Abstract
Background:
Previous studies have demonstrated that children in the United States who were of racial and ethnic minorities have inferior waitlist and post-heart transplant (HT) outcomes. Whether these disparities still exist in the contemporary era of increased ventricular assist device use remains unknown.
Methods:
All children (age <18 years) in the Scientific Registry of Transplant Recipients database listed for HT from December 2011 to February 2019 were included and were separated into 5 races/ethnicities: Caucasian, African American, Hispanic, Asian, and Other. Differences in clinical characteristics and survival among children of different racial/ethnic groups were compared at listing and at HT.
Results:
The waitlist cohort consisted of 2134 (52.2%) Caucasian, 840 (20.5%) African American, 808 (19.8%) Hispanic, 161 (3.9%) Asian, and 146 children of Other races (3.6%). At listing, Asian children mostly had cardiomyopathy (70.8%), whereas Caucasian children had congenital heart disease (58.7%). African American children were most likely to be listed as Status 1A and to have renal dysfunction and hypoalbuminemia at listing. African American and Hispanic children were most likely to be on Medicaid. After multivariable analysis, it was found that only African American children were at increased risk for waitlist mortality as compared to Caucasian children (adjusted hazard ratio = 1.25; P = 0.029). Post-HT, there were no disparities in early and midterm graft survival among groups, but African American children had increased numbers of rejection episodes compared to Caucasian and Hispanic children.
Conclusion:
African American children continue to experience increased waitlist mortality and have increased rejection episodes post-HT. Studies exploring barriers to health care access and implicit bias as reasons for these disparities need to be conducted.
Racial and ethnic disparities among adults with advanced heart failure are well recognized. African American adults with heart failure have higher mortality rates,1,2 are less likely to receive left ventricular assist devices (VADs)3 and have increased post-heart transplant mortality rates when compared to Caucasian patients.4,5
Pediatric studies evaluating racial and ethnic disparities in heart transplantation, however, are sparse.6–9 One study of waitlist outcomes among children with heart failure in the United States (U.S.) by Singh et al.6 found that compared to Caucasian children, African American children had a 60% increased risk of waitlist mortality, after adjusting for clinical confounders. Other pediatric studies focused on post-heart transplant outcomes in the U.S. have shown that African American children had increased post-heart transplant morbidity and mortality compared to Caucasian children.7–9 All prior pediatric studies highlighting racial and ethnic differences in heart transplant outcomes were conducted using data from national transplant registries from longer than a decade ago (before 2009). Thus, they all preceded the approval of the first pediatric ventricular assist device, the Berlin EXCOR (Berlin, Germany), by the U.S. Food and Drug Administration (FDA).10 It has been shown in the U.S. national transplant and VAD registries that since that time, there has been a rise in VAD use in children with advanced heart failure.11–15 This rise in VAD use is attributable to a variety of advancements in the pediatric heart failure landscape, such as the FDA approval of the Berlin EXCOR, increased comfort by pediatric providers in implanting adult devices, such as the HeartWare HVAD (Medtronic; Minneapolis, MN) and the HeartMate (Abbott; Abbott Park, IL) VADs, formation of the Advanced Cardiac Therapies Improving Outcomes Network, and improved VAD outcomes by standardization of post-VAD implant-management practices.13–17
Given these recent advances, we wanted to evaluate racial and ethnic differences in waitlist and post-heart transplant outcomes for children with advanced heart failure in the contemporary era.
Methods
Study Population
All pediatric patients (age <18 years) listed for primary heart transplantation in the national Scientific Registry of Transplant Recipients (SRTR) database from December 16, 2011 (date of FDA approval of Berlin EXCOR), to February 28, 2019, were included in this study. We excluded patients listed for heart-lung transplantation (n = 43), lung transplantation (n = 394) and retransplantation (n = 243).
SRTR Database
The SRTR data system includes data for all donors, waitlist candidates and transplant recipients in the U.S., as submitted by the members of the Organ Procurement and Transplantation Network. The Health Resources and Services Administration, U.S. Department of Health and Human Services, provides oversight of the activities of the Organ Procurement and Transplantation Network contractor. This study was approved by the Cleveland Clinic internal review board, and informed consent was waived because the data obtained from routine care were completely deidentified by SRTR prior to their transmission to the investigators.
End-points
The primary endpoint of waitlist survival analysis was time from initial listing to a composite outcome of pretrans-plant death or becoming too sick for transplantation (removal from the waitlist due to clinical deterioration), censored at transplantation or last date of follow-up (May 31, 2019). This analysis was based on intent–to–treat such that deaths following removal from the waiting list were included in the analysis.
The primary endpoint of post-transplant survival analysis was time to graft loss (death or retransplantation), censored at last graft follow-up.
Statistical Analysis
The cohort was stratified by race/ethnicity into 5 groups based on data available in SRTR: Caucasian, African American, Hispanic, Asian, and Other. Other races included American Indian/Alaska Native (n = 31), Native Hawaiian/Pacific Islander (n = 17), and multiracial (n = 98). Children from these racial/ethnic groups were combined as Other, given the limited sample size in each of these 3 cohorts. Data at time of listing and time of transplantation were summarized with continuous variables expressed as medians (25th, 75th percentile), and categorical variables expressed as number of candidates and percent. For comparisons of waitlist and transplant characteristics by race/ethnicity, the Kruskal-Wallis test was used for continuous/ordinal characteristics; the Pearson χ2 test or the Fisher exact test was used for categorical characteristics, as appropriate. For variables in which the overall group comparison was statistically significant at the 0.05 level, pairwise tests were performed among all racial/ethnic groups with a Bonferroni multiple comparison adjustment to the significance criteria (α = 0.05/10 = 0.005). To assess whether racial/ethnic distribution changed over time, the multinomial logistic regression model of race/ethnicity was used, with a main effect for year of listing treated as a continuous variable.
Survival analysis was performed on imputed datasets. Multiple imputation was performed using the multiple imputation procedure in SAS 9.4 software (SAS Institute) to address missing values for variables with <5% missing data (5 imputations were performed separately for waitlist and post-transplant analysis cohorts). Variables imputed using multiple imputation for waitlist and post-transplant analyses are listed in the Supplementary Statistics document. No covariates at listing or transplant were missing >5% but <30% of the data. There were very few variables with >30% missing data. These were intensive care unit at listing (59.6% missing); mean pulmonary arterial pressure at listing and transplant (44.2% and 37.3% missing, respectively); mean pulmonary capillary wedge pressure at listing; and transplant (47.9% and 39.3% missing, respectively); panel reactive antibodies at transplant (65.5% missing); chest drain >2 weeks (68.2% missing); hospitalized for infection in the first 3 years (45.7% missing), hospitalized for rejection in the first 3 years (45.6% missing) and cardiac reoperation (66.6% missing). The variables that had >30% missing data were summarized in the descriptive tables but excluded from the regression analyses. Competing risk analysis was performed to evaluate outcomes after heart transplant listing (transplant, death or removal from the waitlist due to clinical deterioration). Cumulative death/deterioration and transplant rates were compared among racial/ethnic groups using the Gray’s test. Kaplan-Meier plots were constructed for both the waitlist and the post-transplant time to endpoints, and the log-rank test was used to compare time-to-event curves among racial/ethnic groups.
Cox proportional hazards survival analysis was performed to assess the associations between race/ethnicity and death/deterioration at waitlist and between race/ethnicity and graft loss after transplant, and to estimate unadjusted and adjusted hazard ratios (aHRs) with their 95% confidence intervals. An automated stepwise variable selection method (significance criteria for entry and removal 0.1 and 0.05, respectively) performed on 1000 bootstrap samples was used to choose final multivariable models after excluding variables causing multicollinearity. The variables used in the bootstrap variable selection process for Cox proportional hazards models are listed in the Supplementary Statistics document. The variables with inclusion rates larger than 50% and consistent signs of parameter coefficients were selected for the final models. Race/ethnicity was not selected in the variable selection procedure for the post-transplant model and was, therefore, added later. All tests were 2-tailed and were performed at an overall significance level of 0.05. SAS 9.4 software (SAS Institute) was used for all analyses and plots.
Results
Waitlist Cohort
A total of 4089 pediatric patients listed for heart transplantation were included in our analysis. Of these, 2134 (52.2%) were Caucasian, 840 (20.5%) African American, 808 (19.8%) Hispanic, 161 (3.9%) Asian, and 146 Other (3.6%). From 2012 to 2018, the relative percentage of children from various races and ethnicities listed for heart transplantation remained the same, except in 2014, 2015 and 2017, when there were more Hispanic than African American children (Fig. 1).
Fig. 1.
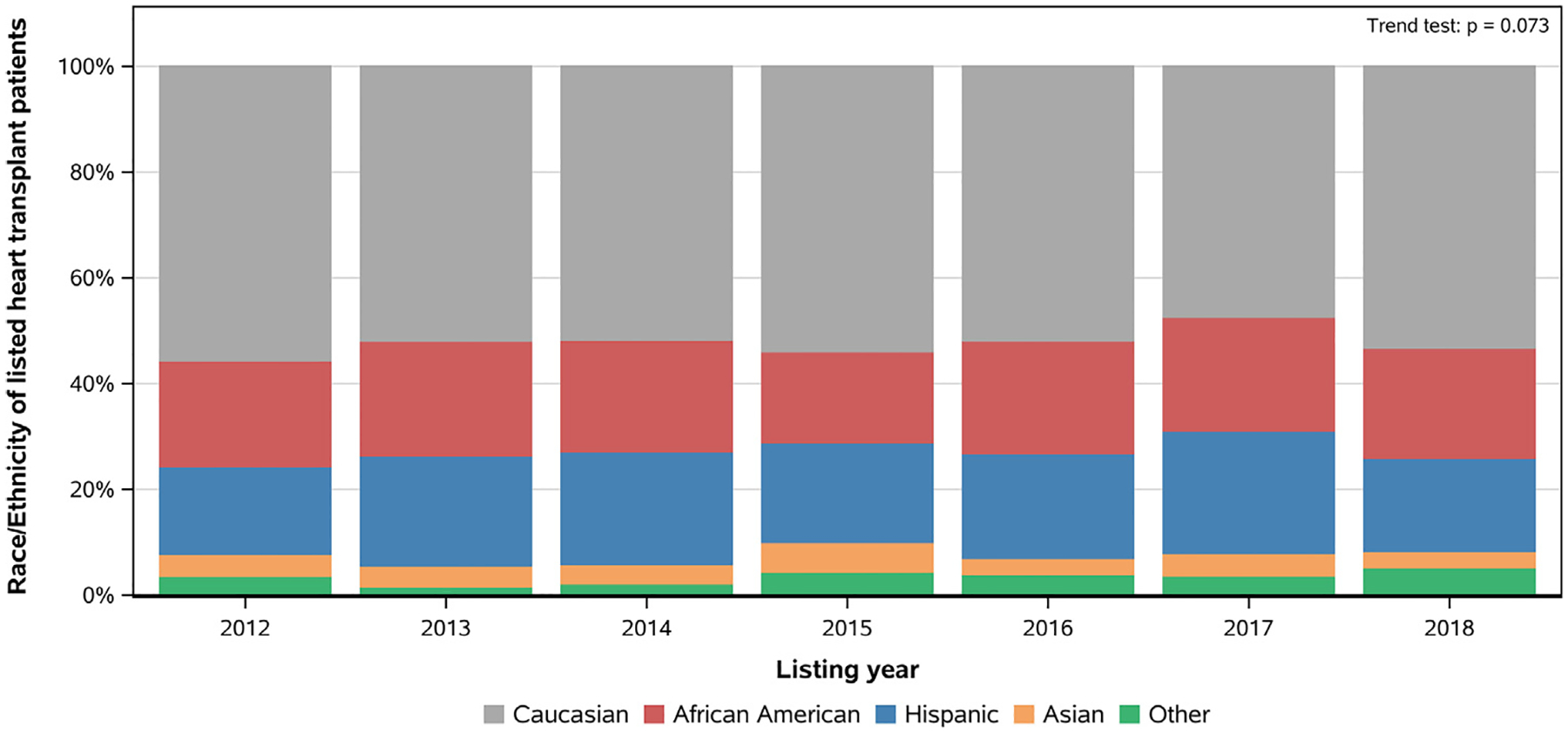
Race/ethnicity distribution of children listed for heart transplantation in the current era.
Differences in Characteristics Among Children of Differing Races/Ethnicities at Listing
The majority of children listed for heart transplantation were male and were listed for urgent transplantation (Status 1A) while in the intensive care unit. Few had an implantable cardioverter defibrillator or required extracorporeal membrane oxygenation support. Asian children were most likely to have cardiomyopathy (70.8%), whereas Caucasian children were most likely to have congenital heart disease (58.7%). African American children were most likely to be listed as Status 1A (70.1%), to have renal dysfunction (estimated glomerular filtration rate <90 mL/min/1.73m2) (52.7%) and to have hypoalbuminemia (albumin ≤3.5g/dL) (52.7%) at listing. African American and Hispanic children were most likely to be on Medicaid at listing (64.4% and 63.7%, respectively) (P < 0.05 for all) (Table 1).
Table 1.
Differences in Baseline Characteristics at Listing in Patients of Differing Races/Ethnicities
| African American | Caucasian | Hispanic | Asian | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or n (%) | p-value |
| Age at listing (years) | 840 | 2134 | 808 | 161 | 146 | 0.013 b | |||||
| <1 | 300 (35.7) | 799 (37.4) | 309 (38.2)¶ | 36 (22.4)§ | 65 (44.5) | ||||||
| 1–10 | 276 (32.9) | 710 (33.3) | 302 (37.4) | 82 (50.9) | 43 (29.5) | ||||||
| 11–17 | 264 (31.4) | 625 (29.3) | 197 (24.4) | 43 (26.7) | 38 (26.0) | ||||||
| Weight (kg) | 838 | 14.8 (5.5, 44.2) | 2132 | 12.6 (5.2, 38.1) | 808 | 12.1 (5.7, 34.3) | 161 | 15.0 (7.5, 30.9) | 146 | 10.0 (4.8, 34.4) | 0.059b |
| BMI (kg/m2) | 832 | 16.0 (14.0, 19.0)¶ | 2120 | 16.0 (14.0, 18.0) | 803 | 16.0 (14.0, 19.0)¶ | 159 | 15.0 (14.0, 17.0)*§ | 144 | 16.0 (14.0, 18.0) | <0.001 b |
| Sex | 840 | 2134 | 808 | 161 | 146 | 0.12c | |||||
| Female | 390 (46.4) | 894 (41.9) | 362 (44.8) | 78 (48.4) | 66 (45.2) | ||||||
| Male | 450 (53.6) | 1240 (58.1) | 446 (55.2) | 83 (51.6) | 80 (54.8) | ||||||
| Underlying diagnosis | 840 | 2134 | 808 | 161 | 146 | <0.001 c | |||||
| CMP | 413 (49.2)#¶ | 855 (40.1)*§¶ | 392 (48.5)#¶ | 114 (70.8)*#§Ω | 66 (45.2)¶ | ||||||
| CHD | 414 (49.3) | 1253 (58.7) | 406 (50.2) | 43 (26.7) | 78 (53.4) | ||||||
| Initial status | 840 | 2134 | 808 | 161 | 146 | 0.013 c | |||||
| Status 1A | 589 (70.1)§ | 1404 (65.8) | 500 (61.9) * | 104 (64.6) | 99 (67.8) | ||||||
| Status 1B | 114 (13.6) | 349 (16.4) | 143 (17.7) | 33 (20.5) | 24 (16.4) | ||||||
| Status 2 | 117 (13.9) | 341 (16.0) | 136 (16.8) | 21 (13.0) | 23 (15.8) | ||||||
| ICU | 349 | 226 (64.8) | 876 | 528 (60.3) | 325 | 198 (60.9) | 64 | 40 (62.5) | 39 | 24 (61.5) | 0.70c |
| ICD use | 833 | 51 (6.1) | 2119 | 183 (8.6) | 806 | 63 (7.8) | 161 | 18 (11.2) | 145 | 9 (6.2) | 0.089c |
| Blood type | 840 | 2134 | 808 | 161 | 146 | <0.001 c | |||||
| A | 221 (26.3)#§¶Ω | 855 (40.1)*§¶ | 245 (30.3)*#¶Ω | 45 (28.0)*#§Ω | 61 (41.8) *§¶ | ||||||
| AB | 29 (3.5) | 75 (3.5) | 14 (1.7) | 16 (9.9) | 5 (3.4) | ||||||
| B | 163 (19.4) | 253 (11.9) | 76 (9.4) | 45 (28.0) | 22 (15.1) | ||||||
| O | 427 (50.8) | 951 (44.6) | 473 (58.5) | 55 (34.2) | 58 (39.7) | ||||||
| ECMO | 840 | 55 (6.5) | 2134 | 156 (7.3) | 808 | 45 (5.6) | 161 | 10 (6.2) | 146 | 14 (9.6) | 0.32c |
| Ventilator | 840 | 177 (21.1) | 2134 | 452 (21.2) | 808 | 167 (20.7) | 161 | 21 (13.0) | 146 | 31 (21.2) | 0.19c |
| IV inotropes | 840 | 394 (46.9) | 2134 | 1060 (49.7)§ | 808 | 332 (41.1)# | 161 | 67 (41.6) | 146 | 67 (45.9) | <0.001c |
| VAD | 840 | 118 (14.0) | 2,134 | 240 (11.2)¶ | 808 | 96 (11.9)¶ | 161 | 35 (21.7)#§ | 146 | 17 (11.6) | 0.001 c |
| eGFR <90 mL/min/1.73 m2 | 831 | 438 (52.7)#¶ | 2115 | 973 (46.0)* | 801 | 374 (46.7) | 156 | 55 (35.3)* | 144 | 64 (44.4) | <0.001c |
| Dialysis | 840 | 18 (2.1) | 2131 | 38 (1.8) | 808 | 14 (1.7) | 161 | 3 (1.9) | 146 | 4 (2.7) | 0.82d |
| Albumin ≤3.5 g/dL | 819 | 432 (52.7)¶ | 2069 | 1051 (50.8) | 786 | 378 (48.1) | 155 | 62 (40.0)* | 142 | 73 (51.4) | 0.035 c |
| Mean PAP (mmHg) | 461 | 22.0 (17.0, 32.0) | 1217 | 21.0 (16.0, 30.0) | 445 | 21.0 (16.0, 28.0) | 83 | 23.0 (17.0, 34.0) | 76 | 22.0 (16.5, 30.0) | 0.015 b |
| Mean PCWP (mmHg) | 418 | 15.0 (11.0, 22.0) | 1149 | 15.0 (10.0, 20.0) | 414 | 15.0 (10.0, 20.0) | 81 | 16.0 (12.0, 22.0) | 68 | 15.0 (10.5, 20.5) | 0.11b |
| Insurance | 840 | 2133 | 808 | 161 | 146 | <0.001c | |||||
| Private | 243 (28.9)#§¶Ω | 1222 (57.3)*§¶Ω | 165 (20.4)*#¶Ω | 73 (45.3)*#§Ω | 55 (37.7) *#§¶ | ||||||
| Medicaid | 541 (64.4) | 739 (34.6) | 515 (63.7) | 48 (29.8) | 71 (48.6) | ||||||
| Other | 56 (6.7) | 172 (8.1) | 128 (15.8) | 40 (24.8) | 20 (13.7) | ||||||
P values <0.05:
Kruskal-Wallis test,
Pearson χ2,
Fisher exact test. Post hoc pairwise comparisons used Bonferroni adjustment.
Significantly different from African American.
Significantly different from Caucasian.
Significantly different from Hispanic.
Significantly different from Asian.
Significantly different from Other.
Boldface P values: statistically significant (P < 0.05).
BMI, body mass index; CHD, congenital heart disease; CMP, cardiomyopathy; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate; ICU, intensive care unit; ICD, implantable cardioverter defibrillator; IV, intravenous; PAP, pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; VAD, ventricular assist device.
Differences in Heart Transplant Waitlist Outcomes by Race/Ethnicity
There was no significant difference in waitlist survival among children of any race/ethnicity in unadjusted analysis (P = 0.10) (Fig. 2). However, after adjusting for confounding clinical risk factors, only African American children were at increased risk of heart transplant waitlist mortality compared to Caucasian children (aHR = 1.25, 95% CI 1.02 to 1.53; P = 0.029) (Table 2).
Fig. 2.
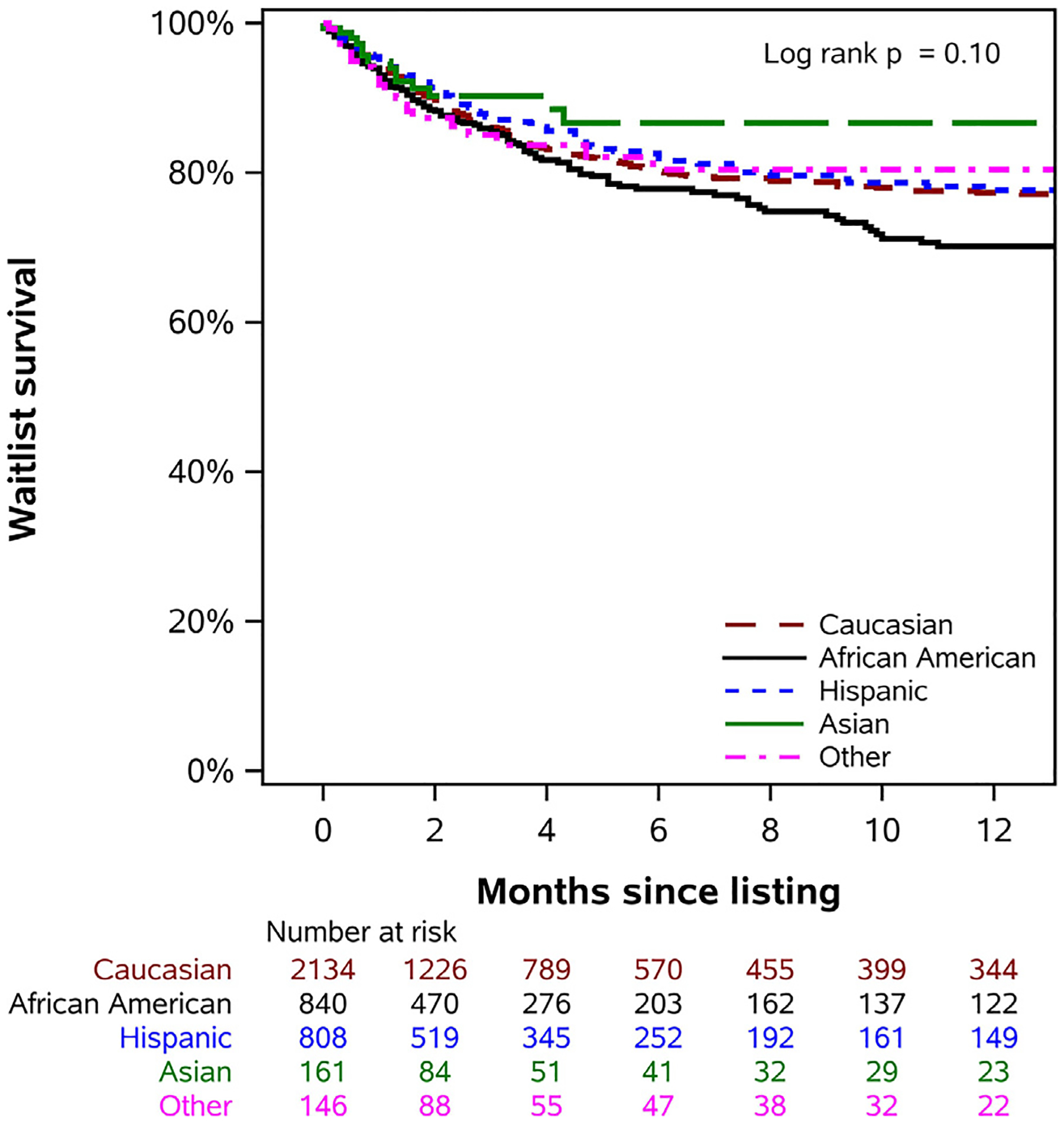
Differences in waitlist survival between children of different races/ethnicities in the current era.
Table 2.
Multivariable Risk Factors of Waitlist Mortality in the Current Era
| Risk factors | HR | 95% CI | p value |
|---|---|---|---|
| Race (reference: Caucasians) | |||
| African American | 1.25 | 1.02–1.53 | 0.029 |
| Hispanic | 1.07 | 0.86–1.33 | 0.53 |
| Asian | 0.82 | 0.48–1.38 | 0.45 |
| Other | 1.17 | 0.75–1.81 | 0.49 |
| Age (reference: 11–17 years) | |||
| <1 | 1.77 | 1.33–2.35 | <0.001 |
| 1–10 | 1.46 | 1.10–1.93 | 0.009 |
| Female | 1.22 | 1.04–1.43 | 0.017 |
| Diagnosis (reference: CMP) | |||
| CHD | 1.89 | 1.57–2.28 | <0.001 |
| Other | 0.99 | 0.46–2.12 | 0.98 |
| Status 1A | 1.82 | 1.45–2.30 | <0.001 |
| Ventilator | 1.73 | 1.43–2.09 | <0.001 |
| ECMO | 2.13 | 1.69–2.69 | <0.001 |
| eGFR (reference : eGFR ≥ 90 mL/min/1.73 m2) | |||
| 60-<90 | 1.27 | 1.03–1.57 | 0.023 |
| 30-<60 | 1.94 | 1.55–2.42 | <0.001 |
| <30 | 2.35 | 1.66–3.32 | <0.001 |
| Dialysis | 1.54 | 1.04–2.27 | 0.031 |
| Albumin ≤3.5 g/dL | 1.35 | 1.12–1.63 | 0.002 |
| BMI (10 kg/m2 increase) | 1.00 | 1.00–1.01 | <0.001 |
Boldface P values: statistically significant (P < 0.05).
BMI, body mass index; CHD, congenital heart disease; CMP, cardiomyopathy; ECMO, extracorporeal membrane oxygenation; eGFR, estimated glomerular filtration rate.
Racial/Ethnic Differences in Competing Outcomes
There were no significant differences in rates of transplantation between children of different races/ethnicities (P = 0.054), (Fig. S1). There were also no differences in unadjusted rates of waitlist death or delisting due to clinical deterioration among children of any race/ethnicity (P = 0.12) (Fig. S2).
Differences in Characteristics Among Children of Different Races/Ethnicities at Transplant
A total of 2865 children in the waitlisted cohort underwent heart transplantation. Of these, 563 were African American, 1509 Caucasian, 570 Hispanic, 125 Asian, and 98 children of Other races. African American children were most likely to be listed as Status 1A (72.8%), to be in the intensive care unit (65.7%) and to have hypoalbuminemia (52.0%). Intravenous inotrope use at time of transplantation was more common in African American and Caucasian children than in Hispanic children (65.5% vs 65.8% vs 56.7%) (P < 0.05 for all). Renal dysfunction and liver dysfunction (bilirubin ≥2 mg/dL) was similar in all racial/ethnic groups. (Table S1).
Differences in Donor Characteristics by Race/Ethnicity
African American children were more likely to have higher donor-recipient age differences than Asian and Caucasian children (P < 0.05). There was no difference in causes of donors’ death, use of Centers for Disease Control and Prevention increased-risk donors or donor left ventricular ejection fraction in any groups (P > 0.05) (Table S2)
Differences in Post-Heart Transplant Mortality and Morbidity
There were no significant racial/ethnic differences in post-heart transplant survival in unadjusted analysis (P = 0.41) (Fig. 3). On multivariable adjusted analysis, there was no significant difference in post-heart transplant survival for African American children compared to Caucasian children (aHR = 1.12, 95% CI 0.86 to 1.47; P = 0.39), (Table 3). There were no significant racial/ethnic differences noted in early post-transplant morbidity, such as post-transplant stroke, dialysis, permanent pacemaker placement, cardiac reoperation, acute rejection episodes prior to discharge, or length of stay in transplanted children (P > 0.05 for all). However, in the first 3 years post-heart transplant, African American children were more likely to have acute rejection episodes than were Caucasian and Hispanic children (36.6% vs 27.5% vs 24.5%) and to be hospitalized for rejection (31.2% vs 21.5% vs 20.3%) (P < 0.05 for both) (Table 4).
Fig. 3.
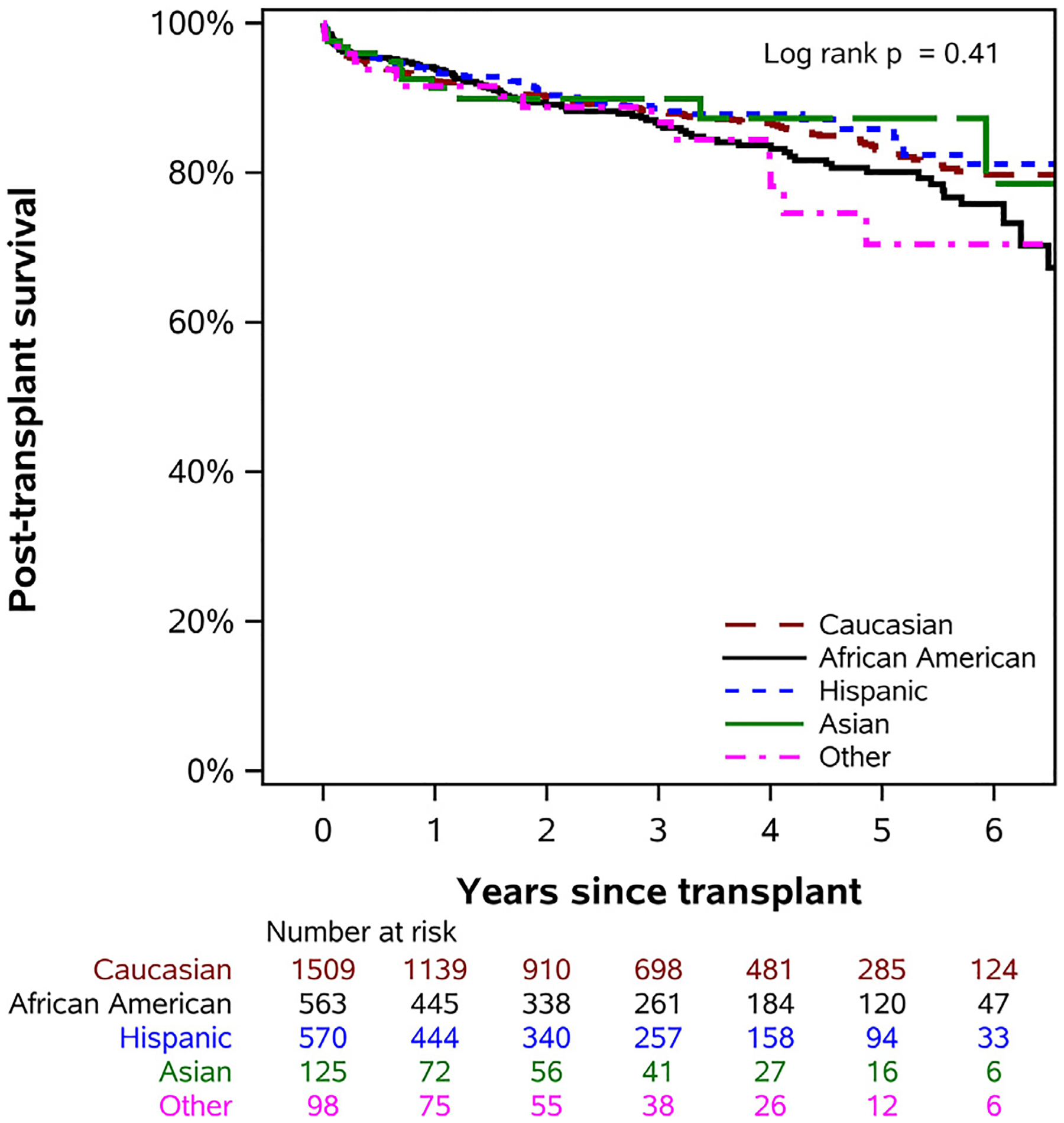
Differences in post-heart transplant survival in children of different races/ethnicities in the current era.
Table 3.
Multivariable Risk Factors of Post-transplant Graft Loss in the Current Era
| Risk factors | HR | 95% CI | p-value |
|---|---|---|---|
| Race (reference: Caucasians) | |||
| African American | 1.12 | 0.86–1.47 | 0.39 |
| Hispanic | 0.87 | 0.65–1.16 | 0.33 |
| Asian | 1.09 | 0.60–1.96 | 0.78 |
| Other | 1.12 | 0.67–1.88 | 0.66 |
| Diagnosis (reference: CMP) | |||
| CHD | 2.07 | 1.65–2.59 | <0.001 |
| Other | 1.01 | 0.37–2.73 | 0.99 |
| Bilirubin ≥2 mg/dL | 1.71 | 1.32–2.21 | <0.001 |
| Albumin ≤3.5 g/dL | 1.27 | 1.03–1.58 | 0.029 |
| ECMO | 1.53 | 1.09–2.14 | 0.015 |
| Infection requiring IV drug therapy within 2 weeks prior to transplant | 1.47 | 1.16–1.87 | 0.001 |
| Medicaid | 1.36 | 1.10–1.69 | 0.005 |
| Male donor | 1.33 | 1.07–1.65 | 0.010 |
| Ischemic time >3.5 hours | 1.37 | 1.10–1.70 | 0.004 |
Boldface P values are statistically significant at P < 0.05.
CMP, cardiomyopathy; CHD, congenital heart disease; ECMO, extracorporeal membrane oxygenation; IV, intravenous.
Table 4.
Differences in Post-transplant Morbidity Among Children of Different Races/Ethnicities Undergoing Transplantation in the Current Era
| African American | Caucasian | Hispanic | Asian | Other | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or n (%) | n | Median (P25, P75) or N (%) | n | Median (P25, P75) or n (%) | p-value |
| Stroke | 558 | 18 (3.2) | 1491 | 49 (3.3) | 565 | 27 (4.8) | 122 | 8 (6.6) | 96 | 3 (3.1) | 0.22d |
| Dialysis | 563 | 44 (7.8) | 1507 | 91 (6.0) | 570 | 43 (7.5) | 124 | 8 (6.5) | 98 | 4 (4.1) | 0.42c |
| Chest drain >2 weeks | 192 | 23 (12.0) | 489 | 53 (10.8) | 174 | 17 (9.8) | 33 | 3 (9.1) | 23 | 3 (13.0) | 0.94d |
| Permanent pacemaker | 561 | 3 (0.5) | 1497 | 17 (1.1) | 569 | 4 (0.7) | 123 | 0 (0) | 96 | 1 (1.0) | 0.59d |
| Cardiac reoperation | 200 | 19 (9.5) | 515 | 44 (8.5) | 180 | 17 (9.4) | 38 | 5 (13.2) | 24 | 2 (8.3) | 0.85d |
| Length of stay | 559 | 21.0 (14.0, 36.0) | 1506 | 19.0 (12.0, 36.0) | 569 | 19.0 (13.0, 34.0) | 124 | 20.5 (14.0, 36.0) | 98 | 21.0 (14.0, 40.0) | 0.33b |
| Acute rejection episodes prior to discharge | 563 | 78 (13.9) | 1509 | 230 (15.2) | 570 | 74 (13.0) | 125 | 20 (16.0) | 98 | 9 (9.2) | 0.36c |
| Acute rejection episodes in the first 3 years | 492 | 180 (36.6)#§ | 1279 | 352 (27.5)* | 497 | 122 (24.5)* | 89 | 20 (22.5) | 88 | 28 (31.8) | <0.001c |
| Hospitalized for rejection in the first 3 years | 337 | 105 (31.2)#§ | 797 | 171 (21.5)* | 320 | 65 (20.3)* | 45 | 12 (26.7) | 59 | 15 (25.4) | 0.005 c |
| Hospitalized for infection in the first 3 years | 336 | 192 (57.1) | 796 | 455 (57.2) | 320 | 197 (61.6) | 45 | 22 (48.9) | 59 | 38 (64.4) | 0.34c |
P values:
Kruskal-Wallis test;
Pearson χ2 test;
Fisher exact test.
Significantly different from African American.
Significantly different from Caucasian.
Significantly different from Hispanic.
Significantly different from Asian.
Significantly different from Other.
Boldface P values, statistically significant (P < 0.05).
Discussion
There are 3 important findings in our contemporary analysis of pediatric candidates listed for heart transplantation in the U.S. First, African American children listed for heart transplantation in the current era have higher severity of illness at listing and at transplantation. Second, in the current era of rising VAD use in children with advanced heart failure, African American children continue to experience higher waitlist mortality than Caucasian children. Finally, although early and midterm post-heart transplant survival is similar in children of varying races and ethnicities, African American children continue to experience higher numbers of rejection episodes than Caucasian children.
In our study, African American children were in more advanced heart failure at listing and at transplant. Structural racism is recognized to be an important driver of health disparities in adult cardiovascular disease.18,19 Adult studies have noted that implicit physician bias exists when treating African American patients with heart failure. They are less likely to be evaluated by a cardiologist when admitted with heart failure,20 to receive a left VAD3 or to be listed for heart transplantation.21 Studying racial/ethnic disparities in the timely diagnosis and management of children with heart failure is important. Also, evaluating implicit physician bias among pediatric providers caring for children with advanced heart failure, especially heart transplant listing practices and use of advanced heart failure therapies, is vital. It is also important to recognize barriers to health care access for children of racial/ethnic minorities. In our study, African American and Hispanic children were most likely to be on Medicaid. Perceived discrimination is a known barrier to health care access,22 especially among people from racial/ethnic minorities and lower socioeconomic status, and efforts should be made to understand whether this concern affects access to care for children of racial/ethnic minorities with advanced heart failure.
A previous study evaluated waitlist outcomes for children from the same national transplant database (study period: 1999–2006).6 The authors found that compared to Caucasian children, the risk for waitlist mortality was increased by 60% for African American, 50% for Hispanic, 100% for Asian, and 130% for children of Other races. We found that currently, African American children continue to be at increased risk for waitlist mortality compared to Caucasian children, but such disparities are not noted among children of other races/ethnicities. These differences in survival persisted despite adjusting for clinical variables captured by SRTR. It is known that children with heart failure who have hyponatremia, elevated natriuretic peptides and lower left ventricular ejection fraction are more likely to die.23–25 Unfortunately, the SRTR does not have detailed information concerning these clinical variables that are known to affect outcomes in children with advanced heart failure. We speculate that differences in these important parameters that are not captured may account for some of the disparities noted in waitlist outcomes, and they need to be evaluated in future studies. Also, the progression of heart failure during the waitlist period may differ by race/ethnicity, and evaluating these factors may explain the reason for inferior survival rates among African American children currently.
Prior pediatric studies evaluating post-heart transplant outcomes have found that African American children have a 67% increased risk of graft failure8 compared to children of other races/ethnicities and a 120% increased risk of long-term death or retransplantation compared to Caucasian children.7 We did not find any differences in early or midterm graft survival in children of different races/ethnicities undergoing heart transplantation currently. We surmise that this is because at time of transplantation, there were no racial/ethnic differences in known post-transplant mortality risk factors, such as liver dysfunction, ECMO use or pro-longed ischemic times. Moreover, the proportion of African American children with congenital heart disease was lower and those supported by VADs higher among African American children undergoing heart transplantation; this may explain the lack of differences in post-heart transplant survival compared to Caucasian children. Whether the lack of racial/ethnic differences in graft survival pan out long-term for children currently undergoing transplantation remains to be seen. We did note that African American children continue to experience increased episodes of rejection. It has been shown, in other pediatric studies using similar and other national transplant databases, that African American children are at increased risk for rejection, hemodynamic compromising rejection and cardiac allograft vasculopathy.26–28
Study Limitations
Although our cohort included all children listed in the national heart transplant database, our study was subject to limitations, such as variables included in the database, accuracy of data, and human error upon entry of data. For instance, the racial/ethnic profiles used may be misclassified by the reporting center, thus wrongly adjudicating the true racial/ethnic profile of a child listed for heart transplantation, and this could have affected our results. Another limitation is the heterogeneity of different races/ethnicities, making generalizations inaccurate. For instance, Asian children represent children with descent from South Asia (Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, and Sri Lanka) as well as East Asia (China, Japan, North Korea, South Korea, Outer Mongolia, and Taiwan). Hispanic children include people from Spanish-speaking areas such as Cuba, Puerto Rico and Mexico. Given the diversity within these racial/ethnic groups, any racial/ethnic disparities in health care outcomes are unlikely to be genetic and will be of concern for both children and adults. It is known that African American children have a higher rates of post-transplant hemodynamic-compromising rejection and cardiac allograft vasculopathy,26,27 but we were unable to evaluate this in our study because these outcomes are not captured in great detail in the SRTR database.
In conclusion, in a contemporary cohort of pediatric patients listed for heart transplantation, African American children continue to be at increased risk for waitlist mortality compared to Caucasian children. Post-transplant early and midterm graft survival are similar for children of all races/ethnicities; however, African American children continue to experience increased rejection episodes post-heart transplant.
Supplementary Material
Acknowledgments
The data reported here have been supplied by the Hennepin Healthcare Research Institute as the contractor for the SRTR. SW and WL had full access to the data in the study and take responsibility for the integrity of the data and the accuracy of the analysis. All authors contributed to the design, interpretation of data and drafting of the manuscript along with revisions and participated in the final approval of the manuscript submitted. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. government.
Disclosures
Dr. Hsich is supported in part by HL141892 from the National Institute of Health. No other authors have any disclosures to report.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.cardfail.2021.05.027.
References
- 1.Carnethon MR, Pu J, Howard G, Albert MA, Anderson CA, Bertoni AG, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association 2017;136:e393–423. [DOI] [PubMed] [Google Scholar]
- 2.Sharma A, Colvin-Adams M, Yancy CWJCCJM. Heart failure in African Americans: disparities can be overcome 2014;81:301–11. [DOI] [PubMed] [Google Scholar]
- 3.Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DCJJoSR. Disparities in access to left ventricular assist device therapy. 2009;152:111–7. [DOI] [PubMed] [Google Scholar]
- 4.Kilic A, Higgins RS, Whitson BA, Kilic AJC. Racial disparities in outcomes of adult heart transplantation. 2015;131:882–9. [DOI] [PubMed] [Google Scholar]
- 5.Lui C, Fraser III CD, Zhou X, Suarez-Pierre A, Kilic A, Zehr KJ, et al. Racial disparities in patients bridged to. heart transplantation with left ventricular assist devices 2019;108:1122–6. [DOI] [PubMed] [Google Scholar]
- 6.Singh TP, Gauvreau K, Thiagarajan R, Blume ED, Piercey G, Almond C. Racial and ethnic differences in mortality in children awaiting heart transplant in the United States. Am J Transplant 2009;9:2808–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh T, Almond C, Gauvreau KT. Improved survival in pediatric heart transplant recipients: have white, black and Hispanic children benefited equally? 2011;11:120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mahle WT, Kanter KR, Vincent RJ. Disparities in outcome for Black patients after pediatric heart transplantation. 2005;147:739–43. [DOI] [PubMed] [Google Scholar]
- 9.Green D, Brooks M, Burckart G, Chinnock R, Canter C, Addonizio L, et al. The influence of race and common genetic variations on outcomes after pediatric heart transplantation. 2017;17:1525–39. [DOI] [PubMed] [Google Scholar]
- 10.FDA Humanitarian Device Exemption of Berlin EXCOR ventricluar assist device. Available at: https://www.accessdata.fda.gov/cdrh_docs/pdf10/H100004A.pdf. Accessed: 12/26/2020.
- 11.Zafar F, Castleberry C, Khan MS, Mehta V, Bryant III R, Lorts A, et al. Pediatric heart transplant waiting list mortality in the era of ventricular assist devices. 2015;34:82–8. [DOI] [PubMed] [Google Scholar]
- 12.Amdani S, Boyle G, Saarel EV, Godown J, Liu W, Worley S, et al. Waitlist and Post-Heart Transplant Outcomes for Children With Nondilated Cardiomyopathy. Ann Thorac Surg 2021;112:188–96. [DOI] [PubMed] [Google Scholar]
- 13.Blume ED, VanderPluym C, Lorts A, Baldwin JT, Rossano JW, Morales DL, et al. Second annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) report: pre-implant characteristics and outcomes. J Heart Lung Transplant 2018;37:38–45. [DOI] [PubMed] [Google Scholar]
- 14.Morales DL, Adachi I, Peng DM, Sinha P, Lorts A, Fields K, et al. Fourth Annual Pediatric Interagency Registry for Mechanical Circulatory Support (Pedimacs) Report. Ann Thorac Surg 2020;110:1819–31. [DOI] [PubMed] [Google Scholar]
- 15.Morales DL, Rossano JW, VanderPluym C, Lorts A, Cantor R, Louis JDS, et al. Third annual pediatric interagency registry for mechanical circulatory support (PediMACS) report: preimplant characteristics and outcomes. Ann Thorac Surg 2019;107:993–1004. [DOI] [PubMed] [Google Scholar]
- 16.Lorts A, Smyth L, Gajarski RJ, VanderPluym CJ, Mehegan M, Villa CR, et al. The creation of a pediatric health care learning network: the ACTION quality improvement collaborative. Asaio J 2020;66:441–6. [DOI] [PubMed] [Google Scholar]
- 17.VanderPluym CJ, Cantor RS, Machado D, Boyle G, May L, Griffiths E, et al. Utilization and outcomes of children treated with direct thrombin inhibitors on paracorporeal ventricular assist device support. Asaio J 2020;66:939–45. [DOI] [PubMed] [Google Scholar]
- 18.Churchwell K, Elkind MS, Benjamin RM, Carson AP, Chang EK, Lawrence W, et al. Call to action: structural racism as a fundamental driver of health disparities: a presidential advisory from the. American Heart Association. Circulation 2020;142:e454–68. [DOI] [PubMed] [Google Scholar]
- 19.Brewer LC, Cooper LA. State of the art and science: race, discrimination, and cardiovascular disease. Virt Ment 2014;16:455. [PMC free article] [PubMed] [Google Scholar]
- 20.Breathett K, Liu WG, Allen LA, Daugherty SL, Blair IV, Jones J, et al. African Americans are less likely to receive care by a cardiologist during an intensive care unit admission for heart failure. JACC 2018;6:413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Breathett K, Yee E, Pool N, Hebdon M, Crist JD, Knapp S, et al. Does race influence decision making for advanced heart failure therapies? J Am Heart Assoc 2019;8:e013592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allen EM, Call KT, Beebe TJ, McAlpine DD, Johnson PJ. Barriers to care and healthcare utilization among the publicly insured. Med Care 2017;55:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price JF, Kantor PF, Shaddy RE, Rossano JW, Goldberg JF, Hagan J, et al. Incidence, severity, and association with adverse outcome of hyponatremia in children hospitalized with heart failure. Am J Cardiol 2016;118:1006–10. [DOI] [PubMed] [Google Scholar]
- 24.Auerbach SR, Richmond ME, Lamour JM, Blume ED, Addonizio LJ, Shaddy RE, et al. BNP levels predict outcome in pediatric heart failure patients: post hoc analysis of the Pediatric Carvedilol Trial. Circulation 2010;3:606–11. [DOI] [PubMed] [Google Scholar]
- 25.Rusconi P, Wilkinson JD, Sleeper LA, Lu M, Cox GF, Towbin JA, et al. Differences in presentation and outcomes between children with familial dilated cardiomyopathy and children with idiopathic dilated cardiomyopathy: a report from the Pediatric Cardiomyopathy Registry Study Group. Circulation 2017;10:e002637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Everitt MD, Pahl E, Schechtman KB, Zheng J, Ringewald JM, L’ecuyer T, et al. Rejection with hemodynamic compromise in the current era of pediatric heart transplantation: a multi-institutional study. J Heart Lung Transplant 2011;30:282–8. [DOI] [PubMed] [Google Scholar]
- 27.Kleinmahon JA, Gralla J, Kirk R, Auerbach SR, Henderson HT, Wallis GA, et al. Cardiac allograft vasculopathy and graft failure in pediatric heart transplant recipients after rejection with severe hemodynamic compromise. J Heart Lung Transplant 2019;38:277–84. [DOI] [PubMed] [Google Scholar]
- 28.Ameduri RK, Zheng J, Schechtman KB, Hoffman TM, Gajarski RJ, Chinnock R, et al. Has late rejection decreased in pediatric heart transplantation in the current era? A multi-institutional study. J Heart Lung Transplant 2012;31:980–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


