Abstract
Background:
Historically, women have had less access to advanced heart failure therapies, including temporary and permanent mechanical circulatory support and heart transplantation (HT), with worse waitlist and post-transplant survival compared with men. This study evaluated for improvement in sex differences across all phases of HT in the 2018 allocation system.
Methods and Results:
The United Network for Organ Sharing registry was queried to identify adult patients (≥18 years) listed for HT from October 18, 2016, to October 17, 2018 (old allocation), and from October 18, 2018, to October 18, 2020 (new allocation). The outcomes of interest included waitlist survival, pretransplant use of temporary and durable mechanical circulatory support, rates of HT, and post-transplant survival. There were 15,629 patients who were listed for HT and included in this analysis; 7745 (2039 women, 26.3%) in the new and 7875 patients (2074 women, 26.3%) in the old allocation system. When compared with men in the new allocation system, women were more likely to have lower priority United Network for Organ Sharing status at time of transplant, and less likely to be supported by an intra-aortic balloon pump (27.1% vs 32.2%, P < .001), with no difference in the use of venoarterial extracorporeal membrane oxygenation (5.5% vs 6.3%, P = .28). Despite these findings, when transplantation was viewed in the context of risk for death or delisting, the cumulative incidence of transplant within 6 months of listing was higher in women than men in the new allocation system (62.4% vs 54.9%, P < .001) with no differences in post-transplant survival. When comparing women in the old with the new allocation system, the distance traveled for organ procurement was 187.5 ± 207.0 miles vs 272.8 ± 233.7 miles (P < .001).
Conclusions:
Although the use of temporary mechanical circulatory support in women remains lower than in men in the new allocation system, more women are being transplanted with comparable waitlist and post-transplant outcomes as men. Broader sharing may be making its greatest impact on improving transplant opportunities for women.
Keywords: Heart transplantation, sex, women, mechanical circulatory support, disparities
Lay Summary
The heart allocation system has changed the way that organs are prioritized. In the old system, some studies showed that women were at a disadvantage and were more likely to die while waiting for a heart. The findings of the current study show that women are more likely to receive an organ than men after the change of the system and there is no difference in death while waiting on the list or survival after transplant.
Historically, women with advanced heart failure and those in cardiogenic shock have been less likely to receive intensive medical therapy, invasive interventions including temporary mechanical circulatory support (MCS), and advanced therapies including durable left ventricular assist device (LVAD) support or heart transplantation (HT).1–4 Sex differences in survival while awaiting HT have also been reported.5 In the previous 3-tiered heart allocation system, women listed as United Network for Organ Sharing (UNOS) status 1A and 1B had a significantly increased risk of waitlist mortality compared with their male counterparts.5,6 However, an analysis using the International Society for Heart and Lung Transplantation Registry found that, when male and female heart transplant recipients were matched for recipient and donor characteristics, there was no difference in survival.7 Factors influencing sex differences in waitlist and transplant outcomes include donor–recipient sex mismatch, allosensitization, the underlying etiology of heart failure, size-based barriers in receiving temporary MCS (cannula size) and durable MCS (pump size), and psychosocial factors, including caregiver availability.8
On October 18, 2018, the new HT organ allocation system was implemented with the goals of better stratifying medically urgent candidates, decreasing waitlist mortality, and minimizing geographic disparities by instituting broader sharing for the sickest patients.9,10 The new system is composed of 6 tiers, defined by need for temporary and/or durable mechanical support as well as type of cardiomyopathy (Supplementary Table 1). As a result of these changes, the use of temporary MCS, particularly intra-aortic balloon pumps (IABPs), has increased for men and women, leading to decreased waitlist time but longer ischemic times—a result of broader geographic sharing.11,12 Although early reports demonstrated decreased survival at 90 and 180 days after HT, more recent studies with longer follow-up periods suggest similar post-transplant survival when compared with the older allocation system.11–14 The goals of the current study were to examine sex differences in (1) the use of waitlist strategies for temporary and durable MCS, (2) waitlist survival or time to transplantation, (3) post-transplant survival in the new allocation system, and (4) the impact of broader sharing for women in the new allocation system compared with women in the old allocation system.
Methods
Patient Population
The UNOS registry includes data on all donors, waitlisted candidates, and transplant recipients in the United States. The registry was queried to identify adult patients (≥18 years old) listed for primary HT from October 18, 2016, through October 18, 2020. Patients in the old allocation system group included those waitlisted as of October 18, 2016, until October 17, 2018. Patients in the new allocation system group included those waitlisted on or after October 18, 2018 (the implementation date of the new allocation), through October 18, 2020. These dates were chosen to balance the sample sizes. Waitlist outcomes, including transplantation and death or delisting for worsening clinical status, were assessed in the first 180 days on the waitlist to ensure balanced follow-up time between the old and new allocation systems. Patients were considered as delisted for worsening status if their reason for waitlist removal was deterioration of condition or medical unsuitability. Those that were removed for other reasons (eg, condition improved, patient refused transplant) were censored on the day of waitlist removal. Supplementary Fig. 1 provides a CONSORT diagram of the study population. When analyzing waitlist outcomes, patients listed for HT in the old allocation system who remained on the waitlist as of October 18, 2018, were censored on this date for an analysis of waitlist outcomes. Patients transplanted after October 18, 2018, who were still alive after 180 days were censored at 180 days for the post-transplant survival analysis. Because the data were obtained as part of routine care and de-identified by UNOS, institutional review board approval was not required.
Statistical Analysis
Sex-specific baseline characteristics included demographics, type of heart disease, comorbid conditions, functional status, UNOS status at listing, and pretransplant hemodynamics at listing. Similarly, the rates of temporary MCS including IABP and venoarterial extracorporeal membrane oxygenation (VA-ECMO) as well as durable MCS (ie, LVADs) were obtained at time of listing and time of transplant. Continuous variables were presented as means and standard deviations and categorical variables were expressed as counts and percentages. Groups compared included women in the old allocation system, women in the new allocation system, and men in the new allocation system. Differences between groups were quantified using independent t test and the χ2 test when appropriate. The primary outcome of interest was transplantation in the new allocation system. Cumulative incidences of transplant with death or delisting as a competing event were calculated by gender and compared using the modified χ2 statistic as proposed by Gray.15 Fine–-Gray subdistribution hazard models were used to model the effect of sex on the incidence of transplant after accounting for the competing risk of death.16 The cumulative incidences of death or delisting with transplant as a competing event were also compared. A multivariable competing risk regression was also performed with additional covariates including age at listing, emergent listing status, Black race, and sex. Kaplan–Meier curves were generated to estimate post-transplant survival and log-rank tests used to compare groups. Multivariable Cox proportional hazard modeling for post-transplant mortality in the new allocation system was performed with a priori defined covariates including recipient and donor age, LVAD at transplant, VA-ECMO at transplant, heart failure etiology, ischemic time, recipient ethnicity, and dual organ status. Proportional hazard assumptions were checked to ensure no violation. All P values were based on 2-sided tests with P < .05 considered statistically significant. R, Version 3.6.3 was used to perform statistical analyses.17
Results
Between October 18, 2018, and October 18, 2020, a total of 7745 patients (2039 women, 26.3%) were actively listed for HT in the new allocation system. Comparatively, 7875 patients (2074 women, 26.3%) were listed in the old allocation system.
Characteristics of Women Listed in the New vs the Old Allocation System
We first compared characteristics of women listed in the new allocation system with those of women listed in the old system. Women added to the waitlist were of similar age and race during both periods (Table 1). There were no differences in burden of comorbidities such as diabetes, cerebrovascular disease, or former tobacco smoking. Similarly, hemodynamics including pulmonary pressures, pulmonary capillary wedge pressure, cardiac output, and pulmonary vascular resistance were not significantly different in each era. More women were listed for multiorgan transplantation in the new allocation system (6.8% vs 4.5%, P = .002), reflective of increased multiorgan transplantation overall in the contemporary era.
Table 1.
Sex-Specific Baseline Characteristics at Time of Addition to Waitlist
| Variable | Women in the Old Allocation System (n = 2074) | Women in the New Allocation System (n = 2039) | P Value |
|---|---|---|---|
| Demographics | |||
| Age (y) at listing, mean | 50.7 ± 13.7 | 50.0 ± 13.7 | .107 |
| Race | .524 | ||
| White | 1214 (58.5%) | 1158 (56.8%) | |
| Black | 593 (28.6%) | 605 (29.7%) | |
| Other | 267 (12.9%) | 276 (13.5%) | |
| BSA (m2) | 1.81 ± 0.23 | 1.82 ± 0.23 | .043 |
| BMI (kg/m2) | 27.2 ± 5.4 | 27.6 ± 5.5 | .007 |
| HF etiology | .027 | ||
| Nonischemic | 1251 (60.3%) | 1201 (58.9%) | |
| Ischemic | 352 (17.0%) | 294 (14.4%) | |
| HCM | 103 (5.0%) | 105 (5.1%) | |
| RCM | 69 (3.3%) | 99 (4.9%) | |
| Congenital | 101 (4.9%) | 117 (5.7%) | |
| Allograft failure | 83 (4.0%) | 88 (4.3%) | |
| Other | 115 (5.5%) | 135 (6.6%) | |
| Blood type (O) | 949 (45.8%) | 901 (44.2%) | .327 |
| Diabetes | 502 (24.2%) | 496 (24.3%) | .943 |
| CVA | 137 (6.6%) | 148 (7.3%) | .427 |
| Tobacco use | 711 (34.3%) | 664 (32.6%) | .266 |
| ICD at listing | 1471 (71.8%) | 1383 (69.4%) | .091 |
| Hemodynamics | |||
| PA systolic (mm Hg) | 39.7 ± 13.5 | 39.7 ± 13.7 | .853 |
| PA diastolic (mm Hg) | 19.2 ± 8.4 | 19.2 ± 8.4 | .972 |
| PA mean (mm Hg) | 27.1 ± 10.0 | 26.9 ± 9.9 | .525 |
| PCWP mean (mm Hg) | 17.5 ± 8.6 | 17.8 ± 8.7 | .262 |
| CO (L/min) | 3.88 ± 1.19 | 3.85± 1.21 | .518 |
| Cardiac index (L/min/m2) | 2.16 ± 0.67 | 2.13 ± 0.71 | .208 |
| PVR (WU) | 2.72 ± 1.89 | 2.62 ± 1.87 | .094 |
| Creatinine (mg/dL) | 1.20 ± 0.93 | 1.22 ± 0.96 | .562 |
| Prior dialysis | 67 (4.6%) | 68 (4.7%) | 1.00 |
| Multiorgan Transplant | 93 (4.5%) | 138 (6.8%) | .002 |
| Tier at Listing | <.001 | ||
| Status 1A | 508 (24.5%) | — | |
| Status 1B | 867 (41.8%) | — | |
| Status 2 (old) | 631 (30.4%) | — | |
| Status 1 | — | 85 (4.2%) | |
| Status 2 | — | 392 (19.2%) | |
| Status 3 | — | 245 (12.0%) | |
| Status 4 | — | 780 (38.3%) | |
| Status 5 | — | 29 (1.4%) | |
| Status 6 | — | 475 (23.3%) | |
| Inactive (801) | 68 (3.3%) | 33 (1.6%) | |
| Inotrope | 715 (34.5%) | 661 (32.4%) | .172 |
| Temporary MCS | |||
| IABP | 102 (4.9%) | 256 (12.6%) | <.001 |
| ECMO | 46 (2.2%) | 68 (3.3%) | .037 |
| Durable LVAD | 467 (22.5%) | 418 (20.5%) | .129 |
BSA, body surface area; BMI, body mass index; CO, cardiac output; CVA, cerebrovascular disease; ECMO, extracorporeal membrane oxygenation; HCM, hypertrophic cardiomyopathy; HF, heart failure; IABP, intra-aortic balloon pump; ICD, implantable cardioverter-defibrillator; LVAD, left ventricular assist device; MCS, mechanical circulatory support; PA, pulmonary artery; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance; RCM, restrictive cardiomyopathy.
With respect to temporary MCS, women in the new allocation system were significantly more likely to receive an IABP (12.6% vs 4.9%, P < .001) as well as VA-ECMO (3.3% vs 2.2%, P = .037) compared with women in the old system. There was no difference in the proportion of women on a durable LVAD who were listed as bridge to transplant (20.5% vs 22.5%, P = .129).
Characteristics of Women Transplanted in the New Allocation System vs the Old Allocation System
We then investigated differences among the subset of women who were transplanted under the new and old allocation systems. There was a 2.2% increase in the percentage of women who received a transplant in the new system (n = 1503, 73.7%) compared with the old system (n = 1483, 71.5%). Women in the new system were significantly more likely to have an IABP in place at time of transplant (27.1% vs 9.0%, P < .001) or VA-ECMO support (5.5% vs 1.6%, P < .001) compared with women in the old allocation system (Table 2). Women in the new allocation system were also significantly less likely to have a durable LVAD (21.8% vs 31.8%, P < .001) at transplantation. Trends over time in ventricular support strategies by sex at time of transplant are shown in Fig. 1. Women were less likely to receive an organ from a female donor in the new system compared with women in the old system (P = .012). Ischemic times (3.14 ± 1.10 hours vs 3.40 ± 1.02 hours, P < .001) and distance from transplant center (187.5 ± 207.0 miles vs 272.8 ± 233.7; P <.001) were significantly increased for women in the new system. These are previously reported trends overall and undoubtedly the result of broader sharing, which was a new component to the 2018 heart allocation policy.
Table 2.
Recipient and Donor Characteristics of Transplanted Women, Stratified by Allocation System
| Variable | Women in the Old Allocation System (n = 1483) | Women in the New Allocation System (n = 1503) | P Value |
|---|---|---|---|
| Recipient characteristics | |||
| Age (y), mean | 51.4 ± 13.7 | 50.6 ± 13.5 | .094 |
| BMI, mean | 27.0 ± 5.3 | 27.3 ± 5.5 | .094 |
| Status at transplant* | <.001 | ||
| Status 1A | 753 (50.8%) | — | |
| Status 1B | 401 (27.0%) | — | |
| Status 2 (old) | 58 (3.9%) | — | |
| Status 1 | 8 (0.5%) | 114 (7.5%) | |
| Status 2 | 68 (4.6%) | 610 (40.6%) | |
| Status 3 | 68 (4.6%) | 282 (18.8%) | |
| Status 4 | 99 (6.7%) | 381 (25.3%) | |
| Status 5 | 3 (0.2%) | 15 (1.9%) | |
| Status 6 | 23 (1.6%) | 99 (6.6%) | |
| Ventricular support | |||
| Inotrope | 606 (41.5%) | 600 (40.2%) | .489 |
| Temporary MCS | |||
| IABP | 132 (9.0%) | 405 (27.1%) | <.001 |
| ECMO | 23 (1.6%) | 82 (5.5%) | <.001 |
| Durable LVAD | 465 (31.8%) | 319 (21.8%) | <.001 |
| Donor | |||
| Donor age (y) | 32.5 ± 11.5 | 31.9 ± 10.6 | .058 |
| Female donor sex | 938 (63.3%) | 910 (60.5%) | .012 |
| White donor race | 944 (64.7%) | 942 (63.1%) | .586 |
| Ischemic time (h) | 3.14 ± 1.10 | 3.40 ± 1.02 | <.001 |
| Distance traveled (miles) | 187.50 ± 207.0 | 272.84 ± 233.7 | <.001 |
Patients with status 1–6 were listed in the old allocation system but transplanted in the new allocation system.
HCV, hepatitis C virus. Other abbreviations as in Table 1.
Fig. 1.
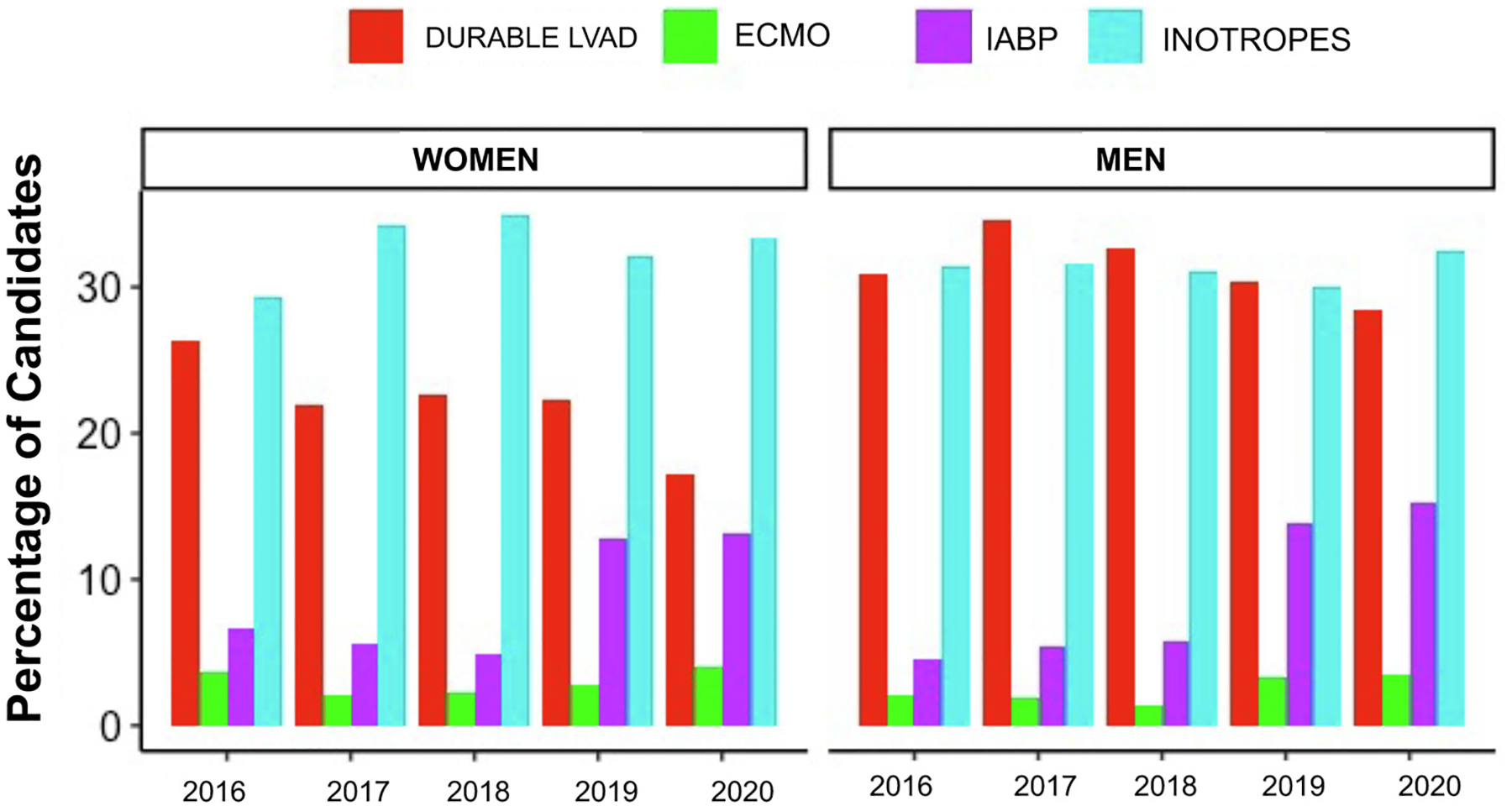
Trends in the use of ventricular support by sex at the time of transplantation, 2016–2020. Trends in the use of ventricular support at the time of transplant are shown stratified by sex with a durable left ventricular assist device (LVAD) in red, venoarterial extracorporeal membrane oxygenation (ECMO) in green, intra-aortic balloon pump (IABP) in purple, and inotrope support in light blue.
Characteristics of Women and Men Listed in the New Allocation System
Subsequently, we compared the characteristics of women and men listed in the new allocation system. Women listed in new allocation system were significantly younger at listing and were more likely to be Black compared with men (Supplementary Table 2). Additionally, women were less likely than men to use tobacco and had a lower prevalence of diabetes. There was no difference in the frequency of women with blood type O or UNOS status at listing between sexes. At the time of listing in the new allocation system, nearly 30% of both women and men were on inotropic support [women 661, 32.4%, vs men 1780, 31.1%, P = .301). Although there were no differences in the use of temporary MCS, there were significantly more durable LVADs in men (29.7% vs 20.5% in women, P < .0001) at listing.
Characteristics of Women and Men Transplanted in the New Allocation System
Next, we examined the characteristics of the subset of women and men who underwent transplantation under the new allocation system. When compared with men, at the time of transplant, women were more likely to have lower priority UNOS status (Table 3), and thus less likely to be supported by an IABP (27.1% vs 32.2% P < .001), with no difference in the use of VA-ECMO (5.5% vs 6.3%, P = .28) (Table 3). Women were significantly less likely than men to have a durable LVAD at the time of transplant (21.8% vs 29.7%, P < .001).
Table 3.
Recipient and Donor Characteristics of Transplanted Patients by Sex in the New Allocation System
| Variable | Women (n = 1503, 28.3%) | Men (n = 3807, 71.7%) | P Value |
|---|---|---|---|
| Recipient | |||
| Status at transplant | <.001 | ||
| Status 1 | 114 (7.5%) | 369 (9.7%) | |
| Status 2 | 610 (40.6%) | 1943 (51.0%) | |
| Status 3 | 282 (18.8%) | 723 (19.0%) | |
| Status 4 | 381 (25.3%) | 604 (15.9%) | |
| Status 5 | 15 (1.9%) | 26 (0.7%) | |
| Status 6 | 99 (6.6%) | 133 (3.5%) | |
| Ventricular assistance at transplant | |||
| Inotrope only | 600 (40.2%) | 1582 (41.9%) | .27 |
| Temporary MCS | |||
| IABP | 405 (27.1) | 1218 (32.2) | <.001 |
| ECMO | 82 (5.5) | 239 (6.3) | .28 |
| Durable LVAD | 319 (21.8) | 1105 (29.7) | <.001 |
| Donor | |||
| Donor age (y) | 31.9 ± 10.6 | 32.3 ± 10.4 | .218 |
| Female donor sex | 910 (60.5%) | 584 (15.3%) | <.001 |
| White donor race | 942 (63.1%) | 2391 (63.3%) | .456 |
| Ischemic time (h) | 3.40 ± 1.02 | 3.45 ± 1.06 | .148 |
| Distance traveled, miles | 272.8 ± 233.7 | 268.2 ± 235.3 | .521 |
Abbreviations as in Table 1.
Increased Transplantation for Women in New Allocation System
When transplantation was viewed in the context of risk for death or delisting, the cumulative incidence of transplant within 6 months of listing was higher in women than men in the new allocation system (62.4% vs 54.9%, P < .001) (Fig. 2). As compared with the old allocation system, women in the new system had an increased cumulative incidence of transplant at 6 months (62.4% vs 55.2%, P < .001) (Fig. 3). The cumulative incidence of transplant in women remained higher than men even after adjustment for age at listing, UNOS status 1 at listing, and Black race (adjusted hazard ratio [HR] 1.21, 95% confidence interval 1.13–1.29, P < .001).
Fig. 2.

Comparison of sex-specific outcomes in the new allocation system. Despite the relatively lower use of intra-aortic balloon pump (IABP) and durable left ventricular assist device (LVAD) in women compared with men at the time of transplant, women were more likely to receive a heart transplant (HT) in the new allocation system with no difference in death or delisting in competing risk analysis. The x axis represents waitlist time.
Fig. 3.
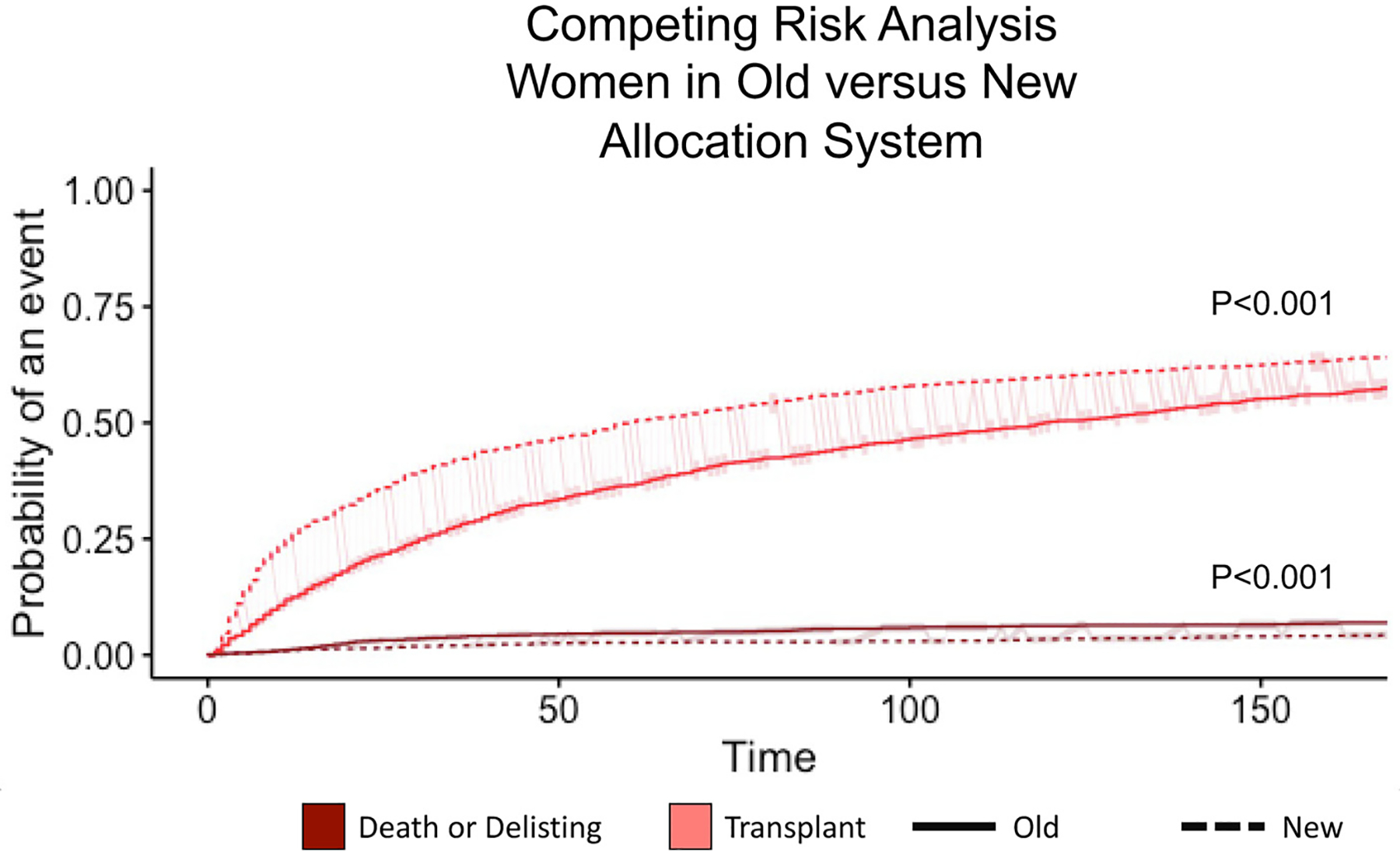
Competing Risk analysis of transplant versus death or delisting among women in both allocation systems. The competing risk analysis shows that women in the new allocation system had a significantly increased probability of heart transplant and reduced probability of death or delisting compared with women in the old allocation system (P < .001).
Waitlist Outcomes in the New Allocation System: Death or Delisting
The new allocation system was associated with a lower cumulative incidence of waitlist removal for deterioration in the presence of the competing risk of transplant in the first 6 months of listing for women compared with the old allocation system (4.3% vs 6.9%, P ±.001) (Fig. 3). There was no difference between death or delisting for worsening status between men and women in new allocation (Take Home Graphic).
Post-Transplant Survival in the New Allocation System
We compared post-transplant survival between women and men transplanted in the new allocation system. Among the 4551 patients who underwent transplant in new allocation system, the median follow-up time was 369 days (interquartile range 181–733 days). A total of 148 patients did not have follow-up data available and were excluded from the post-transplant survival analysis. A total of 478 patients (10.5%) died during study follow-up, with 362 deaths (75.7%) within the first 180 days. The 6-month survival was 92.2% for men and 91.4% for women (P = .51) (Fig. 4A). When univariate and multivariable Cox proportional hazard modeling was used to identify risk factors associated with 6-month mortality, including recipient and donor age, recipient sex, MCS at transplant, and ischemic time, sex was not significantly associated with 6-month mortality rate (Table 4).
Fig. 4.

Post-transplantation graft survival by sex at 180 days. (A) Kaplan–Meier estimates are shown for the subset of men and women who underwent heart transplantation in the new allocation period. The red line represents women, and the blue line represents men. (B) Estimates are shown for post-transplant survival for women in the old allocation system vs the new allocation system.
Table 4.
Cox Proportional Hazards Modeling for Predictors of Mortality Post-Transplant in the New Allocation System
| Variable | Unadjusted HR (95% CI) | Adjusted HR* (95% CI) | P Value |
|---|---|---|---|
| Male sex | 0.93 (0.74–1.16) | 0.80 (0.64–1.02) | .073 |
| Recipient age (y) | 1.02 (1.01–1.03) | 1.03 (1.02–1.04) | <.001 |
| Recipient race | |||
| Black | (Reference) | (Reference) | .129 |
| White | 1.16 (0.89–1.5) | 1.06 (0.81–1.39) | |
| Other | 1.32 (0.94–1.85) | 1.4 (0.99–1.98) | |
| Heart failure etiology | <.001 | ||
| Congenital | (Reference) | (Reference) | |
| HCM/RCM | 0.80 (0.47–1.35) | 0.57 (0.33–0.99) | |
| Ischemic | 0.75 (0.47–1.18) | 0.41 (0.25–0.68) | |
| Nonischemic | 0.50 (0.32–0.78) | 0.33 (0.20–0.54) | |
| Other | 0.58 (0.31–1.08) | 0.42 (0.22–0.80) | |
| Retransplant | 0.71 (0.36–1.41) | 0.51 (0.25–1.02) | |
| Durable LVAD at transplant | 1.51 (1.21–1.87) | 1.71 (1.32–2.22) | <.001 |
| ECMO at transplant | 1.63 (1.16–2.30) | 2.04 (1.43–2.90) | <.001 |
| IABP at transplant | 0.69 (0.55–0.88) | 0.91 (0.69–1.19) | .485 |
| Ischemic time (h) | 1.12 (1.02–1.23) | 1.12 (1.02–1.23) | .016 |
| Donor age (y) | 1.01 (1.00–1.02) | 1.01 (1.00–1.02) | .272 |
| Dual organ transplant | 1.81 (1.36–2.41) | 1.79 (1.33–2.41) | <.001 |
CI, confidence interval. Other abbreviations as in Table 1.
Adjusted for recipient and donor age, LVAD at transplant, ECMO at transplant, heart failure etiology, ischemic time, recipient ethnicity, and dual organ status.
Last, we compared post-transplant survival between women transplanted in the new and old allocation systems. Women transplanted under the new allocation system had a slightly lower post-transplant survival at 6 months compared with women under the old system: 91.4% vs 93.6% (P = .045) (Fig. 4B).
Discussion
Prior reports have demonstrated increased waitlist mortality among women compared with men in the old allocation system. The new heart allocation policy was written to heavily prioritize candidates supported with temporary MCS; women have historically had lower rates of temporary MCS use. Therefore, our initial hypothesis for this study was that rates of transplant for women in new allocation may be (1) worse than in the prior allocation and (2) worse compared with men in the new allocation system, despite broader sharing, which was in part aimed to provide increased opportunities for hard-to-transplant candidates, such as those highly sensitized or at the extremes of body size. However, we found the opposite trends. Our major findings are that, although IABP and VA-ECMO use has significantly increased overall in the new allocation system, women were significantly less likely than men to be supported by an IABP or a durable LVAD at the time of transplantation. Nevertheless, women experienced higher rates of HT as compared with women in the old allocation system and as compared with men in the new allocation system, with improved waitlist survival and no difference in post-transplant survival when controlling for other factors that can negatively affect post-transplant survival. These are positive trends, suggesting that the new policy may be improving transplant opportunities for women.
In the new system, the use of IABP significantly increased in both sexes between the time of listing and the time of transplantation, yet remained relatively lower in women as compared with men. The reasons for the avoidance of temporary MCS by sex are unclear. Although sex-specific data on VA-ECMO are limited, some reports highlight female sex as a risk factor for vascular complications and poor outcomes more generally.18,19 Additionally, vessel caliber and size or concerns about bleeding may be factors contributing to differences in temporary MCS devices. Notably, there were no differences in VA-ECMO use by sex in our analysis. We and other investigators have also demonstrated previously that durable MCS is used less often among women compared with men.4,20 In the prior heart transplant allocation system, women listed as UNOS status 1A were less likely to be on MCS and more likely to be on inotropic support as a bridge to transplantation.6,21,22 In the new allocation system, women were still significantly less likely to be supported on durable MCS at the time of listing and at time of transplantation. The persistently lower use of durable MCS in women may be due to risk of further allosensitization and the higher burden of sex-specific adverse events (ie, stroke) on LVAD support.4,23
Prior reports of the old allocation system demonstrated worse waitlist outcomes for women than men. Increased waitlist mortality was observed for women listed as UNOS 1A (adjusted HR 1.14) and UNOS 1B (adjusted HR 1.17) from 2004 to 2015.6 For patients listed as UNOS status 2, female sex was protective for time to death (adjusted HR 0.85) in the old allocation system. When these differences were stratified by era of transplantation, sex differences in waitlist mortality for Status 1A patients were no longer present for patients transplanted more recently, from 2012 to 2015, but remained for UNOS status 1B patients during that same 3-year time period. Similarly, female sex was no longer protective for UNOS status 2 patients in the 2012–2015 period.6 With the old allocation system, there was concern that women were referred with more advanced disease and were less likely to undergo LVAD implantation as bridge to transplant than men, which may have contributed to their poorer survival.8,24
In the current study, women were more likely to be transplanted than men in the new allocation system without any difference in death or delisting. The percentage of women getting transplanted increased by 2.2% and the percentage of men getting transplanted from the old to new system decreased by 0.9%, suggesting that the new allocation system has preferentially benefited women. Yet, only 48% of women are status 1 or 2 at transplant compared with more than 60% of men. How do we reconcile these findings? Potential explanations include the combination of a change in the sex or size of the donor pool, broader geographic sharing, and the increased use of donor preservation technology such as Transmedics and SherpaPak, which may allow for longer travel times, especially to obtain hearts for hard-to-transplant candidates (eg, highly sensitized and/or extreme body sizes). In our analysis, donor graft ischemic times and distance traveled were not significantly different between men and women in the new allocation system. However, the distance traveled was significantly increased for women in the new allocation system compared with the old system, suggesting that the increase in transplantation in women may be driven, at least in part, by broader sharing. Women were also significantly less likely in the new allocation system to be on a bridge to transplant LVAD compared with the old allocation system, which suggests that women may be benefiting from a direct-to-transplant strategy. Further studies are needed to determine if there is geographic variation by sex and other factors driving transplantation in women.
We found no difference in post-transplant graft survival at 180 days between men and women in the new allocation system. A recent study using the Scientific Registry of Transplant Recipients evaluated 3 phases of mortality post-transplant (early procedure-related mortality, constant, and late phase).25 Women had improved long-term survival compared with men, mainly owing to their younger age, decreased incidence of diabetes mellitus, and lower incidence of obesity. Therefore, based on these findings one would not be expected to see any sex differences in mortality in the current study.
Our study has limitations that should be acknowledged. This study was a retrospective analysis using registry data. Discrepancies in UNOS data submission requirements may introduce ascertainment bias where all events are accounted for, but surviving patients are more likely to be censored. Data regarding the specific types of percutaneous ventricular assist device used (ie, Impella) are not easily captured as compared with the use of IABP and ECMO. Strengths of our analysis include using a similar duration of time in both the old and new allocation system with a similar duration of time, as well as the competing risks analysis. In the current study, patients through October of 2020 were included, providing 2 years of experience with the new allocation system.
In conclusion, although there has been relatively lower use of temporary MCS in women in the new donor heart allocation system, women had preferentially higher incidence of transplantation without differences in waitlist mortality or post-transplant survival compared with men.
Supplementary Material
Footnotes
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cardfail.2022.03.354.
References
- 1.Habal MV, Axsom K, Farr M. Advanced therapies for advanced heart failure in women. Heart Fail Clin 2019;15:97–107. [DOI] [PubMed] [Google Scholar]
- 2.Galvao M, Kalman J, DeMarco T, et al. Gender differences in in-hospital management and outcomes in patients with decompensated heart failure: analysis from the Acute Decompensated Heart Failure National Registry (ADHERE). J Card Fail 2006;12:100–7. [DOI] [PubMed] [Google Scholar]
- 3.Stretch R, Sauer CM, Yuh DD, Bonde P. National trends in the utilization of short-term mechanical circulatory support: incidence, outcomes, and cost analysis. J Am Coll Cardiol 2014;64:1407–15. [DOI] [PubMed] [Google Scholar]
- 4.DeFilippis EM, Truby LK, Garan AR, et al. Sex-related differences in use and outcomes of left ventricular assist devices as bridge to transplantation. JACC Heart Fail 2019;7:250–7. [DOI] [PubMed] [Google Scholar]
- 5.Hsich EM, Starling RC, Blackstone EH, et al. Does the UNOS heart transplant allocation system favor men over women? JACC Heart Fail 2014;2:347–55. [DOI] [PubMed] [Google Scholar]
- 6.Hsich EM, Blackstone EH, Thuita L, et al. Sex differences in mortality based on United Network for Organ Sharing status while awaiting heart transplantation. Circ Heart Fail 2017;10:e003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moayedi Y, Fan CPS, Cherikh WS, et al. Survival Outcomes after heart transplantation: does recipient sex matter? Circ Heart Fail 2019;12:e006218. [DOI] [PubMed] [Google Scholar]
- 8.Melk A, Babitsch B, Borchert-Morlins B, et al. Equally interchangeable? How sex and gender affect transplantation. Transplantation 2019;103:1094–110. [DOI] [PubMed] [Google Scholar]
- 9.Meyer DM, Rogers JG, Edwards LB, et al. The future direction of the adult heart allocation system in the United States. Am J Transplant 2015;15:44–54. [DOI] [PubMed] [Google Scholar]
- 10.Hoosain J, Hankins S. Time is a precious commodity: 2018 OPTN policy change and the potential to lower heart transplant waitlist time in the sickest patients. Curr Cardiol Rep 2019;21:67. [DOI] [PubMed] [Google Scholar]
- 11.Jawitz OK, Fudim M, Raman V, et al. Reassessing recipient mortality under the new heart allocation system. JACC Heart Fail 2020;8:548–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parker WF, Chung K, Anderson AS, Siegler M, Huang ES, Churpek MM. Practice changes at U.S. transplant centers after the new adult heart allocation policy. J Am Coll Cardiol 2020;75:2906–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goff RR, Uccellini K, Lindblad K, et al. A change of heart: preliminary results of the US 2018 adult heart allocation revision. Am J Transplant 2020;20:2781–90. [DOI] [PubMed] [Google Scholar]
- 14.Cogswell R, John R, Estep J, et al. An early investigation of outcomes with the new 2018 donor heart allocation system in the United States. J Heart Lung Transplant 2019;39:1–4. [DOI] [PubMed] [Google Scholar]
- 15.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat 1988;16:1141–54. [Google Scholar]
- 16.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496–509. [Google Scholar]
- 17.R Development Core Team. A language and environment for statistical computing: reference index. Vienna: R Foundation for Statistical Computing; 2010. Available at: http://www.polsci.wvu.edu/duval/PS603/Notes/R/fullrefman.pdf Accessed May 2, 2021. [Google Scholar]
- 18.Lo Coco V, Lorusso R, Raffa GM, et al. Clinical complications during veno-arterial extracorporeal membrane oxygenation in post-cardiotomy and non post-cardiotomy shock: still the Achille’s heel. J Thorac Dis 2018;10:6993–7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keebler ME, Haddad EV, Choi CW, et al. Venoarterial extracorporeal membrane oxygenation in cardiogenic shock. JACC Heart Fail 2018;6:503–16. [DOI] [PubMed] [Google Scholar]
- 20.Gruen J, Caraballo C, Miller PE, et al. Sex differences in patients receiving left ventricular assist devices for end-stage heart failure. JACC Heart Fail 2020;8:770–9. [DOI] [PubMed] [Google Scholar]
- 21.Morris AA, Cole RT, Laskar SR, et al. Improved outcomes for women on the heart transplant wait list in the modern era. J Cardiac Fail 2015;21:555–60. [DOI] [PubMed] [Google Scholar]
- 22.Hsich EM. Sex differences in advanced heart failure therapies. Circulation 2019;139:1080–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi AA, Lerman JB, Sajja AP, et al. Sex-based differences in left ventricular assist device utilization: insights from the nationwide inpatient sample 2004 to 2016. Circ Heart Fail 2019;12:e006082. Available at https://www.ahajournals.org/doi/10.1161/CIRCHEART-FAILURE.119.006082 Accessed July 13, 2020. [DOI] [PubMed] [Google Scholar]
- 24.Regitz-Zagrosek V, Petrov G, Lehmkuhl E, et al. Heart transplantation in women with dilated cardiomyopathy. Transplantation 2010;89:236–44. [DOI] [PubMed] [Google Scholar]
- 25.Hsich EM, Blackstone EH, Thuita LW, et al. Heart transplantation: an in-depth survival analysis. JACC Heart Fail 2020;8:557–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


