Abstract
Background and aims
Recessive dystrophic epidermolysis bullosa (RDEB) is a hereditary, rare, devastating and life-threatening skin fragility disorder with a high unmet medical need. In a recent international, single-arm clinical trial, treatment of 16 patients (aged 6–36 years) with 3 intravenous infusions of 2×106 immunomodulatory ABCB5+ dermal mesenchymal stromal cells (MSCs)/kg on days 0, 17 and 35 reduced disease activity, itch and pain. A post-hoc analysis was undertaken to assess the potential effects of treatment with ABCB5+ MSCs on the overall skin wound healing in patients suffering from RDEB.
Methods:
Documentary photographs of the affected body regions taken on days 0, 17, 35 and at 12 weeks were evaluated regarding proportion, temporal course and durability of wound closure as well as development of new wounds.
Results:
Of 168 baseline wounds in 14 patients, 109 (64.9%) wounds had closed at week 12, of which 63.3% (69 wounds) had closed already by day 35 or day 17. Conversely, 74.2% of the baseline wounds that had closed by day 17 or day 35 remained closed until week 12. First-closure ratio within 12 weeks was 75.6%. The median rate of newly developing wounds decreased significantly (p=0.001) by 79.3%.
Conclusions:
Comparison of the findings with published data from placebo arms and vehicle-treated wounds in controlled clinical trials suggests potential capability of ABCB5+ MSCs to facilitate wound closure, prolongate wound recurrence and decelerate formation of new wounds in RDEB. Beyond suggesting therapeutic efficacy for ABCB5+ MSCs, the analysis might stimulate researchers who develop therapies for RDEB and other skin fragility disorders to not only assess closure of preselected target wounds but pay attention to the patients’ dynamic and diverse overall wound presentation as well as to the durability of achieved wound closure and the development of new wounds.
Trial registration:
Clinicaltrials.gov NCT03529877; EudraCT 2018–001009-98.
Keywords: ABCB5, Inflammation, Mesenchymal stromal cells, Recessive dystrophic epidermolysis bullosa, Wound healing
Introduction
Recessive dystrophic epidermolysis bullosa (RDEB) is a rare, devastating, and life-threatening inherited skin fragility disorder caused by biallelic mutations in the COL7A1 gene [1]. Lack of functional type VII collagen causes an extremely impaired mechanical cutaneous stability, which manifests with recurrently blistering and non-healing chronic wounds [2, 3]. Persistent skin inflammation significantly contributes to symptom severity and disease complications [4, 5]. Dermal mesenchymal stromal cells (MSCs) expressing the ABC transporter ABCB5 [6] are capable, upon systemic administration, of efficiently migrating and homing to skin wounds [7] and dampening IL-1-driven skin inflammation [8]. They can also secrete basement membrane proteins including type VII collagen [7] and facilitate healing of acute and chronic wounds [8–12].
A recently published international clinical trial of intravenous infusions of ABCB5+ MSCs to RDEB patients [13] demonstrated statistically significant reductions in EBDASI activity [14] iscorEB clinician [15] disease severity scores and in an itch numeric rating score, along with good tolerability and manageable safety [13]. The beneficial effect of ABCB5+ MSCs on disease severity was mainly attributable to a decrease in skin activity, with patients whose EBDASI activity score contained a high portion of skin activity at baseline responding best to treatment (supplementary Figure 1).
Both from patient and drug regulatory perspective [16–19], skin wound closure is considered one of the most clinically meaningful outcomes in RDEB. Moreover, wound closure is a particularly robust outcome, which can be independently and objectively measured [19] and is thus substantially less prone to bias and placebo effects than patient-perceived outcomes such as pain or itch. The need to generate robust data on wound healing in the rare disease RDEB has stimulated us to conduct an in-depth wound closure analysis using patient photographs taken at each trial visit, taking into account the complex heterogenous wound presentation in RDEB, which is characterized by coexistence of recurrently healing/re-opening and of chronically open wounds [2, 3]. In contrast to previous RDEB trials, which have typically focused on individual wounds per patient and followed up the durability of achieved wound closure either not all or only over 1–2 weeks, we present a unique analysis, which evaluated all wound types and followed up the durability of achieved wound closure up to more than 9 weeks.
Methods
Clinical trial
The design of the trial, inclusion and exclusion criteria, and the results for all pre-defined outcome measures have been reported previously [13]. Briefly, 16 patients with genotypically and phenotypically confirmed RDEB enrolled at five study sites in Germany, Austria, France, United Kingdom and USA received three intravenous infusions of 2×106 ABCB5+ MSCs/kg, provided as a highly standardized GMP-conforming advanced-therapy medicinal product [20, 21], on day 0, 17, and 35. The infusion scheme was based on previous studies of other MSC types to treat RDEB, intending to slightly extent the intervals between infusions over those used in the previous studies (i.e., day 0, 7 or 14, and 28) [22–24] in view of the anticipated need of RDEB patients for lifelong treatment with disease-modifying therapies as long as curative therapies are not available. The patients were followed up for 12 weeks regarding efficacy and 1 year regarding and safety [13].
The trial was conducted in accordance with the ethical principles of the Declaration of Helsinki. The protocol and all other relevant documents had been approved by the competent local drug regulatory authorities and independent ethics committees/institutional review boards. Prior to any procedures, all patients or, in case of children, their parent gave written informed consent.
Photograph assessments
At each efficacy visit (day 0, day 17, day 35, week 12), photographs of the affected body regions had been taken for documentary purposes. In situations where this would have imposed an undue stress on the patient, the investigator was allowed to desist from photographing the respective body area(s) at that visit. For the present analysis, all wounds in all body regions of which photographs were taken at least at baseline and at week 12 were used. If a photograph for the day-17 or day-35 visit had been missed out, the results from the photographs of the previous visit were assumed also for that visit. For the rationale and validation of the missing data imputation method see supplementary Figure 2.
The photographs were evaluated by an expert wound care specialist to follow up the number of baseline wounds, defined as all distinct open wounds present at day 0, in each patient across all visits. Changes in wound size were estimated by three independent reviewers by visual comparison of each post-baseline photograph against the corresponding photograph from the preceding visit. Changes against the preceding visit were rated semi-quantitatively as described and validated in earlier RDEB trials [25–27], using a numeric scale ranging from −3 to 3 (defined in supplementary Table 1). In cases of different ratings between the evaluators, the mean score is presented. In addition, the photographs were independently evaluated by three reviewers to record the number of new wounds, defined as wounds that were open at any post-baseline visit but had not been open at day 0. For exemplary series of evaluated photographs see Figure 1.
Fig. 1.
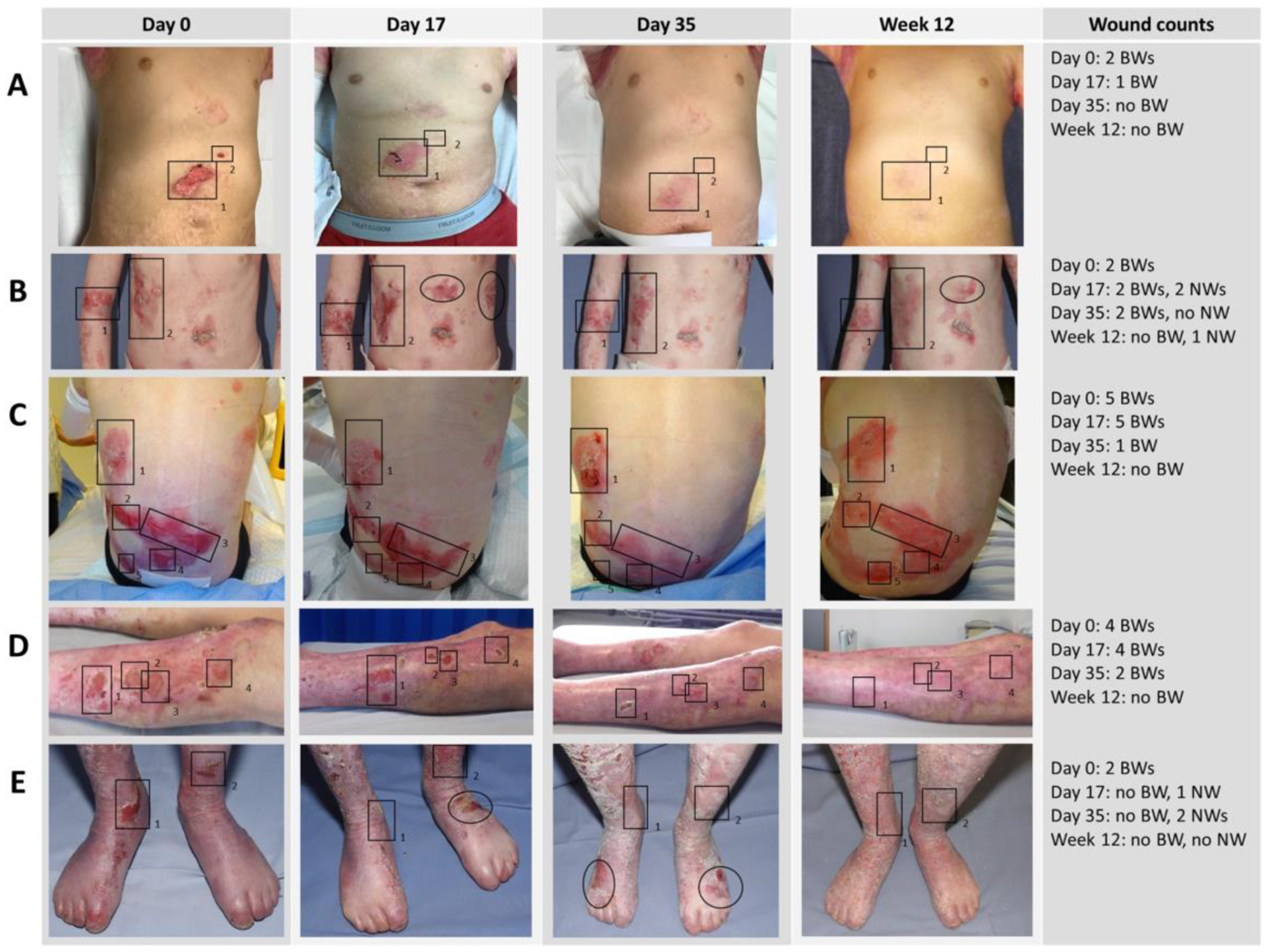
Exemplary series of photographs. Baseline wounds (BWs) were marked with rectangles, new wounds (NWs) with circles/ellipses. (A) Male, 10 years. (B) Female, 13 years. (C) Female, 7 years. (D) Male, 34 years. (E) Female, 13 years. All patients or, in case of children, their parent consented to the publication of their photographs.
Calculations and statistical analyses
The numbers of open wounds were used to calculate, across all post-baseline visits, the overall wound closure ratios (i.e., numbers and percentages of baseline wounds being closed at the visit), the first-closure ratios (i.e., numbers and percentages of baseline wounds having shown first closure until the visit) and the median time to first wound closure. New wound counts were used to calculate new-wound development rates (new wounds per day).
Statistical analyses were performed using GraphPad Prism 9 software (GraphPad, San Diego, CA, USA). Normally distributed data (D’Agostini-Pearson test) are presented as means with SD, not normally distributed data as medians with IQR. The tests used for inferential statistics are given in the figure legends.
Results
Patients
Of the 16 patients, 14 (6 male, 8 female, 6–36 years) had attended the baseline and all three post-baseline visits and were included in the present analysis.
Baseline wound closure
Totally 168 wounds were included in the baseline wound analysis. During treatment, the number of baseline wounds decreased significantly (p<0.001) within patients on average by 28%, 51% and 66% on day 17, day 35 and at week 12, respectively, from a median wound count of 10.5 (range 6–25) to 4 (range 0–14) per patient (Figure 2A, B; supplementary Figure 3). Median time to first wound closure was 35 days (Figure 2C).
Fig. 2.
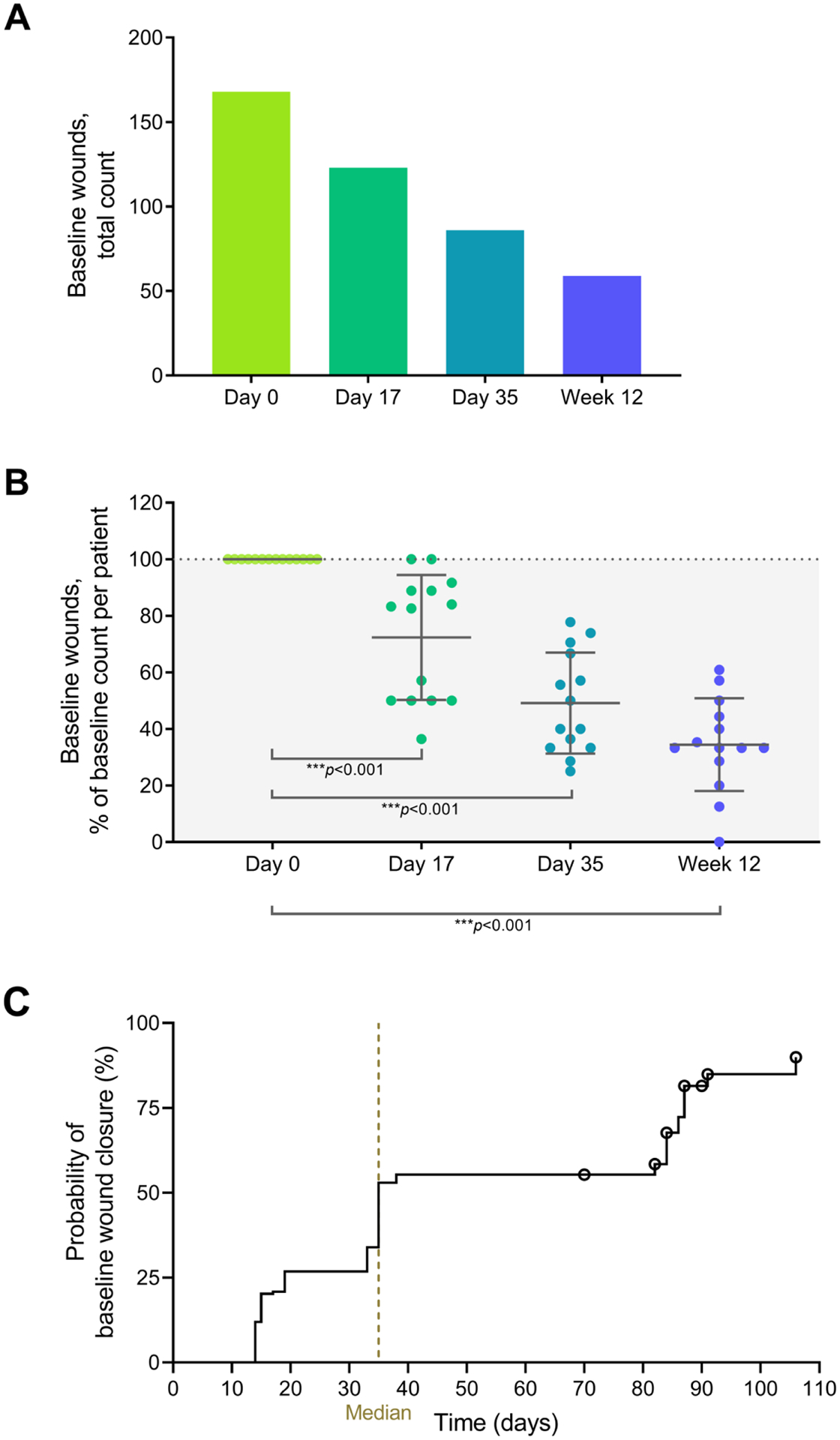
Baseline wound follow-up. (A) Total number of evaluated baseline wounds from 14 patients. (B) Reduction in baseline wound numbers, expressed as percentage of the baseline count. Error bars show means with SD from 14 patients. Statistical significance of the reductions was tested against the null hypothesis (mean = 100%) using one-sample t-tests. (c) Kaplan–Meier plot for the time to first wound closure (N=168 wounds). Wounds that did not close during efficacy follow-up were censored at the date of the last visit (denoted by a circle).
Overall wound closure ratios and first-closure ratios are given in Table 1. On day 17, 45 (26.8%) of the 168 baseline wounds had closed (Figure 3A, Day 17, blue-shaded slices). Of these closed wounds, 31 wounds (68.9%) were closed also on both subsequent visits, while the remaining had reopened on day 35 and/or week 12. On day 35, 82 (48.8%) baseline wounds were closed, of which 69 wounds (84.1% of the wounds that had closed) were also closed at week 12 (Figure 3A, Day 35, blue-shaded slices). At week 12, 109 (64.9%) baseline wounds were closed (Figure 3A, Week 12, blue-shaded slices).
Table 1.
Closure ratios and first-closure ratios of baseline woundsa
| Visit | Closure ratios Baseline wounds found closed at visit |
First-closure ratios Baseline wounds with first closure (irrespective of later reopening) occurring until visit |
|---|---|---|
| No. (%) N=168 |
No. (%) N=168 |
|
| Day 17 | 45 (26.8%) | 45 (26.8%) |
| Day 35 | 82 (48.8%) | 93 (55.4%) |
| Week 12 | 109 (64.9%) | 127 (75.6%) |
Baseline wounds were defined as distinct open wounds present at the baseline visit (day 0).
Fig. 3.
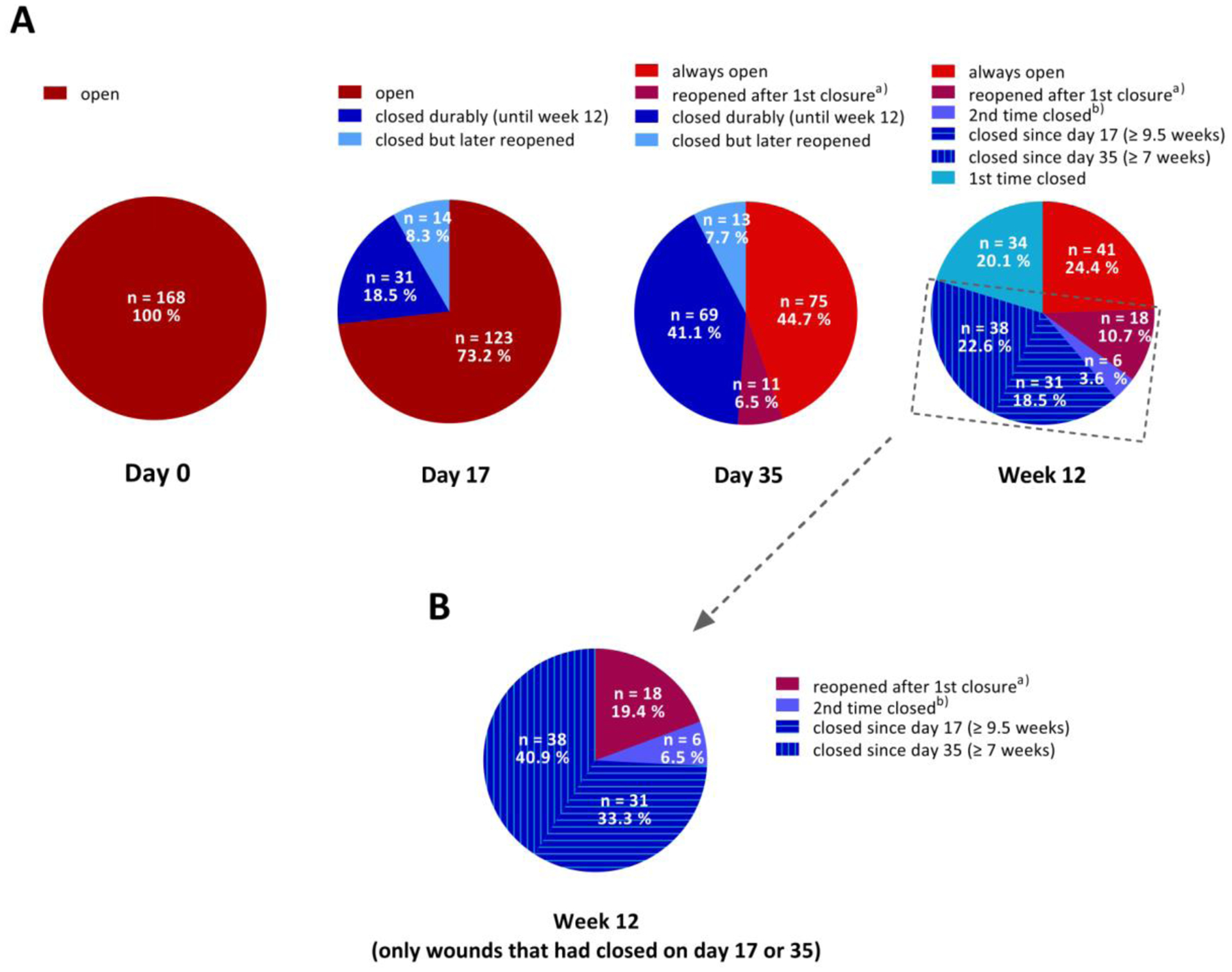
Baseline wound closure. (A) Numbers and percentages of open, closed, reopened and reclosed baseline wounds (N=168) by visit. (B) Wound closure outcome at week 12 of the baseline wounds that had closed on day 17 and/or day 35 (n=93). Shades of blue color refer to wounds that were closed, shades of red/brown color refer to wounds that were open at the respective visit.
a Refers to wounds that were closed at a previous visit but open at the respective visit.
b Refers to wounds that were closed on Day 17, open again on Day 35, and closed again at Week 12.
Durability of achieved wound closure
Of the 109 wounds that were closed at week 12 (Figure 3A, Week 12, blue-shaded slices), 69 wounds (corresponding to 63.3% of the 109 closed wounds and to 41.1% of the 168 total baseline wounds) had closed already by day 35 or even day 17 (Figure 3A, Week 12, “closed since day 35” and “closed since day 17”).
Conversely, 93 baseline wounds had shown first closure until day 35 (Figure 3A, Week 12, encircled by dashed line). Of these, 69 wounds (74.2%) were still closed at the week-12 visit (Figure 3B, “closed since day 17” and “closed since day 35”).
Representativeness of individual wound closure
To retrospectively estimate whether the outcome of a single target wound per patient would have reflected the overall proportion of closed target wounds at week 12, in each patient all baseline wounds were numbered consecutively in a random order. Then the outcome was individually assessed for the wounds No. 1–6 (which were, at baseline, present in all patients, because each patient had at least 6 baseline wounds) across all patients. Statistical comparison revealed that the observed proportion of closed wounds at week 12 for each single wound did not significantly differ from the overall proportion of closed wounds at week 12 (supplementary Figure 4).
Wound size changes
Semi-quantitative rating of the wound size changes using a −3 to +3 scale, revealed significant median changes by 1.4, 1.8 and 1.9 score points on day 17, day 35 and at week 12, respectively (supplementary Figure 5A). Smaller, but still significant increases in the wound size change scores over baseline were also seen when only the wounds that never closed during the 12-week follow-up period were considered (supplementary Figure 5B).
New wound development
The median (IQR) new-wound development rate significantly decreased by 79.3% from 0.29 (0.12–0.49) wounds/day between day 0 and day 17 to 0.06 (0.04–0.12) wounds/day between day 35 and week 12, and by 76.0% from 0.25 (0.16–0.33) wounds/day between day 17 and day 35 to 0.06 (0.04–0.12) wounds/day between day 35 and week 12 (Figure 4).
Fig. 4.
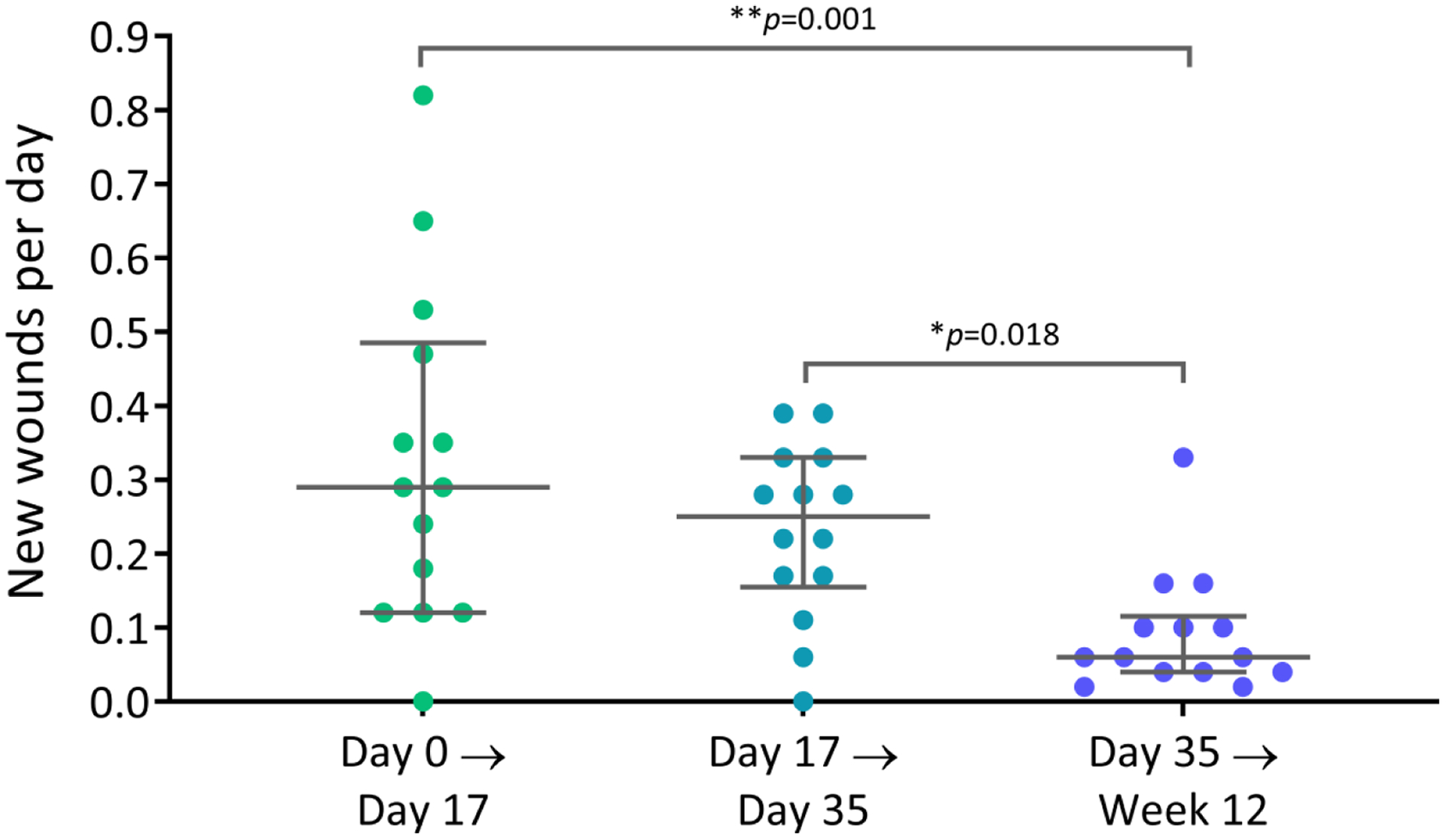
New-wound development rates, calculated as the increase in the number of new wounds since the previous visit divided by the number of days since the previous visit. Error bars show medians with IQR of n=14 patients; p values (Kruskal-Wallis test followed by Dunn’s multiple comparison tests) indicate statistically significant differences between the time intervals evaluated.
Discussion
Studies investigating the natural history of RDEB have identified two distinct wound types: recurrent wounds that heal within 6 weeks and subsequently reopen within 3 weeks on average coexisting with chronic open wounds that do not heal for years [2, 3]. Thus, trials evaluating individual target wounds per patient [28–31] bear the risk of selecting wounds that would close irrespective of the intervention. Moreover, any observed wound closure at a single time point does not necessarily indicate a meaningful sustained benefit if it was not confirmed that the wound remained closed over a period of time that at least reflects or even exceeds the natural reopening time. Here we present a more bespoke analysis, which, by recording all wounds present at any time point in each patient and following up the durability of achieved wound closure over up to 9.5 weeks, considers the complex dynamic and diverse wound presentation in RDEB more comprehensively.
ABCB5+ MSCs increased the proportion of wound closure
Chronic RDEB wounds are accompanied by inflammation, fibrosis, scarring and mitten deformities [32], are significantly more painful [2] and particularly predisposed to infection and development of aggressive metastatic squamous cell carcinomas [32–34]. Therefore, healing of chronic wounds would improve both life quality and life expectancy [34]. A commonly used parameter to estimate treatment effects on wound healing in RDEB is the first-closure ratio, i.e. the proportion of wounds that have closed at a defined time point, irrespective of their further development. Since a cutoff of 12 weeks is assumed to distinguish between recurrent and chronic wounds [2], 12-week first-closure ratios reflect the proportion of healing (irrespective of later recurring) wounds as opposed to the chronic, non-healing wounds.
In the present study, 75.6% of baseline wounds showed first closure within 12 weeks (Table 1; Figure 3A, Week 12, all wounds except “always open”). This ratio distinctly exceeds publicly available data reporting that during treatment with placebo or vehicle only 44.0% or 50.8% of wounds showed first closure within 12 weeks [35, 36]. Thus, during treatment with ABCB5+ MSCs a greater proportion of wounds have healed as can naturally be expected within 12 weeks, suggesting that the treatment has stimulated chronic, otherwise non-healing wounds to close.
ABCB5+ MSCs accelerated wound healing
A shorter the median time to wound closure of 35 days (Figure 2C) as compared with published control data of 57 days [36] suggests that ABCB5+ MSCs infusions have increased the velocity of wound healing. This can be expected to further reduce distressing symptoms related to open wounds, such as pain and itch, and to decrease the risk of wound infections [17, 32].
ABCB5+ MSCs enhanced the durability of wound closure
Undoubtedly, treatment success in RDEB wound care does not solely depend on facilitation of wound closure, but also on the period during which a wound, once closed, remains closed thereafter. Remarkably, while natural-history data have revealed that healed RDEB wounds commonly reopen, on average, within 3 weeks after healing [2], in the present trial 74.2% of the baseline wounds that had closed on day 17 and/or day 35 were still closed at week 12, i.e. had remained closed over at least 7 or even 9.5 weeks (Figure 3B).
In contrast, to the best of our knowledge, almost all controlled trials on RDEB wound healing published to date have disregarded the further development of the wounds that closed during treatment. One exception is the GEM-3 trial, in which wound closure needed to be confirmed at a subsequent visit 2 weeks apart [31]. Moreover, the durability of wound closure was followed up across 3 further months [31]. In that trial, at 12 weeks 20% of the placebo-treated wounds had been closed over (at least) 2 weeks [31], as compared with a more than twice as high proportion (41.1%) of total baseline wounds having been closed over even 7 or 9.5 weeks in the present trial (Figure 3A, Week 12). Moreover, 35% of the placebo-treated wounds that were found closed at week 12 in the GEM-3 trial remained closed until month 6, i.e. over 3 months [31]. While we have not followed up closed wounds beyond week 12, this result may be compared with the more than twice as high proportion of 74.2% of wounds closed on day 17 and/or day 35 being still being closed at week 12, i.e. having remained closed over (at least but possibly longer than) 7 or 9.5 weeks (Figure 3B). Together, treatment with ABCB5+ seem to result in durable wound closure.
In wounds that did not reach full closure ABCB5+ MSCs decreased the wound size
Since wound pain, pruritus and overall skin disease severity have been found significantly associated with larger wound size [3, 37], even partial wound closure would supply some benefit to the patient. Semi-quantitative assessment revealed significant mean decreases in wound size, even when only the wounds that never fully closed during the 12-week follow-up period (i.e., those with the poorest healing tendency) were considered (supplementary Figure 5). These observations point to a potential supplementary treatment benefit, which might have contributed to the previously reported significant reductions in disease severity and itch score during treatment with ABCB5+ MSCs [13].
ABCB5+ MSCs decelerated the development of new wounds
Rather unexpectedly, we observed a significant decrease in the median new-wound development rate by 79.3% across 12 weeks (Figure 4). Compared with a previous trial reporting ≥20% decreases in the mean numbers of new blisters per day across 4 months in 31% of the patients in the placebo arm [38], the presently observed decrease suggest an additional dampening effect of the treatment with ABCB5+ MSCs on the development of new wounds.
From a mechanistic perspective, the here described effects substantiate the modes of action supposed to mediate the decrease in RDEB disease activity seen with ABCB5+ MSCs infusions [13]. Specifically, ABCB5+ MSCs have emerged capable of facilitating healing of chronic venous ulcers [8–10], which has been ascribed to an effective attenuation of persistent IL-1-driven skin inflammation via adaptive secretion of IL-1 receptor antagonist [8]. Moreover, in a murine vasculitis model, ABCB5+ MSCs have ameliorated unrestrained neutrophil activation [39]. Given that IL-1 has been identified among the primary drivers of sustained skin inflammation in RDEB [4, 5, 40] and progression of RDEB wounds to a chronic state has been found associated with excessive accumulation of neutrophils [41], it seems conceivable that ABCB5+ MSCs might have supported healing of RDEB wounds in overcoming their pro-inflammatory non-healing state and entering into a healing cycle.
Clearly, these modes of action are not unique to ABCB5+ dermal MSCs, and clinical trials of MSCs derived from other niches than the skin, including bone marrow (BM) [22, 23, 42] and umbilical cord blood [24], have also achieved beneficial effects on the wound presentation in RDEB patients. While different infusion protocols, follow-up periods and outcome variables impede comparisons of the observed clinical efficacy between the different cell sources, a recent non-clinical study has directly compared ABCB5+ dermal MSCs and BM-MSCs regarding characteristics with potential importance in the treatment of RDEB, including homing skin homing and transcriptional profiles [7]. This study revealed superior homing to skin and wound engraftment in mice as well as increased expression of vascular cell adhesion molecule (important in homing to the perivascular skin niche), several homeobox genes including HOXA3 (a master coordinator of wound healing) and MHC class II (important in immune evasion) for human ABCB5+ dermal MSCs over BM-MSCs [7]. Whether these findings in fact constitute a superior therapeutic efficacy of ABCB5+ MSCs over BM-MSCs in RDEB remains to be studied in future clinical trials.
Beyond alleviating skin inflammation and facilitating wound healing, ABCB5+ MSCs have been shown capable of secreting basement membrane proteins including type VII collagen [7]. It may thus be hypothesized that treatment with ABCB5+ MSCs could even enhance skin structural integrity via deposition and substitution of type VII collagen, the lack of which represents the causative factor underlying the wound development in RDEB. However, at present this hypothesis is speculative and needs to be supported by evaluation of investigational skin biopsies taken pre- and post-treatment.
Unequivocally, the conclusions from this analysis are limited by several factors. First, the trial lacked a control group, which is why the present results could only be compared with historical control data from other clinical trials. Second, the present study was an unplanned post-hoc analysis, which inherently bears a certain risk of introducing selection bias. Third, the photographs had been taken for documentary purposes and were not standardized, which did not enable actual wound size measurements. A major strength of the present analysis is the high number (N=168) of wounds that were followed up in patients suffering from the rare disease REDB. Further strengths include the robustness of the endpoint of full wound closure, which is less prone to bias and placebo effects than patient-perceived outcomes, and, not least, the inclusion of all evaluable wounds without any further selection, as opposed to the commonly applied assessment of carefully selected target wounds that had met pre-defined inclusion criteria regarding age, size and location of the wound [28–31, 35, 36]. Given that a significant proportion of wounds in an RDEB patient are chronic wounds that usually exist for years [2, 3], a considerable proportion of the wounds evaluated in the present trial were likely such chronic wounds with an extremely poor healing tendency. Thus, in the light of observations that higher wound age, larger wound size and wound locations in body sites that are more easily exposed to trauma negatively affect RDEB wound healing [32, 36, 37], it could even be speculated that the herein applied procedure of evaluating all evaluable wounds might have disfavored the outcomes when compared with trials that have investigated preselected target wounds. Thus, the present results can be expected to reflect the real-life situation of RDEB patients more closely and might thus be even more promising.
Together, we find it reasonable to conclude that, in addition to previously reported effects on RDEB disease activity, itch and pain [13], systemic treatment with ABCB5+ MSCs is capable of facilitating wound closure, prolongating wound recurrence, and decelerating the formation of new wounds. A larger trial with a randomized, placebo-controlled design, a longer efficacy period covering more MSC doses, and refined pre-defined outcome parameters using standardized photography is needed to confirm these conclusions. Moreover, beyond indicating therapeutic efficacy for ABCB5+ MSCs on RDEB wound healing, the present study might stimulate other researchers who develop therapies for skin fragility disorders to pay attention to the patients’ dynamic and diverse overall wound presentations. Although single-wound closure endpoints can uncover potential efficacy of investigational interventions while minimizing the amount of stress posed on the highly vulnerable patients, evaluation of all wound types as well as the durability of achieved wound closure and development of new wounds will predict the expectable patient benefit more closely.
Supplementary Material
Highlights.
ABCB5+ MSCs facilitated wound closure in recessive dystrophic epidermolysis bullosa
The wounds that closed during treatment showed a prolonged time to recurrence
Wounds that did not reach full closure during treatment decreased in size
ABCB5+ MSC treatment decelerated the formation of new wounds
Acknowledgements
The authors thank Dr. Thomas E. Serena, Medical Director of SerenaGroup®, Cambridge, MA, USA, for expert contribution and support to the evaluation of the wound photographs.
Funding
Contributions by NYF and MHF to this work were supported by the NIH/National Eye Institute (NEI) grants RO1EY025794 and R24EY028767, NIH National Heart, Lung, and Blood Institute (NHLBI) grant 1R01HL161087, and NIH National Institute on Aging 1P01AG071463. Contributions by DK were supported by the Berta-Ottenstein Advanced Clinician Scientist Programme of the University of Freiburg and by the German Research Foundation (DFG) through KI1795/2-1, the CRC1160/2 - project B03(N) and the CRC-1479 - Project ID: 441891347. The funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest
NYF and MHF are inventors or co-inventors of U.S. and international patents assigned to Brigham and Women’s Hospital, Boston Children’s Hospital, and/or the VA Boston Healthcare System, Boston, MA, licensed to TICEBA GmbH, Heidelberg, Germany, and RHEACELL GmbH & Co. KG, Heidelberg, Germany. MHF serves as scientific advisor and holds stock in TICEBA GmbH and RHEACELL GmbH & Co. KG. DK and JT serve as scientific advisors to RHEACELL. KD, CD and SF are employees of RHEACELL. ENR is employee of TICEBA. CG is CEO, and MAK is CSO of TICEBA and RHEACELL. JAM declares that he has no conflicts of interest.
References
- [1].Bardhan A, Bruckner-Tuderman L, Chapple ILC, Fine JD, Harper N, Has C, et al. Epidermolysis bullosa. Nat Rev Dis Primers 2020;6:78. 10.1038/s41572-020-0210-0 [DOI] [PubMed] [Google Scholar]
- [2].Solis DC, Teng C, Gorell ES, Barriga M, Nazaroff J, Li S, et al. Classification of 2 distinct wound types in recessive dystrophic epidermolysis bullosa: A retrospective and cohort natural history study. J Am Acad Dermatol 2021;85:1296–8. 10.1016/j.jaad.2020.08.118 [DOI] [PubMed] [Google Scholar]
- [3].Eng VA, Solis DC, Gorell ES, Choi S, Nazaroff J, Li S, et al. Patient-reported outcomes and quality of life in recessive dystrophic epidermolysis bullosa: A global cross-sectional survey. J Am Acad Dermatol 2021;85:1161–7. 10.1016/j.jaad.2020.03.028 [DOI] [PubMed] [Google Scholar]
- [4].Yamanaka K, Nakanishi T, Saito H, Maruyama J, Isoda K, Yokochi A, et al. Persistent release of IL-1s from skin is associated with systemic cardio-vascular disease, emaciation and systemic amyloidosis: the potential of anti-IL-1 therapy for systemic inflammatory diseases. PLoS One 2014;9:e104479. 10.1371/journal.pone.0104479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Annicchiarico G, Lopalco G, Morgese MG, Cantarini L, Iannone F. Canakinumab in recessive dystrophic epidermolysis bullosa: a novel unexpected weapon for non-healing wounds? Clin Exp Rheumatol 2016;34:961–2. [PubMed] [Google Scholar]
- [6].Frank NY, Pendse SS, Lapchak PH, Margaryan A, Shlain D, Doeing C, et al. Regulation of progenitor cell fusion by ABCB5 P-glycoprotein, a novel human ATP-binding cassette transporter. J Biol Chem 2003;278:47156–65. 10.1074/jbc.M308700200 [DOI] [PubMed] [Google Scholar]
- [7].Riedl J, Pickett-Leonard M, Eide C, Kluth MA, Ganss C, Frank NY, et al. ABCB5+ dermal mesenchymal stromal cells with favorable skin homing and local immunomodulation for recessive dystrophic epidermolysis bullosa treatment. Stem Cells 2021;39:897–903. 10.1002/stem.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vander Beken S, de Vries JC, Meier-Schiesser B, Meyer P, Jiang D, Sindrilaru A, et al. Newly defined ATP-binding cassette subfamily B member 5 positive dermal mesenchymal stem cells promote healing of chronic iron-overload wounds via secretion of interleukin-1 receptor antagonist. Stem Cells 2019;37:1057–74. 10.1002/stem.3022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kerstan A, Niebergall-Roth E, Esterlechner J, Schröder HM, Gasser M, Waaga-Gasser AM, et al. Ex vivo-expanded highly pure ABCB5(+) mesenchymal stromal cells as Good Manufacturing Practice-compliant autologous advanced therapy medicinal product for clinical use: process validation and first in-human data. Cytotherapy 2021;23:165–75. 10.1016/j.jcyt.2020.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kerstan A, Dieter K, Niebergall-Roth E, Dachtler A-K, Kraft K, Stücker M, et al. Allogeneic ABCB5(+) mesenchymal stem cells for treatment-refractory chronic venous ulcers: a phase I/IIa clinical trial. JID Innovations 2022;2:100067. 10.1016/j.xjidi.2021.100067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Singh K, Maity P, Koroma AK, Basu A, Pandey RK, Beken SV, et al. Angiogenin Released from ABCB5(+) Stromal Precursors Improves Healing of Diabetic Wounds by Promoting Angiogenesis. J Invest Dermatol 2021;142:1725–36. 10.1016/j.jid.2021.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kerstan A, Dieter K, Niebergall-Roth E, Klingele S, Jünger M, Hasslacher C, et al. Translational development of ABCB5+ dermal mesenchymal stem cells for therapeutic induction of angiogenesis in non-healing diabetic foot ulcers. Stem Cell Res Ther 2022;13:455. 10.21203/rs.3.rs-1508134/v1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kiritsi D, Dieter K, Niebergall-Roth E, Fluhr S, Daniele C, Esterlechner J, et al. Clinical trial of ABCB5+ mesenchymal stem cells for recessive dystrophic epidermolysis bullosa. JCI Insight 2021;6:e151922. 10.1172/jci.insight.151922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Loh CC, Kim J, Su JC, Daniel BS, Venugopal SS, Rhodes LM, et al. Development, reliability, and validity of a novel Epidermolysis Bullosa Disease Activity and Scarring Index (EBDASI). J Am Acad Dermatol 2014;70:89–97. 10.1016/j.jaad.2013.09.041 [DOI] [PubMed] [Google Scholar]
- [15].Schwieger-Briel A, Chakkittakandiyil A, Lara-Corrales I, Aujla N, Lane AT, Lucky AW, et al. Instrument for scoring clinical outcome of research for epidermolysis bullosa: a consensus-generated clinical research tool. Pediatr Dermatol 2015;32:41–52. 10.1111/pde.12317 [DOI] [PubMed] [Google Scholar]
- [16].Bruckner AL, Losow M, Wisk J, Patel N, Reha A, Lagast H, et al. The challenges of living with and managing epidermolysis bullosa: insights from patients and caregivers. Orphanet J Rare Dis 2020;15:1. 10.1186/s13023-019-1279-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Schräder NHB, Korte EWH, Duipmans JC, Stewart RE, Bolling MC, Wolff AP. Identifying Epidermolysis Bullosa Patient Needs and Perceived Treatment Benefits: An Explorative Study Using the Patient Benefit Index. J Clin Med 2021;10:5836. 10.3390/jcm10245836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].U.S. Department of Health and Human Services, Food and Drug Administration. Epidermolysis Bullosa: Developing Drugs for Treatment of Cutaneous Manifestations – Guidance for Industry, https://www.fda.gov/media/128419/download; 2019. [Accessed 16 November 2022].
- [19].U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Chronic Cutaneous Ulcer and Burn Wounds – Developing Products for Treatment, https://www.fda.gov/media/71278/download; 2006. [Accessed 16 November 2022].
- [20].Ballikaya S, Sadeghi S, Niebergall-Roth E, Nimtz L, Frindert J, Norrick A, et al. Process data of allogeneic ex vivo-expanded ABCB5+ mesenchymal stromal cells for human use: off-the-shelf GMP-manufactured donor-independent ATMP. Stem Cell Res Ther 2020;11:482. 10.1186/s13287-020-01987-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tappenbeck N, Schröder HM, Niebergall-Roth E, Hassinger F, Dehio U, Dieter K, et al. In vivo safety profile and biodistribution of GMP-manufactured human skin-derived ABCB5-positive mesenchymal stromal cells for use in clinical trials. Cytotherapy 2019;21:546–60. 10.1016/j.jcyt.2018.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Petrof G, Lwin SM, Martinez-Queipo M, Abdul-Wahab A, Tso S, Mellerio JE, et al. Potential of systemic allogeneic mesenchymal stromal cell therapy for children with recessive dystrophic epidermolysis bullosa. J Invest Dermatol 2015;135:2319–21. 10.1038/jid.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rashidghamat E, Kadiyirire T, Ayis S, Petrof G, Liu L, Pullabhatla V, et al. Phase I/II open-label trial of intravenous allogeneic mesenchymal stromal cell therapy in adults with recessive dystrophic epidermolysis bullosa. J Am Acad Dermatol 2020;83:447–54. 10.1016/j.jaad.2019.11.038 [DOI] [PubMed] [Google Scholar]
- [24].Lee SE, Lee SJ, Kim SE, Kim K, Cho B, Roh K, et al. Intravenous allogeneic umbilical cord blood-derived mesenchymal stem cell therapy in recessive dystrophic epidermolysis bullosa patients. JCI Insight 2021;6:e143606. 10.1172/jci.insight.143606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Woodley DT, Cogan J, Hou Y, Lyu C, Marinkovich MP, Keene D, et al. Gentamicin induces functional type VII collagen in recessive dystrophic epidermolysis bullosa patients. J Clin Invest 2017;127:3028–38. 10.1172/jci92707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Eichstadt S, Barriga M, Ponakala A, Teng C, Nguyen NT, Siprashvili Z, et al. Phase 1/2a clinical trial of gene-corrected autologous cell therapy for recessive dystrophic epidermolysis bullosa. JCI Insight 2019;4:e130554. 10.1172/jci.insight.130554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Dutt-Singkh Y, Barriga M, Nazaroff J, Solis D, Li S, Marinkovich M, et al. 50% wound healing correlates with RDEB patient reported outcomes in pain, itch and skin durability. J Invest Dermatol 2018;138:S56. 10.1016/j.jid.2018.03.334 [DOI] [Google Scholar]
- [28].Petrof G, Martinez-Queipo M, Mellerio JE, Kemp P, McGrath JA. Fibroblast cell therapy enhances initial healing in recessive dystrophic epidermolysis bullosa wounds: results of a randomized, vehicle-controlled trial. Br J Dermatol 2013;169:1025–33. 10.1111/bjd.12599 [DOI] [PubMed] [Google Scholar]
- [29].Paller AS, Browning J, Nikolic M, Bodemer C, Murrell DF, Lenon W, et al. Efficacy and tolerability of the investigational topical cream SD-101 (6% allantoin) in patients with epidermolysis bullosa: a phase 3, randomized, double-blind, vehicle-controlled trial (ESSENCE study). Orphanet J Rare Dis 2020;15:158. 10.1186/s13023-020-01419-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kern JS, Schwieger-Briel A, Löwe S, Sumeray M, Davis C, Martinez AE. Oleogel-S10 Phase 3 study “EASE” for epidermolysis bullosa: study design and rationale. Trials 2019;20:350. 10.1186/s13063-019-3362-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Guide SV, Gonzalez ME, Bağcı IS, Agostini B, Chen H, Feeney G, et al. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N Engl J Med 2022;387:2211–9. 10.1056/NEJMoa2206663 [DOI] [PubMed] [Google Scholar]
- [32].Cianfarani F, Zambruno G, Castiglia D, Odorisio T. Pathomechanisms of altered wound healing in recessive dystrophic epidermolysis bullosa. Am J Pathol 2017;187:1445–53. 10.1016/j.ajpath.2017.03.003 [DOI] [PubMed] [Google Scholar]
- [33].Condorelli AG, Dellambra E, Logli E, Zambruno G, Castiglia D. Epidermolysis Bullosa-Associated Squamous Cell Carcinoma: From Pathogenesis to Therapeutic Perspectives. Int J Mol Sci 2019;20:5707. 10.3390/ijms20225707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huitema L, Phillips T, Alexeev V, Igoucheva O. Immunological mechanisms underlying progression of chronic wounds in recessive dystrophic epidermolysis bullosa. Exp Dermatol 2021;30:1724–33. 10.1111/exd.14411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Kern JS, Sprecher E, Fernandez MF, Schauer F, Bodemer C, Cunningham T, et al. Efficacy and safety of Oleogel-S10 (birch triterpenes) for epidermolysis bullosa: results from the phase III randomized double-blind phase of the EASE study. British Journal of Dermatology 2022. 10.1093/bjd/ljac001 [DOI] [PubMed] [Google Scholar]
- [36].Murrell DF, Paller AS, Bodemer C, Browning J, Nikolic M, Barth JA, et al. Wound closure in epidermolysis bullosa: data from the vehicle arm of the phase 3 ESSENCE Study. Orphanet J Rare Dis 2020;15:190. 10.1186/s13023-020-01435-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Solis DC, Gorell ES, Teng C, Barriga M, Nazaroff J, Li S, et al. Clinical characteristics associated with increased wound size in patients with recessive dystrophic epidermolysis bullosa. Pediatr Dermatol 2021;38:704–6. 10.1111/pde.14576 [DOI] [PubMed] [Google Scholar]
- [38].Chiaverini C, Roger C, Fontas E, Bourrat E, Bourdon-Lanoy E, Labrèze C, et al. Oral epigallocatechin-3-gallate for treatment of dystrophic epidermolysis bullosa: a multicentre, randomized, crossover, double-blind, placebo-controlled clinical trial. Orphanet J Rare Dis 2016;11:31. 10.1186/s13023-016-0411-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Jiang D, Muschhammer J, Qi Y, Kugler A, de Vries JC, Saffarzadeh M, et al. Suppression of neutrophil-mediated tissue damage - a novel skill of mesenchymal stem cells. Stem Cells 2016;34:2393–406. 10.1002/stem.2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Matsushima Y, Mizutani K, Goto H, Nakanishi T, Kondo M, Habe K, et al. Emaciation, Congestive Heart Failure, and Systemic Amyloidosis in Severe Recessive Dystrophic Epidermolysis Bullosa: Possible Internal Complications Due to Skin-Derived Inflammatory Cytokines Derived from the Injured Skin. Dermatopathology (Basel) 2020;7:41–7. 10.3390/dermatopathology7020007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Phillips T, Huitema L, Cepeda R, Cobos DL, Perez RIM, Garza MS, et al. Aberrant recruitment of leukocytes defines poor wound healing in patients with recessive dystrophic epidermolysis bullosa. J Dermatol Sci 2020;100:209–16. 10.1016/j.jdermsci.2020.10.009 [DOI] [PubMed] [Google Scholar]
- [42].El-Darouti M, Fawzy M, Amin I, Abdel Hay R, Hegazy R, Gabr H, et al. Treatment of dystrophic epidermolysis bullosa with bone marrow non-hematopoeitic stem cells: a randomized controlled trial. Dermatol Ther 2016;29:96–100. 10.1111/dth.12305 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


