Abstract
The drivers of cognitive change following first-episode psychosis remain poorly understood. Evidence regarding the role of antipsychotic medication is primarily based on naturalistic studies or clinical trials without a placebo arm, making it difficult to disentangle illness from medication effects. A secondary analysis of a randomised, triple-blind, placebo-controlled trial, where antipsychotic-naive patients with first-episode psychotic disorder were allocated to receive risperidone/paliperidone or matched placebo plus intensive psychosocial therapy for 6 months was conducted. A healthy control group was also recruited. A cognitive battery was administered at baseline and 6 months. Intention-to-treat analysis involved 76 patients (antipsychotic medication group: 37; 18.6Mage [2.9] years; 21 women; placebo group: 39; 18.3Mage [2.7]; 22 women); and 42 healthy controls (19.2Mage [3.0] years; 28 women). Cognitive performance predominantly remained stable (working memory, verbal fluency) or improved (attention, processing speed, cognitive control), with no group-by-time interaction evident. However, a significant group-by-time interaction was observed for immediate recall (p = 0.023), verbal learning (p = 0.024) and delayed recall (p = 0.005). The medication group declined whereas the placebo group improved on each measure (immediate recall: p = 0.024; ηp2 = 0.062; verbal learning: p = 0.015; ηp2 = 0.072 both medium effects; delayed recall: p = 0.001; ηp2 = 0.123 large effect). The rate of change for the placebo and healthy control groups was similar. Per protocol analysis (placebo n = 16, medication n = 11) produced similar findings. Risperidone/paliperidone may worsen verbal learning and memory in the early months of psychosis treatment. Replication of this finding and examination of various antipsychotic agents are needed in confirmatory trials. Antipsychotic effects should be considered in longitudinal studies of cognition in psychosis.
Trial registration: Australian New Zealand Clinical Trials Registry (http://www.anzctr.org.au/; ACTRN12607000608460).
Subject terms: Clinical pharmacology, Schizophrenia
Introduction
Widespread cognitive impairments are a core feature of psychotic disorders, being present prior to [1] and during the first-episode of psychosis (FEP) [2, 3]. In both antipsychotic-naive and antipsychotic-exposed FEP patients, medium-to-large impairments are routinely observed. The most severe impairments are in verbal learning and memory, processing speed and working memory [2, 3]. Generally, cognitive impairments remain fairly stable [4, 5], although recent longitudinal studies have shown decline in specific domains years after FEP [6–8].
The causes of cognitive change in psychosis remain poorly understood. One contentious factor is the role of antipsychotic medication. It is unclear whether antipsychotics ameliorate, exacerbate, or have negligible effects on cognitive impairments and whether effects differ by medication type. Clinical trials investigating the cognitive effects of atypical antipsychotics in first-episode or recent-onset schizophrenia-spectrum samples have documented mild cognitive improvements over a 3-to-6-month period (regardless of antipsychotic type) [9–13]. These improvements were typically modest, with further examination suggesting improvements were mostly due to test practice [9, 13]. Small improvements in cognitive functioning following antipsychotic treatment may also occur due to symptom improvement [10]. It is unknown whether cognitive improvements would also be observed with symptom improvement in the absence of antipsychotic medication. Most trials examining the cognitive effects of antipsychotics have been head-to-head (e.g., typical vs. atypical or atypical comparisons) without a placebo or healthy control group [9, 10, 12]. Thus, the effect of antipsychotics on cognitive functioning could not be clearly disentangled from the illness itself. Older trials were also compromised by inequitable dosing (i.e., first-generation prescribed at higher equivalent dose compared to second-generation) potentially inflating the beneficial effects of second-generation antipsychotics on cognition [14]. Given cognitive functioning is among the most robust predictors of functional recovery following FEP [15, 16], it is critical to understand what effect antipsychotic treatment has on cognition.
Most cognitive impairment seems to occur prior to or during the development of full-threshold psychotic disorder [17, 18]. However, because antipsychotics are usually the first-line treatment for all psychotic disorders, understanding the ongoing course of cognitive functioning post-treatment is complicated. Specifically, do antipsychotics prevent or exacerbate further cognitive decline or lag? Do their effects differ by cognitive domain? One early randomised placebo-controlled trial involving people with acute ‘functional psychosis’ showed that, at 2.5-year follow-up the cognitive functioning of those with an initial 4-week non-medication period (placebo) was no different from those who were immediately prescribed antipsychotics [19]. In other words, a 4-week delay in introducing antipsychotic medication did not result in long-term adverse cognitive effects. Meta-analyses of the relationship between duration of untreated psychosis (DUP), usually defined as the time between psychotic symptom onset and adequate treatment with antipsychotic medication, and cognitive functioning suggest DUP is not associated with cognitive performance [20, 21]. Moreover, naturalistic studies have shown higher cumulative use of antipsychotics is associated with poorer cognitive functioning [22–24], but these findings may be confounded by illness severity and cohort effects.
The primary aim of this study was to disentangle the effects of psychotic illness from those of antipsychotic medication on cognition over the first 6 months of treatment for a first-episode psychotic disorder. We analysed cognition data collected within a randomised, triple-blind placebo-controlled trial [25]. A healthy control group was also recruited to account for typical cognitive development and practice effects. The cognition outcomes reported here were a pre-planned secondary analysis of the Staged Treatment and Acceptability Guidelines in Early Psychosis (STAGES) trial [26], where we previously reported that the placebo group had comparable functional outcomes (primary outcome) and clinical outcomes to the group receiving antipsychotic medication [25], but showed different trajectories of brain volume and function [27, 28].
Methods and materials
Study design
STAGES was a randomised, triple-blind, placebo-controlled trial comparing the effects of antipsychotic medication plus CBCM (medication group) with placebo plus CBCM (placebo group) in FEP [25, 26]. FEP participants were randomised 1:1, stratified by sex and DUP, within six permuted blocks. DUP was included as a three-level factor (0–30, 31–90, >90 days). Clinicians, patients, and assessors remained blind to treatment allocation throughout the trial. The active treatment phase was 6 months, with assessments conducted at baseline (prior to treatment allocation) and 6 months. To account for practice effects, a healthy control group also completed baseline and 6-month assessments. The Melbourne Health Human Research Ethics Committee approved the study (MH-HREC: #2007:616). Trial registration was with the Australian New Zealand Clinical Trials Registry (ACTRN12607000608460).
Participants
FEP patients were aged 15–25 years and presented for treatment at the Early Psychosis Prevention and Intervention Centre, a specialist public early psychosis programme in Melbourne’s northern-western suburbs, Australia. Participants met criteria for a DSM-IV psychotic disorder, including schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder, depression with psychosis, substance-induced psychotic disorder, or psychosis NOS, confirmed using the Structured Clinical Interview for DSM-IV (SCID-IV) for Axis I disorders. Inclusion criteria were: ability to provide informed consent; English language comprehension; DUP < 6 months; low past exposure to antipsychotic medication (<7 days or lifetime maximum 1750mg chlorpromazine equivalent); no previous lithium/anticonvulsant treatment; and to minimise risk: living in stable accommodation; low risk to self/others [25, 26].
Sex- and age-matched healthy controls were recruited via flyers, word-of-mouth, and social media advertisements. Inclusion criteria for healthy controls were aged 15–25 years and English language comprehension. Exclusion criteria included: history of psychotic disorder or any current mental disorder (screened using the SCID-IV Research Non-Patient Edition); head injury or neurological disorder; and studied psychology at university (to mitigate cognitive test exposure). All participants gave written informed consent, including parent/guardian consent for participants <18 years. All participants were reimbursed $50AUD per assessment.
Treatment
FEP patients received weekly cognitive behavioural case management, a manualised formulation-based psychological and psychoeducation treatment for early psychosis [25]. Patients also saw a medical doctor weekly and received close monitoring, family therapy, vocational support, and 24-hour crisis support as required. Patients randomised to the medication group received either 1 mg risperidone (n = 33) or 3 mg paliperidone (n = 4), depending on the availability of medication and matched placebo [25]. The starting dose was titrated according to clinical response by the blinded study doctor. The same procedure was followed for the placebo group; allocated patients received a placebo pill that was identical to the active medication in taste, appearance, and packaging. To ensure participant safety, strict discontinuation criteria were applied in situations of insufficient clinical improvement, increased risk to self/others or worsening mental state/functioning and an alternative open-label antipsychotic medication was offered [26].
Cognitive battery
The Information and Picture Completion subtests of the Wechsler Adult Intelligence Scale–Third Edition (WAIS-III) were administered at baseline to estimate current IQ. The repeated cognitive battery included measures of cognitive domains known to be most affected in psychotic disorders [3], including attention (WAIS-III Digit Span: forward), working memory (WAIS-III Digit Span: backward), processing speed (WAIS-III Digit Symbol-Coding), processing speed and inhibition (Golden Stroop: word, colour, colour-word subtests), verbal fluency (Controlled Oral Word Association Test, animal fluency), and verbal learning and memory (Paired-Associate Learning). All cognitive tasks are standard tasks [29] except for the Paired-Associate Learning task, described in more detail below. Raw scores were used in the analysis.
The verbal Paired-Associate Learning task used in the current study is a variant of a novel verbal associative learning task and was chosen because it has previously shown to be sensitive to medial temporal lobe dysfunction and to load minimally on attention and working memory [30]. This paired-associate learning paradigm uses arbitrary word pairs as opposed to words that are semantically related. Each trial included eight word pairs: four concrete word pairs (i.e., with high imageability, e.g., horse-forest) and four abstract word pairs (i.e., with low imageability, e.g., motion-honest). Imageability has been shown to be sensitive for assessing verbal learning, where durable memories are formed for highly imageable words in both healthy controls and neurological patients [30, 31]. The first part of the task is akin to a list learning paradigm, where the participant is presented with the eight words that form the first half of each word pair and asked to recall as many words as they can (List A). This is repeated a further two times. Then, List B is presented (i.e., the eight words that form the second part of each word pair) and learnt over three trials. Next, the assessor conducts the paired-associate learning part of the task, and reads the eight word pairs (i.e., each word pair is comprised of one word from List A and one word from List B) to the participant. After which the participant is presented with the first word and is asked to provide the second word that goes with it (i.e., cued recall). This is repeated a further two times, resulting in three paired-associate learning trials. The concrete and abstract pairs were presented in different orders for each trial. After a 20-minute delay, the participant again completed cued-recall, where they were given the first word and had to provide the second word of the previously learned pair. In the current study, the primary outcome variables from this task were Trial 1 of paired-associate learning (immediate recall: score out of 8), paired-associate learning (Trials 1–3 total; score out of 24) and delayed cued recall (score out of 8). Alternate forms of the task were used at baseline and 6 months.
Statistical analysis
Baseline comparison of the three groups was conducted using analysis of variance, followed by pairwise comparison using Fisher’s least significant difference test. To assess rate of change in cognitive performance, group comparisons on each cognitive outcome were conducted using linear mixed-effects (LME) modelling analysis with random effects for intercept and slope and group-by-time interaction. Time was expressed as number of weeks from baseline to follow-up. The LME analysis included all cases with data on at least one timepoint (of 118 total participants, 84 had cognition data at both timepoints, and 34 only had data at baseline). Significant group-by-time interactions were followed by pairwise comparisons with effect size indexed using partial eta squared (ηp2), indicating the proportion of variance explained by a given term in a model after accounting for variance explained by the other terms in the model (ηp2 = 0.01 is considered small, 0.06 medium and 0.14 a large effect [32]). In accordance with CONSORT recommendations [33], a per protocol LME analysis was also performed, i.e., only including participants who remained on their allocated trial medication (placebo/medication) throughout the 6-month trial. A significance level of p < 0.05 was used (two-sided). Adjustment for multiple testing was not applied as this analysis was considered exploratory (not confirmatory) and was not powered for the cognition outcomes. In this scenario, priority is given to minimising type II error and generating evidence to inform future hypothesis-driven studies [34, 35]. The analysis was conducted using the nlme and effectsize R packages [32, 36].
Results
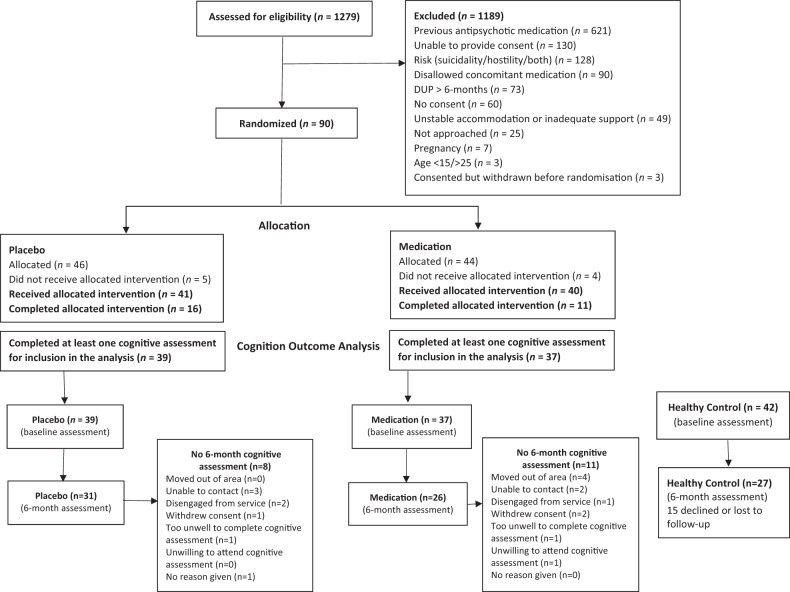
Figure 1 shows participant flow into the study. The placebo and medication groups did not significantly differ in number of missing 6-month assessments (p = 0.431) and there were few baseline differences between those who did and did not complete the 6-month assessment (Table S1).
Fig. 1.
A diagram of participant flow into the study.
The three groups were well matched on age and sex (Table 1). The placebo and medication groups were comparable on all baseline variables, including cognition. As expected, healthy controls had a significantly higher mean education, IQ, social and occupational functioning, and lower symptomatology than the FEP groups. Healthy controls also performed better than the FEP groups at baseline on all cognitive measures (Table S2).
Table 1.
Participant characteristics at baseline.
| First-episode psychosis | Healthy control (N = 42) | ||
|---|---|---|---|
| Placebo (N = 39) | Medication (N = 37) | ||
| Age, years (SD) | 18.3 (2.7) | 18.6 (2.9) | 19.2 (3.0) |
| Women, N (%) | 22 (56.4%) | 21 (56.8%) | 28 (66.7%) |
| Education, years (SD) | 11.8 (1.8) | 12.0 (3.1) | 13.4 (2.2) |
| Estimated IQ | 89.4 (14.1) | 91.8 (14.3) | 107.6 (10.0) |
| BPRS Total, mean (SD) | 58.7 (9.1) | 57.5 (10.1) | 31.6 (3.6) |
| BPRS Psychotic, mean (SD) | 15.0 (3.1) | 13.9 (3.7) | 4.1 (0.3) |
| SOFAS, mean (SD) | 53.8 (13.1) | 53.3 (10.7) | 80.5 (8.9) |
| SANS Total, mean (SD) | 26.8 (15.1) | 25.9 (15.0) | – |
| DUP, N (%) | |||
| 0–30 days | 6 (15.4%) | 6 (16.2%) | – |
| 31–90 days | 14 (35.9%) | 12 (32.4%) | – |
| >90 days | 19 (48.7%) | 19 (51.4%) | – |
| Psychotic diagnosis, N (%) | |||
| Major depression with psychosis | 7 (17.9%) | 8 (21.6%) | – |
| Schizophreniform disorder | 6 (15.4%) | 8 (21.6%) | – |
| Psychotic disorder NOS | 12 (30.8%) | 8 (21.6%) | – |
| Schizophrenia | 7 (17.9%) | 5 (13.6%) | – |
| Substance-induced psychotic disorder | 6 (15.4%) | 4 (10.8%) | – |
| Delusional disorder | 1 (2.6%) | 4 (10.8%) | – |
BPRS Brief Psychiatric Rating Scale, SOFAS Social and Occupational Functioning Assessment Scale, SANS Scale for the Assessment of Negative Symptoms, DUP duration of untreated psychosis, NOS not otherwise specified.
Primary analysis
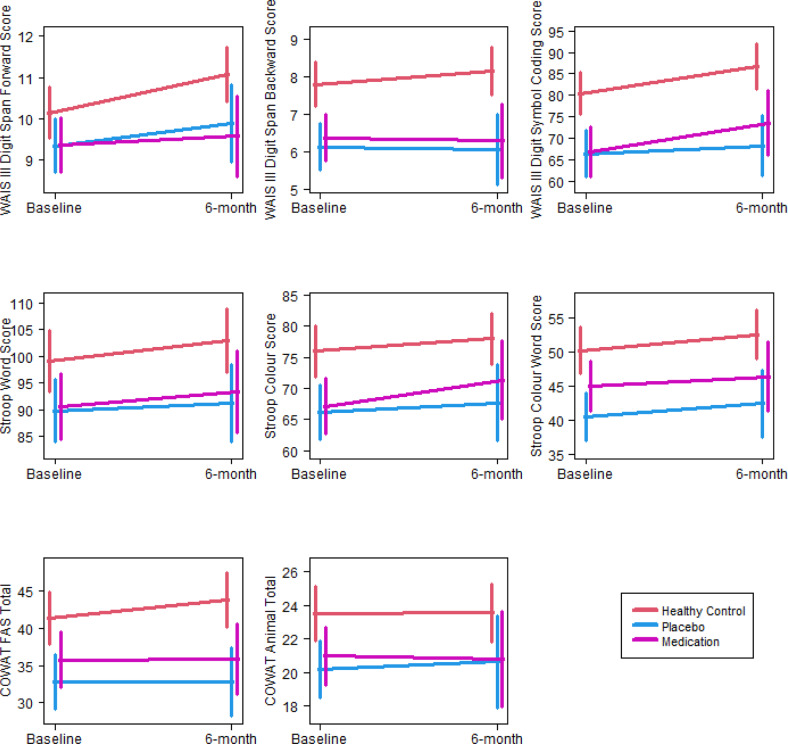
The three groups were compared on rate of change in cognitive functioning using LME analyses (Table 2). A significant effect of time was observed on tasks of attention, processing speed and cognitive control (i.e., Digit Span: forward, Digit Symbol-Coding, Stroop: colour, word, colour-word); the estimated rates of change were all positive, indicating there was a significant improvement from baseline to 6 months on these measures regardless of group. Stable cognitive performance was observed in working memory (i.e., Digit Span: backward) and verbal fluency (letter/animal). There were no group-by-time interactions on any of these measures (Fig. 2).
Table 2.
Results of LME analysis comparing the three groups on the rate of cognitive change from baseline to 6 months.
| p values | Estimated rate of change | N | ||||
|---|---|---|---|---|---|---|
| Group × time interaction | Group | Time | Coefficient | Standard error | ||
| Digit Span: forward | 0.180 | 0.029 | <0.001 | 0.023 | 0.006 | 118 |
| Digit Span: backward | 0.507 | <0.001 | 0.654 | 0.003 | 0.006 | 118 |
| Digit Symbol-Coding | 0.126 | <0.001 | <0.001 | 0.185 | 0.043 | 107 |
| Stroop: words | 0.605 | 0.026 | 0.015 | 0.107 | 0.039 | 117 |
| Stroop: colours | 0.452 | 0.001 | 0.006 | 0.096 | 0.035 | 117 |
| Stroop: colour-word | 0.879 | <0.001 | 0.016 | 0.073 | 0.030 | 117 |
| Letter fluency | 0.295 | 0.002 | 0.152 | 0.036 | 0.025 | 118 |
| Animal fluency | 0.889 | 0.011 | 0.853 | 0.003 | 0.019 | 118 |
| Immediate recall (Trial 1) | 0.023 | <0.001 | 0.138 | 0.014 | 0.009 | 118 |
| Verbal learning (Trials 1–3) | 0.024 | <0.001 | 0.457 | 0.014 | 0.020 | 118 |
| Delayed recall | 0.005 | <0.001 | 0.772 | 0.0004 | 0.007 | 117 |
Note. The p values for ‘Group × time interaction’ indicates if the three groups differ in terms of the rate of change from baseline to 6-month assessment. The p values for ‘Group’ indicate the significance of the group main effect, i.e., the overall difference between the group means. For those measures with no significant group × time interaction, this is a similar test to an ANOVA of the baseline values. The p values for ‘Time’ indicate the significance of the overall rate of change assuming there is no group × time interaction, i.e., if all the groups have the same rate of change. When there is significant group × time interaction (i.e., when there is significant difference in rate of change between the groups), then ‘Time’ may not be meaningful.
Bold values denote significant p values.
Fig. 2. Plots of estimated trends for cognitive measures with no significant group × time interaction from baseline to 6 months and associated 95% confidence intervals for the means at endpoints.
Each panel represents a different cognitive test score, with the test score shown on the y-axis and the timepoint shown on the x-axis.
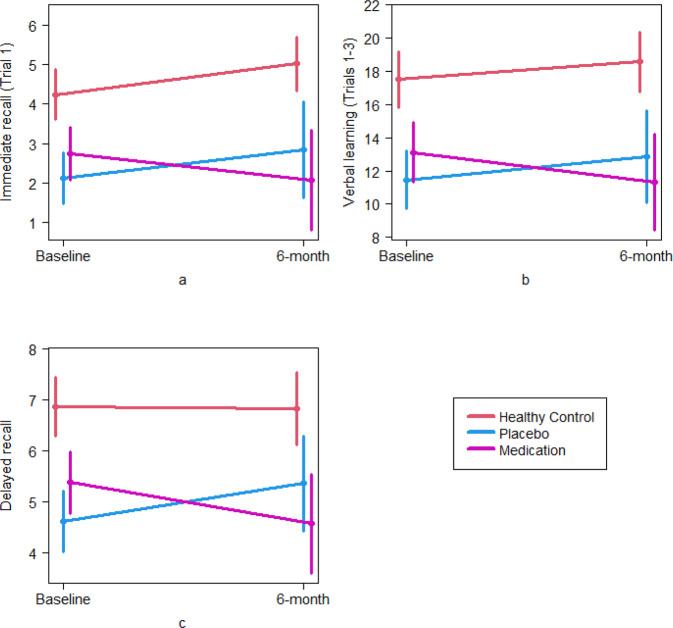
Significant group-by-time interactions were found on the verbal learning and memory task, including immediate recall (p = 0.023), verbal learning (p = 0.024) and delayed recall (p = 0.005) (Table 2). Figure 3 plots estimated trends for the three groups from baseline to 6 months on these measures. Figure 3a illustrates the difference in rate of change in immediate recall, where the healthy control and placebo groups show an improvement, and the medication group shows a deterioration. Similar patterns can be observed in Fig. 3b, c. Pairwise comparisons showed a significant decline in the medication group compared to the healthy control (p = 0.013; ηp2 = 0.074 medium effect) and placebo (p = 0.024; ηp2 = 0.062 medium effect) groups for immediate recall, as well as verbal learning (healthy control vs. medication p = 0.022; ηp2 = 0.064 medium effect; placebo vs. medication p = 0.015; ηp2 = 0.072 medium effect). For delayed recall, the medication group again showed significant decline compared to the placebo group (p = 0.001; ηp2 = 0.123 large effect); however, neither group showed a significant difference in rate of change compared to healthy controls (healthy control vs. placebo p = 0.072; ηp2 = 0.040 medium effect; healthy control vs. medication p = 0.090; ηp2 = 0.036 small effect).
Fig. 3. Plots of estimated trends for significant group × time interaction for verbal paired associate learning and memory from baseline to 6 months and associated 95% confidence intervals for the means at the endpoints.
Each panel represents a different cognitive test score (a immediate recall; b verbal learning; and c delayed recall), with the test score shown on the y-axis and the timepoint shown on the x-axis.
Per protocol analysis
During the 6-month trial the mean cumulative dose of antipsychotics (olanzapine equivalent mg) was 858.2 (SD = 512.1; range 4–2105) in the medication group and 314.5 (SD = 490.0; range 0–1773) in the placebo group, a significant difference (p < 0.001). Nineteen patients from the placebo group and 26 from the medication group stopped their allocated medication (placebo or risperidone/paliperidone) during the 6-month trial and many commenced a different medication (Table S3). Reasons for stopping were similar between groups [25] (Fig. S1).
To test the reliability of the intention-to-treat findings, a per protocol analysis was conducted including only trial completers (placebo n = 16, medication n = 11, healthy controls n = 42; Table S4; Fig. S2). Results remained similar, with significant group-by-time interactions on the verbal learning and memory task, specifically verbal learning (p = 0.025) and delayed recall (p < 0.001); with immediate recall just non-significant (p = 0.051). Pairwise comparisons showed that all three groups differed from each other in rate of change for delayed recall (healthy control vs. placebo p = 0.004; ηp2 = 0.154; healthy control vs. medication p = 0.012; ηp2 = 0.123; placebo vs. medication p < 0.001; ηp2 = 0.297; all large effects). There was a significant difference in the rate of change between the medication group and the other two groups for immediate recall (healthy control vs. medication p = 0.030; ηp2 = 0.093; placebo vs. medication p = 0.029; ηp2 = 0.093; medium–large effects) and verbal learning (healthy control vs. medication p = 0.018; ηp2 = 0.109; placebo vs. medication p = 0.010; ηp2 = 0.129; medium–large effects). Additionally, a group-by-time interaction was seen for digit symbol-coding (p = 0.016), where the healthy control group improved, but the medication and placebo groups remained stable (healthy control vs. placebo p = 0.028; ηp2 = 0.099; healthy control vs. medication p = 0.019; ηp2 = 0.111; medium–large effects).
Discussion
This pre-planned secondary analysis of a triple-blind, placebo-controlled, randomised clinical trial, with a demographically matched healthy control group, enabled us to determine the effects of psychotic disorder, antipsychotic medication, and typical changes on cognitive performance over 6 months. The prospective randomised design ensured the medication and placebo groups did not significantly differ at baseline on any cognitive measure, length of DUP, or other illness variables. Two key findings emerged. First, change in cognitive functioning was similar across the placebo, medication, and healthy control groups in most cognitive domains. This suggests that the cognitive improvement or stability observed was not specifically associated with the effects of medication or illness, as the same pattern of change was also seen in healthy controls with no history of psychosis or antipsychotic exposure. The second key finding was that allocation to the risperidone/paliperidone over 6 months was associated with a decline in verbal learning and memory. In contrast, patients who received placebo improved in verbal learning and memory at a similar rate to healthy controls. The effect sizes were medium to large. These findings were broadly similar when only trial completers were included in the analysis.
A significant implication of the findings is that partially or completely withholding antipsychotics is not harmful to cognitive functioning and may be specifically beneficial for verbal learning and memory in the early course of treatment. Experimentally lengthening the DUP (in the placebo group) was not associated with worsening of cognition or a slower rate of improvement in any domain measured. This finding is consistent with previous meta-analyses showing negligible associations between DUP and cognition [20, 21]. On most measures, the change in cognition was similar across the placebo, medication, and healthy control groups, suggesting improvements over 6 months are likely explained by typical development or practice effects [13] and not symptom improvement or medication effects.
A second key implication of the findings is that risperidone/paliperidone may worsen verbal learning and memory at least for some young FEP patients not highly suicidal or hostile and with short DUP. This finding contrasts with earlier randomised head-to-head trials showing mildly improved cognitive functioning, including verbal learning and memory, following 3–6 months of treatment with second-generation antipsychotics, including risperidone [9, 10, 12, 13]. Differences between previous studies and our study may account for this. First, previous studies did not include a placebo arm [13]. Second, participants in previous studies were older on average [9, 10, 12]. Third, we enrolled patients diagnosed with any psychotic disorder, whereas only people diagnosed with schizophrenia-spectrum disorders were included in previous trials. Perhaps most importantly, all previous trials employed wordlist learning memory tasks comprising nouns that can be imagined and semantically-encoded [9, 10, 12, 13] (one study also included a story memory task [13]). We used an associate learning task, involving concrete and abstract word-pairs, where the latter are more difficult to imagine and encode [31] (see Table S5); therefore, our task was arguably more sensitive than those used previously [29, 37]. We tested this proposition in a post hoc LME analysis of the wordlist learning component of the task and found no significant group × time interaction (Table S6). Thus, risperidone/paliperidone treatment for some young people with early course psychosis may impair novel effortful associate learning and memory.
These effects of risperidone/paliperidone on learning and memory may arise because of their strong antagonism at dopamine (D2) receptors [38]. D2 receptors are prominently expressed in brain regions involved in learning and memory, particularly the striatum, prefrontal cortex, and hippocampus [39, 40]. Previous research shows significant negative correlations between risperidone-associated extrastriatal D2/3 occupancy and cognitive performance [41] and striatal D2 occupancy and subjective cognitive effects [42]. Given antipsychotics as a group differ in their off-target effects outside of dopamine (D2) antagonism and may differ in effects on cognition as well [43], our findings cannot be generalised to all antipsychotic types.
Side-effects such as sedation, movement disorders, blurred vision and amotivation may also arise in response to taking risperidone/paliperidone [44–47], all of which can impair cognitive functioning and performance on cognitive tasks [47]. This explanation seems unlikely in this study because we found no significant difference between the FEP groups in terms of antipsychotic side-effects (including cognitive and sedative effects) as rated by the study doctors [25]. It is worth noting that we did not assess subjective effects. Subjective side-effects as listed above may have affected cognitive capacity or cognitive effort on the verbal paired-associate learning task, which was the most effortful and difficult task within the cognitive battery. Future research should measure subjective medication effects as potential mechanisms of cognitive impairment (or improvement) in psychotic disorders.
An anticholinergic mechanism could also plausibly explain the observed decline in verbal learning and memory [48–50]; however, risperidone/paliperidone have a relatively low anticholinergic profile [51]. Furthermore, the doses prescribed here, while still considered therapeutic [25], were relatively low. Use of other medications, such as antidepressants and benzodiazepines also have low anticholinergic activity [51] and did not differ between groups [25]. The anticholinergic benztropine was prescribed PRN to three cases in the medication group, but they were excluded from the completer analysis. Thus, anticholinergic effects are an unlikely explanation for the findings.
The specificity of the negative effect of risperidone/paliperidone to learning and memory is notable given impairment in this domain is associated with poorer community/vocational and social functioning in early psychosis [15], and shows decline in longitudinal studies of FEP (including in paired-associate learning) [6–8]. The contribution of medication to cognitive decline cannot be accurately determined in these cohort studies, since cumulative antipsychotic exposure is confounded with illness severity; however the current findings suggest antipsychotic medication may play a role [52]. In support of this supposition, antipsychotic dose reduction has been associated with improved cognitive functioning (including learning and memory) in naturalistic longitudinal studies [22] and RCTs [53, 54] involving FEP and schizophrenia patients.
These cognition findings must be considered within the context of the full risk: benefit profile of withholding or prescribing antipsychotics in the early stages of psychosis treatment, including symptomatology, functioning, physical and metabolic health, brain health outcomes, in addition to patient-driven personal recovery goals [55]. The STAGES study is one of the few placebo-controlled studies to examine the risks and benefits of antipsychotic treatment in FEP [56]. We have previously reported that the placebo group had comparable functioning (primary outcome), symptomatology, quality of life, adverse events and physical and metabolic outcomes to the group receiving antipsychotic medication [25, 57]. We also showed an increase in right pallidum volume in the risperidone/paliperidone group and decline in the placebo group over the first three months of the trial, suggestive of a potentially early protective effect of antipsychotic medication [27]. However, increased connectivity was observed in different brain regions across the placebo and medication groups, suggesting psychosocial treatment alone can lead to improved brain function [28]. More high-quality clinical trials are needed to support evidence-based shared decision-making among clinicians, patients, and their caregivers.
It is important to acknowledge that, in addition to the small sample, this was a distinctive and highly selective FEP sample due to the inclusion/exclusion criteria, so findings may not generalise to all FEP individuals and certainly require replication in confirmatory trials. Nevertheless, the degree of baseline cognitive impairment in the sample was comparable to other FEP samples diagnosed with schizophrenia [3]. We were not able to report on medication adherence due to a large amount of missing adherence data. Another limitation is the high percentage of participants who did not complete the trial as planned. However, the per protocol analysis was mostly consistent with the primary analysis (apart from immediate recall), suggesting results are quite robust. Finally, while regression to the mean may be an explanation for the verbal learning and memory findings, this is unlikely because the differences between the placebo and medication groups at baseline were small and non-significant (Table S2) and randomisation controls for this effect across groups [58, 59].
In conclusion, the findings highlight the importance of accounting for the potential longitudinal cognitive effects of antipsychotic medication following FEP. Careful consideration of the risks and benefits of antipsychotic initiation and maintenance is critical and should occur within a shared decision-making framework. Indeed, a recent study showed that of various antipsychotic side effects, memory impairment had the strongest influence on the medication preferences of people with schizophrenia [60]. Future research must investigate class differences in the effects and mechanisms of action of antipsychotics on various domains of cognition. If this confirms what is reported here, then efforts to address cognitive impairment in FEP should consider medication effects, alongside a focus on developing novel agents and psychosocial treatments with pro-cognitive effects.
Supplementary information
Author contributions
KA: conceptualisation, writing—original draft, methodology, funding acquisition. HPY: formal analysis, funding acquisition, writing—review and editing. LB: investigation, project administration. BO: investigation, writing—review and editing. AF: funding acquisition, writing—review and editing. SC: writing—review and editing. BN: funding acquisition, writing—review and editing. JG: investigation, project administration, writing—review and editing. MJK: investigation, project administration. TMP: methodology, writing—review and editing. AR: writing—review and editing. MAJ: funding acquisition, writing—review and editing. SH: formal analysis, funding acquisition, writing—review and editing. EB: writing—review and editing. ADT: investigation, writing—review and editing. CP: writing—review and editing. MB: writing—review and editing. PDM: funding acquisition, investigation, writing—review and editing. SMF: funding acquisition, investigation, writing—review and editing. SJW: conceptualisation, writing—review and editing.
Funding
The study was supported by Janssen-Cilag, Australia and a Project Grant from the National Health and Medical Research Council, Australia (1064704). Janssen-Cilag partially supported the early years of this study with an unrestricted investigator-initiated grant and provided risperidone, paliperidone and matched placebo for the first 30 participants. The study was then funded by an Australian National Health and Medical Research Project grant (1064704). The funders had no role in study design, data collection, data analysis, data interpretation, writing, approval, or submission of this manuscript.
Competing interests
KA was supported by a NHMRC Career Development Fellowship (1141207) and a University of Melbourne Dame Kate Campbell Fellowship. KA has received funding from the NHMRC, MRFF and Wellcome Trust—all unrelated to this work. MB is supported by a NHMRC Senior Principal Research Fellowship and Leadership 3 Investigator grant (1156072 and 2017131). MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier—all unrelated to this work. ADT has received honoraria for lectures for Otsuka Pty, Janssen and Janssen and Servier and has served on an advisory board for Servier—all unrelated to this work. BN is supported by a NHMRC Senior Research Fellowship (1137687) and a University of Melbourne Dame Kate Campbell Fellowship—all unrelated to this work. MA-J was supported by an Investigator Grant (APP1177235) from the NHMRC and a Dame Kate Campbell Fellowship from The University of Melbourne—all unrelated to this work. CP was supported by a NHMRC Senior Principal Research Fellowship (1105825), and NHMRC L3 Investigator Grant (1196508). Over the past three years, CP has received funding from NHMRC, Lundbeck Foundation, MRFF, and has received honoraria for talks at educational meetings and as a member of an advisory board for Lundbeck, Australia Pty Ltd—all unrelated to this work. The other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shona M. Francey, Stephen J. Wood.
Supplementary information
The online version contains supplementary material available at 10.1038/s41398-023-02501-7.
References
- 1.Catalan A, Salazar de Pablo G, Aymerich C, Damiani S, Sordi V, Radua J, et al. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78:859–67. doi: 10.1001/jamapsychiatry.2021.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L. Meta-analysis of cognitive performance in drug-naive patients with schizophrenia. Schizophr Res. 2014;158:156–62. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 3.Mesholam-Gately R, Giuliano AJ, Faraone SV, Goff KP, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology. 2009;23:315–36. doi: 10.1037/a0014708. [DOI] [PubMed] [Google Scholar]
- 4.Bozikas VP, Andreou C. Longitudinal studies of cognition in first episode psychosis: a systematic review of the literature. Aust NZ J Psychiatry. 2011;45:93–108. doi: 10.3109/00048674.2010.541418. [DOI] [PubMed] [Google Scholar]
- 5.Szoke A, Trandafir A, Dupont M-E, Meary A, Schurhoff F, Leboyer M. Longitudinal studies of cognition in schizophrenia: meta-analysis. Br J Psychiatry. 2008;192:248–57. doi: 10.1192/bjp.bp.106.029009. [DOI] [PubMed] [Google Scholar]
- 6.Wannan CMJ, Bartholomeusz CF, Cropley VL, Van Rheenen TE, Panayiotou A, Brewer WJ, et al. Deterioration of visuospatial associative memory following a first psychotic episode: a long-term follow-up study. Psychol Med. 2018;48:132–41. doi: 10.1017/S003329171700157X. [DOI] [PubMed] [Google Scholar]
- 7.Zanelli J, Mollon J, Sandin S, Morgan C, Dazzan P, Pilecka I, et al. Cognitive change in schizophrenia and other psychoses in the decade following the first episode. Am J Psychiatry. 2019;176:811–9. doi: 10.1176/appi.ajp.2019.18091088. [DOI] [PubMed] [Google Scholar]
- 8.Fett A-KJ, Velthorst E, Reichenberg A, Ruggero CJ, Callahan JL, Fochtmann LJ, et al. Long-term changes in cognitive functioning in individuals with psychotic disorders: findings from the Suffolk County Mental Health Project. JAMA Psychiatry. 2020;77:387–96. doi: 10.1001/jamapsychiatry.2019.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keefe RSE, Sweeney JA, Hongbin G, Hamer RM, Perkins DO, McEvoy JP, et al. Effects of olanzapine, quetiapine, and risperidone on neurocognitive function in early psychosis: a randomized, double-blind 52-week comparison. Am J Psychiatry. 2007;164:1061–71. doi: 10.1176/ajp.2007.164.7.1061. [DOI] [PubMed] [Google Scholar]
- 10.Davidson M, Galderisi S, Weiser M, Werbeloff N, Fleischhacker WW, Keefe RS, et al. Cognitive effects of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: a randomized, open-label clinical trial (EUFEST) Am J Psychiatry. 2009;166:675–82. doi: 10.1176/appi.ajp.2008.08060806. [DOI] [PubMed] [Google Scholar]
- 11.Ayesa-Arriola R, Rodríguez-Sánchez JM, Pérez-Iglesias R, Roiz-Santiáñez R, Martínez-García O, Sánchez-Moreno J, et al. Long-term (3-year) neurocognitive effectiveness of antipsychotic medications in first-episode non-affective psychosis: randomized comparison of haloperidol, olanzapine & risperidone. Psychopharmacology. 2013;227:615–25. doi: 10.1007/s00213-013-2994-z. [DOI] [PubMed] [Google Scholar]
- 12.Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry. 2005;162:1888–95. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg TE, Goldman RS, Burdick KE, Malhotra AK, Lencz T, Patel RC, et al. Cognitive improvement after treatment with second-generation antipsychotic medications in first-episode schizophrenia. Is it a practice effect? Arch Gen Psychiatry. 2007;64:1115–22. doi: 10.1001/archpsyc.64.10.1115. [DOI] [PubMed] [Google Scholar]
- 14.Harvey PD, Keefe RSE. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–84. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- 15.Cowman M, Holleran L, Lonergan E, O’Connor K, Birchwood M, Donohoe G. Cognitive predictors of social and occupational functioning in early psychosis: a systematic review and meta-analysis of cross-sectional and longitudinal data. Schizophr Bull. 2021;47:1243–53. doi: 10.1093/schbul/sbab033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santesteban-Echarri O, Paino M, Rice S, Gonzalez-Blanch C, McGorry P, Gleeson J, et al. Predictors of functional recovery in first-episode psychosis: a systematic review and meta-analysis of longitudinal studies. Clin Psychol Rev. 2017;58:59–75. doi: 10.1016/j.cpr.2017.09.007. [DOI] [PubMed] [Google Scholar]
- 17.Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40:744–55. doi: 10.1093/schbul/sbt085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mollon J, David AS, Zammit S, Lewis G, Reichenberg A. Course of cognitive development from infancy to early adulthood in the psychosis spectrum. JAMA Psychiatry. 2018;75:270–9. doi: 10.1001/jamapsychiatry.2017.4327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnstone EC, Owens DGC, Crow TJ, Davis JM. Does a four-week delay in the introduction of medication alter the course of functional psychosis? J Psychopharmacol. 1999;13:238–44. doi: 10.1177/026988119901300305. [DOI] [PubMed] [Google Scholar]
- 20.Allott K, Fraguas D, Bartholomeusz CF, Díaz-Caneja CM, Wannan C, Parrish EM, et al. Duration of untreated psychosis and neurocognitive functioning in first-episode psychosis: a systematic review and meta-analysis. Psychol Med. 2018;48:1592–607. doi: 10.1017/S0033291717003002. [DOI] [PubMed] [Google Scholar]
- 21.Bora E, Yalincetin B, Akdede BB, Alptekin K. Duration of untreated psychosis and neurocognition in first-episode psychosis: a meta-analysis. Schizophr Res. 2018;193:3–10. doi: 10.1016/j.schres.2017.06.021. [DOI] [PubMed] [Google Scholar]
- 22.Albert N, Randers L, Allott K, Jensen HD, Melau M, Hjorthøj C, et al. Cognitive functioning following discontinuation of antipsychotic medication. A naturalistic sub-group analysis from the OPUS II trial. Psychol Med. 2019;49:1138–47. doi: 10.1017/S0033291718001836. [DOI] [PubMed] [Google Scholar]
- 23.Husa A, Moilanen J, Murray GK, Marttila R, Haapea M, Rannikko I, et al. Lifetime antipsychotic medication and cognitive performance in schizophrenia at age 43 years in a general population birth cohort. Psychiatry Res. 2017;247:130–8. doi: 10.1016/j.psychres.2016.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh A, Kumar V, Pathak H, Jacob AA, Venkatasubramanian G, Varambally S, et al. Effect of antipsychotic dose reduction on cognitive function in schizophrenia. Psychiatry Res. 2022;308:114383. doi: 10.1016/j.psychres.2021.114383. [DOI] [PubMed] [Google Scholar]
- 25.Francey SM, O’Donoghue B, Nelson B, Graham J, Baldwin L, Yuen HP, et al. Psychosocial intervention with or without antipsychotic medication for first-episode psychosis: randomized noninferiority clinical trial. Schizophr Bull Open. 2020;1:sgaa015.
- 26.O’Donoghue B, Francey SM, Nelson B, Ratheesh A, Allott K, Graham J, et al. Staged treatment and acceptability guidelines in early psychosis study (STAGES): a randomized placebo controlled trial of intensive psychosocial treatment plus or minus antipsychotic medication for first-episode psychosis with low-risk of self-harm or aggression. Study protocol and baseline characteristics of participants. Early Interv Psychiatry. 2019;13:953–60. doi: 10.1111/eip.12716. [DOI] [PubMed] [Google Scholar]
- 27.Chopra S, Fornito A, Francey SM, O’Donoghue B, Cropley V, Nelson B, et al. Differentiating the effect of antipsychotic medication and illness on brain volume reductions in first-episode psychosis: a longitudinal, randomised, triple-blind, placebo-controlled MRI study. Neuropsychopharmacology. 2021;46:1494–501. doi: 10.1038/s41386-021-00980-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopra S, Francey S, O’Donoghue B, Sabaroedin K, Arnatkeviciute A, Cropley V, et al. Functional connectivity in antipsychotic-treated and antipsychotic-naive patients with first-episode psychosis and low risk of self-harm or aggression: secondary analysis of a randomized clinical trial. JAMA Psychiatry. 2021;78:994–1004. doi: 10.1001/jamapsychiatry.2021.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MD Lezak, DB Howieson, DW Loring, Neuropsychological assessment, 4th edition. New York, NY: Oxford University Press; 2004.
- 30.Savage GR, Saling MM, Davis CW, Berkovic SF. Direct and indirect measures of verbal relational memory following anterior temporal lobectomy. Neuropsychologia. 2002;40:302–16. doi: 10.1016/S0028-3932(01)00092-6. [DOI] [PubMed] [Google Scholar]
- 31.Marschark M, Hunt RR. A reexamination of the role of imagery in learning and memory. J Exp Psychol Learn Mem Cogn. 1989;15:710–20. doi: 10.1037/0278-7393.15.4.710. [DOI] [PubMed] [Google Scholar]
- 32.Ben-Shachar M, Ludecke D, Makowski D. effectsize: Estimation of effect size indices and standardized parameter. J Open Sci Softw. 2020;5:2815. doi: 10.21105/joss.02815. [DOI] [Google Scholar]
- 33.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c869. [DOI] [PMC free article] [PubMed]
- 34.Rothman KJ, Greenland S, Lash T. Modern epidemiology, 3rd edition. Philadelphia, PA: Wolters Kluwer Health; 2008.
- 35.Li G, Taljaard M, Van den Heuvel ER, Levine MA, Cook DJ, Wells GA, et al. An introduction to multiplicity issues in clinical trials: the what, why, when and how. Int J Epidemiol. 2017;46:746–55. doi: 10.1093/ije/dyw320. [DOI] [PubMed] [Google Scholar]
- 36.Pinheiro J, Bates D, DebRoy S, Sarker D, R Core Team. nlme: Linear and nonlinear mixed effects models. R package version 3. 2020;1–148.
- 37.Harvey PD. Domains of cognition and their assessment. Dialog Clin Neurosci. 2019;21:227–37. doi: 10.31887/DCNS.2019.21.3/pharvey. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lako IM, van den Heuvel ER, Knegtering H, Bruggeman R, Taxis K. Estimating dopamine D2 receptor occupancy for doses of 8 antipsychotics: a meta-analysis. J Clin Psychopharmacol. 2013;33:675–81. doi: 10.1097/JCP.0b013e3182983ffa. [DOI] [PubMed] [Google Scholar]
- 39.Suhara T, Sudo Y, Okauchi T, Maeda J, Kawabe K, Suzuki K, et al. Extrastriatal dopamine D2 receptor density and affinity in the human brain measured by 3D PET. Int J Neuropsychopharmacol. 1999;2:73–82. doi: 10.1017/S1461145799001431. [DOI] [PubMed] [Google Scholar]
- 40.Goldman-Rakic PS. Dopamine-mediated mechanisms of the prefrontal cortex. Semin Neurosci. 1992;4:149–59. doi: 10.1016/1044-5765(92)90013-R. [DOI] [Google Scholar]
- 41.Nørbak-Emig H, Ebdrup B, Fagerlund B, Svarer C, Rasmussen H, Friberg L, et al. Frontal D2/3 receptor availability in schizophrenia patients before and after their first antipsychotic treatment: Relation to cognitive functions and psychopathology. Int J Neuropsychopharmacol. 2016;19:006. doi: 10.1093/ijnp/pyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Haan L, Lavalaye J, Linszen D, Dingemans PM, Booij J. Subjective experience and striatal dopamine D(2) receptor occupancy in patients with schizophrenia stabilized by olanzapine or risperidone. Am J Psychiatry. 2000;157:1019–20. doi: 10.1176/appi.ajp.157.6.1019. [DOI] [PubMed] [Google Scholar]
- 43.Baldez D, Biazus T, Rabelo-da-Ponte F, Nogaro G, Martins D, Kunz M, et al. Effect of antipsychotics on the cognitive performance of individuals with psychotic disorders: network meta-analyses of randomized controlled trials. Neurosci Biobehav Rev. 2021;126:265–75. doi: 10.1016/j.neubiorev.2021.03.028. [DOI] [PubMed] [Google Scholar]
- 44.Leucht S, Cipriani A, Spineli L, Mavridis D, Orey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382:951–62. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 45.Thompson J, Stansfeld J, Cooper R, Morant N, Crellin N, Moncrieff J. Experiences of taking neuroleptic medication and impacts on symptoms, sense of self and agency: systematic review and thematic synthesis of qualitative data. Soc Psychiatry Psychiatr Epidemiol. 2020;55:151–64. doi: 10.1007/s00127-019-01819-2. [DOI] [PubMed] [Google Scholar]
- 46.Read J, Williams J. Positive and negative effects of antipsychotic medication: an international online survey of 832 recipients. Curr Drug Saf. 2019;14:173–81. doi: 10.2174/1574886314666190301152734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moncrieff J, Cohen D, Mason JP. The subjective experience of taking antipsychotic medication: a content analysis of Internet data. Acta Psychiatr Scand. 2009;120:102–11. doi: 10.1111/j.1600-0447.2009.01356.x. [DOI] [PubMed] [Google Scholar]
- 48.Ballesteros A, Sanchez-Torres AM, Lopez-Ilundain JM, Cabrera B, Lobo A, Gonzalez-Pinto AM, et al. Is cognitive impairment associated with antipsychotic dose and anticholinergic equivalent loads in first-episode psychosis? Psychol Med. 2018;48:2247–56. doi: 10.1017/S0033291717003774. [DOI] [PubMed] [Google Scholar]
- 49.Baitz HA, Thornton AE, Procyshyn RM, Smith GN, MacEwan GW, Kopala LC, et al. Antipsychotic medications: linking receptor antagonism to neuropsychological functioning in first episode psychosis. J Int Neuropsychol Soc. 2012;18:717–27. doi: 10.1017/S1355617712000343. [DOI] [PubMed] [Google Scholar]
- 50.Verdoux H, Quiles C, Bon L, Chéreau-Boudet I, Dubreucq J, Fiegi L, et al. Impact of anticholinergic load on functioning and cognitive performances of persons with psychosis referred to psychosocial rehabilitation centers. Psychol Med. 2021;51:2789–97. [DOI] [PubMed]
- 51.Salahudeen MS, Duffull SB, Nishtala PS. Anticholinergic burden quantified by anticholinergic risk scales and adverse outcomes in older people: a systematic review. BMC Geriatr. 2015;15:31. doi: 10.1186/s12877-015-0029-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jonas K, Abi-Dargham A, Kotov R. Two hypotheses on the high incidence of dementia in psychotic disorders. JAMA Psychiatry. 2021;78:1305–6. doi: 10.1001/jamapsychiatry.2021.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faber G, Smid H, Van Gool AR, Wiersma D, Van den Bosch RJ. The effects of guided discontinuation of antipsychotics on neurocognition in first onset psychosis. Eur Psychiatry. 2012;27:275–80. doi: 10.1016/j.eurpsy.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi H, Suzuki T, Remington G, Bies RR, Abe T, Graff-Guerrero A, et al. Effects of risperidone and olanzapine dose reduction on cognitive function in stable patients with schizophrenia: open-label, randomized, controlled, pilot study. Schizophr Bull. 2013;39:993–8. doi: 10.1093/schbul/sbt090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Francey SM, Nelson B, Thompson A, Parker AG, Kerr M, Macneil C, et al. Who needs antipsychotic medication in the earliest stages of psychosis? A reconsideration of benefits, risks, neurobiology and ethics in the era of early intervention. Schizophr Res. 2010;119:1–10. doi: 10.1016/j.schres.2010.02.1071. [DOI] [PubMed] [Google Scholar]
- 56.Jauhar S, Lawrie SM. What is the evidence for antipsychotic medication and alternative psychosocial interventions for people with acute, non-affective psychosis? Lancet Psychiatry. 2022;9:253–60. doi: 10.1016/S2215-0366(21)00293-5. [DOI] [PubMed] [Google Scholar]
- 57.O’Donoghue B, Allott K, Harrigan S, Scalzo F, Ward J, Mallawaarachchi S, et al. Isolating the impact of antipsychotic medication on metabolic health: secondary analysis of a randomized controlled trial of antipsychotic medication versus placebo in antipsychotic medication naïve first-episode psychosis (the STAGES study). Early Interv Psychiatry. 2022. 10.1111/eip.13353. [DOI] [PMC free article] [PubMed]
- 58.Linden A. Assessing regression to the mean effects in health care initiatives. BMC Med Res Methodol. 2013;13:119. doi: 10.1186/1471-2288-13-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol. 2004;34:215–20. doi: 10.1093/ije/dyh299. [DOI] [PubMed] [Google Scholar]
- 60.McCrone P, Mosweu I, Yi D, Ruffell T, Dalton B, Wykes T. Patient preferences for antipsychotic drug side effects: a discrete choice experiment. Schizophr Bull Open. 2021;2:sgab046.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.