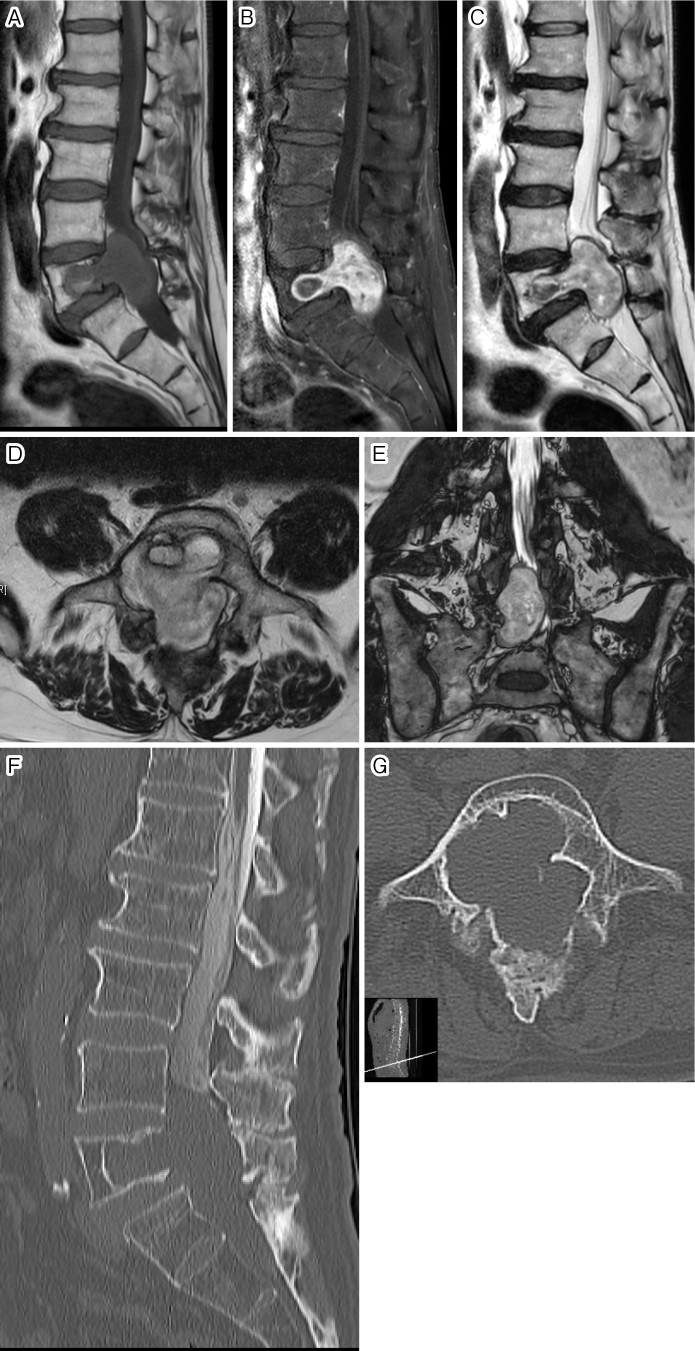
A 66-year-old man was presented at another hospital with increased urinary frequency and lower limb numbness and was diagnosed with lumbar spinal canal stenosis (LSCS). Although he received prostaglandin as treatment for cauda equine caused by LSCS, the symptoms did not resolve. Results of the subsequent magnetic resonance imaging (MRI) revealed a solid tumor in the fifth lumbar vertebra (L5) and spinal canal (Fig. 1A-E). Interestingly, the tumor did not extend to the intervertebral foramen and did not form a dumbbell shape. He was referred to our hospital for surgical treatment. A computed tomography scan after myelogram was performed to investigate the dural sac in the spinal canal. The tumor was observed to completely fill the canal such that the dural sac was not detectable (Fig. 1F and G). There was no other tumor in the canal. Transpedicular biopsy revealed a cluster of spindle cells and the partial presence of a necrotic lesion, which was suspected to be intraosseous schwannoma. Because the facet joint at L5/S1 was disrupted due to scalloping caused by the tumor, L4-S1 posterior lateral fusion (PLF) with instrumentation as well as L5 and S1 laminectomy were performed to remove the giant tumor. After fenestration, remarkable compression of the dural sac caused by the giant tumor was confirmed (Fig. 2A). The tumor contents were completely removed using an ultrasonic aspirator (CUSA ClarityⓇ, Integra LifeSciences, Tokyo, Japan). Although foraminotomy was performed after a sufficient mass reduction in order to identify the inlet nerve root, we could not locate the nerve tissue that resembled a spinal nerve root. Finally, a capsule of the tumor and an adhesive band linked to the right L5 nerve root were isolated (Fig. 2B). Histological examination revealed schwannoma with 2.1% Ki-67-positive cells (Fig. 2C). Artificial bone cement containing polymethyl methacrylate (SpinePlexⓇ; Stryker Corporation, Kalamazoo, MI) was placed into the defect, and PLF was performed (Fig. 3A-D). Postoperative MRI showed that the tumor was completely removed, while scar tissue remained in front of the dural sac (Fig. 3E). His lower limb numbness gradually improved, and there was no tumor recurrence during the 2-year follow-up period; however, the urinary disorder persisted.
Figure 1.
Midsagittal T1-weighted image (A), gadolinium-enhanced T1-weighted image (B), and T2-weighted image (C) on preoperative magnetic resonance imaging. Axial view at the L5 vertebral level (D) and coronal view (E) on T2-weighted images. Midsagittal reconstructive image (F) and axial image at the L5 vertebral level (G) on computed tomography after myelogram.
Figure 2.
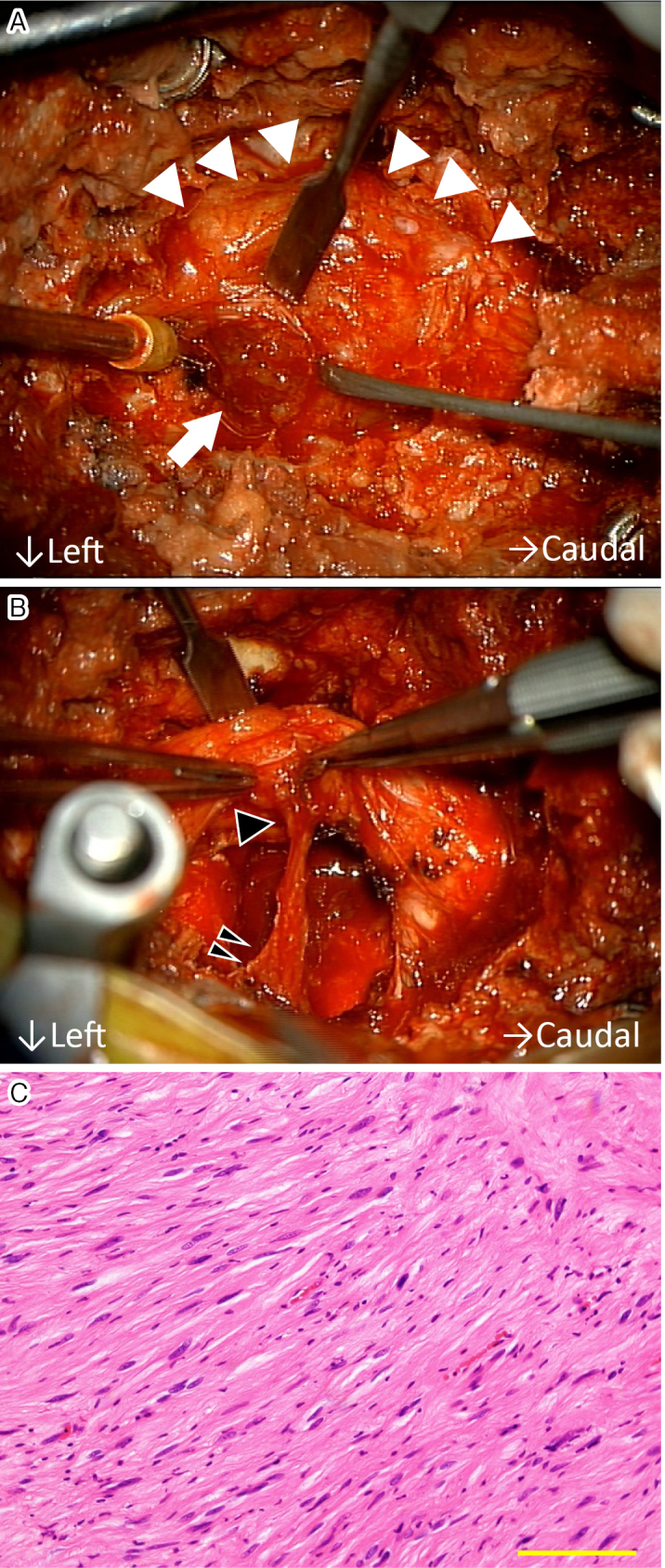
A: Intraosseous tumor (arrow) was observed behind the dural sac (arrowheads). B: Capsule of the tumor connected (double black arrowheads) to the L5 root (a single black arrowhead). C: Hematoxylin/eosin staining demonstrated a cluster of spindle cells without idioblasts (scale bar: 50 μm).
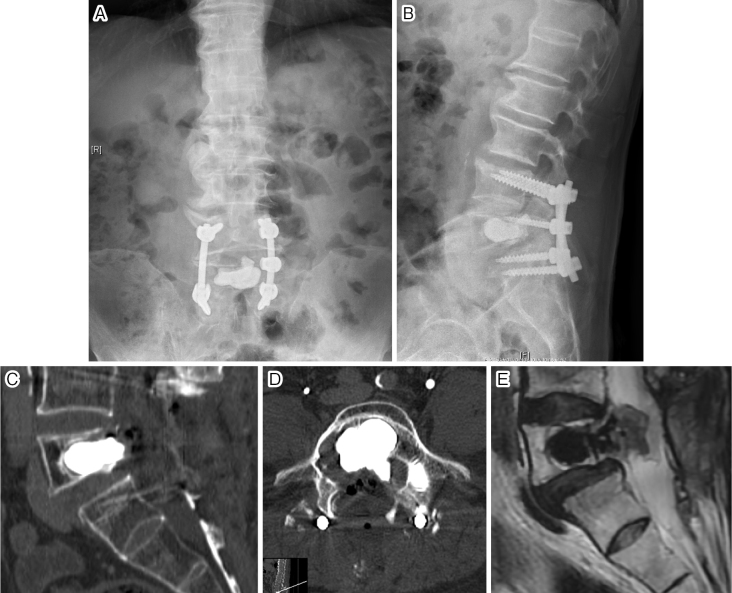
Figure 3.
Postoperative anteroposterior (A) and lateral (B) X-rays of lumbar spine. Midsagittal (C) and axial (D) images on postoperative computed tomography. Midsagittal T2-weighted image (E) on magnetic resonance imaging.
Clinically, although schwannoma is a benign neural tumor and can occur anywhere in the body, intraosseous presentation is extremely rare, accounting for 0.2% of primary tumors1,2). Notably, only 10 cases with aggressive intraosseous schwannoma in the lumbar spine have been reported to date (Table 1)3). Although spinal nerve sheath schwannoma often grows into the spinal canal and intervertebral foramen4), thereby forming a dumbbell tumor usually with sclerotic and clear margins5), aggressive intraosseous schwannoma can lead to invasive and osteolytic bone destruction in the vertebrae. Therefore, it is important in terms of radiological evaluation to understand the difficulty in differentiating between malignant tumors and osteolytic lesions caused by aggressive intraosseous schwannoma6). Fortunately, in most cases, total resection is performed with posterior instrumentation, and there is no recurrence during the approximately 5-year follow-up period3). Taken together, this evidence suggests the necessity of excluding malignancy based on preoperative biopsy in the differential diagnosis of such tumors.
Table 1.
Demographics and Operative Details of the Previous Reports and Present Case.
| No. | Author/year | Age/sex | Level | Symptom | Approach | Stabilization | Resection | Follow-up (months) | Prognosis |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Dickson/1971 | 51/F | L3 | Pain in bilateral lower limbs | Abdominal approach | Iliac crest bone graft | Total | 24 | No recurrence |
| 2 | Chang/1998 | 58/M | L4-L5 | Pain and numbness | Anterolateral +posterior | Fusion+fixation | Total | 1 | Unknown |
| 3 | Inaoka/2001 | 39/M | L5 | Local pain | Unknown | Unknown | Total | 36 | No recurrence |
| 4 | Gupta/2005 | 30/F | L2 | Local pain and motor weakness | Unknown | Iliac crest bone graft | Total | 6 | No recurrence |
| 5 | Park/2009 | 46/F | L4 | Local pain | Unknown | Fusion+fixation | Total | 21 | No recurrence |
| 6 | Youn/2012 | 65/M | L2 | Local pain and numbness | Posterior | Fusion+fixation | Total | 12 | No recurrence |
| 7 | Zhang/2012 | 71/M | L4 | Radicular pain and motor weakness | Posterior | Fusion+fixation | Total | 24 | No recurrence |
| 8 | Song/2014 | 44/M | L5 | Radicular pain | Anterior | Fusion+fixation | Total | 12 | No recurrence |
| 9 | Wang/2018 | 50/M | L5 | Asymptomatic | Posterior | Fusion+fixation | Subtotal | 132 | Residual |
| 10 | Wang/2018 | 36/F | L5 | Local pain and numbness | Posterior | Fusion+fixation | Total | 48 | No recurrence |
| 11 | Present case | 66/M | L5 | Increased urinary frequency and lower limb numbness | Posterior | Fusion+fixation with artificial bone cement (PMMA) | Total | 24 | No recurrence |
L, lumbar; PMMA, polymethyl methacrylate
The defect in the L5 vertebrae was filled with artificial bone cement. Although schwannoma is generally a benign tumor, we used bone cement to increase the intensity of the vertebral structure7) and to cauterize the residual schwannoma cells via the heat of solidification. To our knowledge, this is the first case in which bone cement was used to fill a vertebral defect caused by an aggressive schwannoma (Table 1). Additionally, we applied PLF for reconstruction in the lower lumbar spine. Because we preserved as much of the bilateral facet joints as possible with the aim of limiting surgical invasion, we selected PLF instead of posterior lumbar interbody fusion in this case.
In the present case, we were able to identify the outlet branch connecting to the L5 root but not the inlet nerve sheath. Although most schwannomas have inlet and outlet rootlets, the schwannoma in the present case had a dead-end shape, suggesting that the intraosseous schwannoma may have been invasion by an extraosseous nerve root tumor with extension along the sinuvertebral nerves that innervate vertebral body8,9). Although further anatomical studies are required, it is important for spine surgeons to recognize such abnormal structures in aggressive intraosseous schwannoma prior to surgery.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Sources of Funding: None
Author Contributions: T.H.: study design, data acquisition, drafting of the manuscript, and critical revision of the manuscript; T.Y.: measurement of radiologic data and data acquisition; H.I.: data acquisition and software; Y.M.: data acquisition and software; S.E.: data acquisition and software; K.U.: data acquisition and critical revision of the manuscript; J.H.: data acquisition and software; M.N.: data acquisition and interpretation of the data; K.Y.: data acquisition and interpretation of the data; A.O.: supervision and critical revision of the manuscript.
Ethical Approval: Ethical approval was waived by the ethics committee because this is not required for case reports.
Informed Consent: Informed consent for publication was obtained by the patient described in this study.
References
- 1.Kashima TG, Gibbons MR, Whitwell D, et al. Intraosseous schwannoma in schwannomatosis. Skeletal Radiol. 2013;42(12):1665-71. [DOI] [PubMed] [Google Scholar]
- 2.Ida CM, Scheithauer BW, Yapicier O, et al. Primary schwannoma of the bone: a clinicopathologic and radiologic study of 17 cases. Am J Surg Pathol. 2011;35(07):989-97. [DOI] [PubMed] [Google Scholar]
- 3.Wang QY, Hu XJ, Yang MS, et al. Intraosseous schwannoma of the mobile spine: a report of twenty cases. Eur Spine J. 2018;27(12):3092-104. [DOI] [PubMed] [Google Scholar]
- 4.Inomata K, Iizuka Y, Koshi H, et al. Sporadic hybrid neurofibroma-schwannoma arising from a spinal nerve root in the cervical spine: a case report. Spine Surg Relat Res. 2021;6(1):86-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi T, Hirai T, Yoshii T, et al. Risk factors for recurrence and regrowth of spinal schwannoma. J Orthop Sci. S0949-2658(22). Forthcoming 2022. [DOI] [PubMed] [Google Scholar]
- 6.Agha PF, Lilienfeld MR. Roentgen features of osseous neurilemmoma. Radiology. 1972;102(2):325-6. [DOI] [PubMed] [Google Scholar]
- 7.Turner TM, Urban RM, Singh K, et al. Vertebroplasty comparing injectable calcium phosphate cement compared with polymethylmethacrylate in a unique canine vertebral body large defect model. Spine J. 2008;8(3):482-7. [DOI] [PubMed] [Google Scholar]
- 8.Inaoka T, Takahashi K, Hanaoka H, et al. Paravertebral neurinoma associated with aggressive intravertebral extension. Skeletal Radiol. 2001;30(5):286-9. [DOI] [PubMed] [Google Scholar]
- 9.Song D, Chen Z, Song D, et al. Lumbar intraosseous schwannoma: case report and review of the literature. Turk Neurosurg. 2014;24(6):982-6. [DOI] [PubMed] [Google Scholar]