Abstract
Herein, a new heterogeneous catalytic system through modification of urea functionalized magnetic nanoparticles with choline chloride [Fe3O4@SiO2@urea-riched ligand/Ch-Cl] was designed and synthesized. Then, the synthesized Fe3O4@SiO2@urea-riched ligand/Ch-Cl was characterized by using FT-IR spectroscopy, FESEM, TEM, EDS-Mapping, TGA/DTG and VSM techniques. After that, the catalytic usage of Fe3O4@SiO2@urea-riched ligand/Ch-Cl was investigated for the synthesis of hybrid pyridines with sulfonate and/or indole moieties. Delightfully, the outcome was satisfactory and the applied strategy represents several advantages such as short reaction times, convenience of operation and relatively good yields of obtained products. Moreover, the catalytic behavior of several formal homogeneous DESs was investigated for the synthesis of target product. In addition, a cooperative vinylogous anomeric-based oxidation pathway was suggested as rational mechanism for the synthesis of new hybrid pyridines.
Subject terms: Catalysis, Organic chemistry, Chemical synthesis
Introduction
Deep eutectic solvents (DESs) as an emerging alternative to ionic liquids and organic solvents have a brilliant breakthrough in many scientific areas1–4. Low vapor pressure, tunable physiochemical properties, high polarity, biodegradability, greener and excellent catalytic activity of DESs are usually highlighted5–8. Recently, DESs linked to heterogeneous supports, as a new subclass of heterogeneous systems, have a creating and implementing beneficial and neoteric chemical transformations9–11. DESs have applied for modification of the surface of some materials such as magnetic nanoparticles (MNPs), metal organic frameworks (MOFs), silicates, covalent organic frameworks (COFs), biopolymers and etc.12–16. So, it can be said that these compounds are the forefront of post-synthetic modifications17. In this respect, nanomagnetic supported DESs (MDESs) is oriented toward fundamental and synthesis researches. MDESs due to easy reusing and recoverability and workup simplicity create an ingenious insight in many academic and industrial areas18–21. These materials have significant potentials and applications in several outlines including solar cells22, electrochemistry23, redox flow batteries24, supercapacitors25, biosensors26 and chemical separation processes27. Also, due to the simultaneous presence of acidic and basic functional groups and response to external magnetic field, MDESs are a prosperous assortment as sustainable media as well as catalysts in many of organic transformations such as coupling reactions28–30 and multi-component reactions31,32. Recently, there are several studies from catalytic applications of MDESs in multi-component reactions33–35. A number of catalytic applications of DESs in multi-component reactions are sketched in below (Fig. 1)33,36–40.
Figure 1.
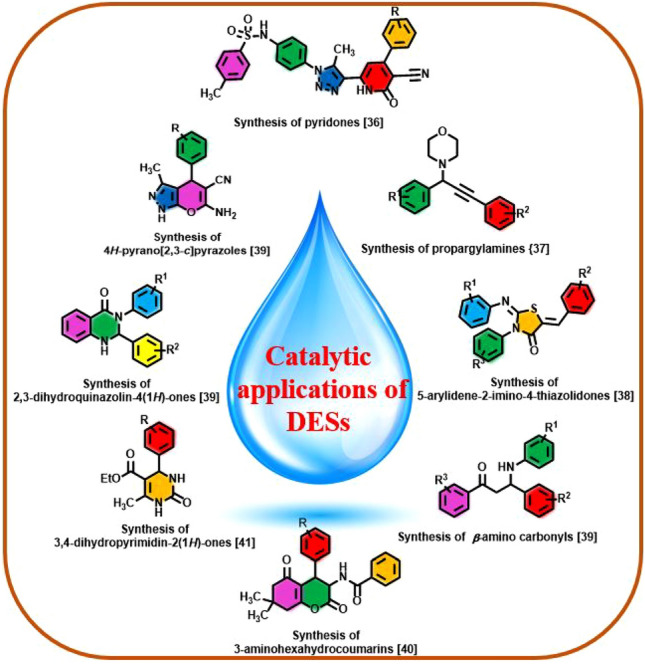
A number of catalytic applications of DESs in multi-component reactions.
Despite extensive research on magnetic nanoparticles, these particles are still being researched by scientists due to their unique features and capabilities. These particles have the ability to be a suitable substrate for connecting organic groups. Magnetic nanoparticles have unique properties such as low toxicity, low cost, high magnetic property, compatibility with the environment, high surface area, chemical stability41–43.
These particles are used as sensors, absorption of organic substances (paints, pharmaceuticals, etc.), water disinfection, wastewater treatment, as well as in the field of biomedicine and cancer treatment44,45.
Another prominent application of magnetic nanoparticles is their usage as catalysts, which attracted a lot of attention among scientists due to their availability, easy recovery, and reusability46,47. Photocatalysis, oxidation/reduction reactions, multi-component reactions, photoelectrochemical catalysis, coupling reactions and chiral catalysis are examples of catalytic applications of MNPs48–50. Therefore, immobilizing catalytically active species on magnetic substrates while maintaining or improving their activity also allows for easy separation. For this reason, these catalytic systems are superior to homogeneous systems.
Molecular hybridization due to its compositional characterization is an interesting structural modification approach which are including of the incorporation of two or more pharmacophores into a single molecule51,52. The unique performance of hybrid heterocycles is based on the recognition of pharmacophoric moieties in two or more biologically active molecules which preserved or promoted pre-selected properties of the original templates52,53. Hybrid pyridines as distinguished scaffolds of heterocyclic compounds not only are extensively present in pharmaceutical active molecules, agricultural compounds and functional materials54–58 but also are one of the most top-selling drugs and can serve as treatment of Alzheimer’s diseases59, anticancer60 and antihypertensive61. Intriguingly, hybrid molecules bearing pyridine, indole and sulfonate moieties have exceptional potential in pharmaceutical and medicinal chemistry such as antioxidant, antiapoptosis, antidyslipidemia, antitumorigenic, antiinflammatory and can enhance the stability and solubility of drugs62–67. A number of biologically active hybrid molecules bearing pyridine, indole and sulfonate moieties are sketched in Fig. 2.
Figure 2.
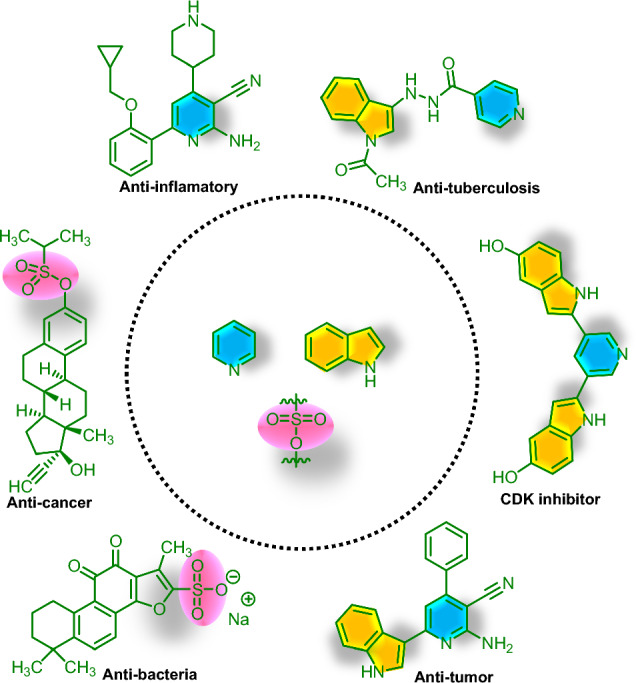
A number of biologically active hybrid molecules.
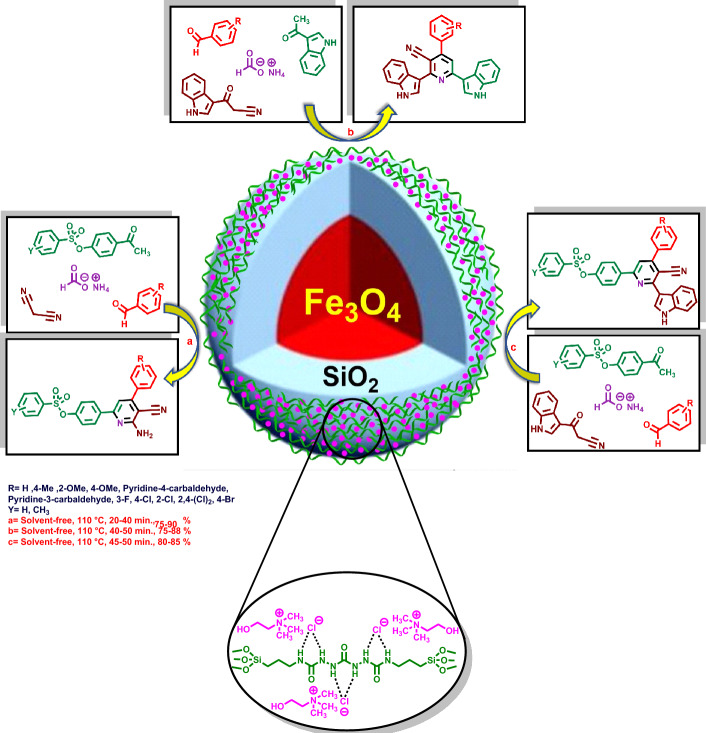
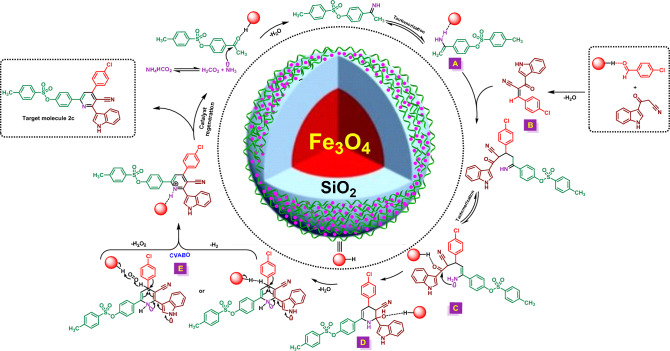
In this investigation, after synthesis of Fe3O4@SiO2@urea-riched ligand/Ch-Cl, pyridines with indole and/or sulfonate moieties were synthesized in the presence of Fe3O4@SiO2@urea-riched ligand/Ch-Cl via a multi-component reaction strategy68,69 (Figs. 3, 4). Also, a cooperative vinylogous anomeric-based oxidation pathway was suggested as plausible mechanism for the synthesis of new hybrid pyridines70–84.
Figure 3.
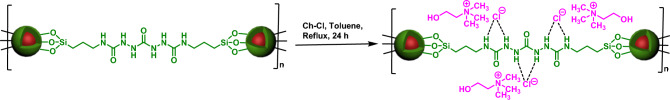
General procedure for the synthesis of Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
Figure 4.
Synthesis of hybrid pyridines bearing indole and/or sulfonate moieties by using Fe3O4@SiO2@urea-riched ligand/Ch-Cl as catalyst.
Results and discussion
A literature survey shows that in heterocyclic chemistry as a significant category of organic chemistry, pyridine plays as same as benzene in the concept of aromaticity. On the other hand, hybrid pyridines are the most heterocycle molecules which have been used for various purposes such as medicinal drugs, agricultural adducts, dyes, polymers etc.85–88. Therefore, development of hybrid pyridines is one of our main research interests. With this aim, herein we wish to report a new catalytic system for preparation of new hybrid pyridines with aryl, indole and sulfonate moieties.
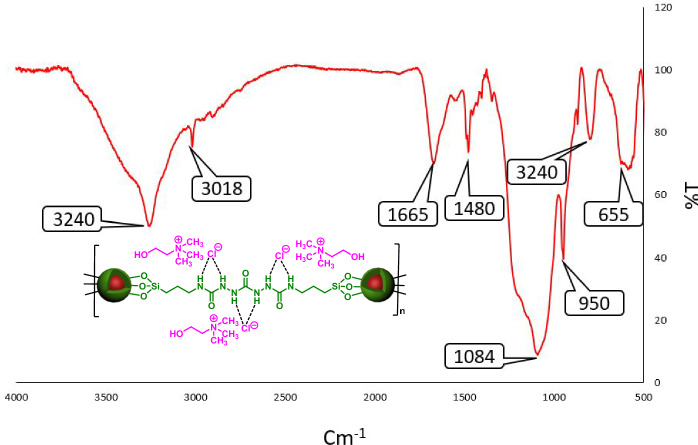
After synthesis of Fe3O4@SiO2@urea-riched ligand/Ch-Cl, we focused on the precise characterization of its structure. Firstly, FT-IR spectrum of catalyst were investigated (Fig. 5). According to FT-IR spectrum of Fe3O4@SiO2@urea-riched ligand/Ch-Cl, the clear peak of C=O is appeared at 1665 cm−1. The vibrational modes of Fe–O, Si–O and NH groups are respectively shown at 655, 1084 and 3240 cm−1. Moreover, the broad peak about of 3200 cm−1 confirmed the existence of hydroxy group of Ch-Cl and free hydroxy groups in the surface of Fe3O4.
Figure 5.
FT-IR spectrum of Fe3O4@SiO2@urea-riched ligand /Ch-Cl.
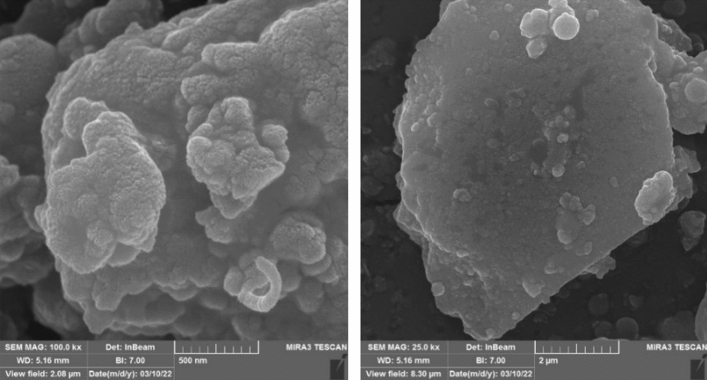
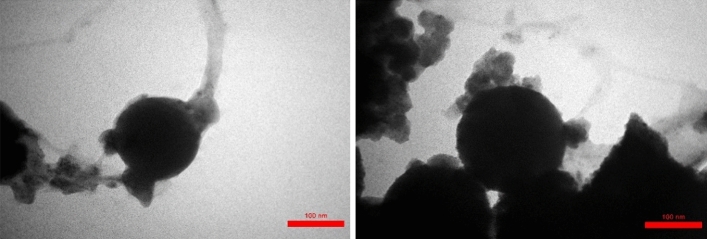
Field emission scanning electron microscopy (FESEM) analysis was recorded to check the morphology of Fe3O4@SiO2@urea-riched ligand /Ch-Cl. According to relevant images (Fig. 6), catalyst has a spherical and uniform shapes and its size is in the range of nanometers. Also, TEM analysis was investigated to confirm the formation of the catalyst with spherical morphology and the presence of organic layers on the surface of magnetic nanoparticles is well confirmed (Fig. 7).
Figure 6.
FESEM images Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
Figure 7.
TEM images of Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
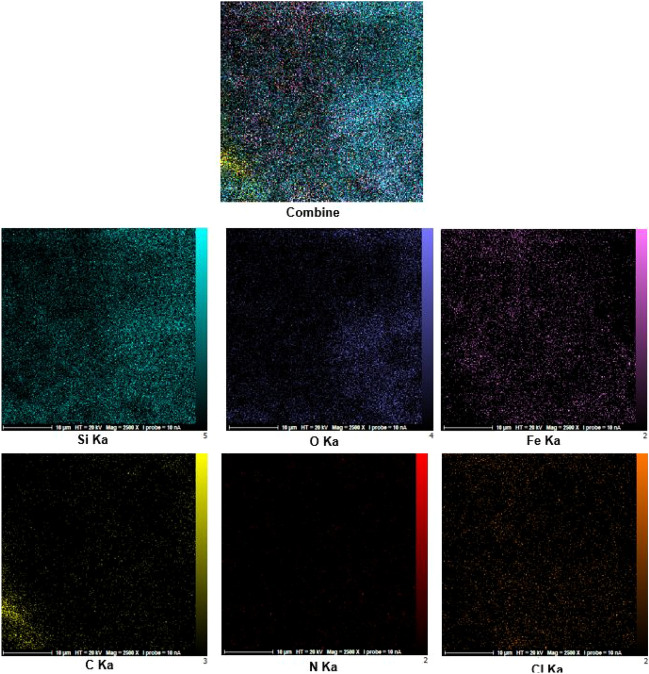
Energy-dispersive X-ray spectroscopy (EDS) analysis was used for the examination of expected elements within the catalyst structure. As predicted, the elements of C, N, O, Fe, Cl and Si are presented in the structure of desired catalyst (Fig. 8). In addition, elemental mapping analysis shows how the elements are dispersed and confirmed the existence of abovementioned expected elements in the structure of catalyst (Fig. 9).
Figure 8.
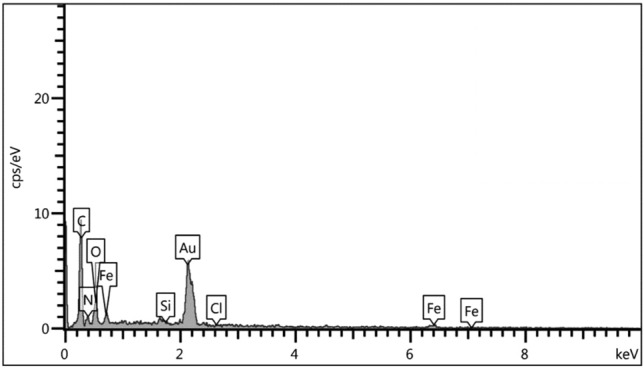
EDS analysis diagram of Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
Figure 9.
Elemental mapping analysis of Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
For investigation of magnetic properties of target catalyst, VSM technique was performed for Fe3O4@SiO2@urea-riched ligand/Ch-Cl. According to revealed results, the saturation magnetization of Fe3O4@SiO2@urea-riched ligand/Ch-Cl is about 27 emu/g which is enough for the easy separation of the catalyst from the reaction mixture (Fig. 10).
Figure 10.
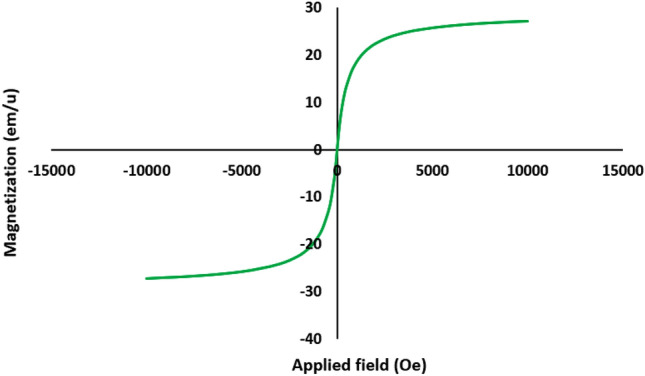
Magnetization curves of Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
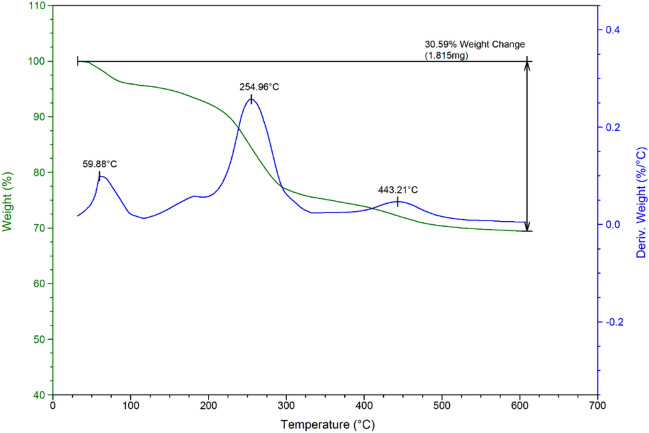
Thermal stability is another important factor for MDES systems. Therefore, we investigated the thermal stability of the catalyst by using TGA/DTG analysis (Fig. 11). When the catalyst is exposed to the thermal conditions up to 600 °C, two main weight losses are observed at temperatures 255 and 443 °C. The poor weight losses below 110 °C is related to removing of trapped solvents and the significant weight losses at 255 °C is related to decomposition of organic layers. Therefore, it can be said that the catalyst is thermally stable up to this temperature. In an overview, the decrease in the weight of the catalyst by 30.59% indicates the presence of a significant amount of organic ligand on the Fe3O4 surface.
Figure 11.
TGA/DTG curves of Fe3O4@SiO2@urea-riched ligand/Ch-Cl.
At the outset of the synthesis of hybrid pyridines, benzaldehyde, 4-acetylphenyl 4-methylbenzenesulfonate, malononitrile and ammonium format were chosen as model substrates for multi-component synthesis of pyridine derivatives. At the first, the reaction conducted in the presence of different amounts of catalyst such as 5, 10 and 20 mg. Also, the model reaction was tested in the absence of any catalyst. Anyway, the best result was obtained by using 10 mg of catalyst. Subsequent study on the effect of the temperature parameter displayed that 110 °C is the most suitable temperature to provide activation energy for the model reaction. After that, for the investigation of solvent effect, the model reaction was performed in several formal polar and nonpolar solvents and also, solvent free conditions. Nonetheless, due to high yield and low toxicity of reaction and simplicity of work up, solvent free conditions were chosen as proper conditions. More details are given in the Table 1. The bold values indicates the optimal reaction conditions.
Table 1.
Optimization of reaction conditions for synthesis of 1aa.
| Entry | Solvent | Temperature (°C) | Catalyst loading (mg) | Time (min.) | Yield (%)b |
|---|---|---|---|---|---|
| 1c | – | 110 | 10 | 30 | 75 |
| 2 | – | 110 | 20 | 40 | 60 |
| 3 | – | 110 | 5 | 70 | 50 |
| 4 | – | 110 | – | 60 | 20 |
| 5 | – | 110 | – | 120 | 38 |
| 6 | – | 100 | 10 | 50 | 80 |
| 7 | – | 90 | 10 | 70 | 70 |
| 8 | – | 80 | 10 | 100 | 40 |
| 9 | – | 70 | 10 | 180 | 35 |
| 10 | H2O | Reflux | 10 | 60 | – |
| 11 | EtOH | Reflux | 10 | 60 | 50 |
| 12 | n-Hexane | Reflux | 10 | 60 | – |
| 13 | EtOAc | Reflux | 10 | 60 | – |
| 14 | CHCl3 | Reflux | 10 | 60 | – |
aReaction conditions: 4-acetylphenyl 4-methylbenzenesulfonate (1 mmol, 0.290 g), malononitrile (1 mmol, 0.066 g), ammonium format (1.5 mmol, 0.094 g), benzaldehyde (1 mmol, 0.106 g), bIsolated yields. cOptimal data.
Significant values are given in bold.
In a separate study, the model reaction was performed in the presence of formal homogeneous DESs. For this purpose, several selected homogeneous DESs was prepared89–94 and were used as catalyst for the model reaction. Distinguishingly, these materials have a good response to the synthesis of target molecule and all of products have a relatively good yield (Table 2). Nevertheless, devoid of suitable recycling and reusing of the catalyst in homogeneous systems is one of the defecting of these systems, while Fe3O4@SiO2@urea-riched ligand/Ch-Cl as heterogeneous catalyst can easily recycled and reused.
Table 2.
Investigation of catalytic performance of selected homogeneous DESs upon model reaction for 1aa.
| Entry | Catalyst | The amount of catalyst | Yield (%) |
|---|---|---|---|
| 1 | Fe3O4@SiO2@urea-riched ligand/Ch-Cl | 10 mg | 75 |
| 2 | Ch-Cl/urea | 10 mol% | 77 |
| 3 | Ch-Cl/thiourea | 10 mol% | 66 |
| 4 | Ch-Cl/Citric acid | 10 mol% | 70 |
| 5 | Ch-Cl/Benzamide | 10 mol% | 60 |
| 6 | Ch-Cl/ Ascorbic acid | 10 mol% | 65 |
| 7 | Ch-Cl/ Benzoic acid | 10 mol% | 70 |
| 8 | Ch-Cl/Acetamide | 10 mol% | 60 |
| 9 | Ch-Cl/ Glycerol | 10 mol% | 55 |
aReaction conditions: 4-acetylphenyl 4-methylbenzenesulfonate (1 mmol, 0.290 g), malononitrile (1 mmol, 0.066 g), ammonium format (1.5 mmol, 0.094 g), benzaldehyde (1 mmol, 0.106 g), solvent-free, 110 °C, 30 min.
For the validation of the importance of target catalyst, the model reaction was also performed in the presence of relative intermediates of Fe3O4@SiO2@urea-riched ligand/Ch-Cl and some of formal catalysts such as Lewis acids, protic acids, hydrogen bond and basic catalysts. Using Fe3O4@SiO2@urea-riched ligand/Ch-Cl as catalyst gave the best yield. It goes without saying that using of urea, thiourea and K2CO3 as catalyst have relatively good yield. Nevertheless, these catalysts do not have the ability to recycle and reuse, which are the basic capabilities of a complete catalyst. (Table 3).
Table 3.
Comparative investigation of catalytic performance of Fe3O4@SiO2@urea-riched ligand/Ch-Cl and its relative intermediates as well as some known catalysts upon model reaction for 1aa.
| Entry | Catalyst | The amount of catalyst | Yield (%) |
|---|---|---|---|
| 1 | Fe3O4 | 10 mg | 40 |
| 2 | Fe3O4@SiO2 | 10 mg | 40 |
| 3 | Fe3O4@SiO2@urea-riched ligand | 10 mg | 70 |
| 4 | Fe3O4@SiO2@urea-riched ligand/Ch-Cl | 10 mg | 75 |
| 5 | Ch-Cl | 10 mol% | 50 |
| 6 | FeCl3 | 10 mol% | 50 |
| 7 | Al(HSO4)3 | 10 mol% | 45 |
| 8 | Ca(HSO4)2 | 10 mol% | 35 |
| 9 | Zn(HSO4)2 | 10 mol% | 35 |
| 10 | Fe(HSO4)3 | 10 mol% | 40 |
| 11 | NH2SO3H | 10 mol% | 45 |
| 12 | H3BO3 | 10 mol% | 40 |
| 13 | Urea | 10 mol% | 75 |
| 14 | Thiourea | 10 mol% | 60 |
| 15 | K2CO3 | 10 mol% | 70 |
| 16 | NaOH | 10 mol% | Trace |
| 17 | C5H5N | 10 mol% | 45 |
aReaction conditions: 4-acetylphenyl 4-methylbenzenesulfonate (1 mmol, 0.290 g), malononitrile (1 mmol, 0.066 g), ammonium format (1.5 mmol, 0.094 g), benzaldehyde (1 mmol, 0.106 g), solvent-free, 110 °C, 30 min.
In a comparative and precise study, for the investigation of the ability of ammonium format as reagent, we used several ammonium sources including ammonium format, ammonium acetate, ammonium sulfate, ammonium carbonate, ammonium florid, ammonium dichromate, ammonium chloride and ammonium nitrate upon model reaction. According to revealed results (Table 4), ammonium format revealed better performance for the synthesis of 1a molecule.
Table 4.
Comparison of different ammonium sources as reagent upon model reaction for 1aa.
| Entry | Reagent | Yield (%) |
|---|---|---|
| 1 | NH4HCO2 | 75 |
| 2 | NH4OAc | 70 |
| 3 | (NH4)2SO4 | – |
| 4 | (NH4)2CO3 | 60 |
| 5 | NH4F | – |
| 6 | (NH4)2Cr2O7 | 50 |
| 7 | NH4Cl | – |
| 8 | NH4NO3 | 35 |
aReaction conditions: 4-acetylphenyl 4-methylbenzenesulfonate (1 mmol, 0.290 g), malononitrile (1 mmol, 0.066 g), benzaldehyde (1 mmol, 0.106 g), ammonium source (1.5 mmol), solvent-free, 110 °C, 30 min.
Significant values are given in bold.
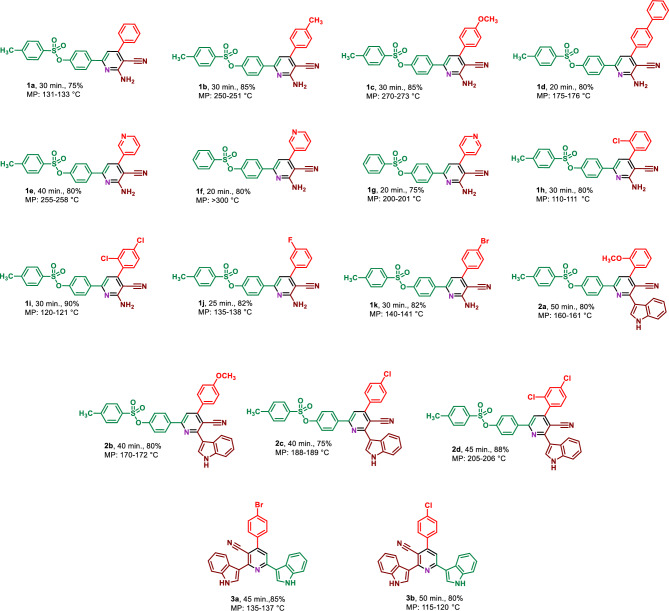
Based on the in-hand results of optimization reactions, the generality of the reaction for synthesis of various hybrid pyridines was investigated. For this purpose, variety of aromatic aldehydes with electron-poor or electron-rich aryl groups, three different methyl ketones bearing indole or sulfonate groups and malononitrile or 3-(1H-indol-3-yl)-3-oxopropanenitrile were applied for the synthesis of hybrid pyridine derivatives. The tolerance of the reaction to diverse starting materials displayed the broad application scope of the present route in the synthesis of complex hybrid pyridines (Table 5).
Table 5.
Synthesis of hybrid pyridines with indole and sulfonate moieties by using Fe3O4@SiO2@urea-riched ligand /Ch-Cla.
aReaction conditions: 4-acetylphenyl 4-methylbenzenesulfonate (1 mmol, 0.290 g) or 3-acetyl indole (1 mmol, 0.1590) malononitrile (1 mmol, 0.066 g) or 3-(1H-indol-3-yl)-3-oxopropanenitrile (1 mmol, 0.184 g), ammonium format (1.5 mmol, 0.094 g), benzaldehyde (1 mmol, 0.106 g), catalyst (10 mg), solvent-free, 110 °C.
Based on our knowledge from the synthesis of hybrid pyridine rings, we suggested a plausible mechanism for the synthesis of 2c (Fig. 12). At the first step, the carbonyl functional group of 4-acetylphenyl 4-methylbenzenesulfonate is activated with catalyst and reacted with ammonia (arisen from thermal dissociation of ammonium format) and via a tautomerization process gives intermediate A. In another part of the reaction, aldehyde was activated by the catalyst and by a condensation reaction with 3-(1H-indol-3-yl)-3-oxopropanenitrile yields Knoevenagel intermediate (B). Subsequently, intermediate A was reacted with intermediate B and then via a tautomerization process, intermediate C was formed. Then, intermediate C undergoes intramolecular cyclization to gives intermediate D. In the next step, this intermediate undergoes a H2O removing process which leads the formation of intermediate E. Finally, molecular H2 (inert conditions) or H2O2 (air conditions) was released based on a cooperative vinylogous anomeric-based oxidation (CVABO)70–84 to yields target molecule 2c.
Figure 12.
The plausible mechanism for the synthesis of 2c.
Magnetic substrates serve as ideal systems in recoverable catalysts and investigation of recycling and reusing potential of nanomagnetic catalyst is very important. Therefore, we examined the recycling and reusing ability of Fe3O4@SiO2@urea-riched ligand/Ch-Cl for the synthesis of 1a which leads to acceptable results. After running and performing each of reactions, the mixture of reaction was dissolved in CH2Cl2 and insoluble catalyst was separated from the reaction mixture and washed with CH2Cl2 (3 10 mL) and air dried. This work was conducted five times without significant reduction in yield of the reaction (Fig. 13). In addition, FT-IR spectrum was used for investigation the stability of recovered catalyst (See ESI).
Figure 13.
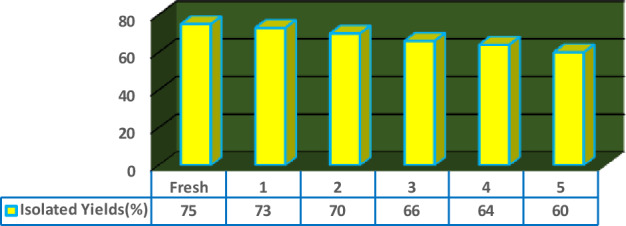
Diagram of recycling and reusing of Fe3O4@SiO2@urea-riched ligand/Ch-Cl in the synthesis of 1a.
Experimental section
Experimental procedure for the synthesis of Fe3O4@SiO2@urea-riched ligand/Ch-Cl
At the first, Fe3O4@SiO2@urea-riched ligand was synthesized according to our previous report70–76. Then, in a 100 mL round-bottomed flask 1 g of Fe3O4@SiO2@urea-riched ligand and choline chloride (6 mmol, 0.837 g) and 100 mL of toluene as solvent were added and was refluxed for 24 h. After completing of reaction, the desired catalyst was separated by using external magnet and washed with n-hexane and EtOH several times and dried in air condition.
General experimental route for the synthesis of hybrid pyridine derivatives
In 10 mL round-bottomed flask methyl ketones (1 mmol), aromatic aldehydes (1 mmol), malononitrile (1 mmol, 0.066 g) or 3-(1H-indol-3-yl)-3-oxopropanenitrile (1 mmol, 0.184 g), ammonium format (1.5 mmol, 0.094 g) and 10 mg of catalyst were added and the mixture of reaction was stirred at 110 °C for appropriate times as indicated in Table 2. The progress of reactions was monitored by TLC techniques (n-hexane/ethylacetate, 6/4). After completing of each reaction, the mixture of reaction was dissolved in CH2Cl2 and the catalyst was separated from organic mixture. Then, each of products was purified by TLC plate techniques with n-hexane/ethyl acetate.
Spectral data
4-(6-Amino-5-cyano-4-phenylpyridin-2-yl)phenyl 4-methylbenzenesulfonate (1a)
M.P. 131–133 °C, FT-IR (KBr, υ, cm−1): 3489, 3367, 2209, 1597, 1493, 1091. 1H NMR (250 MHz, DMSOd6) δppm 8.10 (d, J = 10 Hz, 2H, Aromatic), 7.73 (d, J = 10 Hz, 2H, Aromatic), 7.65–7.62 (m, 2H, Aromatic), 7.52–7.43 (m, 5H, Aromatic), 7.23 (s, 1H, Aromatic), 7.15–7.03 (m, 4H, Aromatic and NH2), 2.39 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 161.2, 157.5, 155.5, 150.7, 146.4, 137.0, 130.70, 130.10, 129.40, 129.2, 128.8, 122.7, 117.4, 109.8, 87.4, 21.6. MS (m/z) = calcd. for C25H19N3O3S: 441.5, found: 441.3.
4-(6-Amino-5-cyano-4-(p-tolyl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (1b)
M.P. 250–251 °C, FT-IR (KBr, υ, cm−1): 3508, 3391, 2922, 2206, 1367, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.14 (d, J = 9 Hz, 2H, Aromatic), 7.78 (d, J = 9 Hz, 2H, Aromatic), 7.59 (d, J = 9 Hz, 2H, Aromatic), 7.50 (d, J = 9 Hz, 2H, Aromatic), 7.37 (d, J = 6 Hz, 2H, Aromatic), 7.25 (s, 1H, Aromatic), 7.15 (d, J = 6 Hz, 2H, Aromatic), 7.04 (s, 2H, NH2), 2.43 (s, 3H, CH3), 2.41 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 161.3, 157.5, 155.5, 150.7, 146.4, 140.0, 137.1, 134.4, 131.7, 130.8, 129.8, 129.4, 128.8, 128.7, 122.7, 117.5, 109.7, 87.3, 21.7, 21.4. MS (m/z) = calcd. for C26H21N3O3S: 455.5, found: 455.2.
4-(6-Amino-5-cyano-4-(4-methoxyphenyl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (1c)
M.P. 270–273 °C, FT-IR (KBr, υ, cm−1): 3447, 3390, 2925, 2206, 1608, 1515, 1368, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.14 (d, J = 9 Hz, 2H, Aromatic), 7.79 (d, J = 9 Hz, 2H, Aromatic), 7.67 (d, J = 9 Hz, 2H, Aromatic), 7.50 (d, J = 9 Hz, 2H, Aromatic), 7.26 (s, 1H, Aromatic), 7.17 (s, 1H, Aromatic), 7.14 (s, 2H, Aromatic), 7.11 (s, 1H, Aromatic), 7.01 (s, 2H, NH2), 3.86 (s, 3H, OCH3), 2.44 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 161.4, 161.0, 157.4, 155.1, 150.6, 146.4, 140.9, 137.2, 131.7, 130.8, 130.4, 129.4, 128.8, 122.7, 117.7, 114.6, 109.6, 87.2, 55.9, 21.7. MS (m/z) = calcd. for C26H21N3O4S: 471.5, found: 471.2.
4-(4-([1,1'-Biphenyl]-4-yl)-6-amino-5-cyanopyridin-2-yl)phenyl 4-methylbenzenesulfonate (1d)
M.P. 175–176 °C, FT-IR (KBr, υ, cm−1): 3502, 3402, 2205, 1366, 1154. 1H NMR (301 MHz, DMSOd6) δppm 8.17 (d, J = 9 Hz, 2H, Aromatic), 7.88 (d, J = 9 Hz, 2H, Aromatic), 7.81–7.77 (m, 5H, Aromatic), 7.56–7.44 (m, 5H, Aromatic), 7.35 (s, 1H, Aromatic), 7.17 (d, J = 9 Hz, 2H), 7.10 (s, 2H, NH2), 2.44 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 161.3, 157.6, 155.1, 150.7, 146.5, 141.9, 139.7, 137.1, 136.3, 131.7, 130.8, 129.6, 129.5, 129.5, 128.8, 128.5, 127.4, 127.3, 122.7, 117.5, 109.8, 87.3, 21.7. MS (m/z) = calcd. for C31H23N3O3S: 517.6, found: 517.3.
4-(6'-Amino-5'-cyano-[3,4'-bipyridin]-2'-yl)phenyl 4-methylbenzenesulfonate (1e)
M.P. 255–258 °C, FT-IR (KBr, υ, cm−1): 3442, 3373, 2925, 2213, 1369, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.88 (dd, J = 3, 0.8 Hz, 1H), 8.75 (dd, J = 6, 3 Hz, 1H), 8.17 (m, 2H), 8.12 (m, 1H, Aromatic), 7.97 (s, 1H, Aromatic), 7.79 (d, J = 9 Hz, 2H, Aromatic), 7.61 (ddd, J = 7.9, 4.8, 0.9 Hz, 1H, Aromatic), 7.50 (d, J = 6 Hz, 2H), 7.38 (s, 1H, Aromatic), 7.18 (s, 2H, NH2), 7.15 (s, 1H, Aromatic), 2.44 (s, 3H, CH3). MS (m/z) = calcd. for C24H18N4O3S: 442.5, found: 442.2.
4-(6'-Amino-5'-cyano-[3,4'-bipyridin]-2'-yl)phenyl benzenesulfonate (1f)
M.P. > 300 °C, FT-IR (KBr, υ, cm−1): 3426, 3317, 3168, 2212, 1642, 1448, 1354, 1152. 1H NMR (250 MHz, DMSOd6) δppm 8.84–8.80 (m, 1H, Aromatic), 8.70 (d, J = 5 Hz, 1H, Aromatic), 8.13 (d, J = 7.5 Hz, 2H, Aromatic), 7.92–7.79 (m, 3H, Aromatic), 7.69–7.66 (m, 3H, Aromatic), 7.60–7.54 (m, 1H, Aromatic), 7.34 (s, 1H, Aromatic), 7.20–7.11 (m, 4H, Aromatic and NH2). 13C NMR (76 MHz, DMSOd6) δppm 161.0, 157.8, 151.0, 149.1, 136.6, 136.5, 135.6, 132.1, 130.8, 130.3, 129.5, 128.7, 124.0, 122.7, 119.1, 117.1, 116.1, 109.9, 87.4. MS (m/z) = calcd. for C23H16N4O3S: 428.5, found: 428.3.
4-(6-Amino-5-cyano-[4,4'-bipyridin]-2-yl)phenylbenzenesulfonate (1g)
M.P. 200–201 °C, FT-IR (KBr, υ, cm−1): 3442, 3291, 3170, 2216, 1375, 1153. 1H NMR (301 MHz, DMSO) δppm 8.75 (d, J = 6 Hz, 2H, Aromatic), 7.94 (dd, J = 6, 1.3 Hz, 2H, Aromatic), 7.74–7.65 (m, 8H, Aromatic), 7.23 (d, J = 9 Hz, 2H, Aromatic), 7.03 (s, 2H, NH2). 13C NMR (76 MHz, DMSOd6) δppm 154.5, 150.5, 150.2, 149.2, 147.6, 145.2, 136.8, 135.7, 134.8, 131.1, 130.5, 128.7, 123.7, 122.8, 118.7, 95.6. MS (m/z) = calcd. for C23H16N4O3S: 428.5, found: 428.4.
4-(6-Amino-4-(2-chlorophenyl)-5-cyanopyridin-2-yl)phenyl 4-methylbenzenesulfonate (1h)
M.P. 110–111 °C, FT-IR (KBr, υ, cm−1): 3489, 3373, 3168, 2918, 2213, 1370, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.13 (d, J = 9 Hz, 2H, Aromatic), 7.77 (d, J = 6 Hz, 2H, Aromatic), 7.68–7.65 (m, 1H, Aromatic), 7.53–7.48 (m, 5H, Aromatic), 7.23 (s, 1H, Aromatic), 7.20–7.13 (m, 4H, Aromatic and NH2), 2.43 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 160.1, 157.5, 153.8, 150.8, 146.5, 136.8, 136.5, 131.7, 131.4, 131.1, 130.8, 130.2, 129.4, 129.1, 128.8, 128.0, 122.8, 116.4, 110.3, 89.3, 21.7. MS (m/z) = calcd. for C25H18ClN3O3S: 475.9, found: 475.2.
4-(6-Amino-5-cyano-4-(2,4-dichlorophenyl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (1i)
M.P. 120–121 °C, FT-IR (KBr, υ, cm−1): 3485, 3376, 2925, 2213, 1502, 1447, 1371, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.12 (d, J = 9 Hz, 2H, Aromatic), 7.88 (d, J = 1.9 Hz, 1H, Aromatic), 7.77 (d, J = 6 Hz, 2H, Aromatic), 7.63 (dd, J = 9, 3 Hz, 1H, Aromatic), 7.57 (s, 1H, Aromatic), 7.50 (d, J = 9 Hz, 2H, Aromatic), 7.25 (s, 1H, Aromatic), 7.21 (s, 2H, NH2), 7.15 (d, J = 9 Hz, 2H, Aromatic), 2.43 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 160.5, 157.7, 152.7, 150.8, 146.5, 136.7, 135.5, 135.3, 133.0, 132.5, 131.7, 130.8, 129.7, 129.4, 128.8, 128.3, 122.8, 116.3, 110.2, 89.1, 21.7. MS (m/z) = calcd. for C25H17Cl2N3O3S: 510.4, found: 510.3.
4-(6-Amino-5-cyano-4-(3-fluorophenyl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (1j)
M.P. 135–138 °C, FT-IR (KBr, υ, cm−1): 3475, 3382, 2211, 1615, 1447, 1369, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.16 (d, J = 9 Hz, 2H, Aromatic), 7.79 (d, J = 9 Hz, 2H, Aromatic), 7.59 (s, 1H, Aromatic), 7.55 (s, 1H, Aromatic), 7.51–7.49 (m, 3H, Aromatic), 7.43–7.37 (m, 1H, Aromatic), 7.32 (s, 1H, Aromatic), 7.17 (s, 1H, Aromatic), 7.14–7.13 (m, 3H, Aromatic and NH2), 2.44 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 164.1, 161.2, 157.8, 154.1, 150.8, 146.5, 139.5, 139.4, 136.9, 131.7, 130.8, 129.5, 128.8, 125.1, 122.7, 117.2, 116.8, 116.1, 115.7, 109.9, 87.4, 21.7. MS (m/z) = calcd. for C25H18FN3O3S: 459.5, found: 459.2.
4-(6-Amino-4-(4-bromophenyl)-5-cyanopyridin-2-yl)phenyl 4-methylbenzenesulfonate (1k)
M.P. 140–141 °C, FT-IR (KBr, υ, cm−1): 3479, 3380, 2923, 2213, 1594, 1492, 1370, 1153. 1H NMR (301 MHz, DMSOd6) δppm 8.14 (d, J = 9 Hz, 2H, Aromatic), 7.78 (d, J = 6 Hz, 4H, Aromatic), 7.65 (d, J = 9 Hz, 2H, Aromatic), 7.50 (d, J = 9 Hz, 2H, Aromatic), 7.29 (s, 1H, Aromatic), 7.17–7.12 (m, 4H, Aromatic, NH2), 2.44 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 161.2, 157.7, 154.4, 150.7, 146.5, 137.0, 136.4, 132.2, 131.7, 131.0, 130.8, 129.5, 128.8, 123.8, 122.7, 117.2, 116.2, 109.7, 87.2, 21.7. MS (m/z) = calcd. for C25H18BrN3O3S: 520.4, found: 521.1.
4-(5-Cyano-6-(1H-indol-3-yl)-4-(2-methoxyphenyl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (2a)
M.P. 160–161 °C, FT-IR (KBr, υ, cm−1): 3396, 2928, 2223, 1374, 1168. 1H NMR (301 MHz, DMSOd6) δppm 11.92 (s, 1H, NH), 8.34 (d, J = 9 Hz, 4H, Aromatic), 7.90 (s, 1H, Aromatic), 7.82 (d, J = 9 Hz, 2H, Aromatic), 7.59–7.56 (m, 2H, Aromatic), 7.54–7.48 (m, 3H, Aromatic), 7.28– 7.23 (m, 5H, Aromatic), 7.17 (t, J = 9 Hz, 1H, Aromatic), 3.85 (s, 3H, OCH3), 2.44 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 158.6, 157.2, 157.0, 156.6, 153.6, 150.9, 146.5, 137.1, 136.9, 131.8, 130.9, 129.6, 128.9, 128.7, 126.4, 126.2, 123.1, 122.9, 121.7, 121.3, 121.2, 118.9, 118.0, 113.3, 112.6, 112.3, 104.3, 56.1, 21.7. MS (m/z) = calcd. for C34H25N3O4S: 571.6, found: 571.3.
4-(5-Cyano-6-(1H-indol-3-yl)-4-(4-methoxyphenyl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (2b)
M.P. 170–172 °C, FT-IR (KBr, υ, cm−1): 3402, 2900, 2211, 1368, 1153. 1H NMR (301 MHz, DMSOd6) δppm 11.89 (s, 1H, NH), 8.38–8.32 (m, 4H, Aromatic), 7.94 (s, 1H, Aromatic), 7.84–7.80 (m, 4H, Aromatic), 7.58–7.50 (m, 4H, Aromatic), 7.27 (d, J = 9 Hz, 3H, Aromatic), 7.19 (d, J = 9 Hz, 2H, Aromatic), 3.89 (s, 3H, OCH3), 2.45 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 161.1, 158.0, 157.2, 155.3, 150.9, 146.5, 137.2, 136.8, 131.8, 131.0, 130.8, 129.7, 129.3, 128.8, 126.5, 123.1, 122.9, 121.7, 121.3, 119.6, 116.9, 114.7, 113.4, 112.6, 102.1, 55.9, 21.7. MS (m/z) = calcd. for C34H25N3O4S: 571.6, found: 571.3.
4-(4-(4-Chlorophenyl)-5-cyano-6-(1H-indol-3-yl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (2c)
M.P. 188–189 °C, FT-IR (KBr, υ, cm−1): 3362, 2922, 2213, 1596, 1433, 1365, 1150. 1H NMR (301 MHz, DMSOd6) δppm 11.90 (s, 1H, NH), 8.39–8.32 (m, 4H, Aromatic), 7.98 (s, 1H, Aromatic), 7.88–7.81 (m, 4H, Aromatic), 7.71 (d, J = 9 Hz, 2H, Aromatic), 7.58 (d, J = 9 Hz, 1H, Aromatic), 7.51 (d, J = 9 Hz, 2H, Aromatic), 7.28–7.24 (m, 4H, Aromatic), 2.44 (s, 3H, CH3). MS (m/z) = calcd. for C33H22ClN3O3S: 576.1, found: 576.2.
4-(5-Cyano-4-(2,4-dichlorophenyl)-6-(1H-indol-3-yl)pyridin-2-yl)phenyl 4-methylbenzenesulfonate (2d)
M.P. 205–206 °C, FT-IR (KBr, υ, cm−1): 3424, 2221, 1619, 1432, 750. 1H NMR (301 MHz, DMSOd6) δppm 12.00 (s, 1H, NH), 8.39– 8.34 (m, 4H, Aromatic), 8.00 (s, 1H, Aromatic), 7.95 (s, 1H, Aromatic), 7.82 (d, J = 9 Hz, 2H, Aromatic), 7.71 (s, 2H, Aromatic), 7.58 (d, J = 6 Hz, 1H, Aromatic), 7.51 (d, J = 9 Hz, 2H, Aromatic), 7.29–7.26 (m, 4H, Aromatic), 2.44 (s, 3H, CH3). 13C NMR (76 MHz, DMSOd6) δppm 162.3, 157.5, 152.7, 151.1, 146.5, 136.9, 135.6, 135.2, 133.2, 132.9, 131.8, 130.8, 129.7, 129.2, 128.8, 128.4, 126.3, 123.2, 121.8, 121.5, 118.2, 117.5, 113.1, 112.7, 103.4, 21.7.
4-(4-Bromophenyl)-2,6-di(1H-indol-3-yl) nicotinonitrile (3a)
M.P. 135–137 °C, 1H NMR (301 MHz, DMSOd6) δppm 11.94 (s, 1H, NH), 11.82 (s, 1H, NH), 8.63 (d, J = 9 Hz, 1H, Aromatic), 8.53 (d, J = 3 Hz, 1H, Aromatic), 8.37 (d, J = 9 Hz, 1H, Aromatic), 8.30 (d, J = 3 Hz, 1H, Aromatic), 7.90 (s, 1H, Aromatic), 7.85 (d, J = 9 Hz, 2H, Aromatic), 7.78 (d, J = 9 Hz, 2H, Aromatic), 7.61 (d, J = 9 Hz, 1H, Aromatic), 7.54 (d, J = 9 Hz, 1H, Aromatic), 7.32–7.20 (m, 3H, Aromatic), 7.10 (t, J = 9 Hz, 1H, Aromatic). 13C NMR (76 MHz, DMSOd6) δppm 158.4, 158.1, 152.6, 137.8, 136.9, 132.1, 131.5, 129.9, 128.7, 126.5, 125.8, 123.6, 122.8, 122.6, 121.9, 121.1, 120.8, 119.7, 115.8, 115.3, 114.1, 112.5, 98.8. MS (m/z) = calcd. for C28H17BrN4: 489.4, found: 489.1.
4-(4-Chlorophenyl)-2,6-di(1H-indol-3-yl) nicotinonitrile (3b)
M.P. 115–120 °C, FT-IR (KBr, υ, cm−1): 3391, 2959, 2217, 731. 1H NMR (301 MHz, DMSOd6) δppm 11.92 (s, 1H, NH), 11.81 (s, 1H, NH), 8.61 (d, J = 9 Hz, 1H, Aromatic), 8.53 (d, J = 9 Hz, 1H, Aromatic), 8.34 (d, J = 9 Hz, 1H, Aromatic), 8.28 (d, J = 6 Hz, 1H, Aromatic), 7.90 (s, 1H, Aromatic), 7.86 (d, J = 9 Hz, 2H, Aromatic), 7.71 (d, J = 6 Hz, 2H, Aromatic), 7.59 (d, J = 6 Hz, 1H, Aromatic), 7.52 (d, J = 9 Hz, 1H, Aromatic), 7.30–7.16 (m, 3H, Aromatic), 7.09 (t, J = 9 Hz, 1H, Aromatic). MS (m/z) = calcd. for C28H17ClN4: 444.9, found: 444.2.
Conclusion
In summary, we reported the design, synthesis and characterization of a new heterogeneous catalytic system namely [Fe3O4@SiO2@urea-riched ligand/Ch-Cl]. The revealed results from characterization of this compound such as FT-IR, FESEM, TEM, EDS-Mapping, TGA/DTG and VSM analysis show its successful synthesis. This system has an excellent catalytical potential for synthesis of hybrid pyridines containing sulfonate or indole sections. In this method several starting materials were used for the synthesis of hybrid pyridine rings which yield divers products in mild reaction conditions. Besides, cooperative vinylogous anomeric-based oxidation pathway was suggested as a rational mechanism for the synthesis of hybrid pyridine (Supplementary Information S1).
Supplementary Information
Acknowledgements
We thank the Bu-Ali Sina University for the financial support to our research group.
Author contributions
N.Z.: methodology, validation, investigation, writing—original draft. M.A.Z.: supervision, resources, project administration, funding acquisition, conceptualization, writing—review and editing. M.T.: methodology, validation, investigation, writing—original draft. M.Y.: methodology, validation, investigation, writing—original draft.
Data availability
All data generated or analyzed during this study are included in this published article [and its supplementary information files].
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Ali Zolfigol, Email: mzolfigol@yahoo.com.
Meysam Yarie, Email: myarie.5266@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-35849-3.
References
- 1.Hansen BB, Spittle S, Chen B, Poe D, Zhang Y, Klein JM, Sangoro JR. Deep eutectic solvents: A review of fundamentals and applications. Chem. Rev. 2020;121:1232–1285. doi: 10.1021/acs.chemrev.0c00385. [DOI] [PubMed] [Google Scholar]
- 2.Smith EL, Abbott AP, Ryder KS. Deep eutectic solvents (DESs) and their applications. Chem. Rev. 2014;114:11060–11082. doi: 10.1021/cr300162p. [DOI] [PubMed] [Google Scholar]
- 3.Vilková M, Płotka-Wasylka J, Andruch V. The role of water in deep eutectic solvent-base extraction. J. Mol. Liq. 2020;304:112747. doi: 10.1016/j.molliq.2020.112747. [DOI] [Google Scholar]
- 4.Atilhan M, Aparicio S. Review and perspectives for effective solutions to grand challenges of energy and fuels technologies via novel deep eutectic solvents. Energy Fuels. 2021;35:6402–6419. doi: 10.1021/acs.energyfuels.1c00303. [DOI] [Google Scholar]
- 5.Van Osch DJ, Dietz CH, Warrag SE, Kroon MC. The curious case of hydrophobic deep eutectic solvents: A story on the discovery, design, and applications. ACS Sustain. Chem. Eng. 2020;8:10591–10612. [Google Scholar]
- 6.Ge X, Gu C, Wang X, Tu J. Deep eutectic solvents (DESs)-derived advanced functional materials for energy and environmental applications: Challenges, opportunities, and future vision. J. Mater. Chem. A. 2017;5:8209–8229. doi: 10.1039/C7TA01659J. [DOI] [Google Scholar]
- 7.Hooshmand SE, Afshari R, Ramón DJ, Varma RS. Deep eutectic solvents: Cutting-edge applications in cross-coupling reactions. Green Chem. 2020;22:3668–3692. doi: 10.1039/D0GC01494J. [DOI] [Google Scholar]
- 8.García-Álvarez J. Deep eutectic mixtures: Promising sustainable solvents for metal-catalysed and metal-mediated organic reactions. Eur. J. Inorg. Chem. 2015;2015:5147–5157. doi: 10.1002/ejic.201500892. [DOI] [Google Scholar]
- 9.Zamani P, Ozdemir J, Ha Y, Benamara M, Kuchuk AV, Wang T, Beyzavi MH. Magnetic nanoparticle anchored deep eutectic solvents as a catalyst for the etherification and amination of naphthols. Adv. Synth. Catal. 2018;360:4372–4380. doi: 10.1002/adsc.201800743. [DOI] [Google Scholar]
- 10.Wu S, Cai C, Li F, Tan Z, Dong S. Deep eutectic supramolecular polymers: Bulk supramolecular materials. Angew. Chem. Int. Ed. 2020;59:11871–11875. doi: 10.1002/anie.202004104. [DOI] [PubMed] [Google Scholar]
- 11.Akbarian M, Sanchooli E, Oveisi AR, Daliran S. Choline chloride-coated UiO-66-Urea MOF: A novel multifunctional heterogeneous catalyst for efficient one-pot three-component synthesis of 2-amino-4H-chromenes. J. Mol. Liq. 2021;325:115228–115239. doi: 10.1016/j.molliq.2020.115228. [DOI] [Google Scholar]
- 12.Aguirre MÁ, Canals A. Magnetic deep eutectic solvents in microextraction techniques. Trends Analyt. Chem. 2022;146:116500. doi: 10.1016/j.trac.2021.116500. [DOI] [Google Scholar]
- 13.Le Nguyen TT. An efficient multicomponent synthesis of 2, 4, 5-trisubstituted and 1, 2, 4, 5-tetrasubstituted imidazoles catalyzed by a magnetic nanoparticle supported Lewis acidic deep eutectic solvent. RSC Adv. 2019;9:38148–38153. doi: 10.1039/C9RA08074K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maia RA, Louis B, Baudron SA. Deep eutectic solvents for the preparation and post-synthetic modification of metal-and covalent organic frameworks. CrystEngComm. 2021;23:5016–5032. doi: 10.1039/D1CE00714A. [DOI] [Google Scholar]
- 15.Santana-Mayor Á, Rodríguez-Ramos R, Socas-Rodríguez B, Afonso MDM, Palenzuela JA. Combinations of nanomaterials and deep eutectic solvents as innovative materials in food analysis. Processes. 2021;9:2131. doi: 10.3390/pr9122131. [DOI] [Google Scholar]
- 16.Xu K, Wang Y, Ding X, Huang Y, Li N, Wen Q. Magnetic solid-phase extraction of protein with deep eutectic solvent immobilized magnetic graphene oxide nanoparticles. Talanta. 2016;148:153–162. doi: 10.1016/j.talanta.2015.10.079. [DOI] [PubMed] [Google Scholar]
- 17.Kalaj M, Cohen SM. Postsynthetic modification: An enabling technology for the advancement of metal-organic frameworks. ACS cent. Sci. 2020;6:1046–1057. doi: 10.1021/acscentsci.0c00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sadiq AC, Olasupo A, Rahim NY, Ngah WSW, Suah FBM. Comparative removal of malachite green dye from aqueous solution using deep eutectic solvents modified magnetic chitosan nanoparticles and modified protonated chitosan beads. J. Environ. Chem. Eng. 2021;9:106281. doi: 10.1016/j.jece.2021.106281. [DOI] [Google Scholar]
- 19.Bide Y, Shokrollahzadeh S. Toward tailoring of a new draw solute for forward osmosis process: Branched poly (deep eutectic solvent)-decorated magnetic nanoparticles. J. Mol. Liq. 2020;320:114409. doi: 10.1016/j.molliq.2020.114409. [DOI] [Google Scholar]
- 20.Li L, Liu K, Xing H, Li X, Zhang Q, Han D, Tang B. Deep eutectic solvents functionalized polymers for easily and efficiently promoting biocatalysis. J. Catal. 2019;374:306–319. doi: 10.1016/j.jcat.2019.05.006. [DOI] [Google Scholar]
- 21.Li Z, Sun W, Chen C, Guo Q, Li X, Gu M, Guan G. Deep eutectic solvents appended to UiO-66 type metal organic frameworks: Preserved open metal sites and extra adsorption sites for CO2 capture. Appl. Surf. Sci. 2019;480:770–778. doi: 10.1016/j.apsusc.2019.03.030. [DOI] [Google Scholar]
- 22.Cruz H, Pinto AL, Jordão N, Neves LA, Branco LC. Alkali iodide deep eutectic solvents as alternative electrolytes for dye sensitized solar cells. Sustain. Chem. 2021;2:222–236. doi: 10.3390/suschem2020013. [DOI] [Google Scholar]
- 23.Pateli IM, Abbott AP, Jenkin GR, Hartley JM. Electrochemical oxidation as alternative for dissolution of metal oxides in deep eutectic solvents. Green Chem. 2020;22:8360–8368. doi: 10.1039/D0GC03491F. [DOI] [Google Scholar]
- 24.Ma Q, Zhao L, Xu J, Su H, Zhang W, Yang W, Xu Q. Electrochim. Acta. 2020;353:136486–136495. doi: 10.1016/j.electacta.2020.136486. [DOI] [Google Scholar]
- 25.Mahanta U, Choudhury S, Venkatesh RP, SarojiniAmma S, Ilangovan SA, Banerjee T. Ionic-liquid-based deep eutectic solvents as novel electrolytes for supercapacitors: COSMO-SAC predictions, synthesis, and characterization. ACS Sustain. Chem. Eng. 2019;8:372–381. doi: 10.1021/acssuschemeng.9b05596. [DOI] [Google Scholar]
- 26.Svigelj R, Dossi N, Grazioli C, Toniolo R. Deep eutectic solvents (DESs) and their application in biosensor development. Sensors. 2021;21:4263–4281. doi: 10.3390/s21134263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang X, Zou Q, Zhao T, Chen P, Liu Z, Liu F, Lin Q. Deep eutectic solvents as efficient catalysts for fixation of CO2 to cyclic carbonates at ambient temperature and pressure through synergetic catalysis. ACS Sustain. Chem. Eng. 2021;9:10437–10443. doi: 10.1021/acssuschemeng.1c03187. [DOI] [Google Scholar]
- 28.Shaabani A, Afshari R. Magnetic Ugi-functionalized graphene oxide complexed with copper nanoparticles: efficient catalyst toward Ullman coupling reaction in deep eutectic solvents. J. Colloid Interface Sci. 2018;510:384–394. doi: 10.1016/j.jcis.2017.09.089. [DOI] [PubMed] [Google Scholar]
- 29.Kiani, A. Alinezhad, H. & Ghasemi, S. Embedded palladium nanoparticles on metal–organic framework/covalently sulfonated magnetic SBA‐15 mesoporous silica composite: As a highly proficient nanocatalyst for Suzuki–Miyaura coupling reaction in amino acid‐based natural deep eutectic solvent. Appl. Organomet. Chem. e6912 (2022).
- 30.Makoś-Chełstowska P, Kaykhaii M, Płotka-Wasylka J, de la Guardia M. Magnetic deep eutectic solvents—Fundamentals and applications. J. Mol. Liq. 2022;365:120158–120170. doi: 10.1016/j.molliq.2022.120158. [DOI] [Google Scholar]
- 31.Bakhtiarian M, Khodaei MM. Synthesis of 2, 3-dihydro-4 (1H) quinazolinones using a magnetic pectin-supported deep eutectic solvent. Colloids Surf. A Physicochem. Eng. Asp. 2022;641:128569. doi: 10.1016/j.colsurfa.2022.128569. [DOI] [Google Scholar]
- 32.Shaibuna M, Theresa LV, Sreekumar K. Neoteric deep eutectic solvents: History, recent developments, and catalytic applications. Soft Matter. 2022;18:2695–2721. doi: 10.1039/D1SM01797G. [DOI] [PubMed] [Google Scholar]
- 33.Longo LS, Jr, Craveiro MV. Deep eutectic solvents as unconventional media for multicomponent reactions. J. Braz. Chem. Soc. 2018;29:1999–2025. [Google Scholar]
- 34.Tran PH. One-pot multicomponent synthesis of thieno [2, 3-b] indoles catalyzed by a magnetic nanoparticle-supported [Urea] 4 [ZnCl2] deep eutectic solvent. RSC Adv. 2020;10:9663–9671. doi: 10.1039/D0RA00773K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu P, Hao JW, Mo LP, Zhang ZH. Recent advances in the application of deep eutectic solvents as sustainable media as well as catalysts in organic reactions. RSC Adv. 2015;5:48675–48704. doi: 10.1039/C5RA05746A. [DOI] [Google Scholar]
- 36.Anizadeh MR, Zolfigol MA, Torabi M, Yarie M, Notash B. J. Mol. Liq. 2022;364:120016–120027. doi: 10.1016/j.molliq.2022.120016. [DOI] [Google Scholar]
- 37.Abtahi B, Tavakol H. (2020) Choline chloride-urea deep eutectic solvent as an efficient media for the synthesis of propargylamines via organocuprate intermediate. Appl. Organometal. Chem. 2020;34:e5895. doi: 10.1002/aoc.5895. [DOI] [Google Scholar]
- 38.Lin YF, Sun IW. Electrodeposition of zinc from a Lewis acidic zinc chloride-1-ethyl-3-methylimidazolium chloride molten salt. Electrochim. Acta. 1999;44:2771–2777. doi: 10.1016/S0013-4686(99)00003-1. [DOI] [Google Scholar]
- 39.Zamani P, Khosropour AR. A combination of natural deep eutectic solvents and microflow technology: A sustainable innovation for the tandem synthesis of 3-aminohexahydrocoumarins. Green Chem. 2016;18:6450–6455. doi: 10.1039/C6GC02642G. [DOI] [Google Scholar]
- 40.Cui Y, Li C, Bao M. Deep eutectic solvents (DESs) as powerful and recyclable catalysts and solvents for the synthesis of 3, 4-dihydropyrimidin-2 (1H)-ones/thiones. Green Process. Synth. 2019;8:568–576. doi: 10.1515/gps-2019-0026. [DOI] [Google Scholar]
- 41.Ma Z, Mohapatra J, Wei K, Liu JP, Sun S. Magnetic nanoparticles: Synthesis, anisotropy, and applications. Chem. Rev. 2021;123:3904–3943. doi: 10.1021/acs.chemrev.1c00860. [DOI] [PubMed] [Google Scholar]
- 42.Garcia-Merino B, Bringas E, Ortiz I. Synthesis and applications of surface-modified magnetic nanoparticles: Progress and future prospects. Rev. Chem. Eng. 2022;38:821–842. doi: 10.1515/revce-2020-0072. [DOI] [Google Scholar]
- 43.Liu S, Yu B, Wang S, Shen Y, Cong H. Preparation, surface functionalization and application of Fe3O4 magnetic nanoparticles. Adv. Colloid Interface Sci. 2020;281:102165. doi: 10.1016/j.cis.2020.102165. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed Noor MH, Wong S, Ngadi N, Mohammed Inuwa I, Opotu LA. Assessing the effectiveness of magnetic nanoparticles coagulation/flocculation in water treatment: A systematic literature review. Int. J. Sci. Environ. Technol. 2022;19:6935–6956. doi: 10.1007/s13762-021-03369-0. [DOI] [Google Scholar]
- 45.Mittal A, Roy I, Gandhi S. Magnetic nanoparticles: An overview for biomedical applications. Magnetochemistry. 2022;8:107. doi: 10.3390/magnetochemistry8090107. [DOI] [Google Scholar]
- 46.Zhou K, Zhou X, Liu J, Huang Z. Application of magnetic nanoparticles in petroleum industry: A review. J. Pet. Sci. Eng. 2020;188:106943. doi: 10.1016/j.petrol.2020.106943. [DOI] [Google Scholar]
- 47.Verma S, Kujur S, Sharma R, Pathak DD. Cucurbit [6] uril-supported Fe3O4 magnetic nanoparticles catalyzed green and sustainable synthesis of 2-substituted benzimidazoles via acceptorless dehydrogenative coupling. ACS Omega. 2022;7:9754–9764. doi: 10.1021/acsomega.1c07350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu L, Mendoza-Garcia A, Li Q, Sun S. Organic phase syntheses of magnetic nanoparticles and their applications. Chem. Rev. 2016;116:10473–10512. doi: 10.1021/acs.chemrev.5b00687. [DOI] [PubMed] [Google Scholar]
- 49.Xiong, G. Chen, X. L., You, L. X., Ren, B. Y., Ding, F., Dragutan, I., & Sun, Y. G. La-metal-organic framework incorporating Fe3O4 nanoparticles, post-synthetically modified with Schiff base and Pd. A highly active, magnetically recoverable, recyclable catalyst for CC cross-couplings at low Pd loadings. J. Catal.361, 116–125 (2018).
- 50.Qi B, Wu C, Liu Y, Liu J, Zhang H. Self-assembled magnetic Pt nanocomposites for the catalytic reduction of nitrophenol. ACS Appl. Nano Mater. 2019;2:4377–4385. doi: 10.1021/acsanm.9b00794. [DOI] [Google Scholar]
- 51.Viegas-Junior, C., Danuello, A., da Silva Bolzani, V., Barreiro, E. J., & Fraga, C. A. M. Molecular hybridization: A useful tool in the design of new drug prototypes. Curr. Med. Chem.14, 1829–1852 (2007). [DOI] [PubMed]
- 52.Eldehna WM, Altoukhy A, Mahrous H, Abdel-Aziz HA. Design, synthesis and QSAR study of certain isatin-pyridine hybrids as potential anti-proliferative agents. Eur. J. Med. Chem. 2015;90:684–694. doi: 10.1016/j.ejmech.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 53.Fortin S, Bérubé G. Advances in the development of hybrid anticancer drugs. Expert Opin. Drug Discov. 2013;8:1029–1047. doi: 10.1517/17460441.2013.798296. [DOI] [PubMed] [Google Scholar]
- 54.Khidre RE, Radini IAM. Design, synthesis and docking studies of novel thiazole derivatives incorporating pyridine moiety and assessment as antimicrobial agents. Sci. Rep. 2021;11:7846. doi: 10.1038/s41598-021-86424-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bull JA, Mousseau JJ, Pelletier G, Charette AB. Synthesis of pyridine and dihydropyridine derivatives by regio-and stereoselective addition to N-activated pyridines. Chem. Rev. 2012;112:2642–2713. doi: 10.1021/cr200251d. [DOI] [PubMed] [Google Scholar]
- 56.Rashdan HR, Shehadi IA, Abdelmonsef AH. Synthesis, anticancer evaluation, computer-aided docking studies, and ADMET prediction of 1, 2, 3-triazolyl-pyridine hybrids as human aurora B kinase inhibitors. ACS Omega. 2021;6:1445–1455. doi: 10.1021/acsomega.0c05116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gygi D, Hwang SJ, Nocera DG. Scalable syntheses of 4-substituted pyridine–diimines. J. Org. Chem. 2017;82:12933–12938. doi: 10.1021/acs.joc.7b02571. [DOI] [PubMed] [Google Scholar]
- 58.Torabi, M., Yarie, M., Baghery, S., & Zolfigol, M. A. Recent advances in catalytic synthesis of pyridine derivatives 503–580 (2023).
- 59.Zafar R, Naureen H, Zubair M, Shahid K, Jan MS, Akhtar S, Sadiq A. Prospective application of two new pyridine-based Zinc (II) amide carboxylate in management of alzheimer's disease: Synthesis, characterization, computational and in vitro approaches. Drug Des. Devel. Ther. 2021;15:2679. doi: 10.2147/DDDT.S311619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mrozek-Wilczkiewicz A, Malarz K, Rejmund M, Polanski J, Musiol R. Anticancer activity of the thiosemicarbazones that are based on di-2-pyridine ketone and quinoline moiety. Eur. J. Med. Chem. 2019;171:180–194. doi: 10.1016/j.ejmech.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 61.Dawood DH, Srour AM, Saleh DO, Huff KJ, Greco F, Osborn HM. RSC Adv. 2021;11:29441–29452. doi: 10.1039/D1RA04758B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Salar U, Khan KM, Iqbal J, Ejaz SA, Hameed A, Al-Rashida M, Shahnaz T, Tahir MN. Coumarin sulfonates: New alkaline phosphatase inhibitors; in vitro and in silico studies. Eur. J. Med. Chem. 2017;131:29–47. doi: 10.1016/j.ejmech.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 63.Vieira BM, da Thurow S, Costa M, Casaril AM, Domingues M, Schumacher RF, Lenardão EJ. Ultrasound-assisted synthesis and antioxidant activity of 3-selanyl-1H-indole and 3-selanylimidazo [1, 2-a] pyridine derivatives. Asian J. Org. Chem. 2017;6:1635–1646. doi: 10.1002/ajoc.201700339. [DOI] [Google Scholar]
- 64.Desai, N., Jadeja, D., Mehta, H., Khasiya, A. K., & Shah, U. Pandit, N-Heterocycles. Springer, Singapore 143–189 (2022).
- 65.Alizadeh SR, Ebrahimzadeh MA. Antiviral activities of pyridine fused and pyridine containing heterocycles, a review (from 2000 to 2020) Mini-Rev. Med. Chem. 2021;21:2584–2611. doi: 10.2174/1389557521666210126143558. [DOI] [PubMed] [Google Scholar]
- 66.Mermer A, Keles T, Sirin Y. Bioorg. Chem. 2021;114:105076–105124. doi: 10.1016/j.bioorg.2021.105076. [DOI] [PubMed] [Google Scholar]
- 67.Rodríguez I, Saavedra ED, Rosario H, Perdomo J, Quintana J, Prencipe F, Estévez F. Apoptosis pathways triggered by a potent antiproliferative hybrid chalcone on human melanoma cells. Int. J. Mol. Sci. 2021;22:13462. doi: 10.3390/ijms222413462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Toure, B. B. & Hall, D. G. Natural product synthesis using multicomponent reaction strategies. Chem. l Rev.109, 4439–4486 (2009). [DOI] [PubMed]
- 69.MacParland SA, Liu JC, Ma XZ, Innes BT, Bartczak AM, Gage BK, McGilvray ID. Single cell RNA sequencing of human liver reveals distinct intrahepatic macrophage populations. Nat. commun. 2018;9:4383. doi: 10.1038/s41467-018-06318-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Torabi M, Yarie M, Zolfigol MA, Rouhani S, Azizi S, Olomola TO, Msagati TA. Synthesis of new pyridines with sulfonamide moiety via a cooperative vinylogous anomeric-based oxidation mechanism in the presence of a novel quinoline-based dendrimer-like ionic liquid. RSC Adv. 2021;11:3143–3152. doi: 10.1039/D0RA09400E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karimi F, Yarie M, Zolfigol MA. Synthesis and characterization of Fe3O4@SiO2@(CH2)3NH(CH2)2O2P(OH)2 and its catalytic application in the synthesis of benzo-[h] quinoline-4-carboxylic acids via a cooperative anomeric based oxidation mechanism. Mol. Catal. 2020;489:110924. doi: 10.1016/j.mcat.2020.110924. [DOI] [Google Scholar]
- 72.Ghasemi P, Yarie M, Zolfigol MA, Taherpour AA, Torabi M. Ionically tagged magnetic nanoparticles with urea linkers: application for preparation of 2-aryl-quinoline-4-carboxylic acids via an anomeric-based oxidation mechanism. ACS Omega. 2020;5:3207–3217. doi: 10.1021/acsomega.9b03277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Karimi F, Yarie M, Zolfigol MA. RSC Adv. 2020;10:25828. doi: 10.1039/D0RA04461J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zarei N, Torabi M, Yarie M, Zolfigol MA. Functionalized hybrid magnetic catalytic systems on micro- and nanoscale utilized in organic synthesis and degradation of dyes. Polycycl. Aromat. Compd. 2022;4:1263–1307. doi: 10.1039/d1na00818h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Fazl F, Torabi M, Yarie M, Zolfigol MA. Synthesis and application of novel urea–benzoic acid functionalized magnetic nanoparticles for the synthesis of 2,3-disubstituted thiazolidin-4-ones and hexahydroquinolines. RSC Adv. 2022;12:16342–16353. doi: 10.1039/D2RA02205B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Torabi M, Yarie M, Zolfigol MA, Azizian S, Gu Y. A magnetic porous organic polymer: Catalytic application in the synthesis of hybrid pyridines with indole, triazole and sulfonamide moieties. RSC Adv. 2022;12:8804–8814. doi: 10.1039/D2RA00451H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yarie M. Catalytic anomeric based oxidation. Iran. J. Catal. 2017;7:85–88. [Google Scholar]
- 78.Yarie M. Spotlight: Catalytic vinylogous anomeric based oxidation (Part I) Iran. J. Catal. 2020;10:79–83. [Google Scholar]
- 79.Torabi M, Zolfigol MA, Yarie M, Gu Y. Application of ammonium acetate as a dual rule reagent-catalyst in synthesis of new symmetrical terpyridines. Mol. Catal. 2021;516:110959. [Google Scholar]
- 80.Kalhor S, Yarie M, Torabi M, Zolfigol MA, Rezaeivala M, Gu Y. Synthesis of 2-amino-6-(1H-indol-3-yl)-4-phenylnicotinonitriles and bis(indolyl) pyridines using a novel acidic nanomagnetic catalyst via a cooperative vinylogous anomeric-based oxidation mechanism. Polycycl. Aromat. Compd. 2021;11:4270–4285. [Google Scholar]
- 81.Torabi, M., Zolfigol, M. A., Yarie, M., Notash, B., Azizian, S., & Mirzaei Azandaryani, M. Synthesis of triarylpyridines with sulfonate and sulfonamide moieties via a cooperative vinylogous anomeric-based oxidation. Sci. Rep.11, 16846 (2021). [DOI] [PMC free article] [PubMed]
- 82.Dashteh M, Afsar J, Baghery S, Zolfigol MA, Khazaei A, Moosavi-Zare AR, Alonso DA, Khoshnood A. A convenient catalytic method for the synthesis of pyridines with henna and pyrazole moieties using cooperative vinylogous anomeric-based oxidation. Chem. Select. 2022;7:e202200346. [Google Scholar]
- 83.Alabugin IV, Kuhn L, Medvedev MG, Krivoshchapov NV, Vil VA, Yaremenko IA, Zolfigol MA. Stereoelectronic power of oxygen in control of chemical reactivity: the anomeric effect is not alone. Chem. Soc. Rev. 2021;50:10253–10345. doi: 10.1039/D1CS00386K. [DOI] [PubMed] [Google Scholar]
- 84.Alabugin IV, Kuhn L, Krivoshchapov NV, Mehaffy P, Medvedev MG. Chem. Soc. Rev. 2021;50:10212–10252. doi: 10.1039/D1CS00564B. [DOI] [PubMed] [Google Scholar]
- 85.Arlan FM, Marjani AP, Javahershenas R, Khalafy J. Recent developments in the synthesis of polysubstituted pyridines via multicomponent reactions using nanocatalysts. New J. Chem. 2021;45:12328–12345. doi: 10.1039/D1NJ01801A. [DOI] [Google Scholar]
- 86.Akhmadiev NS, Akhmetova VR, Ibragimov AG. 2-Amino-3, 5-dicarbonitrile-6-sulfanylpyridines: Synthesis and multiple biological activity–a review. RSC Adv. 2021;11:11549–11567. doi: 10.1039/D1RA00363A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gebre, S. H. Recent developments of supported and magnetic nanocatalysts for organic transformations: an up-to-date. Appl. Nanosci. (2021).
- 88.Asressu KH, Chan CK, Wang CC. One-pot synthesis of 1, 5-diketones under a transition-metal-free condition: Application in the synthesis of 2, 4, 6-triaryl pyridine derivatives. ACS Omega. 2021;6:7296–7311. doi: 10.1021/acsomega.0c05328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Makoś P, Fernandes A, Przyjazny A, Boczkaj G. Sample preparation procedure using extraction and derivatization of carboxylic acids from aqueous samples by means of deep eutectic solvents for gas chromatographic-mass spectrometric analysis. J. Chromatogr. A. 2018;1555:10–19. doi: 10.1016/j.chroma.2018.04.054. [DOI] [PubMed] [Google Scholar]
- 90.Shafie MH, Yusof R, Gan CY. J. Mol. Liq. 2019;288:111081–111087. doi: 10.1016/j.molliq.2019.111081. [DOI] [Google Scholar]
- 91.Figueiredo M, Gomes C, Costa R, Martins A, Pereira CM, Silva F. Differential capacity of a deep eutectic solvent based on choline chloride and glycerol on solid electrodes. Electrochim. Acta. 2009;54:2630–2634. doi: 10.1016/j.electacta.2008.10.074. [DOI] [Google Scholar]
- 92.Ling, J. K. U., San Chan, Y., Nandong, J., Chin, S. F., & Ho, B. K. Lwt, 133, 110096–110106 (2020).
- 93.Silva JM, Reis RL, Paiva A, Duarte ARC. ACS Sustain. Chem. Eng. 2018;6:10355–10363. doi: 10.1021/acssuschemeng.8b01687. [DOI] [Google Scholar]
- 94.Subba N, Sahu P, Das N, Sen P. Rational design, preparation and characterization of a ternary non-ionic room-temperature deep eutectic solvent derived from urea, acetamide, and sorbitol. J. Chem. Sci. 2021;133:25. doi: 10.1007/s12039-020-01866-2. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article [and its supplementary information files].