Abstract
The endogenous signaling roles of carbon monoxide (CO) have been firmly established at the pathway level. For CO’s molecular mechanism(s) of actions, hemoproteins are generally considered as possible targets. Importantly, soluble guanylyl cyclase (sGC) is among the most widely referenced molecular targets. However, the affinity of CO for sGC (Kd: 240 μM ) is much lower than for other highly abundant hemoproteins in the body, such as myoglobin (Kd: 29 nM) and hemoglobin (Kd: 0.7 nM-4.5 μM), which serve as CO reservoirs. Further, most of the mechanistic studies involving sGC activation by CO were based on in-vitro or ex-vivo studies using CO concentrations not readily attenable in vivo and in the absence of hemoglobin as a competitor in binding. As such, whether such in-vitro/ex-vivo results can be directly extrapolated to in-vivo studies is not clear because of the need for CO to be transferred from a high-affinity binder (e.g., hemoglobin) to a low-affinity target if sGC is to be activated in vivo. In this review, we discuss literature findings of sGC activation by CO and the experimental conditions; examine the myths in the disconnect between the low affinity of sGC for CO and the reported activation of sGC by CO; and finally present several possibilities that may lead to additional studies to improve our understanding of this direct CO-sGC axis, which is yet to be convincingly established as playing generally critical roles in CO signaling in vivo.
Keywords: carbon monoxide, soluble guanylyl cyclase, nitrogen oxide, gasotransmitter, signaling molecule, hemoprotein
1. Introduction
After decades of research since the pioneering and foundational work of many in the early days including Sjöstrand,[1] Engstedt,[2] Gydell,[3] Coburn,[4] and Ludwig[5] among many others, the endogenous signaling roles of carbon monoxide (CO) are now firmly established.[6-10] A recent book on CO comprehensively examines various aspects of CO including endogenous production, pharmacokinetic behaviors, possible mechanisms of actions, CO’s roles in various physiological processes, CO’s therapeutic effects in animal models of various pathological conditions, and clinical trials of CO.[11] Also included are chapters on CO donors as research tools and potential therapeutic agents as well as fluorescent probes for in-vitro and possibly in-vivo detection of CO. One of the intriguing aspects of CO’s pharmacological actions is its pleiotropic effects and the involvement of multiple targets. In terms of CO’s mechanism(s) of actions, much more is known at the pathway level than at the molecular level, even though there has been much rigorous work on studying the binding constants and binding kinetics between CO and various targets.[12-16] CO’s established molecular targets are largely hemoproteins because of its known ability to bind to the ferrous form of iron with high but varying affinities depending on the other ligands for the metal. At this time no less than 25 molecular targets have been identified with affinity spanning 8 orders of magnitude in the range of low pM to high μM in terms of Kd values. Furthermore, it is believed that CO largely exists in the hemoglobin-bound form, i.e., carboxyhemoglobin (COHb); thus hemoglobin (Hb) is believed to serve as a carrier molecule for CO. However, this statement on CO being mostly in the bound form may only be true under “near-equilibrium” conditions when CO’s concentration is low. Rapid administration of CO via inhalation may lead to fairly high concentrations of CO in the unbound form, which becomes readily accessible by other molecular targets. This is a different subject that is covered elsewhere.[17] Further, the gaseous nature of CO as a signaling molecule makes it hard to examine a precise dose-response relationship and detailed mechanisms in a spatial-temporal fashion, especially in in-vivo studies. CO is highly diffusible across biologic membranes; its concentration across the space is dependent on the partial pressure difference, distance, medium property, and solubility.[18] The endogenous generation rate of CO is not a constant but a highly regulated enzymatic process, leading to significant fluctuations.[19] All these add to the difficulty in understanding CO’s molecular mechanism(s) of actions.
Among all the identified molecular targets, soluble guanylyl cyclase (sGC) is one of the most widely recognized.[20-26] It has been reported to be involved in regulating muscle contraction, platelet functions, vasorelaxation, and neutrophil migration, among many others. However, if one looks at the engagement of sGC by CO, there seem to be many missing pieces to allow for direction connection of CO with its biological functions while bound to sGC (i.e., a direct CO-sGC signaling axis), except under certain unique circumstances. For example, the affinity of CO for sGC has been reported to be about 240 μM,[27, 28] while CO’s affinity for hemoglobin is in the nM range in the high-affinity R-state and low μM range in the low-affinity T-state.[12] If one accepts Hb being the carrier protein for CO, how would CO go up the huge energy hill to be transferred from a high-affinity binder (Hb) to a low-affinity target (sGC) to trigger a response? Further, most other known and abundant CO targets have a much higher affinity for CO than sGC. For example, the affinity for CO is 28 nM for myoglobin, 0.2 nM for neuroglobin, and 0.3 μM for cytochrome c oxidase.[12] The availability of all these molecular targets with higher affinity than sGC begs the question as to how CO could “bypass” these targets to bind a low-affinity target in sGC and trigger a biological response. Below we present experimental findings by many, analyze the disconnection and propose possible explanations, which we hope will help future research.
2. Soluble guanylyl cyclase
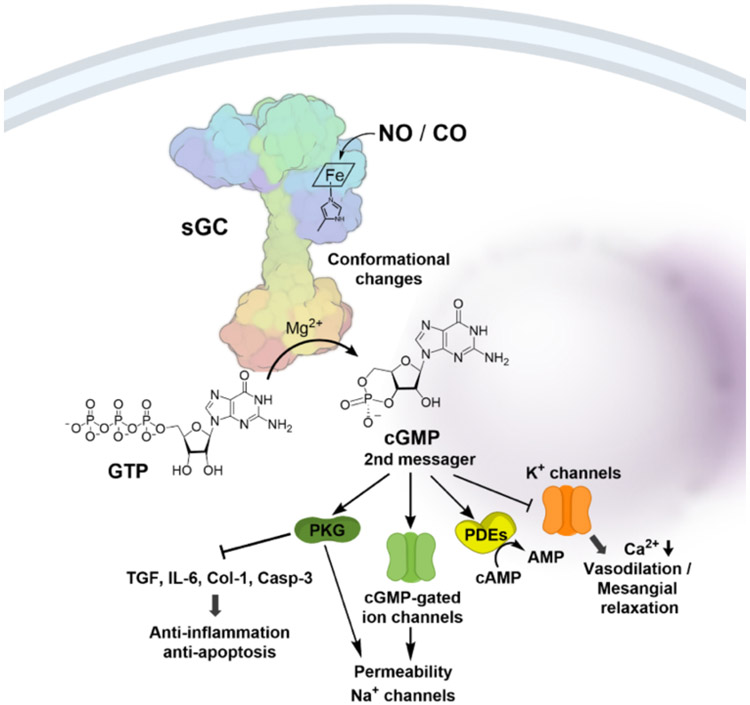
To start, we give a brief description of sGC. Guanylyl cyclase is responsible for the synthesis of the second messenger, cyclic GMP (cGMP) from GTP.[29] There are two forms of guanylyl cyclase: namely particulate guanylate cyclase (pGC) and soluble guanylate cyclase (sGC). pGC is membrane-bound and responds to extracellular signal molecules such as natriuretic peptides. sGC is cytosolic and exists in two forms, NO-dependent heme-containing form and NO-independent heme-free/oxidized form. In the presence of a ferrous heme moiety, sGC catalyzes cGMP production at a low rate. Upon binding with NO (Kd 4.2 pM),[30] an intrinsic sGC stimulant, sGC increases the catalytic activity by about 200 fold through allosteric regulations (Fig. 1).[31]
Fig. 1.
Structure and function of sGC. NO or CO binds to the prosthetic heme group which induce conformational changes to the sGC thus activates the enzymatic conversion of GTP to the 2nd messager cGMP which mediates downstream signaling pathways.
Structurally speaking, sGC is a heterodimeric complex consisting of two subunits, α and β, each of which has two isoforms.[32] The most common sGC combination is α1/β1, but α2/β1 is highly expressed in some tissues (e.g. brain).[33] Each subunit contains three common domains. The first one is the N-terminal heme-binding domain (HBD), which belongs to the H-NOX (heme-nitric oxide/oxygen binding) family[32] and mediates NO sensitivity for the enzyme. The second is a dimerization domain, which is a helical region found in the middle of the structure of each subunit, and is crucial for the transduction of the NO-heme binding signal to the activation of the C-terminal catalytic domain through conformational changes. The third is a C-terminal catalytic domain, which is the most highly conserved region among the subunits and is responsible for the enzymatic conversion of GTP to cGMP.[34] As a secondary messenger, cGMP mediates three major pathways including cGMP-dependent protein kinase, cGMP-regulated phosphodiesterase, and cGMP-gated ion channels (Fig. 1).[35] These signaling pathways, in turn, lead to various effects including vasodilation, inhibition of smooth muscle proliferation, blockade of leukocyte infiltration and inhibition of platelet aggregation, anti-inflammation, anti-fibrosis, anti-apoptosis.[32, 36, 37] Activation of the GC/cGMP signaling pathway offers protective effect in cardiovascular diseases[36] and kidney injury,[38, 39] among others.[32, 40, 41]
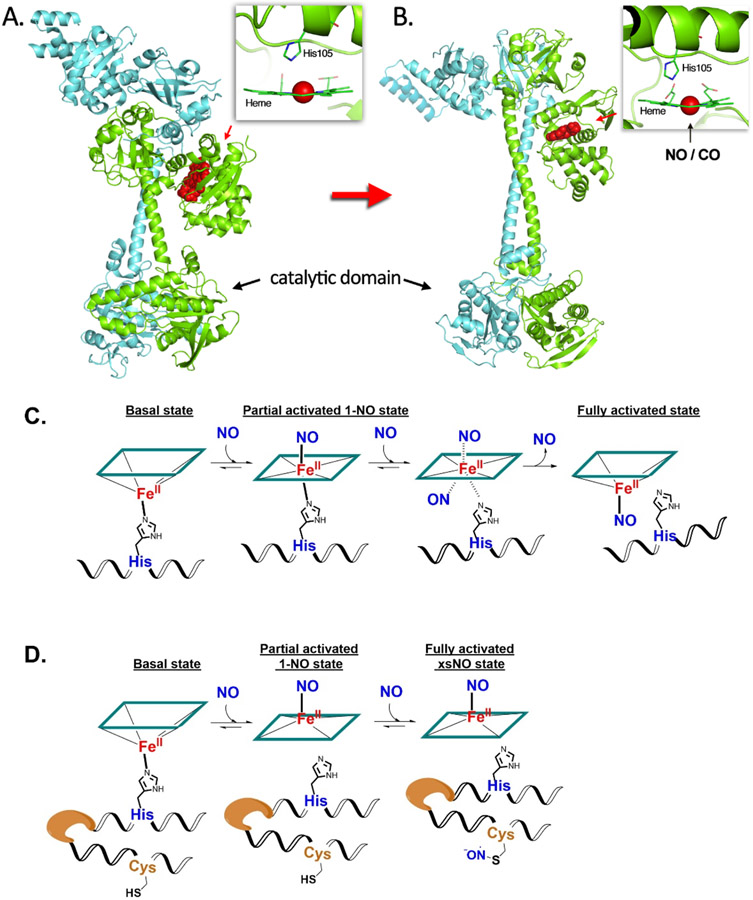
NO is known to bind to the five-coordinated ferrous iron of the heme located in the β1 subunit of the non-activated form of sGC, where the iron is coordinated to the imidazole of His105 (Fig. 2A). In fully activated sGC, the iron is also in a five-coordinated state with only one NO being the coaxal ligand. The formation of this fully activated complex has been found to adopt two distinct NO binding processes, which vary in kinetic profiles depending on the stoichiometric ratio between NO and sGC.[42] There are two possible mechanisms to explain such anomalous kinetic behavior. In one explanation, NO binds to sGC at the distal side of heme and forms the 6-coordinate complex; this is followed by binding by a second NO molecule to the proximal side to substitute the His105 residue, forming a transient bisnitrosyl heme species (bis-NO-heme), which quickly loses the distal NO leading to a proximal 5-coordinate NO-heme as the activated sGC (Fig 2C). This mechanism is supported by the interconversion of NO-coordination from the distal to the proximal heme face.[43] The other mechanism involves protein modification (presumably via S-nitrosation) by NO after binding the first NO to heme. Presumably, this nitrosation induces conformational changes favoring the formation of a five-coordinate activated form (Fig 2D).[44] A detailed analysis on the NO’s stoichiometry responsiveness showed that upon binding with one equivalent NO, the catalytic activity of the 1-NO state of sGC only increased by about 10-fold,[45, 46] even though this state encompasses the induced proximal histidine dissociation. X-ray scattering and cryoEM analyses of an insect (Manduca sexta) sGC also structurally confirms the of semi-activated configuration the 1-NO state of sGC adopting a state in between the bent inactivated conformation and the extended fully activated conformation. Upon treatment with additional NO to transform to the xsNO state (the state after binding of additional NO), its catalytic activity has been found to increase by up to 200-fold from the basal level.[44] Further conformational changes induced by nitrosation of a cysteine residue have been proposed to be the mechanism of the final activation.[44] Such discoveries challenge the initial understanding that Fe-His dissociation resulting from NO binding as the only mechanism of sGC activation. Nevertheless, these two proposed mechanisms do not contradict each other; they may both hold true and be involved in the activation of sGC, depending on the specific physiological and pathological conditions in the cell.
Fig. 2.
Conformational changes of sGC before (A) and after (B) NO binding with heme (modeled from PDB file 6jt0 and 6jt2); multi-NO activation mechanisms proposed in the literature, including distal histidine displacement (C) and protein nitrosation (D).
The conformational changes induced by NO binding to the heme-binding H-NOX domain allosterically led to large conformational changes of the catalytic domain, favoring enzyme activation through enhanced GTP binding and catalytic activity (Fig. 2B). A cryo-EM study shows the drastic changes of protein conformation upon NO binding.[47] The same N-terminal HBD of sGC that mediates NO sensitivity of the enzyme also responds to CO binding, but to a much lesser extent in terms of conformational changes compared to NO binding, leading to activation by only 3-6-fold based on various in-vitro studies.[31, 48, 49]
3. Experimental findings involving CO’s possible effects on sGC: The facts
3.1. The initial establishment of sGC as a target of CO.
Again, NO binds to sGC with high affinity (4.2 pM),[30] increasing the catalytic activity of sGC by about 200 fold.[31] Due to the ferrous heme in sGC, naturally CO seems to be a logical ligand as well. The ability for CO to activate sGC was first discovered in 1987 in a study of the anti-platelet aggregation activity of CO.[50] It was found that pure CO gas was able to induce a 30% increase in platelet cGMP level. Though the ability to activate sGC by CO was much lower than that of NO, the increased cGMP concentration was sufficient to block platelet aggregation in vitro as verified by using nitroprusside as a guanylate cyclase activator.[50] It should be noted that the experiments were conducted with pure CO gas, a concentration unlikely to be achieved in vivo except in rare conditions. In later studies of the vasodilation effects of NO and CO, CO was found to be at least 1000-fold less potent than NO in dilating the rabbit aorta. Specifically, CO at about 100 μM concentration led to a similar dilation effect (80% relaxation) as 100 nM NO in preconstructed rabbit aorta.[51] After treatment with 100 μM CO for 30 min, the concentration of cGMP in the endothelium-free rabbit aorta tissue increased from 60 pmol/g protein to about 90 pmol/g protein by a radioimmunoassay, which was in line with earlier findings. A breakthrough in suggesting CO as a neural transmitter that works through the sGC-cGMP pathway was reported by Snyder et al in 1993.[52] It demonstrated the constitutive expression of heme oxygenase-2 (HO-2) with a high degree of co-localization with sGC in parts of the mouse brain, such as in the olfactory epithelium, the neuronal and granule cell layer of the olfactory bulb, the pyramidal cell layer and dentate gyrus of the hippocampus, the granule and Purkinje cell layer of the cerebellum, and the coronal sections, among others. The mRNA of a rate-limiting enzyme for porphyrin biosynthesis, 5-aminolevulinate synthase (ALAS), and cytochrome P-450 which is the electron provider for HO-2 catalytic activity, were both found to be highly co-localized with HO-2 mRNA. These findings suggested a well-established CO production pathway through HO-2. Equally important, NO synthase was found to be absent in some parts of the brain including tenia tecta, habenula, islands of Callejae, and olfactory tubercle where there is a high degree of HO-2/sGC co-localization. Considering the short-lived nature of NO, these findings led to the suggestion that the NO-sGC pathway may not be the dominant mechanism for cGMP production in these regions. By using cultured primary olfactory neuron cells, it was found that HO inhibitor ZnPP-9 significantly decreased cGMP concentration with clear dose-dependency (10 nM – 10 μM). While 0.5 mM NOS inhibitor L-NAME did not change the inhibitory effect of ZnPP-9, indicating a low likelihood of an alternative NO pathway to subdue cGMP deficiency caused by HO inhibition. Further, externally supplied CO (100% CO gas bubbled in the culture medium for 1min) completely reversed the inhibitory effect of ZnPP-9 and restored cGMP level to that of the control in the cell culture. Along with other experiments to confirm the observed effects caused by constitutive CO production through sGC activation, it was suggested that CO could be a neurotransmitter functioning through the sGC/cGMP pathway depending on the localization of the HO-2 in the brain.[52] The results also indicated that CO generated in-situ near sGC could be sufficient for its activation.
There has been extensive work in determining CO’s binding kinetics and affinity to sGC. Using kinetic constants from flash photolysis studies, the dissociation constant of CO binding with guanylate cyclase was calculated to be about 240 μM.[53] It is obvious that the binding affinity of CO towards sGC is low compared with that of hemoglobin (Hb) and myoglobin (Mb), largely due to some unique kinetic features. Specifically, the association rate constant ka for CO-sGC binding was determined to be 1.2 ± 0.1 × 105 M−1s−1, similar to that of CO binding with the T-state of Hb. Whereas the dissociation rate constant kd for CO-sGC was determined to be 28 ± 2 s−1, which is significantly higher than that of CO-Hb or CO-Mb. The high dissociation rate constant not only renders sGC low affinity for CO but also indicates marked differences in binding mode between CO-sGC and CO-Hb/Mb. The high dissociation rate constant was shown[54] to be associated with the five-coordinate binding mode instead of six-coordinate binding by which most hemoproteins in the Hb family adopt. In the initial deduction of the binding mode based on kinetic analyses, it was proposed that the higher positive trans effect (defined as reinforcing the affinity of the trans ligand) of CO compared to the higher negative trans effect (defined as reducing the affinity of the trans ligand and more likely to expel the proximal imidazole ligand and form a 5-coordinate complex) of NO in binding with the porphyrin iron is the reason that CO induces much less activation of sGC than NO.[53] The dissociation of the proximal histidine of the heme-binding site initiates the conformational changes towards the activated sGC protein. The proposed mechanism was further supported by findings from different labs using structural analysis methods such as electronic and magnetic circular dichroism,[55] and resonance Raman spectroscopy.[56]
3.2. Examples of in-vitro, ex-vivo, and in-vivo experiments and concentrations of CO used
Since the initial demonstration of sGC’s involvement as a molecular target for CO, there have been many studies on this subject. Some of these studies included experimental work to confirm the involvement of sGC and others only suggested such involvement. Below, we summarized some reported studies of CO’s biological effects that were proposed to involve sGC as at least one of the molecular targets. These reports show the volume and variety of experiments that have been devoted to studying sGC as a CO target. In summarizing the data, we separately list and discuss results from CO gas and from CO donors, which are largely metal-carbonyl complexes with the exception of Entry 22, which is a metal-free organic prodrug.
It can be seen from the table that ex-vivo experiments have shown the potential signaling role of endogenously generated CO for maintaining the basal cGMP level in cells of specific tissues such as olfactory neuronal cells,[52] liver Ito cells,[58] and smooth muscle.[75] On the other hand, externally introduced CO also showed moderate sGC activation effects compared to that of NO. For example, in rabbit aorta dilation studies,[51] 20 nM NO increased cGMP level from a control level of 60 pmol/g protein to 1580 pmol/g protein within 30 s (about 26-fold increase). In another similar experimental setting, 100 μM CO increased cGMP level from 60 pmol/g protein to about 92 pmol/g protein within 30 s and a maximum of 110 pmol/g protein in 120 s (about 1.8-fold increase). Compared to the 90% relaxation effect induced by 20 nM NO in 3 min, 100 μM CO induced about 50% relaxation in 2 min. Therefore, in the endothelium-free rabbit aorta ring, exogenous CO treatment is much less effective in increasing cGMP level, presumably due to less activation of sGC. Further, the relaxation effect of NO was rapidly reversed with the addition of 10 μM free radical inducer LY-83583 (also known as an sGC inhibitor) as an NO scavenger.[51] On the other hand, LY-83583 did not affect the relaxation induced by CO, indicating the independent nature of CO’s effect. The pronounced effect of exogenous CO in aorta relaxation made the case of demonstrating bioactivity of metal-based CO releasing molecules such as CORM-2,[63] CORM-3,[66] CORM-A1,[67] and Mn2(CO)10.[68] In such studies, CORMs induced a similar vasodilation effect as CO gas used at comparable concentrations in previous studies. However, their effects in regulating cGMP level were diverse or not available, thus leaving the question open as to whether such vasodilation effects are solely dependent on the CO/sGC pathway.
Though many studies suggested sGC involvement under a given set of experimental conditions, directly or indirectly, there are also studies that raised questions. For example, Mn2(CO)10 showed dose-dependent dilation of the pial arterioles but did not affect the cGMP level (Table 1, Entry 16).[68] In yet another study, 4 mg/kg CORM-3 was shown to increased liver tissue cGMP from 40 pmol/mg protein to about 70 pmol/mg protein, while at the same time increasing the liver tissue CO concentration from 0.5 μmol/mg to 1.2 μmol/mg (Table 1, Entry 17).[69] Given its molecular weight of 294.6, 4 mg/kg of CORM-3 translates into 0.0136 pmol/mg as the theoretical maximum if CO is distributed evenly throughout. The extremely large discrepancy between the measured value of CO concentration and the theoretical maximum seems to indicate additional sources of CO or unexpected enrichment of CO at an exceedingly high magnitude at the sampling site. It is hard to find an explanation of such observations based on known mechanism and pharmacokinetic behaviors of CO. Further, a recent study revealed that the widely used sGC inhibitor ODQ is not a selective sGC inhibitor, rather it also inhibits nitric oxide synthase and other cytochrome P-450 enzymes involved in NO-mediated pathways.[76] Another widely used sGC inhibitor LY-83583 is a quinone derivative. The sGC inhibition activity of LY-83583 was found to be dependent on its reactivity in generating hydroxyl radical, leading to neutralization of NO from endogenous or exogenous sources.[77] Further, some CORMs have been found to be reactive toward thiols, hydrogen peroxide, and free radicals.[78-84] All these could convolute the interpretation of data. Such issues are discussed in the later sections. It should be noted that some studies that proposed the involvement of the CO-sGC axis in vasodilation effects also at the same time suggested the distinct possibility of the existence of alternative mechanisms[58, 68] such as the modulation of the expression of endothelin and growth factors independently of cGMP,[85] increase in potassium current corneal epithelial cells[57, 86, 87] and intestinal smooth muscle cells,[88] among other possibilities such as inhibition of p-450-mediated products.[89]
Table 1.
Select Examples of CO-sGC research
| Entry | Model | CO delivery | Results in brief relevant to sGC | Ref |
|---|---|---|---|---|
| CO gas | ||||
| 1 | Human anti-platelet aggregation, in vitro/ex vivo | Pure CO gas bubbled for 20 s | CO induced 30% increase in cGMP level. | [50] |
| 2 | Rabbit aorta dilation, in vitro/ex vivo | CO in solution | 100 μM CO achieved a similar 80% relaxation effect as 100 nM NO. The concentration of cGMP in the endothelium-free rabbit aorta tissue increased from 60 pmol/g protein to about 90 pmol/g protein. | [51] |
| 3 | Rabbit cornea1 epithelial cells, in vitro | 20 μM CO in Ringer solution | CO increase cGMP levels from 0.41 ± 0.24 pmol/106 cells to 0.55 ± 0.27 pmol/106 cells. | [57] |
| 4 | Rat olfactory neuronal cells, in vitro/ex vivo | cell culture medium bubbled with 100% CO gas for 1 min | CO gas completely restored cGMP level (102%) from 15% level caused by inhibition of heme oxygenase-2 (HO-2) by 1 μM ZnPP-9. | [52] |
| 5 | Rat Ito cells, in vitro/ex vivo | CO in solution | HO inhibitor Zn PP-9 at 1 μM concentration did not alter the cGMP level. CO at 50 μM concentration led to an increase in cGMP level similar to that elicited by 100 μM NO donor SNP. The minimum concentration of CO to significantly increase the cGMP level was 20 μM. | [58] |
| 6 | Human neutrophil cells, in vitro | 10 μM CO in solution | 10 μM CO increased cGMP concentration from about 2.3 pmol/107 cells to 3.8 pmol/107 cells in 30 seconds, then decreased to the basal line within 2 minutes. A higher concentration of CO did not give a further elevation of cGMP. | [26] |
| 7 | Raw 264.7 cells, in vitro | 250 ppm CO gas | Raw cells showed no significant increase in cGMP level after being exposed to 250 ppm CO gas for 2h. | [59] |
| 8 | Human placentas perfusion, in vitro/ex vivo | 0.1 – 1% diluted CO gas saturated perfusion medium | CO saturated medium decreased perfusion pressure in a dose-dependent manner. Such activity can be partially abrogated by 1 μM ODQ, and can be augmented by 100 μM sGC stimulator YC-1. | [60] |
| 9 | Isolated mouse heart, in vitro/ex vivo | CO gas solution (96 μM – 480 μM) | CO gas solution decreased action potential duration in atrial and ventricular tissue and accelerated pace-making activity in sinoatrial node. Such effects could be blocked by 10 μM ODQ thus presumably sGC dependent. | [61] |
| 10 | Human platelets and mouse thoracic aorta, in vitro/ex vivo | CO gas solution and CORM-A1 | 60% CO gas saturated buffer reduced platelet aggregation by about 37%: 30 μM CORM-A1 reduced platelet aggregation by about 65%. 10 μM sGC inhibitor ODQ pretreatment completely blocked aggregation inhibition effect of CO gas but not CORM-A1. Both 60% gas saturated buffer and 500 μM CORM-A1 induced murine aorta by about threefold, which could be blocked by ODQ. | [62] |
| CO donors | ||||
| 11 | Isolated rat aortas, in vitro/ex vivo | CORM-2 | 222 μM CORM-2 induced 50% vasorelaxation which was partially blocked by a sGC inhibitor ODQ at 10 μM. | [63] |
| 12 | Neuronal pyroptosis in rat cortical tissue, in vitro | CORM-3 | CORM-3 at a dosage of 4 mg/kg post-treatment protected rats against neuronal pyroptosis after hemorrhagic shock and resuscitation. CORM-3 was also shown to partially restore sGC activity and cellular cGMP level, while pretreatment with sGC inhibitor NS2028 abolished the restoration effect. | [64] |
| 13 | Ischemia-reperfusion induced gastric mucosal injury in rats, in vivo | CORM-2 | sGC inhibitor ODQ (10 mg/kg) was able to block the protective effect of CORM-2 (5 mg/kg), suggesting sGC activation by CORM-2 as a possible mechanism. | [65] |
| 14 | Isolated rabbit afferent arterioles, in vitro/ex vivo | CORM-3 | microperfusion with 50 μM CORM-3 attenuated TGF levels by 1/3 to 1/2 which was blocked by 1 μM sGC inhibitor LY-83583. | [66] |
| 15 | Isolated rat aortas relaxation, in vitro/ex vivo | CORM-A1 | 80 μM CORM-A1 induced 40% vasodilation effect, which was partially inhibited by a sGC inhibitor ODQ at 30 μM to about 10% dilation level. | [67] |
| 16 | Piglet pial arterioles, in vitro/ex vivo/in vivo | Mn2(CO)10 | 1 nM to 10 μM Mn2(CO)10 showed dose-dependent dilation of the pial arterioles but did not affect the cGMP level. | [68] |
| 17 | Rat hemorrhagic shock/resuscitation (HSR)-induced liver Kupffer cells pyroptosis, in vivo | CORM-3 | 4 mg/kg CORM-3 increased liver tissue cGMP from 40 pmol/mg protein to about 70 pmol/mg protein, while iCORM control showed no effect. The anti-inflammatory activity of CORM-3 in HSR induced Kupffer cells was partially blocked by 10 mg/kg sGC inhibitor NS2028. | [69] |
| 18 | Neuroblastoma cells (SH-SY5Y), in vitro | ALF-186 | ALF-186 (100 μM) significantly increased cellular cGMP from 23 nM to 33 nM, and such effect could be blocked by 10 μM ODQ. ALF-186 (50 – 100 μM) also induced expression of sGC β1 subunit. | [70] |
| 19 | Rat retinal ischemia-reperfusion injury model, in vivo | ALF-186 | 10 mg/kg ALF-186 increased sGC-β1 protein expression significantly. sGC inhibitor ODQ (2.5 mg/kg) pretreatment reduced ALF-186 mediated sGC protein expression. Inhibition of sGC-β1 with ODQ abrogated ALF-186’s anti-inflammatory effects regarding NF-κB, IL-6 and TNF-α expression. | [71] |
| 20 | Rat model of gastric injury, in vivo | DMDC | DMDC treatment (9-81 μmol/kg) prevented alendronate-induced macroscopic and microscopic gastric damage. | [72] |
| 21 | Rat model of gastric injury, in vivo | CORM-2 | CORM-2 at 1-10 mg/kg (i.g.) increased mucosal CO contents and dose-dependently reduced chemically-induced gastric injury. sGC involvement was examined using an inhibitor. | [73] |
| 22 | Gastric protection, in vivo (rats) | BW-CO-111, a metal-free organic prodrug | Intragastric administration of 0.1 mg/kg led to an increase of gastric mucosal CO contents by over 100% and significant reduction of chemical-induced gastric damage. The effective dose was very low with local prodrug application being a possible contributing factor. | [74] |
3.3. Possible interplays between NO and CO in regulating sGC activity
There are emerging findings that CO functions as a modulator of NO’s activity in controlling sGC. Such modulating activities include both agonistic and antagonistic effects depending on the specific circumstance. For example, a study using a monoclonal antibody that specifically interacts with activated sGC suggested the role of CO being different depending on whether NO is present.[90] In this study, exogenous CO exhibited dual effects on the activation of bovine purified sGC induced by a NO donor S-nitroso-N-acetylpenicillamine (SNAP) in the in-vitro assay. Without SNAP treatment, 30 μM CO increased the cGMP production rate from 45 nmol/min/mg protein to 80 nmol/min/mg protein. In the presence of SNAP at 1~100 nM with 30 μM CO, the cGMP generation rate was dose-dependently elevated from 80 nmol/min/mg protein to about 280 nmol/min/mg protein. However, when SNAP concentration was greater than 100 nM, CO was found to attenuate the NO-induced cGMP generation rate modestly but significantly. Specifically, 30 μM of CO was found to attenuate NO induced cGMP production rate by 40% when SNAP concentration was 1 μM. A sGC antibody mAb3221 was used to map the sGC activity in different rat retinal layers.[90] The results of immunoactivities showed that sGC activity was enhanced by the HO inhibitor, ZnPP, and was repressed by a NO synthase inhibitor, L-NAME, suggesting an antagonistic effect of endogenous CO on NO-activated sGC’s activity. The sGC activation was further suppressed by concomitant treatment of ZnPP and L-NAME indicating that endogenous CO plays a role in maintenance of the basal sGC level. Moreover, the effects of endogenous CO in modulating sGC activity were found to be different among different retinal layers depending on their distances to the NO source – neuronal NOS (nNOS), endothelial NOS (eNOS), and NO neutralizer – oxygen. In the inner plexiform layer and inner nuclear layer where NO concentration is higher, CO generated by the constitutive heme oxygenase-2 (HO-2) was suggested to act as a partial antagonist of sGC activation. In the outer plexiform layer where NO is largely neutralized by molecular oxygen, and in the external limiting membrane, which is far from the NO source of the microvascular, endogenous CO may act as a weak sGC activator to keep a basal cGMP level needed in these cells.[90] This retina-based study indicates that CO is endowed with a modulation effect to sGC, which is likely dependent on the specific tissue, responsible for their production, and the environments. A similar phenomenon was found in other tissues such as liver[58] and the brain.[52, 91, 92] In an in-vitro study in perfused rat liver, HO-1 inhibitor ZnPP at 1 μM decreased CO concentration in the tissue effluent from about 0.23 μM to an undetectable level. Treatment with ZnPP exhibited a significant (30%) sinusoidal constriction.[58] However, continuous infusion with CO solution at 1 μM concentration abolished the constriction effect of ZnPP and restored the vascular resistance to the same level without ZnPP treatment. NO inhibitors such as L-NAME and aminoguanidine did not change the vascular resistance, indicating minimal influence from the endogenous NO in the tissue preparation. On the other hand, a cGMP analog 8-Br-cGMP at 1 μM together with ZnPP also abolished the vascular constriction effect of ZnPP. Such results were interpreted to suggest a role of endogenous CO in the vascular tone of the liver sinus where NOS expression is known to be low. However, one cannot rule out the possibility that the vasorelaxation effect of the exogenic CO treatment results from other mechanisms such as the activation of the KB channel.
Along a similar line, the same group also investigated the regulatory effects of CO in rat cerebral microcirculation and found an antagonistic role of CO production against NO-induced vasodilation.[91, 93] Immunohistochemical studies indicated HO-2, NO synthase 1, and NO synthase 3 were detectable in rat cerebral; this colocalization suggest a potential interplay of CO and NO in the same region. The in-vivo study in rat cerebral arterioles showed that inhibiting the HO activity by ZnPP induced significant arteriole dilation, while supplementation with exogenous CO at a concentration of 10 μM significantly reduced the vasodilation effect of ZnPP. Inhibition of endogenous NO by NOS inhibitor L-NAME at 1 mM resulted in significantly vascular constriction, while supplementation with 10 μM CO alone induced vasoconstriction to a similar level of L-NAME. The vasodilation effect by ZnPP was partially reversed by L-NAME but not pseudo-inhibitor D-NAME, suggesting a critical role of NO in inducing vasodilation. Therefore, the authors demonstrated that in the cerebral tissue where NOS is highly expressed, constitutive CO production is a negative regulator to the vasodilation effect of NO.[91] However, sGC activity was not examined in the same studying, leaving the question open as to whether the observed effects can be solely attributed to the direct modulation of sGC by CO. Other indirect pathways that mediate such vascular tone modulation by CO need to be considered.
With the current understanding of the interplay between NO and CO under physiological conditions, the intriguing mechanisms lie within the endogenous generation pathways of NO and CO depending on the tissue phenotype. Based on their own research and literature reports, Suematzu, et al summarized three representative scenarios.[94] First, in the hepatic sinusoid tissue, where NO concentration is low due to the presence of basal level of superoxide produced by Kupffer cells,[58] CO produced by the constitutive HO-2 in the hepatocyte could take the major regulatory role in modestly stimulating sGC, thereby reducing the tonic contractile tension of vascular wall. Second, in resistance arterioles, in which NO concentration in the endothelium cells is as high as 500-600 nM,[95] CO generated by HO-1 and HO-2 in vascular smooth muscle cells competitively attenuates the vasodilation effect of NO and works as a partial inhibitor of sGC, thus rendering tonic contractile action. Third, in the cerebral microcirculation, where CO is generated by HO-2 in endothelium cells and neuron/trabecular cells at high concentrations, CO inhibits eNOS in the same cells thus lowering NO production, and acts as a tonic vasoconstrictor. The overall effects of CO in regulating vascular tone were proposed to be multifaced where low tissue availability of NO renders CO a vasodilator, high tissue NO availability renders CO a constrictor.[68, 94]
4. The myths
Central to the myth of how CO binds a seemingly low-affinity binder in sGC in the presence of hemoproteins of much higher affinity and abundance is the question of Kd values and concentration of CO. In addition, there is also the issue of competition in binding between CO and NO with a much higher affinity for sGC as well as their biologically relevant concentrations. Below we present a few questions.
4.1. How does the competition between NO and CO in binding with sGC affect the possible pathophysiological functions of CO?
As discussed above, the Kd for sGC binding is 4.2 pM for NO and 260 μM for CO.[30] The difference is on the order of 108 in pure binding affinity. In addition, sGC is activated by about 200 fold by NO and about 4 fold by CO.[31] Combined, these numbers mean, in a theoretical sense, that it takes CO concentration of at least 9 orders of magnitude higher in order to compete for NO in terms of leading to meaningful biological responses. Such results further indicate that to compete with 1 pM of NO, it would need 1 mM CO by dissolving pure CO gas at 1 atmospheric pressure under physiological conditions. With these analyses, one has to ask the question as to under what circumstance would CO afford meaningful pathophysiological effects by activating sGC? Would this be in the absence of NO synthesis as in the case of certain regions of the brain as discussed earlier?[52] In a binding study, Vogel already raised the question of whether CO is able to activate sGC in vivo, unless CO is at a very high concentration and/or when NO synthesis is severely inhibited.[96] Of course, one also needs to keep in mind the short-lived nature of NO, which may come into play when examining the competition for binding with sGC between CO and NO.
At this point, it is helpful to examine the experimental conditions used in studying CO’s effect on sGC. In determining the 4-fold activation of sGC by CO, the experiment was conducted in vitro. In the very first report of CO’s effect on sGC, elegant work was performed by studying CO’s inhibitory effect on platelet aggregation and 10,000 ×g centrifuge supernatant from platelet homogenates was used.[50] Most of the experiments were done with pure CO, and it was found hat platelet inhibition effects were observed in the range of 20-80 μM of CO. Such numbers are consistent with the known Kd of 240 μM for sGC for CO. Further, in determining the activation of sGC from the bovine lung by NO (128 ± 17 folds) and CO (4.4 ± 0.4 folds), these experiments were performed using 0.5% of NO in argon and 100% CO, respectively.[48] One wonders whether the CO concentration created with 100% CO can be realized in vivo.[96] As a result, even the modest 4-fold activation of sGC by CO might only be the biochemical maximum and is likely a significant over-estimation of what is achievable in vivo.
4.2. How does the presence of hemoproteins with much higher affinities for CO than sGC affect CO’s ability to bind and activate sGC?
In the general field of drug design, the examination of binding, binding constants, and dose response is the first step in understanding the mechanism of action at the molecular level. Therefore, it is important to examine the binding competitions in terms of other hemoprotein targets in considering CO’s ability to bind and activate sGC. Because carboxyhemoglobin (COHb) is considered the carrier molecule of CO, it is critical to first examine the relative affinity of CO for sGC and Hb. CO has varying affinities for Hb depending on its conformational states. The Kd for CO binding to the α- and β-subunits of Hb in the low-affinity T-state is 1.8 μM and 4.5 μM respectively, which are far below the Kd for CO binding with sGC. In the high affinity R-state, the Kd of CO binding to Hb is 1.7 nM for the α subunit and 0.7 nM for the β subunit. As such, the likelihood for sGC to “extract” CO from COHb is very small under physiological conditions; and the maximum saturation level of sGC in an equilibrated binary system would only reach about 0.3% at a COHb level of 14%, which was the upper limit for human clinical trials on kidney transplantation set by the US Food and Drug Administration (FDA). When the COHb level is about 1% or lower, it seems unlikely to afford a CO level that is close to its Kd with sGC for meaningful activation.
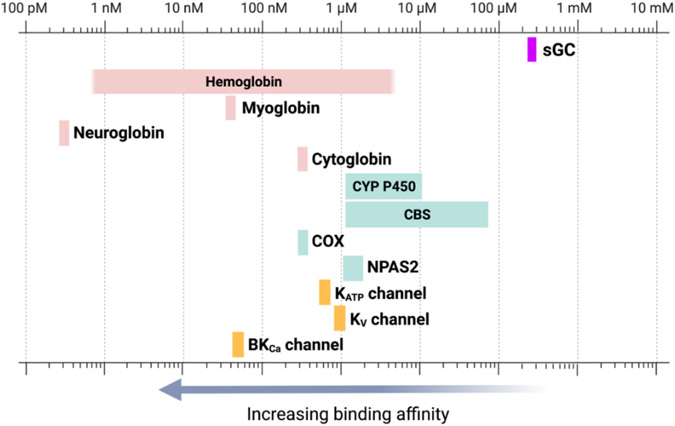
Along a similar line, it is important to note that hemoglobin is the most abundant hemoprotein with myoglobin being second. Combined, these two proteins form the “reservoir” for CO under normal conditions.[12] Further, there are many other hemoproteins with a much higher affinity for CO than does sGC; these include cytochrome c oxidase, p450, neuroglobin, among others.[12] Fig. 3 shows the affinity (Kd values) of some identified CO targets in a diagram. All these hemoproteins could pose as competitions for CO binding, making sGC “extraction” of CO an uphill battle energetically. Therefore, if CO is to play a pathophysiological or a therapeutic role by engaging sGC, there need to have other factors at play, as has been suggested by Vogel.[96] This aspect needs further examination.
Fig. 3.
Binding affinities (Kd) of CO to hemoproteins in comparison to sGC.
5. Intriguing Possibilities
With all the questions related to the disconnect in some cases between binding constants, level of sGC activation, activities attributed to sGC activation, and biologically relevant CO concentrations, one thing still trumps all others: i.e., the experimental findings reported in various publications that attributed the observed pharmacological and/or in-vitro/ex-vivo biological activities to sGC activation. What factors might help connect all the dots? Below, we analyze various factors for consideration. In doing so, we do not strive to be comprehensive. Instead, we try to use examples to show that there may need to be further research in various areas to help establish the missing link(s) that go(es) beyond simple sGC binding and activation.
5.1. Is it possible that CO indirectly regulates NO functions?
As discussed above, CO and NO bind to sGC with different affinities and adopt different binding modes with the prosthetic heme group, thereby inducing enzyme activation to various degrees. These general understandings are mostly based on in-vitro experiments with purified enzyme or isolated tissue with exogenous NO or CO sources. Therefore, NO was identified to be the dominant activator while CO was regarded as an insufficient activator and probably in a non-essential role in the sGC pathway. However, due to the pharmacological effects attributable to increased cGMP production by CO, is it possible that CO indirectly regulates sGC activity?
As NO synthase (NOS) is a hemoprotein, CO can also bind to NOS and inhibit its activity, thus decrease NO production. The Kd for CO binding with substrate-free nNOS was determined to be about 1 μM; while addition of a tetrahydrobiopterin (BH4) cofactor increased the Kd value to about 16 μM in the presence of arginine.[97, 98] However, it was suggested that to sufficiently inhibit NOS, a much higher concentration (1 mM) of CO would be needed.[98] On the other hand, at low concentrations, CO (1000 ppm, about 1 μM) may increase NO production, possibly due to CO’s stabilizing effect on the ferrous iron of NOS through catalytic cycle. Nevertheless, the possibility of high local CO concentration near NOS in the intracellular space may not be ruled out.
Circadian rhythm protein Period 2 (PER2) protein is a key part of the core timekeeping mechanism in eukaryotic cells,[99] and has been shown to be an important modulator of organ function and susceptibility to injury such as ischemia-reperfusion injury (IRI).[100, 101] Studies have also shown that PER2 plays an important role in the maintenance of normal cardiovascular functions.[102] Specifically, Per2 gene loss-of-function mutation is associated with decreased NO production and vasodilatory prostaglandin (PGI2) synthesis and increased vasoconstrictive prostaglandin (PGs).[102] Stabilization of functional PER2 may increase NO production and conceivably activate sGC. Interestingly, it was found that in a mouse kidney model of ischemia reperfusion injury, CO in the form of gas (250 ppm), from an organic CO prodrug (BW-CO-101), or dissolved in a liquid formulation (HBI-002) offered protective effect via upregulating PER2 expression, presumably through activating a heme-containing transcription factor NPAS2.[103] Therefore, CO may indirectly regulate sGC activity through circadian rhythm modulation.
Although it may be not directly relevant to the eukaryotic system, a heme-containing phosphodiesterase (PDE) in E. coli. (Ec DOS) was found to bind O2 and CO with Kd of 340 μM and 3.1 μM respectively.[104] Binding to CO was found to suppress its catalytic activity in converting 3’, 5’-cAMP to 5’-AMP and in converting cyclic di-GMP to linear di-GMP.[105] The implication of such findings in the mammalian are yet to be understood; possibilities exist that CO may regulate gut microbiota through interaction with the Ec DOS or its isozymes in the gut microbiome thus indirectly participating in pathophysiologic processes in the host system.[106] An even bolder suggestion is whether there are any analogous effects of heme on mammalian PDE that modulates cGMP signaling pathways by binding to NO and/or CO.
5.2. Is it possible that non-equilibrium conditions exist in CO inhalation treatments that lead to high concentrations of CO in the non-bound form?
In an in-vivo study of the CNS toxicity of CO poisoning in rats,[92] it was found that acute CO exposure (2400 ppm for 1h) decreased cGMP content and reduced activation of sGC by SNAP (a NO donor). At the 24-h point post exposure, cGMP in the cerebellum tissue decreased from 65 ± 30 pmol/mg protein to 46 ± 20 pmol/mg protein. Within 5 min after the addition of SNAP (100 μM) in the cerebellum tissue homogenates, cGMP production decreased to 1323 ± 737 pmol/g protein in the CO treatment group from the control-group level of 2128 ± 317 pmol/g protein. Chronic exposure (450-500 ppm for 6 h per day over 4 weeks) did not induce such significant changes of cGMP production with or without SNAP 24 h after CO exposure. However, the response to SNAP did decrease by half after the CO exposure for 7 days. Though the mechanism for this delayed response was not clear, an alteration in the sGC expression was observed. Beyond the scope of the original study for neurotoxicity, the inhibitory effect of CO in sGC activity of cerebellum tissue may corroborate previous assumption that in neuro tissues, CO could be a negative regulator of sGC in the presence of the much stronger activator – NO. Judging from the concentration of CO in the cerebellum tissue, acute CO exposure at 2500 ppm may give about 70% COHb level according to another study in rat.[107] Chronic exposure with 500 ppm CO could lead to about 30% COHb in rat.[108] They are undoubtedly non-physiological conditions. However, due to the much higher binding affinity of Hb for CO than sGC, most likely CO-sGC binding is realized via free CO present in the circulation during inhalation treatment as has been analyzed before,[109] which could reach the mid-micromolar range, sufficient for sGC binding. Thus, sGC engagement is feasible under acute CO exposure conditions. However, under chronic CO exposure that only leads to 30% COHb, the free CO carried by the blood may not be sufficient for sGC engagement. This may explain why the cGMP level was not significantly changed by chronic CO exposure in the aforementioned study.
5.3. Binding affinity varies among different species depending on conditions?
Structural studies in the literature also have come to the conclusion that CO predominantly forms a 6-coordinate heme complex with sGC rather than a 5-coordinate complex. Therefore, CO was proposed as “unlikely to be a natural effector of the sGC”[55] at least in human cells. Specifically, it has been proposed that the protein structure where the distal histidine forms the six-coordinate heme complex in the inactivated sGC could dictate the kinetic property of CO-sGC binding, especially in the association process.[53] Is it possible that sGC from different species could have different selectivity and binding affinity towards CO? Since the pioneering studies in demonstrating the ability for CO to inhibit platelet aggregation,[50] there is new evidence that seems to indicate some species difference. For example, in the aforementioned aorta relaxation study using CO and NO, Vedernikov et al. found that the dog arterial relaxation activity of CO was similar to that of NO. However, the detailed conditions and concentrations were not available in the publication.[110] In a study by Furchgott and Jothianandan, it was also found that CO and NO induced approximately the same dilation effect in dog coronary artery.[51] Recently, a new type of sGC (Cyg11) was found in the microbiome Chlamydomonas reinhardtii.[111] It has a much higher kinetic association rate (ka, 237 ± 21 × 104 M−1s−1) for CO binding compared to the rat sGC (4 × 104 M−1s−1) while the kinetic dissociation rate is similar (kd, 6.04 s−1 for Cyg11 vs. 10.7 s−1 for rat sGC). The overall result showed Cyg11 has a significantly higher binding affinity to CO than other sGC found so far, with a Kd of about 2.5 μM. In addition to the marked increase in CO binding affinity, Cyg11 also showed about 2-fold higher enzymatic activation upon binding to CO than NO. All these features make Cyg11 an sGC phenotype that is responsive to both CO and NO under near physiological conditions, which could be important for the survival of C. reinhardtii. However, at this time there is an insufficient amount of data in analyzing species differences in terms of the binding studies reported so far as well as their possible implications.
5.4. The complicated interplay among CO, NO, and H2S.
It is also possible that much of the pharmacological effects observed attributable to sGC are through intricate interplays among the three gasotransmitters. They may compete in binding with hemoprotein targets depending on the selectivity of the target. Interestingly, these gasotransmitters may interfere with each other’s production pathways through the regulation of critical enzymes, suggesting a convoluted interplay among these gasotransmitters.[112] For example, CO binds and inhibit cystathionine β-synthase, which is critical for H2S production;[113] NO has been reported to stimulate HO-1 gene transcription;[114-116] and CO has been reported to inhibit NO synthesis.[117, 118] For discussion on the interplay between these three gasotransmitters, readers are referred to comprehensive reviews.[94, 119-123]
5.5. High local concentrations?
Though the affinity of CO for sGC is low (Kd 240 μM), it is conceivable that high levels of local CO production could lead to high micromolar concentrations of CO, which in turn could activate sGC. The pioneering work of Snyder is a good example of possibly high local CO concentrations for binding with sGC without competition from NO because of a lack of NOS activity at the same location.[52] One way to analyze this issue is the different chemical stability of NO and CO. In general, the stability of CO is much higher than that of NO. Specifically, the half-life of NO in solution is less than two seconds,[124] while CO is very stable in solution and has a biological half-life of about 4 h in the body, which is largely the result of elimination in its unchanged form through exhalation.[18] In light of these considerations, assessing the concentration of each signaling molecule at the target (i.e., sGC) location is essential to analyzing the physiological and pathological functions of the signaling molecules. Three major determinants of the effective gas concentration were proposed:[22] 1) the physicochemical properties of the gas itself, 2) the properties of the local environment, including the surrounding media through which gas travels including viscosity, pH, temperature, and tissue composition, and 3) scavenging systems including chemical reactions that consume the gas. The apparent CO concentration in the tissue is relatively low. According to Vreman’s studies using gas chromatogram (GC), CO concentration was determined to be 1-10 pmol/mg (about 1-10 μM assuming tissue density is about 1 g/ml) in rat tissue.[108, 125] CO in rat cerebrospinal fluid was determined to be 1 μM.[91] However, in cell culture studies by using a cyclodextrin-heme based CO probe, the intracellular CO concentration was determined to be about 250 pmol/106 HepG2 cells.[126] Assuming cell volume of about 2000 μm3,[127] the intracellular CO concentration is estimated to be 125 μM. Such analyses may suggest intracellular CO concentrations being much higher than the apparent tissue CO concentration, probably due to the wash-out effect by binding to the hemoglobin of the tissue blood circulation. However, it needs to be noted that such results do not mean that the concentration numbers correspond to “free” CO concentrations. One still needs to consider the various binding equilibria in the presence other targets with higher affinity than sGC.
5.6. The involvement of an allosteric regulator? -The story of YC-1 and analogs as well as its implication in the endogenous roles of CO.
In addition to the effector effect of nitrosylation, studies have also indicated a critical role in the allosteric activation of sGC. Similar to the further activation of low activation 1-NO state, sGC can be further activated by allosteric regulators. There are synthetic compounds that have been shown to enhance sGC’s affinity for NO and CO. Below are some examples.
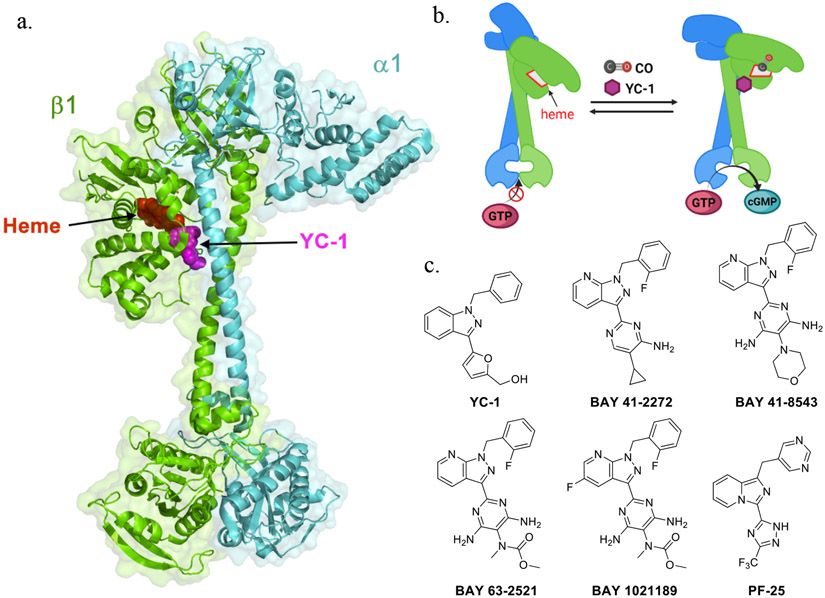
Small molecules such as YC-1,[128] BAY 41-2272,[129] BAY 41-8543[130], BAY 63-2521 (riociguat),[131] BAY 1021189 (vericiguat),[132] and PF-25[133] (Fig. 4) have been discovered to significantly potentiate sGC activity. Specifically, YC-1 was found to bind to the sGC-CO complex by altering the heme geometry and by weakening the positive trans effect to facilitate Fe-His dissociation.[132, 134] YC-1 alone is a moderate stimulator that can boost sGC activity by 12-fold.[49] In the presence of YC-1 at 100 μM, CO’s stimulatory effect (using CO solution) on bovine sGC has been reported to reach a similar maximum magnitude as NO.[135] BAY 41-2272 and PF-25 have been found to increase CO’s binding affinity to that of sGC and vice versa.[132, 135] In the presence of BAY 41-2272, the dissociation rate constant (kd) of CO towards bovine sGC was reported to decrease to 4.6 ± 0.7 s−1, which is significantly lower than the one without the stimulator (9.0 ± 0.8 s−1).[129] As a result, the Kd was calculated to be about 100 μM. However, binding of BAY 41-2272 had little effect on the association rate constant for CO (4.35 × 104 M−1 s−1 after binding and 3.26 × 104 M−1 s−1 before binding), suggesting the stabilization effect of the compound on CO-bound sGC being through an allosteric effect. The affinity of sGC for these stimulators is also drastically augmented by CO. For example, YC-1 binds sGC with a Kd of 9–21 μM in the absence of CO and 0.6–1.1 μM in the presence of CO. Some sGC stimulators with good drug-like properties have been studied in clinical trials for various indications in treating vascular disease.[136, 137] Through many years of research to understand the sensitization mechanisms of these stimulators,[129, 132, 138-145] it was recently confirmed with high resolution cryoEM that YC-1 and riociguat bind to the cleft between the N- and C-terminal subdomains of the β1 H-NOX and middle CC domain (Fig 3a).[146] The YC-1 binding site is adjacent to the heme pocket further stabilizing the extended conformation for maximum catalysis activity (Fig 4a,b). Although how the YC-1-family stimulators bind to the sGC-CO complex has not been resolved yet, it is reasonable to assume that they adopt a similar binding mode to activate sGC.[129] The cryoEM studies show that the YC-1 binding site is adjacent to the heme binding pocket, indicating YC-1 binding might perturb the heme environment. This assessment is consistent with results from earlier spectroscopy studies showing that YC-1 shifts the Soret peak position of sGC-CO complex, indicating Fe-His dissociation of sGC-CO complex through YC-1 binding.[27]
Fig. 4.
Allosteric stimulators of sGC. (a) Binding model of YC-1 in activated sGC (based on PDB: 7d9s); (b) Schematic view of the conformational changes induced by CO and YC-1; (c) Reported sGC stimulators.
The discovery of synthetic allosteric regulators of sGC raises the intriguing question of whether there are yet to be identified endogenous regulators, which could play a role in binding of sGC with CO or other molecules. It is our belief that if nature has built sGC with a “regulatory” pocket, it would make sense for nature to have used it for regulatory purposes. Only future experiments will be able to tell.
5.7. Other possibilities including CO-independent effects from CO donors
There may be other ways of explaining the disconnect between binding constants and sGC activation attributed to CO. For example, ruthenium-based CORMs have been widely used as CO donors with the assumption that their pharmacological functions come directly and solely from CO release. However, there have been a large number of recent reports of CO-independent biological effects for these CORMs.[79, 81-84, 147-156] For example, an early study reported that ruthenium and CORMs (CORM-2 and CORM-3) exerted anti-bacterial activity against E.coli and S. aureus.[157] However, in the same study, the antimicrobial activity of CO gas was significantly weaker than the CORMs, despite being at a higher concentration (saturated in the culture medium) compared to the CORMs (250 - 500 μM) used. Further, antimicrobial activities were not observed in other studies using CO gas[158] or metal-free CO prodrugs.[159] Years later, it was found that the antimicrobial activity of CORM-2 should largely be attributed to ruthenium toxicity but not CO per se.[82] Further, Van de Voorde described the different effects of CO from CORM-2 and CO gas in vasodilation.[160, 161] Then there is the question as to how to figure the CO-independent effects into the assessments of activities attributed to CO binding to sGC when such metal-based CORMs are used. Furthermore, there have also been reports of chemical reactivities for these ruthenium-based CORMs under physiological conditions.[78-84] After years of acknowledging the roles of these CORMs solely as CO donors, many pharmacological functions are eventually attributed to the Ru metal.[81, 83, 148-151] Aside from the ruthenium carbonyl complex CO donors, a boranecarboxyl derivative, CORM-A1 also showed activities different from using CO gas. Specifically, Kaczara et al. showed CO gas (60% saturated buffer) inhibited platelet aggregation by about 37% while CORM-A1 (30 μM) suppressed aggregation by about 65%.[62] Interestingly, an sGC inhibitor ODQ (10 μM) blocked platelet inhibitory effect by CO gas but not by CORM-A1. Myoglobin assay showed that CO delivery from 30 μM CORM-A1 was less than 25% of that from CO gas (60%) saturated medium. Energy metabolism assessed using a Seahorse also showed that CO gas did not affect platelet oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) while CORM-A1 at 30 μM and 300 μM concentrations significantly reduced OCR and increased ECAR of human platelet. These differences clearly showed CO gas and CORM-A1 inhibited platelet aggregation by distinct mechanisms, in which sGC activation was unlikely to be the common mechanism of action.[62] Considering the ability for CORM-A1 to consume ROS such as free radicals and hydrogen peroxide,[80] to inhibit oxidative phosphorylation, and to deplete NAD+,[67] it seems CORM-A1 also endows CO-independent activities potentially due to its chemical reactivity. More experimental work may be needed to truly understand the implications, if any, of these new findings in the interpretation of the above results related to sGC and such metal-based CORMs. It should be noted that the need to consider the effects of the “donor” molecules after CO release is not limited to metal-based CORMs. For organic CO prodrugs, the same issue also needs to be examined. For example, some “carrier” portion of organic CO prodrugs or the prodrug itself have cytotoxicity at high concentrations.[162-164] Thus, such effects need to be considered in designing experiments and interpreting results.
Overall, the question of whether sGC is a key target for NO and CO to intersect under a given set of conditions still needs much more work. On a related note, the effect of CO on vasodilation is supported by results from many studies, though the proposed mechanism(s) of actions go beyond sGC and may include modulation of ROS-related responses, ion channels, p-450 actions, and expression of endothelin and growth factors.[7, 57, 58, 68, 85-89, 160, 161, 165-169]
5. Conclusions
With the above examples and analyses, several points are clear. First sGC is a very important target, and some of observed pharmacological effects of CO can be explained through sGC activation. Second, in providing such an explanation, the binding constant issue and biologically relevant CO concentration issue do not always agree with the proposed involvement of sGC as the sole mechanism, especially for in-vivo actions. Third, there have been other targets proposed that can help the explanation including indirect actions. Fourth, the issue of an endogenous allosteric regulator is very intriguing. It would make sense to have such an endogenous regulator(s), because exploiting the existing regulatory pocket demonstrated by synthetic compounds would offer nature an efficient way of regulating sGC’s functions. If such endogenous regulators do exist, they would help to explain some of the discrepancies observed. However, none has been identified. Fifth, future studies will need to look at sGC activation by CO in the context of its binding affinity, biologically relevant concentrations of CO, dose-response relationships, and the competition for CO binding among different hemoproteins. With these points, we hope this review will help stimulate future discussions and interests in CO-related work including molecular mechanism(s) of actions.
Acknowledgment
CO-related work in the authors’ lab has been supported by the National Institutes of Health (R01DK119202), the Georgia Research Alliance through an Eminent Scholar (BW) endowment, and other and much more work is needed internal resources. Figures were Created with BioRender.com.
References
- [1].Sjostrand T, Endogenous formation of carbon monoxide in man, Nature 164 (1949) 580. [DOI] [PubMed] [Google Scholar]
- [2].Engstedt L, Endogenous formation of carbon monoxide in hemolytic disease; with special regard to quantitative comparisons to other hemolytic indices, Acta Med Scand Suppl 332 (1957) 1–63. [PubMed] [Google Scholar]
- [3].Gydell K, Transient effect of nicotinic acid on bilirubin metabolism and formation of carbon monoxide, Acta Med Scand 167 (1960) 431–41. [DOI] [PubMed] [Google Scholar]
- [4].Coburn RF, Blakemore WS, Forster RE, Endogenous carbon monoxide production in man, J Clin Invest 42(7) (1963) 1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ludwig GD, Blakemore WS, Drabkin DL, Production of carbon monoxide by hemin oxidation, J. Clin. Invest 36 (1957) 912. [Google Scholar]
- [6].Yang X, Lu W, Hopper CP, Ke B, Wang B, Nature’s marvels endowed in gaseous molecules I: carbon monoxide and its physiological and therapeutic roles, Acta Pharm Sin B 11 (2021) 1434–1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wang R, Resurgence of carbon monoxide: an endogenous gaseous vasorelaxing factor, Can J Physiol Pharmacol 76(1) (1998) 1–15. [DOI] [PubMed] [Google Scholar]
- [8].Motterlini R, Otterbein LE, The therapeutic potential of carbon monoxide, Nat Rev Drug Discov 9 (2010) 728–743 and references cited therein. [DOI] [PubMed] [Google Scholar]
- [9].Wang R, Wang Z, Wu L, Carbon monoxide-induced vasorelaxation and the underlying mechanisms, Br J Pharmacol 121(5) (1997) 927–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ndisang JF, Tabien HE, Wang R, Carbon monoxide and hypertension, J Hypertens 22(6) (2004) 1057–1074. [DOI] [PubMed] [Google Scholar]
- [11].Wang B, Otterbein LE, Carbon monoxide in drug discovery: basics, pharmacology, and therapeutic potential, in: Wang B (Ed.) Wiley Series in Drug Discovery and Development, John Wiley and Sons, Hoboken, New Jersey, 2022, p. 608. [Google Scholar]
- [12].Yang X, Wang M, Tan C, Lu W, Wang B, Pharmacokinetics of Carbon Monoxide, in: Wang B, Otterbein LE (Eds.), Carbon Monoxide in Drug Discovery: Basics, Pharmacology, and Therapeutic Potential, John Wiley and Sons, Hoboken, New Jersey, 2022. [Google Scholar]
- [13].Rose EJ, Venkatasubramanian PN, Swartz JC, Jones RD, Basolo F, Hoffman BM, Carbon monoxide binding kinetics in "capped" porphyrin compounds, Proc Natl Acad Sci USA 79 (1982) 5742–5745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Collman JP, Brauman JI, Doxsee KM, Carbon monoxide binding to iron porphyrins, Proc Natl Acad Sci USA 76 (1979) 6035–6039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Collman JP, Brauman JI, Halbert TR, Suslick KS, Nature of O2 and CO binding to metalloporphyrins and heme proteins, Proc Natl Acad Sci USA 73 (1976) 3333–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Tsai AL, Berka V, Martin E, Olson JS, A "sliding scale rule" for selectivity among NO, CO, and O2 by heme protein sensors, Biochemistry 51 (2012) 172–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang X, Lu W, Wang M, Tan C, Wang B, “CO in a pill”: Towards oral delivery of carbon monoxide for therapeutic applications, J. Controlled release 338 (2021) 593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Levitt DG, Levitt MD, Carbon monoxide: a critical quantitative analysis and review of the extent and limitations of its second messenger function, Clin Pharmacol 7 (2015) 37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].De La Cruz LK, Wang B, Carbon Monoxide Production: In Health and in Sickness, in: Wang B, Otterbein LE (Eds.), Carbon Monoxide in Drug Discovery: Basics, Pharmacology, and Therapeutic Potential, John Wiley and Sons, Hoboken, New Jersey, 2022. [Google Scholar]
- [20].Hartsfield CL, Cross talk between carbon monoxide and nitric oxide, Antioxid Redox Signal 4 (2002) 301–7. [DOI] [PubMed] [Google Scholar]
- [21].Ingi T, Cheng J, Ronnett GV, Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system, Neuron 16 (1996) 835–42. [DOI] [PubMed] [Google Scholar]
- [22].Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M, Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology, Antioxid Redox Signal 13 (2010) 157–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Montfort WR, Wales JA, Weichsel A, Structure and activation of soluble guanylyl cyclase, the nitric oxide sensor, Antioxid Redox Signal 26 (2017) 107–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Ryter SW, Otterbein LE, Carbon monoxide in biology and medicine, Bioessays 26 (2004) 270–80. [DOI] [PubMed] [Google Scholar]
- [25].Dal-Secco D, Freitas A, Abreu MA, Garlet TP, Rossi MA, Ferreira SH, Silva JS, Alves-Filho JC, Cunha FQ, Reduction of ICAM-1 expression by carbon monoxide via soluble guanylate cyclase activation accounts for modulation of neutrophil migration, Naunyn Schmiedebergs Arch Pharmacol 381 (2010) 483–493. [DOI] [PubMed] [Google Scholar]
- [26].VanUffelen BE, de Koster BM, VanSteveninck J, Elferink JG, Carbon monoxide enhances human neutrophil migration in a cyclic GMP-dependent way, Biochem Biophys Res Commun 226(1) (1996) 21–6. [DOI] [PubMed] [Google Scholar]
- [27].Kharitonov VG, Sharma VS, Magde D, Koesling D, Kinetics and equilibria of soluble guanylate cyclase ligation by CO: effect of YC-1, Biochemistry 38(33) (1999) 10699–10706. [DOI] [PubMed] [Google Scholar]
- [28].Kharitonov VG, Sharma VS, Pilz RB, Magde D, Koesling D, Basis of guanylate cyclase activation by carbon monoxide, Proc Natl Acad Sci USA 92 (1995) 2568–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hofmann F, The cGMP system: components and function, Biol Chem 401 (2020) 447–469. [DOI] [PubMed] [Google Scholar]
- [30].Martin E, Berka V, Bogatenkova E, Murad F, Tsai A-L, Ligand selectivity of soluble guanylyl cyclase: effect of the hydrogen-bonding tyrosine in the distal heme pocket on binding of oxygen, nitric oxide, and carbon monoxide, J Biol Chem 281(38) (2006) 27836–27845. [DOI] [PubMed] [Google Scholar]
- [31].Ma X, Sayed N, Beuve A, van den Akker F, NO and CO differentially activate soluble guanylyl cyclase via a heme pivot-bend mechanism, EMBO J 26(2) (2007) 578–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Derbyshire ER, Marletta MA, Structure and regulation of soluble guanylate cyclase, Annu Rev Biochem 81 (2012) 533–59. [DOI] [PubMed] [Google Scholar]
- [33].Koglin M, Vehse K, Budaeus L, Scholz H, Behrends S, Nitric oxide activates the beta 2 subunit of soluble guanylyl cyclase in the absence of a second subunit, J Biol Chem 276(33) (2001) 30737–30743. [DOI] [PubMed] [Google Scholar]
- [34].Priviero FB, Webb RC, Heme-dependent and independent soluble guanylate cyclase activators and vasodilation, J Cardiovasc Pharmacol 56(3) (2010) 229–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Denninger JW, Marletta MA, Guanylate cyclase and the .NO/cGMP signaling pathway, Biochim Biophys Acta 1411(2-3) (1999) 334–50. [DOI] [PubMed] [Google Scholar]
- [36].Hoffmann LS, Kretschmer A, Lawrenz B, Hocher B, Stasch JP, Chronic activation of heme free guanylate cyclase leads to renal protection in Dahl salt-sensitive rats, PLoS One 10(12) (2015) e0145048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Friebe A, Sandner P, Schmidtko A, cGMP: a unique 2nd messenger molecule - recent developments in cGMP research and development, Naunyn Schmiedebergs Arch Pharmacol 393(2) (2020) 287–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen Y, Burnett JC, Particulate guanylyl cyclase A/cGMP signaling pathway in the kidney: physiologic and therapeutic indications, Int J Mol Sci 19(4) (2018) 1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Yang X, de Caestecker M, Otterbein LE, Wang B, Carbon monoxide: an emerging therapy for acute kidney injury, Med Res Rev 40 (2020) 1147–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ingi T, Cheng J, Ronnett GV, Carbon monoxide: an endogenous modulator of the nitric oxide-cyclic GMP signaling system, Neuron 16(4) (1996) 835–42. [DOI] [PubMed] [Google Scholar]
- [41].Feil R, Kemp-Harper B, cGMP signalling: from bench to bedside. Conference on cGMP generators, effectors and therapeutic implications, EMBO Rep 7(2) (2006) 149–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhao Y, Brandish PE, Ballou DP, Marletta MA, A molecular basis for nitric oxide sensing by soluble guanylate cyclase, Proc Natl Acad Sci USA 96(26) (1999) 14753–14758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Martin E, Berka V, Sharina I, Tsai AL, Mechanism of binding of NO to soluble guanylyl cyclase: implication for the second NO binding to the heme proximal site, Biochemistry 51(13) (2012) 2737–2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Fernhoff NB, Derbyshire ER, Marletta MA, A nitric oxide/cysteine interaction mediates the activation of soluble guanylate cyclase, Proc Natl Acad Sci USA 106(51) (2009) 21602–21607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Cary SPL, Winger JA, Marletta MA, Tonic and acute nitric oxide signaling through soluble guanylate cyclase is mediated by nonheme nitric oxide, ATP, and GTP, Proc Natl Acad Sci USA 102(37) (2005) 13064–13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Horst BG, Marletta MA, Physiological activation and deactivation of soluble guanylate cyclase, Nitric Oxide 77 (2018) 65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Kang Y, Liu R, Wu JX, Chen L, Structural insights into the mechanism of human soluble guanylate cyclase, Nature 574(7777) (2019) 206–210. [DOI] [PubMed] [Google Scholar]
- [48].Stone JR, Marletta MA, Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states, Biochemistry 33(18) (1994) 5636–5640. [DOI] [PubMed] [Google Scholar]
- [49].Friebe A, Schultz G, Koesling D, Sensitizing soluble guanylyl cyclase to become a highly CO-sensitive enzyme, EMBO J 15(24) (1996) 6863–6868. [PMC free article] [PubMed] [Google Scholar]
- [50].Brune B, Ullrich V, Inhibition of platelet aggregation by carbon monoxide is mediated by activation of guanylate cyclase, Mol Pharmacol 32(4) (1987) 497–504. [PubMed] [Google Scholar]
- [51].Furchgott RF, Jothianandan D, Endothelium-dependent and -independent vasodilation involving cyclic GMP: relaxation induced by nitric oxide, carbon monoxide and light, Blood Vessels 28(1-3) (1991) 52–61. [DOI] [PubMed] [Google Scholar]
- [52].Verma A, Hirsch DJ, Glatt CE, Ronnett GV, Snyder SH, Carbon monoxide: a putative neural messenger, Science 259(5093) (1993) 381–4. [DOI] [PubMed] [Google Scholar]
- [53].Kharitonov VG, Sharma VS, Pilz RB, Magde D, Koesling D, Basis of guanylate cyclase activation by carbon monoxide, Proc Natl Acad Sci U S A 92(7) (1995) 2568–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].White DK, Cannon JB, Traylor T, A kinetic model for R-and T-state hemoglobin. Flash photolysis of heme-imidazole-carbon monoxide mixtures, J Am Chem Soc 101(9) (1979) 2443–2454. [Google Scholar]
- [55].Burstyn JN, Yu AE, Dierks EA, Hawkins BK, Dawson JH, Studies of the heme coordination and ligand binding properties of soluble guanylyl cyclase (sGC): characterization of Fe(II)sGC and Fe(II)sGC(CO) by electronic absorption and magnetic circular dichroism spectroscopies and failure of CO to activate the enzyme, Biochemistry 34 (1995) 5896–5903. [DOI] [PubMed] [Google Scholar]
- [56].Deinum G, Stone JR, Babcock GT, Marletta MA, Binding of nitric oxide and carbon monoxide to soluble guanylate cyclase as observed with Resonance raman spectroscopy, Biochemistry 35 (1996) 1540–1547. [DOI] [PubMed] [Google Scholar]
- [57].Rich A, Farrugia G, Rae JL, Carbon monoxide stimulates a potassium-selective current in rabbit corneal epithelial cells, Am J Physiol 267(2 Pt 1) (1994) C435–C442. [DOI] [PubMed] [Google Scholar]
- [58].Suematsu M, Goda N, Sano T, Kashiwagi S, Egawa T, Shinoda Y, Ishimura Y, Carbon monoxide: an endogenous modulator of sinusoidal tone in the perfused rat liver, J Clin Invest 96(5) (1995) 2431–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Otterbein LE, Bach FH, Alam J, Soares M, Tao Lu H, Wysk M, Davis RJ, Flavell RA, Choi AM, Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway, Nat Med 6(4) (2000) 422–8. [DOI] [PubMed] [Google Scholar]
- [60].Bainbridge SA, Farley AE, McLaughlin BE, Graham CH, Marks GS, Nakatsu K, Brien JF, Smith GN, Carbon monoxide decreases perfusion pressure in isolated human placenta, Placenta 23(8-9) (2002) 563–9. [DOI] [PubMed] [Google Scholar]
- [61].Abramochkin DV, Konovalova OP, Kamkin A, Sitdikova GF, Carbon monoxide modulates electrical activity of murine myocardium via cGMP-dependent mechanisms, J Physiol Biochem 71(1) (2015) 107–19. [DOI] [PubMed] [Google Scholar]
- [62].Kaczara P, Przyborowski K, Mohaissen T, Chlopicki S, Distinct pharmacological properties of gaseous CO and CO-releasing molecule in human platelets, Int J Mol Sci 22(7) (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Motterlini R, Clark JE, Foresti R, Sarathchandra P, Mann BE, Green CJ, Carbon monoxide-releasing molecules, Circ Res 90(2) (2002) e17–e24. [DOI] [PubMed] [Google Scholar]
- [64].Zhang LM, Zhang DX, Fu L, Li Y, Wang XP, Qi MM, Li CC, Song PP, Wang XD, Kong XJ, Carbon monoxide-releasing molecule-3 protects against cortical pyroptosis induced by hemorrhagic shock and resuscitation via mitochondrial regulation, Free Radic Biol Med 141 (2019) 299–309. [DOI] [PubMed] [Google Scholar]
- [65].Magierowska K, Korbut E, Hubalewska-Mazgaj M, Surmiak M, Chmura A, Bakalarz D, Buszewicz G, Wojcik D, Sliwowski Z, Ginter G, Gromowski T, Kwiecien S, Brzozowski T, Magierowski M, Oxidative gastric mucosal damage induced by ischemia/reperfusion and the mechanisms of its prevention by carbon monoxide-releasing tricarbonyldichlororuthenium (II) dimer, Free Radic Biol Med 145 (2019) 198–208. [DOI] [PubMed] [Google Scholar]
- [66].Ren Y, D'Ambrosio MA, Wang H, Falck JR, Peterson EL, Garvin JL, Carretero OA, Mechanisms of carbon monoxide attenuation of tubuloglomerular feedback, Hypertension 59(6) (2012) 1139–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Motterlini R, Sawle P, Hammad J, Bains S, Alberto R, Foresti R, Green CJ, CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule, FASEB J 19(2) (2005) 284–6. [DOI] [PubMed] [Google Scholar]
- [68].Koneru P, Leffler CW, Role of cGMP in carbon monoxide-induced cerebral vasodilation in piglets, Am J Physiol Heart Circ Physiol 286(1) (2004) H304–309. [DOI] [PubMed] [Google Scholar]
- [69].Wang XP, Zheng WC, Bai Y, Li Y, Xin Y, Wang JZ, Chang YL, Zhang LM, Carbon monoxide-releasing molecule-3 alleviates Kupffer cell pyroptosis induced by hemorrhagic shock and resuscitation via sGC-cGMP signal pathway, Inflammation 44(4) (2021) 1330–1344. [DOI] [PubMed] [Google Scholar]
- [70].Schallner N, Romao CC, Biermann J, Lagreze WA, Otterbein LE, Buerkle H, Loop T, Goebel U, Carbon monoxide abrogates ischemic insult to neuronal cells via the soluble guanylate cyclase-cGMP pathway, PLoS One 8(4) (2013) e60672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Ulbrich F, Hagmann C, Buerkle H, Romao CC, Schallner N, Goebel U, Biermann J, The Carbon monoxide releasing molecule ALF-186 mediates anti-inflammatory and neuroprotective effects via the soluble guanylate cyclase ss1 in rats' retinal ganglion cells after ischemia and reperfusion injury, J Neuroinflammation 14(1) (2017) 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Costa NR, Silva RO, Nicolau LA, Lucetti LT, Santana AP, Aragão KS, Soares PM, Ribeiro RA, Souza MH, Barbosa AL, Medeiros JV, Role of soluble guanylate cyclase activation in the gastroprotective effect of the HO-1/CO pathway against alendronate-induced gastric damage in rats, Eur J Pharmacol 700 (2013) 51–59. [DOI] [PubMed] [Google Scholar]
- [73].Magierowska K, Magierowski M, Hubalewska-Mazgaj M, Adamski J, Surmiak M, Sliwowski Z, Kwiecien S, Brzozowski T, Carbon Monoxide (CO) Released from Tricarbonyldichlororuthenium (II) Dimer (CORM-2) in Gastroprotection against Experimental Ethanol-Induced Gastric Damage, PLoS One 10 (2015) e0140493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bakalarz D, Surmiak M, Yang X, Wójcik D, Korbut E, Śliwowski Z, Ginter G, Buszewicz G, Brzozowski T, Cieszkowski J, Głowacka U, Magierowska K, Pan Z, Wang B, Magierowski M, Organic carbon monoxide prodrug, BW-CO-111, in protection against chemically-induced gastric mucosal damage, Acta Pharm Sin B 11 (2021) 456–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Morita T, Perrella MA, Lee ME, Kourembanas S, Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP, Proc Natl Acad Sci USA 92 (1995) 1475–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Feelisch M, Kotsonis P, Siebe J, Clement B, Schmidt HH, The soluble guanylyl cyclase inhibitor 1H-[1,2,4]oxadiazolo[4,3,-a] quinoxalin-1-one is a nonselective heme protein inhibitor of nitric oxide synthase and other cytochrome P-450 enzymes involved in nitric oxide donor bioactivation, Mol Pharmacol 56(2) (1999) 243–53. [DOI] [PubMed] [Google Scholar]
- [77].Kontos HA, Wei EP, Hydroxyl radical-dependent inactivation of guanylate cyclase in cerebral arterioles by methylene blue and by LY83583, Stroke 24(3) (1993) 427–34. [DOI] [PubMed] [Google Scholar]
- [78].Yuan Z, Yang X, Ye Y, Tripathi R, Wang B, Chemical reactivities of two widely used ruthenium-based CO-releasing molecules with a range of biologically important reagents and molecules, Anal. Chem 93(12) (2021) 5317–5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Yuan Z, Yang X, De La Cruz LKC, Wang B, Nitro reduction-based fluorescent probes for carbon monoxide require reactivity involving a ruthenium carbonyl moiety, Chem Commun 56(14) (2020) 2190–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Yuan Z, Yang X, Wang B, Redox and Catalase-like Activities of Four Widely Used Carbon Monoxide Releasing Molecules (CO-RMs), Chem Sci 12 (2021) 13013–13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Southam HM, Smith TW, Lyon RL, Liao C, Trevitt CR, Middlemiss LA, Cox FL, Chapman JA, El-Khamisy SF, Hippler M, Williamson MP, Henderson PJF, Poole RK, A thiol-reactive Ru(II) ion, not CO release, underlies the potent antimicrobial and cytotoxic properties of CO-releasing molecule-3, Redox Biol 18 (2018) 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Southam HM, Williamson MP, Chapman JA, Lyon RL, Trevitt CR, Henderson PJF, Poole RK, ‘Carbon-Monoxide-Releasing Molecule-2 (CORM-2)’ is a misnomer: ruthenium toxicity, not CO release, accounts for its antimicrobial effects, Antioxidants 10(6) (2021) 915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Nielsen VG, The anticoagulant effect of Apis mellifera phospholipase A(2) is inhibited by CORM-2 via a carbon monoxide-independent mechanism, J Thromb Thrombolysis 49 (2020) 100–107. [DOI] [PubMed] [Google Scholar]
- [84].Gessner G, Sahoo N, Swain SM, Hirth G, Schönherr R, Mede R, Westerhausen M, Brewitz HH, Heimer P, Imhof D, Hoshi T, Heinemann SH, CO-independent modification of K(+) channels by tricarbonyldichlororuthenium(II) dimer (CORM-2), Eur J Pharmacol 815 (2017) 33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Morita T, Kourembanas S, Endothelial cell expression of vasoconstrictors and growth factors is regulated by smooth muscle cell-derived carbon monoxide, J Clin Invest 96 (1995) 2676–2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Jaggar JH, Leffler CW, Cheranov SY, Tcheranova D, E S, Cheng X, Carbon monoxide dilates cerebral arterioles by enhancing the coupling of Ca2+ sparks to Ca2+-activated K+ channels, Circ Res 91 (2002) 610–617. [DOI] [PubMed] [Google Scholar]
- [87].Wang R, Wu L, The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells, J Biol Chem 272 (1997) 8222–8226. [DOI] [PubMed] [Google Scholar]
- [88].Rattan S, Chakder S, Inhibitory effect of CO on internal anal sphincter: heme oxygenase inhibitor inhibits NANC relaxation, Am J Physiol 265 (1993) G799–804. [DOI] [PubMed] [Google Scholar]
- [89].Coceani F, Control of the ductus arteriosus--a new function for cytochrome P450, endothelin and nitric oxide, Biochem Pharmacol 48 (1994) 1315–1318. [DOI] [PubMed] [Google Scholar]
- [90].Kajimura M, Shimoyama M, Tsuyama S, Suzuki T, Kozaki S, Takenaka S, Tsubota K, Oguchi Y, Suematsu M, Visualization of gaseous monoxide reception by soluble guanylate cyclase in the rat retina, FASEB J 17(3) (2003) 506–8. [DOI] [PubMed] [Google Scholar]
- [91].Ishikawa M, Kajimura M, Adachi T, Maruyama K, Makino N, Goda N, Yamaguchi T, Sekizuka E, Suematsu M, Carbon monoxide from heme oxygenase-2 Is a tonic regulator against NO-dependent vasodilatation in the adult rat cerebral microcirculation, Circ Res 97(12) (2005) e104–e114. [DOI] [PubMed] [Google Scholar]
- [92].Hernandez-Viadel M, Castoldi AF, Coccini T, Manzo L, Erceg S, Felipo V, In vivo exposure to carbon monoxide causes delayed impairment of activation of soluble guanylate cyclase by nitric oxide in rat brain cortex and cerebellum, J Neurochem 89(5) (2004) 1157–1165. [DOI] [PubMed] [Google Scholar]
- [93].Li A, Xi Q, Umstot ES, Bellner L, Schwartzman ML, Jaggar JH, Leffler CW, Astrocyte-derived CO is a diffusible messenger that mediates glutamate-induced cerebral arteriolar dilation by activating smooth muscle Cell KCa channels, Circ Res 102 (2008) 234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Kajimura M, Goda N, Suematsu M, Organ design for generation and reception of CO: lessons from the liver, Antioxid Redox Signal 4(4) (2002) 633–7. [DOI] [PubMed] [Google Scholar]
- [95].Bohlen HG, Nase GP, Dependence of intestinal arteriolar regulation on flow-mediated nitric oxide formation, Am J Physiol Heart Circ Physiol 279(5) (2000) H2249–2258. [DOI] [PubMed] [Google Scholar]
- [96].Vogel KM, Hu S, Spiro TG, Dierks EA, Yu AE, Burstyn JN, Variable forms of soluble guanylyl cyclase: protein-ligand interactions and the issue of activation by carbon monoxide, J Biol Inorg Chem 4(6) (1999) 804–13. [DOI] [PubMed] [Google Scholar]
- [97].Sato H, Nomura S, Sagami I, Ito O, Daff S, Shimizu T, CO binding studies of nitric oxide synthase: effects of the substrate, inhibitors and tetrahydrobiopterin, FEBS Letters 430(3) (1998) 377–380. [DOI] [PubMed] [Google Scholar]
- [98].Scheele JS, Kharitonov VG, Martasek P, Roman LJ, Sharma VS, Masters BS, Magde D, Kinetics of CO ligation with nitric-oxide synthase by flash photolysis and stopped-flow spectrophotometry, J Biol Chem 272(19) (1997) 12523–12528. [DOI] [PubMed] [Google Scholar]
- [99].Bryant AJ, Ebrahimi E, Nguyen A, Wolff CA, Gumz ML, Liu AC, Esser KA, A wrinkle in time: circadian biology in pulmonary vascular health and disease, Am J Physiol Lung Cell Mol Physiol 322(1) (2022) L84–L101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kung TA, Egbejimi O, Cui J, Ha NP, Durgan DJ, Essop MF, Bray MS, Shaw CA, Hardin PE, Stanley WC, Young ME, Rapid attenuation of circadian clock gene oscillations in the rat heart following ischemia-reperfusion, J Mol Cell Cardiol 43(6) (2007) 744–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Eckle T, Hartmann K, Bonney S, Reithel S, Mittelbronn M, Walker LA, Lowes BD, Han J, Borchers CH, Buttrick PM, Kominsky DJ, Colgan SP, Eltzschig HK, Adora2b-elicited Per2 stabilization promotes a HIF-dependent metabolic switch crucial for myocardial adaptation to ischemia, Nat Med 18(5) (2012) 774–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Viswambharan H, Carvas JM, Antic V, Marecic A, Jud C, Zaugg CE, Ming XF, Montani JP, Albrecht U, Yang Z, Mutation of the circadian clock gene Per2 alters vascular endothelial function, Circulation 115(16) (2007) 2188–2195. [DOI] [PubMed] [Google Scholar]
- [103].Correa-Costa M, Gallo D, Csizmadia E, Gomperts E, Lieberum JL, Hauser CJ, Ji X, Wang B, Camara NOS, Robson SC, Otterbein LE, Carbon monoxide protects the kidney through the central circadian clock and CD39, Proc Natl Acad Sci USA 115(10) (2018) E2302–E2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Taguchi S, Matsui T, Igarashi J, Sasakura Y, Araki Y, Ito O, Sugiyama S, Sagami I, Shimizu T, Binding of oxygen and carbon monoxide to a heme-regulated phosphodiesterase from Escherichia coli. Kinetics and infrared spectra of the full-length wild-type enzyme, isolated PAS domain, and Met-95 mutants, J Biol Chem 279(5) (2004) 3340–3347. [DOI] [PubMed] [Google Scholar]
- [105].Shimizu T, The heme-based oxygen-sensor phosphodiesterase Ec DOS (DosP): structure-function relationships, Biosensors (Basel) 3(2) (2013) 211–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Hopper CP, De La Cruz LK, Lyles KV, Wareham LK, Gilbert JA, Eichenbaum Z, Magierowski M, Poole RK, Wollborn J, Wang B, Role of Carbon Monoxide in Host-Gut Microbiome Communication, Chem Rev 120 (2020) 13273–13311. [DOI] [PubMed] [Google Scholar]
- [107].Levin BC, Paabo M, Gurman JL, Harris SE, Braun E, Toxicological interactions between carbon monoxide and carbon dioxide, Toxicology 47(1-2) (1987) 135–64. [DOI] [PubMed] [Google Scholar]
- [108].Vreman HJ, Wong RJ, Kadotani T, Stevenson DK, Determination of carbon monoxide (CO) in rodent tissue: effect of heme administration and environmental CO exposure, Anal Biochem 341(2) (2005) 280–9. [DOI] [PubMed] [Google Scholar]
- [109].Yang X, Lu W, Wang M, Tan C, Wang B, "CO in a pill": Towards oral delivery of carbon monoxide for therapeutic applications, J Control Release 338 (2021) 593–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Vedernikov YP, Graser T, Vanin AF, Similar endothelium-independent arterial relaxation by carbon monoxide and nitric oxide, Biomed Biochim Acta 48(8) (1989) 601–3. [PubMed] [Google Scholar]
- [111].Horst BG, Stewart EM, Nazarian AA, Marletta MA, Characterization of a carbon monoxide-activated soluble guanylate cyclase from chlamydomonas reinhardtii, Biochemistry 58 (2019) 2250–2259. [DOI] [PubMed] [Google Scholar]
- [112].Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M, Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology, Antioxid Redox Signal 13(2) (2010) 157–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Kawahara B, Sen S, Mascharak PK, Reaction of carbon monoxide with cystathionine β-synthase: implications on drug efficacies in cancer chemotherapy, Future Med Chem 12 (2020) 325–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Liu XM, Peyton KJ, Ensenat D, Wang H, Hannink M, Alam J, Durante W, Nitric oxide stimulates heme oxygenase-1 gene transcription via the Nrf2/ARE complex to promote vascular smooth muscle cell survival, Cardiovasc Res 75(2) (2007) 381–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Motterlini R, Green CJ, Foresti R, Regulation of heme oxygenase-1 by redox signals involving nitric oxide, Antioxid Redox Signal 4 (2002) 615–24. [DOI] [PubMed] [Google Scholar]
- [116].Leffler CW, Balabanova L, Fedinec AL, Parfenova H, Nitric oxide increases carbon monoxide production by piglet cerebral microvessels, Am J Physiol Heart Circ Physiol 289 (2005) H1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Hervera A, Leánez S, Negrete R, Motterlini R, Pol O, Carbon monoxide reduces neuropathic pain and spinal microglial activation by inhibiting nitric oxide synthesis in mice, PLoS One 7 (2012) e43693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].White KA, Marletta MA, Nitric oxide synthase is a cytochrome P-450 type hemoprotein, Biochemistry 31 (1992) 6627–6631. [DOI] [PubMed] [Google Scholar]
- [119].Farrugia G, Szurszewski JH, Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract, Gastroenterology 147(2) (2014) 303–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Andreadou I, Iliodromitis EK, Rassaf T, Schulz R, Papapetropoulos A, Ferdinandy P, The role of gasotransmitters NO, H2S and CO in myocardial ischaemia/reperfusion injury and cardioprotection by preconditioning, postconditioning and remote conditioning, Br J Pharmacol 172(6) (2015) 1587–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Wang B, Huang C, Chen L, Xu D, Zheng G, Zhou Y, Wang X, Zhang X, The emerging roles of the gaseous signaling molecules NO, H2S, and CO in the regulation of stem cells, ACS Biomater Sci Eng 6(2) (2020) 798–812. [DOI] [PubMed] [Google Scholar]
- [122].Bianco CL, Toscano JP, Fukuto JM, Chapter 2 - an integrated view of the chemical biology of NO, CO, H2S, and O2, in: Ignarro LJ, Freeman BA (Eds.), Nitric Oxide (Third Edition), Academic Press; 2017, pp. 9–21. [Google Scholar]
- [123].Wang R, Gasotransmitters: growing pains and joys, Trends Biochem Sci 39(5) (2014) 227–32. [DOI] [PubMed] [Google Scholar]
- [124].Thomas DD, Liu X, Kantrow SP, Lancaster JR Jr., The biological lifetime of nitric oxide: implications for the perivascular dynamics of NO and O2, Proc Natl Acad Sci USA 98(1) (2001) 355–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Kadotani T, Vreman HJ, Wong RJ, Stevenson DK, Concentration of Carbon Monoxide (CO) in Tissue, Pediatr Res 45(7) (1999) 67–67. [Google Scholar]
- [126].Minegishi S, Yumura A, Miyoshi H, Negi S, Taketani S, Motterlini R, Foresti R, Kano K, Kitagishi H, Detection and removal of endogenous carbon monoxide by selective and cell-permeable hemoprotein model complexes, J Am Chem Soc 139(16) (2017) 5984–5991. [DOI] [PubMed] [Google Scholar]
- [127].Guo M, Pegoraro AF, Mao A, Zhou EH, Arany PR, Han Y, Burnette DT, Jensen MH, Kasza KE, Moore JR, Mackintosh FC, Fredberg JJ, Mooney DJ, Lippincott-Schwartz J, Weitz DA, Cell volume change through water efflux impacts cell stiffness and stem cell fate, Proc Natl Acad Sci USA 114(41) (2017) E8618–E8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ko FN, Wu CC, Kuo SC, Lee FY, Teng CM, YC-1, a novel activator of platelet guanylate cyclase, Blood 84(12) (1994) 4226–4233. [PubMed] [Google Scholar]
- [129].Makino R, Obata Y, Tsubaki M, Iizuka T, Hamajima Y, Kato-Yamada Y, Mashima K, Shiro Y, Mechanistic insights into the activation of soluble guanylate cyclase by carbon monoxide: a multistep mechanism proposed for the BAY 41-2272 induced formation of 5-coordinate CO-heme, Biochemistry 57 (2018) 1620–1631. [DOI] [PubMed] [Google Scholar]
- [130].Stasch JP, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Minuth T, Perzborn E, Schramm M, Straub A, Pharmacological actions of a novel NO-independent guanylyl cyclase stimulator, BAY 41-8543: in vitro studies, Br J Pharmacol 135(2) (2002) 333–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Mittendorf J, Weigand S, Alonso-Alija C, Bischoff E, Feurer A, Gerisch M, Kern A, Knorr A, Lang D, Muenter K, Radtke M, Schirok H, Schlemmer KH, Stahl E, Straub A, Wunder F, Stasch JP, Discovery of riociguat (BAY 63-2521): a potent, oral stimulator of soluble guanylate cyclase for the treatment of pulmonary hypertension, ChemMedChem 4(5) (2009) 853–65. [DOI] [PubMed] [Google Scholar]
- [132].Purohit R, Fritz BG, The J, Issaian A, Weichsel A, David CL, Campbell E, Hausrath AC, Rassouli-Taylor L, Garcin ED, Gage MJ, Montfort WR, YC-1 binding to the beta subunit of soluble guanylyl cyclase overcomes allosteric inhibition by the alpha subunit, Biochemistry 53(1) (2014) 101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Roberts LR, Bradley PA, Bunnage ME, England KS, Fairman D, Fobian YM, Fox DN, Gymer GE, Heasley SE, Molette J, Smith GL, Schmidt MA, Tones MA, Dack KN, Acidic triazoles as soluble guanylate cyclase stimulators, Bioorg Med Chem Lett 21(21) (2011) 6515–6518. [DOI] [PubMed] [Google Scholar]
- [134].Ibrahim M, Derbyshire ER, Marietta MA, Spiro TG, Probing soluble guanylate cyclase activation by CO and YC-1 using resonance Raman spectroscopy, Biochemistry 49 (2010) 3815–3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Stone JR, Marletta MA, Synergistic activation of soluble guanylate cyclase by YC-1 and carbon monoxide: implications for the role of cleavage of the iron-histidine bond during activation by nitric oxide, Chem Biol 5(5) (1998) 255–61. [DOI] [PubMed] [Google Scholar]
- [136].Xiao S, Li Q, Hu L, Yu Z, Yang J, Chang Q, Chen Z, Hu G, Soluble guanylate cyclase stimulators and activators: where are we and where to go?, Mini Rev Med Chem 19(18) (2019) 1544–1557. [DOI] [PubMed] [Google Scholar]
- [137].Armstrong PW, Roessig L, Patel MJ, Anstrom KJ, Butler J, Voors AA, Lam CSP, Ponikowski P, Temple T, Pieske B, Ezekowitz J, Hernandez AF, Koglin J, O'Connor CM, A multicenter, randomized, double-blind, placebo-controlled trial of the efficacy and safety of the oral soluble guanylate cyclase stimulator: the VICTORIA trial, JACC Heart Fail 6(2) (2018) 96–104. [DOI] [PubMed] [Google Scholar]
- [138].Becker EM, Alonso-Alija C, Apeler H, Gerzer R, Minuth T, Pleiss U, Schmidt P, Schramm M, Schroder H, Schroeder W, Steinke W, Straub A, Stasch JP, NO-independent regulatory site of direct sGC stimulators like YC-1 and BAY 41-2272, BMC Pharmacol 1 (2001) 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Stasch JP, Becker EM, Alonso-Alija C, Apeler H, Dembowsky K, Feurer A, Gerzer R, Minuth T, Perzborn E, Pleiss U, Schroder H, Schroeder W, Stahl E, Steinke W, Straub A, Schramm M, NO-independent regulatory site on soluble guanylate cyclase, Nature 410(6825) (2001) 212–5. [DOI] [PubMed] [Google Scholar]
- [140].Lamothe M, Chang FJ, Balashova N, Shirokov R, Beuve A, Functional characterization of nitric oxide and YC-1 activation of soluble guanylyl cyclase: structural implication for the YC-1 binding site?, Biochemistry 43(11) (2004) 3039–3048. [DOI] [PubMed] [Google Scholar]
- [141].Yazawa S, Tsuchiya H, Hori H, Makino R, Functional characterization of two nucleotide-binding sites in soluble guanylate cyclase, J Biol Chem 281(31) (2006) 21763–21770. [DOI] [PubMed] [Google Scholar]
- [142].Agullo L, Buch I, Gutierrez-de-Teran H, Garcia-Dorado D, Villa-Freixa J, Computational exploration of the binding mode of heme-dependent stimulators into the active catalytic domain of soluble guanylate cyclase, Proteins 84(10) (2016) 1534–1548. [DOI] [PubMed] [Google Scholar]
- [143].Mota F, Allerston CK, Hampden-Smith K, Garthwaite J, Selwood DL, Surface plasmon resonance using the catalytic domain of soluble guanylate cyclase allows the detection of enzyme activators, Bioorg Med Chem Lett 24(4) (2014) 1075–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Denninger JW, Schelvis JP, Brandish PE, Zhao Y, Babcock GT, Marletta MA, Interaction of soluble guanylate cyclase with YC-1: kinetic and resonance Raman studies, Biochemistry 39(14) (2000) 4191–4198. [DOI] [PubMed] [Google Scholar]
- [145].Wales JA, Chen CY, Breci L, Weichsel A, Bernier SG, Sheppeck JE 2nd, Solinga R, Nakai T, Renhowe PA, Jung J, Montfort WR, Discovery of stimulator binding to a conserved pocket in the heme domain of soluble guanylyl cyclase, J Biol Chem 293(5) (2018) 1850–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Liu R, Kang Y, Chen L, Activation mechanism of human soluble guanylate cyclase by stimulators and activators, Nat Commun 12(1) (2021) 5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Santos-Silva T, Mukhopadhyay A, Seixas JD, Bernardes GJ, Romão CC, Romão MJ, Towards improved therapeutic CORMs: understanding the reactivity of CORM-3 with proteins, Curr Med Chem. 18 (2011) 3361–3366. [DOI] [PubMed] [Google Scholar]
- [148].Juszczak M, Kluska M, Wysokiński D, Woźniak K, DNA damage and antioxidant properties of CORM-2 in normal and cancer cells, Sci Rep 10 (2020) 12200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nielsen VG, Wagner MT, Frank N, Mechanisms responsible for the anticoagulant properties of neurotoxic dendroaspis Venoms: a viscoelastic analysis, Int J Mol Sci 21(6) (2020) 2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Nielsen VG, Ruthenium, not carbon monoxide, inhibits the procoagulant activity of atheris, echis, and pseudonaja venoms, Int J Mol Sci 21(8) (2020) 2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Stucki D, Krahl H, Walter M, Steinhausen J, Hommel K, Brenneisen P, Stahl W, Effects of frequently applied carbon monoxide releasing molecules (CORMs) in typical CO-sensitive model systems - A comparative in vitro study, Arch Biochem Biophys 687 (2020) 108383. [DOI] [PubMed] [Google Scholar]
- [152].Rossier J, Delasoie J, Haeni L, Hauser D, Rothen-Rutishauser B, Zobi F, Cytotoxicity of Mn-based photoCORMs of ethynyl-α-diimine ligands against different cancer cell lines: The key role of CO-depleted metal fragments, J Inorg Biochem 209 (2020) 111122. [DOI] [PubMed] [Google Scholar]
- [153].Nobre LS, Jeremias H, Romao CC, Saraiva LM, Examining the antimicrobial activity and toxicity to animal cells of different types of CO-releasing molecules, Dalton Trans 45(4) (2016) 1455–1466. [DOI] [PubMed] [Google Scholar]
- [154].Wareham LK, Poole RK, Tinajero-Trejo M, CO-releasing metal carbonyl compounds as antimicrobial agents in the post-antibiotic era, J. Biol. Chem 290(31) (2015) 18999–19007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Dong DL, Chen C, Huang W, Chen Y, Zhang XL, Li Z, Li Y, Yang BF, Tricarbonyldichlororuthenium (II) dimer (CORM2) activates non-selective cation current in human endothelial cells independently of carbon monoxide releasing, Eur J Pharmacol 590(1-3) (2008) 99–104. [DOI] [PubMed] [Google Scholar]
- [156].Nielsen VG, Garza JI, Comparison of the effects of CORM-2, CORM-3 and CORM-A1 on coagulation in human plasma, Blood Coagul Fibrinolysis 25(8) (2014) 801–5. [DOI] [PubMed] [Google Scholar]
- [157].Nobre LS, Seixas JD, Romão CC, Saraiva LM, Antimicrobial Action of Carbon Monoxide-Releasing Compounds, Antimicrobial Agents and Chemotherapy 51(12) (2007) 4303–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Wegiel B, Larsen R, Gallo D, Chin BY, Harris C, Mannam P, Kaczmarek E, Lee PJ, Zuckerbraun BS, Flavell R, Soares MP, Otterbein LE, Macrophages sense and kill bacteria through carbon monoxide-dependent inflammasome activation, J Clin Invest 124 (2014) 4926–4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].De La Cruz LKC, Benoit SL, Pan Z, Yu B, Maier RJ, Ji X, Wang B, Click, Release, and Fluoresce: A Chemical Strategy for a Cascade Prodrug System for Codelivery of Carbon Monoxide, a Drug Payload, and a Fluorescent Reporter, Org Lett 20 (2018) 897–900. [DOI] [PubMed] [Google Scholar]
- [160].Decaluwé K, Pauwels B, Boydens C, Van de Voorde J, Divergent molecular mechanisms underlay CO- and CORM-2-induced relaxation of corpora cavernosa, J Sex Med 9(9) (2012) 2284–2292. [DOI] [PubMed] [Google Scholar]
- [161].Decaluwé K, Pauwels B, Verpoest S, Van de Voorde J, Divergent mechanisms involved in CO and CORM-2 induced vasorelaxation, Eur J Pharmacol 674(2) (2012) 370–377. [DOI] [PubMed] [Google Scholar]
- [162].Ji X, Wang B, Strategies toward Organic Carbon Monoxide Prodrugs, Acc Chem Res. 51 (2018) 1377–1385. [DOI] [PubMed] [Google Scholar]
- [163].Wang D, Viennois E, Ji K, Damera K, Draganov A, Zheng Y, Dai C, Merlin D, Wang B, A Click-and-Release Approach to CO Prodrugs, Chem Commun 50 (2014) 15890–15893. [DOI] [PubMed] [Google Scholar]
- [164].Kueh JTB, Stanley NJ, Hewitt RJ, Woods LM, Larsen L, Harrison JC, Rennison D, Brimble MA, Sammut IA, Larsen DS, Norborn-2-en-7-ones as physiologically-triggered carbon monoxide-releasing prodrugs, Chem Sci 8 (2017) 5454–5459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Lamon BD, Zhang FF, Puri N, Brodsky SV, Goligorsky MS, Nasjletti A, Dual pathways of carbon monoxide–mediated vasoregulation, Circ. Res 105(8) (2009) 775–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].US-EPA, Pharmacokinetics and mechanisms of action of carbon monoxide, Air Quality Criteria for Carbon Monoxide, Office of Research and Development, Washington, D. C., 2000, pp. 5-1–5-30. [Google Scholar]
- [167].Kaczara P, Przyborowski K, Mohaissen T, Chlopicki S, Distinct Pharmacological Properties of Gaseous CO and CO-Releasing Molecule in Human Platelets, Intl J Mol Sci 22(7) (2021) 3584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Bae H, Kim T, Lim I, Carbon monoxide activates large-conductance calcium-activated potassium channels of human cardiac fibroblasts through various mechanisms, Korean J Physiol Pharmacol 25(3) (2021) 227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Ryan MJ, Jernigan NL, Drummond HA, McLemore GR, Rimoldi JM, Poreddy SR, Gadepalli RSV, Stec DE, Renal vascular responses to CORM-A1 in the mouse, Pharmacol Res 54(1) (2006) 24–29. [DOI] [PubMed] [Google Scholar]