Abstract
Background
The role of central and/or peripheral nervous system dysfunction is basically fundamental in fibromyalgia.
Aim
The aim of this position statement on behalf of the Neuropathic Pain Study Group of the Italian Society of Neurology is to give practical guidelines for the clinical and instrumental assessment of fibromyalgia (FM) in the neurological clinical practice, taking into consideration recent studies.
Methods
Criteria for study selection and consideration were original studies, case-controls design, use of standardized methodologies for clinical practice, and FM diagnosis with ACR criteria (2010, 2011, 2016).
Results
ACR criteria were revised. For diagnostic procedure of small-fiber pathology, 47 studies were totally considered.
Recent diagnostic criteria should be applied (ACR, 2016). A rheumatologic visit seems mandatory. The involvement of small fibers should request at least 2 among HRV + SSR and/or laser-evoked responses and/or skin biopsy and/or corneal confocal microscopy, eventually followed by monitoring of metabolic and/or immunological/ and or/paraneoplastic basis, to be repeated at 1-year follow-up.
Conclusions
The correct diagnostic approach to FM could promote the exclusion of the known causes of small-fiber impairment. The research toward common genetic factors would be useful to promote a more specific therapeutic approach.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10072-023-06836-3.
Keywords: Fibromyalgia, Diagnostic criteria, Small-fiber neuropathy, Laser-evoked potentials, Skin biopsy, Corneal confocal microscopy, Heart rate variability
Introduction
Fibromyalgia (FM) is a complex and common syndrome, whose main clinical element is represented by chronic non-specific pain associated with numerous and various other symptoms, linked to the involvement of the nervous system such as fatigue, insomnia, and emotional distress. Pain in FM is most typically classified as nociplastic; the terminological definition of nociplastic pain was formulated by the IASP in 2017 and defined as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” [1]. The need to classify pain in a new third category originates from the observation of a more elusive subtype of pain with mechanisms that are not yet fully understood, compared to the two classic typologies of pain, nociceptive and neuropathic pain, caused respectively by non-neural tissue damage, producing an activation of nociceptors with a normal functioning of the somatosensory nervous system, and by damage to the somatosensory system [2]. Nonetheless, it is evident that FM patients may experience overlap between the three different types of pain, nociceptive and neuropathic in addition to nociplastic pain [2], even in the presence of normal functioning of the somatosensory nervous system [3]. A more complete understanding of the pathophysiological mechanisms of pain may in the future lead to an overcoming of the current pain classification.
Impaired pain processing, consisting of central pain amplification or decreased pain modulation along the descending pathway, or a combination of these two types of dysfunctions, is the most distinctive element in FM [2]. However, it is currently not clear whether the detectable alterations have a primary or secondary origin at the level of the central or the peripheral nervous system, indeed such alterations being identifiable at both levels. At the same time, a predominantly non-length-dependent neuropathy, of a non-developmental type, frequently accompanies patients with FM [4].
Considering that FM is frequently comorbid with autoimmune pathologies and since in patients with FM there are measurable altered cytokine levels, it has been hypothesized that this syndrome may be due to autoimmune processes, as demonstrated in mice by transfer of purified IgG from individuals with FM [5].
The complex and only partially known pathophysiological basis in addition to diagnostic criteria based only on subjective symptoms in the absence of specific clinical signs and recognized biomarkers means that FM is underdiagnosed and undertreated. For these reasons, an aura of stigma and the burden of inadequate medical and social support still weigh on the affected patients.
The clinical features included in the diagnostic criteria are largely attributable to central and/or peripheral nervous system dysfunction; so far, the role of the neurologist is of evident utility in the diagnosis and clinical management of FM.
The aim of this position statement on behalf of the Neuropathic Pain Study Group of the Italian Society of Neurology (SIN) is to give practical guidelines for the clinical and instrumental assessment of FM in the neurological clinical practice, taking into consideration recent studies.
Methods
This is a position statement on diagnostic assessment of fibromyalgia, based on a not structured review.
Eligibility criteria and search strategy
For clinical criteria, we took into consideration the American College of Rheumatology criteria for adult and childhood fibromyalgia.
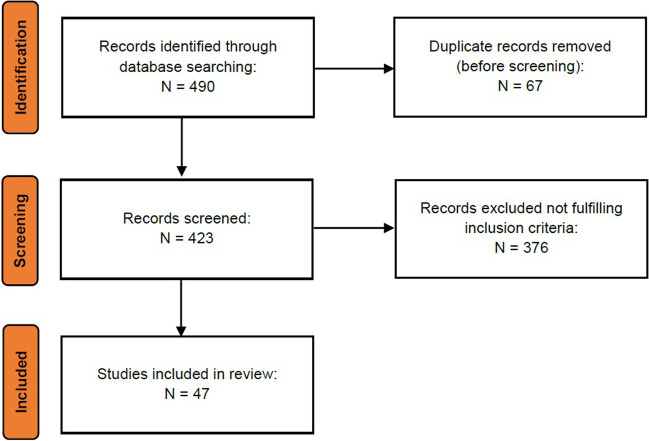
For instrumental assessment, studies on neurophysiological, psychophysical, autonomic, and other instrumental techniques applied to clinical assessment of FM, with special regard to sensory and autonomic neuropathy, were searched on PubMed. The search strings are reported in Table 1. The search was not filtered for study type. Two authors independently assessed studies and any disagreement was planned to be solved by consensus with a third author. Main original data were thus revised. Criteria for study selection and consideration were: original studies, case-controls design, use of standardized methodologies for clinical practice, FM diagnosis with ACR criteria (2010, 2011, 2016). Reviews and case reports were not considered. Figure 1 reports the flow chart showing the total number of identified, screened, included, and excluded records. We thus took into consideration studies in the last 10 years, and summarized publications within 5 years (2018–2022) in tables.
Table 1.
Total number of studies selected with the key words. Main studies are described in detail in the next paragraphs. When a study included more than one technique, it was reported only as the first occurrence
| Instrumental technique | Search string | Retrieved and selected records |
|---|---|---|
| Nerve conduction study | (fibromyalgia) AND (nerve conduction studies OR electroneurography) |
Identified 26 Screened 26 |
| Laser–nociceptive evoked potential or pain-related evoked potentials | (fibromyalgia) AND (laser-evoked potentials or LEP or” nociceptive evoked potentials” or “pain related evoked potentials”) |
Identified 32 Screened 32 |
| Autonomic assessment | (fibromyalgia) AND ("sympathetic dysfunction" OR "autonomic assessment" OR "heart rate variability" OR "Quantitative Sudomotor Axon Reflex Test" OR “QSART” OR Valsalva OR Tilt test OR Sudoscan OR "sympathetic skin response" OR "SSR" NOT SSR [journal]) |
Identified 147 Screened 147 |
| Skin biopsy | (fibromyalgia) AND ("skin biopsy" OR small-fiber neuropathy OR "intraepidermal nerve fiber density" OR IENFD) |
Identified 99 Screened 99 |
| Corneal confocal microscopy | (fibromyalgia) AND ("corneal confocal microscopy" OR CCM OR "corneal innervation") |
Identified 14 Screened 14 |
| Microneurography | (fibromyalgia) AND (microneurography) | Identified 5 |
| Screened 5 | ||
| Included 2 [45, 46] | ||
| Quantitative sensory testing | (fibromyalgia) AND (quantitative sensory testing OR psychophysics OR "QST" NOT QST [journal]) |
Identified 167 Screened 167 |
Fig. 1.
Flow chart showing the total number of identified, screened, included, and excluded records
Search strings and retrieved records are reported separately for each considered instrumental technique in Table 1.
Diagnostic criteria
Fibromyalgia in adult age
The first studies on FM date back to sixteenth century observations, which saw slow progress toward the formulation of recognized diagnostic criteria. The term FM was coined only in 1976, from the union of the words “fibro” and “mio,” to highlight the involvement of the fibrous and muscular tissue and the word “algia,” indicating pain as an essential element for the diagnosis. The first diagnostic criteria were formulated by a scientific society only in 1990, thanks to the lack of prejudices and the farsightedness of the College of Rheumatology (ACR), which, despite the absence of clinical tests to objectify the pathology, believed it could not ignore this condition. The 1990 diagnostic criteria emphasized the role of tender points, tenderness of at least 11 of 18 specific body sites, and the role of diffuse pain defined as pain persisting for at least 3 months in both supra-pelvic and subpelvic regions in addition to axial pain [51].
A strong limitation of these criteria was represented by the fact that at least 25% of patients affected by FM do not fully satisfy the ACR criteria proposed, since in addition to the subjectivity of the response to the pressure exerted, as well as the difficulty for clinicians to apply 4 kg of strength, it has been widely experienced that acupressure tenderness in male subjects appears less evident than in female subjects, leaving many male FM patients undiagnosed. Despite the evident limitations of the first diagnostic criteria, they are considered instrumental in the recognition of the pathology and in its inclusion by the consensus group of the Declaration of Copenhagen (CD) among non-articular rheumatism in the International Classification of Disease of the World Health Organization Healthcare with the ICD code M 79.0 (ICD 10th revision).
Hence, in 2010, the ACR also published the first revision of the criteria, in which the search for the positivity of tender points has seen its diagnostic role disappear, replaced by a greater weight given to subjective symptoms [52]. Subsequently, in 2011, the same authors proposed a modified version of the ACR criteria to allow diagnosis through a self-assessment based on six somatic symptoms considered predominant (non-refreshing sleep, fatigue, cognitive deficits, headache, depression, and abdominal pain) among a list of 41 symptoms proposed in the Symptom Severity Score (SS) of the 2010 ACR criteria to be reserved for research purposes only and to introduce a quantitative measure of disease severity, the Fibromyalgia Severity Scale (FS score, range from 0 to 31) resulting from the sum of the widespread pain index (WPI) score and the modified SS score [53]. The last revision of the ACR criteria dates back to 2016 and changes the definition of widespread pain, defined as present in at least four of five regions (excluding the jaw, chest, and abdomen); specifies somatic symptoms such as headache, pain, or cramps abdominal pain and depression; adds the FS scale, and introduces the criterion according to which the diagnosis of FM should be done regardless of other diagnoses and that the diagnosis of FM must not lead to the exclusion of the presence of other relevant concomitant pathologies [54].
Fibromyalgia in children
Although fibromyalgia is highly prevalent in adulthood, it can be present also in the developmental age. In children and adolescents, its prevalence is poorly studied and varies between 7 and 15% of new visits to pediatric rheumatology clinics [55]. Girls/boys ratio is 4:1 and age at diagnosis ranges from 13 to 15 years, although diagnoses at younger ages are reported [56].
Diagnostic criteria for juvenile fibromyalgia (JFM) were first described in 1985 by Yunus and Masi [57] and subsequently revised in 2010 [58], with peculiarities as compared to the criteria for adulthood. Laboratory and diagnostic tests are often negative [56]. Clinically, JFM is characterized by chronic and widespread pain, which can be spontaneous or evoked by painful or non-painful stimuli. It is associated with fatigue and highly disabling symptoms [59]: headache in two-thirds of cases, abdominal pain [60], sleep disorders, such as periodic limb movement [61], and psychiatric disorders, such as depression and anxiety [62, 63].
Growing pains can be considered precursors of JFM [64].
Diagnostic procedures
In the following paragraph, we summarize the main studies on technical procedures of small-fiber function and autonomic system assessment that fulfilled our inclusion criteria (Fig. 1). Nerve conduction studies-NCS- and electromyography-EMG- Apart from few studies in favor of electroneurography signs compatible with sensory neuropathy [6, 7], nerve conduction study and electromyography are generally within normal limits in FM patients [8-11]. Lawson et al. found a mild sural and medial plantar (MP) response amplitude reduction in those subjects reporting symptoms of neuropathic pain, distal small-fiber neuropathy, and markers of metabolic syndrome [7]. The lack of a previous neurological assessment [6] or the presence of signs of neuropathic pain [7] could suggest cautiousness in interpreting abnormal NCV findings, but their routine execution in the presence of clinical signs of polyneuropathies or pain of neuropathic origin seems strongly recommended.
Laser and nociceptive evoked potentials
Ten studies tested pain-related evoked potentials in patients with FM. Four studies showed increased amplitude of laser-evoked potentials (LEPs) and reduced habituation in patients with FM, supporting an abnormal central elaboration of pain and pain matrix hyper excitability [12-15]. Accordingly, Aδ LEP amplitude, conditioned by a preceding C-fiber LEP, was significantly higher in patients with FM than in healthy controls, supporting the hypothesis of pain matrix hyper excitability [16].
Three studies used pain-related evoked potentials (PREPs) to detect small-fiber pathology in FM with contrasting findings [3, 17, 18]. In a case-control study involving 25 patients with FM, Üçeyler and colleagues reported reduced amplitudes of pain-related evoked potentials elicited by surface concentric electrodes in patients with FM compared to control subjects, supporting abnormalities of small fibers [17]. Two studies, using LEPs, did not confirm Aδ fiber function abnormalities, suggesting that small-fiber pathology negligibly affects somatosensory system function in FM and is not a significant contributor to the pathophysiology of this condition [3, 18]. In the study of Fasolino and colleagues in 57 patients with FM, LEP parameters fell within normative ranges and did not differ in patients with or without small-fiber pathology, detected through skin biopsy [3]. In the study of Van Assche and colleagues, none of the 92 patients enrolled showed signs of loss of function of the nociceptive responses evoked by Aδ fiber activations [18].
Two recent studies assessing multichannel LEPs in patients with FM and small-fiber impairment showed abnormal N2P2 habituation index, more marked in patients with reduced IENFD, and reduced amplitude of the P2 component, not coherent with the site of denervation [11, 19] (Table 2). In summary, laser-evoked potentials reflect abnormal pain processing at both peripheral and central levels in FM, with amplitude amplification occurring in the presence of peripheral denervation. Nevertheless, clear amplitude loss or even the absence of cortical components should be considered as a sign of severe Aδ fiber impairment (Table 2).
Table 2.
Studies about laser evoked potentials (LEPs) in fibromyalgia (FM) and healthy controls (HC) published within the last 5 years. In bracket, disease duration in years is reported, when available
| Authors | Methods | Subjects | Results | Significance |
|---|---|---|---|---|
| De Tommaso et al. (2017) | CO2 laser stimulation | 50 FM (7.2 ± 6.21 y) and 30 HC | Reduced habituation of vertex LEPs | Finding supporting abnormal central elaboration of pain |
| Fasolino et al. (2020) | Nd:YAP laser stimulation | 57 FM (7 ± 8.5 y) | Normal LEP parameters in patients with and without small-fiber pathology | Finding suggesting that small-fiber pathology has a negligible impact on somatosensory system function in fibromyalgia |
| Van Assche et al. (2020) | CO2 laser stimulation | 92 FM 39 HC | Normal LEP parameters | Finding suggesting that small-fiber neuropathy is not a significant contributor to the pathophysiology of fibromyalgia |
| Vecchio et al. (2020) | CO2 laser stimulation | 81 FM (10.69 ± 8.16 y) | Abnormal habituation index and correlation with reduced IENFD at the thigh | Finding suggesting central impairment of pain processing and association with mild proximal small-fiber pathology |
| Vecchio et al. (2022) | CO2 laser stimulation | 37 FM (10.27 ± 7.17 y) (22 patients with proximal denervation, 18 with normal skin biopsy, and 7 with proximal and distal IENFD reduction |
Reduced amplitude of the P2 component, not coherent with the site of denervation Decreased habituation of P2 prevailing in patients with reduced IENFD |
Finding suggesting that LEP abnormalities are not the expression of small-fiber impairment in fibromyalgia |
Sympathetic skin response — SSR — and autonomic assessment (heart rate variability-HRV)
Patients with FM, beside pain, often present a constellation of complaints, like persistent fatigue, gastrointestinal dysmotility, dizziness, and syncope that can be expression of a dysfunction of the autonomic nervous system (ANS). Also, in patients with a history of vasovagal syncope, a higher prevalence of FM compared to the general population has been reported [20]. Different hypotheses have been formulated to explain autonomic disturbances in fibromyalgia, including abnormal hypothalamic–pituitary axis functioning and autonomic small-fiber impairment. Many studies, most of them based on the analysis of heart rate variability (HRV) at rest or following physical challenges, have found ANS dysfunctions in FM [21-28]. The idea has prevailed that FM is characterized by an altered sympatho-vagal balance with an increase in sympathetic and decrease in parasympathetic activity. Few studies assessed sudomotor function in FM.
Sympathetic skin response amplitude in patients is not generally different from controls [15, 29]. However, it correlates with disease severity as assessed by clinical questionnaires [29]. Conversely, SSR latency has been found to be significantly longer in FM compared to controls and the response totally absent in a percent varying from 15 to 18% of patients [15]. Moreover, using electrochemical skin conductance analysis, sudomotor function has been found to be significantly impaired in patients with FM [29]. Autonomic symptoms are more severe in women affected by FM who also complain of more intense pain [27]. Moreover, a significant correlation has been found between the prevalence of autonomic symptoms and the impact of FM on general health status [25].
In conclusion, the data present in literature are strongly oriented toward an association between FM and dysautonomia that further deteriorates patients’ quality of life, so the evaluation of autonomic involvement could offer information on the severity of the disease (Table 3).
Table 3.
Studies about autonomic system assessment with hearth rate variability and sympathetic skin response published within the last 5 years. In bracket, disease duration is reported, when available. FM, fibromyalgia; HC, healthy controls
| Authors | Methods | Subjects | Results | Significance |
|---|---|---|---|---|
| Reyes-Manzano et al. (2018) [23] | Cardiovascular reflexes |
30 FM patients 30 HC (not reported) |
Reduction of multifractality of RR fluctuations | Dysfunction of ANS |
| Pickering et al. (2020) [29] | Electrochemical skin conductance |
50 FM patients 50 HC (12 ± 3 years) |
Reduced ESC values in FM patients | Impaired sudomotor function |
| Hazra et al. (2020) [24] | Cardiovascular reflexes |
50 FM patients 50 HC (42.8 ± 37.1 months) |
Normal parasympathetic activity Sympathetic hyperactivity in FM |
Altered sympatho-vagal balance |
| Singh et al. (2020) [25] | Cardiovascular reflexes |
30 FM patients 30 HC |
Autonomic dysfunction in 40% of FM patients | Dysfunction of ANS |
| Schamne et al. (2021) [26] | Cardiovascular reflexes |
23 FM patients 17 HC (7.7 ± 7.1 years) |
Lower HR max, HR reserve, and chronotropic reserve in FM during exercise | Impaired parasympathetic activity |
| Rost et al. (2021) [27] | Cardiovascular reflexes |
46 FM patients 46 HC (189.9 ± 117.8 months) |
Reduced HRV in FM patients | Impaired parasympathetic activity |
| Sochodolak et al. (2022) [28] | Cardiovascular reflexes |
35 FM patients 17 HC (moderate FM 8.50 ± 6.07 years; severe FM 8.57 ± 6.68 years) |
Reduced HRV in FM patients | Impaired parasympathetic activity |
| Papadopoulou et al. (2022) | SSR |
21 FM patients 28 HC |
SSR latency correlated to pain intensity | Association between pain and autonomic impairment |
Skin biopsy
In the last decade, several studies showed evidence of small-fiber pathology in a variable proportion of patients with FM, with lower intraepidermal nerve fiber density (IENFD) compared with healthy subjects [3, 10, 11, 14, 17, 30-32]. A similar finding has also been reported in JFM [33]. Heterogeneity between studies and, often, small population size prevented conclusive findings. Skin denervation was mostly reported as not length-dependent pattern [3, 17, 34]; however, in a subset of patients, a length-dependent distribution has been described [10, 11], leading to a challenging distinction from the small-fiber neuropathy. The presence of a more severe pain phenotype and anxiety associated with generalized denervation has been also reported [4, 10]. Myelinated dermal fibers have been found to be usually spared [14, 17], although a loss of Meissner corpuscles at fingertips has been observed [14]. In addition to nerve loss, morphological changes such as lower density of regenerating nerve fiber positive to growth-associated protein (GAP) 43 [17] and a reduction of diameter in dermal nerve fibers have also been reported [8]. The observation of increased peptidergic innervation of cutaneous arteriole-venues shunts in the glabrous skin of patients with fibromyalgia [34] suggested a neurogenic microvascular dysfunction that may imply the coexistence of peripheral mechanisms underlying pain [17, 35-38]. Along with cutaneous denervation at proximal leg, an aberrant cutaneous and systemic miRNA expression pattern was found in patients with fibromyalgia [34]. Finally, the finding of reduced mitochondrial chain activities and bioenergetics levels and increased levels of oxidative stress in the skin biopsies of 20 women with FM compared to controls [39] suggests a role of oxidative stress, mitochondrial dysfunction, and inflammation in the pathophysiology of pain in fibromyalgia. (Table 4). Skin biopsy is the elective test for small-fiber pathology. Its evaluation in patients with FM is suggested to ensure small-fiber involvement.
Table 4.
Studies on skin biopsy in fibromyalgia (FM) patients published within the last 5 years. In bracket, disease duration is reported, when available. FM, fibromyalgia; HC, healthy controls
| Authors | Methods | Subjects | Results | Significance |
|---|---|---|---|---|
| Lawson et al. (2018) [31] |
Skin biopsy NCS |
155 FM |
Reduced IENFD 43 patients (28%) at distal site 19 (12%) at proximal site. Sensory amplitudes correlation with distal IENFD |
Identification of subset of FM with small fiber early or mild sensory neuropathy and metabolic disease |
| Evdokimov et al. (2019) [10] | Skin biopsy, corneal confocal microscopy, microneurography, quantitative sensory nociceptive evoked potentials |
117 FM (mean 12 years, range 0.8–56) 54 HC 11 women with major depressive disorder and chronic widespread pain (mean 5 years, range 1–44) |
4 distinct FM subgroups: normal skin innervation (FM 37%, controls: 82%), reduced distal IENFD (FM: 17%, controls: 13%), reduced proximal IENFD (FM: 31%, controls: 2%) proximal and distal reduction in IENFD (FM: 15%, controls: 2%) | Higher pain intensity and anxiety in FM patients with generalized IENFD reduction |
| Evdokimov et al. (2020) [38] |
Skin biopsy Epidermal and dermal nerve quantification |
86 FM (mean 4 years, range 0.5–35) 35 HC |
IENF reduction in 38 FM with reduced nerve length in close proximity to blood vessels | Altered peripheral circulation in FMS patients |
| Evdokimov et Al. (2020) [36] | Skin biopsy (IENFD and primary fibroblast and keratinocyte cell cultures). PCR (gene expression of selected pro-and anti-inflammatory cytokines, nociception-associated ion channels, and axon guidance cues) |
128 FM 26 controls |
In FM, higher expression of TGF-ß1 gene, HCN2, EFNA4, and EPHA4 in fibro-blasts and IL-10 gene in Keratinocytes | Membrane bound and soluble pain mediators and axon pathfinders as contributors to cutaneous nociception in FM |
| Fasolino et al. (2020) [37] |
Skin biopsy QST LEP |
57 FM (mean 5 years, range 2–9.5) | 18 patients with non-length-dependent loss of IENFD but without abnormalities in QST and LEP | Negligible impact of small-fiber pathology on somatosensory system function in fibromyalgia |
| Vecchio et al. (2021) [11] |
Skin biopsy LEP NCS |
81 FM (mean 10.7 years, range 1–20) |
59 FM proximal IENF loss; 10 with IENFD distal and proximal loss No association among IENFD, LEP, clinical features, and comorbidities. Normal NCV |
Central impairment of pain processing and mild proximal small-fiber pathology in the most of FM |
| Bonepart et al. (2021) [32] | Skin biopsy |
15 patients with juvenile fibromyalgia (range 21–26 months) 23 controls |
Reduced IENFD in 8 out of 15 FM patients | IENF reduction is present in adolescents with FM, with similar prevalence as adults |
| Quitadamo et al. (2022) [4] | Skin biopsy | 62 FM (mean ± SD 9.69 ± 7.21 years) clinical and skin biopsy 18-month follow-up | Stable IENFD at follow-up. Length-dependent small-fiber reduction predicted more severe disability and reduced response to drugs and physical exercise | Small-fiber impairment seems stable in medium term in FM, but it can predict FM outcome |
CCM
Considering the elective innervation of the cornea by C fibers, corneal confocal bio-microscopy is a reliable method to assess small nerve fiber pathology. While the study by Oudejans clearly reported diagnostic cut-off values and relied on widely agreed parameters including nerve fiber length, nerve fiber density, and branching, the study by Ramirez investigated different parameters (stromal nerve thickness and corneal sub-basal plexus nerve density) and did not provide diagnostic cut-off values [40, 41]. More recent studies confirmed utility of corneal confocal microscopy in FM with abnormalities in most of the patients, frequently in association with severity of clinical expression [10, 42]. Changes in corneal innervation and Langerhans cells were detected in FM patients and those with small-fiber neuropathy [43]. To summarize, corneal confocal microscopy confirmed the presence of small-fiber impairment in FM, although the clinical picture did not resemble neuropathic pain, especially in patients with severe anxiety and depression [44]. Its evaluation in FM patients could reliably confirm impairment of small afferents (Table 5).
Table 5.
Studies on corneal confocal microscopy (CCM) in fibromyalgia (FM) patients published within the last 5 years. In bracket, disease duration is reported, when available. FM, fibromyalgia; HC, healthy controls
| Authors | Methods | Subjects | Results | Significance |
|---|---|---|---|---|
| Erkan Turan et al. (2018) [42] | (CCM) Nerve fiber density and tortuosity |
34 FM 42 HC (6.9 + 5.1 years) |
Lower epithelial cell density in FM, correlated with increased widespread pain index (WPI) | Utility of corneal confocal microscopy in clinical assessment of FM |
| Van de Donk et al. (2018) [43] | CCM fiber length and density, double-blind placebo controlled trial with tapentadol |
34 FM (tapentadol group 5.4 + 4.9, placebo 4.8 + 3.8 years) Laboratory reference |
13 FM patients with corneal fiber density and length abnormalities Prevalence in tapentadol not responders |
CCM could predict therapeutic response to tapentadol |
| Evdokimov et al. (2019) [10] | CCM fiber density, branching density, and nerve fiber length. (microneurography, QST, PREPs, skin biopsy) |
117 FM (mean 12 years, range 0.8–56) Laboratory reference 54 HC 11 patients with depression and widespread pain |
Corneal nerve fiber density reduced in FM, not in patients with depression. Correlation with severity of intrapidermal nerve density reduction | Corneal denervation parallels skin denervation and is associated with severe FM |
| Klitsch et al. (2020) [44] | CCM Langerhans cells-dendritic and non-dendritic, corneal nerve fiber density, length, and branch density |
134 FM (12; 0.75–56 years) 41 SFN (4; 0–20 years) 60 HC |
Fewer dendritic Langerhans cells, nerve fiber length and density in FMS and SFN, branch density reduced in SFN patients | Changes in corneal innervation and Langerhans cell distribution in FMS and SFN |
| Ramirez et al. (2020) [41] | CCM fiber density, length, and branch density (correlation with neuropathic pain scores: assessment of anxiety and depression) | 28 FM (4.71 + 6.2 years) | Strong negative correlation between neuropathic pain scored and corneal nerve density in the subgroup of FM women without severe anxiety or depression (n = 13) | Severe anxiety or depression distorts fibromyalgia symptoms |
Microneurography
Microneurography represents an ideal technique to explore the peripheral nociceptor system and to record intraneural single C-fiber action potentials from nociceptors. Multiple C-fibers can be recorded simultaneously for hours [65], thereby allowing the assessment of individual firing behavior to various external stimuli.
The application of microneurography in FM is unfortunately limited. Using this technique, C-nociceptors were recorded in female FM patients [45]. The results were compared with those collected in female patients with small-fiber neuropathy and controls. The majority of FM patients showed abnormal firing of C-nociceptors. Many silent nociceptors exhibit hyper excitability resembling that in small-fiber neuropathy, but high activity—dependent slowing of conduction velocity is more common in FM patients, and may constitute a hallmark. The main conclusion of this study was that abnormal peripheral C-nociceptor ongoing activity and increased mechanical sensitivity could contribute to the pain and tenderness referred by FM patients [45]. Essentially, the same results were found in a second paper from the same group [10] showing that FM patients showed hyper excitability and sensitization of mechanoresponsive (1A fibers) and mechano-insensitive (1B) nociceptors. If confirmed this is an important contribution as it may suggest that peripheral nociceptors are functionally abnormal in FM in agreement with the morphological abnormalities disclosed by skin biopsy. An important limitation of these papers is the lack of correlation with morphological analysis of peripheral nociceptors that prevent to correlate the functional abnormalities highlighted by microneurography with the morphological abnormalities disclosed by skin biopsy. Presently, microneurography seems a promising tool to evaluate nociceptors abnormalities, but the procedure is of limited diffusion in clinical practice with few application in patients with FM.
QST
Quantitative sensory testing (QST), a standardized method used to assess somatosensory system function, if a comprehensive protocol is applied, allows to detect sensory and pain profiles, and to hypothesize the underlying mechanisms (i.e., peripheral versus central nervous involvement, focal versus generalized sensory dysfunction) [46].
In fibromyalgia, QST findings showed a certain heterogeneity in the sensory threshold abnormalities. However, a common result between different studies is the presence of hypersensitivity or gain of function to a broad of standardized sensory stimuli [8, 10, 17, 33, 47, 48]. In particular, increased mechanical pain sensitivity (MPS) and reduced pressure pain thresholds (PPT) are commonly reported [3, 17], that suggest prevalent mechanism of central sensitization [46]. Moreover, a high rate of aftersensation after mechanical stimuli is also described [47] while, less commonly, mechanical dynamic allodynia is reported [3, 40, 47]. Additionally, in a subset of patients, also, mechanical detection thresholds (MDT) can be increased, that, unrelated to other evidence of large nerve fiber impairment, probably is supposed to be due to impaired C-tactile afferents in the context of a small-fiber pathology [10].
Regarding thermal stimuli, several studies described cold pain hyperalgesia [10], and increased cold and warm detection thresholds [3, 8, 10, 17], although other authors reported normal thermal thresholds [48].
Compared with small-fiber neuropathy, patients with FM with or without epidermal denervation have different sensory phenotypes [48], suggesting that despite the coexistence of skin denervation and small-fiber pathology, SFN and FM have different mechanisms underlying pain [3, 48, 49]
Finally, the application of dynamic protocols of temporal summation and conditioned pain modulation (CPM) showed significant dysfunction of the diffuse noxious inhibitory control (DNIC) [29, 50], rising the hypothesis of altered endogenous pain mechanisms, compared with healthy controls. (Table 6)
Table 6.
Studies on quantitative sensory testing application in fibromyalgia published within the last 5 years. In bracket, disease duration is reported, when available. FM, fibromyalgia; HC, healthy controls; SFN, small-fiber neuropathy
| Authors | Methods | Subjects | Results | Significance |
|---|---|---|---|---|
| Evdokimov et al. (2019) [10] | QST battery (13 parameters) (skin biopsy, CCM microneurography, PREP) |
117 FM (mean 12 years, range 0.8–56) 34 HC |
Different subgroups; worse hyperalgesia in generalized IENFD reduction | Existence of different clinical features, more severity related to small-fiber pathology |
| Wodehouse et al. (2018) [50] | QST (only mechanical pressure stimuli) and CPM using tonic and phasic mechanical pressure pain |
25 FM 14 completed follow-up |
Pressure pain sensitivity Diffuse noxious inhibitory controls (DNIC) less efficient Both improve after treatment 12 weeks of pregabalin treatment |
Modulation of pressure pain and DNIC efficacy after pharmacological treatment (pregabalin) |
| Pickering et al. (2019) [29] |
QST (cold and warm thresholds) CPM to assess DNICs ESC |
(12 + 3 years) | In fibromyalgia, DNIC is less functional, cold and warm detection threshold higher, thermal pain thresholds reduced | Altered endogenous pain mechanisms |
| Fasolino et al. (2020) [3] | QST battery 10 parameters (skin biopsy, voltage-gated channel genotyping) | 57 FM (mean 5 years, range 2–9.5) | Increased mechanical pain sensitivity, no difference between pts with or without skin denervation |
Central sensitization as a major mechanism Small-fiber pathology does not affect somatosensory system |
| Rehm et al. (2021) [49] | QST battery (13parameters) | 87 FM (12.95 + 9.03) |
Different somatosensory pattern Hypersensitivity for noxious mechanical and thermal stimuli. Pinprick hyperalgesia or dynamic mechanical allodynia |
Central sensitization as a major pathophysiological mechanism |
| Berwick et al. (2022) [47] | QST mechanical pain thresholds; pressure pain thresholds; brushstroke pain | 44 FM (10.6; 8.912.3 years)16 HC | Pain after pressure aftersensation to mechanical not painful stimuli (77%), and dynamic mechanical allodynia in 10% | Evidence of central sensitization |
| Leone et al. (2022) [48] | QST 10 parameters |
64 FM 20 SFN (5.1; 0.4–20 years), 44 without SFN (6.4; 0.5–14 years) 30 pure SFN |
Different sensory phenotype between FM (with or without SFN) and SFN. In FM, presence of hyperalgesia phenotypes (both thermal and mechanical) or normal profile, compared with SFN (sensory loss and mechanical hyperalgesia) |
The study supports the present of a complex mechanisms underlying pain Small-fiber pathology does not significantly affect somatosensory system |
In conclusion, considering the consistent methodological heterogeneity between studies, future research needs to investigate with a multimodal approach, the potential role of QST-based algorithm in stratification of FM patients for clinical and research purposes.
Position statement
Neurological management of FM patient-clinical criteria are as follows:
Patients with generalized pain should be taken into consideration for FM diagnosis.
Recent diagnostic criteria should be applied (ACR, 2016). Diagnosis of FM is based on the sum of WPI (WPI) > 7 and symptom severity scale (SSS) score > 5 OR WPI of 4–6 and SSS score > 9. Severity of FM is also based on the sum between WPI and SSS scores. For fatigue, waking unrefreshed, and cognitive symptoms, the questionnaire should be followed. For headache comorbidity, International Headache Society criteria [66] should be applied. For depression, neurologist should consider the presence of depressive symptoms, using clinical impression, sustained by clinical criteria (DSM V). For abdominal pain, the description of such symptom since at least 3 months should be considered. For juvenile age, the same criteria should be applied, with the recommendation to be administered by a clinician or health care professional. In addition, the WPI would consider the last 3 months, instead of the last week [58].
A careful investigation of personal and familiar history of neurological conditions, as well as a detailed objective examination of neurological deficit, with particular regard to multimodal sensations, including tactile, vibration, nociceptive, and thermal ones, seems mandatory in the clinical approach of patients with suspected FM. The huge expression of central sensitization phenomena could contribute to hide myopathies and central and peripheral neurogenic pathologies. In the new classification, other conditions leading to pain do not exclude FM, so the definition of associated syndromes outside those included in the classification should complete the diagnosis. The clinical neurological examination would contribute to define deficit in central and peripheral nervous system, and to proceed with the adequate diagnostic measures. In the supplementary section, the neurological diseases with diffuse pain with possible association with FM are described (Supplementary section).
A rheumatologic visit should also be requested, if lacking, to clarify frequent comorbid conditions
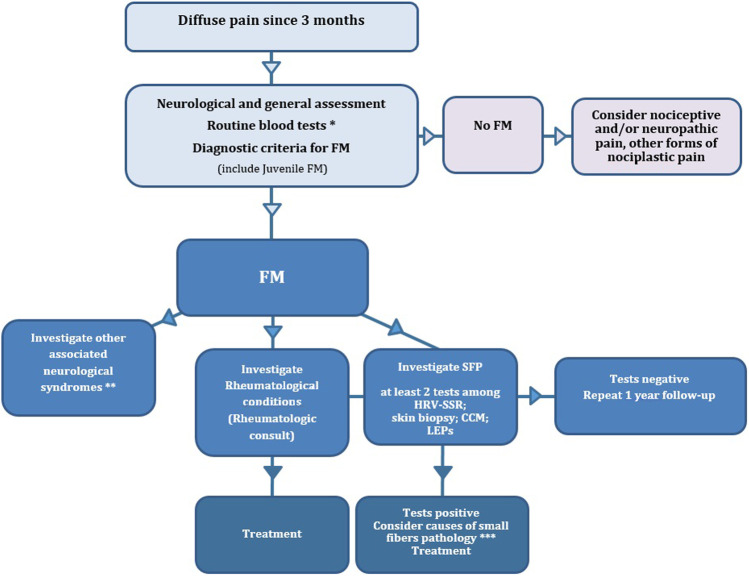
In the case of FM diagnosis, the involvement of small somatic and autonomic fibers, frequently not associated to objective signs of sensitive and autonomic dysfunctions, would request the diagnostic procedures detailed in the above reported paragraph. At least two among HRV + SSR and/or laser-evoked responses and/or skin biopsy and/or corneal confocal microscopy should be suggested. Microneurography is rarely available. The QST could also be suggested, although this is a psychophysical technique, based on subjective responses, that may not reflect small nerve fibers dysfunctions. The presence of objective signs of small-fiber impairment should be followed by monitoring of possible metabolic and/or immunological and/or paraneoplastic basis, with basal assessment of blood tests [67]. Such tests could be repeated at 1-year follow-up (Fig. 2).
Fig. 2.
Flow chart reporting the proposal simplified diagnostic assessment for patients with FM. *Glucose dysmetabolism assessment: renal, thyroid, and liver function tests; B12 vitamin and folate blood levels; erythrocyte sedimentation rate; blood cell count. **For associated neurological conditions, consider the supplementary section. ***For tests useful to consider causes of small-fiber neuropathy, consider Gemignani et al. [66]
Limitations
This position statement was based on an expert opinion taking into consideration recent literature. However, we did not perform a systematic review, and considered main studies exploring only the last 10 years in PubMed.
Conclusions
This shared document has the function of helping clinical neurologists and neurophysiologists in the diagnosis of FM disease although we are still far from understanding mechanisms that could sustain FM clinical picture in single cases. To clarify these mechanisms will definitively improve FM treatment with a precision medicine approach.
The altered pain processing at central level and the frequent involvement of somatic and autonomic small fibers justify the interest and the competence of the neurologists, who may give an important aid to the diagnosis of associated central and peripheral nervous system conditions, as well as novel input to the research about the fundamental causes of the disease. Recent studies on voltage-gated ionic channels [3, 68] showed genetic abnormalities in several patients with FM, similar to those found in patients with neuropathic pain.
The correct diagnostic approach to FM could promote the exclusion of the known causes of small-fiber impairment, and the research toward common genetic factors, useful to promote a more specific therapeutic approach.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thanked the Neuropathic Pain Study Group-Italian Society of Neurology (SIN).
Author contribution
MdT: study design, manuscript editing, expert consensus, literature revise, table preparation.
Funding
Open access funding provided by Università degli Studi di Bari Aldo Moro within the CRUI-CARE Agreement.
Declarations
Ethical approval
It was a consensus paper, no patients’ data were employed. No Ethical statement was required.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
G. Devigili, G. Di Stefano, V. Donadio, I. Frattale, E. Mantovani, M. Nolano, G. Occhipinti, V. Provitera, S. Quitadamo, S. Tamburin and A. Toscano equally contributed to this work.
References
- 1.International Association for the Study of Pain (IASP) (201). IASP Terminology. Accessed 28 March 2019; Available online: https://www.iasppain.org/terminology?navItemNumber=576#Centralsensitization
- 2.Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. 2021;397(10289):2098–2110. doi: 10.1016/S0140-6736(21)00392-5. [DOI] [PubMed] [Google Scholar]
- 3.Fasolino A, Di Stefano G, Leone C, Galosi E, Gioia C, Lucchino B, Terracciano A, Di Franco M, Cruccu G, Truini A. Small-fibre pathology has no impact on somatosensory system function in patients with fibromyalgia. Pain. 2020;161(10):23852393. doi: 10.1097/j.pain.0000000000001920. [DOI] [PubMed] [Google Scholar]
- 4.Quitadamo SG, Vecchio E, Delussi M, Libro G, Clemente L, Lombardi R, Modena D, Giannotta M, Iannone F, de Tommaso M. Outcome of small fibers pathology in fibromyalgia: a real life longitudinal observational study. Clin Exp Rheumatol. 2022 doi: 10.55563/clinexprheumatol/ld0lxn. [DOI] [PubMed] [Google Scholar]
- 5.Goebel A, Krock E, Gentry C, Israel MR, Jurczak A, Urbina CM, Sandor K, Vastani N, Maurer M, Cuhadar U, Sensi S, Nomura Y, Menezes J, Baharpoor A, Brieskorn L, Sandström A, Tour J, Kadetoff D, Haglund L, Kosek E, Bevan S, Svensson CI, Andersson DA. Passive transfer of fibromyalgia symptoms from patients to mice. J Clin Invest. 2021;131(13):e144201. doi: 10.1172/JCI144201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caro XJ, Galbraith RG, Winter EF. Evidence of peripheral large nerve involvement in fibromyalgia: a retrospective review of EMG and nerve conduction findings in 55 FM subjects. Eur J Rheumatol. 2018;5(2):104–110. doi: 10.5152/eurjrheum.2018.17109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawson VH, Grewal J, Hackshaw KV, Mongiovi PC, Stino AM. Fibromyalgia syndrome and small fiber, early or mild sensory polyneuropathy. Muscle Nerve. 2018;58(5):625–630. doi: 10.1002/mus.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doppler K, Rittner HL, Deckart M, Sommer C. Reduced dermal nerve fiber diameter in skin biopsies of patients with fibromyalgia. Pain. 2015;156(11):2319–2325. doi: 10.1097/j.pain.0000000000000285. [DOI] [PubMed] [Google Scholar]
- 9.Solmaz V, Yavuz S, İnanr A, Aksoy D, Pektaş E, Tekataş A, Kurt SG. Investigation of nerve conduction studies of carpal tunnel syndrome cases with different risk factors: an electrodiagnostic study. J Clin Neurophysiol. 2017;34(2):139–143. doi: 10.1097/WNP.0000000000000352. [DOI] [PubMed] [Google Scholar]
- 10.Evdokimov D, Frank J, Klitsch A, Unterecker S, Warrings B, Serra J, Papagianni A, Saffer N, Altenschildesche MZ, Kampik CD, Malik RA, Sommer C, Üçeyler N. Reduction of skin innervation is associated with a severe fibromyalgia phenotype. Ann Neurol. 2019;86(4):504–516. doi: 10.1002/ana.25565. [DOI] [PubMed] [Google Scholar]
- 11.Vecchio E, Lombardi R, Paolini M, Libro G, Delussi M, Ricci K,Quitadamo SG, Gentile E, Girolamo F, Iannone F, Lauria G, de Tommaso M (2020) Peripheral and central nervous system correlates in fibromyalgia. Eur J Pain. 6(1). [DOI] [PubMed]
- 12.Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58:185–193. doi: 10.1016/0304-3959(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 13.de Tommaso M, Federici A, Santostasi R, Calabrese R, Vecchio E, Lapadula G, Iannone F, Lamberti P, Livrea P. Laser-evoked potentials habituation in fibromyalgia. J Pain. 2011;12:116–124. doi: 10.1016/j.jpain.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 14.de Tommaso M, Nolano M, Iannone F, Vecchio E, Ricci K, Lorenzo M, Delussi M, Girolamo F, Lavolpe V, Provitera V, Stancanelli A, Lapadula G, Livrea P. Update on laser-evoked potential findings in fibromyalgia patients in light of clinical and skin biopsy features. J Neurol. 2014;261:461–472. doi: 10.1007/s00415-013-7211-9. [DOI] [PubMed] [Google Scholar]
- 15.de Tommaso M, Ricci K, Libro G, Vecchio E, Delussi M, Montemurno A, Lopalco G, Iannone F (2017) Pain processing and vegetative dysfunction in fibromyalgia: a study by sympathetic skin response and laser evoked potentials. Pain Res Treat. 9747148. 10.1155/2017/9747148 [DOI] [PMC free article] [PubMed]
- 16.Truini A, Gerardi MC, Di Stefano G, La Cesa S, Iannuccelli C, Pepe A, SarziPuttini P, Cruccu G, Di Franco M. Hyperexcitability in pain matrices in patients with fibromyalgia. Clin Exp Rheumatol. 2015;33:S68–72. [PubMed] [Google Scholar]
- 17.Üçeyler N, Zeller D, Kahn AK, Kewenig S, Kittel-Schneider S, Schmid A, CasanovaMolla J, Reiners K, Sommer C. Small fibre pathology in patients with fibromyalgia syndrome. Brain. 2013;136(Pt 6):1857–1867. doi: 10.1093/brain/awt053. [DOI] [PubMed] [Google Scholar]
- 18.Van Assche DCS, Plaghki L, Masquelier E, Hatem SM. Fibromyalgia syndrome—a laser-evoked potentials study unsupportive of small nerve fibre involvement. Eur J Pain. 2020;24:448–456. doi: 10.1002/ejp.1501. [DOI] [PubMed] [Google Scholar]
- 19.Vecchio E, Quitadamo SG, Ricci K, Libro G, Delussi M, Lombardi R, Lauria G, de Tommaso M. Laser evoked potentials in fibromyalgia with peripheral small fiber involvement. Clin Neurophysiol. 2022;135:96–106. doi: 10.1016/j.clinph.2022.01.001. [DOI] [PubMed] [Google Scholar]
- 20.Vallejo M, Martínez-Martínez LA, Grijalva-Quijada S, Olguín-Ruvalcaba HM, Salas E, Hermosillo AG, Cárdenas M, Martínez-Lavín M. Prevalence of fibromyalgia in vasovagal syncope. J Clin Rheumatol. 2013;19(3):111–114. doi: 10.1097/RHU.0b013e318289bbbb. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow A, Stone TM, Kingsley JD. Resistance exercise training on disease impact, pain catastrophizing and autonomic modulation in women with fibromyalgia. Int J Exerc Sci. 2017;10(8):1184–1195. [PMC free article] [PubMed] [Google Scholar]
- 22.Rizzi M, Radovanovic D, Santus P, Airoldi A, Frassanito F, Vanni S, Cristiano A, Masala IF, Sarzi-Puttini P. Influence of autonomic nervous system dysfunction in the genesis of sleep disorders in fibromyalgia patients. Clin Exp Rheumatol. 2017;35 Suppl 105(3):74–80. doi: 10.1136/annrheumdis-2017-eular.3365. [DOI] [PubMed] [Google Scholar]
- 23.Reyes-Manzano CF, Lerma C, Echeverría JC, Martínez-Lavin M, Martínez-Martínez LA, Infante O, Guzmán-Vargas L. Multifractal analysis reveals decreased non-linearity and stronger anticorrelations in heart period fluctuations of fibromyalgia patients. Front Physiol. 2018;17(9):1118. doi: 10.3389/fphys.2018.01118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hazra S, Venkataraman S, Handa G, Yadav SL, Wadhwa S, Singh U, Kochhar KP, Deepak KK, Sarkar KA. Cross-sectional study on central sensitization and autonomic changes in fibromyalgia. Front Neurosci. 2020;14:788. doi: 10.3389/fnins.2020.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Rai NK, Rastogi A, Endukuru C, Joshi A, Mishra SS. Impact of sleep disturbances and autonomic dysfunction on the quality of life of patients with fibromyalgia. J Basic Clin Physiol Pharmacol. 2021;32(6):1021–1029. doi: 10.1515/jbcpp-2020-0007. [DOI] [PubMed] [Google Scholar]
- 26.Schamne JC, Ressetti JC, Lima-Silva AE, Okuno NM. Impaired cardiac autonomic control in women with fibromyalgia is independent of their physical fitness. J Clin Rheumatol. 2021;27(6S):S278–S283. doi: 10.1097/RHU.0000000000001518. [DOI] [PubMed] [Google Scholar]
- 27.Rost S, Crombez G, Sütterlin S, Vögele C, Veirman E, Van Ryckeghem DML. (2021) Altered regulation of negative affect in patients with fibromyalgia: a diary study. Eur J Pain. 2021;25(3):714–724. doi: 10.1002/ejp.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sochodolak RC, Schamne JC, Ressetti JC, Costa BM, Antunes EL, Okuno NM. A comparative study of heart rate variability and physical fitness in women with moderate and severe fibromyalgia. J Exerc Rehabil. 2022;18(2):133–140. doi: 10.12965/jer.2244070.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pickering G, Achard A, Corriger A, Sickout-Arondo S, Macian N, Leray V, Lucchini C, Cardot JM, Pereira B. Electrochemical skin conductance and quantitative sensory testing on fibromyalgia. Pain Pract. 2020;20(4):348–356. doi: 10.1111/papr.12857. [DOI] [PubMed] [Google Scholar]
- 30.Lodahl M, Treister R, Oaklander AL. Specific symptoms may discriminate between fibromyalgia patients with vs without objective test evidence of small- fiber polyneuropathy. Pain Rep. 2017;3(1):e633. doi: 10.1097/PR9.0000000000000633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lawson VH, Grewal J, Hackshaw KV, Mongiovi PC, Stino AM. Fibromyalgia syndrome and small fiber, early or mild sensory polyneuropathy. Muscle Nerve. 2018;58(5):625–630. doi: 10.1002/mus.26131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boneparth A, Chen S, Horton DB, Moorthy LN, Farquhar I, Downs HM, Lee H, Oaklander AL. Epidermal neurite density in skin biopsies from patients with juvenile fibromyalgia. J Rheumatol. 2021;48(4):575–578. doi: 10.3899/jrheum.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leinders M, Doppler K, Klein T, Deckart M, Rittner H, Sommer C, Üçeyler N. Increased cutaneous miR-let-7d expression correlates with small nerve fiber pathology in patients with fibromyalgia syndrome. Pain. 2016;157(11):2493–2503. doi: 10.1097/j.pain.0000000000000668. [DOI] [PubMed] [Google Scholar]
- 34.Albrecht PJ, Hou Q, Argoff CE, Storey JR, Wymer JP, Rice FL. Excessive peptidergic sensory innervation of cutaneous arteriole-venule shunts (AVS) in the palmar glabrous skin of fibromyalgia patients: implications for widespread deep tissue pain and fatigue. Pain Med. 2013;14(6):895–915. doi: 10.1111/pme.12139. [DOI] [PubMed] [Google Scholar]
- 35.Oaklander AL, Herzog ZD, Downs HM, Klein MM. Objective evidence that small-fiber polyneuropathy underlies some illnesses currently labeled as fibromyalgia. Pain. 2013;154(11):2310–2316. doi: 10.1016/j.pain.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Evdokimov D, Dinkel P, Frank J, Sommer C, Üçeyler N. Characterization of dermal skin innervation in fibromyalgia syndrome. PLoS One. 2020;15(1):e0227674. doi: 10.1371/journal.pone.0227674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Evdokimov D, Kreß L, Dinkel P, Frank J, Sommer C, Üçeyler N. Pain associated mediators and axon pathfinders in fibromyalgia skin cells. J Rheumatol. 2020;47(1):140–148. doi: 10.3899/jrheum.190248. [DOI] [PubMed] [Google Scholar]
- 38.Sánchez-Domínguez B, Bullón P, Román-Malo L, Marín-Aguilar F, Alcocer-Gómez E, Carrión AM, Sánchez-Alcazar JA, Cordero MD. Oxidative stress, mitochondrial dysfunction and inflammation common events in skin of patients with Fibromyalgia. Mitochondrion. 2015;2015:69–75. doi: 10.1016/j.mito.2015.01.010. [DOI] [PubMed] [Google Scholar]
- 39.Tavakoli M, Marshall A, Pitceathly R, Fadavi H, Gow D, Roberts ME, et al. Corneal confocal microscopy: a novel means to detect nerve fibre damage in idiopathic small fibre neuropathy. Exp Neurol. 2010;223:245–250. doi: 10.1016/j.expneurol.2009.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oudejans L, He X, Niesters M, Dahan A, Brines M, van Velzen M. Cornea nerve fiber quantification and construction of phenotypes in patients with fibromyalgia. Sci Rep. 2016;6:23573. doi: 10.1038/srep23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ramirez M, Martinez-Martinez LA, Hernandez-Quintela E, Velazco-Casapia J, Varga A, Martinez-Lavin M. Small fiber neuropathy in women with fibromyalgia An in vivo assessment using corneal confocal bio-microscopy. Semin Arthritis Rheum. 2015;45(2):214–219. doi: 10.1016/j.semarthrit.2015.03.003. [DOI] [PubMed] [Google Scholar]
- 42.ErkanTuran K, Kocabeyoglu S, Unal-Cevik I, Bezci F, Akinci A, Irkec M. Ocular surface alterations in the context of corneal in vivo confocal microscopic characteristics in patients with fibromyalgia. Cornea. 2018;37(2):205–210 73. doi: 10.1097/ICO.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 43.van de Donk T, van Velzen M, Dahan A. Niesters M (2019) Cornea nerve fibre state determines analgesic response to tapentadol in fibromyalgia patients without effective endogenous pain modulation. Eur J Pain. 2019;23(9):1586–1595. doi: 10.1002/ejp.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klitsch A, Evdokimov D, Frank J, Thomas D, Saffer N, Altenschildesche MZ, Sisignano CM, Kampik D, Malik RA, Sommer C, Üçeyler N. Reduced association between dendritic cells and corneal sub-basal nerve fibers in patients with fibromyalgia syndrome. J Peripher Nerv Syst. 2020;25(1):9–18. doi: 10.1111/jns.12360. [DOI] [PubMed] [Google Scholar]
- 45.Serra J, Collado A, Solà R, Antonelli F, Torres X, Salgueiro M, Quiles C, Bostock H. Hyperexcitable C nociceptors in fibromyalgia. Ann Neurol. 2014;75(2):196–208. doi: 10.1002/ana.24065. [DOI] [PubMed] [Google Scholar]
- 46.Baron R, Maier C, Attal N, et al. Peripheral neuropathic pain: a mechanism-related organizing principle based on sensory profiles. Pain. 2017;158(2):261–272. doi: 10.1097/j.pain.0000000000000753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berwick RJ, Andersson DA, Goebel A, Marshall A. After sensations and lingering pain after examination in patients with fibromyalgia syndrome. Pain Med. 2022;23(12):1928–1938. doi: 10.1093/pm/pnac089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Leone C, Galosi E, Esposito N, Falco P, Fasolino A, Di Pietro G, Di Stefano G, Camerota F, Vollert J, Truini A. Small-fibre damage is associated with distinct sensory phenotypes in patients with fibromyalgia and small-fibre neuropathy. Eur J Pain. 2022;27(1):163–173. doi: 10.1002/ejp.2049. [DOI] [PubMed] [Google Scholar]
- 49.Rehm S, Sachau J, Hellriegel J, Forstenpointner J, Børsting Jacobsen H, Harten P, Gierthmühlen J, Baron R. Pain matters for central sensitization: sensory and psychological parameters in patients with fibromyalgia syndrome. Pain Rep. 2021;6(1):e901. doi: 10.1097/PR9.0000000000000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wodehouse T, Poply K, Ramaswamy S, Snidvongs S, Bourke J, Tahir H, Ullrich K, Mehta V. A pilot study investigating whether quantitative sensory testing alters after treatment in patients with fibromyalgia. Br J Pain. 2018;12(4):250–256. doi: 10.1177/2049463718776336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, Tugwell P, Campbell SM, Abeles M, Clark P, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia Report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33(2):160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 52.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB, Yunus MB. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis Care Res (Hoboken) 2010;62(5):600–610. doi: 10.1002/acr.20140. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RS, Mease P, Russell AS, Russell IJ, Winfield JB. Fibromyalgia criteria and severity scales for clinical and epidemiological studies: a modification of the ACR Preliminary Diagnostic Criteria for Fibromyalgia. J Rheumatol. 2011;38(6):1113–1122. doi: 10.3899/jrheum.100594. [DOI] [PubMed] [Google Scholar]
- 54.Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Häuser W, Katz RL, Mease PJ, Russell AS, Russell IJ, Walitt B. 2016 Revisions to the 2010/2011 fibromyalgia diagnostic criteria. Semin Arthritis Rheum. 2016;46(3):319–329. doi: 10.1016/j.semarthrit.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 55.Siegel DM, Janeway D, Baum J. (1998) Fibromyalgia syndrome in children and adolescents: clinical features at presentation and status at follow-up. Pediatrics. 1998;101(3 Pt 1):377–382. doi: 10.1542/peds.101.3.377. [DOI] [PubMed] [Google Scholar]
- 56.Weiss JE, Kashikar-Zuck S. Juvenile fibromyalgia. Rheum Dis Clin North Am. 2021;47(4):725–736. doi: 10.1016/j.rdc.2021.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Yunus MB, Masi AT. Juvenile primary fibromyalgia syndrome A clinical study of thirty-three patients and matched normal controls. Arthritis Rheum. 1985;28(2):138–45. doi: 10.1002/art.1780280205. [DOI] [PubMed] [Google Scholar]
- 58.Ting TV, Barnett K, Lynch-Jordan A, Whitacre C, Henrickson M, Kashikar-Zuck S. 2010 American College of Rheumatology Adult Fibromyalgia Criteria for Use in an Adolescent Female Population with Juvenile Fibromyalgia. J Pediatr. 2016;169:181–7.e1. doi: 10.1016/j.jpeds.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Varni JW, Burwinkle TM, Limbers CA, Szer IS. The PedsQL as a patient reported outcome in children and adolescents with fibromyalgia: an analysis of OMERACT domains. Health Qual Life Outcomes. 2007;12(5):9. doi: 10.1186/1477-7525-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gedalia A, García CO, Molina JF, Bradford NJ, Espinoza LR. Fibromyalgia syndrome: experience in a pediatric rheumatology clinic. Clin Exp Rheumatol. 2000;18(3):415–419. [PubMed] [Google Scholar]
- 61.Tayag-Kier CE, Keenan GF, Scalzi LV, Schultz B, Elliott J, Zhao RH, Arens R. (2000) Sleep and periodic limb movement in sleep in juvenile fibromyalgia. Pediatrics. 2000;106(5):E70. doi: 10.1542/peds.106.5.e70. [DOI] [PubMed] [Google Scholar]
- 62.Ting TV, Hashkes PJ, Schikler K, Desai AM, Spalding S, Kashikar-Zuck S. The role of benign joint hypermobility in the pain experience in Juvenile Fibromyalgia: an observational study. Pediatr Rheumatol Online J. 2012;10(1):16. doi: 10.1186/15460096-10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cunningham NR, Tran ST, Lynch-Jordan AM, Ting TV, Sil S, Strotman D, Noll JG, Powers SW, Arnold LM, Kashikar-Zuck S. Psychiatric disorders in young adults diagnosed with juvenile fibromyalgia in adolescence. J Rheumatol. 2015;42(12):2427–2433. doi: 10.3899/jrheum.141369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Uziel Y, Chapnick G, Jaber L, Nemet D, Hashkes PJ. Five-year outcome of children with “growing pains”: correlations with pain threshold. J Pediatr. 2010;156(5):838–840. doi: 10.1016/j.jpeds.2009.11.078. [DOI] [PubMed] [Google Scholar]
- 65.Schmelz M, Schmidt R. Microneurographic single-unit recordings to assess receptive properties of afferent human C-fibers. Neurosci Lett. 2010;470(3):158–161. doi: 10.1016/j.neulet.2009.05.064. [DOI] [PubMed] [Google Scholar]
- 66.Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38(1):1–211. doi: 10.1177/0333102417738202. [DOI] [PubMed] [Google Scholar]
- 67.Gemignani F, Bellanova MF, Saccani E, Pavesi G. Non-length-dependent small fiber neuropathy: not a matter of stockings and gloves. Muscle Nerve. 2022;65(1):10–28. doi: 10.1002/mus.27379. [DOI] [PubMed] [Google Scholar]
- 68.Salvi E, Marchi M, Andelic M, Mehmeti E, D’Amato I, Cazzato D, Chiappori F, Lombardi R, Cartelli D, Devigili G, Dalla Bella E, Gerrits M, Almomani R, Malik RA, Ślęczkowska M, Mazzeo A Gentile L, Dib-Hajj S, Waxman S,G., Faber CG, Vecchio E, de Tommaso M, Lauria G (2023) TRPA1 gene rare variants in chronic neuropathic and nociplastic pain patients Pain, accepted for publication, in press [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.