Abstract
The COVID-19 survivors and long-term steroid administered patients exhibit a variety of fungal co-infections. The lives of COVID-19 patients and survivors are hampered by fungal species of the genera Candida, Aspergillus, and Mucor. There have been cases of mucormycosis, aspergillosis, and candidiasis in COVID-19 patients. The treatments given to these opportunistic fungal infections include polyene like amphotericin B, azoles including imidazoles like ketoconazole, miconazole, and triazoles like fluconazole, voriconazole, itraconazole, Echinocandin derivatives like- caspofungin, micafungin, immunomodulatory therapy, granulocyte transfusion, etc. A successful recovery and the reduction of fatalities depend on prompt diagnosis and treatment. To reduce mortality, advanced techniques to identify such uncommon infections at a very early stage are necessary. This review's goal is to provide a summary of the systemic and superficial opportunistic fungal infections that the COVID-19 survivors were dealing with, including information on illness incidence, pathogenicity, and treatment.
Keywords: Opportunistic fungal infections, Polyenes, Azoles, Diabetes, Post-COVID-19
Introduction
Opportunistic fungi are often non-pathogenic, but given the right conditions, they can develop into pathogens that can cause illness and even death. Opportunistic fungal infections are one of the most prevalent illnesses that have emerged in the recent years and are now a serious health concern for the general public (Chakrabarti 2008; Ravi Kant 2015; Singh 2014). Local fungal infections can affect any region of the body; systemic fungal infections, on the other hand, can spread throughout the bloodstream and affect several organs, including the liver, kidneys, skin, brain, and so on. These opportunistic fungi are frequently found in the environment and primarily harm immune-suppressed people (Chakrabarti 2008).
Aspergillosis, Zygomycosis, Blastomycosis, Candidimia, Rhinosinositis, Histoplasmosis, Penicillosis, Cryptococcosis, Phaeohyphomycosis, etc. are some examples of opportunistic fungal infections (Chakrabarti 2008). Invasive candidiasis and invasive aspergillosis are the two most prevalent mycosis types in India, respectively (Singh 2014). The construction taking place within and outside hospitals, as well as through hospital workers, is to be blamed for the spread of various fungal infections in hospitals (Chakrabarti 2008).
Mucormycosis, often known as the black fungus, is an opportunistic fungal infection that has spread throughout the world and in India as a part of the pandemic epidemic, particularly during this crisis of coronavirus and COVID-19. Its etiological agents are the Rhizopus and Rhizomucor. Mucormycosis is more likely to affect people with diabetes and those taking steroids. Aspergillosis, candidiasis, and blastomycosis are additional COVID-19 side effects that can afflict patients receiving steroid therapy and immune suppression (Baddley 2021). Not just the co-infections have been connected to a detrimental effect on cognition, but also the high levels of inflammation caused by COVID-19 and the potential for intubation. A higher risk of post-COVID-19 infections exists in people with severe mental problems, such as ADHD. Last but not least, because of their potential to affect cognition, the pandemic's particular psychological impacts have raised concerns (De Berardis 2020; Ali Awan 2021).
According to certain research, a healthy person can potentially get the illness if they are exposed to larger concentrations of opportunistic fungal spores. Dimorphs, which live in soil, reproduce by sporulation, and infect hosts through the respiratory system, are responsible for some of the diseases mentioned. The majority of these nosocomial opportunistic fungal illnesses are encountered in intensive care units, where candidemia consequences are the most lethal (Chakrabarti 2008; Singh 2014). However, mucormycosis has lately been a problem among COVID-19 patients who are hypoxic and receiving intensive care (Baddley 2021). This is thought to have resulted from poor cleanliness practices by medical staff members and a shortage of space during the pandemic. The second wave of COVID-19 primarily revealed this worldwide.
Diabetes patients frequently have morbidity and mortality, and steroid therapy is used to treat COVID-19, which is the main cause for concern (Dubey 2022). One strategy to deal with fungal diseases is to monitor the individuals who have the infection, practice good hygiene, watch for the fungus to develop drug resistance, and research and identify the pathology and clinical manifestations of the diseases (Ravikant 2015).
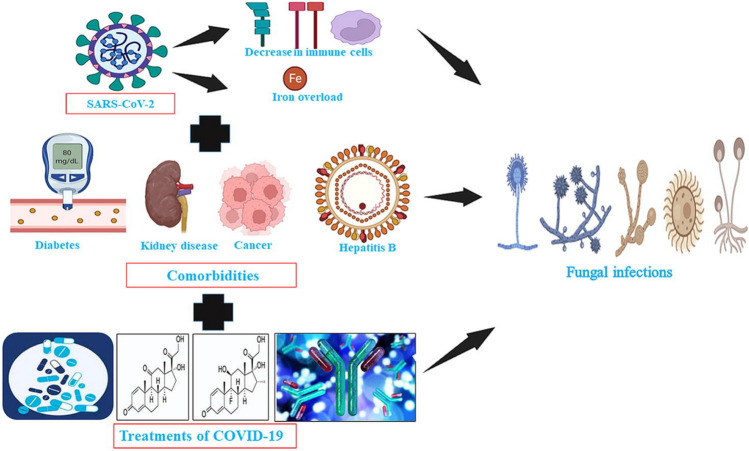
Figure 1 clearly illustrates the comorbidities and risk factors (steroids) that increase the likelihood that COVID-19 patients would develop opportunistic fungal infections.
Fig. 1.
Graphical data representation of COVID-19 co-infections associated with co-morbidities.
Adopted from: Nahid Akhtar, Atif Khurshid Wani, Surya Kant Tripathi, Ajit Prakash, M.Amin-ul Mannan. The role of SARS-CoV-2 immunosuppression and the therapy used to manage COVID-19 disease in the emergence of opportunistic fungal infections: A review. Current Research in Biotechnology.2022;4.337–349 https://doi.org/10.1016/j.crbiot.2022.08.001
Antifungal treatment
Amphotericin B
Amphotericin B, a polyene antifungal agent, has been one of the most remarkable discoveries in scientific history (Cavassin 2021). It was produced from the organism Streptomyces nodosus, which is found in soil (Sanjay 2021). Amphotericin B binds to ergosterol, which is an indispensable component of the fungal cell membrane, and causes depolarization of the membrane, thereby modifying the permeability of the cell membrane (Cavassin 2021). Because of this, the outflow of essential intracellular components causes rupturing of cells, which leads to the death of the cell. It has been used to treat many diseases as it has a broad spectrum of activity despite its side effects and toxicity (Cavassin 2021). So, to reduce its toxic nature, there are modified forms of amphotericin B that have been developed (Cavassin 2021). One of the most popularly used ones is the amphotericin B lipid complex. This modified form is known to show fewer side effects and is better tolerated by a remarkable number of patients (Cavassin 2021).
The original form of Amphotericin B is the deoxycholate formulation, and there are other formulations like lipid-based formulations (e.g., Amphotericin B lipid complex and liposomal Amphotericin B) and amphotericin B colloidal dispersion, which is currently being studied (Nett 2016).
AZOLES
Azoles are compounds having a five-ring structure and containing nitrogen atoms in their azole rings. The azoles include imidazoles like ketoconazole and miconazole and triazoles like fluconazole, voriconazole, and itraconazoles (Nett 2016). This anti-mycotic agent inhibits the synthesis of ergosterol, and hence the integrity of the cell membrane is disrupted. The original form of azoles like ketoconazole is known to cause toxicity during treatment, so newer formulations like fluconazole, voriconazole, itraconazole, etc. have been developed, which have improved and have less toxic characteristics (Nett 2016).
Amphotericin B and azoles are used as treatments against many mycotic diseases as they are effective against many pathogens like Candida spp., including Candida albicans, Candida krusei, Candida tropicalis, and Candida parapsilosis, which cause candidiasis. It is also effective against Aspergillus spp., which causes aspergillosis, and other infections like cryptococcosis, histoplasmosis, mucormycosis, penicillosis, etc. (Nett 2016; Ghannoum 1999).
Risk factors
Opportunistic fungal infections are associated with COVID-19 and diabetes, and co-infections are widespread in critically ill COVID-19 patients and those with diabetes. A COVID-19 infection can be complicated by many invasive fungal diseases due to many factors. And it has been at its peak during the second wave of COVID-19, especially in India (Baddley 2021). These issues have happened as a result of the virus's extreme harm or its treatment against numerous diseases (Baddley 2021). The most frequent complication of respiratory failure is invasive pulmonary aspergillosis (7% to 30%) (Baddley 2021). But recently, it was found that not only aspergillosis but also emerging cases of mucormycosis had taken over the patients with severe COVID-19 in India. Patients with COVID-19 are at risk for invasive fungal infections if they have the following symptoms: leucopenia, neutropenia, immune suppression, poorly controlled diabetes, lung diseases, and also if they are under antibiotic treatment for fungal colonization or are taking COVID-19 medications such as corticosteroids or immune modulators. In the intensive care unit, the percentage of invasive aspergillosis is about 2% to 33% (Baddley 2021).
SARS-CoV-2 infection suppresses the production of type 1 and type III interferons, which affect the normal innate immunity against Aspergillus as interferon III acts as an innate immunity against Aspergillus (Baddley 2021). Also, there is disturbance in the patient’s iron metabolism, which increases the iron level, leading to the predisposition to mucormycosis. Moreover, the treatment of COVID-19 includes immune therapies that may increase the risk of opportunistic fungal diseases. These immune therapies consist of corticosteroids (like dexamethasone), interleukin-6 inhibitors, etc. (Baddley 2021).
Invasive candidiasis was mostly found in the intensive care units, ranging from 0.8 percent to 14% (Baddley 2021). Certain studies have shown that candidemia in COVID-19 patient’s shows an increase of 3–eightfold as compared to non-COVID-19 patients. This alarmingly increasing rate of candidemia may reflect increased risk, including prolonged ICU stays, prolonged invasive mechanical ventilation, ECMO, broad-spectrum antimicrobial therapy, etc. (Baddley 2021).
According to recent studies, it was found that high-resolution computed tomography (HRCT) of the thorax helps in the early detection of these dreadful conditions, following the protocol for patients with chest pain and breathing problems during this pandemic. With the use of HRCT, the cases of the patients were studied, and the following data could be obtained (Dubey 2022).
Case 1: a 55-year-old patient suffering from pyrexia, a sore throat, and a breathing problem with RT-PCR positivity. He was a diabetic patient on hypoglycemic drugs. His HRCT scan showed diffuse ground glass opacities in both lungs with patchy consolidations and a reverse halo sign. The CT severity showed 22/25, which is typical for COVID-19. He was under treatment for COVID-19. A sputum sample collected from the patient was sent for clinical analysis and was found to be positive for mucormycosis. The fungus was sensitive to amphotericin B and voriconazole, whereas intermediate sensitivity was seen toward fluconazole and was resistant to caspofungin. A repeat scan showed the progression of mucormycosis, but the RTPCR report was negative. Then he was under observation for further treatment at the high-dependency unit (Dubey 2022).
Case 2: a 32-year-old patient who had a fever and cough and was diabetic with chronic kidney disease. The patient had completed an anti-tubercular treatment a few years ago. The patient was treated with oxygen, intravenous antibiotics, and COVID-19-related drug injections. His CT scan showed fibro-cavitary lesions, patchy consolidation, and bronchiectatic changes in the lungs. After a few days, his oxygen requirement was at its maximum, and he developed respiratory distress, which led him to the intensive care unit. A repeated CT scan of the chest showed fresh growth of the organism. The sputum sample was collected and was positive for mucormycosis, and treatment with amphotericin B was initiated, but unfortunately, the patient succumbed to death on the 14th day (Dubey 2022).
Case 3: a 55-year-old patient who recovered from COVID-19 and was diabetic. The RT-PCR report showed negative results but showed symptoms like breathlessness and coughing with a sore throat that was worsening. His HRCT scan showed a large number of lesions and cavities with ground glass opacities in both lungs. A sputum sample collected showed positive results for Mucor sp. The patient was in critical condition and was receiving antifungal and antibiotic therapy (Dubey 2022).
Case 4: a 74-year-old diabetic patient who recovered from COVID-19. This patient was on steroid treatment two times per day. His HRCT scan showed large, thick-walled lesions with cavities. This was a suspect of the fungal infection. In addition to this, there were ground-glass opacities in both lungs. The sputum sample showed positive results for Mucor sp. The patient was under observation in the intensive care unit and received antifungal and antibiotic treatment recommended by antibiotic susceptibility tests (Dubey 2022).
Case 5: a 52-year-old non-diabetic patient with RT-PCR positivity, fever, burning urination, and joint pain. He was advised for home isolation but was admitted after a month due to breathlessness, a sore throat, and a cough. His HRCT scan showed ground-glass opacities in all the lobes of the lungs. There were many cavitary lesions, which showed the possibility of an invasive fungal infection. Sputum samples collected showed negative for acid-fast bacteria and positive for Candida tropicalis, which showed resistance to amphotericin B and fluconazole and sensitivity to caspofungin, flucytosin, and micafungin. The organism was intermediately sensitive to voriconazole (Dubey 2022).
Case 6: 45-year-old diabetic patient, RT-PCR positive, suffering from fever, cough, and breathlessness. HRCT showed patches of ground glass opacities with cavities. The sputum sample showed positive for Candida albicans, which are sensitive to amphotericin B, caspofungin, fluconazole, flucytosine, and micafungin but resistant to voriconazole (Dubey 2022).
From the above study, it is evident that the fungal co-infections are caused by Aspergillus, Candida, and Mucor. It was found that the HRCT thorax was useful in the detection of fungal characteristics. Five of the six patients in this study were diabetics receiving corticosteroid therapy for the COVID-19 infection. The drawback of this method was that the result of radiological findings almost overlapped with the result of COVID-19, and it was difficult to differentiate the results. The prevailing infectious fungal diseases, however, appear likely based on close observation by skilled radiologists and some clues. Opportunistic fungal infections could therefore be misdiagnosed. Generally, pulmonary aspergillosis and candidiasis were the more common ones as compared to mucormycosis, but during this pandemic, there was a sudden emergence of mucormycosis (Dubey 2022).
So according to this case study, the factors that affect fungal infection in COVID-19 patients are:
If the patient is diabetic, the development of mucormycosis is most likely.
Usage of corticosteroids can result in uncontrollable hyperglycemia.
Low pH supports the growth of mucormycosis, i.e., acidosis.
The COVID-19 infection suppresses the immunity of the patient by decreasing CD4 + and CD8 + levels, which increases the risk of opportunistic fungal infections, etc. (Dubey 2022).
The most typical opportunistic invasive infections are discussed below.
Aspergillosis
Aspergillosis is a pulmonary disease infecting the respiratory system, especially in immune-compromised patients where the lung tissues are invaded by the fungal hyphae. The etiological organism of this disease is Aspergillus sp., where the most common isolate is Aspergillus fumigatus. The etiological agent Aspergillus sp. is a ubiquitous fungus that (Kosmidis 2015) is found in the environment naturally and can be isolated from the outdoor environment like air, soil, and dead and decaying vegetation (Dagenais 2009; Challa 2018) and indoor environments like hospitals (Kosmidis 2015). This saprophytic fungus plays a significant role in the carbon and nitrogen cycles of the earth. It produces conidia that are hydrophobic, easily dispersible into the air, and able to survive many environmental conditions (Dagenais 2009). Aspergillosis can be invasive or non-invasive.
Invasive pulmonary aspergillosis caused by Aspergillus fumigatus (Dagenais 2009) is one of the common diseases of aspergillosis in immune-compromised patients including lung transplant recipients, critically ill patients in the intensive care units, and patients who are under steroid treatment (Kosmidis 2015). The most important risk factor is neutropenia, which may be due to chemotherapy (Kosmidis 2015). Non-nutropenic patients like those under corticosteroids, liver failure, etc. can also develop aspergillosis (Kosmidis 2015). Invasive aspergillosis is the invasion of the tissues of the lungs by the hyphae of the etiological agent, which can be demonstrated by histology (Kosmidis 2015).
The spectrum of infection with pulmonary aspergillosis can be one of the following:
A healthy individual will not develop aspergillosis as long as the individual has not inhaled the spores in an excessive amount.
If the Aspergillus spores enter the cavitary lung of a diseased person, then an aspergilloma is developed, which is a ball of fungus that develops in an already existing cavity inside the parenchyma of the lungs.
If a patient with chronic lung disease or a mildly immune-compromised patient inhales the Aspergillus spores, then chronic necrotizing aspergillosis is developed.
If an immune-compromised patient inhales the Aspergillus spores, then invasive pulmonary aspergillosis is developed.
If an asthma or cystic fibrosis patient inhales the Aspergillus spores, then allergic broncho pulmonary aspergillosis is developed.
Invasive pulmonary aspergillosis is not very common in HIV patients, as they are on high doses of antiretroviral treatment. But patients who are suffering from chronic obstructive pulmonary disease are more susceptible to this infection because they have changes in their lung structure, long-term usage of corticosteroid therapy, broad-spectrum antibiotic therapy, etc. Studies show that in chronic lung disease, Aspergillus spp. could colonize the airways and transform into an invasive disease (Kosmidis 2015).
Detection of aspergillosis
Generally, the Aspergillus spores inhaled are found in the lower respiratory tract. Finding it in the skin, sinuses, and gastrointestinal tract is not very common. The symptoms of the disease are not very specific and are found to be fever, coughing, and sputum production with chest pain that is unresponsive to antibiotics. This infection can be spread through the blood and reach other organs like the brain (where seizures, meningitis, and epidural abscesses are caused), the kidney, the heart, and the liver, which are not very common. The unique feature of invasive aspergillosis is Aspergillus tracheobronchitis, where the isolated tracheobronchial tree is invaded by the organism. Findings by radiological methods are not specified, but there is evidence that supports segmental or lobar collapsing. The diagnosis is made with the combined result of findings from bronchoscopy and analysis of the respiratory specimens.
The standard diagnosis of invasive pulmonary aspergillosis is made by histopathological examination of the lung tissue, which is obtained using methods like a thoracoscopic or open lung biopsy. The positive result is indicated by the presence of septate, acute hyphae branches invading the tissues of the lungs, and at the same time, the positive culture for Aspergillus at the same location. Chest radiography is not useful; however, chest computed tomography along with high-resolution images is useful. The CT findings of this disease show the halo sign with multiple nodules and an air crescent sign in the area of necrosis. Through HRCT chest findings, it was shown that the positive result had ill-defined nodules, ground glass nodules, and consolidation in the pneumonic neutropenic patient. Another useful diagnosis of invasive aspergillosis is by bronchoscopy with bronchoalveolar lavage (BAL), but it is not very consistent, and there are reports of lower yields.
The most advanced diagnosis of invasive pulmonary aspergillosis is made by the detection of antigens produced by Aspergillus in the body fluids, which are galactomannan and (13) b-D-glucan. The double sandwich ELISA method is approved by the FDA for the detection of galactomannan in the serum of the sample, which indicates the confirmation of the diagnosis as this substance is detected in the serum before any clinical signs are reported. The b-D-glucan test requires blood samples, whereas other tests require respiratory samples. Polymerase chain reaction (PCR) is another way to detect Aspergillus spp. using the BAL fluid and serum to detect the DNA of the organism. More than one positive test indicates the existence of the disease (Kosmidis 2015).
Treatment
In order to protect high-risk patients from invasive fungal infections, azole prophylaxis is used (Alastruey 2018). Another effective drug Amphotericin B is used to treat severe and potentially fatal fungal infections. Amphotericin B injection belongs to the group of drugs known as antifungals. It functions by inhibiting the development of infection-causing fungus.
The first line of treatment for aspergillosis is amphotericin B with a dosage of 1–1.5 mg/kg/day. But side effects like nephrotoxicity and hypersensitivity can occur. So a newer formulation of amphotericin B that is lipid-based is introduced is needed in higher concentrations for its efficacy (Alastruey 2018). According to studies, it was found that voriconazole has fewer side effects and is better tolerated by patients than amphotericin B (Spanakis 2006; Kosmidis 2015; Walsh 2008). Voriconazole can be used either orally or intravenously. Posaconazole, a broad-spectrum antibiotic called triazole, is also used for salvage therapy as a standard anti-fungal treatment. However, side effects like heart failure, peripheral neuropathy, elevated liver enzymes, etc. are seen with the use of first-line drugs (Alastruey 2018).
If the patients are unable to tolerate the first line of treatment, then Echinocandin derivatives like caspofungin and micafungin are also used as an effective treatment against invasive aspergillosis. It is recommended to use a combination of antifungal therapy along with salvage therapy for a higher rate of recovery. Immune modulatory therapy can also be used as one of the methods of treatment as it decreases the degree of immune suppression. Granulocyte transfusion can also be used for patients who have neutropenia and are not candidates for conventional therapy (Kousha 2011; Walsh 2008).
In lung transplant patients, prophylaxis using amphotericin B, voriconazole, or itraconazole is used for those who are at risk for invasive aspergillosis. For non-neutropenic patients, the duration of the treatment is 12 weeks; for immune-compromised patients, the continuation of the antifungal treatment throughout the duration of immune suppression has a more favorable result (Kosmidis 2015; Walsh 2008). Patients who have developed lesions that are contagious with the pericardium hemoptysis from a single cavity to the invasion of the chest wall may need surgical therapy (Walsh 2008).
COVID-19-associated aspergillosis
Aspergillosis, which complicates the existing COVID-19 situation, is one of the most emerging cases in and around the world. The symptoms of this infection are very different from the usual invasive aspergillosis. The complication of COVID-19 with aspergillosis results in blockage of airways, inflammation due to epithelial damage, lung disease, etc. The patients who developed these complications are immune-compromised patients, mainly due to systemic or inhaled steroids. The symptoms of the hospitalized patients included inflammation, acute respiratory distress, and lung injury, and the patients in the intensive care units required respiratory support. For diagnosis, the results of the CT scans showed a mixed report of the viral infection and the fungal infection. Inflammation in the respiratory tract, mucus, large nodules with necrosis, and cavitations can also be detected using radiographic findings. A tracheal aspirate culture was used for diagnosis. The methods used for diagnosis are similar to those used for classical aspergillosis. Intravenous anti-fungal drugs like voriconazole, posaconazole, and liposomal amphotericin B were used as the treatment (Marr 2021).
Pathogenesis of invasive aspergillosis
The infectious life cycle of the causative organism initiates with the production of conidia (which are the asexual spores), which are dispersed into the air. The primary route for the causative organism to enter the host is through inhalation of the conidia, which gets deposited into the bronchioles or the alveolar spaces. In a healthy individual, the mucous membranes of the sinopulmonary cavity are the first line of defense for the exclusion of Aspergillus conidia by phagocytosis. Macrophages and gathered neutrophils at the site of infection with invasive aspergillosis also act as host defense mechanisms (Challa 2018).
The colonization of the lungs is a result of the dysfunction of the host defense mechanism, which allows colonization of the pulmonary system. In immune-compromised patients, the risk of invasive aspergillosis increases due to neutropenia and corticosteroid therapy (Kousha 2011). Corticosteroids disable the phagocytic activity of the phagocytes to kill the conidia and hyphae, which is seen in non-neutropenic patients. The characteristics of invasive aspergillosis are thrombosis and hemorrhage due to extensive hyphal growth. When the conidia escape the host defense mechanism and reach the alveoli, the swelling begins (Challa 2018).
The invasion happens in the following route:
Airway colonization due to inhalation of the conidia enters the lungs by escaping the first line of defense and interacting with the components of the lungs. Then it interacts with the epithelial cells of the lungs, which secrete antimicrobial components as a host defense mechanism. Alveolar macrophages also phagocytose the conidia (Challa 2018). But if the conidia escape all these host defense mechanisms, they start to colonize the lung of the host, which results in tissue degradation. It then starts nutrient biosynthesis for survival. The fungi have the ability to degrade various forms of nitrogen sources, so nitrogen metabolism plays a significant role in the pathogenesis of the organism.
After this, the etiological agent can be transported to different parts of the body through the bloodstream. The growing hyphae can invade the epithelial cells of the blood vessel and enter the vasculature. Sometimes, due to the breakage of the hyphae, they invade other organs. The hyphal growth may become uncontrollable and disseminate throughout the body if the host is highly immune-suppressed (Dagenais 2009). The fungal conidia have alpha- and beta-glucans, chitins, and other polysaccharides on their cell surface. It also consists of a pigment called DHN melanin, which helps in escaping the host defense mechanism and is resistant to high temperatures. After it is phagocytosed by the alveolar macrophages, it becomes active and forms germ tubes and hyphae inside. In immune-compromised patients, there is a delayed or weak response by the macrophages, which prevents the macrophages from stopping the germination process of the spores. The fungi have the ability to inhibit the phagocytic function of the macrophages by secreting various mycotoxins (Challa 2018).
Blastomycosis
Blastomycosis is a pulmonary and systemic mycosis that is geographically restricted, caused by the organism Blastomyces dermatitis.
The fungi Blastomyces dermatitidis is a dimorphic fungus that exists as a filamentous (McBride 2017) mold in its asexual form. The mycelium produces conidia, which can be exposed to the air. When humans inhale the conidia and they reach the alveoli, they are then transformed into yeasts by oxidative phosphorylation with the use of heat, which causes the disease blastomycosis, which is predominant in North America. In India, there was a case of blastomycosis in a 27-year-old female patient in 2010. The study showed that she acquired the infection while traveling to the USA (Randhawa 2013; McBride 2017).
Pathology
The infection starts when the conidia enter the host through inhalation (McBride 2017). The host defense mechanisms in the lungs, like the alveolar macrophages, neutrophils, and monocytes in the lungs, like the alveolar macrophages, neutrophils, and monocytes (McBride 2017), have the ability to provide resistance to the conidia and inhibit their transformation into yeast. This yeast form becomes difficult to phagocytose as it possesses a thick capsule. Also, poly-morpho-nuclear leukocytes have a greater capacity to tackle the inhaled conidia than macrophages, which become less effective against the yeast form. If the host defense mechanism fails, the development of the yeast cells in the alveoli happens; this eventually spreads to other parts of the body. To prevent the progression of blastomycosis, cell-mediated immunity is very much required (Patel 2010).
The symptoms of blastomycosis are similar to those of influenza, including fever, cough, myalgia, pleurisy, etc. If left untreated, the disease may spread beyond the lungs and lead to the patient's death before the start of the antifungal treatment. The chest radiography of patients with blastomycosis shows mass lesions of fibro-nodular infiltrate (Patel 2010). Cavitation is not commonly seen, but small effusions are. Blastomycosis may be pulmonary, which occurs in the lungs, or extra pulmonary, where the skin is involved. It can also be developed on the bones, where long bones are mostly affected (Patel 2010). In immune-compromised patients, the disease becomes more aggressive as the spreading of the disease reaches the central nervous system easily and results in early death (Saccente 2010).
Diagnosis
The radiographic findings of the fungal disease overlap with other diseases such as tuberculosis, so it becomes indistinguishable (Saccente 2010). Therefore, isolation and visualization of the yeast form, which contains 8–12 nuclei and has thick, refractile cell walls, or the hyphal growth of the fungus, are much needed for diagnosis (Patel 2010). Direct observation, such as wet preparation of the culture, is often performed with the specimen collected through biopsy (bronchial washings, bronchial brushings, BALs, and alveolar lavages) (Patel 2010), and characteristic histopathology of the organism is observed. A potassium hydroxide solution is used to enhance the visibility of the fungal cultures (Saccente 2010). Special stains such as Gomori methenamine silver and periodic acid Schiff are used for morphological diagnosis (Patel 2010). Selective media can also be used for the identification of the organism, which forms a white or off-white color with a waxy appearance that eventually becomes gray with the development of hyphae (Saccente 2010).
The microscopic view of the fungi shows delicate septate hyphae with oval or pyriform single-celled conidia on the conidiophores. The commercially available and most commonly used confirmatory test is a chemiluminescent DNA probe. However, it may give a false-positive result if other fungi are present. Repetitive sequence-based PCR can also be used. Antibody testing for blastomycosis is available, but it is not adequate for diagnosis because of its low sensitivity and specificity (Saccente 2010).
Treatment
All the patients diagnosed with blastomycosis are given antifungal treatment. The choice of treatment and its duration depends on the severity, condition, and advancement of the disease. Amphotericin B is used in the majority of cases where patients' immune systems are compromised and their central nervous systems are affected. A lipid formulation of amphotericin B can also be used. Itraconazole is used for patients with mild to moderate disease, and oral solutions are preferred over capsules. Fluconazole is considered and used as a second-line agent, which serves as an option for step-down treatment after the patient has been exposed to amphotericin for a CNS infection. Ketoconazole was the first azole to be proven effective, but it is not used nowadays because other forms of azoles, like itraconazole, are better tolerated and more effective. Voriconazole is also used as a step-down therapy after amphotericin exposure (Saccente 2010).
Candidiasis
Candidiasis is a disease that affects the skin, mucous membranes, and deep-seated organs at any age of the host. The etiological organism of this disease is Candida albicans. The causative organism of the disease candidiasis is a common microflora of the skin and the gastrointestinal tract. These commensals become pathogenic when they are given the opportunity to invade the host. Patients with COVID-19 were first diagnosed with C. auris bloodstream infections in February 2020. Since then, 13 outbreaks with 2–12 infections per outbreak and several colonized patients have been documented worldwide (Hoenigl 2022). Candida spp. have the capacity to exhibit polymorphism, which can grow either as budding yeast, which is oval-shaped, or as hyphae, which are elongated septate cells (Mayer 2013). The factors that contribute to the pathogenic potential of this microbe include the molecules that allow adhesion to and invasion of the host cells, the secretion of the enzyme hydrolases, the morphological conversion from yeast to hyphae, the formation of biofilms, phenotypic switching, etc. (Mayer 2013). The fungus Candida albicans has the ability to exist in filamentous form with pseudo-hyphae and hyphae and also interconvert into unicellular yeast form. Candidiasis is a disease that affects the skin, mucous membranes, and deep-seated organs at any age in the host. Invasive candidiasis is one of the most common fungal infections caused by the Candida species.
The following are the species of Candida that cause invasive candidiasis: C. albicans, C. tropicalis, C. parapsilosis, and C. krusei. These species have distinct characteristics related to their ability to invade, virulence factor, antifungal susceptibility, etc.). The multiple drug resistance has drastically affected the anti-fungal susceptibility of each disease-causing organism. Candida species are one of the most common healthcare-associated bloodstream infections in and around the world. The risk factors include patients with indwelling catheters, exposure to broad-spectrum antibiotics, which kill the gut microflora and introduce overgrowth of Candida spp. in the gut, patients staying in the intensive care units for a long time, patients under ventilation, immune suppression, such as chemotherapy, corticosteroid treatment, neutropenia, etc., which suppress the innate immunity of the host, patients under treatment using cytotoxic chemotherapy, etc. (Mayer 2013).
Pathology
Candida spp. are present on the mucosal surface of a healthy person in amounts estimated to be 50–70%. It can be detected on the mucosal surface. However, as an example, if it can cross the intestinal barrier after gastrointestinal surgery, it can proliferate into the abdominal cavity and enter directly into the bloodstream. The suppressed immune response can affect the growth of the microorganism by promoting fungal overgrowth, which can lead to deep-seated opportunistic invasive candidiasis. The presence of different compositions like manno-proteins, chitin, and glucans in their cell wall and their ability to change their morphological forms affect the identification of the pathogen by the host defense mechanism. The first step in the host response to the candidiasis infection is the immune response by the membrane-bound pattern recognition receptors, which are expressed by myeloid phagocytes, C-type lectin receptors, etc. Neutrophils also act as an effective defense against the pathogen by accumulating at the site of infection.
Mononuclear phagocytes like monocytes, macrophages, etc. also play an important role against the pathogen (Mayer 2013; Pappas 2018). The factors that contribute to the pathogenic potential of this microbe include the molecules that allow adhesion to and invasion of the host cells, the secretion of the enzyme hydrolases, the morphological conversion from yeast to hyphae, the formation of biofilms, phenotypic switching, etc. (Mayer 2013). Candida spp. have the capacity to exhibit polymorphism, which can grow either as budding yeast (which are oval-shaped) or hyphae (which are elongated septate cells). This polymorphism is an important factor in pathogenesis. In most cases, it is seen that the hyphal forms are more invasive than the yeast form. However, the yeast forms are the ones that are involved in dissemination. The ability of the organism to adhere to the host cell is possible because of the presence of a set of specialized proteins that enable the cell to adhere to other cells of the same type, to the host cell surface, or to any other organism. When Candida sp. invades the host cell, it uses two types of mechanisms:
induced endocytosis which is mediated by Als3 and
Ssa1 and active penetration which is mediated by an unknown molecular mechanism.
Another significant virulence factor for this etiological agent is the ability to form biofilms on any surface. E.g., it can form biofilms in the catheters (inanimate) and mucosal cell surfaces (living). The biofilm formed on these surfaces multiplies and matures. The mature biofilms become resistant to the antimicrobial agents and the host's innate defense mechanisms. These organisms secrete the enzyme hydrolase, which aids in active penetration and improves the effectiveness of extracellular nutrient uptake. These hydrolases include proteases, phospholipases, and lipases. It can also survive and adapt to a wide range of pH. The organism has a wide range of metabolic flexibility and has also evolved in such a way that it has the ability to escape the macrophages by inhibiting the production of antimicrobial effectors and causing the formation of hyphae inside the phagocytic cells. These hyphae can break through the host immune cells and escape using mechanical forces. The organisms also store heat shock proteins that help them survive under unfavorable conditions (Mayer 2013; Pappas 2018).
When the illness spreads through the bloodstream, various organs may be impacted, including.
Abdominal cavity—where abscess, pancreatitis, peritonitis occur.
In the bone, it causes osteomyelitis, spondylodiscitis.
In the brain, it causes brain abscess, meningo encephalitis
If the eye is infected, it can cause choriditis, retinitis, and endophthalmitis.
In the heart, it causes endocarditis,
In the kidney, it causes candiduria, pylonephritis, pyonephrosis, and renal abscess
In the lungs, it causes focal abscess (Mayer 2013; Pappas 2018).
Diagnosis
There are no clinical symptoms or signs related to candidiasis, so it is required that patients who are already known to be at risk for candidiasis be identified. The patient may develop an unexplained fever that cannot be cured by antibacterial treatment. The diagnosis should be done periodically so that it can be treated at an earlier stage, as a delay in the antifungal treatment for a short period of time doubles the rate of mortality. However, the detection of the disease is challenging. The classical methods used in microbiology, like light microscopy, culture, etc., can be used for the detection of Candida spp., but they may not be easily recognized as bacterial growth can outgrow the fungal species. Therefore, special media or selective media like blood cultures should be used to isolate and confirm the disease. So, combinations of different methods for diagnosis result in early detection and a favorable outcome. Histopathological data and PCR also help in the diagnosis. Different fungal stains, like fluorescent brighteners and Grocott–Gomori methamine silver stains, are used (Pappas 2018).
Antigen–antibody detection of the Candida spp. is also useful in the diagnosis and detection of the disease. It is essential to develop a susceptibility profile for the identified species once the diagnosis of invasive candidiasis is confirmed. The antifungal susceptibility test, like the disk or tablet diffusion method, the gradient strip agar diffusion method (E-test), and the minimum inhibitory concentration (MIC) test, can be performed to identify the appropriate antifungal drug, its doses, and its prophylaxis (Pappas 2018).
Treatment
The change in the epidemiology of Candida infection has affected the treatment of the disease due to the difference in susceptibility to azoles and echinocandins. The shift in the species distribution and the MICs of the available antifungal substances is due to pre-exposure to antifungal agents (Pappas 2018). The initial treatment of the disease should be based on the patient’s history of exposure to antibiotics, the severity of the illness, the involvement of other organs, and the susceptibility data of the particular organism identified and confirmed during diagnosis (Pappas 2018).
Echinocandins (Bassetti 2016) like anidulafungin, caspofungin (Spanakis 2006), and micafungin are used as first-line therapies. These antifungal agents act on the fungal cell wall and inhibit the synthesis of 1,3-B-glucan. It also has a strong and broad fungicidal action that can even act on biofilm formation. Azoles (Bassetti 2016) are also used, which have broad activity against most Candida spp. Azoles target the enzyme that helps in the conversion of lanosterol to ergosterol, which is present on the cell wall of the fungus (Bassetti 2016). This drug is tolerated by most patients but may have drug-drug interactions. This drug can be taken orally or intravenously. Fluconazole is used as a second line of treatment. For step-down treatment, voriconazole is used (Pappas 2018).
Amphotericin B, a polyene antifungal agent that can bind to the fungal cell wall, is also used for treatment. But due to its extensive side effects and toxicity, a modified form of amphotericin B in a lipid formulation is used. Antifungal prophylaxis should be restricted to patients who are at high risk. Since invasive candidiasis is associated with high morbidity and mortality, the antifungal treatment should be started as soon as possible according to the patient's risk factors, and early therapies that are inappropriate should be stopped. The duration of the therapy should be checked regularly. Itraconazole can also be used as a treatment option and is available as an intravenous preparation (Bassetti 2016; Pappas 2004).
Zygomycosis
Rare fungal disease zygomycosis poses a major risk if not promptly addressed. This illness is brought on by a mucormycete-related organism, a class of mold. Rhizopus oryzae is the most common isolate of the disease. Zygomycosis is an emerging disease that causes fatalities at a high rate (Roden 2005). The most common organism that is found in patients suffering from mucormycosis is Rhizopus oryzae (Ibrahim 2012). The people who are at risk include patients who are receiving transplants, immune-compromised patients, patients suffering from diabetes mellitus in ketoacidosis (Ibrahim 2012), drug abusers, cancer patients, patients with hematological malignancies, neutropenia, etc. In this disease, if the pathogen has infected the pulmonary system, the infection may extend to the chest wall, pulmonary artery, aorta, or heart. According to the case study, diabetes was one of the most common underlying conditions, and patients were also associated with sinusitis, which is the second most common pattern of this disease. However, these symptoms have a different pattern in each case relating to their underlying issues (Roden 2005). By the first week of June 2021, India had recorded over 20,000 cases of mucormycosis (Yasmin 2021).
Detection
Histopathological findings and culture were used as testing methods. The positive test includes the organism Rhizopus species, and Rhizopusoryzae is the most commonly detected pathogen. It was observed that the disease was found to affect men more than women (Roden 2005). The basic tools for the identification of this disease include a CT scan, positron emission tomography, computed tomography with 18F-fluorodeoxyglucose, microscopy, mass spectroscopy, and serological tests like ELISA, PCR, DNA sequencing, etc. For the diagnosis of pulmonary mucormycosis, quantitative PCR, a histopathological study, was found to be the most effective method for the detection of the causative organism (Hassan 2019; Skiada 2018).
Pathogenesis
The pathogen of humans can cause disease to the host on the basis of two steps:
The ability of the micro-organism to escape the host immune defense and survive inside the host cell.
Agitation of the host immune system and damage the host cell (Hassan 2019).
Mucormycosis can cause the following diseases.
Rhinocerebral mucormycosis- where the sinuses and brain are affected leading to fever, swelling of facial organ, black lesions in the mouth or the face, headache.
Pulmonary mucormycosis- which affects the lungs causing chest pain, breathing problems, fever, cough etc.
Cutaneous mucormycosis- which cause skin infection with ulcers, redness, and swelling.
Gastrointestinal mucormycosis – leading to nausea, vomiting, abdominal pain.
Disseminated mucormycosis- leading to medical complications.
Uncommon renal infection (Hassan 2019).
When the causative organisms invade the body, they are attacked by the host immune system. These interactions are of the following types:
Bronchial alveolar macrophages—they act as the first line of defense. These cells phagocytose the pathogen, but they are unable to suppress or kill the spores.
The fungal pathogens initially come into contact with epithelial cells. The epithelial cells are harmed by these infections.
Antigen-specific T lymphocytes participate in this pathogenesis. These T cells serve as a diagnostic tool for the condition because they are only produced in mucormycosis patients.
Natural killer cells: Natural killer cells, aid in protection against the mucormycosis infection by limiting dissemination. These cells can recognize infected cells because they express a number of receptors that block the histocompatibility complex.
Platelets: These cells aid in hemostasis and aid in identifying and eliminating pathogens. Although platelet cells can adhere to the pathogen's spores and hyphae, they can only harm the hyphal structure.
Endothelial cells: Found in the inner layers of blood arteries, these cells aid in pathogen identification and phagocytose it, potentially harming even spores.
Dendritic cells—because they exist between the epithelium and the interstitium, these cells are also referred to as the "linker" between innate immunity and adaptive immunity. Following the release of the microbial antigen, these cells migrate toward the infection site, enhancing the host cell's immunity (Hassan 2019; Ibrahim 2012).
Treatment
Once the infection has been identified, it is important to administer antifungal medicine at the right time and dosage, remove the contaminated tissues, and employ adjunctive therapy. In order to achieve the desired objectives, ketoacidosis can be reduced using sodium bicarbonate (Skiada 2018).
Amphotericin B, a polyene, is used as the first choice of treatment, and it was found that it was highly effective against most of the species causing mucormycosis (Rogers 2008; Spellberg 2009). Posaconazole is the second-most effective antifungal drug used for the same treatment. Azoles like itraconazole and terbinafine were also found to be effective (Hassan 2019; Rogers 2008). Popular anti-fungal treatment drugs like fluconazole and voriconazole are not effective against the pathogen, whereas caspofungin and flucytosine showed little activity against the pathogen (Rogers 2008).
Suggested treatment methods for mucormycosis include the following:
Early diagnosis
Prevention of underlying disease
Surgical management
Introduction of primary antifungal therapy
Salvage therapy (Spellberg 2009)
Current management of mucormycosis includes the following:
Rapid diagnosis-to identify the patients at risk so that early therapy with the antifungal drugs decreases the rate of mortality (Rogers 2008; Spellberg 2009).
Elimination or mitigation of risk factors, such as quitting therapy, which may impair a person's immunity.
Antifungal treatment based on the patient's data.
Adjunctive therapies, such as the use of hyperbaric oxygen to treat zygomycosis (Rogers 2008).
Treatment challenges with opportunistic fungal infections:
The rise in the number of opportunistic fungal infections is due to the increase in the number of patients who are on immune suppressive therapy or whose immune systems are affected by certain diseases. Before the COVID pandemic, 19 patients who are suffering from AIDS were the most common patients having secondary infections with opportunistic fungal infections. However, with the increasing incidence of patients suffering from COVID-19 due to the pandemic, opportunistic fungal infections have become uncontrollable complications. The risk factors include patients with diabetes who are on steroid therapy. Also, non-diabetic patients are seen with complications since the treatment of COVID-19 includes steroid drug therapy (Armstrong 1989).
These complications by the opportunistic fungal infections can be classified into two types:
The infection due to neutrophil defects like candidiasis, aspergillosis, mucormycois
The infection due to defects of the T cells phagocyte like cryptococcosis, histoplasmosis etc.
Treatment challenges seen with Candidiasis
The most commonly isolated and identified species that causes candidiasis is Candida albicans. These pathogens, in most cases, cause invasive diseases, especially in neutropenia patients. This condition is a result of using broad-spectrum antibiotics for other suspected or identified bacterial infections. The source of the cause of the infection is not known. The diagnosis and confirmation of Candida species are not very common, as they are commensals of the gastrointestinal tract and the gynecologic tract. These species may not be able to be isolated during the first time of culturing but only appear after the patient has been on antibiotic therapy for about a week. Moreover, the infection doesn’t exhibit specific symptoms except for the fever, which is common in most diseases. The blood culture shows a positive result only when the skin lesions and myositis begin to appear. But by this time the infection would have advanced to the point that it is difficult to treat and fails to respond to the therapy (Armstrong 1989).
The mortality rate for candidiasis patients lies in the range of 50% to 80%, and the serological tests that have been developed so far show false-positive and false negative results so frequently that it makes it even more complicated. Also, the duration of the antifungal treatment is uncertain. There are antifungal drugs that work against the pathogen, but due to the lack of better methods for detection and diagnosis, the mortality rate is high in patients with secondary infection (Armstrong 1989). Candida spp. are now known to have resistance against echinocandin, which targets the enzyme that helps in cell wall synthesis. The organism had undergone mutations that made it resistant. These organisms also show resistance to fluconazole (Pappas 2018).
Challenges and problems have been there since the infections were first recognized. This is because these infections are difficult to detect and diagnose, and effective treatments for these complications are scarce. Also, once the disease is diagnosed and the treatment starts, the duration of the therapy is difficult to find (Armstrong 1989).
Treatment challenges of aspergillosis
Invasive aspergillosis is the most common form of aspergillosis, found mostly in immunocompromised patients. Studies have found that this infection is due to the inhalation of large amounts of spores of the pathogen due to poor ventilation. The highest risk factors are associated with neutropenic patients, corticosteroid therapy, HIV-infected patients, etc. The symptoms shown by pulmonary aspergillosis are similar to those of mucormycosis when a sinus infection occurs. It also infects other organs in the body, but the signs and symptoms start to show mostly when the brain cells are infected. Pulmonary hemorrhage due to arterial blockage, which arises due to clotting of the vessels, makes the treatment difficult to reach the site of infection, leading to mortality. Studies have suggested that the result of the chest roentgenogram may show negative results, so it cannot be used for diagnosis purposes.
Despite severe pathogen infection, just a little portion of the patient's sputum can be used to isolate the organisms for identification. Treatment options should be examined for the severe infection indicated by the hyphae seen on the wet mount stained with KOH. Antigen antibody testing, which would usually yield false negative findings, can be used for diagnosis when performed carefully. When samples from bronchial wash and serums were employed, very few encouraging outcomes were seen. The diagnosis of infections like candidiasis is minimal due to the restricted and less sensitive diagnostic tools. Amphotericin B and other antifungal medications are utilized in the treatment, however the length of time required varies from patient to patient (Armstrong 1989).
Treatment challenges in mucormycosis
The disease mucormycosis is most often associated with immune-suppressed patients, patients under steroid therapy, neutropenic patients, etc. (Skiada2018). Recently, during the second wave of COVID-19, the incidence of mucormycosis has increased to a certain level. Studies have shown that the secondary complication of mucormycosis is associated with patients with COVID-19 and diabetes. The reason was that both the treatment of the primary and secondary infection complications included the use of steroid drugs. When the patient’s lungs were infected, the symptoms were not prominent, but when the brain was infected, the symptoms started to show. If the nasopharynx is infected, then the sample for diagnosis can be taken from the black necrotic lesions on the hard or soft palate.
A biopsy should be done if the scrapings do not provide proper diagnostic results. Since there is no reliable serological testing method, the identification of the disease is challenging. If there is a suspect for the pathogen causing the disease, treatment should start at the earliest possible time. It is seen that the control of the underlying immune defects is more important when treating the disease. Surgery is also seen as one of the most effective methods of treatment (Armstrong 1989).
Conclusion
Drugs including azoles, echinocandins, allylamines, and polyenes are used as the standard treatment for opportunistic fungal infections. The azoles, such as fluconazole, itraconazole, and voriconazole, are thought to be the medicines that are utilized as the first line of treatment among these (Vandeputte et al. 2012). Therefore, due to mutations, organisms that are resistant to certain antibiotics have emerged as a result of the extended use of these drugs. This is primarily observed with the Candida spp. that cause candidiasis. Due to the rise in opportunistic fungal infections worldwide, developing new antifungal medications or enhancing the treatment approach to treat these infections has therefore become one of the key priorities (Shrestha 2015).
To combat the virus, scientists are looking for solutions in natural resources. Natural products can be exploited as substitute sources of novel compounds, lowering their toxicity and side effects while increasing cost effectiveness (Soliman 2017).
Schinus terebinthifolius leaf ethanol extracts have been proven to exhibit antimycotic action against Candida spp. Given the short half-lives and low efficiency of antibiotics, it is evident that microorganisms can evolve a resistance to therapeutic chemical agents. Therefore, there is a critical need for novel and enhanced anti-mycotic chemicals.
Combination therapy has better success in treating invasive fungal infections and has fewer adverse effects, according to recent studies. Azoles with various chemicals, such as tacrolimus, or any other drug against Candida spp., have demonstrated promising outcomes as one example of combined therapy. Additionally, research has indicated that the synergistic effects of C12 or C14 and four azoles have been successful. The treatment of fungi infections may benefit from this combination because it is not harmful to mammalian cells (Shrestha 2015).
Rezafungin, encochleated, and amphotericin B are recently identified medications for mucormycosis that are currently being tested (Brunet 2020).
The use of another triazole is an alternative treatment for aspergillosis that can be used if the first line of treatment is unsuccessful, provided that the serum concentration of the antifungal medication is regularly monitored and that the pathogen is not resistant to the triazole that was initially used. Posaconazole, which is well tolerated by the majority of patients, is to be used as a third line of treatment if the first line of medication exhibits side effects or resistance (Alastruey 2018).
Isaconazole, a triazole, has recently been found to exhibit a wide variety of antifungal activities. The ECIL-16 guidelines advise using this medication as the first line of treatment for invasive aspergillosis, and the IDSA also suggests using it as a fallback option if other treatments are ineffective (Alastruey 2018).
It has been demonstrated that luliconazole works against Aspergillus terreus, which causes non-invasive aspergillosis. Therefore, this recently found medication can be utilized as a complementary therapy (Zargaran 2017). The rise in opportunistic fungal infections during COVID-19 demonstrated the necessity for risk factor identification in COVID-19 afflicted patients, prompt diagnosis and prompt treatment if necessary, alternate treatment for the effective disease management and mortality prevention efforts (Akhtar et al. 2022).
The researchers put a lot of attention on a high index of suspicion, early diagnosis, and appropriate therapy for patient survival because of a link with a very high death rate. To provide the finest, most individualized therapy, it is essential to assess the risk factors and various forms of invasive mycosis. To comprehend the impact of opportunistic infections in COVID-19 patients, extensive research is required in order to handle aspergillosis, candidiasis, mucormycosis, in COVID-19 patients (Bhatt 2021).
Acknowledgements
We would like to thank Prof. Shilpa B.R for the support and motivation. We are grateful to my colleagues at REVA University for providing their valuable insights and suggestions during the revision of the manuscript. Without their support, it would not have been possible for us to complete the publication process of this manuscript.
Author contributions
Contributions of corresponding author: Introduction to fungal infections, treatment, risk factors, case studies, conclusion are written by the corresponding author. And overall review revision was made by compiling co-author's concepts. Contributions of second author: Did literature survey and wrote on Aspergillosis, blastomycosis, Candidiasis, Zygomycosis, mucormycosis—pathogeneses, detection, and treatment.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability
Not applicable.
Declarations
Conflict of interest
We declare no conflicts of interest.
Ethical standards
This work did not require ethical clearance since it is a review article.
Contributor Information
Navidita Kangabam, Email: navihyun@gmail.com.
V. Nethravathy, Email: nethraprasad08@gmail.com, Email: Nethravathi.v@reva.edu.in
References
- Akhtar N, Wani AK, Tripathi SK, Prakash A, Mannan MA. The role of SARS-CoV-2 immunosuppression and the therapy used to manage COVID-19 disease in the emergence of opportunistic fungal infections: A review. Curr Res Biotechnol. 2022;4:337–349. doi: 10.1016/j.crbiot.2022.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alastruey-Izquierdo A, Cadranel J, Flick H, Godet C, Hennequin C, Hoenigl M, Kosmidis C, Lange C, Munteanu O, Page I, Salzer HJF, on behalf of CPAnet Treatment of chronic pulmonary aspergillosis: current standards and future perspectives. Respiration. 2018;96(2):159–170. doi: 10.1159/000489474. [DOI] [PubMed] [Google Scholar]
- Ali Awan H, Najmuddin Diwan M, Aamir A, Ali M, Di Giannantonio M, Ullah I, Shoib S, De Berardis D. SARS-CoV-2 and the brain: What do we know about the causality of ‘cognitive COVID? J Clin Med. 2021;10(15):3441. doi: 10.3390/jcm10153441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D. Problems in management of opportunistic fungal diseases. Rev Infect Dis. 1989;11(Suppl 7):S1591–S1599. doi: 10.1093/clinids/11.supplement_7.s1591. [DOI] [PubMed] [Google Scholar]
- Baddley JW, Thompson GR, 3rd, Chen SC, White PL, Johnson MD, Nguyen MH, Schwartz IS, Spec A, Ostrosky-Zeichner L, Jackson BR, Patterson TF, Pappas PG. Coronavirus disease 2019-associated invasive fungal infection. Open Forum Infect Dis. 2021 doi: 10.1093/ofid/ofab510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassetti M, Peghin M, Timsit JF. The current treatment landscape: candidiasis. J Antimicrob Chemother. 2016;71(Suppl 2):ii13–ii22. doi: 10.1093/jac/dkw392. [DOI] [PubMed] [Google Scholar]
- De Berardis D. How concerned should we be about neurotropism of SARS-Cov-2? A brief clinical consideration of the possible psychiatric implications. CNS Spectrum. 2020 doi: 10.1017/S1092852920002175. [DOI] [PubMed] [Google Scholar]
- Bhatt K, Agolli A, Patel MH, et al. (2021) High mortality co-infections of COVID-19 patients: mucormycosis and other fungal infections. Discoveries (Craiova). 9(1):e126. 10.15190/d.2021.5 [DOI] [PMC free article] [PubMed]
- Brunet K, Rammaert B. Mucormycosis treatment: recommendations, latest advances, and perspectives. J Mycol Med. 2020;30(3):101007. doi: 10.1016/j.mycmed.2020.101007. [DOI] [PubMed] [Google Scholar]
- Cavassin FB, Baú-Carneiro JL, Vilas-Boas RR, Queiroz-Telles F. Sixty years of Amphotericin B: an overview of the main antifungal agent used to treat invasive fungal infections. Infect Dis Ther. 2021;10(1):115–147. doi: 10.1007/s40121-020-00382-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti A, Chatterjee SS, Shivaprakash MR. Overview of opportunistic fungal infections in India. Nippon Ishinkin Gakkai Zasshi. 2008;49(3):165–172. doi: 10.3314/jjmm.49.165. [DOI] [PubMed] [Google Scholar]
- Challa S. Pathogenesis and pathology of invasive aspergillosis. Curr Fungal Infect Rep. 2018;12:23–32. doi: 10.1007/s12281-018-0310-4. [DOI] [Google Scholar]
- Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive aspergillosis. Clin Microbiol Rev. 2009;22(3):447–465. doi: 10.1128/CMR.00055-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey R, Sen KK, Mohanty SS, et al. The rising burden of invasive fungal infections in COVID-19, can structured CT thorax change the game. Egypt J Radiol Nucl Med. 2022;53:18. doi: 10.1186/s43055-022-00694-3. [DOI] [Google Scholar]
- Ghannoum MA, Rice LB. Antifungal agents: mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin Microbiol Rev. 1999;12(4):501–517. doi: 10.1128/CMR.12.4.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan MIA, Voigt K (2019) Pathogenicity patterns of mucormycosis: epidemiology, interaction with immune cells and virulence factors. Med Mycol. 57(Supplement_2):S245–S256. 10.1093/mmy/myz011 [DOI] [PMC free article] [PubMed]
- Hoenigl M, Seidel D, Sprute R, et al. COVID-19-associated fungal infections. Nat Microbiol. 2022;7:1127–1140. doi: 10.1038/s41564-022-01172-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AS, Spellberg B, Walsh TJ, Kontoyiannis DP (2012) Pathogenesis of mucormycosis. Clin Infect Dis 54 (Suppl 1):S16–22. 10.1093/cid/cir865 [DOI] [PMC free article] [PubMed]
- Kosmidis C, Denning DW. The clinical spectrum of pulmonary aspergillosis. Thorax. 2015;70(3):270–277. doi: 10.1136/thoraxjnl-2014-206291. [DOI] [PubMed] [Google Scholar]
- Kousha M, Tadi R, Soubani AO. Pulmonary aspergillosis: a clinical review. Eur Respir Rev. 2011;20(121):156–174. doi: 10.1183/09059180.00001011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marr KA, Platt A, Tornheim JA, Zhang SX, Datta K, Cardozo C, Garcia-Vidal C. Aspergillosis complicating severe coronavirus disease. Emerg Infect Dis. 2021;27(1):18–25. doi: 10.3201/eid2701.202896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–28. doi: 10.4161/viru.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride JA, Gauthier GM, Klein BS. Clinical manifestations and treatment of blastomycosis. Clin Chest Med. 2017;38(3):435–449. doi: 10.1016/j.ccm.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nett JE, Andes DR. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect Dis Clin North Am. 2016;30(1):51–83. doi: 10.1016/j.idc.2015.10.012. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Prim. 2018;4:18026. doi: 10.1038/nrdp.2018.26. [DOI] [PubMed] [Google Scholar]
- Pappas PG, Rex JH, Sobel JD, Filler SG, Dismukes WE, Walsh TJ, Edwards JE, Infectious Diseases Society of America Guidelines for treatment of candidiasis. Clin Infect Dis. 2004;38(2):161–89. doi: 10.1086/380796. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Gattuso P, Reddy VB. Diagnosis of blastomycosis in surgical pathology and cytopathology: correlation with microbiologic culture. Am J Surg Pathol. 2010;34(2):256–261. doi: 10.1097/PAS.0b013e3181ca48a5. [DOI] [PubMed] [Google Scholar]
- Randhawa HS, Chowdhary A, Kathuria S, Roy P, Misra DS, Jain S, Chugh TD. Blastomycosis in India: report of an imported case and current status. Med Mycol. 2013;51(2):185–192. doi: 10.3109/13693786.2012.685960. [DOI] [PubMed] [Google Scholar]
- Ravikant KT, Gupte S, et al (2015) A review on emerging fungal infections and their significance. J Bacteriol Mycol Open Access 1(2):39–41. 10.15406/jbmoa.2015.01.00009
- Roden MM, Zaoutis TE, Buchanan WL, Knudsen TA, Sarkisova TA, Schaufele RL, Sein M, Sein T, Chiou CC, Chu JH, Kontoyiannis DP, Walsh TJ. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin Infect Dis. 2005;41(5):634–653. doi: 10.1086/432579. [DOI] [PubMed] [Google Scholar]
- Rogers TR. Treatment of zygomycosis: current and new options. J Antimicrob Chemother. 2008;61(Suppl 1):i35–40. doi: 10.1093/jac/dkm429. [DOI] [PubMed] [Google Scholar]
- Saccente M, Woods GL. Clinical and laboratory update on blastomycosis. Clin Microbiol Rev. 2010;23(2):367–381. doi: 10.1128/CMR.00056-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjay G. Revankar (2021) Overview of fungal infections, last full review/revision
- Shrestha SK, Fosso MY, Garneau-Tsodikova S. A combination approach to treating fungal infections. Sci Rep. 2015;5:17070. doi: 10.1038/srep17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh T, Kashyap AK, Ahluwalia G, Chinna D, Sidhu SS. Epidemiology of fungal infections in critical care setting of a tertiary care teaching hospital in North India: a prospective surveillance study. J Clin Scient Res. 2014;3:14–25. doi: 10.15380/2277-5706.JCSR.13.050. [DOI] [Google Scholar]
- Skiada A, Lass-Floerl C, Klimko N, Ibrahim A, Roilides E, Petrikkos G (2018) Challenges in the diagnosis and treatment of mucormycosis. Med Mycol 56(suppl_1):93–101. 10.1093/mmy/myx101 [DOI] [PMC free article] [PubMed]
- Soliman S, Alnajdy D, El-Keblawy AA, Mosa KA, Khoder G, Noreddin AM. Plants’ natural products as alternative promising anti-Candida drugs. Phcog Rev. 2017;11:104–122. doi: 10.4103/phrev.phrev_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis EK, Aperis G, Mylonakis E (2006) New agents for the treatment of fungal infections: clinical efficacy and gaps in coverage. Clin Infect Dis 43(8):1060–1068. 10.1086/507891. Erratum in: Clin Infect Dis. 43(9):1232 [DOI] [PubMed]
- Spellberg B, Walsh TJ, Kontoyiannis DP, Edwards J, Jr, Ibrahim AS. Recent advances in the management of mucormycosis: from bench to bedside. Clin Infect Dis. 2009;48(12):1743–1751. doi: 10.1086/599105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeputte P, Ferrari S, Coste AT. Antifungal resistance and new strategies to control fungal infections. Int J Microbiol. 2012 doi: 10.1155/2012/713687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF, Infectious Diseases Society of America Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46(3):327–60. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- Yasmin F, Najeeb H, Naeem A, Dapke K, Phadke R, Asghar MS, Shah SMI, De Berardis D, Ullah I. COVID-19 associated mucormycosis: a systematic review from diagnostic challenges to management. Diseases. 2021;9(4):65. doi: 10.3390/diseases9040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zargaran M, Taghipour S, Kiasat N, Aboualigalehdari E, Rezaei-Matehkolaei A, Zarei Mahmoudabadi A, Shamsizadeh F. Luliconazole, an alternative antifungal agent against Aspergillus terreus. J Mycol Med. 2017;27(3):351–356. doi: 10.1016/j.mycmed.2017.04.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.