Abstract
BACKGROUND
The efficacy and safety of treatment with cabozantinib in combination with nivolumab and ipilimumab in patients with previously untreated advanced renal-cell carcinoma are unknown.
METHODS
In this phase 3, double-blind trial, we enrolled patients with advanced clear-cell renal-cell carcinoma who had not previously received treatment and had intermediate or poor prognostic risk according to the International Metastatic Renal-Cell Carcinoma Database Consortium categories. Patients were randomly assigned to receive 40 mg of cabozantinib daily in addition to nivolumab and ipilimumab (experimental group) or matched placebo in addition to nivolumab and ipilimumab (control group). Nivolumab (3 mg per kilogram of body weight) and ipilimumab (1 mg per kilogram) were administered once every 3 weeks for four cycles. Patients then received nivolumab maintenance therapy (480 mg once every 4 weeks) for up to 2 years. The primary end point was progression-free survival, as determined by blinded independent review according to Response Evaluation Criteria in Solid Tumors, version 1.1, and was assessed in the first 550 patients who had undergone randomization. The secondary end point was overall survival, assessed in all patients who had undergone randomization.
RESULTS
Overall, 855 patients underwent randomization: 428 were assigned to the experimental group and 427 to the control group. Among the first 550 patients who had undergone randomization (276 in the experimental group and 274 in the control group), the probability of progression-free survival at 12 months was 0.57 in the experimental group and 0.49 in the control group (hazard ratio for disease progression or death, 0.73; 95% confidence interval, 0.57 to 0.94; P = 0.01); 43% of the patients in the experimental group and 36% in the control group had a response. Grade 3 or 4 adverse events occurred in 79% of the patients in the experimental group and in 56% in the control group. Follow-up for overall survival is ongoing.
CONCLUSIONS
Among patients with previously untreated, advanced renal-cell carcinoma who had intermediate or poor prognostic risk, treatment with cabozantinib plus nivolumab and ipilimumab resulted in significantly longer progression-free survival than treatment with nivolumab and ipilimumab alone. Grade 3 or 4 adverse events were more common in the experimental group than in the control group. (Funded by Exelixis; COSMIC-313 ClinicalTrials.gov number, NCT03937219.)
Tyrosine kinase inhibitors and immune checkpoint inhibitors are standard treatments for clear-cell, advanced renal-cell carcinoma as single agents or in combination.1–9 In the phase 3 CheckMate 214 trial, which included patients with advanced renal-cell carcinoma who had intermediate or poor prognostic risk according to the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) categories, first-line therapy with nivolumab and ipilimumab resulted in higher overall survival and objective response rates than with sunitinib.6 However, 20% of the patients who received nivolumab and ipilimumab had progressive disease as the best response.10–12
Cabozantinib is a tyrosine kinase inhibitor that targets multiple receptor tyrosine kinases involved in tumor growth, angiogenesis, metastasis, and immune regulation, including vascular endothelial growth factor receptor, MET, and the TAM family of kinases (TYRO3, AXL, MER).13 Cabozantinib improved outcomes as compared with sunitinib in previously untreated patients with advanced renal-cell carcinoma when administered as a single agent in the CABOSUN trial5 and in combination with nivolumab in the phase 3 CheckMate 9ER trial.8 An exploratory analysis in the CheckMate 9ER trial that included a small cohort of patients who received cabozantinib with nivolumab and ipilimumab showed that this triplet regimen had clinical activity and an acceptable safety profile.14
The phase 3 COSMIC-313 trial is evaluating cabozantinib in addition to nivolumab and ipilimumab as compared with placebo in addition to nivolumab and ipilimumab in patients with previously untreated advanced renal-cell carcinoma who have intermediate or poor risk according to the IMDC categories. Here, we report the first results, including the results for progression-free survival.
METHODS
PATIENTS
Eligible patients were 18 years of age or older and had histologically confirmed advanced or metastatic renal-cell carcinoma with a clear-cell component. Patients were required to have an IMDC risk of intermediate or poor (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org); measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1; a Karnofsky performance-status score of 70 or greater (on a scale from 0 to 100, with lower scores reflecting greater disability); and archival or fresh tumor tissue for programmed death ligand 1 (PD-L1) quantification.
Patients were excluded if they had received previous systemic anticancer therapy for advanced renal-cell carcinoma. One previous systemic adjuvant therapy was allowed, except for combination regimens with PD-L1 or programmed death 1 and cytotoxic T-lymphocyte antigen 4 inhibitors. Additional exclusion criteria included brain metastases or cranial epidural disease, unless the disease was adequately treated and stable; uncontrolled, clinically significant illnesses, including autoimmune disease; and use of immunosuppressive medications (>10 mg of prednisone or equivalent per day) within 14 days before randomization.
TRIAL DESIGN
In this phase 3, randomized, double-blind, placebo-controlled trial, patients were randomly assigned in a 1:1 ratio to receive cabozantinib in addition to nivolumab and ipilimumab (experimental group) or placebo in addition to nivolumab and ipilimumab (control group). Randomization was stratified according to IMDC risk (intermediate vs. poor) and region (United States, Canada, Europe, Australia, or New Zealand vs. Latin America or Asia).
Both groups received nivolumab (3 mg per kilogram of body weight) and ipilimumab (1 mg per kilogram) intravenously every 3 weeks for four cycles, followed by nivolumab maintenance therapy (480 mg every 4 weeks) for up to 2 years. Cabozantinib (40 mg) or placebo was administered orally once daily. Crossover between groups was prohibited. Dose delays were permitted for all agents, but dose reductions were permitted only for cabozantinib (to 20 mg per day, then 20 mg every other day) and placebo. Dose reescalation was permitted. Patients were treated until a loss of clinical benefit was observed or unacceptable toxic effects occurred. Discontinuation of one component of a trial regimen did not mandate discontinuation of other components, and patients could be treated beyond disease progression.
END POINTS AND ASSESSMENTS
The primary end point was progression-free survival, assessed according to RECIST, version 1.1, in the first 550 patients who had undergone randomization (the progression-free survival population). Progression-free survival was assessed by an independent radiology committee whose members were unaware of trial-group assignments. The secondary end point was overall survival, assessed in all the patients who had undergone randomization (the intention-to-treat population). Additional end points included an objective tumor response according to RECIST, version 1.1 (as determined by blinded independent review); duration of response; and safety. Tumor assessments with the use of computed tomography or magnetic resonance imaging were performed at baseline, week 10, every 8 weeks through week 50, and then every 12 weeks. Progression-free survival and response were also assessed by investigators. Safety was assessed every 1 to 2 weeks for the first 14 weeks, then every 4 weeks thereafter, with post-treatment follow-up at 30 days and 100 days. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 5.0. PD-L1 status was assessed with the use of the Dako PD-L1 IHC 28–8 pharmDx test.15
TRIAL OVERSIGHT
The protocol (available at NEJM.org) was approved by the institutional review board or ethics committee at each trial center. The trial was conducted in accordance with the principles of the Declaration of Helsinki and Good Clinical Practice guidelines. All the patients provided written informed consent. Safety was monitored by an independent data and safety monitoring committee. The trial was designed by the members of the steering committee in collaboration with the sponsor (Exelixis) and partner (Bristol-Myers Squibb). Data were collected by the investigators and their teams, and analyses were conducted by the sponsor. Investigators agreed to keep all aspects of the trial, including the data, confidential as part of the site agreement. The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. The first draft was written collaboratively by several of the authors with medical writing support funded by the sponsor. All the authors reviewed the manuscript and provided approval for submission of the manuscript for publication.
STATISTICAL ANALYSIS
The trial was designed with a target sample of 840 patients. Because the sample size that was needed to evaluate overall survival was larger than that needed to assess progression-free survival, we planned to assess progression-free survival in the first 550 patients who underwent randomization, which would allow longer follow-up in a smaller population and minimize potential bias from overrepresentation of early progressions among the planned events. For the analysis of progression-free survival (the primary end point), we estimated that 249 events (disease progression or death) would provide 90% power to detect a hazard ratio of 0.66 in the experimental group as compared with the control group, assessed with a two-sided log-rank test at a significance level of 0.05. Initially, progression-free survival was planned to be assessed in the first 440 patients who underwent randomization. However, after 17 months of minimum follow-up, only 209 events (84% information fraction) had occurred, and the event rate had slowed. To allow 249 events to occur in a reasonable time frame, the sample for progression-free survival was expanded to the first 550 patients who underwent randomization, according to a prespecified provision in the protocol. Additional details are provided in the Supplementary Appendix.
For the analysis of overall survival (the secondary end point), we estimated that 433 deaths among 840 patients would provide 90% power to detect a hazard ratio for death of 0.73 in the experimental group as compared with the control group, assessed with a two-sided log-rank test at a significance level of 0.05. A hierarchical testing procedure was used to control inflation of a type I error associated with testing multiple end points, and the critical P value for overall survival would depend on the information fraction at the time of the analysis (the interim or final analysis). A prespecified interim analysis of overall survival was conducted with the primary analysis of progression-free survival; after reviewing efficacy and safety data, the independent data and safety monitoring committee recommended that the trial continue. One additional interim analysis of overall survival is planned under the Lan–DeMets O’Brien–Fleming alpha-spending function. To reduce bias, data on overall survival will remain concealed from investigators and patients, with data access limited to a small group from the trial sponsor until additional analyses of overall survival are completed.
Efficacy evaluations other than the primary and secondary end points are considered to be descriptive, without adjustment for multiplicity; confidence intervals should not be used in place of hypothesis tests. The duration of progression-free survival and the duration of response and associated confidence intervals were estimated with the use of the Kaplan–Meier method. Stratified hazard ratios with 95% confidence intervals were estimated with the use of a Cox proportional-hazards model for progression-free survival. Two-sided confidence intervals for the point estimate of response in each group were calculated with the use of the Clopper–Pearson method.
RESULTS
PATIENTS
Between June 25, 2019, and March 29, 2021, a total of 855 patients underwent randomization (intention-to-treat population); 428 patients were assigned to the experimental group, and 427 were assigned to the control group. The first 550 patients who had undergone randomization were included in the progression-free survival population (276 in the experimental group and 274 in the control group). Baseline demographic and disease characteristics were balanced between the two groups in both populations (Table 1). The representativeness of the trial participants is described in Table S2.
Table 1.
Baseline Characteristics of the Patients.*
| Characteristic | Progression-free Survival Population | Intention-to-Treat Population | ||
|---|---|---|---|---|
| Experimental (N = 276) | Control (N = 274) | Experimental (N = 428) | Control (N = 427) | |
| Median age (range) — yr | 61 (29–82) | 60 (28–85) | 61(19–85) | 60 (28–87) |
| Sex — no. (%) | ||||
| Male | 213 (77) | 204 (74) | 326 (76) | 312 (73) |
| Female | 63 (23) | 70 (26) | 102 (24) | 115 (27) |
| Geographic region — no. (%) | ||||
| United States, Canada, Europe, Australia, or New Zealand | 195 (71) | 192 (70) | 278 (65) | 278 (65) |
| Latin America or Asia | 81 (29) | 82 (30) | 150 (35) | 149 (35) |
| Race or ethnic group — no. (%)† | ||||
| Asian | 19 (7) | 26 (9) | 29 (7) | 33 (8) |
| Black | 1 (<1) | 5 (2) | 3 (1) | 6 (1) |
| Native American or Alaska Native | 2 (1) | 6 (2) | 4 (1) | 10 (2) |
| White | 224 (81) | 207 (76) | 339 (79) | 331 (78) |
| Multiple | 1 (<1) | 1 (<1) | 2 (<1) | 2 (<1) |
| Other | 10 (4) | 9 (3) | 22 (5) | 20 (5) |
| Not reported | 19 (7) | 20 (7) | 29 (7) | 25 (6) |
| IMDC risk category — no. (%)‡ | ||||
| Intermediate | 209 (76) | 208 (76) | 321 (75) | 321 (75) |
| Poor | 67 (24) | 66 (24) | 107 (25) | 106 (25) |
| Karnofsky performance-status score — no. (%)§ | ||||
| 90 or 100 | 162 (59) | 178 (65) | 251 (59) | 269 (63) |
| 70 or 80 | 113 (41) | 95 (35) | 174 (41) | 157 (37) |
| <70 | 0 | 0 | 1 (<1) | 0 |
| Missing data | 1 (<1) | 1 (<1) | 2 (<1) | 1 (<1) |
| PD-L1 tumor proportion score — no. (%)¶ | ||||
| <1% | 182 (66) | 168 (61) | 272 (64) | 264 (62) |
| ≥1% | 50 (18) | 62 (23) | 84 (20) | 94 (22) |
| Could not be determined or data were missing | 44 (16) | 44 (16) | 72 (17) | 69 (16) |
| Previous nephrectomy — no. (%) | 177 (64) | 176 (64) | 278 (65) | 276 (65) |
| No. of organs with target and nontarget lesions — no. (%)|| | ||||
| 1 | 73 (26) | 78 (28) | 116 (27) | 114 (27) |
| >2 | 201 (73) | 194 (71) | 308 (72) | 309 (72) |
| Missing data | 2 (1) | 2 (1) | 4 (1) | 4 (1) |
| Median sum of diameters of target lesions (range) — mm|| | 87.4 (11.5–468.5) | 84.8 (10.8–392.4) | 94.7 (10.1–468.5) | 89.7 (10.8–392.4) |
| Most common sites of target and nontarget lesions — no. (%)|| | ||||
| Lung | 180 (65) | 186 (68) | 277 (65) | 282 (66) |
| Lymph node | 130 (47) | 119 (43) | 203 (47) | 185 (43) |
| Kidney** | 109 (39) | 100 (36) | 168 (39) | 155 (36) |
| Liver | 47 (17) | 39 (14) | 74 (17) | 66 (15) |
| Bone | 33 (12) | 57 (21) | 54 (13) | 83 (19) |
| Adrenal | 33 (12) | 40 (15) | 57 (13) | 55 (13) |
Patients were assigned to receive cabozantinib in addition to nivolumab and ipilimumab (experimental group) or placebo in addition to nivolumab and ipilimumab (control group). The progression-free survival population included the first 550 patients who had undergone randomization, and the intention-to-treat population included all the patients who had undergone randomization. Percentages may not total 100 because of rounding.
Race or ethnic group was reported by the patients.
The International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) risk category was determined with the use of the IxRS interactive voice- and Web-based response system.
Karnofsky performance-status scores range from 0 to 100, with lower scores reflecting greater disability.
The programmed death ligand 1 (PD-L1) tumor proportion score is the percentage of viable tumor cells that show PD-L1 membrane stain ing of any intensity.
The data were determined by an independent radiology committee whose members were unaware of trial-group assignments.
Target and nontarget lesions could include the primary tumor.
The prespecified 249th occurrence of disease progression or death was observed on August 23, 2021, after a median follow-up of 14.9 months (range, 10.8 to 26.0), as determined retrospectively at the time of the expansion of the progression-free survival population in early 2022. These events informed the analyses of the primary end point and the associated subgroup analyses. A more recent cutoff date of January 31, 2022, was used for the analyses of additional efficacy and safety end points, with a median follow-up of 17.7 months (range, 10.2 to 31.3) in the intention-to-treat population and 20.2 months (range, 16.1 to 31.3) in the progression-free survival population. At the time of data cutoff, in the intention-to-treat population, 43% of the patients in the experimental group and 39% in the control group were continuing to receive at least one component of a trial regimen (Fig. S1). Among patients in the intention-to-treat population, including those who were receiving a trial agent at the time of data cutoff, 30% in the experimental group and 32% in the control group were receiving subsequent systemic therapy (Table S3).
EFFICACY
The probability of progression-free survival at 12 months was 0.57 (95% confidence interval [CI], 0.50 to 0.63) in the experimental group and 0.49 (95% CI, 0.42 to 0.55) in the control group (hazard ratio for disease progression or death, 0.73; 95% CI, 0.57 to 0.94; P = 0.01) (Fig. 1). The median progression-free survival was not reached (95% CI, 14.0 months to could not be estimated) in the experimental group and was 11.3 months (95% CI, 7.7 to 18.2) in the control group. In prespecified subgroup analyses, the progression-free survival benefit associated with the addition of cabozantinib to nivolumab and ipilimumab was maintained, except in the subgroup of patients who had poor IMDC risk (Fig. 2 and Fig. S2).
Figure 1. Final Analysis of Progression-free Survival (Progression-free Survival Population).
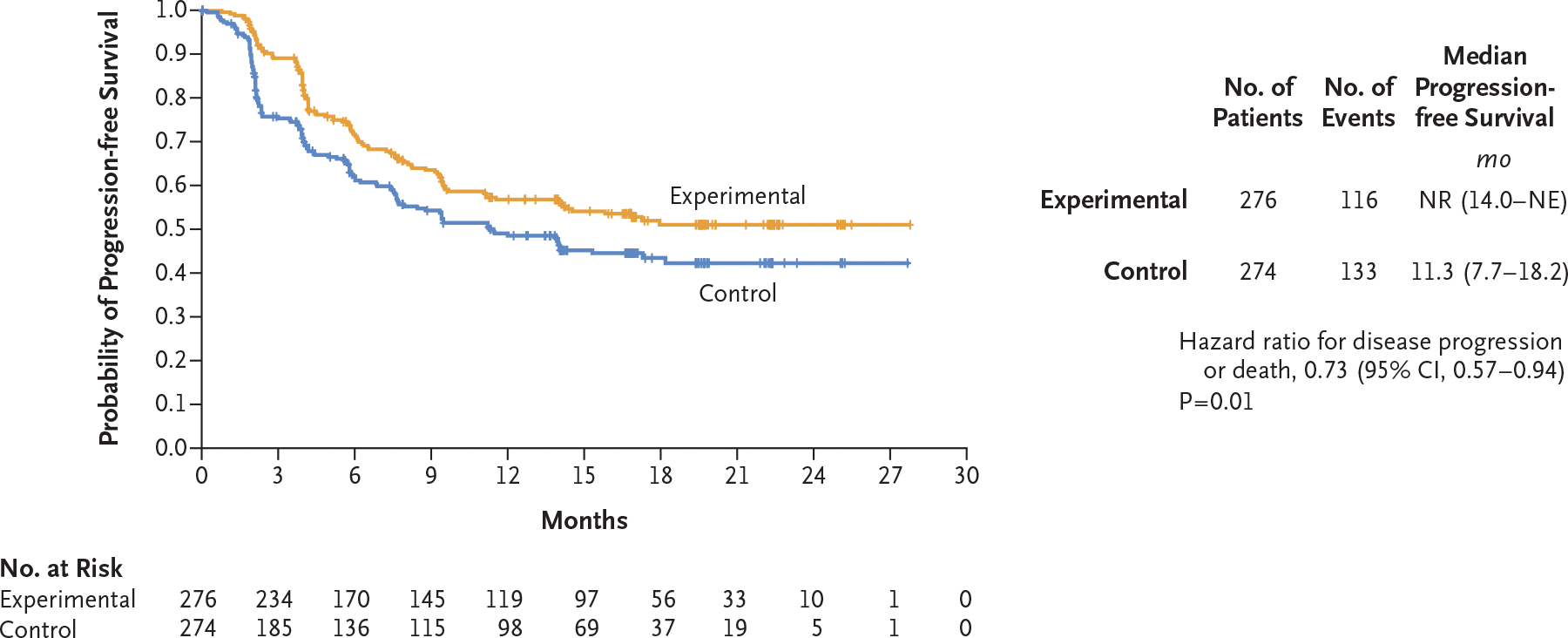
Patients were assigned to receive cabozantinib in addition to nivolumab and ipilimumab (experimental group) or placebo in addition to nivolumab and ipilimumab (control group). The progression-free survival population included the first 550 patients who had undergone randomization. A total of 249 events (disease progression or death) occurred after a median follow-up of 14.9 months. The date of the 249th event was August 23, 2021. Events were adjudicated by an independent radiology committee whose members were unaware of trial-group assignments. NE denotes could not be estimated, and NR not reached.
Figure 2. Progression-free Survival in Prespecified Subgroups (Progression-free Survival Population).
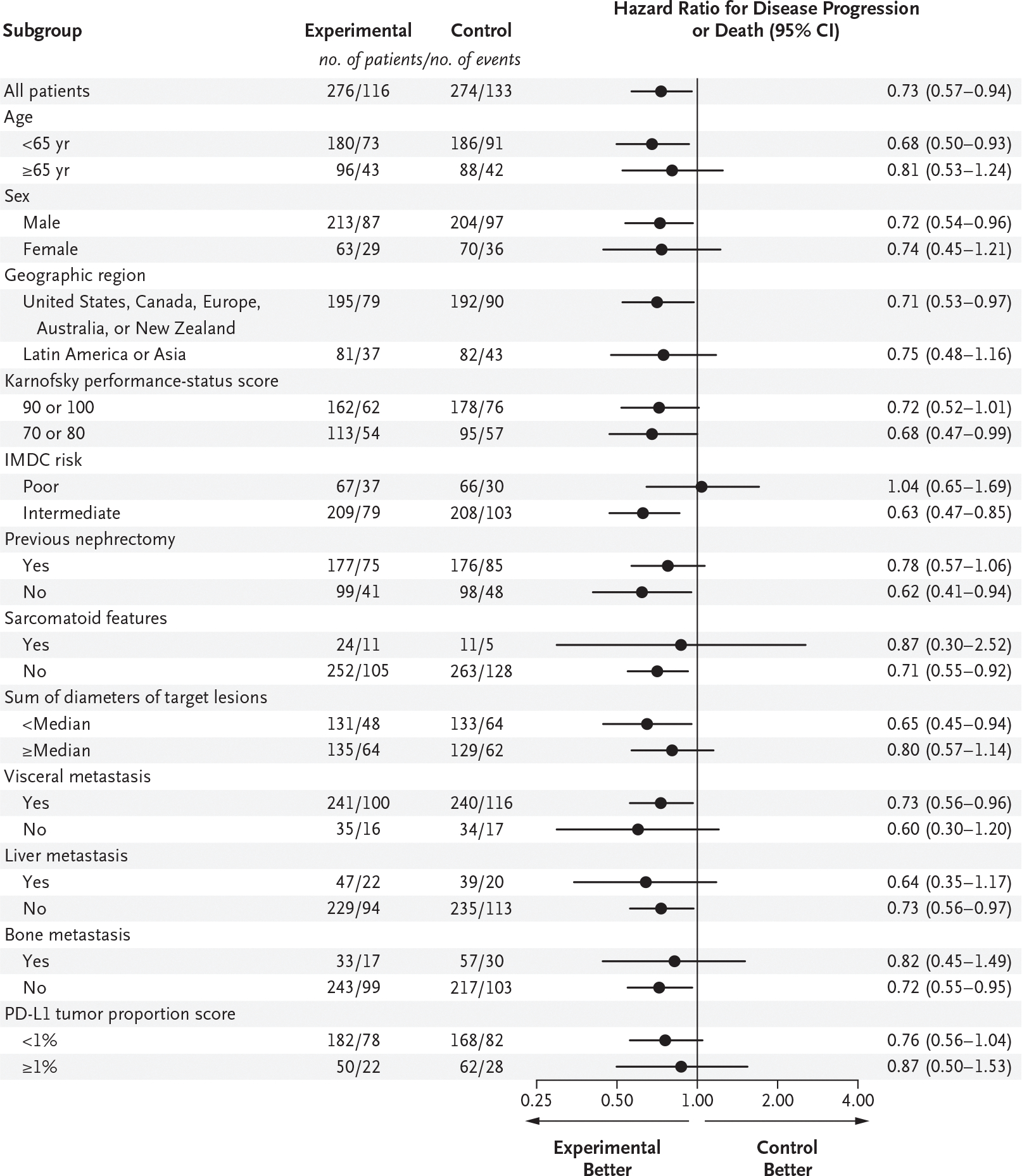
Patients were assigned to receive cabozantinib in addition to nivolumab and ipilimumab (experimental group) or placebo in addition to nivolumab and ipilimumab (control group). The progression-free survival population included the first 550 patients who had undergone randomization. A total of 249 events (disease progression or death) occurred after a median follow-up of 14.9 months. The date of the 249th event was August 23, 2021. Events were adjudicated by an independent radiology committee whose members were unaware of trial-group assignments. No adjustments were made for multiplicity, and confidence intervals should not be used in place of hypothesis tests. Karnofsky performance-status scores range from 0 to 100, with lower scores reflecting greater disability. The International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) risk category was determined with the use of the IxRS interactive voice- and Web-based response system. The programmed death ligand 1 (PD-L1) tumor proportion score is the percentage of viable tumor cells that show PD-L1 membrane staining of any intensity.
In supportive analyses in the progression-free survival population, which included all the events that had occurred by the time of data cutoff, the median progression-free survival as determined by blinded independent review was 16.9 months (95% CI, 11.5 to could not be estimated) in the experimental group and 11.3 months (95% CI, 7.7 to 14.0) in the control group (hazard ratio for disease progression or death, 0.74; 95% CI, 0.58 to 0.94) (Fig. S3). The median progression-free survival as determined by investigators was 13.8 months (95% CI, 9.7 to 15.9) and 11.2 months (95% CI, 7.6 to 14.0), respectively (hazard ratio for disease progression or death, 0.79; 95% CI, 0.63 to 0.98) (Fig. S4). In the intention-to-treat population, the median progression-free survival according to blinded independent review was 15.3 months (95% CI, 12.7 to 22.5) in the experimental group and 11.3 months (95% CI, 9.3 to 14.0) in the control group (hazard ratio for disease progression or death, 0.74; 95% CI, 0.61 to 0.90) (Fig. S5).
In the progression-free survival population, 43% (95% CI, 37 to 49) of the patients in the experimental group and 36% (95% CI, 30 to 42) in the control group had a response, according to blinded independent review; 3% of the patients in both groups had a complete response (Table 2). In prespecified subgroup analyses of response, results were generally consistent with those in the progression-free survival population, with the exception of patients who were 65 years of age or older, had poor IMDC risk, or had a PD-L1 tumor proportion score (the percentage of viable tumor cells that show PD-L1 membrane staining of any intensity) of 1% or greater (Fig. S6).
Table 2.
Tumor Response (Progression-free Survival Population).*
| Variable | Experimental (N = 276) | Control (N = 274) |
|---|---|---|
| Objective response (95% CI) — % | 43 (37–49) | 36 (30–42) |
| Best overall response — no. (%) | ||
| Complete response | 7 (3) | 9 (3) |
| Partial response | 112 (41) | 89 (32) |
| Stable disease | 119 (43) | 100 (36) |
| Progressive disease | 23 (8) | 55 (20) |
| Could not be evaluated or data were missing | 15 (5) | 21 (8) |
| Disease control — no. (%)† | 238 (86) | 198 (72) |
| Median time to response (range) — mo | 2.4 (1.5–17.1) | 2.3 (1.9–16.8) |
| Median duration of response (95% CI) — mo | NR (20.2-NR) | NR (NE-NE) |
Patients were assigned to receive cabozantinib in addition to nivolumab and ipilimumab (experimental group) or placebo in addition to nivolumab and ipilimumab (control group). Responses were assessed by an independent radiology committee according to the Response Evaluation Criteria in Solid Tumors, version 1.1; members of the committee were unaware of trial-group assignments. Complete and partial responses were confirmed. The data-cutoff date was January 31, 2022. Percentages may not total 100 because of rounding. NE denotes could not be estimated, and NR not reached.
Disease control was defined as a complete response, partial response, or stable disease as the best overall response.
Response outcomes as assessed by the investigators were consistent with those as determined by blinded independent review. A response was observed in 50% (95% CI, 44 to 56) of the patients in the experimental group and in 41% (95% CI, 35 to 47) in the control group (Table S4).
EXPOSURE AND SAFETY
The safety population included all the patients who received any component of the assigned regimen and no unassigned agents (426 patients in the experimental group and 424 in the control group). The median duration of exposure to the trial regimen was 10.9 months (range, 0.2 to 28.5) in the experimental group and 10.3 months (range, 0.1 to 28.1) in the control group (Table S5). The median average daily dose of cabozantinib was 23.2 mg, and the median average daily dose of placebo was 36.1 mg. A total of 29% of the patients in the experimental group and 41% in the control group continued to receive trial treatment after disease progression. Dose modifications due to adverse events were more frequent in the experimental group than in the control group (91% vs. 71%), as were dose delays of any trial-regimen component (90% vs. 70%) and dose reductions of cabozantinib or placebo (54% vs. 20%).
An adverse event of any cause occurred in nearly all the patients in the safety population (Table 3). A grade 3 or 4 adverse event occurred in 79% of the patients in the experimental group and in 56% in the control group. Grade 3 or 4 adverse events that occurred more frequently in the experimental group than in the control group included increased alanine aminotransferase level (27% in the experimental group and 6% in the control group), increased aspartate aminotransferase level (20% and 5%, respectively), and hypertension (10% and 3%, respectively).
Table 3.
Adverse Events (Safety Population).*
| Event | Experimental (N = 426) | Control (N = 424) | ||
|---|---|---|---|---|
| Any Grade | Grade 3 or 4 | Any Grade | Grade 3 or 4 | |
| number of patients (percent) | ||||
| Any event | 425 (100) | 337 (79) | 424 (100) | 236 (56) |
| Diarrhea | 212 (50) | 24 (6) | 98 (23) | 15 (4) |
| Alanine aminotransferase increased | 208 (49) | 113 (27) | 82 (19) | 26 (6) |
| Aspartate aminotransferase increased | 195 (46) | 87 (20) | 73 (17) | 21 (5) |
| Fatigue | 123 (29) | 12 (3) | 120 (28) | 10 (2) |
| Palmar-plantar erythrodysesthesia | 119 (28) | 14 (3) | 21 (5) | 0 |
| Hypertension | 116 (27) | 43 (10) | 35 (8) | 13 (3) |
| Hypothyroidism | 113 (27) | 1 (<1) | 70 (17) | 0 |
| Decreased appetite | 109 (26) | 2 (<1) | 74 (17) | 2 (<1) |
| Nausea | 108 (25) | 5 (1) | 103 (24) | 8 (2) |
| Rash | 102 (24) | 10 (2) | 95 (22) | 3 (1) |
| Lipase increased | 100 (23) | 41 (10) | 64 (15) | 27 (6) |
| Asthenia | 99 (23) | 10 (2) | 65 (15) | 8 (2) |
| Pruritus | 99 (23) | 0 | 128 (30) | 1 (<1) |
| Amylase increased | 95 (22) | 23 (5) | 59 (14) | 12 (3) |
| Arthralgia | 76 (18) | 5 (1) | 70 (17) | 7 (2) |
| Pyrexia | 75 (18) | 2 (<1) | 56 (13) | 0 |
| Anemia | 70 (16) | 15 (4) | 84 (20) | 26 (6) |
| Vomiting | 69 (16) | 3 (1) | 67 (16) | 4 (1) |
| Blood creatinine increased | 68 (16) | 4 (1) | 57 (13) | 3 (1) |
| Stomatitis | 68 (16) | 6 (1) | 11 (3) | 0 |
| Abdominal pain | 66 (15) | 8 (2) | 38 (9) | 3 (1) |
| Headache | 64 (15) | 4 (1) | 55 (13) | 3 (1) |
| Constipation | 63 (15) | 0 | 61 (14) | 0 |
| Back pain | 59 (14) | 5 (1) | 59 (14) | 3 (1) |
| Dysphonia | 57 (13) | 0 | 13 (3) | 0 |
| Hyperthyroidism | 54 (13) | 2 (<1) | 50 (12) | 1 (<1) |
| Cough | 52 (12) | 1 (<1) | 50 (12) | 0 |
| Mucosal inflammation | 50 (12) | 2 (<1) | 15 (4) | 1 (<1) |
| Hyponatremia | 49 (12) | 13 (3) | 38 (9) | 12 (3) |
| γ-Glutamyltransferase increased | 45 (11) | 18 (4) | 26 (6) | 11 (3) |
| Blood bilirubin increased | 44 (10) | 7 (2) | 15 (4) | 3 (1) |
| Dyspnea | 44 (10) | 2 (<1) | 45 (11) | 10 (2) |
| Blood alkaline phosphatase increased | 43 (10) | 6 (1) | 15 (4) | 2 (<1) |
| Dysgeusia | 43 (10) | 0 | 16 (4) | 0 |
Patients were assigned to receive cabozantinib in addition to nivolumab and ipilimumab (experimental group) or placebo in addition to nivolumab and ipilimumab (control group). Shown are adverse events of any cause that occurred in at least 10% of the patients in either trial group from the first dose of any trial agent through 30 days after the last dose. The safety population included all the patients who received any amount of any trial agent. Events are listed in descending order of frequency in the experimental group. A grade 5 event of any cause occurred in 27 patients (6%) in the experimental group and in 34 (8%) in the control group. Adverse events were classified according to the Medical Dictionary for Regulatory Activities, version 23.0. The data-cutoff date was January 31, 2022.
Adverse events that were considered by the investigator to be related to the trial regimen occurred in 99% of the patients in the experimental group and in 91% in the control group, with 73% and 41% of the patients in the two groups, respectively, having grade 3 or 4 events (Table S6). Adverse events related to the trial regimen that led to discontinuation of any component occurred in 45% of the patients in the experimental group and in 24% in the control group: 28% in the experimental group discontinued cabozantinib, 14% in the control group discontinued placebo, 26% in the experimental group and 18% in the control group discontinued nivolumab, and 30% in the experimental group and 12% in the control group discontinued ipilimumab. A total of 12% of the patients in the experimental group and 5% in the control group discontinued all components owing to a single adverse event. The most common adverse events related to the trial regimen that led to discontinuation of any component were increased alanine aminotransferase level (19% in the experimental group and 4% in the control group), increased aspartate aminotransferase level (15% and 3%, respectively), and immune-mediated hepatitis (5% and 1%, respectively).
Adverse events of special interest that were related to nivolumab, ipilimumab, or both occurred in 83% of the patients in the experimental group and in 65% in the control group and included increased alanine aminotransferase level (42% and 16%, respectively), increased aspartate aminotransferase level (38% and 14%, respectively), and diarrhea (26% and 13%, respectively) (Table S7). Concomitant high-dose glucocorticoid treatment (≥40 mg of prednisone or equivalent per day) was used for adverse events for any duration in 58% of the patients in the experimental group and in 35% in the control group, and for more than 30 days in 18% and 10%, respectively. Adverse events of special interest that were related to cabozantinib are shown in Table S8.
Deaths that were related to the trial regimen and occurred within 100 days before the last dose of the trial regimen were observed in 5 patients (1%) in the experimental group (one event each of acute hepatic failure, gastrointestinal hemorrhage, hepatic failure, immune-mediated hepatitis, and respiratory failure) and in 4 patients (1%) in the control group (one event each of myocarditis, perforated ulcer, renal failure, and sudden death). Additional details are provided in Table S9.
DISCUSSION
In this randomized, double-blind, phase 3 trial involving patients with previously untreated advanced renal-cell carcinoma who had intermediate or poor IMDC risk, progression-free survival was significantly longer with cabozantinib in combination with nivolumab and ipilimumab than with nivolumab and ipilimumab alone. The results of subgroup analyses in previous studies that evaluated tyrosine kinase inhibitors in combination with anti–PD-1 agents for the first-line treatment of advanced renal-cell carcinoma have suggested that the benefit of immune checkpoint inhibitor combinations as compared with sunitinib is greater for patients with poor risk than for patients with intermediate risk.6,8,9,16 In this trial, the addition of a tyrosine kinase inhibitor to the immune checkpoint inhibitor doublet of nivolumab and ipilimumab did not appear to provide a benefit over nivolumab and ipilimumab alone in the subgroup of patients with poor risk, although interpretability is limited by the small subgroup size, and differences in populations confound comparisons across trials. Exploratory analyses according to individual IMDC risk factors may provide further insight into patient characteristics associated with outcomes.
A total of 43% of the patients in the experimental group and 36% in the control group had a response. Progressive disease as the best response occurred in 8% and 20%, respectively. The percentage of patients with a complete response was similar in the two groups and was relatively low as compared with that for nivolumab plus ipilimumab in the CheckMate 214 trial6,17,18 and that in pivotal studies of tyrosine kinase inhibitor and immune checkpoint inhibitor combinations.8,9,16 In this trial, the proportion of patients who had not undergone nephrectomy was higher than that reported in other phase 3 trials in advanced renal-cell carcinoma,8,9,16 and kidney tumor was a frequent persistent lesion, which may have reduced the percentage of patients with a complete response.19 Whether the percentage of patients with a complete response will increase after longer follow-up remains to be seen.
Adverse events were more frequent and were of higher grade in the experimental group than in the control group. Adverse events associated with both cabozantinib and immune checkpoint inhibitors (e.g., hepatic-enzyme elevation, diarrhea, and skin effects) and discontinuations owing to adverse events were more common in the experimental group than in the control group. Elevated liver aminotransferase levels were the most common grade 3 or 4 adverse events in the experimental group and the most common events related to the trial regimen that led to discontinuation of any component. High-dose glucocorticoids to manage immune-mediated adverse events were used more frequently in the experimental group. Deaths related to the trial regimen were infrequent in both groups. These observations emphasize the importance of frequent routine monitoring after treatment initiation, dose modifications, and supportive care for the management of adverse events.
Limitations of this trial include the relatively short duration of follow-up in the intention-to-treat population and the fact that the data for overall survival (which is an important end point for clinical implementation of the regimen) are not mature, given the number of observed events and duration of follow-up. Follow-up for survival may be protracted given the median overall survival of 47 months among patients who received nivolumab and ipilimumab in the CheckMate 214 trial.18 Other limitations include the difficulty in assigning causality of overlapping adverse effects to cabozantinib or to the immune checkpoint inhibitors, since both can result in hepatic and gastrointestinal effects. The concealment of the trial-group assignments from the investigators may also have posed challenges for management of adverse events. Other ongoing phase 3 trials are evaluating combinations of tyrosine kinase inhibitors and immune checkpoint inhibitors either as a triplet regimen (ClinicalTrials.gov number, NCT04736706) or administered in a sequential manner (NCT03793166), the results of which may further inform the use of combination therapies.
Among patients with previously untreated advanced renal-cell carcinoma who had intermediate or poor prognostic risk, treatment with cabozantinib plus nivolumab and ipilimumab resulted in significantly longer progression-free survival than treatment with nivolumab and ipilimumab alone. Adverse events and discontinuations were more frequent in the experimental group than in the control group. Follow-up for overall survival is ongoing.
Supplementary Material
Acknowledgments
Supported by Exelixis. Bristol-Myers Squibb provided nivolumab and ipilimumab. Dr. Choueiri is supported in part by the Dana–Farber/Harvard Cancer Center Kidney Specialized Program of Research Excellence (2P50CA101942-16), the National Cancer Institute Program Project (5P30CA006516-56), the Kohlberg Chair at Harvard Medical School, the Trust Family, Michael Brigham, Pan Mass Challenge, Hinda and Arthur Marcus, and Loker Pinard Funds for Kidney Cancer Research at the Dana–Farber Cancer Institute. Patients treated at Memorial Sloan Kettering Cancer Center were supported in part by a Memorial Sloan Kettering Cancer Center Support Grant–Core Grant (P30 CA008748).
We thank the patients, their families, and site staff; and Julie Lougheed, Ph.D., of Exelixis and Namiko Abe, Ph.D., and Alexus Rivas, Pharm.D., of Fishawack Communications (part of Fishawack Health) for editorial and medical writing support with an earlier version of the manuscript.
Appendix
The authors’ full names and academic degrees are as follows: Toni K. Choueiri, M.D., Thomas Powles, M.D., Laurence Albiges, M.D., Ph.D., Mauricio Burotto, M.D., Cezary Szczylik, M.D., Ph.D., Bogdan Zurawski, M.D., Ph.D., Eduardo Yanez Ruiz, M.D., Marco Maruzzo, M.D., Ph.D., Alberto Suarez Zaizar, M.D., Luis E. Fein, M.D., Fabio A. Schutz, M.D., Daniel Y.C. Heng, M.D., Fong Wang, M.D., Ph.D., Fabio Mataveli, M.D., Ph.D., Yu-Lin Chang, M.S., Maximiliano van Kooten Losio, M.D., Ph.D., Cristina Suarez, M.D., Ph.D., and Robert J. Motzer, M.D.
The authors’ affiliations are as follows: the Department of Medical Oncology, Lank Center for Genitourinary Oncology, Dana–Farber Cancer Institute, Brigham and Women’s Hospital, and Harvard Medical School — both in Boston (T.K.C.); the Department of Genitourinary Oncology, Barts Cancer Institute, Cancer Research UK Experimental Cancer Medicine Centre, Queen Mary University of London, Royal Free NHS Trust, London (T.P.); the Department of Cancer Medicine, Institut Gustave Roussy, Université Paris-Saclay, Villejuif, France (L.A.); Bradford Hill Clinical Research Center, Santiago (M.B.), and James Lind Centro de Investigación del Cáncer, Temuco (E.Y.R.) — both in Chile; the Postgraduate Medical Center, Department of Oncology, European Health Center, Otwock, Warsaw (C. Szczylik), and the Department of Outpatient Chemotherapy, Professor Franciszek Łukaszczyk Oncology Center, Bydgoszcz (B.Z.) — both in Poland; Oncology Unit 1, Department of Oncology, Istituto Oncologico Veneto IRCCS, Padua, Italy (M.M.); Consultorio de Medicina Especializada, Benito Juárez, Mexico City (A.S.Z.); Instituto de Oncología de Rosario, Rosario, Argentina (L.E.F.); Latin American Cooperative Oncology Group, Porto Alegre, and Beneficência Portuguesa de São Paulo, São Paulo — both in Brazil (F.A.S.); the Department of Medical Oncology, Tom Baker Cancer Centre, University of Calgary, Calgary, AB, Canada (D.Y.C.H.); Exelixis, Alameda, CA (F.W., F.M., Y.-L.C.); Bristol Myers Squibb, Boudry, Switzerland (M.K.L.); the Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, Barcelona (C. Suarez); and the Department of Medicine, Memorial Sloan Kettering Cancer Center, New York (R.J.M.).
Footnotes
Presented in part at the European Society of Medical Oncology Congress, Paris, September 9–13, 2022.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
A data sharing statement provided by the authors is available with the full text of this article at NEJM.org.
The authors’ full names, academic degrees, and affiliations are listed in the Appendix.
Contributor Information
T.K. Choueiri, Department of Medical Oncology, Lank Center for Genitourinary Oncology, Dana–Farber Cancer Institute, Brigham and Women’s Hospital, Boston Harvard Medical School, Boston.
T. Powles, Department of Genitourinary Oncology, Barts Cancer Institute, Cancer Research UK Experimental Cancer Medicine Centre, Queen Mary University of London, Royal Free NHS Trust, London
L. Albiges, Department of Cancer Medicine, Institut Gustave Roussy, Université Paris-Saclay, Villejuif, France
M. Burotto, Bradford Hill Clinical Research Center, Santiago, Chile
C. Szczylik, Postgraduate Medical Center Department of Oncology, European Health Center, Otwock, Warsaw, Poland
B. Zurawski, Department of Outpatient Chemotherapy, Professor Franciszek Łukaszczyk Oncology Center, Bydgoszcz, Poland
E. Yanez Ruiz, James Lind Centro de Investigación del Cáncer, Temuco, Chile
M. Maruzzo, Oncology Unit 1, Department of Oncology, Istituto Oncologico Veneto IRCCS, Padua, Italy
A. Suarez Zaizar, Consultorio de Medicina Especializada, Benito Juárez, Mexico City
L.E. Fein, Instituto de Oncología de Rosario, Rosario, Argentina, Brazil
F.A. Schutz, Latin American Cooperative Oncology Group, Porto Alegre, and Beneficência Portuguesa de São Paulo, São Paulo, Brazil
D.Y.C. Heng, Department of Medical Oncology, Tom Baker Cancer Centre, University of Calgary, Calgary, AB, Canada
F. Wang, Exelixis, Alameda, CA
F. Mataveli, Exelixis, Alameda, CA
Y.-L. Chang, Exelixis, Alameda, CA
M. van Kooten Losio, Bristol Myers Squibb, Boudry, Switzerland
C. Suarez, Department of Medical Oncology, Vall d’Hebron Institute of Oncology, Vall d’Hebron University Hospital, Vall d’Hebron Barcelona Hospital Campus, Barcelona
R.J. Motzer, Department of Medicine, Memorial Sloan Kettering Cancer Center, New York
References
- 1.Choueiri TK, Motzer RJ. Systemic therapy for metastatic renal-cell carcinoma. N Engl J Med 2017;376:354–66. [DOI] [PubMed] [Google Scholar]
- 2.Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 2007;356:115–24. [DOI] [PubMed] [Google Scholar]
- 3.Choueiri TK, Escudier B, Powles T, et al. Cabozantinib versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1814–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med 2015;373:1803–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the Alliance A031203 CABOSUN trial. J Clin Oncol 2017;35:591–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2021;384:829–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motzer R, Alekseev B, Rha S-Y, et al. Lenvatinib plus pembrolizumab or everolimus for advanced renal cell carcinoma. N Engl J Med 2021;384:1289–300. [DOI] [PubMed] [Google Scholar]
- 10.Ravi P, Bakouny Z, Schmidt A, Choueiri TK. Novel therapeutic approaches and the evolution of drug development in advanced kidney cancer. Cancer J 2020;26:464–70. [DOI] [PubMed] [Google Scholar]
- 11.Choueiri TK, Atkins MB, Bakouny Z, et al. Summary from the first Kidney Cancer Research Summit, September 12–13, 2019: a focus on translational research. J Natl Cancer Inst 2021;113:234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun DA, Bakouny Z, Hirsch L, et al. Beyond conventional immune-checkpoint inhibition — novel immunotherapies for renal cell carcinoma. Nat Rev Clin Oncol 2021;18:199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther 2011;10:2298–308. [DOI] [PubMed] [Google Scholar]
- 14.Escudier B Nivolumab plus ipilimumab plus cabozantinib for previously untreated advanced renal cell carcinoma: results from a discontinued study arm of CheckMate 9ER. In: Proceedings and Abstracts of the 2022 European International Kidney Cancer Symposium, April 22–24, 2022. Antwerp, Belgium: Kidney Cancer Association, 2022; (https://www.eventscribe.net/2022/IKCSEU22/fsPopup.asp?PresentationID=1029663&query=escudier&mode=presinfo). abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krigsfeld GS, Prince EA, Pratt J, et al. Analysis of real-world PD-L1 IHC 28–8 and 22C3 pharmDx assay utilisation, turnaround times and analytical concordance across multiple tumour types. J Clin Pathol 2020;73:656–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med 2019;380:1116–27. [DOI] [PubMed] [Google Scholar]
- 17.Albiges L, Tannir NM, Burotto M, et al. Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 2020;5(6):e001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, McDermott DF, Escudier B, et al. Conditional survival and long-term efficacy with nivolumab plus ipilimumab versus sunitinib in patients with advanced renal cell carcinoma. Cancer 2022;128:2085–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer RJ, Rini BI, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 2019;20:1370–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


