INTRODUCTION
Lung cancer is the leading cause of cancer death among both men and women in the United States. It is also the leading cause of cancer death among men and the second leading cause of cancer death among women worldwide.1 In the United States, it accounts for more cancer deaths than colon, prostate, and breast cancers combined.2 Factors contributing to the lethality and increased mortality are grounded on asymptomatic nature of the disease leading to late detection and increased disease burden at the time of diagnosis. Although recent advances in immunotherapy and other systemic therapies have been encouraging, the 5-year survival for all comers have remained humbling at less than 20%.2
Given the ever-increasing prevalence and incidence of this disease, various national and international societies have implemented lung cancer screening guidelines based on current evidence. Although there have been considerable debate in the literature, these screening strategies have been beneficial in detecting early stage disease in high risk patient populations. The National Lung Screening Trial, one of the sentinel trials in this realm, demonstrated decrease in lung cancer and all-cause mortality in patients ages 55 to 74 with a 30 pack-year smoking history who underwent low-dose helical computed tomography (CT) screening.3 This finding has shaped the US Preventative Services Task Force recommendations for lung cancer screening in high-risk patient populations.4
Increase in surveillance imaging as well as the ubiquitous use of cross-sectional imaging in various aspects of health care have made detection and management of solitary pulmonary nodules (SPN) an increasing clinical problem, especially in high-risk smokers. These patients have as high as 50% prevalence of SPNs.5 Although there are institutional and societal variations in the management of these findings, there is a consensus among clinicians that high-risk patients with evidence of SPN growth on serial imaging or lesions larger than 8 mm should have tissue sampling for accurate diagnosis.6
CLINICAL CHALLENGES
Patients who fit the criteria for tissue sampling of concerning radiographic findings should undergo appropriate evaluation for either a transthoracic needle aspiration, transbronchial needle aspiration, or minimally invasive surgical wedge resection. Video-assisted thoracoscopic surgery (VATS) is particularly useful in cases where nonsurgical biopsy is unavailable, not practical, or nondiagnostic. VATS wedge resection provides a superior tissue sample for appropriate pathologic analysis and is therapeutic in those with early stage disease. Management of SPNs via VATS requires the thoracic surgeon to identify the lesion of interest by visual and tactile cues. This procedure can be challenging in patients with numerous, deep, and small lesions. To ameliorate the challenges posed during lesion detection, various adjuncts, including percutaneous wire placement, dye injection, intraoperative imaging, and intraoperative molecular imaging (IMI) devices, can be used.
If the patient undergoes a diagnostic or therapeutic operation, intraoperative margin assessment and attempts at R0 resection should be the paramount concern because this factor has important staging and adjuvant therapy ramifications. Specifically, patients with microscopic tumor involvement of the resection margin (R1) have a markedly worse prognosis than those with negative microscopic margins (R0).7,8 Currently, there are limited methods of intraoperative margin assessment other than vigorous inspection using vision and palpation. Otherwise, surgeon can elect to evaluate the specimen with frozen section analysis, but this can be time consuming and often impractical. Some of the new emerging IMI techniques can alleviate these concerns and subject the patients to minimal parenchymal loss and minimize the time required for anesthesia.
NONSURGICAL BIOPSY ADJUNCTS
The recent implementation of screening guidelines and frequent use of highly accurate cross-sectional imaging techniques have increased the incidence of SPN detection. An increasing number of these patients are being evaluated by thoracic surgeons on an outpatient basis.1 However, optimal strategies for definitive evaluation of these nodules are often heterogenous because this patient population has multiple medical comorbidities that may preclude certain operative interventions. Although VATS wedge resections can yield both therapeutic and diagnostic results, the requirement of single lung ventilation and anesthesia may not be suitable for all patients. These patients often can benefit from minimally invasive methods for SPN biopsy that use bronchoscopic and transthoracic techniques. When indicated, these procedures are generally preferred to more invasive surgery; however, they have imperfect sensitivity and lack therapeutic potential.2,9
With the advent of ever improving endoscopic technology and increased training available for physicians, the use of bronchoscopy in the evaluation of lung malignancies and masses are becoming more used. The bronchoscopic biopsy techniques are often undertaken under conscious sedation and involve instrumentation of airways under direct visualization. Ideal patients suitable for these interventions include those with large, visible, and central lesions. For those without the visible lesions on direct visualization, can benefit from endobronchial ultrasound-guided transbronchial needle aspiration. The addition of endobronchial ultrasound guidance to conventional bronchoscopic evaluation has been shown to substantially increase the diagnostic sensitivity.10
However, transbronchial tissue sampling may not appropriate for patients with more peripheral lesions. These lesions usually are best served by transthoracic needle biopsy (TTNB) or aspiration. TTNB involves percutaneously passing the needle under CT guidance through the chest wall into the lung nodule. These procedures have considerable risk of postintervention pneumothorax development, reaching as high as 19% on various literature analyses. Certain patient factors, including older age, presence of emphysematous or obstructive pulmonary disease, and active smoking, have shown to be risk factors in pneumothorax development after TTNB. Clinicians should also take into account that TTNB have false-negative rate of more than 20%, where a negative result may be discordant with imaging and provide limited value.11,12
The more invasive VATS provides an accurate modality for assessing primary tumors, mediastinal lymph node involvement, and pleural involvement. Although it requires general anesthesia and carries greater morbidity and mortality than minimally invasive techniques, it is often used when alternative procedures are unable to access the primary tumor or are nondiagnostic. Additionally, VATS provides an opportunity for therapeutic intervention at the time of diagnosis.
PREOPERATIVE NODULE LOCALIZATION
Localization and appropriate oncologic resection are keys for the management of any SPNs, especially for those with marginal pulmonary functions where preservation of the parenchyma is of utmost importance. Many surgeons rely on intraoperative palpation and feel, but recent literature data suggest that these techniques are suboptimal for the intraoperative localization of SPNs.13 The difficulty of these techniques are compounded for VATS resections, which are limited by small port sizes and can require conversion to open thoracotomy in more than one-third of cases.14 However, there are various radiologic, endoscopic, and intraoperative techniques available for the practicing thoracic surgeon for optimal localization and resection of lung nodules. These techniques are discussed elsewhere in this article.
Needle Localization
Needle localization techniques have been the mainstay of lesion localization in breast malignancies for many years with a successful track record. Thistlethwaite and associates in their institutional analysis of more than 250 patients over a decade have found that optimal characteristics of SPNs amenable for needle localization includes those greater than 2 cm from the from the pleural surface, less than 1.2 cm in size, and showing interval enlargement on serial scanning.15 Those patients with a pulmonary artery systolic pressure of greater than 50 mm Hg, a forced expiratory volume in 1 second of less than 0.6 L, and lung nodules near vascular structures should have other techniques used for optimal patient safety.
Successful implementation of needle localization requires close collaboration between the interventional radiologists and the thoracic surgeons. Although the technical aspects of the procedure vary among the institutions, the overall steps remain the same. Common equipment used during the procedure, including the needle types, are shown in Fig. 1. First, patients receive light sedation and scout CT imaging is performed. After localization of the skin entry site, adequate local anesthetic is used before needle insertion. Various needles with built-in hook wires could be then inserted and advanced toward the edge of the nodule. A second needle is then inserted using a similar technique. Once appropriate positions are confirmed, the needles are removed with the wires left in place.
Fig. 1.

Commonly used equipment for needle localization including the hook wire types available. Part A: demonstrates common pre-procedural equipment needed; Part B: demonstrates the common hookwires available for use. (From Kalambo M., Basak E. Dogan, Gary J. Whitman. Step by step: Planning a needle localization procedure. Clinical Imaging 2020;60(1); 100–108.)
The success of needle localization has been well-documented in literature. Mayo and associates16 in their analysis of 69 patients showed 97% success rate of localizing SPNs. Similar localization rates were reported by Thistlethwaite and colleagues15 at the University of California San Diego, with 97% of 253 patients having the SPNs appropriately located preoperatively. Comparable success rates have been reproduced among international studies as well. Summary of studies investigation needle localization is presented in Table 1.
Table 1.
Studies investigating needle localization techniques reporting 70% to 90% success rate in nodule detection
| Technique | First Author | Group | Year | Study Size |
|---|---|---|---|---|
| Microcoil | Powell | Vancouver | 2004 | 12 |
| Microcoil | Mayo | Vancouver | 2009 | 69 |
| Spiral wire | Eichfeld | Leipzig, Germany | 2005 | 22 |
| Hook wire | Miyoshi | Japan | 2009 | 108 |
| Hook wire | Dendo | Okayama, Japan | 2002 | 150 |
| Hook wire | Gonfiotti | Florence, Italy | 2007 | 50 |
The success for optimal lesion detection and patient safety depends on technique and the equipment available to the operator. There are several types of guidewires available. In general, needles with established hooks, which are longer and therefore less easily displaced during repositioning or lung deflation, are preferred. Data from Miyoshi and colleagues17 and Dendo and colleagues18 showed a greater than 90% success rates in using established hook wires. It is thought that the hooks on these wires prevent needle dislodgement. Common complications associated with needle localization include pneumothorax, hemothorax, and dislodgement. These complications are readily managed by conventional postoperative care, including short-term tube thoracostomy, a without significant increase in additional surgical procedures or conversion to open thoracotomy. A literature analysis shows around a 32% risk of postprocedure pneumothorax and a 15% risk of developing a hemothorax.11,15 The majority of study participants across studies did not require additional surgical interventions.
In summary, needle localization, where available, is a safe approach for preoperative nodule localization in those patients without significant pulmonary hypertension whose primary lesions are more than 2 cm deep and are not near major vasculature. Expertise among the operator and interventional radiologist can minimize postprocedural risks. Additionally, needles are inserted under local anesthesia while the patient is awake and can be associated with patient discomfort and uncooperativeness.
Dye, Radioisotope, and Fiducial Preoperative Localization
There are several novel and emerging techniques using dye injection, which can be used both preoperatively and intraoperatively for nodule localization. Many types of injectable dyes are used, including methylene blue, lipiodol, cyanoacrylate, India ink, and barium. These dyes are injected into or adjacent to the nodule. One should note that methylene blue, in particular, uses coaxial needle insertion into the parenchyma adjacent to the nodule of interest. Second, after the injection of methylene blue, it rapidly diffuses into the surrounding tissues, requiring a rapid transport into the operating room and swift intraoperative localization. This factor can be limiting in large hospital settings with increased operating room turnover times. To mitigate this limiting factor, McConnell and associates19 have used an autologous blood binder, which rendered the dye useful for several hours after injection.
Conversely, to minimize adjacent dye diffusion, barium and lipiodol could be used. These agents are identified using intraoperative fluoroscopy and do not necessitate rapid patient transport into the operating room.5 Watanabe and associates20 described 100% success in localization of SPNs (10 ± 6 mm) using lipiodol. Similar results were reported by Fumimoto and colleagues21 in their institutional analysis of lipiodol use for SPN detection. Although fluoroscopic guidance seems to be a feasible strategy for SPN localization before VATS resection, it is associated with an intense tissue inflammatory reaction that alters the histologic analysis. Additionally, various anaphylactoid reactions have been reported in the literature, which should be included in the preoperative informed consent. Other complications associated with lipiodol include chest wall pain (11%), hemoptysis (6%), pneumothorax (17%), and hemothorax (0.6%), and the complications and were associated with needle insertion rather than the lipiodol agent itself.5
Similar to gamma probe localization in breast surgery, radioisotopes can be used in comparable fashion in SPN localization. The most common radioisotope marker used for preoperative localization is technetium-99M–labeled macroaggregated albumin (traditionally used in perfusion studies). Radioisotope is coupled with noniodinated contrast and its position is confirmed with cross-sectional imaging. Subsequently, a Geiger counter is used intraoperatively during VATS to confirm the location of the nodule. The marker has a half-life of 6 to 9 hours, necessitating VATS to be performed 6 hours after injection. Various studies in literature compared technetium-99M versus needle localization techniques. Gonfiotti and associates22 observed that both techniques were superior in locating SPNs (84% vs 96%) compared with finger palpation (26%) and there was no statistical difference in localization between them. However, as mentioned elsewhere in this article, needle localization is associated with a greater than 20% risk of developing a pneumothorax.22 These findings were echoed by a single-institutional experience of 147 patients by Daniel and colleagues,23 who noted greater than 95% successful localization rate with minimal complications. Similar findings were reported in various small sample case series across multiple institutions.24
Fiducial localization is an important adjunct for nodule localization, particularly if there is going to be a significant delay in performing definitive VATS resection. These localizers consist of 3- and 5-mm gold seeds and are placed adjacent to the nodule using a coaxial needle, similar to methylene blue injection. The advantage for the gold seeds is that they are inert and can be localized days to months after the injection. They can readily be localized intraoperatively using fluoroscopic techniques and the specimen can be confirmed with postresection back table imaging. However, these seeds can migrate and embolize into vessels and airways. Caution should be taken in deploying these seeds near critical structures.25,26
Three-Dimensional Navigational Printing
Increasing availability and decreased cost of operation of 3-dimensional printing have made the technology accessible for medical research. Zheng and associates27 have published a small case series involving 16 patients where they obtained preoperative cross-sectional imaging and created a 3-dimensional digital anatomic template, which they then printed in 4 to 6 hours. The cost of each template ranged from $US80 to $US 100. This navigational template then allowed for accurate percutaneous localization based on the patient’s anatomic limitations. They report a 100% success rate in nodule localization and subsequent VATS resection.27 Although the sample size is small and the technology is in its infancy, one can expect an increased use of 3-dimensional printing in the future.
Navigational Bronchoscopy
Advances in endoscopic techniques and improvements in novel lesion detection software are improving the ability to perform minimally invasive thoracic surgery. One of these technologies that is becoming popular at large volume centers is electromagnetic navigational bronchoscopy. Electromagnetic navigational bronchoscopy combines traditional and virtual bronchoscopy for the localization of the lung nodules and allows the guidance of diagnostic as well as dye marking instruments. Furthermore, electromagnetic navigational bronchoscopy has expanded the use of bronchoscopic localization for small peripheral lesions that were previously unattainable with conventional endoscopic techniques.
The SuperDimension system (Medtronic, Minneapolis, MN) is the most commonly used electromagnetic navigational bronchoscopy system reported in the literature (Fig. 2). In the first phase, also known as the planning phase, the patient’s CT scan is loaded into the navigational software, which allows the surgeon to identify the target lesion and pick the most appropriate bronchial pathway. A standard bronchoscopy is performed, and the landmarks are registered into the software system. The navigational system then links the images from the initial CT scan and the bronchoscopy, creating virtual images. The surgeon then navigates toward the lesion using both standard and virtual images. When the bronchoscope is wedged in the segmental bronchus and cannot advance further, the operator advances the extended working channel, which has a built-in locatable guide using the virtual image. This technique allows one to obtain cytologic brushings, place fiducials/dyes for marking, and guide stereotactic body radiation therapies.
Fig. 2.
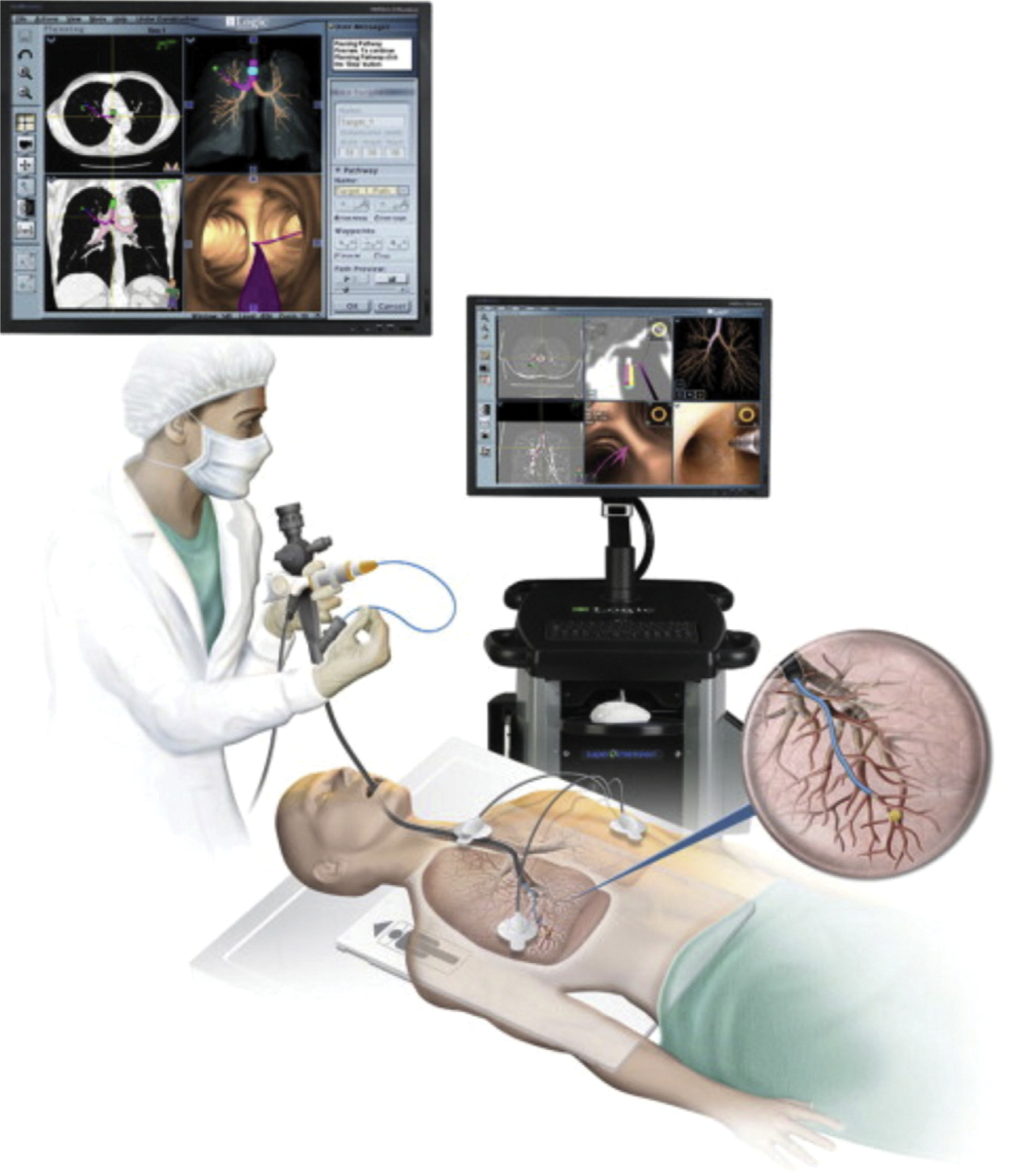
The Ilogic electromagnetic navigation bronchoscopy by super dimension. (From Christie S., Electromagnetic Navigational Bronchoscopy and Robotic-Assisted Thoracic Surgery. AORN Journal 2007–2017; 99(6); 750–763.)
Electromagnetic navigational bronchoscopy has been shown to effective in the diagnosis and preoperative localization of lung nodules with a lower complication profile than that of more invasive techniques. However, the diagnostic yield reported in literature ranges between 59% and 94%.28 The reason for this large variability stems from lack of uniform selection criteria and standardized protocols. Overall, lesions larger greater than 2 cm and those that exhibit bronchus sign on the CT scan have the greatest diagnostic yield.
INTRAOPERATIVE IMAGING-GUIDED NODULE LOCALIZATION
Preoperative localization techniques are useful adjuncts that can enhance the arsenal of the thoracic surgeon, but these procedures tend to be operator dependent, have varying rates of complications, and cause discomfort to the patients. Therefore, one can use various intraoperative imaging techniques to mitigate those risks in a less costly and invasive manner. Various options for intraoperative imaging include intraoperative ultrasound examination, intraoperative CT scans or fluoroscopy, near-infrared fluorescence, and IMI.
Intraoperative Ultrasound Examination
Performing an intraoperative ultrasound examination is a real-time and alternative approach to localizing small, nonvisible, and nonpalpable pulmonary lesions without injury to lung parenchyma. It is a preferred method because it is noninvasive, nonionizing, and affordable. In fact, it is one of the earliest methods for noninvasive nodule detection, dating back to the inception of minimally invasive thoracoscopic surgery. However, using ultrasound examination to detect small nodules is heavily operator dependent because air in the lung parenchyma inhibits proper detection in conventional ultrasound probes. Nevertheless, the use of high-frequency probes has surpassed this limitation and is accurate in detecting SPNs smaller than 20 mm. Small lung lesions can be found by intraoperative ultrasound examination when they are superficial, fixed in lung parenchyma, and solid. Deeper lesions, however, require complete collapse of the lung, which can be challenging if the anesthesia team is not comfortable with single lung ventilation and/or limited by underlying patient lung mechanics, such as emphysematous disease.
Although there is no widespread use of intraoperative ultrasound examination, several small institutional studies have observed its efficacy in detecting SPNs. Hsia and colleagues29 reported a 81.7% success rate among 153 patients over a 24-month period. Piolanti and colleagues30 reported a 92.6% success rate in 35 patients with a mean nodule size of 13.2 mm with no reported complications. Additionally, when ultrasound examination was compared with video imaging and palpation during VATS alone in 23 patients with nodules smaller than 30 mm, ultrasound imaging was found to be 100% effective, palpation 88% effective, and imaging alone 60% effective for nodule localization (P = .012).30
Intraoperative Fluoroscopy and Computed Tomography Scans
With the advent of screening guidelines, more and more subcentimeter SPNs are detected on an annual basis. As the size of the lesion decreases, various techniques including needle and dye localization become limited in accurately detecting the lesion of interest. These challenges can be mitigated by the use of fluoroscopy intraoperatively. A common dye used for fluoroscopic detection is lipiodol, which is a lipid soluble contrast medium. Real-time CT fluoroscopy has been shown to be valuable during preoperative lipiodol marking of ground-glass opaque and deep, small, solid nodules.
Swensen and colleagues31 reported a 97% success rate of nodule localization in 107 patients analyzed over a 17-month period. After CT-guided needle localized injection of lipiodol, they used a C-arm–shaped fluoroscopic unit to detect and resect regions of interest (Fig. 3). The specimen adequacy was confirmed by reimaging the resected parenchyma, looking for the presence of the lipiodol.
Fig. 3.
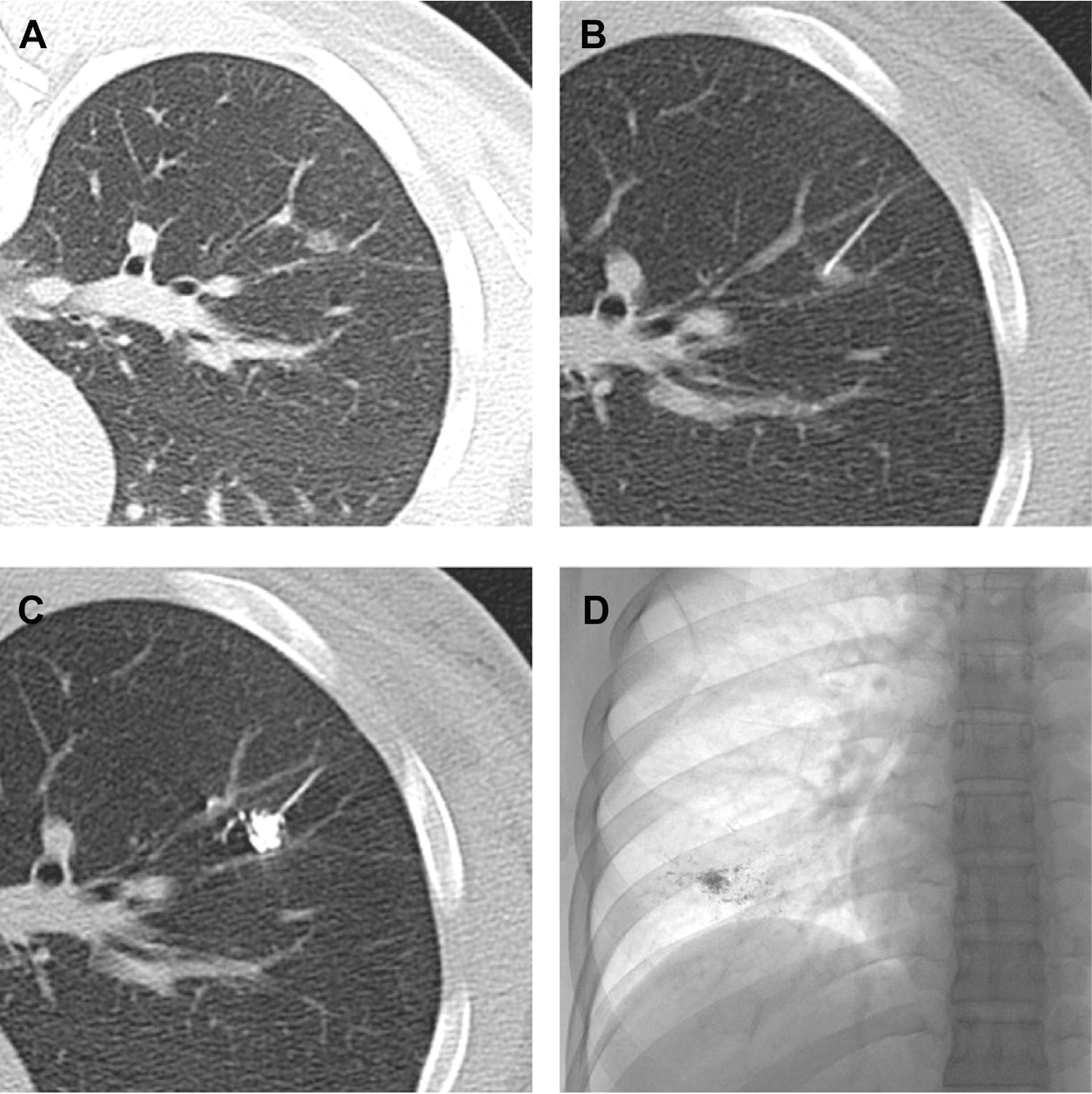
Computed tomographic fluoroscopic guided lipiodol-marking procedure for ground-glass opaque (GGO) nodules. (A) A small GGO in the right lower lobe. (B) A percutaneous transhepatic cholangiography needle (23 gauge) is inserted in the center of the GGO nodule. (C) Lipiodol 0.3 mL is then injected into the nodule. (D) A radiopaque spot is clearly demonstrated on the plain chest radiograph. (Obtained from Thoracic Surgery Clinics. Elsevier.)
One of the earlier studies looking at the application of this technology was performed by Nomori and colleagues32 with similar technical aspects to Yamashita’s methods. Although a small sample size, this study showed 18 nodules in 16 patients were detected using this procedure with an average size of 7 mm. One patient had a pneumothorax that required drainage before going to the operating room. As described elsewhere in this article, the use of fluoroscopic CT guidance can be enhanced by using fiducial gold seeds, but this technique requires operator expertise.
One of the limiting and inconvenient factors associated with fluoroscopic guidance is that the patient has to undergo the needle localization technique before VATS. Depending on the technique used, this process can lead to multiple trips to the health care institution, or require patient transport between procedure suites. Similar to vascular surgery, this situation has led to the development of a hybrid technique, termed image-guided VATS, by Gill and colleagues.33 The study combines placement of fiducials using intraoperative C-arm CT guidance with standard thoracoscopic resection technique using image-guided VATS. In their phase I and II trial, they successfully resected all the specimens in 23 patients enrolled in the study with minimal complications.
Intraoperative Molecular Imaging
IMI is a novel technique aimed at localization, diagnosis and margin assessment of lung nodules. IMI involves 2 key components: fluorescent contrast agents and specialized imaging devices. A fluorescent contrast agent is given systemically and binds to tumor cells. When used in combination with a specialized imaging device, pulmonary tumors may be assessed via either thoracotomy or minimally invasive thoracoscopic techniques for fluorescence.
Currently there is only commercially available agent that has been approved by the US Food and Drug Administration, namely, indocyanine green (ICG); however, several additional contrast agents are currently being developed. Despite the limited availability of other agents, ICG has shown promise in intraoperative SPN detection. The use of electromagnetic navigational bronchoscopy using near-infrared fluorescence with ICG contrast has emerged as an accurate and efficient method for localizing pulmonary nodules. ICG is a fluorophore contrast that illuminates in the near infrared spectrum, has a high signal-to-noise ratio, is inexpensive, and is relatively nontoxic. The theory behind the use of ICG relies on the fact that malignant cells retain the dye by a nonspecific inflammatory permeability. Furthermore, infiltration of ICG into the tissues does not distort its cellular integrity, thereby allowing for accurate histopathologic analysis, including margin assessment. Since the initial 2001 study by Sakamoto and colleagues,34 interest in ICG use for pulmonary nodule localization has increased dramatically. Early studies reported 79% to 100% success rate in nodule localization. A number of groups have since reported institutional studies regarding the accuracy of electromagnetic navigational bronchoscopy-guided near-infrared fluorescence using ICG for intraoperative localization of pulmonary lesions. Our institutional pilot study in 2015 looked at localization after administering preoperative intravenous ICG (5 mg/kg) followed by open thoracotomy within 24 hours.9 We reported that near-infrared fluorescence detected 16 of 18 nodules (88%), which were as small as 0.2 cm in size and 1.3 cm from the pleural surface. Additionally, this approach discovered 5 subcentimeter metastatic deposits that were resected by wedge excision.
Although ICG is nontargeted and is thought to localize to the tumors through increased vasculature and dysfunctional lymphatics, other recently studied agents are targeted to tumor surface receptors. Okusanya and associates in their pilot study looking at folate-targeted dyes in tumor nodule localization demonstrated that optical fluorescent molecular imaging can be used to visually identify lung adenocarcinomas.9,35,36 These folate targeted dyes localize to pulmonary adenocarcinomas that highly express folate receptor alpha. The folate dye used in the study was a conjugate between folate and a near-infrared dye. This conjugate forms a negatively charged fluorescent molecule that binds weakly and nonspecifically to serum proteins. In addition, in normal lung pneumocytes, folate receptor alpha is expressed on the apical (luminal) side of polarized epithelial cells; thus, it has no access to systemically administered folate.
A phase I and more recent multi-institutional phase II study have been completed and the results reported. Patients with lung nodules received the folate–near infrared tracer preoperatively. The primary goal was to determine if the tracer improved surgeons’ ability to localize hard to find nodules, identify occult cancers, and discriminate close margins. Assessments were made in 3 phases: (i) lung inspection, in which the hemithorax was evaluated for other pathology, (ii) tumor resection, in which the primary tumor was resected, and (iii) the specimen check, in which the specimen was assessed on the back table for positive margins. The gold standard was pathology. One hundred ten patients were recruited; 92 were eligible for analysis. There were no drug-related serious adverse events. During the inspection phase, IMI found 10 additional cancers that were not suspected by the surgeon in 7 patients (8%). During the resection phase, IMI located 11 lesions (12%) that the surgeon could not palpate or visualize. During the specimen check phase, although the surgeon felt the margins were adequate, IMI revealed 8 positive margins (9%). The IMI learning curve was 6 cases. Benefits of IMI were most pronounced in patients undergoing sublobar pulmonary resections and those with ground-glass opacities.37,38
Although this finding represents an exciting avenue for intraoperative nodule localization, much work needs to be done for mainstream integration. However, early works suggest that this emerging technology is useful for real-time subcentimeter pulmonary nodule localization, margin assessment, and intraoperative diagnosis.
INTRAOPERATIVE MARGIN ASSESSMENT
Positive margins after surgery for non-small cell lung cancer occurs in approximately 5% to 15% of patients, which impacts long-term outcomes by causing local and distant disease recurrence. Numerous studies spanning multiple decades have placed importance on R0 resection as the key prognostic indicator. Therefore, every attempt should be made for sound oncologic resection of non-small cell lung cancer lesions. Despite the clinical ramifications, there are few dedicated reports that discuss intraoperative methods of margin assessment or the implications of positive margins after surgery for non-small cell lung cancer. Currently, there are limited methods whereby pulmonary tumor margins are assessed intraoperatively for both bronchial margins after pulmonary lobectomy or sublobar pulmonary resections. The ideal surgical margin for sublobar resections remains controversial. Reports suggest that for small tumors (<2 cm), the ideal surgical margin is 1.5 cm with additional resection providing no additional benefit. In larger tumors, the optimal distance from the tumor to the surgical margin is less evident, however, larger resection margin may be more favorable. Specifically, larger tumors should be resected with a margin of at least the size of the tumor in largest diameter. Most frequently, margins are assessed using gross examination either by the surgeon and/or pathologist. Additional methods of intraoperative margin assessment include frozen section, cytologic lavage analysis, intraoperative ultrasound examination, and molecular imaging.
Frozen Section
Frozen sections are one of the frequently used means for assessing margin positivity for non-small cell lung cancer. Advances in preoperative imaging and tissue sampling has led to an overall decreased use of frozen sections. However, thoracic frozen sections are still frequently used, although their indication has changed with the most common request being the characterization of margins. Margin assessment in lung sparing procedures has become a more frequently requested frozen section. With the recognition that multiple pulmonary nodules may reflect (1) synchronous primary tumors, (2) invasive cancer plus a synchronous preinvasive or minimally invasive lesion, or (3) carcinoma plus an inflammatory nodule, intraoperative sampling of ipsilateral nodules in a separate lobe or segment for lesion identification and margin assessment may be needed as part of a limited resection. Additionally, although small, there are significant false-positive and false-negative rates associated with frozen section analysis. Reasons for the false-negative rates, which are as high as 40% in some reports, include sampling error and interpretation error (misnaming malignant cells for lymphocytes, glandular tissue, metaplasia, or cautery artifact).39,40 Furthermore, the timing required for optimal analysis by an experienced thoracic pathologist requires that the patient remain under anesthesia. This factor is particularly important, because this patient population has above average rates of significant lung and cardiovascular disease.
Data from Owen and colleagues,40 analyzing frozen sections of bronchial margins in 270 patients, have shown that frozen section analysis rarely yields a positive result and infrequently changes intraoperative management of patients undergoing non-small cell lung cancer resection. In light of these conclusions, the National Comprehensive Cancer Network guidelines do not currently recommend frozen section analysis when performing a lobectomy or pneumonectomy.
Nevertheless, it is important to recognize that frozen section analysis is important for those undergoing sublobar resection because a positive margin can impact the exact extent of surgical resection. Despite this indication, frozen section remains costly, time consuming, and predisposes the patient to additional anesthesia. There has not been a clinical study assessing frozen section for margin assessment in this scenario.
Cytologic Lavage Analysis
Cytologic analysis of lung margins has been developed in an attempt to predict retained tumor margins at the cut end during sublobar resections. Confirmation of negative margins is thought to decrease local recurrence rates and allow for optimal oncologic resection. Miyoshi and colleagues17 in their 2019 analysis built on techniques and findings by Higashiyama and colleagues,41 who initially published their results on intraoperative lavage cytology dating back to 2002. In fact, they report that intraoperative lavage cytology is their primary means of assessing margin positivity for more than decade and has supplanted intraoperative frozen section analysis. The technique as described by the authors is as follows:
All autostapling cartridges used for wedge or segmental resection of pulmonary malignancies are rinsed with 50 mL saline. The washing saline is centrifuged, and the sediment is stained using Papanicolaou’s method to examine for cancer cells. The result is reported to the operating room within approximately 30 minutes, and in case of a positive result, additional wedge resection is usually attempted. If there is an anatomic restriction, additional segmental resection, lobectomy, or even pneumonectomy is performed if the patient is not at high risk.
Among the 262 patients who underwent intraoperative lavage cytology, 22 (7%) had positive cytology, of whom 19 underwent additional resection. Slightly higher positive margins were detected by Higashiyama and associates (11%). During the median follow-up period of 42 months, 2% of patients with negative initial cytology developed margin recurrence.17,41
Intraoperative Ultrasound Examination
With the availability and cost efficacy associated with an ultrasound examination, effort has been made to use both intraoperative endoscopic and transthoracic ultrasound examinations to guide resection margins in lung tumors. Tatsumura42 describes a technique whereby both transesophageal endoscopic ultrasound examination and intraoperative ultrasound examination were used in 71 patients with preoperatively diagnosed primary lung cancers to assess involvement of pericardium, aorta, vena cava, pulmonary vessels, left atrium, chest wall, and diaphragm. Intraoperative ultrasound examination outperformed endoscopic ultrasound examination for both the sensitivity and specificity of tumor organ involvement; however, both methods outperformed preoperative CT scan and MRI for prediction of surrounding structure involvement.42 Although an effort has been made to use intraoperative ultrasound examination for tumor localization and assessment of surrounding structure involvement, this technique has not been described for use in determining adequate margins during sublobar resections.
Intraoperative Molecular Imaging
As detailed elsewhere in this review, intraoperative molecular imaging represents a new frontier for tumor localization and same can be said for real time intraoperative margin localization. De Jesus and colleagues43,44 described their institutional experience using a novel folate-targeted dye (folate–fluorescein) in mice models where compared with traditional inspection (30%), IMI identified 80% of residual tumor deposits. These findings were echoed by Newton and colleagues45 in their review of intraoperative fluorescence imaging in thoracic surgery. As these techniques and molecular tracers were identified at authors institution, there are multiple prospective trials currently underway to ascertain the efficacy of folate-based dyes in tumor localization and margin assessment.37 There have been numerous iterations to the types of dyes used. Currently, our group is analyzing the efficacy of folate–near infrared examination, which has shown decreased autofluorescence and increased depth of penetration compared with folate-fluorescein examination, which was used in initial studies. Additionally, folate–near infrared examination had a high specificity for folate receptor alpha–expressing tumor cells lines in vitro, that folate–near infrared examination localized to murine lung cancer xenografts and spontaneous lung cancers in dogs in vivo, and that tumor margins correlated with fluorescence. With increased development of both tumor specific dyes, as well as intraoperative imaging devices, we foresee the ability of IMI to provide an accurate optical biopsy for assessment of tumor margins in the operating room in the near future.
SUMMARY
With the ever-increasing implementation of lung cancer screening programs by high-quality cross-sectional imaging, more and more SPNs are identified in this high-risk patient population. Ideally, these lesions would be managed by minimally invasive and nonsurgical techniques. However, anatomic constraints and patient comorbidities may preclude these approaches or fail in providing a diagnosis. These patients are best served by VATS surgical biopsy, which can be both diagnostic and therapeutic. For this reason, intraoperative localization of pulmonary nodules and margin assessment are challenging, although crucial, to thoracic surgeons for management of these early stage lung cancers. Several methods have been developed to improve localization and decrease time to diagnosis and rate of conversion to thoracotomy; however, none are 100% sensitive or without complications.
Depending on institutional practice patterns, equipment availability, and surgeon preference, several preoperative methods can be used. These include percutaneous CT-guided wire placement techniques, including the placement of microcoils, hook wires, and spiral wires, which can help with nodule localization during VATS and may also be used in conjunction with intraoperative fluoroscopy or ultrasound. Notably, all of these techniques require 2 procedures and have considerable complications including pneumothorax, hemothorax, and patient discomfort. Additionally, patient comorbidities such as emphysematous disease and severe pulmonary hypertension, may preclude them from these preoperative localizations.
To further enhance localization, surgeons can use preoperative dye marking Nevertheless, this again requires 2 procedures for diagnosis, which can complicate operative schedule, and remains complicated by risk of pneumothorax, dye embolism, and cases of anaphylaxis to the dye of choice. Owing to the cumbersome nature of those procedures, most thoracic surgeons still rely on finger palpation to locate nodules intraoperatively.
R0 resection is the mainstay of sound oncologic resection in non-small cell lung cancer; it has been shown that these patients have lower rates of local recurrence and better prognosis than those who do not get complete oncologic resection. With the advent of sublobar resections, which preserve lung parenchyma while performing adequate resection, margin assessment remains a cardinal step. Currently, thoracic surgeons can assess surgical margins using frozen section, cytologic analysis, and intraoperative imaging. However, currently available methods are time consuming, costly, and inaccurate. Therefore, currently, methods beyond traditional visualization and palpation are not frequently used. Although challenges for intraoperative margin assessment remain, there have been significant strides in the realm of IMI with promising early results.
KEY POINTS.
R0 resection is key in lung cancer management, R1 resection patients have overall worse survival prognosis versus R0 resection.
Preoperative nodule localization techniques include wire placement, dye marking, ultrasound examination, fluoroscopy, and intraoperative molecular imaging.
Currently, there are limited methods of intraoperative margin assessment other than vigorous inspection using vision and palpation.
Minimally invasive approaches can provide accurate assessment of primary tumors, mediastinal lymph node involvement, and pleural involvement.
Folate-targeted dye detection of retained tumor cells after pulmonary adenocarcinoma isan emerging technique with the potential of transforming of intraoperative margin assessment.
REFERENCES
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics. Adv Exp Med Biol 2016; 893:1–19. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet 2017;389(10066):299–311. [DOI] [PubMed] [Google Scholar]
- 3.The National Lung Screening Trial: overview and study design1. Radiology 2011; 258(1):243–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Final Recommendation Statement: Lung Cancer: screening - US Preventive Services Task Force. Available from: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/lung-cancer-screening. Accessed January 20, 2020. [Google Scholar]
- 5.Kawanaka K, Nomori H, Mori T, et al. Marking of small pulmonary nodules before thoracoscopic resection: injection of lipiodol under CT-fluoroscopic guidance. Acad Radiol 2009;16(1):39–45. [DOI] [PubMed] [Google Scholar]
- 6.Sánchez M, Benegas M, Vollmer I. Management of incidental lung nodules <8 mm in diameter. J Thorac Dis 2018;10(Suppl 22):S2611–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hofmann HS, Taege C, Lautenschläger C, et al. Microscopic (R1) and macroscopic (R2) residual disease in patients with resected non-small cell lung cancer. Eur J Cardiothorac Surg 2002;21(4):606–10. [DOI] [PubMed] [Google Scholar]
- 8.Riquet M, Achour K, Foucault C, et al. Microscopic residual disease after resection for lung cancer: a multifaceted but poor factor of prognosis. Ann Thorac Surg 2010;89(3):870–5. [DOI] [PubMed] [Google Scholar]
- 9.Keating J, Singhal S. Novel Methods of Intraoperative Localization and Margin Assessment of Pulmonary Nodules. Semin Thorac Cardiovasc Surg 2016;28(1): 127–36. [DOI] [PubMed] [Google Scholar]
- 10.Marchand C, Medford ARL. Relationship between endobronchial ultrasound-guided (EBUS)-transbronchial needle aspiration utility and computed tomography staging, node size at EBUS, and positron emission tomography scan node standard uptake values: a retrospective analysis. Thorac Cancer 2017;8(4): 285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hsu H-H, Shen C-H, Tsai W-C, et al. Localization of nonpalpable pulmonary nodules using CT-guided needle puncture. World J Surg Oncol 2015;13. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4536773/. Accessed January 26, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Needle localization of small pulmonary nodules: lessons learned- ClinicalKey.Available at: https://www.clinicalkey.com/#!/content/journal/1-s2.0-S0022522318300552?scrollTo5%231-s2.0-S0022522318300552-fx2. Accessed January 26, 2020. [DOI] [PubMed]
- 13.Mattioli S, D’Ovidio F, Daddi N, et al. Transthoracic endosonography for the intraoperative localization of lung nodules. Ann Thorac Surg 2005;79(2):443–9. [DOI] [PubMed] [Google Scholar]
- 14.Suzuki K, Nagai K, Yoshida J, et al. Video-assisted thoracoscopic surgery for small indeterminate pulmonary nodules: indications for preoperative marking. Chest 1999;115(2):563–8. [DOI] [PubMed] [Google Scholar]
- 15.Thistlethwaite PA, Gower JR, Hernandez M, et al. Needle localization of small pulmonary nodules: lessons learned. J Thorac Cardiovasc Surg 2018;155(5): 2140–7. [DOI] [PubMed] [Google Scholar]
- 16.Mayo JR, Clifton JC, Powell TI, et al. Lung nodules: CT-guided placement of microcoils to direct video-assisted thoracoscopic surgical resection. Radiology 2009;250(2):576–85. [DOI] [PubMed] [Google Scholar]
- 17.Miyoshi K, Toyooka S, Gobara H, et al. Clinical outcomes of short hook wire and suture marking system in thoracoscopic resection for pulmonary nodules. Eur J Cardiothorac Surg 2009;36(2):378–82. [DOI] [PubMed] [Google Scholar]
- 18.Dendo S, Kanazawa S, Ando A, et al. Preoperative localization of small pulmonary lesions with a short hook wire and suture system: experience with 168 procedures. Radiology 2002;225(2):511–8. [DOI] [PubMed] [Google Scholar]
- 19.McConnell PI, Feola GP, Meyers RL. Methylene blue-stained autologous blood for needle localization and thoracoscopic resection of deep pulmonary nodules. J Pediatr Surg 2002;37(12):1729–31. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, Nomori H, Ohtsuka T, et al. Usefulness and complications of computed tomography-guided lipiodol marking for fluoroscopy-assisted thoracoscopic resection of small pulmonary nodules: experience with 174 nodules. J Thorac Cardiovasc Surg 2006;132(2):320–4. [DOI] [PubMed] [Google Scholar]
- 21.Fumimoto S, Sato K, Koyama M, et al. Combined lipiodol marking and video-assisted thoracoscopic surgery in a hybrid operating room. J Thorac Dis 2018; 10(5):2940–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonfiotti A, Davini F, Vaggelli L, et al. Thoracoscopic localization techniques for patients with solitary pulmonary nodule: hookwire versus radio-guided surgery. Eur J Cardiothorac Surg 2007;32(6):843–7. [DOI] [PubMed] [Google Scholar]
- 23.Daniel TM, Altes TA, Rehm PK, et al. A novel technique for localization and excisional biopsy of small or ill-defined pulmonary lesions. Ann Thorac Surg 2004; 77(5):1756–62 [discussion: 1762]. [DOI] [PubMed] [Google Scholar]
- 24.Lin M-W, Chen J-S. Image-guided techniques for localizing pulmonary nodules in thoracoscopic surgery. J Thorac Dis 2016;8(Suppl 9):S749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sancheti MS, Lee R, Ahmed SU, et al. Percutaneous fiducial localization for thoracoscopic wedge resection of small pulmonary nodules. Ann Thorac Surg 2014; 97(6):1914–8 [discussion: 1919]. [DOI] [PubMed] [Google Scholar]
- 26.Sharma A, McDermott S, Mathisen DJ, et al. Preoperative localization of lung nodules with fiducial markers: feasibility and technical considerations. Ann Thorac Surg 2017;103(4):1114–20. [DOI] [PubMed] [Google Scholar]
- 27.Zhang L, Li M, Li Z, et al. Three-dimensional printing of navigational template in localization of pulmonary nodule: a pilot study. J Thorac Cardiovasc Surg 2017; 154(6):2113–9.e7. [DOI] [PubMed] [Google Scholar]
- 28.Muñoz-Largacha JA, Litle VR, Fernando HC. Navigation bronchoscopy for diagnosis and small nodule location. J Thorac Dis 2017;9(Suppl 2):S98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsia DW, Jensen KW, Curran-Everett D, et al. Diagnosis of lung nodules with peripheral/radial endobronchial ultrasound-guided transbronchial biopsy. J Bronchology Interv Pulmonol 2012;19(1):5–11. [DOI] [PubMed] [Google Scholar]
- 30.Piolanti M, Coppola F, Papa S, et al. Ultrasonographic localization of occult pulmonary nodules during video-assisted thoracic surgery. Eur Radiol 2003; 13(10):2358–64. [DOI] [PubMed] [Google Scholar]
- 31.Swensen SJ, Yamashita K, McCollough CH, et al. Lung nodules: dual-kilovolt peak analysis with CT–multicenter study. Radiology 2000;214(1):81–5. [DOI] [PubMed] [Google Scholar]
- 32.Nomori H, Horio H, Naruke T, et al. Fluoroscopy-assisted thoracoscopic resection of lung nodules marked with lipiodol. Ann Thorac Surg 2002;74(1):170–3. [DOI] [PubMed] [Google Scholar]
- 33.Gill RR, Zheng Y, Barlow JS, et al. Image-guided video assisted thoracoscopic surgery (iVATS) - phase I-II clinical trial. J Surg Oncol 2015;112(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sakamoto T, Takada Y, Endoh M, et al. Bronchoscopic dye injection for localization of small pulmonary nodules in thoracoscopic surgery. Ann Thorac Surg 2001; 72(1):296–7. [DOI] [PubMed] [Google Scholar]
- 35.Okusanya OT, Deshpande C, Barbosa EM, et al. Molecular imaging to identify tumor recurrence following chemoradiation in a hostile surgical environment. Mol Imaging 2015;14(1). 7290.2014.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kennedy GT, Okusanya OT, Keating JJ, et al. The optical biopsy: a novel technique for rapid intraoperative diagnosis of primary pulmonary adenocarcinomas. Ann Surg 2015;262(4):602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Predina JD, Newton AD, Keating J, et al. A phase I clinical trial of targeted intraoperative molecular imaging for pulmonary adenocarcinomas. Ann Thorac Surg 2018;105(3):901–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Predina JD, Newton AD, Keating J, et al. Intraoperative molecular imaging combined with positron emission tomography improves surgical management of peripheral malignant pulmonary nodules. Ann Surg 2017;266(3):479–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sienko A, Allen TC, Zander DS, et al. Frozen section of lung specimens. Arch Pathol Lab Med 2005;129(12):1602–9. [DOI] [PubMed] [Google Scholar]
- 40.Owen RM, Force SD, Gal AA, et al. Routine intraoperative frozen section analysis of bronchial margins is of limited utility in lung cancer resection. Ann Thorac Surg 2013;95(6):1859–65 [discussion: 1865–6]. [DOI] [PubMed] [Google Scholar]
- 41.Higashiyama M, Kodama K, Takami K, et al. Intraoperative lavage cytologic analysis of surgical margins in patients undergoing limited surgery for lung cancer. J Thorac Cardiovasc Surg 2003;125(1):101–7. [DOI] [PubMed] [Google Scholar]
- 42.Tatsumura T Preoperative and intraoperative ultrasonographic examination as an aid in lung cancer operations. J Thorac Cardiovasc Surg 1995;110(3):606–12. [DOI] [PubMed] [Google Scholar]
- 43.De Jesus E, Keating JJ, Kularatne SA, et al. Comparison of folate receptor targeted optical contrast agents for intraoperative molecular imaging. Int J Mol Imaging 2015;2015. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4600912/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keating JJ, Okusanya OT, De Jesus E, et al. Intraoperative molecular imaging of lung adenocarcinoma can identify residual tumor cells at the surgical margins. Mol Imaging Biol 2016;18(2):209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton AD, Predina JD, Nie S, et al. Intraoperative fluorescence imaging in thoracic surgery. J Surg Oncol 2018;118(2):344–55. [DOI] [PMC free article] [PubMed] [Google Scholar]


