 | 0.00 | 106.64 |
| CqGSTH1 | 10 | − 3.26 | 0.00 | 179.61 |
| CqGSTMi1 | 7 | + 6.7 | 0.00 | 115.18 |
| CqGSTGHR1 | 1 | − 3.7 | 0.00 | 113.82 |
Discussion
GST genes have been found to be involved in various physiological activities in plants that include plant growth and development, signal transduction pathways, tetrapyrrole signaling, hormone responses and importantly in plant’s biotic and abiotic stress metabolism (Csiszár et al. 2019). Therefore, GSTs have become a potential target for plant breeders. The comprehensive genome-wide identification studies of various gene families using the genomic information available at different databases is precise and highly significant (Vaish et al. 2022). In the present investigation, genome wide analysis of GST genes in C. quinoa have been reported for the first time. This study identified 120 GST genes in quinoa that are higher in number as compared to those reported in several other species like 31 in V. radiata (Vaish et al. 2018), 60 in A. thaliana (Lallement et al. 2014a, b), 65 in Brassica oleracea (Vijayakumar et al. 2016), 81 in S. lycopersicum (Csiszár et al. 2014), 82 in O. sativa (Jain et al. 2010), 84 in H. vulgare (Rezaei et al. 2013), 85 in C. annuum L. (Islam et al. 2019) and 101 in G. max (Liu et al. 2015), but fewer than reported in Triticum aestivum and B. napus having 346 and 179 GST genes, respectively (Wei et al. 2019; Hao et al. 2021).
Among the 11 identified classes in quinoa, tau and phi class GSTs were most represented with 65 and 19 members, respectively followed by EF1B (7), GHR and microsomal (6), hemerythrin and zeta (4) and theta (3). Metaxin, DHAR and lambda were represented by 2 members each. The occurrence of tau and phi class was most abundant that was in accordance with those reported for other crops like O. sativa and C. arietinum (Jain et al. 2010; Ghangal et al. 2020). The high number of tau and phi genes reflect their functional importance in plant growth and development, and therefore can be termed as ‘plant specific GST’s’ due to their dominance over other classes (Kumar et al. 2013). The CqGSTs varied in size, sequence and physicochemical parameters like isoelectric point, molecular weight, GRAVY, aliphatic index which were comparable with GSTs of other plant species.
Subcellular location prediction of proteins is vital for having an in-depth understanding of its function and physicochemical characteristics (Liao et al. 2021; Cong et al. 2022). In the present investigation, subcellular localization prediction showed dominance of proteins in cytoplasm, followed by its presence in other cellular compartments including chloroplast, mitochondria, plastid, plasma membrane, nucleus, extracellular, ER and cytoskeleton. The identified GST genes were found to be distributed over 18 chromosomes at different locations. To study the expanded mechanism of GST genes in quinoa, gene duplication events were analyzed which showed that the origin of new members in a gene family was majorly due to gene duplication. The ratio of non-synonymous to synonymous substitutions (Ka/Ks value) < 1 indicates that a gene pair has purifying selection, the Ka/Ks value > 1 indicates positive selection, while Ka/Ks = 1 indicates neutral selection. In the current study, Ka/Ks was less than 1 for most of the genes which was indicative of dominance of purifying selection over positive selection. In quinoa, tandem and segmental duplication were two major driving force for gene family expansion of GST. Chromosome 7 possessed highest seventeen genes while chromosome 4 possessed the lowest i.e., only one CqGST gene. Segmental duplication has major contribution for rapid expansion of GSTs in quinoa as well as reported in other crops like C. annum (Islam et al. 2019), B. napus (Wei et al. 2019), C. arietinum (Ghangal et al. 2020), S. lycopersicum (Islam et al. 2017) and P. bretschneideri (Wang et al. 2018) while tandem duplication was reported as major event for expansion of GSTs in M. accuminata (Vaish et al. 2022), T. aestivum (Hao et al. 2021), B. oleracea and B. rapa (Wei et al. 2019). Secondary structure prediction of GSTs showed highest percentage of alpha helix, followed by coiled and extended strands.
Phylogenetic relationship revealed that all the tau and phi class GSTs were closely associated with those of G. max, O. sativa and A. thaliana. Well-conserved signature motifs were identified in CqGSTs i.e. W(A/V)S(P/M) in tau, SQPS/C in theta, SSCS/A in zeta, CPF/YA in lambda, CPFC/S in DHAR, and CPWA in GHR (Vaish et al. 2020). Similar results have also been reported in S. lycopersicum (Islam et al. 2017), C. annum (Islam et al. 2019) and T. aestivum (Wang et al. 2019a, b). The presence of conserved motifs validates them as GST proteins and confers their functions in plants. There are 2 exons in most of tau class members, 3- 6 in phi, 5–6 in DHAR, 6–8 in EF1B, 3–6 in GHR, 11–14 in hemerythrin, 6 in metaxin, 4–12 in microsomal prostaglandin E synthetase and 2–9 in zeta class. Similar gene organization (2 exons and 1 intron) have also been reported in cotton and mung bean (Dong et al. 2016; Vaish et al. 2018). Structural heterogeneity was observed in almost all classes along with variable number of exons/introns within the members of the same class. A number of previous studies have also reported the presence of multiple introns in zeta and lambda classes, while fewer in tau (Ding et al. 2017; Islam et al. 2017; Kayum et al. 2018; Han et al. 2018; Vaish et al. 2018).
CARE, a specific motif that exists in the promoter region of a gene, has the unique ability to combine with transcription factors and aid in regulation of the downstream genes (Zhu et al. 2021). Thus, the identification and analysis of CAREs in gene promoter regions is of immense utility in understanding the molecular regulation of these genes (Xiaolin et al. 2022). Promoter analysis revealed the presence of different CAREs in response to hormone, light, cellular and stress (Kaur et al. 2017) as well as core promoters including AT ~ TATA box, TATA box and CAAT box (Rahman et al. 2021). Various regulatory elements like ERE motif, GARE motif, ABRE, AAGAA, TCCC, TCT, TGA, MYB, P box were found in the promoter region of CqGSTs that are responsive to light and various hormones like auxin, gibberellins, abscisic acid, thus playing an important role in plant growth and metabolism. The presence of different stress responsive element like STRE, DRE, TC rich repeats confirmed the role of CqGSTs in biotic and abiotic stress. Circadian elements responsible for circadian control were also found in CqGSTs. Sporadic reports are available regarding the role of this element in plant metabolism. Alderete et al. (2018) extensively studied putative circadian regulation while analyzing the NtGST gene (phi) in tobacco seedlings.
Downy mildew caused by Peronospora farinosa (Fr.) Fr. f. sp. chenopodii is a serious disease in quinoa that leads to considerable drop in crop yields across continents (Colque-Little et al. 2021). Metalaxyl, a member of the phenylamides group of systemic fungicides, has been traditionally used for the control of downy mildew in several economically important crops. But there have been reports of health hazards associated with this fungicide (Gupta 2018). Molecular docking study showed that CqGSTF1 possessed highest affinity with metalaxyl, and therefore can be effectively applied to enhance in vivo GST activity in quinoa. Binding of the fungicide metalaxyl with phi family members could be a potential target for enhancing GST activity for detoxification of fungicides. Although safeners induced GST activity has been reported in variety of crops including arabidopsis, maize, and wheat but there is no such report of fungicide induced GST activity is available in quinoa till date. Results of the present study identified metalaxyl as potential GST inducer that could be beneficial for crop development and stress modulation. Availability of this information might encourage researchers for further functional validation.
Conclusion
The in-silico genome wide identification and characterization of GST genes in quinoa assume significance since a number of these assist the plant in their physiological functions, and enables it to grow through biotic and abiotic stresses. Modern biotechnological tools coupled with conventional breeding approaches can help raise plants adapted to various abiotic stresses. These findings can be used for developing stress tolerant plants with enhance productivity through molecular cloning and characterization, as well as their expression studies under stress conditions. The role of GSTs in crop improvement with special emphasis on plant growth and productivity will open new avenues for genetic improvement of plants.
Author contributions
Conceptualization, ST, MB and AB. Methodology, ST, SV, NS and MB. Analysis, ST, SV, MB and AB. Writing- original draft preparation, ST, MB and AB. Writing- review and editing, ST, SV and MB. Supervision, AB. All authors have read and agreed to the published version of the manuscript.
Funding
No funding, financial or non-financial interests were involved.
Data availability
The datasets were derived from sources in the public domain.
Declarations
Conflict of interest
The authors report there are no competing interests to declare.
Ethical approval
Not applicable.
Research involving human participants and/or animals
Not applicable. No human or animal trials were conducted.
Informed consent
Not applicable. No human trials involved.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
References
- Abdul Kayum M, Nath UK, Park J-I, Biswas MK, Choi EK, Song J-Y et al (2018) Genome-wide identification, characterization, and expression profiling of Glutathione S-Transferase (GST) family in pumpkin reveals likely role in cold-stress tolerance. Genes 9:84. 10.3390/genes9020084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderete LGS, Guido ME, Agostini E, Mas P (2018) Identification and characterization of key circadian clock genes of tobacco hairy roots: putative regulatory role in xenobiotic metabolism. Environ Sci Pollut Res Int 25:1597–1608. 10.1007/s11356-017-0579-9 [DOI] [PubMed] [Google Scholar]
- Alvar-Beltrán J, Verdi L, Marta AD, Dao A, Vivoli R, Sanou J et al (2020) The effect of heat stress on quinoa (cv. Titicaca) under controlled climatic conditions. J Agric Sci 158:1–7. 10.1017/S0021859620000556 [Google Scholar]
- Armenteros JJA, Sønderby CK, Sønderby SK, Nielsen H, Winther O (2017) DeepLoc: prediction of protein subcellular localization using deep learning. Bioinformatics 21:3387–3395. 10.1093/bioinformatics/btx431 [DOI] [PubMed] [Google Scholar]
- Bailey TL, Bodén M, Buske FA, Frith M, Grant CE, Clementi L et al (2009) MEME SUITE: tools for motif discovery and searching. Nucleic Acids Res 37(Web Server Issue):202–208. 10.1093/nar/gkp335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava A, Ohri D (2015) Quinoa in the Indian subcontinent. In: Bazile A et al (eds) FAO and CIRAD: state of the art report of Quinoa in the World in 2013. FAO, Rome, pp 511–523 [Google Scholar]
- Chronopoulou E, Kontouri K, Chantzikonstantinou M, Pouliou F, Perperopoulou F, Voulgari G et al (2014) Plant glutathione transferases: structure, antioxidant catalytic function and in planta protective role in biotic and abiotic stress. Curr Chem Biol 8:58–75. 10.2174/2212796809666150302213733 [Google Scholar]
- Colque-Little C, Amby DB, Andreasen C (2021) A review of Chenopodium quinoa (Willd.) diseases—an updated perspective. Plants (basel, Switzerland) 10:1228. 10.3390/plants10061228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combet C, Blanchet C, Geourjon C, Deléage G (2000) NPS@: network protein sequence analysis. TIBS 25:147–150. 10.1016/s0968-0004(99)01540-6 [DOI] [PubMed] [Google Scholar]
- Cong H, Liu H, Cao Y, Chen Y, Liang C (2022) Multiple protein subcellular locations prediction based on deep convolutional neural networks with self-attention mechanism. Interdiscip Sci Comput Life Sci 14:421–438. 10.1007/s12539-021-00496-7 [DOI] [PubMed] [Google Scholar]
- Csiszár J, Horvath E, Vary Z, Galle A, Bela K, Brunner S, Tari I (2014) Glutathione transferase supergene family in tomato: salt stress-regulated expression of representative genes from distinct GST classes in plants primed with salicylic acid. Plant Physiol Biochem 78:15–26. 10.1016/j.plaphy.2014.02.010 [DOI] [PubMed] [Google Scholar]
- Csiszár J, Hecker A, Labrou NE, Schröder P, Riechers DE (2019) Plant glutathione transferases: diverse, multi-tasking enzymes with yet-to-be discovered functions. Front Plant Sci 10:1304. 10.3389/fpls.2019.01304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarnocka W, Karpiński S (2018) Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic Biol Med 122:4–20. 10.1016/j.freeradbiomed.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Czerniawski P, Bednarek P (2018) Glutathione S-transferases in the biosynthesis of sulfur-containing secondary metabolites in Brassicaceae plants. Front Plant Sci 9:1639. 10.3389/fpls.2018.01639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakhili S, Abdolalizadeh L, Hosseini SM, Shojaee-Aliabadi S, Mirmoghtadaie L (2019) Quinoa protein: composition, structure and functional properties. Food Chem 299:125161. 10.1016/j.foodchem.2019.125161 [DOI] [PubMed] [Google Scholar]
- Danielsen S, Bonifacio A, Ames T (2003) Diseases of quinoa (Chenopodium quinoa). Food Rev Int 19:43–59. 10.1081/FRI-120018867 [Google Scholar]
- Ding N, Wang A, Zhang X, Wu Y, Wang R, Cui H et al (2017) Identification and analysis of glutathione S-transferase gene family in sweet potato reveal divergent GST-mediated networks in aboveground and underground tissues in response to abiotic stresses. BMC Plant Biol 17:225. 10.1186/s12870-017-1179-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Li C, Zhang Y, He Q, Daud MK, Chen J, Zhu S (2016) Glutathione S-Transferase gene family in Gossypium raimondii and G. arboreum: Comparative genomic study and their expression under salt stress. Front Plant Sci 7:139. 10.3389/fpls.2016.00139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafián-Labora JA, Rodríguez-Navarro JA, O’Loghlen A (2020) Small extracellular vesicles have GST activity and ameliorate senescence-related tissue damage. Cell Metab 32:71–86. 10.1016/j.cmet.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, An Y, Zheng J, Shangguan L, Wang L (2020) Genome-wide identification and comparative analysis of GST gene family in apple (Malus domestica) and their expressions under ALA treatment. 3 Biotech 10:307. 10.1007/s13205-020-02299-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes FF, Bhargava A (2011) Morphological analysis of quinoa germplasm grown under lowland desert conditions. J Agron Crop Sci 197:124–134. 10.1111/j.1439-037X.2010.00445.x [Google Scholar]
- Gao J, Chen B, Lin H, Liu Y, Wei Y, Chen F, Li W (2020) Identification and characterization of the glutathione S-transferase (GST) family in radish reveals a likely role in anthocyanin biosynthesis and heavy metal stress tolerance. Gene 743:144484. 10.1016/j.gene.2020.144484 [DOI] [PubMed] [Google Scholar]
- Gasteiger E, Hoogland C, Gattiker A, Duvaud S, Wilkins MR, Appel RD et al (2005) Protein identification and analysis tools on the ExPASy server. The proteomics protocols handbook. Springer Protocols Handbooks, Humana Press, pp 571–607. 10.1385/1-59259-890-0:571 [Google Scholar]
- Ghangal R, Rajkumar MS, Garg R et al (2020) Genome-wide analysis of glutathione S-transferase gene family in chickpea suggests its role during seed development and abiotic stress. Mol Biol Rep 47:2749–2761. 10.1007/s11033-020-05377-8 [DOI] [PubMed] [Google Scholar]
- Gupta PK (2018) Toxicity of fungicides. In: Gupta RC (ed) Veterinary toxicology. Academic Press Elsevier, pp 569–580. 10.1016/B978-0-12-811410-0.00045 [Google Scholar]
- Han XM, Yang ZL, Liu YJ, Yang HL, Zeng QY (2018) Genome-wide profiling of expression and biochemical functions of the Medicago, glutathione S-transferase gene family. Plant Physiol Biochem 126:126–133. 10.1016/j.plaphy.2018.03.004 [DOI] [PubMed] [Google Scholar]
- Hao Y, Xu S, Iyu Z, Wang H, Kong L, Sun S (2021) Comparative analysis of the GLUTATHIONE S-transferase gene family of four Triticeae species and transcriptome analysis of GST Genes in common wheat responding to salt stress. Int J Genomics 18:6289174. 10.1155/2021/6289174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan MS, Singh V, Islam S, Islam MS, Ahsan R, Kaundal A et al (2021) Genome-wide identification and expression profiling of glutathione S-transferase family under multiple abiotic and biotic stresses in Medicago truncatula L. PLoS ONE 16(2):e0247170. 10.1371/journal.pone.0247170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holub EB (2001) The arms race is ancient history in Arabidopsis, the wildflower. Nat Rev Genet 2:516–527. 10.1038/35080508 [DOI] [PubMed] [Google Scholar]
- Hong S, Cheon K, Yoo K, Lee H, Cho K, Suh J et al (2017) Complete chloroplast genome sequences and comparative analysis of C. quinoa and C. album. Front Plant Sci 8:1696. 10.3389/fpls.2017.01696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton P, Park K, Obayashi T, Fujita N, Harada H, Adams-Collier CJ et al (2007) WoLF PSORT: protein localization predictor. Nucleic Acids Res 35(Web Server Issue):W585–W587. 10.1093/nar/gkm259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Jin J, Guo A-Y, Zhang H, Luo J, Gao G (2015) GSDS 2.0: an upgraded gene feature visualization server. Bioinformatics 31:1296–1297. 10.1093/bioinformatics/btu817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Rahman IA, Islam T, Ghosh A (2017) Genome-wide identification and expression analysis of glutathione S-transferase gene family in tomato: gaining an insight to their physiological and stress-specific roles. PLoS ONE 12:e0187504. 10.1371/journal.pone.0187504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Sajib SD, Jui ZS, Arabia S, Islam T (2019) Genome-wide identification of glutathione S-transferase gene family in pepper, its classification, and expression profiling under different anatomical and environmental conditions. Sci Rep 9:910. 10.1038/s41598-019-45320-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain M, Ghanashyam C, Bhattacharjee A (2010) Comprehensive expression analysis suggests overlapping and specific roles of glutathione S-transferases during development and stress responses in rice. BMC Genomics 11:73. 10.1186/1471-2164-11-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis DE, Ho YS, Lightfoot DJ, Schmöckel SM, Li B, Borm TJA et al (2017) The genome of Chenopodium quinoa. Nature 542:307–312. 10.1038/nature21370 [DOI] [PubMed] [Google Scholar]
- Kaur A, Pati PK, Pati AM, Nagpal AK (2017) In-silico analysis of cis-acting regulatory elements of pathogenesis-related proteins of Arabidopsis thaliana and Oryza sativa. PLoS ONE 12:e0184523. 10.1371/journal.pone.0184523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MA, Haubold B, Mitchell-Olds T (2000) Comparative evolutionary analysis of chalcone synthase and alcohol dehydrogenase loci in Arabidopsis, Arabis, and related genera (Brassicaceae). Mol Biol Evol 17:1483–1498. 10.1093/oxfordjournals.molbev.a026248 [DOI] [PubMed] [Google Scholar]
- Kong X, Lv W, Jiang S, Zhang D, Cai G, Pan J, Li D (2013) Genome-wide identification and expression analysis of calcium-dependent protein kinase in maize. BMC Genomics 14:433. 10.3389/fpls.2016.00469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Asif MH, Chakrabarty D, Tripathi RD, Dubey RS, Trivedi PK (2013) Differential expression of rice lambda class GST gene family members during plant growth, development, and in response to stress conditions. Plant Mol Biol Rep 31:569–580. 10.1007/s11105-012-0524-5 [Google Scholar]
- Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. 10.1093/molbev/msw054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement PA, Brouwer B, Keech O, Hecker A, Rouhier N (2014a) The still mysterious roles of cysteine-containing glutathione transferases in plants. Front Pharmacol 5:192. 10.3389/fphar.2014.00192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lallement PA, Meux E, Gualberto JM, Prosper P, Didierjean C, Saul F, Haouz A, Rouhier N, Hecker A (2014b) Structural and enzymatic insights into Lambda glutathione transferases from Populus trichocarpa, monomeric enzymes constituting an early divergent class specific to terrestrial plants. Biochem J 462:39–52. 10.1042/BJ20140390 [DOI] [PubMed] [Google Scholar]
- Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y et al (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30:325–327. 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letunic I, Khedkar S, Bork P (2021) SMART: recent updates, new developments and status in 2020. Nucleic Acids Res 49(D1):D458–D460. 10.1093/nar/gkaa937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Fan Y, Zhou G et al (2022) Genome-wide identification, phylogenetic analysis, and expression profiles of trihelix transcription factor family genes in quinoa (Chenopodium quinoa Willd.) under abiotic stress conditions. BMC Genomics 23:499. 10.1186/s12864-022-08726-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Z, Pan G, Sun C, Tang J (2021) Predicting subcellular location of protein with evolution information and sequence-based deep learning. BMC Bioinform 22(Suppl 10):515. 10.1186/s12859-021-04404-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu YJ, Han XM, Ren LL, Yang HL, Zeng QY (2013) Functional divergence of the glutathione S-transferase supergene family in Physcomitrella patens reveals complex patterns of large gene family evolution in land plants. Plant Physiol 161:773–786. 10.1104/pp.112.205815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Liu Y, Yang X, Tong C, Edwards D, Parkin IA et al (2014) The Brassica oleracea genome reveals the asymmetrical evolution of polyploid genomes. Nat Commun 5:3930. 10.1038/ncomms4930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H-J, Tang Z-X, Han X-M, Yang Z-L, Zhang F-M, Yang H-L et al (2015) Divergence in enzymatic activities in the soybean GST supergene family provides new insight into the evolutionary dynamics of whole-genome duplicates. Mol Biol Evol 32:2844–2859. 10.1093/molbev/msv156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Wang R, Liu W, Zhang H, Guo Y, Wen R (2018) Genome-wide characterization of heat-shock protein 70s from Chenopodium quinoa and expression analyses of Cqhsp70s in response to drought stress. Genes 9:35. 10.3390/genes9020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchler Bauer A, Bo Y, Han L, He J, Lanczycki CJ, Lu S et al (2017) CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res 45:D200–D203. 10.1093/nar/gkw1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marimo P, Hayeshi R, Mukanganyama S (2016) Inactivation of glutathione transferase 2 by Epiphyllocoumarin. Biochem Res Int 2016:1–8. 10.1155/2016/2516092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittler R, Vanderauwera S, Gollery M, Breusegem FV (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9:490 [DOI] [PubMed] [Google Scholar]
- Morales A, Zurita-Silva A, Maldonado J, Silva H (2017) Transcriptional responses of Chilean quinoa (Chenopodium quinoa Willd.) under water deficit conditions uncovers ABA-independent expression patterns. Front Plant Sci 8:216. 10.3389/fpls.2017.00216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS et al (2009) AutoDock4 and Auto-DockTools4: automated docking with selective receptor flexibility. J Comput Chem 30:2785–2591. 10.1002/jcc.21256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nianiou-Obeidat I, Madesis P, Kissoudis C, Voulgari G, Chronopoulou E, Tsaftaris A et al (2017) Plant glutathione transferase-mediated stress tolerance: functions and biotechnological applications. Plant Cell Rep 36:791–805. 10.1007/s00299-017-2139-7 [DOI] [PubMed] [Google Scholar]
- Pizzio GA (2022) Genome-wide identification of the PYL gene family in Chenopodium quinoa: from genes to protein 3D structure analysis. Stresses 2:290–307. 10.3390/stresses2030021 [Google Scholar]
- Rahman MM, Rahman MM, Eom JS et al (2021) Genome-wide identification, expression profiling and promoter analysis of trehalose6-phosphate phosphatase gene family in rice. J Plant Biol 64:55–71. 10.1007/s12374-020-09279-x [Google Scholar]
- Rezaei MK, Shobbar ZS, Shahbazi M, Abedini R, Zare S (2013) Glutathione S-transferase (GST) family in barley: identification of members, enzyme activity, and gene expression pattern. J Plant Physiol 170:1277–1284. 10.1016/j.jplph.2013.04.005 [DOI] [PubMed] [Google Scholar]
- Robert X, Gouet P (2014) Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res 42(Web Server Issue):W320–W324. 10.1093/nar/gku316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen DG, Gibson TJ, Karplus K, Li W et al (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7:539. 10.1038/msb.2011.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Zhou F, Shan C, Zhang Q, Ning M, Liu X et al (2021) Identification of glutathione S-transferase genes in hami melon (Cucumis melo var. saccharinus) and their expression analysis under cold stress. Front Plant Sci 12:672017. 10.3389/fpls.2021.672017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34:W609–W612. 10.1093/nar/gkl315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaish S, Awasthi P, Tiwari S, Tiwari SK, Basantani MK (2018) In silico genome-wide identification and characterization of glutathione S-transferase gene family in Vigna radiata (L.) Wilczek. Genome 61:311–322. 10.1139/gen-2017-0192 [DOI] [PubMed] [Google Scholar]
- Vaish S, Gupta D, Mehrotra R, Mehrotra S, Basantani MK (2020) Glutathione S-transferase: a versatile protein family. 3 Biotech 10:321. 10.1007/s13205-020-02312-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaish S, Praveen R, Gupta D, Basantani MK (2022) Genome-wide identification and characterization of glutathione S-transferase gene family in Musa acuminata L. AAA group and gaining an insight to their role in banana fruit development. J Appl Genet 63:609–631. 10.1007/s13353-022-00707-x [DOI] [PubMed] [Google Scholar]
- Vijayakumar H, Thamilarasan SK, Shanmugam A, Natarajan SK, Jung HJ, Park JI et al (2016) Glutathione transferases superfamily: cold-inducible expression of distinct GST genes in Brassica oleracea. Int J Mol Sci 17:1211. 10.3390/ijms17081211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vita F, Ghignone S, Bazihizina N, Rasouli F, Sabbatini L, Kiani-Pouya A et al (2021) Early responses to salt stress in quinoa genotypes with opposite behavior. Physiol Plant 173:1392–1420. 10.1111/ppl.13425 [DOI] [PubMed] [Google Scholar]
- Wang L, Qian M, Wang R et al (2018) Characterization of the glutathione S-transferase (GST) gene family in Pyrus bretschneideri and their expression pattern upon superficial scald development. Plant Growth Regul 86:211–222. 10.1007/s10725-018-0422-4 [Google Scholar]
- Wang C, Xu H, Lin S, Deng W, Zhou J, Zhang Y, Shi Y, Di P, RY (2019a) GPS 5.0: an update on the prediction of kinase-specific phosphorylation sites in proteins. Genom Proteom Bioinform 18:72–80. 10.1016/j.gpb.2020.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ma J, Zhang Q et al (2019b) Genome-wide identification and expression profiling of glutathione transferase gene family under multiple stresses and hormone treatments in wheat (Triticum aestivum L). BMC Genomics 20:986. 10.1186/s12864-019-6374-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Zhu Y, Liu R, Zhang A, Zhu M, Xu W et al (2019) Genome wide identification and comparative analysis of glutathione transferases (GST) family genes in Brassica napus. Sci Rep 9:9196. 10.1038/s41598-019-45744-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiaolin Z, Baoqiang W, Xian W, Xiaohong W (2022) Identification of the CIPK-CBL family gene and functional characterization of CqCIPK14 gene under drought stress in quinoa. BMC Genomics 23:447. 10.1186/s12864-022-08683-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui Y, Hirakawa H, Oikawa T, Toyoshima M, Matsuzaki C, Ueno M et al (2016) Draft genome sequence of an inbred line of Chenopodium quinoa, an allotetraploid crop with great environmental adaptability and outstanding nutritional properties. DNA Res 23:535–546. 10.1093/dnares/dsw037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C-S, Chen Y-C, Lu C-H, Hwang J-K (2006) Prediction of protein subcellular localization. Proteins 64:643–651. 10.1002/prot.21018 [DOI] [PubMed] [Google Scholar]
- Yue H, Chang Xi, Zhi Y, Wang L, Xing G, Song W, Nie X (2019) Evolution and identification of the WRKY gene family in quinoa (Chenopodium quinoa). Genes 10:131. 10.3390/genes10020131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Zhang C, Li Y, Pearce R, Bell EW, Zhang Y (2021) Folding non-homology proteins by coupling deep-learning contact maps with I-TASSER assembly simulations. Cell Rep Methods 1:100014. 10.1016/j.crmeth.2021.100014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Wang B, Wang X, Zhang C, Wei X (2021) Genome-wide identification, characterization and expression analysis of the LIM transcription factor family in quinoa. Physiol Mol Biol Plants 27:787–800. 10.1007/s12298-021-00988-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets were derived from sources in the public domain.