Abstract
Purpose
In COPD, exacerbation of the disorder causes a deterioration in the quality-of-life and worsens respiratory dysfunction, leading to a poor prognosis. In recent years, nutritional indices have been reported as significant prognostic factors in various chronic diseases. However, the relationship between nutritional indicators and prognosis in elderly subjects with COPD has not been investigated.
Patients and methods
We enrolled 91 subjects who received COPD assessment tests (CAT), spirometry, blood tests, and multidetector computed tomography (MDCT). We divided the subjects into two groups according to age (<75 years (n=57) and ≥ 75 years (n=34)). The prognostic nutritional index (PNI) was used to assess immune-nutritional status and was calculated as 10 x serum albumin + 0.005 x total lymphocyte count. We then examined the relationship between PNI and clinical parameters, including exacerbation events.
Results
There was no significant correlation between the PNI and CAT, the FEV1%pred, or low attenuation volume percentage (LAV%). In the elderly group, there were significant differences between the groups with or without exacerbation in the CAT and PNI (p=0.008, p=0.004, respectively). FEV1%pred, neutrophil-to-lymphocyte ratio (NLR) and LAV% did not differ between the two groups. The analytical model combining CAT and PNI improved the prediction of exacerbations in the elderly subjects (p=0.0068).
Conclusion
In elderly subjects with COPD, CAT were associated significantly with the risk of COPD exacerbation, with PNI also a potential predictor. The combined assessment of CAT and PNI may be a useful prognostic tool in subjects with COPD.
Keywords: chronic obstructive pulmonary disease, exacerbation, prognostic nutritional index
Introduction
Chronic obstructive pulmonary disease (COPD) is the fourth leading cause of death in the world and is associated with increasing economic costs and social burdens.1,2 Although spirometry is the gold standard for the clinical measurement of COPD, it does not provide sufficient essential information on exacerbation, leading to worsening respiratory impairment and prognosis.3
Exacerbation of COPD decreases the quality-of-life, causes respiratory dysfunction, and adversely affects prognosis. Numerous studies have discussed exacerbation and risk factors. The prognostic factors of COPD vary between smoking status, degree of dyspnea, severity of airflow limitation, degree of emphysema, exercise tolerability, and physical inactivity.4–6 Recent reports have also described the impact of the neutrophil-lymphocyte ratio (NLR)7 and the existence of asthmatic components.8
The nutritional status of a patient has also been established as a prognostic factor for various chronic diseases.9 Since Japan and Western countries have entered the era of an unprecedented ultra-aging society,10 there has been a focus on nutritional status as a risk factor for chronic diseases, especially in elderly patients.11 For example, malnutrition is common in elderly patients with heart failure, with evidence showing that nutritional status is strongly linked to their prognosis.12
In 1980, Buzby et al suggested the concept of the prognostic nutritional index (PNI).13 Subsequently, Onodera et al proposed that PNI could be calculated easily using serum albumin level and total lymphocyte count and showed that the index was a risk indicator for postoperative complications and prognosis for patients undergoing gastrointestinal cancer surgery.14 Subsequent studies reported a relationship between PNI and clinical outcomes in various other malignant diseases.15–17
Nutritional status has also been reported to be a significant prognostic indicator in patients with COPD.18 Previously, the body mass index (BMI) was the health indicator mainly used to assess prognosis in COPD.19 It remains unclear, however, whether the PNI is related to COPD exacerbations and prognosis of elderly patients.
Therefore, the aim of the present study was to elucidate whether PNI was associated with exacerbation and to clarify the clinical value of assessing the immune-nutritional status in elderly patients with COPD.
Methods
Subjects
This prospective, observational study enrolled 139 subjects who presented to Chiba University Hospital from March 2014 to June 2019 for management of COPD. The subjects were required to meet all of the following inclusion criteria: (a) ≥ 40 years; (b) smoking history ≥ 10 pack-years; (c) COPD diagnosed or suspected to have COPD based on subjective symptoms/other findings/pulmonary function tests/imaging findings; (d) no history of acute exacerbation or hospitalization within 2 months. Exclusion criteria were any of the following: (a) obvious respiratory diseases other than COPD; (b) any malignancy within the past 3 years; (c) severe heart failure; (d) currently receiving oral systemic corticosteroids; (e) deemed unsuitable for inclusion by investigators for any other reason.
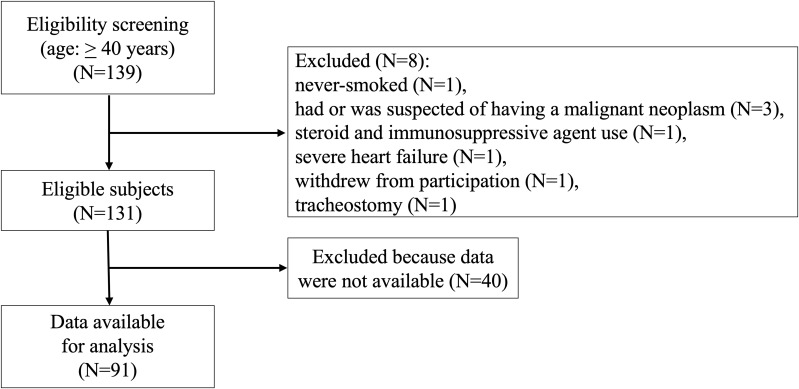
At enrollment, eight subjects were excluded for the following reasons: one never-smoked without a history of smoking; three with malignant neoplasms; one prescribed a steroid and an immunosuppressive agent; one with severe heart failure; and one with a tracheostomy. During the follow-up period, one subject was excluded as they decided to withdraw from participating in the study. 40 subjects were excluded by the end of follow-up due to discrepancies in each data set, such as a discontinuation of hospital visits, confirmation of worsening symptoms, COPD assessment test (CAT), pulmonary function tests, and chest CT scans. Finally, 91 subjects with COPD were enrolled in the study (Figure 1).
Figure 1.
Flow chart of the study subjects.
The diagnosis of COPD was made comprehensively by respiratory specialists according to the recommendations of the American Thoracic Society (ATS) and European Respiratory Society (ERS),20 that included the subject’s smoking history, respiratory symptoms including dyspnea, cough and sputum, physical examination, spirometry results. Pulmonary function tests (PFTs) were performed using a Fudac-60 (Fukuda Denshi, Tokyo, Japan) and included forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1), which were expressed as their predicted values based on the Japanese Respiratory Society (JRS) guidelines.21 The subjects underwent a CAT, PFTs, laboratory tests, and multidetector computed tomography (MDCT) at the time of enrollment. The CAT is a simple tool for comprehensively evaluating the clinical symptoms of subjects with COPD,22,23 and consists of eight items rated on a scale of 0 to 5, and is an excellent indicator for assessing respiratory and extrapulmonary symptoms.
This study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the Chiba University School of Medicine approved the study protocol (approval number, 857). Written, informed consent was obtained from all the study subjects.
Calculation of the Prognostic Nutritional Index
As an immune-nutritional index, the PNI was calculated as follows, in accordance with the original description of Onodera et al14 10 x serum albumin level (g/dL) + 0.005 x total lymphocyte count (×103/μL). Originally, the PNI was used to assess surgical risk for gastrointestinal malignancies, with a resection anastomosis contraindicated in subjects with PNI values ≤ 40.
MDCT Scanning and CT Measurements of Low Attenuation Volume
All CT studies were performed on a 64-MDCT Aquilion ONE and Aquilion PRIME (Canon Medical Systems, Otawara, Tochigi, Japan) at full inspiration, with no contrast medium being used. The CT parameters used were as follows: collimation 120kV; CT-AEC; gantry rotation time, 0.5s; and beam pitch, 0.70–0.83. All the images were reconstructed using standard reconstruction algorithms, with a slice thickness of 0.5 mm and a reconstruction interval of 0.5 mm. The reconstructed CT images were transferred to a commercial workstation (Ziostation2, Ziosoft Ltd., Tokyo, Japan). Total lung volume (LV) and low attenuation volume (LAV) were measured based on a threshold of −950 Hounsfield units (HU). LAV% was calculated as 100%×LAV/LV.24
Clinical Events
We investigated the clinical events during a two-year observation period. The clinical events were identified as COPD exacerbations. An exacerbation was defined as a worsening of COPD requiring a change in therapy, the use of antibiotics or steroids, and/or hospital admission.25
Statistical Analysis
The results were expressed as means ± standard deviation (± SD) or as medians (interquartile range [IQR]) as appropriate. Categorical data were expressed as the number (%). After confirming the normality of the study parameter data, the correlations between PNI and CAT, and LAV%, and FEV1%pred were assessed by Spearman rank correlation analysis, as appropriate. For clinical outcome, comparisons between the subject groups with or without an exacerbation requiring treatment changes were performed using the Mann–Whitney U-test for continuous variables and the chi-square test or Fisher exact test for categorical variables.
For the selection of variables in the multivariate analysis, we used those that showed a causal relationship with COPD exacerbations based on previous reports7,25–27 and those that were significant in our univariate analysis. The association of selected variables with clinical outcome was assessed by univariate logistic regression analysis, and significant variables in this analysis then entered into a multivariate logistic regression analysis.
The variables selected were age, CAT as a comprehensive indicator of subjective symptoms, NLR as an indicator of the level of inflammation, PNI as an indicator of nutrition and immunity, FEV1% pred as an indicator of airflow limitation to measure disease severity, and LAV% as an imaging indicator to determine the degree of emphysema. In accordance with previous reports, CAT was treated as a continuous variable in the analysis.28,29
In the group with subjects aged > 75 years, we also calculated the areas under the receiver operating curve (ROC) analysis for using PNI and CAT to predict COPD exacerbation, with the optimal cut-off of the PNI and CAT determined by the Youden index. In addition, based on Akaike’s information criterion,30 we examined whether PNI improved the predictive accuracy of the analytical model for exacerbations in the elderly group. The original model was constructed with items that were significant in the univariate analysis. A statistically significant increase in the global chi-square of the model was interpreted as indicating an increase in prognostic value.31,32 All the statistical analyses were performed by JMP Pro version 14.0 software (SAS Institute, Cary, NC, USA). For all the statistical analyses, the level of significance was set at p <0.05.
Results
Subject Characteristics
The clinical characteristics of all the subjects are presented in Table 1.
Table 1.
Characteristics of the Total Subjects (Comparison of the Group Aged<75 Years vs the Group Aged ≧75 Years)
| All Subjects (n=91) | Age<75 yr (n=57) | Age ≥75 yr (n=34) | P-value | |
|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | ||
| Age (yr) | 71.3 ± 7.6 | 67.2 ± 6.2 | 78.3 ± 3.2 | <0.0001 |
| Female gender, n (%) | 4 (4.4%) | 3 (5.3%) | 1 (2.9%) | 0.601 |
| Pack-years | 59.6 ± 33.8 | 57.2 ± 30.7 | 63.6 ± 38.5 | 0.447 |
| Body weight (kg) | 63.3 ± 11.7 | 63.9 ± 11.9 | 62.3 ± 11.5 | 0.682 |
| Height (m) | 1.65 ± 0.07 | 1.66 ± 0.07 | 1.641 ± 0.062 | 0.296 |
| BMI (kg/m2) | 23.1 ± 3.8 | 23.2 ± 3.8 | 23.1 ± 3.8 | 1.000 |
| CAT score | 12.0 ± 8.6 | 12.3 ± 8.5 | 11.5 ± 8.8 | 0.665 |
| VC (L) | 3.4 ± 0.8 | 3.6 ± 0.8 | 3.0 ± 0.7 | 0.002 |
| %VC | 95.1 ± 17.6 | 97.6 ± 16.5 | 91.0 ± 18.9 | 0.141 |
| FVC (L) | 3.3 ± 0.9 | 3.5 ± 0.8 | 2.9 ± 0.7 | 0.001 |
| FEV1 (L) | 1.8 ± 0.8 | 1.8 ± 0.8 | 1.7 ± 0.7 | 0.446 |
| FEV1 / FVC (%) | 53.3 ± 15.6 | 51.3 ± 16.2 | 56.7 ± 14.1 | 0.111 |
| FEV1% pred | 64.6 ± 24.8 | 63.2 ± 23.7 | 67.1 ± 26.7 | 0.697 |
| LAV% | 16.3 ± 15.0 | 19.8 ± 16.2 | 10.5 ± 10.7 | 0.01 |
| WBC count (×103/μL) | 6724 ± 1592 | 6647 ± 1515 | 6853 ± 1728 | 0.631 |
| Neutrophil count (×103/μL) | 4116 ± 1327 | 4008 ± 1230 | 4297 ± 1476 | 0.412 |
| Lymphocyte count (×103/μL) | 1853 ± 689 | 1885 ± 687 | 1799 ± 700 | 0.514 |
| Eosinophil count (×103/μL) | 266 ± 292 | 263 ± 295 | 271 ± 290 | 0.838 |
| Serum albumin (g/dL) | 4.1 ± 0.4 | 4.2 ± 0.3 | 4.0 ± 0.5 | 0.02 |
| NLR | 2.5 ± 1.3 | 2.4 ± 1.1 | 2.8 ± 1.5 | 0.365 |
| PNI | 50.8 ± 5.9 | 51.7 ± 5.7 | 49.2 ± 6.0 | 0.09 |
| Treatment | 70 (76.9%) | 45 (78.9%) | 25 (73.5%) | 0.553 |
| LAMA | 14 | 9 | 5 | |
| LABA | 5 | 2 | 3 | |
| ICS | 0 | 0 | 0 | |
| LAMA/LABA | 13 | 9 | 4 | |
| ICS/LABA | 9 | 5 | 4 | |
| ICS/LAMA | 1 | 0 | 1 | |
| ICS/LAMA/LABA | 28 | 20 | 8 | |
| Exacerbations, n (%) | 31 (33.7%) | 21 (36.8%) | 10 (29.4%) | 0.469 |
| Exacerbations per 1 year | 0.4 ± 0.7 | 0.5 ± 0.7 | 0.3 ± 0.5 | 0.345 |
Notes: Data are presented as n or mean ± SD. P-values were determined using the Mann–Whitney U-test for continuous variables and the chi-square test or Fisher exact test for categorical variables. Values in bold indicate statistical significance at the P < 0.05 level.
Abbreviations: yr, year; BMI, body mass index; CAT, COPD assessment test; VC, vital capacity; %VC, vital capacity percentage; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; FEV1 /FVC (%), forced expiratory volume % in one second; FEV1% pred, forced expiratory volume in one second in % predicted; LAV%, low attenuation volume percentage; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; ICS, Inhaled corticosteroids.
In the total subjects (n=91), 34 subjects were aged ≥75 years (33 males and 1 female; mean age, 78.3 ± 3.2 years) and 57 subjects were aged < 75 years (54 males and 3 females; mean age, 67.2 ± 6.2 years). There were no differences between the two groups for sex, body weight, height, BMI, and CAT score. For the pulmonary function tests, VC and FVC were lower in subjects ≥75 years than in those aged <75 years. LAV% in the group aged ≥75 years was also lower than that measured in subjects <75 years. For nutritional status, there was no significant difference in PNI between the two groups, although albumin was slightly lower in the group aged ≥75 years. An exacerbation occurred in 21 of 57 of the younger subjects (36.8%) compared to 10 of the 34 older subjects (29.4%). The median duration of follow-up was 735 days.
Comparisons of the clinical indices in subjects aged ≥75 years with or without an exacerbation are shown in Table 2. In the exacerbation group, the CAT score was significantly higher and PNI was significantly lower compared to those measured in the non-exacerbation group. The serum albumin levels were also significantly different between the two groups.
Table 2.
Characteristics of COPD Subjects in the Group Aged ≧75 Years (Comparison of the Exacerbation and Non-Exacerbation Groups)
| All Subjects (n=34) | Exacerbation + (n=10) | Exacerbation - (n=24) | P-value | |
|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | ||
| Age (yr) | 78.3 ± 3.2 | 78.9 ± 4.7 | 78.0 ± 2.5 | 0.909 |
| Female gender, n (%) | 1 (2.9%) | 1 (10.0%) | 0 (0%) | 0.116 |
| Pack-years | 63.6 ± 38.5 | 66.8 ± 39.2 | 62.3 ± 39.0 | 0.733 |
| Body weight (kg) | 62.3 ± 11.5 | 58.2 ± 12.2 | 64.0 ± 11.0 | 0.168 |
| Height (m) | 1.64 ± 0.06 | 1.65 ± 0.08 | 1.64 ± 0.05 | 0.461 |
| BMI (kg/m2) | 23.1 ± 3.8 | 21.5 ± 4.3 | 23.7 ± 3.4 | 0.219 |
| CAT score | 11.5 ± 8.8 | 18.0 ± 8.7 | 8.8 ± 7.4 | 0.009 |
| VC (L) | 3.0 ± 0.7 | 2.8 ± 0.8 | 3.1 ± 0.6 | 0.374 |
| %VC | 91.0 ± 18.9 | 84.7 ± 20.6 | 93.6 ± 17.9 | 0.265 |
| FVC (L) | 2.9 ± 0.7 | 2.7 ± 0.9 | 3.0 ± 0.7 | 0.307 |
| FEV1 (L) | 1.7 ± 0.7 | 1.5 ± 0.7 | 1.8 ± 0.7 | 0.299 |
| FEV1 / FVC (%) | 56.7 ± 14.1 | 53.7 ± 13.5 | 58.0 ± 14.4 | 0.461 |
| FEV1% pred | 67.1 ± 26.7 | 59.1 ± 27.5 | 70.4 ± 26.2 | 0.219 |
| LAV% | 10.5 ± 10.7 | 11.0 ± 14.5 | 10.3 ± 9.0 | 0.484 |
| WBC count (×103/μL) | 6853 ± 1728 | 7040 ± 1487 | 6775 ± 1843 | 0.664 |
| Neutrophil count (×103/μL) | 4297 ± 1476 | 4702 ± 1599 | 4128 ± 1422 | 0.317 |
| Lymphocyte count (×103/μL) | 1799 ± 700 | 1536 ± 606 | 1909 ± 719 | 0.146 |
| Eosinophil count (×103/μL) | 271 ± 290 | 317 ± 472 | 252 ± 178 | 0.484 |
| Serum albumin (g/dL) | 4.0 ± 0.5 | 3.7 ± 0.6 | 4.2 ± 0.4 | 0.016 |
| NLR | 2.8 ± 1.5 | 3.6 ± 2.1 | 2.4 ± 1.1 | 0.086 |
| PNI | 49.2 ± 6.0 | 44.4 ± 6.6 | 51.2 ± 4.5 | 0.004 |
| Treatment | 25 (73.5%) | 7 (70.0%) | 18 (75.0%) | 0.763 |
| LAMA | 5 | 1 | 4 | |
| LABA | 3 | 0 | 3 | |
| ICS | 0 | 0 | 0 | |
| LAMA/LABA | 4 | 0 | 4 | |
| ICS/LABA | 4 | 2 | 2 | |
| ICS/LAMA | 1 | 0 | 1 | |
| ICS/LAMA/LABA | 8 | 4 | 4 | |
| Exacerbations per 1 year | 0.3 ± 0.5 | 1.1 ± 0.3 | 0 | <0.0001 |
Notes: Data are presented as n or mean ± SD. P-values were determined by the Mann–Whitney U-test for continuous variables and the chi-square test or Fisher exact test for categorical variables. Values in bold indicate statistical significance at the p < 0.05 level.
Abbreviations: yr, year; BMI, body mass index; CAT, COPD assessment test; VC, vital capacity; %VC, vital capacity percentage; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; FEV1 /FVC (%), forced expiratory volume % in one second; FEV1% pred, forced expiratory volume in one second in % predicted; LAV%, low attenuation volume percentage; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; ICS, Inhaled corticosteroids.
Comparisons of the clinical indices in subjects aged <75 years with or without an exacerbation are shown in Table 3. In the exacerbation group, the CAT score, LAV%, WBC count, and neutrophil count were significantly higher than those measured in the non-exacerbation group. The BMI was significantly lower in the exacerbation group than in the non-exacerbation group. Obstructive impairment was also significantly more severe in the exacerbation group compared to that observed in the non-exacerbation group.
Table 3.
Characteristics of COPD Subjects in the Group Aged <75 Years (Comparison of the Exacerbation and Non-Exacerbation Groups)
| All Subjects (n=57) | Exacerbation + (n=21) | Exacerbation - (n=36) | P-value | |
|---|---|---|---|---|
| Mean (±SD) | Mean (±SD) | Mean (±SD) | ||
| Age (yr) | 67.2 ± 6.2 | 66.8 ± 7.5 | 67.4 ± 5.5 | 0.752 |
| Female gender, n (%) | 3 (5.3%) | 1 (4.8%) | 2 (5.4%) | 0.897 |
| Pack-years | 57.2 ± 30.7 | 62.0 ± 34.2 | 54.4 ± 28.6 | 0.267 |
| Body weight (kg) | 63.9 ± 11.9 | 60.2 ± 10.3 | 66.0 ± 12.3 | 0.06 |
| Height (m) | 1.66 ± 0.07 | 1.66 ± 0.08 | 1.65 ± 0.07 | 0.728 |
| BMI (kg/m2) | 23.2 ± 3.8 | 21.6 ± 2.5 | 24.1 ± 4.1 | 0.009 |
| CAT score | 12.3 ± 8.5 | 15.7 ± 9.3 | 10.4 ± 7.4 | 0.044 |
| VC (L) | 3.6 ± 0.8 | 3.4 ± 0.8 | 3.6 ± 0.8 | 0.234 |
| %VC | 97.6 ± 16.5 | 92.3 ± 17.4 | 100.7 ± 15.4 | 0.178 |
| FVC (L) | 3.5 ± 0.8 | 3.3 ± 0.8 | 3.6 ± 0.8 | 0.165 |
| FEV1 (L) | 1.8 ± 0.8 | 1.5 ± 0.7 | 2.0 ± 0.8 | 0.005 |
| FEV1 / FVC (%) | 51.3 ± 16.2 | 43.3 ± 18.0 | 55.9 ± 13.2 | 0.002 |
| FEV1% pred | 63.2 ± 23.7 | 50.3 ± 24.8 | 70.7 ± 19.8 | 0.002 |
| LAV% | 19.8 ± 16.2 | 30.3 ± 16.6 | 13.7 ± 12.6 | 0.0006 |
| WBC count (×103/μL) | 6647 ± 1515 | 7129 ± 1058 | 6367 ± 1677 | 0.027 |
| Neutrophil count (×103/μL) | 4008 ± 1230 | 4342 ± 988 | 3814 ± 1327 | 0.055 |
| Lymphocyte count (×103/μL) | 1885 ± 687 | 1918 ± 682 | 1866 ± 698 | 0.836 |
| Eosinophil count (×103/μL) | 263 ± 295 | 361 ± 442 | 206 ± 136 | 0.15 |
| Serum albumin (g/dL) | 4.2 ± 0.3 | 4.2 ± 0.4 | 4.2 ± 0.3 | 0.803 |
| NLR | 2.4 ± 1.1 | 2.5 ± 1.0 | 2.3 ± 1.2 | 0.237 |
| PNI | 51.7 ± 5.7 | 51.6 ± 6.2 | 51.8 ± 5.4 | 0.980 |
| Treatment | 45 (78.9%) | 19 (90.5%) | 26 (72.2%) | 0.103 |
| LAMA | 9 | 3 | 6 | |
| LABA | 2 | 0 | 2 | |
| ICS | 0 | 0 | 0 | |
| LAMA/LABA | 9 | 4 | 5 | |
| ICS/LABA | 5 | 2 | 3 | |
| ICS/LAMA | 0 | 0 | 0 | |
| ICS/LAMA/LABA | 20 | 10 | 10 | |
| Exacerbations per 1 year | 0.5 ± 0.7 | 1.3 ± 0.5 | 0 | <0.0001 |
Notes: Data are presented as n or mean ± SD. P-values were determined by the Mann–Whitney U-test for continuous variables and the chi-square test or Fisher exact test for categorical variables. Values in bold indicate statistical significance at the P < 0.05 level.
Abbreviations: yr, year; BMI, body mass index; CAT, COPD assessment test; VC, vital capacity; %VC, vital capacity percentage; FVC, forced vital capacity; FEV1, forced expiratory volume in one second; FEV1 /FVC (%), forced expiratory volume % in one second; FEV1% pred, forced expiratory volume in one second in % predicted; LAV%, low attenuation volume percentage; WBC, white blood cell; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; ICS, Inhaled corticosteroids.
Associations Between the Prognostic Nutritional Index and Airflow Limitation and Pulmonary Emphysema
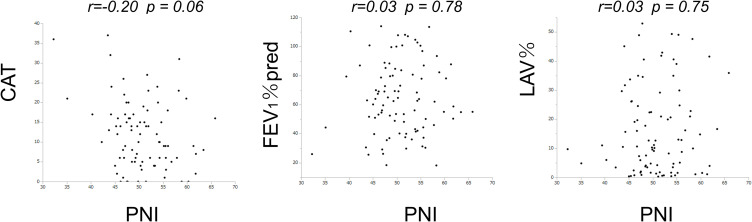
The associations between the PNI and other clinical indices in the entire cohort of subjects are shown in Figure 2. The PNI showed no significant association with CAT (r= −0.20, p=0.06). Similarly, there was no significant correlation between PNI and either FEV1%pred (r= 0.03, p=0.78) or LAV% (r=0.03, p= 0.75).
Figure 2.
The relationship between PNI and CAT and FEV1%pred and LAV% in all the subjects.
Notes: The associations between the PNI and other clinical indices are shown for the entire cohort of subjects (n=91).
Abbreviations: PNI, prognostic nutritional index; CAT, COPD assessment test; FEV1, forced expiratory volume in one second; LAV%, low attenuation volume percentage.
Clinical Factors Associated with Exacerbation Events
The results of the univariate and multivariate analyses for exacerbation in the two groups grouped according to age are shown in Table 4 and Table 5.
Table 4.
Results of the Logistic Analysis of COPD Exacerbation in the ≧75 Year Age Group
| Subjects Age≧75 | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P value | Odds Ratio | 95% CI | P value | |
| Age | 1.08 | 0.86–1.15 | 0.48 | |||
| CAT | 1.15 | 1.03–1.29 | 0.013 | 1.15 | 1.00–1.33 | 0.047 |
| NLR | 1.70 | 1.01–2.86 | 0.046 | 0.99 | 0.45–2.17 | 0.974 |
| PNI | 0.74 | 0.58–0.96 | 0.023 | 0.73 | 0.52–1.02 | 0.063 |
| FEV1% pred | 0.98 | 0.95–1.01 | 0.26 | |||
| LAV% | 1.00 | 0.94–1.08 | 0.87 | |||
Abbreviations: CAT, COPD assessment test; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; FEV1, forced expiratory volume in one second; LAV%, low attenuation volume percentage;
Table 5.
Results of the Logistic Analysis of COPD Exacerbation in the<75 Year Age Group
| Subjects Age<75 | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P value | Odds Ratio | 95% CI | P value | |
| Age | 0.99 | 0.90–1.07 | 0.73 | |||
| CAT | 1.08 | 1.00–1.7 | 0.038 | 1.03 | 0.95–1.12 | 0.43 |
| NLR | 1.22 | 0.76–1.98 | 0.41 | |||
| PNI | 1.00 | 0.90–1.09 | 0.91 | |||
| FEV1% pred | 0.98 | 0.93–0.99 | 0.003 | 1.00 | 0.96–1.95 | 0.91 |
| LAV% | 1.08 | 1.03–1.12 | 0.0007 | 1.07 | 1.00–1.14 | 0.039 |
Abbreviations: CAT, COPD assessment test; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; FEV1, forced expiratory volume in one second; LAV%, low attenuation volume percentage.
In the group aged ≥75 years, univariate analysis showed that CAT (OR = 1.15, 95% CI = 1.03–1.29, p = 0.013), NLR (OR=1.70, 95% CI=1.01–2.86, p =0.046), and PNI (OR = 0.74, 95% CI =0.58–0.96, p = 0.023) were associated significantly with exacerbation. Multivariate analysis identified CAT as an independent factor for exacerbation (OR = 1.15, 95% CI = 1.00–1.33, p = 0.047), while PNI showed a trend of being associated with exacerbation (OR = 0.73, 95% CI =0.52–1.02, p = 0.063).
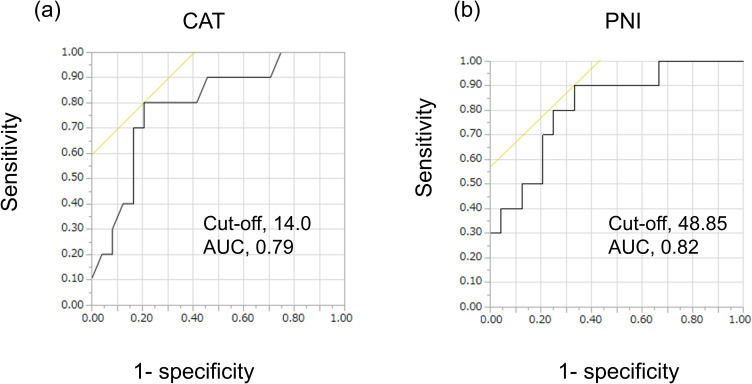
ROC curve analysis was performed for the group ≥75 years of age to evaluate the accuracy of CAT and PNI and to identify the value of the cut-off points. ROC curve analysis in the group ≥75 years of age demonstrated that the optimal cut-off values for CAT and PNI to predict events were 14.0 (sensitivity, 0.80; 1-specificity, 0.21; area under the curve [AUC], 0.79; Figure 3a) and 48.85 (sensitivity, 0.90; 1-specificity, 0.33; AUC, 0.82; Figure 3b), respectively.
Figure 3.
Receiver operating characteristic curve (ROC) analysis of CAT and PNI in the group ≥75 years of age.
Notes: ROC curve analysis was used to evaluate the sensitivity and specificity of CAT (a) and PNI (b) for COPD exacerbation in the group ≥75 years of age. (n=34).
Abbreviations: PNI, prognostic nutritional index; CAT, COPD assessment test.
In the group aged <75 years, univariate analysis showed that CAT (OR = 1.083, 95% CI = 1.00–1.17, p= 0.038), FEV1% pred (OR = 0.957, 95% CI = 0.931–0.986, p = 0.003) and LAV% (OR = 1.077, 95% CI = 1.032–1.123, p = 0.0007) were associated significantly with exacerbation. Multivariate analysis identified LAV% as an independent factor for exacerbation (OR = 1.074, 95% CI = 1.003–1.149, p = 0.031).
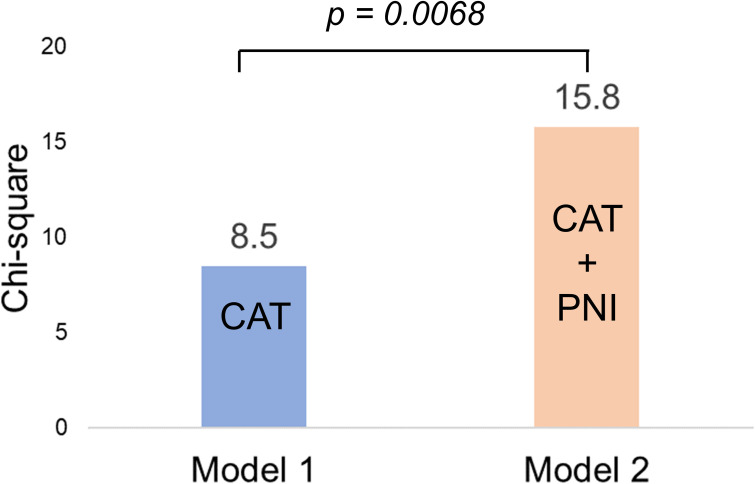
Incremental Value of the PNI in Elderly Subjects with COPD
The incremental value of the PNI in the group ≥75 years of age is shown in Figure 4. CAT was selected as the conventional variable for the prognostic model from Table 4 (Model 1). The addition of PNI to the conventional variable significantly improved the prognostic utility of the model (Model 2, p = 0.0084).
Figure 4.
The incremental benefit of adding PNI to the CAT, to predict exacerbation events in the group ≥75 years of age.
Notes: Model 1, based on CAT, was improved significantly by the addition of the PNI (model 2) in the group ≥75 years of age (n=34).
Abbreviations: CAT, COPD assessment test; PNI, prognostic nutritional index.
Discussion
The key findings of this study were as follows. There were differences in factors related to COPD exacerbations between subjects aged ≥75 years and those aged < 75 years. High CAT values were associated with exacerbation in elderly subjects with COPD, while a combined model of CAT and PNI more accurately predicted COPD exacerbations. In other words, a low PNI in combination with a high CAT increased the risk of an exacerbation in elderly subjects with COPD.
The PNI was calculated using serum albumin levels and the total circulating lymphocyte count. The PNI score has been validated and reported to correlate significantly with subjective global assessment (SGA), a well-established nutritional index and also other nutritional screening tools.33–35 While the SGA is a simple, inexpensive, and quick assessment, it is a subjective assessment that requires skill and experience. In contrast, the PNI is based on the results of peripheral blood tests, thereby allowing a physician to easily and objectively assess the immune-nutritional status of their patients.
Many earlier studies have described that nutrition and immune status are associated closely with tumor progression and prognosis. The PNI reflects both the nutritional and immunological status of patients with a variety of malignancies.36,37 In the respiratory field, the PNI of pretreatment in patients with non-small cell lung cancer has been shown to have prognostic value.38 Recently, it was reported that a lower PNI at the time of admission was related to the risk of mortality in subjects with severe COVID-19.39,40 Regarding chronic respiratory diseases, the present study has shown that the PNI score raised the risk of exacerbation in elderly with COPD.
The CAT is a simple tool for comprehensive evaluation of the clinical symptoms in COPD patients.22 The CAT consists of eight items and is an excellent indicator for assessing respiratory symptoms, such as cough, phlegm, and dyspnea associated with exercise, and extra-pulmonary symptoms, such as activity, insomnia, and energy level. The CAT scores were increased by greater than five points during exacerbations.26 In the present study, the PNI was not associated with CAT in all the subjects. Yoshikawa et al reported that nutritional status using the Mini-nutritional Assessment Short-form predicted COPD exacerbations independently of CAT.41 The present study also showed no significant relationship between PNI and either FEV1%pred and low attenuation volume percentage (LAV%). These findings might indicate that PNI may be an independent predictor from CAT, the degree of obstructive impairment, and emphysema, which have conventionally been reported as predictive factors.42
A cut-off value of 40 for the PNI has been proposed for perioperative evaluation of gastrointestinal cancers.14 In the current study, the cut-off value for PNI for the presence of exacerbations in subjects aged ≥ 75 years was set at 48.85, a value higher than that used for other diseases. A prognostic pretreatment cut-off of 45.5 was used for lung cancer patients receiving immune checkpoint inhibitors.43 Recently, a lower cut-off value of 33 was used for survival in patients with severe COVID-related pneumonia.39 Since COPD is a gradually progressive disease, the serum albumin levels and lymphocyte counts were obtained at the time of consultation, and we speculate that this may be one of the reasons for the higher cut-off values compared to those used for other diseases. Therefore, patients with COPD should be cautioned against exacerbations even if the initial PNI value is higher than previously reported for other diseases.
In the group aged < 75 years, the conventional variables, such as emphysema and the degree of airflow limitation, contributed to exacerbations.25 Most previous studies have focused on relatively young to middle-aged patients, with only a minority carried out in older subjects.44
In the present study we identified prognostic factors for elderly subjects and showed that the complex of subjective symptom scores and immune-nutritional status contributed more to exacerbations than the severity of COPD. The prognosis of elderly patients with chronic diseases is not necessarily determined by the severity of the underlying disease.45 Poor nutritional status is associated with a poor prognosis and exercise intolerance in elderly patients with COPD.41,46 More severe subjective symptoms are associated with a worse prognosis.22 We propose that the combination of CAT and PNI would allow assessment of the risk of exacerbation more accurately in elderly patients with COPD.
Serum albumin level is a conventional marker of nutrition and has been reported to be associated with a poor outcome in many different diseases. On the other hand, the NLR is a reliable indicator of systematic inflammation and has also been investigated as a diagnostic and prognostic marker in COPD.47 Chronic inflammation causes recruitment of the main white blood cell populations, neutrophils and lymphocytes. These factors participate actively in the pathophysiological mechanisms of COPD. A higher NLR has been reported widely to be associated with poor survival in patients with various diseases.48,49 However, the NLR only reflects the inflammation status. In recent studies, the PNI was shown to be superior to the NLR as a prognostic marker in many cancer patients.36,50 PNI as a nutrition plus immunity indicator may therefore be more useful in the evaluation of COPD than either nutrition or immunity alone.
There has been a focus on the role of the asthma-COPD overlap (ACO), including eosinophilia in COPD, and the impact of asthmatic components on the clinical course of COPD.51 Recent studies have reported that eosinophilia in COPD does not affect the clinical course, however, this view is controversial.52 The present study also showed no relationship between eosinophilia and clinical outcome. In recent years, the clinical significance of personalized treatment in COPD has been proposed, with some studies showing that appropriate treatment, such as triple therapy in patients with asthmatic components, decreases symptoms and the risk of exacerbations, thereby contributing to an improved clinical course.53
Limitations
Our study had some limitations. First, the study only enrolled a relatively small number of subjects and was a preliminary and exploratory investigation conducted at a single institute. Second, the observational period was relatively short. Third, because of the small number of subjects we could not fully evaluate the differences in gender, each treatment, or the rate of smoking cessation. Fourth, we could not perform nutritional intervention. Nutritional intervention may be important for preventing COPD exacerbations, especially in elderly patients.54 And finally, previous papers reporting an association between PNI and prognosis used unadjusted multivariate analyses.43,45 The present study also performed unadjusted multivariate analysis of each item. However, this was a single-center, small-group, exploratory study and therefore a larger cohort is needed to confirm the results of the current study that would also require more rigorous examination and exclusion of confounding factors. Further prospective studies on larger study populations and a longer observational period are therefore required to confirm our results.
Conclusions
In elderly subjects with COPD, CAT was associated significantly with the risk of COPD exacerbation, with PNI also a potential predictor. The combined assessment of CAT and PNI may be a useful prognostic tool in patients with COPD.
Acknowledgment
The abstract of this paper was presented at the 2020 European Respiratory Society (ERS) International congress in session “Respiratory viruses in the”pre-COVID-19 “era”, with interim findings. The poster’s abstract was published in “Poster Abstracts” in European Respiratory Journal 2020; 56: Suppl. 64, 5114. https://erj.ersjournals.com/content/56/suppl_64/5114.
Funding Statement
This research was partially supported by the Ministry of Education, Science, Sports and Culture, Grant-in-Aid for Scientific Research (C) (19K12816 and 22K12836), and the Chiba Foundation for Health Promotion & Disease Prevention (No.1272). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
BMI, body mass index; CAT, chronic obstructive pulmonary disease assessment test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FEV1/FVC, forced expiratory volume in 1 second per forced vital capacity; FVC, forced vital capacity; GOLD, Global Initiative for Chronic Pulmonary Obstructive Lung Disease; ICS, inhaled corticosteroid; LAA, low attenuation area; LABA, long-acting β-2 agonist; LAMA, long-acting muscarinic antagonists; LAV, low attenuation volume; LAV%, low attenuation volume percentage; LV, lung volume; MDCT, multi-detector row computed tomography; MRI, magnetic resonance imaging; NLR, neutrophil-to-lymphocyte ratio; PFT, pulmonary function testing; PNI, prognostic nutritional index; TLA, total lung area; VC, vital capacity; %VC, vital capacity percentage; WBC, white blood cell.
Data Sharing Statement
The data sets analyzed during the current study are available from the corresponding author upon reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The author reports no conflicts of interest in this work.
References
- 1.May SM, Li JTC. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc. 2015;36(1):4–10. doi: 10.2500/aap.2015.36.3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh D, Agusti A, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J. 2019;53(5). doi: 10.1183/13993003.00164-2019 [DOI] [PubMed] [Google Scholar]
- 3.Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925–931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nishimura K, Izumi T, Tsukino M, Oga T. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest. 2002;121(5):1434–1440. doi: 10.1378/chest.121.5.1434 [DOI] [PubMed] [Google Scholar]
- 5.Haruna A, Muro S, Nakano Y, et al. CT scan findings of emphysema predict mortality in COPD. Chest. 2010;138(3):635–640. doi: 10.1378/chest.09-2836 [DOI] [PubMed] [Google Scholar]
- 6.Waschki B, Kirsten A, Holz O, et al. Physical activity is the strongest predictor of all-cause mortality in patients with COPD: a prospective cohort study. Chest. 2011;140(2):331–342. doi: 10.1378/chest.10-2521 [DOI] [PubMed] [Google Scholar]
- 7.Taylan M, Demir M, Kaya H, et al. Alterations of the neutrophil-lymphocyte ratio during the period of stable and acute exacerbation of chronic obstructive pulmonary disease patients. Clin Respir J. 2017;11(3):311–317. doi: 10.1111/crj.12336 [DOI] [PubMed] [Google Scholar]
- 8.Miravitlles M, Soriano JB, Ancochea J, et al. Characterisation of the overlap COPD-asthma phenotype. Focus on physical activity and health status. Respir Med. 2013;107(7):1053–1060. doi: 10.1016/j.rmed.2013.03.007 [DOI] [PubMed] [Google Scholar]
- 9.Norman K, Pichard C, Lochs H, Pirlich M. Prognostic impact of disease-related malnutrition. Clin Nutr. 2008;27(1):5–15. doi: 10.1016/j.clnu.2007.10.007 [DOI] [PubMed] [Google Scholar]
- 10.Muramatsu N, Akiyama H. Japan: super-aging society preparing for the future. Gerontologist. 2011;51(4):425–432. doi: 10.1093/geront/gnr067 [DOI] [PubMed] [Google Scholar]
- 11.Kaur D, Rasane P, Singh J, et al. Nutritional interventions for elderly and considerations for the development of geriatric foods. Curr Aging Sci. 2019;12(1):15–27. doi: 10.2174/1874609812666190521110548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng YL, Sung SH, Cheng HM, et al. Prognostic nutritional index and the risk of mortality in patients with acute heart failure. J Am Heart Assoc. 2017;6(6). doi: 10.1161/JAHA.116.004876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buzby GP, Mullen JL, Matthews DC, Hobbs CL, Rosato EF. Prognostic nutritional index in gastrointestinal surgery. Am J Surg. 1980;139(1):160–167. doi: 10.1016/0002-9610(80)90246-9 [DOI] [PubMed] [Google Scholar]
- 14.Onodera T, Goseki N, Kosaki G. ”StageIV・V (Vは大腸癌) 消化器癌の非治癒切除・姑息手術に対するTPNの適応と限界 [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–1005. Japanese. [PubMed] [Google Scholar]
- 15.Zhang Q, Bao J, Zhu ZY, Jin MX. Prognostic nutritional index as a prognostic factor in lung cancer patients receiving chemotherapy: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2021;25(18):5636–5652. doi: 10.26355/eurrev_202109_26783 [DOI] [PubMed] [Google Scholar]
- 16.Kim SI, Kim SJ, Kim SJ, Cho DS. Prognostic nutritional index and prognosis in renal cell carcinoma: a systematic review and meta-analysis. Urol Oncol. 2021;39(10):623–630. doi: 10.1016/j.urolonc.2021.05.028 [DOI] [PubMed] [Google Scholar]
- 17.Demirelli B, Babacan NA, Ercelep O, et al. Modified Glasgow prognostic score, prognostic nutritional index and ECOG performance score predicts survival better than sarcopenia, cachexia and some inflammatory indices in metastatic gastric cancer. Nutr Cancer. 2021;73(2):230–238. doi: 10.1080/01635581.2020.1749290 [DOI] [PubMed] [Google Scholar]
- 18.Landbo C, Prescott E, Lange P, Vestbo J, Almdal TP. Prognostic value of nutritional status in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160(6):1856–1861. doi: 10.1164/ajrccm.160.6.9902115 [DOI] [PubMed] [Google Scholar]
- 19.Raad S, Smith C, Allen K. Nutrition status and chronic obstructive pulmonary disease: can we move beyond the body mass index? Nutr Clin Pract. 2019;34(3):330–339. doi: 10.1002/ncp.10306 [DOI] [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26(2):319–338. doi: 10.1183/09031936.05.00034805 [DOI] [PubMed] [Google Scholar]
- 21.Nishimura M, Aizawa H, Kambe M, et al. 日本呼吸器学会呼吸機能検査ガイドライン ースパイロメトリー,フローボリューム曲線,肺拡散能カー [Guideline of respiratory function tests--spirometry, flow-volume curve, diffusion capacity of the lung]. Nihon Kokyuki Gakkai zasshi. 2004:1–56. Japanese. Japanese: https://europepmc.org/article/MED/15565748. [PubMed] [Google Scholar]
- 22.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 23.Kwon N, Amin M, Hui DS, et al. Validity of the COPD assessment test translated into local languages for Asian patients. Chest. 2013;143(3):703–710. doi: 10.1378/chest.12-0535 [DOI] [PubMed] [Google Scholar]
- 24.Shimada A, Kawata N, Sato H, et al. Dynamic quantitative magnetic resonance imaging assessment of areas of the lung during free-breathing of patients with chronic obstructive pulmonary disease. Acad Radiol. 2022;29(Suppl 2):S215–S225. doi: 10.1016/j.acra.2021.03.034 [DOI] [PubMed] [Google Scholar]
- 25.Tanabe N, Muro S, Hirai T, et al. Impact of exacerbations on emphysema progression in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2011;183(12):1653–1659. doi: 10.1164/rccm.201009-1535OC [DOI] [PubMed] [Google Scholar]
- 26.Mackay AJ, Donaldson GC, Patel AR, Jones PW, Hurst JR, Wedzicha JA. Usefulness of the chronic obstructive pulmonary disease assessment test to evaluate severity of COPD exacerbations. Am J Respir Crit Care Med. 2012;185(11):1218–1224. doi: 10.1164/rccm.201110-1843OC [DOI] [PubMed] [Google Scholar]
- 27.Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax. 2002;57(10):847–852. doi: 10.1136/thorax.57.10.847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim JU, Kim EK, Lim SY, et al. Mixed phenotype of emphysema and airway wall thickening is associated with frequent exacerbation in chronic obstructive pulmonary disease patients. Int J Chron Obstruct Pulmon Dis. 2019;14:3035–3042. doi: 10.2147/copd.S227377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang Y, Ding K, Dai Z, et al. The relationship of low-density-lipoprotein to lymphocyte ratio with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2022;17:2175–2185. doi: 10.2147/copd.S369161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akaike H. A new look at the statistical model identification. IEEE Trans Autom Control. 1974;19(6):8. doi: 10.1109/TAC.1974.1100705 [DOI] [Google Scholar]
- 31.Mochizuki Y, Tanaka H, Matsumoto K, et al. Clinical features of subclinical left ventricular systolic dysfunction in patients with diabetes mellitus. Cardiovasc Diabetol. 2015;14:37. doi: 10.1186/s12933-015-0201-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu B, Kawata T, Daimon M, et al. Prognostic value of a simple echocardiographic parameter, the right ventricular systolic to diastolic duration ratio, in patients with advanced heart failure with non-ischemic dilated cardiomyopathy. Int Heart J. 2018;59(5):968–975. doi: 10.1536/ihj.17-475 [DOI] [PubMed] [Google Scholar]
- 33.Detsky AS, McLaughlin JR, Baker JP, et al. What is subjective global assessment of nutritional status?. JPEN J Parenter Enteral Nutr. 1987;11(1):8–13. doi: 10.1177/014860718701100108 [DOI] [PubMed] [Google Scholar]
- 34.Hu Y, Yang H, Zhou Y, et al. Prediction of all-cause mortality with malnutrition assessed by nutritional screening and assessment tools in patients with heart failure: asystematic review. Nutr Metab Cardiovasc Dis. 2022;32(6):1361–1374. doi: 10.1016/j.numecd.2022.03.009 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Q, Qian L, Liu T, et al. Prevalence and prognostic value of malnutrition among elderly cancer patients using three scoring systems. Front Nutr. 2021;8:738550. doi: 10.3389/fnut.2021.738550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mirili C, Yılmaz A, Demirkan S, Bilici M, Basol Tekin S. Clinical significance of prognostic nutritional index (PNI) in malignant melanoma. Int J Clin Oncol. 2019;24(10):1301–1310. doi: 10.1007/s10147-019-01461-7 [DOI] [PubMed] [Google Scholar]
- 37.Ge K, Fang C, Zhu D, et al. The prognostic value of the Prognostic Nutritional Index (PNI) in radically resected esophagogastric junction adenocarcinoma. Nutr Cancer. 2021;73(11–12):2589–2596. doi: 10.1080/01635581.2020.1841252 [DOI] [PubMed] [Google Scholar]
- 38.Matsubara T, Takamori S, Haratake N, et al. The impact of immune-inflammation-nutritional parameters on the prognosis of non-small cell lung cancer patients treated with atezolizumab. J Thorac Dis. 2020;12(4):1520–1528. doi: 10.21037/jtd.2020.02.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei W, Wu X, Jin C, et al. Predictive significance of the Prognostic Nutritional Index (PNI) in patients with severe COVID-19. J Immunol Res. 2021;2021:9917302. doi: 10.1155/2021/9917302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu G, Zhang S, Mao Z, Wang W, Hu H. Clinical significance of nutritional risk screening for older adult patients with COVID-19. Eur J Clin Nutr. 2020;74(6):876–883. doi: 10.1038/s41430-020-0659-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yoshikawa M, Fujita Y, Yamamoto Y, et al. Mini Nutritional Assessment Short-Form predicts exacerbation frequency in patients with chronic obstructive pulmonary disease. Respirology. 2014;19(8):1198–1203. doi: 10.1111/resp.12380 [DOI] [PubMed] [Google Scholar]
- 42.Shimizu K, Tanabe N, Tho NV, et al. Per cent low attenuation volume and fractal dimension of low attenuation clusters on CT predict different long-term outcomes in COPD. Thorax. 2020;75(2):116–122. doi: 10.1136/thoraxjnl-2019-213525 [DOI] [PubMed] [Google Scholar]
- 43.Shoji F, Takeoka H, Kozuma Y, et al. Pretreatment prognostic nutritional index as a novel biomarker in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2019;136:45–51. doi: 10.1016/j.lungcan.2019.08.006 [DOI] [PubMed] [Google Scholar]
- 44.Beauchamp MK, Ellerton C, Kirkwood R, et al. Feasibility of a 6-month home-based fall prevention exercise program in older adults with COPD. Int J Chron Obstruct Pulmon Dis. 2021;16:1569–1579. doi: 10.2147/copd.S309537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kawata T, Ikeda A, Masuda H, Komatsu S. Changes in prognostic nutritional index during hospitalization and outcomes in patients with acute heart failure. Heart Vessels. 2022;37(1):61–68. doi: 10.1007/s00380-021-01888-x [DOI] [PubMed] [Google Scholar]
- 46.Matsumura T, Mitani Y, Oki Y, et al. Comparison of Geriatric Nutritional Risk Index scores on physical performance among elderly patients with chronic obstructive pulmonary disease. Heart Lung. 2015;44(6):534–538. doi: 10.1016/j.hrtlng.2015.08.004 [DOI] [PubMed] [Google Scholar]
- 47.Pascual-Gonzalez Y, Lopez-Sanchez M, Dorca J, Santos S. Defining the role of neutrophil-to-lymphocyte ratio in COPD: a systematic literature review. Int J Chron Obstruct Pulmon Dis. 2018;13:3651–3662. doi: 10.2147/COPD.S178068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu Y, Du X, Chen J, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. 2020;81(1):e6–e12. doi: 10.1016/j.jinf.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: a systematic review. J Surg Oncol. 2017;115(4):470–479. doi: 10.1002/jso.24523 [DOI] [PubMed] [Google Scholar]
- 50.Wang J, Liu Y, Mi X, Shao M, Liu L. The prognostic value of prognostic nutritional index (PNI) and neutrophil to lymphocyte ratio (NLR) for advanced non-small cell lung cancer treated with platinum-based chemotherapeutics. Ann Palliat Med. 2020;9(3):967–978. doi: 10.21037/apm.2020.04.31 [DOI] [PubMed] [Google Scholar]
- 51.Leung JM, Sin DD. Asthma-COPD overlap syndrome: pathogenesis, clinical features, and therapeutic targets. BMJ. 2017;358:j3772. doi: 10.1136/bmj.j3772 [DOI] [PubMed] [Google Scholar]
- 52.Suzuki M, Makita H, Ito YM, Nagai K, Konno S, Nishimura M. Clinical features and determinants of COPD exacerbation in the Hokkaido COPD cohort study. Multicenter study observational study research support, Non-U.S. Gov’t. Eur Respir J. 2014;43(5):1289–1297. doi: 10.1183/09031936.00110213 [DOI] [PubMed] [Google Scholar]
- 53.Park SY, Kim S, Kim JH, et al. A randomized, noninferiority trial comparing ICS + LABA with ICS + LABA + LAMA in asthma-COPD overlap (ACO) treatment: the ACO Treatment with Optimal Medications (ATOMIC) study. J Allergy Clin Immunol Pract. 2021;9(3):1304–1311.e2. doi: 10.1016/j.jaip.2020.09.066 [DOI] [PubMed] [Google Scholar]
- 54.Sugawara K, Takahashi H, Kasai C, et al. Effects of nutritional supplementation combined with low-intensity exercise in malnourished patients with COPD. Respir Med. 2010;104(12):1883–1889. doi: 10.1016/j.rmed.2010.05.008 [DOI] [PubMed] [Google Scholar]