The International Society for Heart and Lung Transplantation (ISHLT) International Thoracic Organ Transplant (TTX) Registry has gathered data regarding transplant procedures, donor and recipient characteristics, and outcomes from a global community of transplant centres over the last 3 decades. Close to 70,000 adult lung transplant procedures have been reported to the Registry since its initiation, each providing an opportunity for a recipient with end-stage lung disease to regain quality of life and longevity. Each year’s report provides more detailed analyses on a particular focus theme important to recipient outcomes.1 Since 2013, these have been donor and recipient age2; re-transplantation3; early graft failure4; indication for transplant5; allograft ischemic time6; multiorgan transplantation7; and donor and recipient size matching.8 In response to a changing regulatory environment, the ISHLT TTX Registry is undergoing an update in data acquisition, and the patient cohort examined in this report is therefore derived from the same data source or datasets as examined in the 2019 to 2021 annual reports. We refer the reader to the 2019 and prior reports for a detailed description of the baseline characteristics of the cohort and additional core analyses not directly related to the focus explored in this year’s report.
This year’s report aimed to identify important recipient and transplant process characteristics that may influence outcomes in patients receiving transplant for chronic obstructive pulmonary disease (COPD). Due to small numbers, heart-lung transplant recipient characteristics and transplant outcomes have not been included. Thus, this 39th annual adult lung transplant report is based on data submitted to the ISHLT TTX Registry on 67,493 adult recipients of deceased recipient transplants between January 1, 1992, and June 30, 2018.
Data collection, conventions, and statistical methods
National and multinational transplant collectives and individual transplant centers have submitted data to the ISHLT International TTX Registry. Since the Registry’s inception, 481 heart transplant centers, 260 lung transplant centers, and 184 centers that perform combined heart-lung transplants have reported data to the ISHLT TTX Registry.9–11 This report references specific online e-slides when particular data are discussed but not shown due to space limitations; e-slide numbers refer to the online adult lung transplant slides, shortened to “L(a),” and available at https://ishltregistries.org/registries/slides.asp. The ISHLT website also contains slide sets for previous annual reports.
The ISHLT International TTX website (https://ishlt.org/research-data/registries/ttx-registry) provides detailed spread-sheets of the data elements collected in the Registry. The Registry requires submission of core recipient, donor, and transplant procedure variables around the time of transplantation, and at annual follow-up and, thus, has low rates of missing data. Nevertheless, data quality depends on the accuracy and completeness of reporting. Rates of missingness may significantly increase for Registry variables that rely on voluntary reporting. The Registry uses various quality control measures to ensure acceptable data quality and completeness before including data for analyses.
Analytical conventions
The current report includes data on adult recipients of deceased donor, lung alone, transplant procedures. Heart-lung and other combinations involving lung transplants are not included in this report. Additionally, a separate Registry report details pediatric lung and heart-lung transplant outcomes, with this year’s focus on pulmonary vascular disease in children. The Registry does not capture the exact occurrence date for most secondary outcomes, such as bronchiolitis obliterans syndrome (BOS). Still, it does capture a time period for which the event occurred (e.g., the event occurred between the first and the second-year annual follow-up visits). For the report’s analyses, we use the mid-point between the annual follow-ups as a surrogate for the event date. Because deceased subjects no longer contribute to the secondary outcomes, we restrict some analyses to include only surviving recipients to reduce the potential of underestimating event rates or other outcomes. For time-to-event analyses, we censor the follow-up of recipients who have not yet experienced the event at the most recent annual follow-up or the time of re-transplantation. We truncate time-to-event graphs (e.g., survival graphs) when the number of individuals at risk becomes <10. Previous Registry report themes provide more details regarding specific donor and recipient characteristics and outcomes.2,4,6–8
Characteristics of recipients transplanted for COPD
Lung transplantation has evolved from a rare procedure in the 1980s to a well-accepted option for patients with end-stage lung disease in the modern era. Over 3 decades of clinical experience, bolstered by evidence generated from resources such as the ISHLT TTX Registry, on a background of changing demography (an aging population, declining smoking rates, increasing prevalence of diabetes mellitus), has led to changes and refinements in recipient selection, and medical and surgical approaches. Since the beginning of lung transplantation, recipients with COPD have accounted for nearly a third of the cohort. This year’s adult lung transplant Registry report examines the cohort of patients transplanted for COPD.
The changes in adult lung transplant recipient diagnosis by era (1992–2000, 2001–2009, and 2010–2018) are presented in Table 1 (eSlide L(a) 4). The total number of lung transplants has increased over time, from just over 11,000 in the 1990s to nearly 34,000 from 2010 to 2018. The number of lung transplant recipients with COPD has also increased by era, but the proportion of COPD recipients compared to other transplant diagnoses has decreased from 37% to 26% (Table 1, eSlide L(a) 4). In North America, the proportion of lung transplants performed for COPD has dramatically declined over time relative to Europe, where an increase has been seen (Figure 1(eSlide L(a) 9). This seems to be a consequence of the increasing number of transplant recipients with restrictive lung disease transplanted in North America, which was reported as an overall increase in forced expiratory volume in 1 second and a decline in forced vital capacity in last year’s report.12
Table 1.
Diagnosis Distribution by Era (Transplants: January 1992-June 2018)
| Jan 1992-Dec 2000 (N = 11,796) | Jan 2001-Dec 2009 (N = 21,806) | Jan 2010-Jun 2018 (N = 33,891) | p-value | |
|---|---|---|---|---|
| Diagnosis | <0.0001 | |||
| - COPD | 4,162 (37.3%) | 7,102 (33.1%) | 8,917 (26.5%) | |
| - A1ATD | 1,169 (10.5%) | 1,131 (5.3%) | 1,054 (3.1%) | |
| - CF | 1,717 (15.4%) | 3,470 (16.2%) | 4,771 (14.2%) | |
| - IPAH | 578 (5.2%) | 563 (2.6%) | 945 (2.8%) | |
| - IPF | 1,677 (15.0%) | 4,899 (22.8%) | 9,755 (29.0%) | |
| - Retransplant | 394 (3.5%) | 921 (4.3%) | 1,364 (4.1%) | |
| - Other | 1,471 (13.2%) | 3,392 (15.8%) | 6,822 (20.3%) |
Continuous factors are expressed as median (Fifth–95th percentiles).
Summary statistics included transplants with non-missing data.
Diagnosis categories were compared across eras using the chi-square statistic.
A1ATD, alpha-1-antitrypsin deficiency; CF, cystic fibrosis; COPD, chronic obstructive pulmonary disease; IPAH, idiopathic pulmonary arterial hypertension; IPF, idiopathic pulmonary fibrosis.
Figure 1.
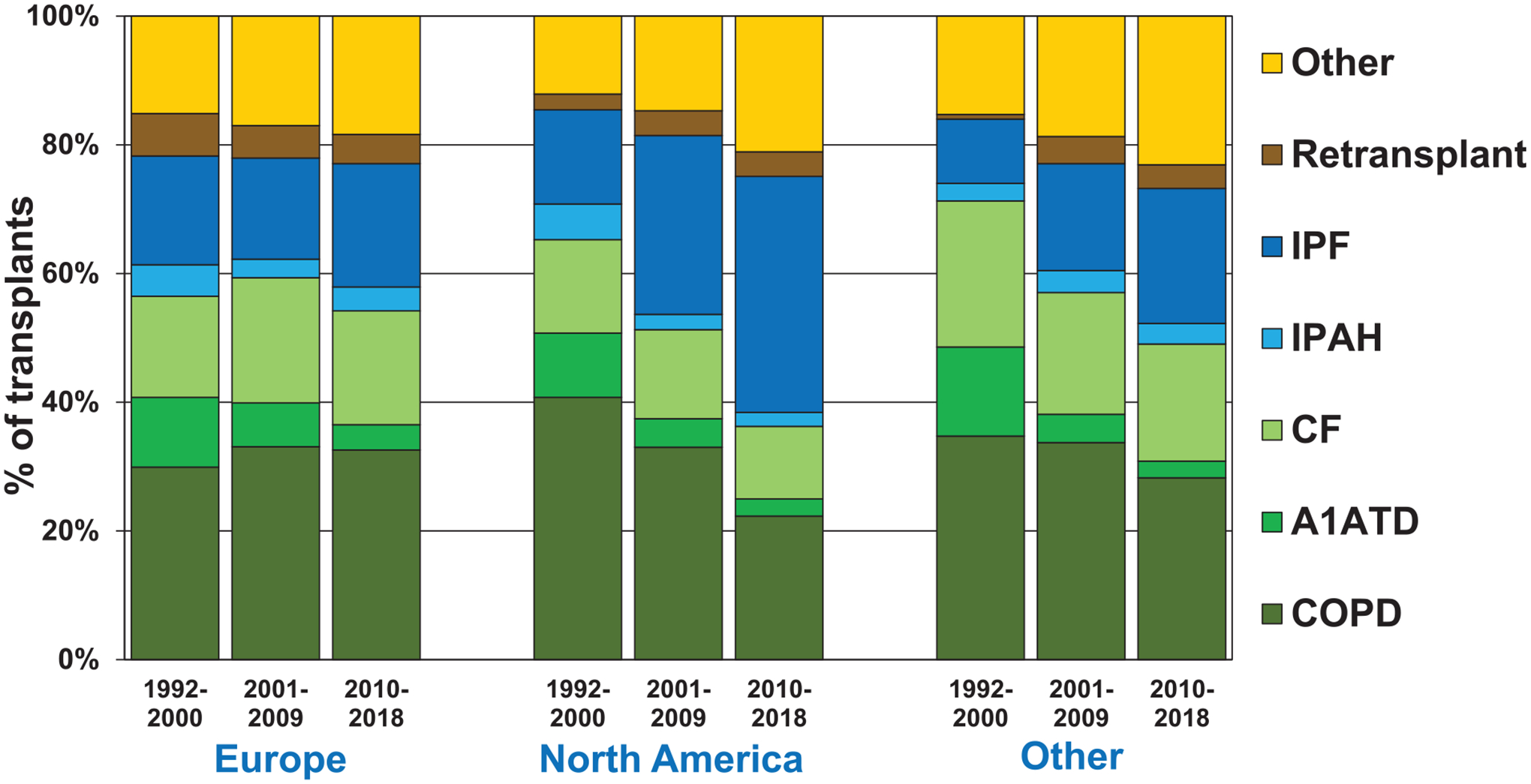
Diagnosis distribution by location and era (transplants: January 1992-June 2018).
Median recipient age has steadily increased from 55 to 60 years (p < 0.0001), reflected by declines in recipients of all age groups below 59 years and an increase in the percentage of 60 to 69-year-old recipients from 23.9% in the first era to 49.2% in the most recent era (Table 2, eSlide L(a) 5). The aging of the recipient population over time likely reflects an increased willingness to accept older candidates, along with the aging of the general population. The proportion of male transplant recipients has increased from 48% to 53% in the most recent era (p < 0.0001). Median weight, and body mass index (BMI) of patients transplanted for COPD have increased from 65 kg and 23 kg/m2 respectively in the 1990s to 68 kg and 24 kg/m2 in the most recent era (p < 0.0001), but are still below the average recipient weight of 80 kg and BMI 26.5 kg/m2 of all adult lung transplant recipients in the most recent era.12 The median recipient height has increased slightly from 168 cm to 169 cm (Table 2, eSlide L(a) 5–8). There have been no changes in the recipient blood type proportion over the 3 eras. Additionally, age and BMI increased in all regions over time (Figure 2, eSlide L(a) 10).
Table 2.
Recipient Characteristics by Era (COPD Transplants: January 1992-June 2018)
| Jan 1992-Dec 2000 (N = 4,162) | Jan 2001-Dec 2009 (N = 7,102) | Jan 2010-Jun 2018 (N = 8,917) | p-value | |
|---|---|---|---|---|
| Geographic Location | ||||
| - Europe | 23.0% | 35.0% | 44.5% | <0.0001 |
| - North America | 71.1% | 58.2% | 46.5% | |
| - Other | 5.8% | 6.8% | 9.0% | |
| Age (years) (continuous) | 55.0 (42.0–64.0) | 58.0 (45.0–66.0) | 60.0 (48.0–69.0) | <0.0001 |
| Age (years) (categorical) | ||||
| - 18–29 | 0.6% | 0.2% | 0.2% | <0.0001 |
| - 30–39 | 2.6% | 1.3% | 0.7% | |
| - 40–49 | 17.9% | 10.8% | 6.4% | |
| - 50–59 | 54.9% | 48.5% | 40.9% | |
| - 60–69 | 23.9% | 38.3% | 49.2% | |
| - ≥70 | 0.2% | 0.7% | 3.5% | |
| Female | 51.7% | 48.5% | 46.7% | <0.0001 |
| Weight (kg) | 64.6 (45.4–91.6) | 66.7 (47.0–93.4) | 68.0 (47.8–94.0) | <0.0001 |
| Height (cm) | 168.0 (153.0–185.0) | 167.9 (154.0–183.0) | 169.0 (154.9–184.0) | 0.0015 |
| BMI (kg/m2) | 22.9 (17.0–30.9) | 23.6 (17.7–30.5) | 23.8 (17.9–30.6) | <0.0001 |
| ABO blood type | ||||
| - A | 42.1% | 43.5% | 43.9% | 0.2239 |
| - AB | 4.9% | 5.0% | 4.6% | |
| - B | 11.0% | 10.8% | 11.5% | |
| - O | 42.0% | 40.6% | 40.0% | |
| PRA ≥ 20% | 3.1% | 5.9% | 13.1% | <0.0001 |
| PRA ≥ 80% | 0.3% | 0.7% | 2.1% | <0.0001 |
| Diabetes | 2.5% | 5.7% | 8.4% | <0.0001 |
| History of malignancy | 3.1% | 5.5% | 8.1% | <0.0001 |
| History of smoking | - | 94.9% | 95.7% | 0.1602 |
| Pre-transplant dialysis | 0.2% | 0.4% | 0.4% | 0.1971 |
| Previous lung surgery | 18.4%a | 12.6% | 7.4% | <0.0001 |
| Hospitalized | 4.9% | 5.6% | 6.6% | 0.0096 |
| Ventilator use | 1.2% | 1.9% | 2.3% | 0.0034 |
| ECMO use | 0.0% | 0.3% | 0.5% | 0.0021 |
| CMV antibody positive | 70.2% | 65.3% | 59.7% | <0.0001 |
| EBV antibody positive | 77.6%b | 86.6% | 93.1% | <0.0001 |
| Hepatitis B antibody positive | 2.2%a | 4.2% | 5.9% | <0.0001 |
| Hepatitis C antibody positive | 1.6%a | 2.1% | 2.6% | 0.022 |
| Bilirubin (mg/dl) | 0.5 (0.2–1.1)a | 0.5 (0.2–1.1) | 0.4 (0.2–1.1) | <0.0001 |
| Creatinine (mg/dl) | 0.8 (0.5–1.3)a | 0.8 (0.5–1.3) | 0.8 (0.5–1.2) | <0.0001 |
| GFR (ml/min/1.73m2)c | 86.7 (51.3–147.0)a | 87.3 (50.5–142.1) | 91.0 (55.0–147.1) | <0.0001 |
| PCW mean (mm Hg) | 11.0 (4.0–21.0)a | 12.0 (5.0–22.0) | 12.0 (5.0–23.0) | <0.0001 |
| PA mean (mm Hg) | 24.0 (15.0–38.0)a | 25.0 (15.0–38.0) | 25.0 (16.0–41.0) | <0.0001 |
| PVR (woods unit) | 2.6 (1.0–5.3)a | 2.5 (1.0–5.5) | 2.6 (1.0–6.1) | 0.0251 |
| FEV1% predicted | 19.0 (11.0–50.0) | 20.0 (12.0–61.0) | 20.0 (12.0–60.0) | <0.0001 |
| FVC% predicted | 50.0 (26.0–79.0) | 51.0 (28.0–83.0) | 53.0 (30.0–87.0) | <0.0001 |
| Donor-recipient sex match | ||||
| - Male-male | 40.1% | 44.0% | 46.5% | <0.0001 |
| - Male-female | 24.5% | 18.6% | 18.9% | |
| - Female-male | 8.1% | 7.5% | 6.9% | |
| - Female-female | 27.4% | 29.9% | 27.8% | |
| Transplant procedure | ||||
| - Single | 74.8% | 47.6% | 23.5% | <0.0001 |
| - Double/bilateral | 25.2% | 52.4% | 76.5% |
Continuous factors are expressed as median (fifth – 95th percentiles).
Summary statistics included transplants with known/non-missing data.
Based on Apr 1994 – Dec 2000 transplants.
Based on Oct 1999– Dec 2000 transplants.
GFR was estimated using the Cockcroft-Gault formula.
BMI, body mass index; CMV, cytomegalovirus; EBV, Epstein-Barr virus; ECMO, extracorporeal membrane oxygenation; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GFR, glomerular filtration rate; PA, pulmonary artery pressure; PCW, pulmonary capillary wedge; PRA, panel reactive antibody; PVR, pulmonary vascular resistance.
Figure 2.
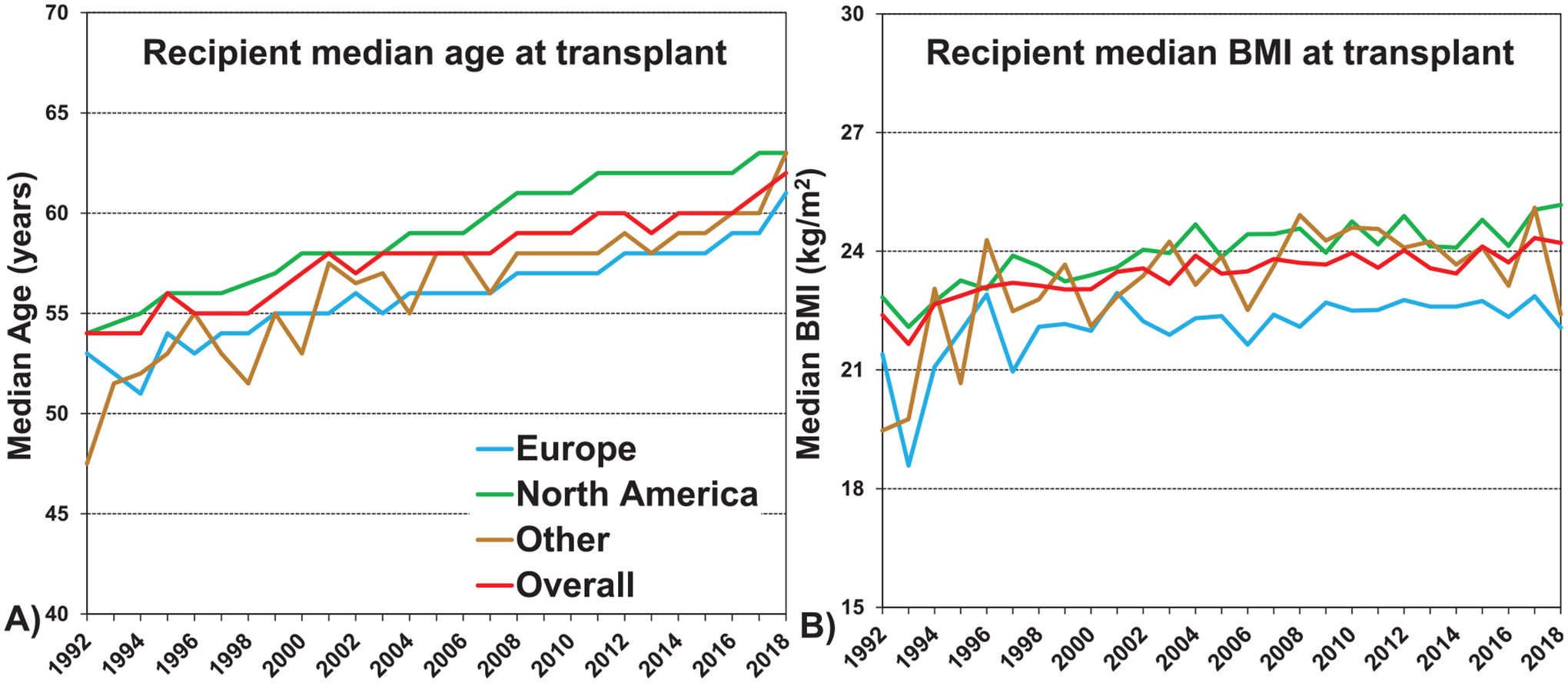
Median recipient A) age and B) body mass index (BMI) by location (COPD transplants: January 1992-June 2018).
The proportion of recipients transplanted for COPD who are allosensitized has steadily increased over time. For instance, candidates with a panel reactive antibody (PRA) ≥20% increased from 3.1% during 1992 to 2000, to 5.9% during 2001 to 2009, and to 13.1% in the most recent era (p < 0.0001). This may reflect a general acceptance for increased immunological risk amongst transplant centers. The same seems to be the case for recipients with diabetes, and a history of previous malignancy, which have increased significantly over time from 2.5% to 8.4%, and 3.1% to 8.1%, respectively. Furthermore, high-risk transplants such as those in hospitalized recipients, recipients on ventilatory support, or ECMO have increased over time, suggesting increasing use of these procedures or an increased candidate urgency (Table 2, eSlide L(a) 5–8), although numbers are still small.
Interestingly, the proportion of recipients transplanted for COPD who had lung surgery before transplantation has declined from 18.4% from 1990 to 2000, to 12.6% from 2001 to 2009, to a low of 7.4% between 2010 and 2018; this is likely due to the changing practice in consideration of lung volume reduction surgery for the treatment of emphysema. Lung volume reduction surgery gained popularity in the 1990s, but after the National Emphysema Treatment Trial in 200313 showed no survival benefit, apart from a specific subgroup, the procedure has mainly been used in highly selected individuals at specialized centers, and the overall number of procedures has decreased.
The proportion of COPD recipients who are cytomegalovirus (CMV) antibody positive has decreased over time from 70.2% to 59.7% in the most recent era (p < 0.0001). This change has occurred despite a steadily aging recipient population, and thus may reflect changes in CMV seroprevalence over time reported in some countries as described in previous reports.12 In contrast, the proportion of Epstein-Barr virus antibody positive recipients has steadily increased from 77.6% to 93.1%. A small but increasing number of recipients are hepatitis B antibody positive (5.9% in the most recent era). The same is the case for the number of seropositive hepatitis C recipients, which has increased from 1.6% to 2.6% (p = 0.022) (Table 2, eSlide L(a) 5–8).
Post-transplant causes of death
Examining the causes of death between 2010 and 2018 in recipients transplanted for COPD by time after transplantation showed infection and graft failure as the most common within the first year after transplantation, chronic lung allograft dysfunction (CLAD) or BOS, and infection as the most common from 1 to 5 years after transplantation, and CLAD/BOS as the most common in the late period after transplantation (Figure 3, eSlide L(a) 17). The cumulative incidence of recipient cause of death is shown in Figure 4, (eSlide L(a) 18).
Figure 3.
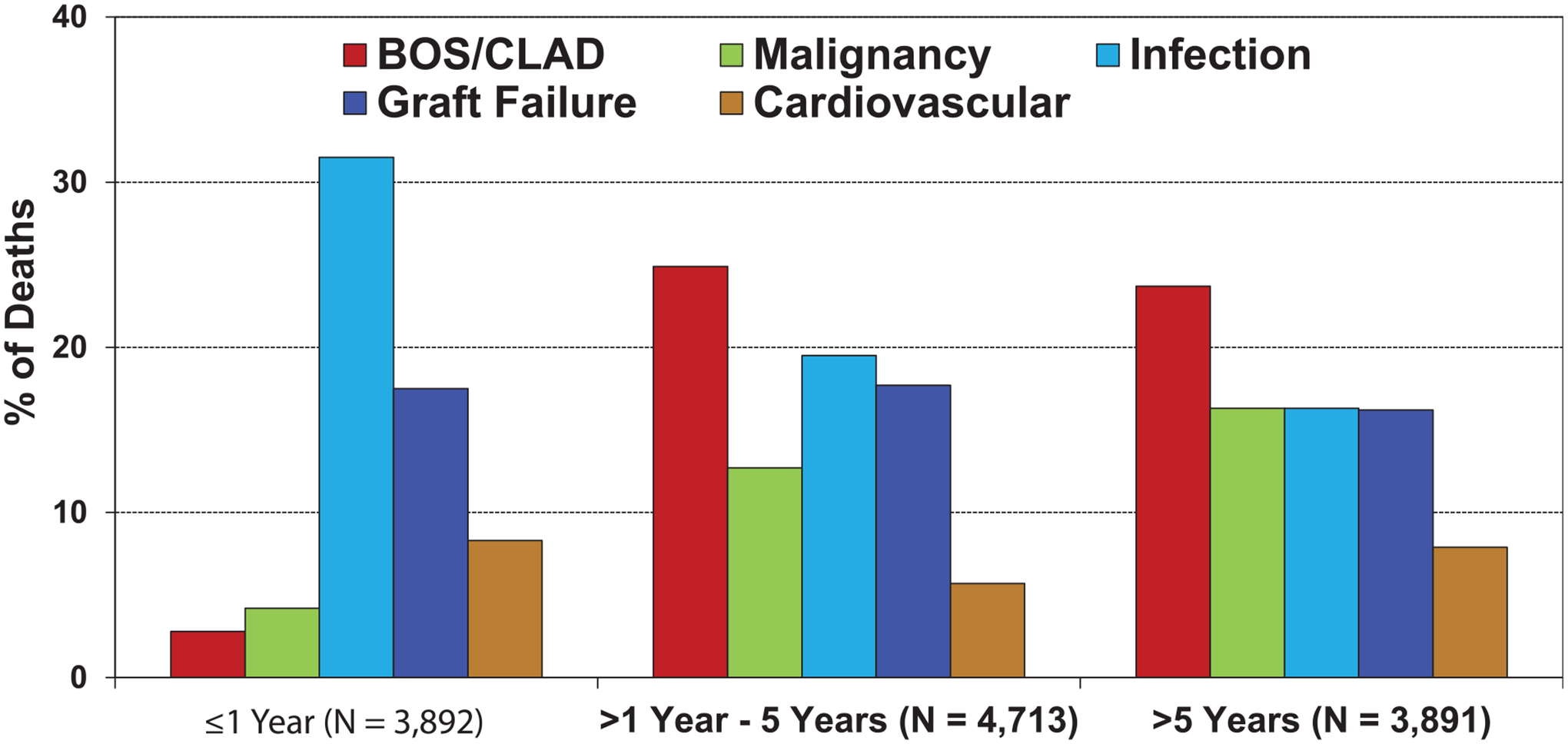
Relative incidence of leading causes of death (COPD transplant deaths: January 2010-June 2018).
Figure 4.
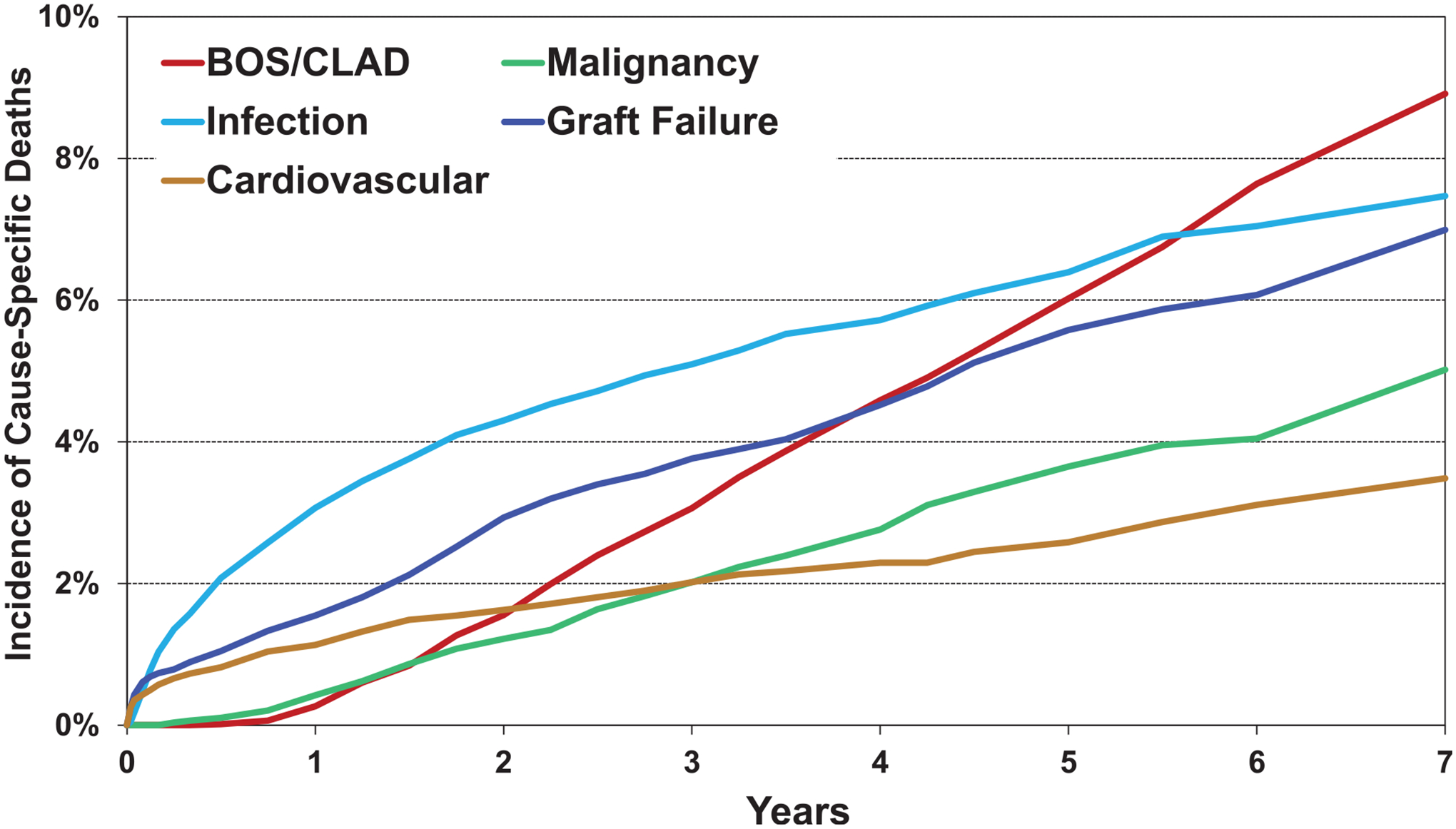
Cumulative incidence of leading causes of death (COPD transplants: January 2010-June 2017).
Survival analyses
We examined associations between COPD recipient risk factors and post-transplant survival. We present several stratified unadjusted analyses, and it should be kept in mind that these analyses have not been adjusted for potential confounders that may account for some of the reported differences. It is also important to remember that when performed, multivariable analyses only contain variables that are reported to the Registry, so that some potential explanatory variables will not appear in the multivariable models. All 5-year survival analyses are conditional on survival to 1 year.
One-year survival
Survival in adults who underwent lung transplantation for COPD from 2000 to 2017 improved over time (Figure 5, eSlide L(a) 20), confirming a previously noted trend towards improved survival in recent eras in the overall cohort of lung transplant recipients. We examined associations between recipient risk factors and post-transplant survival among patients transplanted for COPD. 12-month survival in patients transplanted for COPD was slightly higher compared to survival of recipients transplanted for other diagnoses (Figure 6 (eSlide L(a) 24). There was a significant improvement in survival in the most recent era in North America and Europe, but not in the Other regions (eSlide L(a) 21). This may reflect the commencement of transplantation as a low-volume procedure at centers reporting from outside North America and Europe or could be due to low statistical power.
Figure 5.
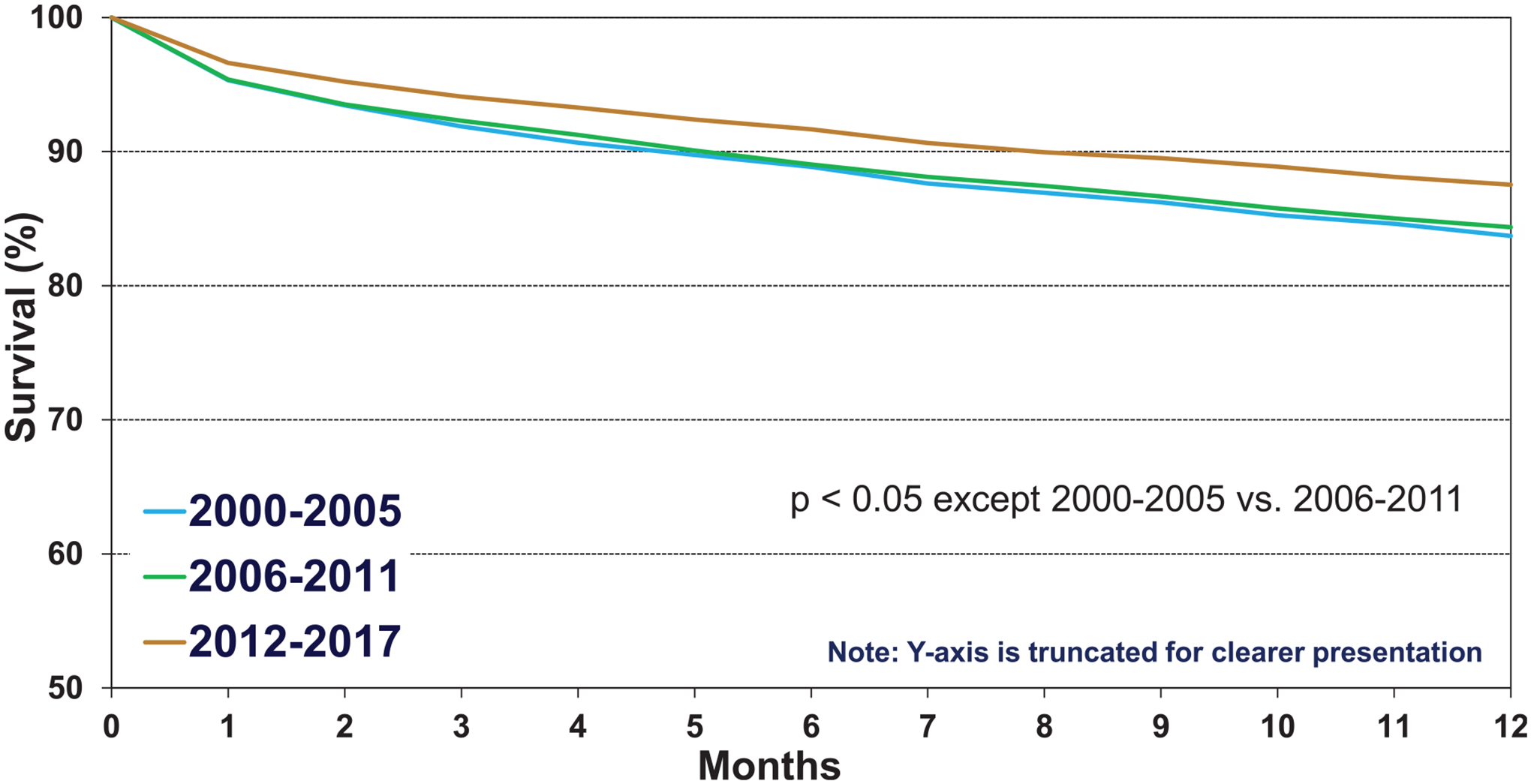
Kaplan-Meier survival within 12 months by era (COPD transplants: January 2000-June 2017).
Figure 6.
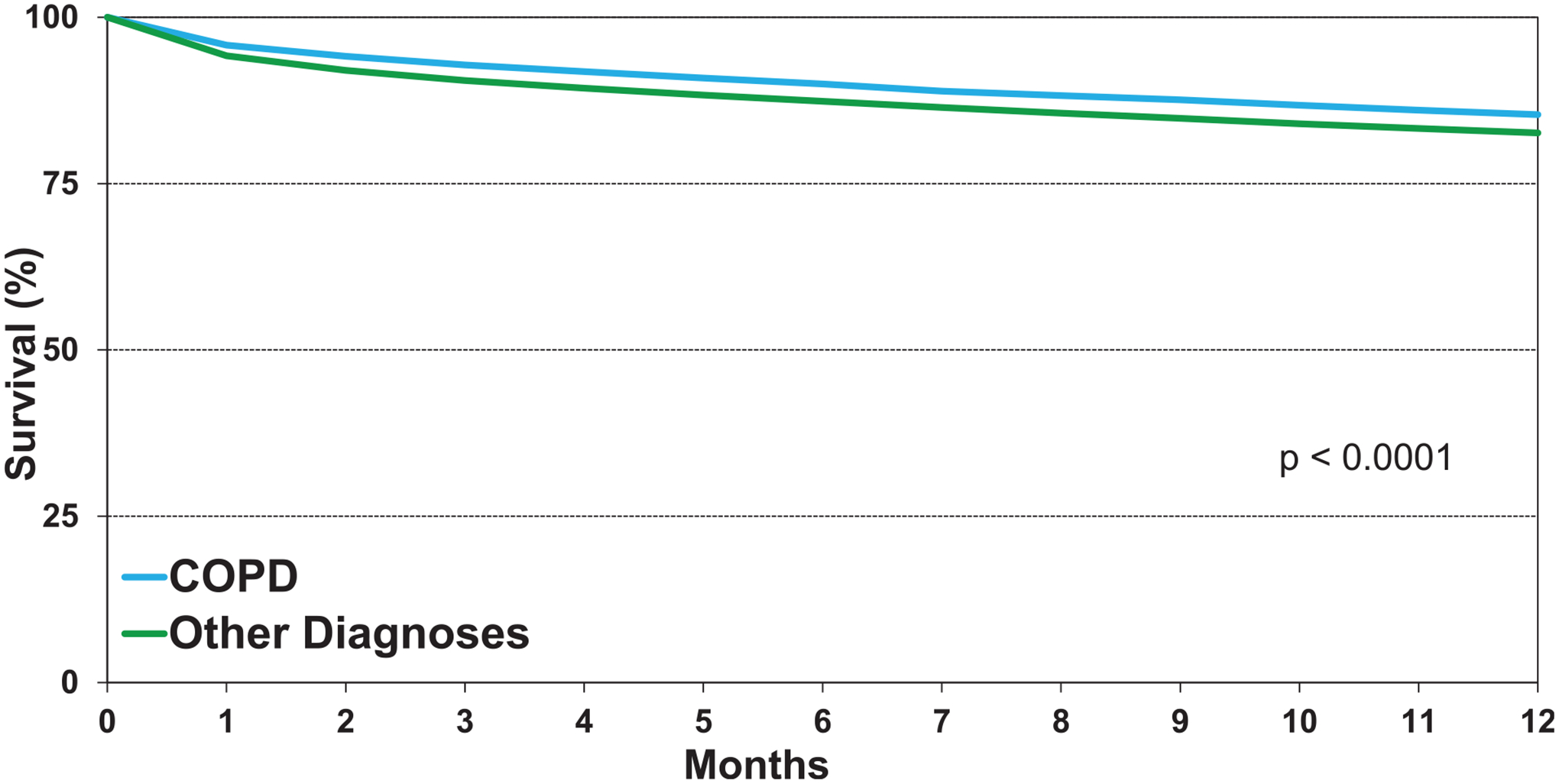
Kaplan-Meier survival within 12 months by diagnosis (transplants: January 2000-June 2017).
Twelve-month survival was significantly higher for recipients 40 to 59 years of age compared to those above 60 years (eSlide L(a) 22). Previous reports have observed that dialysis-dependent renal failure significantly impacts 1- and 5-year survival after lung transplantation. Using the Cockroft-Gault equation to estimate glomerular filtration rate in adult recipients transplanted for COPD, we found significant differences in 1-year survival between the 2 extremes of estimate glomerular filtration rate below 60 and above 90 ml/min/1.73 m2 (eSlide L(a) 27).
Five-year survival conditional on surviving to 1 year
We next examined 5-year survival conditional on surviving to 1 year in adults transplanted for COPD from January 1996 to June 2013. Survival improved significantly from the early era of 1996 to 2001 but was unchanged for the latter 2 eras (eSlide L(a) 29). Stratified by age, 5-year conditional survival for patients transplanted for COPD was 62% in recipients >60 years of age, compared to 70% in recipients 40 to 59 years of age (Figure 7, eSlide L(a) 31). Comparing recipients transplanted for COPD to recipients transplanted for other diagnoses showed a slight but significantly lower 5-year survival, with differences beginning around 2 years and increasing over time (Figure 8, eSlide L(a) 33).
Figure 7.
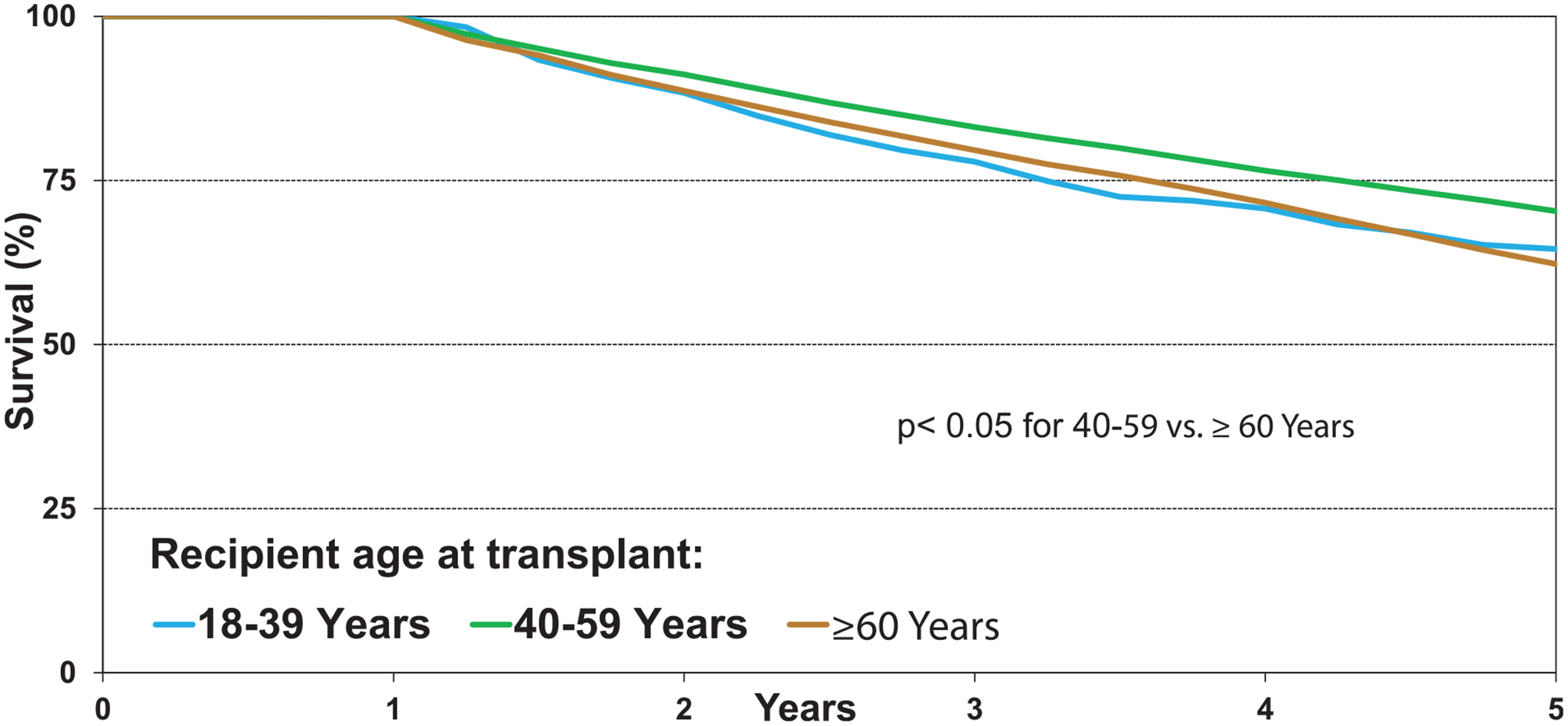
Kaplan-Meier survival within 12 months by age and procedure type (COPD transplants: January 2000-June 2017).
Figure 8.
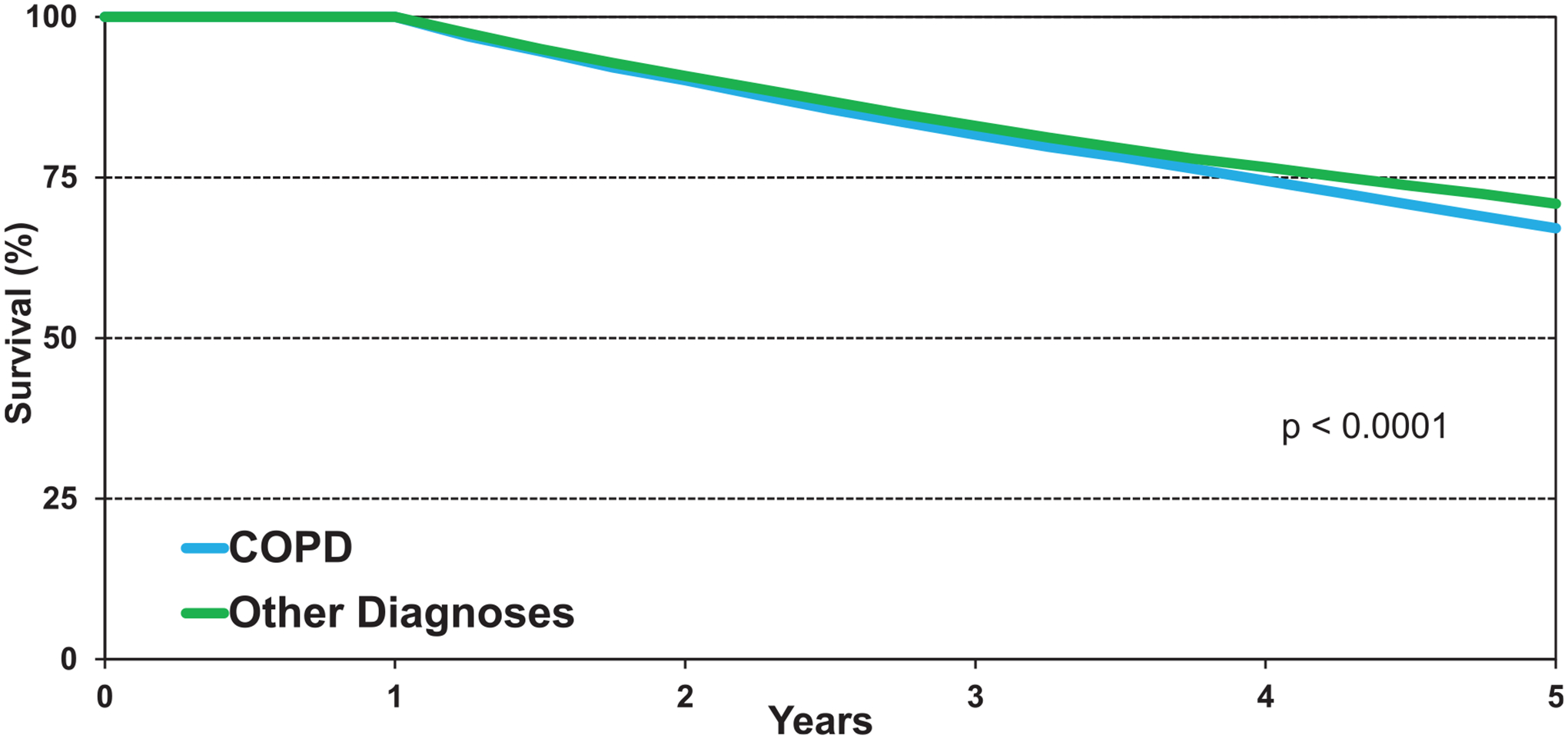
Kaplan-Meier survival within 5 years conditional on survival to 1 year by recipient age (COPD transplants: January 1996-June 2013).
Freedom from bronchiolitis obliterans syndrome (BOS) conditional on survival to discharge
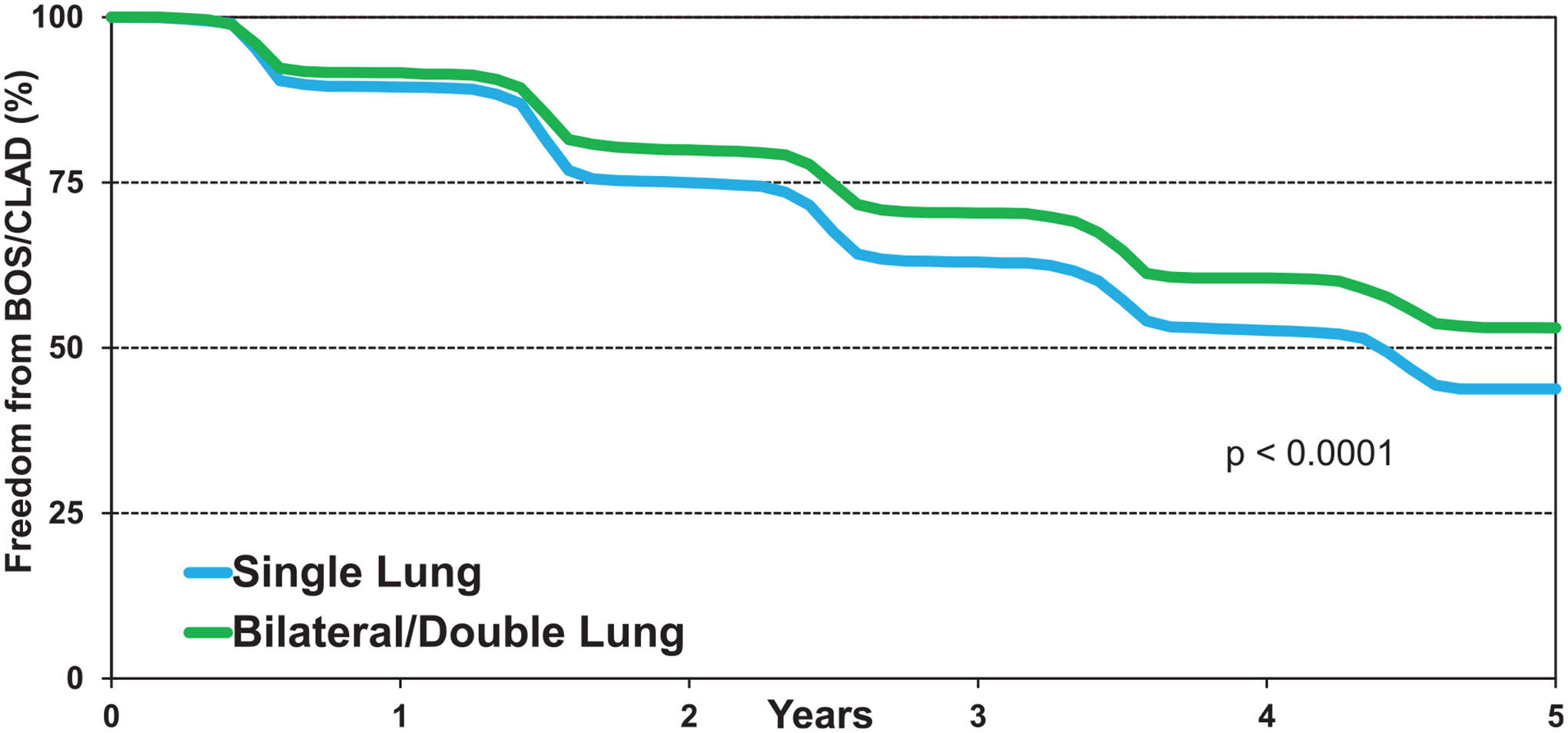
CLAD/BOS remains the major cause of morbidity and mortality after lung transplantation.14 We examined freedom from CLAD/BOS in adult recipients transplanted for COPD who survived the transplant hospitalization. Overall, there was higher freedom from CLAD/BOS at 5 years (56%) in the early 1996 to 2001 period compared with the 2 later time periods, 2002 to 2007 (44%) and 2008 to 2013 (45%) (eSlide L(a) 38). Examining recipient age groups and freedom from CLAD/BOS did not reveal any significant differences (eSlide L(a) 39). We did find a significant difference between freedom from CLAD/BOS in recipients transplanted for COPD with double lungs (53%) vs a single lung (44%) at 5 years (Figure 9, eSlide L(a) 40), and this difference occurred early after transplantation and increased over time. The most likely explanation for this observed difference is the amount of tissue transplanted, making the single lung recipient more vulnerable to tissue damage and development of CLAD, but other reasons might also be possible.
Figure 9.
Kaplan-Meier survival within 5 years conditional on survival to 1 year by diagnosis (transplants: January 1996-June 2013).
It should be kept in mind that these univariate analyses have not been adjusted for other potential confounders that may account for some of the reported differences.
Freedom from malignancy conditional on survival to 1 year
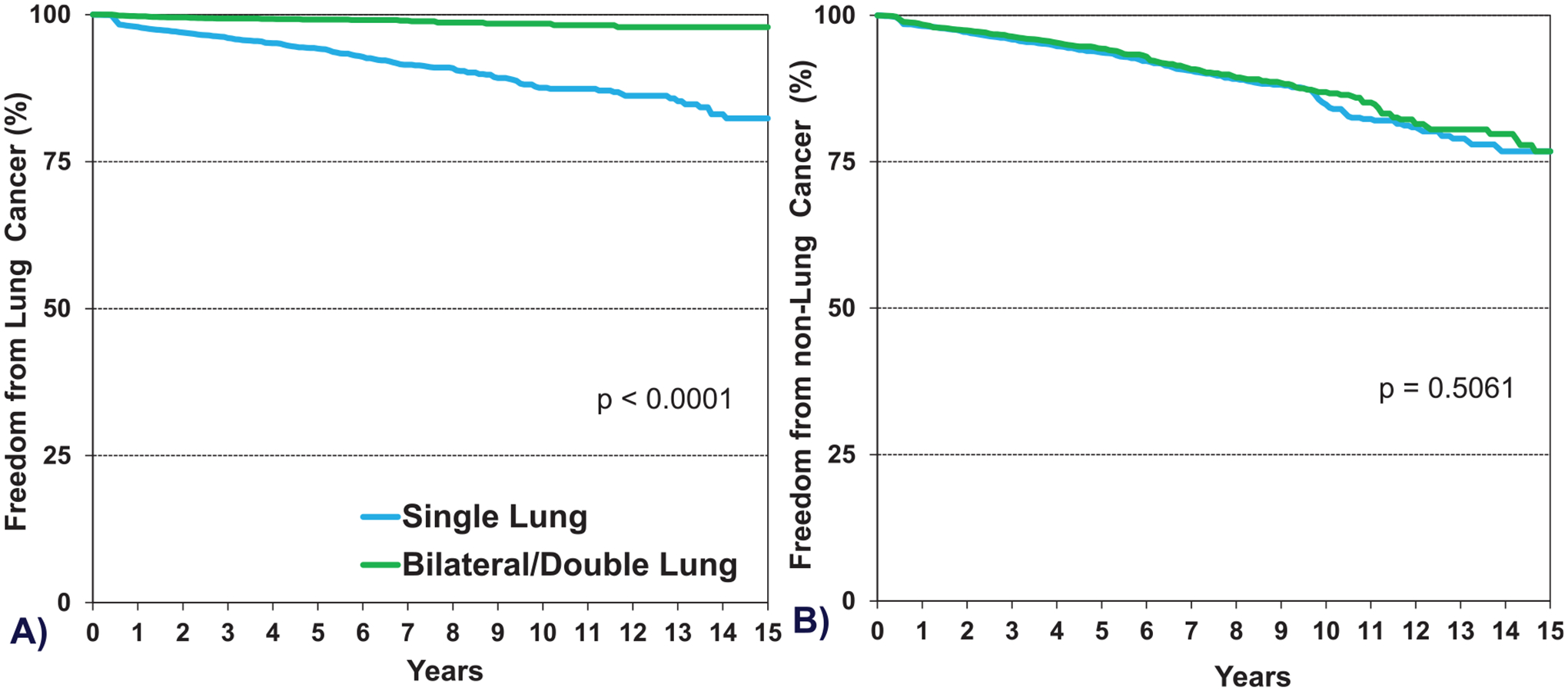
Malignancies are common after lung transplantation and continue to be a frequent cause of death. We examined the freedom from non-skin malignancy conditional to survival to 1 year in recipients transplanted for COPD stratified by a single vs double lung transplant procedure. The freedom from new onset non-skin malignancy in double vs single lung transplants was 94% vs 88%, at 5 years, 86% vs 75%, at 10-years and 76% vs 66%, at 15 years, respectively (eSlide L(a) 42). This difference between double vs single lung transplantation is driven by new onset lung cancer, which is extremely rare in double lung transplants but more frequent in single lung transplant recipients, primarily located in the native COPD lung (Figure 10A, eSlide L(a) 43). All other non-skin malignancies occur with similar frequency in double vs single lung transplantation (Figure 10B, eSlide L(a) 43).
Figure 10.
Kaplan-Meier survival within 5 years conditional on survival to 1 year by recipient age and procedure type (COPD transplants: January 2000-June 2017).
Single vs double lung transplantation
Since the early days of lung transplantation, end-stage COPD has been a common indication for either a double lung or a single lung transplant. There has been a significant change over time in favor of performing double lung transplants, with single lung transplants declining from approximately 75% in the early era to 24% in the most recent era (Table 2, eSlide L(a) 8). Through this period, there have been controversies about the benefit or risk of either as a treatment for the COPD population. Here we review the existing Registry data as they relate to these surgical approaches in the treatment of end-stage COPD.
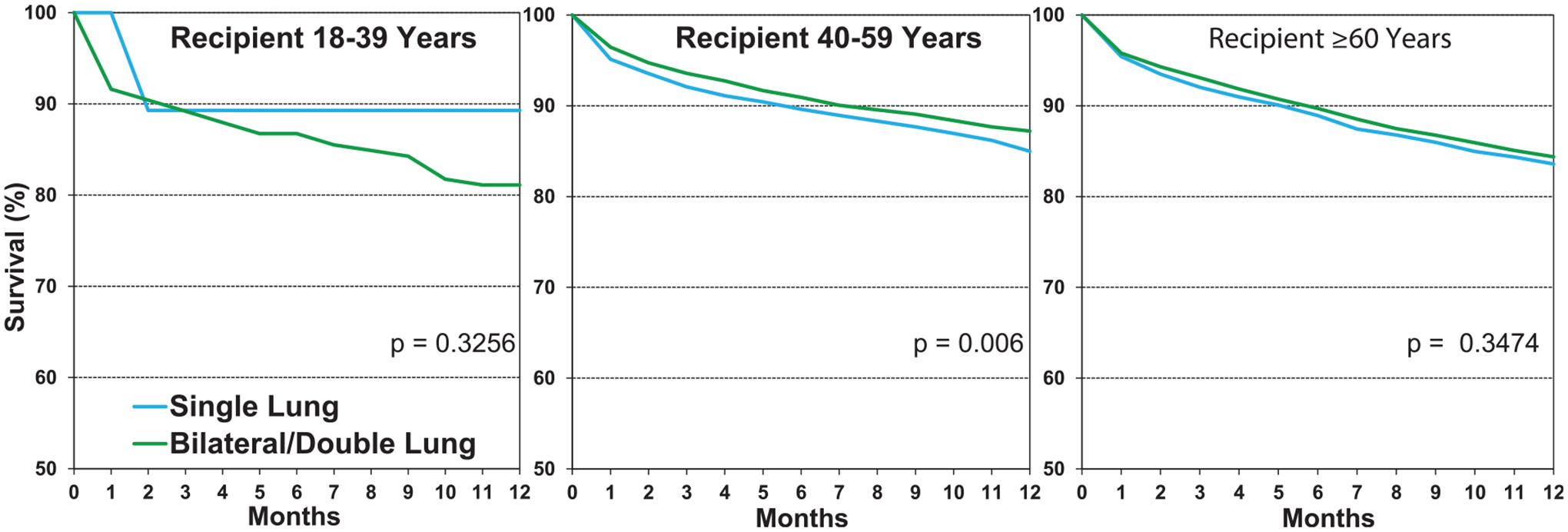
Among patients with COPD transplanted from January 2000 to June 2017 stratified by age group, 1-year survival was higher for double lung transplant among those 40 to 59 years of age, while no difference was seen in the 18 to 39 and ≥60 years age groups (Figure 11, eSlide L(a) 26). Five-year survival conditional on survival to 1 year showed lower survival in single compared to double lung transplant recipients in age groups 40 to 59 years and ≥60 years (Figure 12, eSlide L(a) 35). As noted above, we found a significant difference between freedom from CLAD/BOS in recipients transplanted with a single lung vs double lung at 5 years (Figure 9, eSlide L(a) 40).
Figure 11.
Freedom from BOS/CLAD conditional on survival to discharge by procedure type (COPD transplants: January 1996-June 2013).
Figure 12.
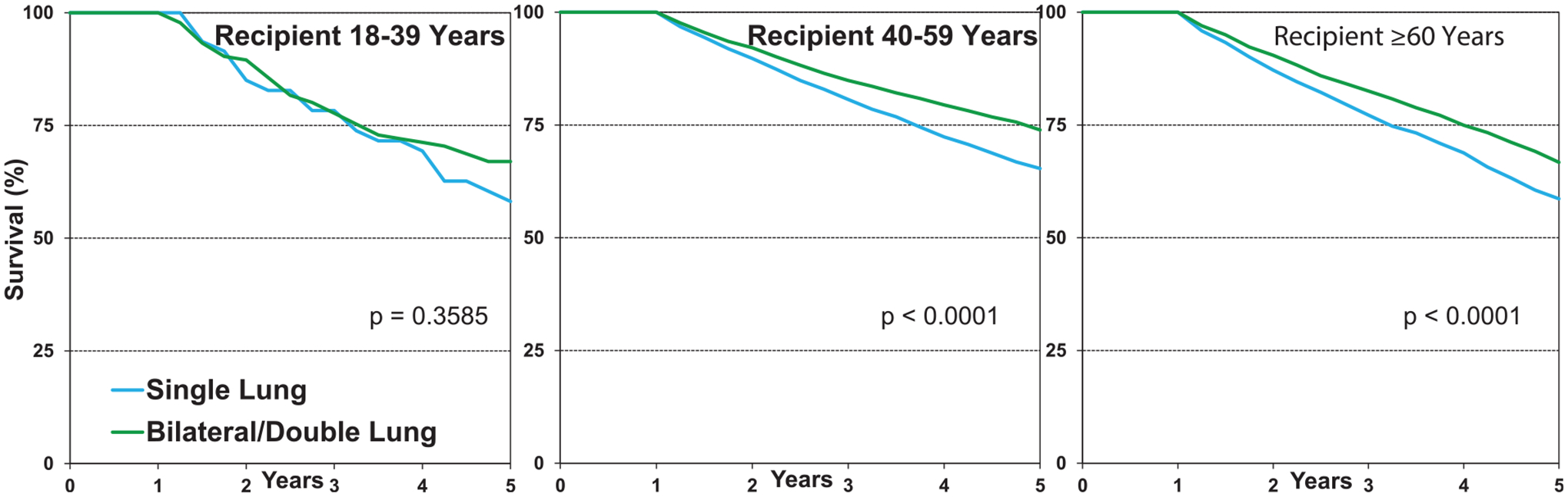
Freedom from new onset of A) lung cancer and B) non-lung cancer, conditional on survival to 1 year by procedure type (COPD transplants: January 1996-June 2017).
Multivariable analyses
We next performed multivariable Cox regression analyses to evaluate risk factors for 1-year mortality, 5-year mortality conditional upon surviving the first post-transplant year, and risk of developing CLAD/BOS conditional upon surviving to hospital discharge. Covariates included in the multivariable models are listed in Supplemental Table 1.
One-year mortality
Statistically significant categorical variables independently associated with the risk of higher 1-year mortality in adult lung transplant recipients transplanted for COPD between January 2000 and June 2017 include being hospitalized and being on mechanical ventilation at the time of transplant. A lower risk of mortality was seen with transplants in the most recent transplant era, and in donor/recipient female to female and male to female sex match as compared to male to male sex match (eSlide L(a) 45).
Continuous variables associated with increased risk of 1-year mortality included older age of recipients (Figure 13, eSlide L(a) 47) and older age of donors (eSlide L(a) 49). Higher pulmonary vascular resistance (PVR) was associated with significantly increased risk of mortality (eSlide L(a) 48), as was lower center volume over the last 3 years (eSlide L(a) 50).
Figure 13.
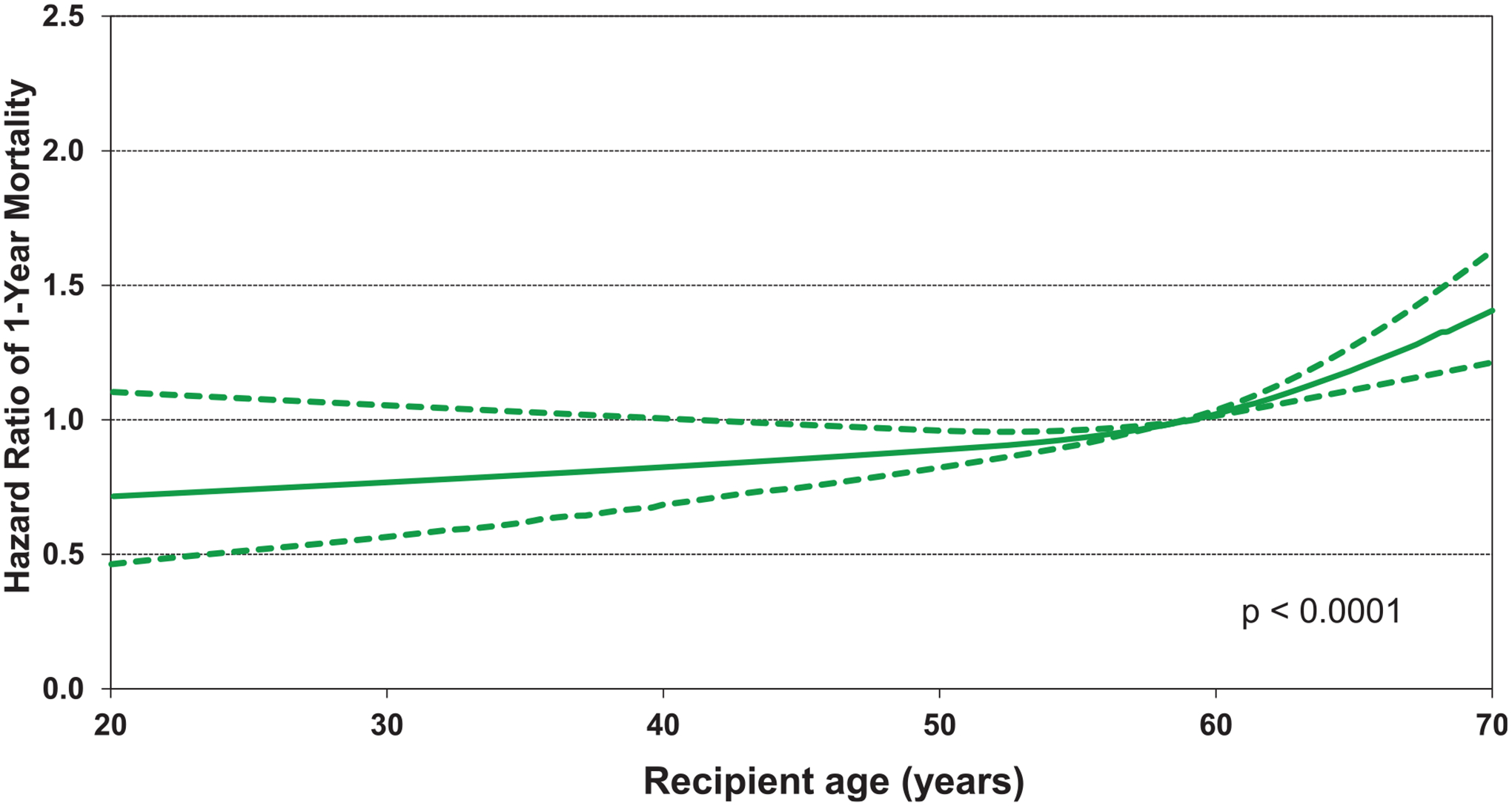
Multivariable hazard ratio plot for 1-year mortality with 95% confidence limits, by recipient age (COPD transplants: January 2000-June 2017; N = 15,643).
Five-year mortality conditional upon surviving the first post-transplant year
Transplantation for COPD in the 2002 to 2007 and 2008 to 2013 eras was independently associated with a lower risk of 5-year mortality than transplantation between 1996 and 2001. In contrast, the following variables were independently associated with a higher risk of 5-year mortality: single lung transplantation, hospitalization at the time of transplant, and recipient diabetes (eSlide L(a) 52).
With respect to continuous variables leading to increased risk for 5-year mortality conditional on 1-year survival, recipient age and BMI were independently associated with mortality, with the hazard ratio increased for older recipients and increasing BMI. We did not see an increased risk with low BMI in this population transplanted for COPD, although the confidence intervals were quite wide for the low BMI range (Figure 14, (eSlide L(a) 54, 55). Similar to 1-year mortality, low center volume increased the risk of 5-year mortality (eSlide L(a) 56). Furthermore, and perhaps counterintuitively, shorter ischemic time increased the risk of 5-year mortality (eSlide L(a) 57), as previously described.
Figure 14.
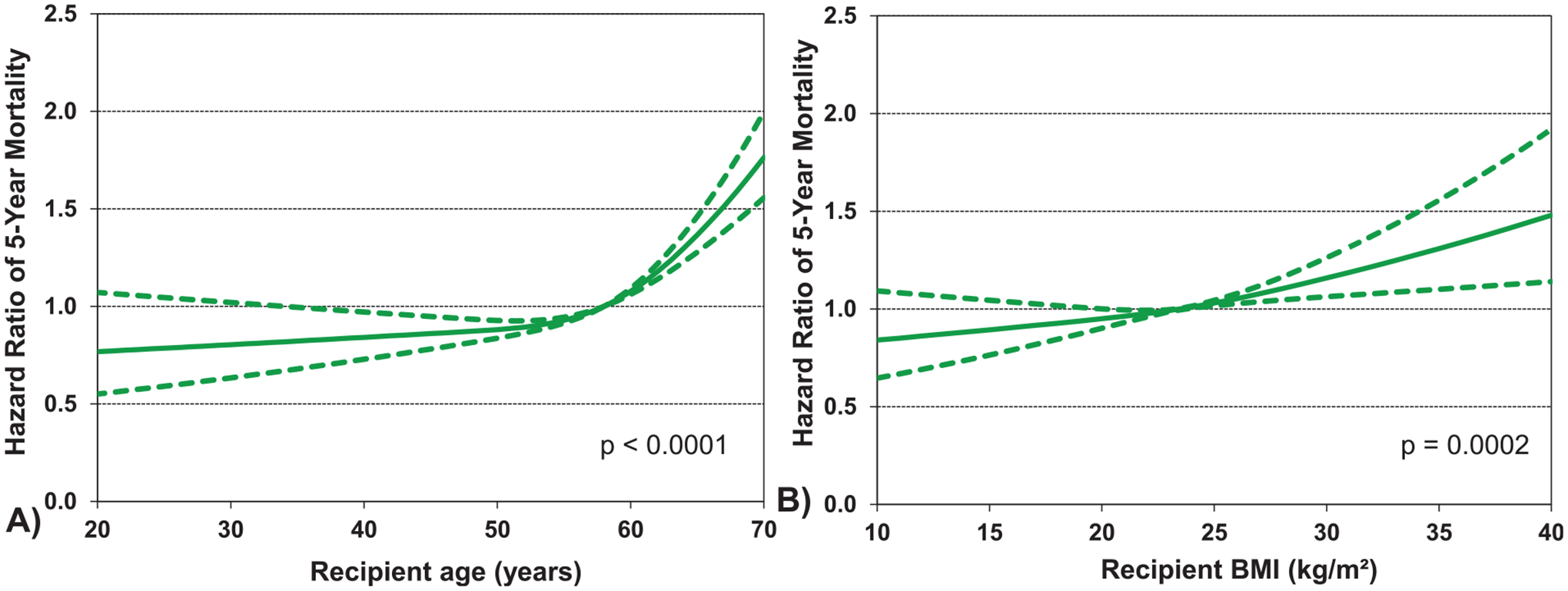
Multivariable hazard ratio plot for 5-year mortality conditional on survival to 1 year with 95% confidence limits, by A) recipient age and B) recipient body mass index (BMI) (COPD transplants: January 1996-June 2013; N = 11,683).
Freedom from bronchiolitis obliterans syndrome (BOS) conditional on survival to discharge
We next investigated categorical risk factors significantly associated with developing CLAD/BOS during the first 5 years after transplant in recipients transplanted for COPD who survived to hospital discharge from January 1996 to June 2013. Again, a single lung transplant procedure and being on a ventilator at the time of transplant were independently associated with an increased risk of developing CLAD/BOS. In contrast to both 1- and 5-year survival where transplants in recent era had improved survival, transplantation in a recent era was associated with higher risk of developing CLAD/BOS (Figure 15, eSlide L(a) 59), possibly due to increasing age and morbidity of recipients transplanted for COPD over time as shown in earlier sections of this report.
Figure 15.
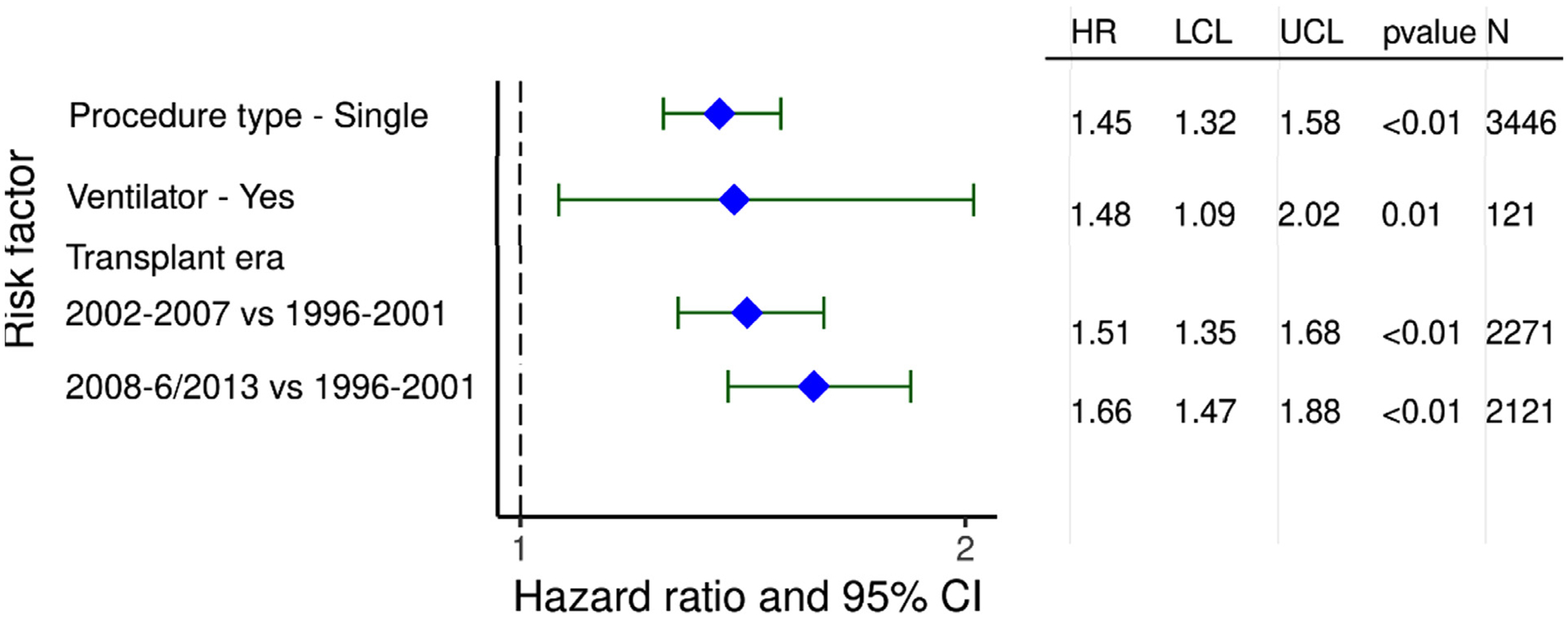
Statistically significant risk factors for BOS/CLAD within 5 years conditional on survival to discharge with 95% confidence limits (COPD transplants: January 1996-June 2013; N = 6,181).
Similar to 5-year mortality, higher BMI, and similar to 1-year mortality, increasing donor age, were also associated with an increased risk of developing CLAD/BOS (eSlides L(a) 61, 65). The association between center volume and risk of CLAD/BOS was complex, suggesting a lower risk of CLAD/BOS in both low and high-volume centers, as was also seen and discussed in last year’s report.12 (eSlide L(a) 66). Lower PVR was associated with lower risk of CLAD/BOS (eSlide L(a) 63). Additional risk factors at the time of transplant significantly associated with developing CLAD/BOS were higher PRA (Figure 16, eSlide L(a) 64) and higher pulmonary capillary wedge pressure (eSlide L(a) 62).
Figure 16.
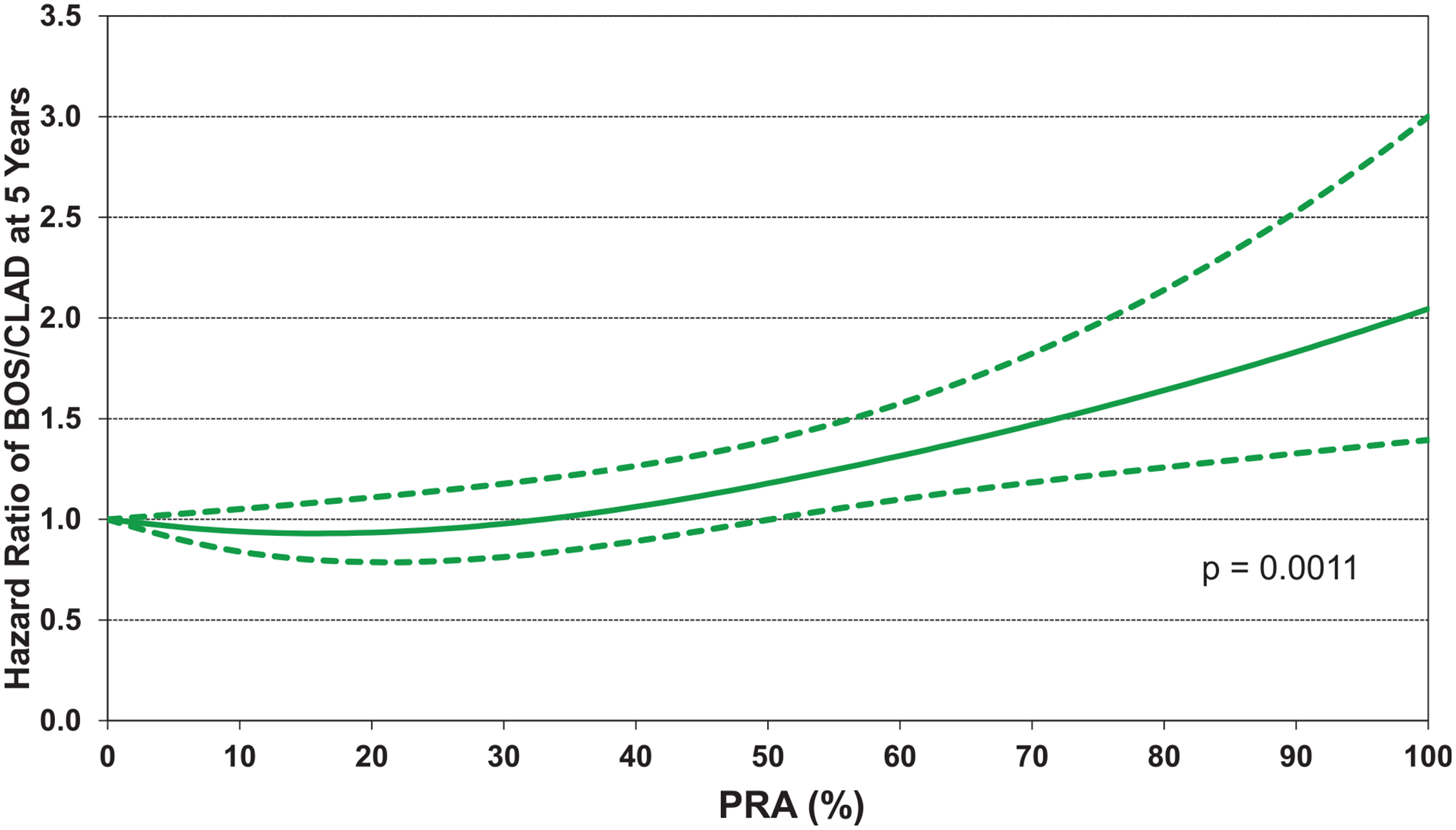
Statistically significant risk factors for BOS/CLAD within 5 years conditional on survival to discharge with 95% confidence limits, by PRA (COPD transplants: January 1996-June 2013; N = 6,181).
Conclusions
The 2022 ISHLT Adult Lung Transplant Report focuses on recipients who received lung transplant for COPD to determine trends and outcomes over time. We observed many important changes over the years, reflecting evolution of clinical practice and changes in demographics of recipients transplanted for COPD. Lung transplant recipients transplanted for COPD are older and more likely to be hospitalized or on mechanical ventilation at the time of transplant. Furthermore, they are more likely to have comorbidities, including diabetes mellitus, allosensitization, and a history of malignancy, while being less likely to be CMV seropositive and less likely to have had previous lung surgery than patients transplanted for other types of lung disease. This year’s Registry report presented specific outcomes comparing single vs double lung transplants in recipients transplanted for COPD and the impact of select recipient factors on post-transplant outcomes. Additionally, we identified transplant era, donor age, PRA, BMI, and lung transplant center volume as factors associated with CLAD/BOS. This report provides a perspective on the practice of adult lung transplantation in patients transplanted for COPD, highlights important trends, and elucidates controversies that have arisen as we aim to improve transplant outcomes in this patient population in the years to come.
Supplementary Material
Disclosure statement
Michael Perch received an institutional research grant from Roche, Ambu, Zambon, consultant fees from Takeda, Zambon, PulmonX, Mallinkrodt, GSK, Novartis, Boeringer-Ingelheim. Michael Harhay received consulting fees from Trinity life sciences. Luciano Potena received consulting fees from Biotest, Novartis, Takeda, Sandoz and CareDx. Andreas Zuckermann served on the speakers’ bureau of Paragonix, Mallinckrodt, Medtronik and Franz Kohler Chemie, and received research grants from Biotest and Xvivo. Josef Stehlik received consulting fees for Medtronic, Natera, Sanofi-Aventis, Transmedics and research support from Natera; Wida S. Cherikh, Aparna Sadavarte, Kelsi Lindblad received funding from ISHLT; Don Hayes, Eileen Hsich and Tajinder P. Singh do not have any relevant financial disclosures.
The authors wish to thank Ms. Lyna Cherikh, United Network of Organ Sharing Research Intern, for her assistance with preparing the figures/table for the manuscript and reviewing the manuscript.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.healun.2022.08.007.
References
- 1.Stehlik J, Christie JD, Goldstein DR, et al. The evolution of the ISHLT transplant registry. Preparing for the future. J Heart Lung Transplant 2021;40:1670–81. [DOI] [PubMed] [Google Scholar]
- 2.Yusen RD, Christie JD, Edwards LB, et al. The Registry of the International Society for Heart and Lung Transplantation: Thirtieth Adult Lung and Heart-Lung Transplant Report–2013; focus theme: age. J Heart Lung Transplant 2013;32:965–78. [DOI] [PubMed] [Google Scholar]
- 3.Lund LH, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first official adult heart transplant report–2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:996–1008. [DOI] [PubMed] [Google Scholar]
- 4.Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-second Official Adult Lung and Heart-Lung Transplantation Report–2015; focus theme: early graft failure. J Heart Lung Transplant 2015;34:1264–77. [DOI] [PubMed] [Google Scholar]
- 5.Yusen RD, Edwards LB, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-third Adult Lung and Heart-Lung Transplant Report-2016; focus theme: primary diagnostic indications for transplant. J Heart Lung Transplant 2016;35:1170–84. [DOI] [PubMed] [Google Scholar]
- 6.Chambers DC, Yusen RD, Cherikh WS, et al. The registry of the International Society for Heart and Lung Transplantation: Thirty-fourth Adult Lung and Heart-Lung Transplantation Report-2017; focus theme: allograft ischemic time. J Heart Lung Transplant 2017;36:1047–59. [DOI] [PubMed] [Google Scholar]
- 7.Chambers DC, Cherikh WS, Goldfarb SB, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth adult lung and heart-lung transplant report-2018; focus theme: multiorgan transplantation. J Heart Lung Transplant 2018;37:1169–83. [DOI] [PubMed] [Google Scholar]
- 8.Chambers DC, Cherikh WS, Harhay MO, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth Adult Lung and Heart-Lung Transplantation Report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khush KK, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-sixth adult lung and heart-lung transplantation Report-2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1042–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossano JW, Singh TP, Cherikh WS, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric heart transplantation report –2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1028–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayes D, Cherikh WS, Chambers DC, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Twenty-second pediatric lung and heart-lung transplantation report—2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chambers DC, Perch M, Zuckermann A, et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-eighth adult lung transplantation report — 2021; focus on recipient characteristics. J Heart Lung Transplant 2021;40:1060–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fishman A, Martinez F, Piantadosi S, Wise R, Hopkins Uni-versity J, Ries A. A randomized trial comparing lung-volume-reduction surgery with medical therapy for severe Emphysema. The writing committee for the National Emphysema Treatment Trial. N Engl J Med 2003;348:2059–73. [DOI] [PubMed] [Google Scholar]
- 14.Kulkarni HS, Cherikh WS, Chambers DC, et al. Bronchiolitis obliterans syndrome-free survival after lung transplantation: an International Society for Heart and Lung Transplantation Thoracic Transplant Registry analysis. J Heart Lung Transplant 2019;38:5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


