Abstract
A 78-year-old Japanese woman with no history of rheumatic disease received 2 doses of the BNT162b2 COVID-19 mRNA vaccine. Two weeks later, she noticed bilateral swelling in the submandibular region. Blood tests showed hyper-immunoglobulin (Ig)G4emia, and 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) revealed the strong accumulation of FDG in the enlarged pancreas. She was diagnosed with IgG4-related disease (IgG4-RD) according to the American College of Rheumatology (ACR)/the European League Against Rheumatism (EULAR) classification criteria. Treatment was started with prednisolone at 30 mg/day, and the organ enlargement improved. We herein report a case of IgG4-RD that may have been associated with an mRNA vaccine.
Keywords: COVID-19, IgG4-related disease, mRNA vaccination
Introduction
The severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) pandemic that began in Wuhan, China, in December 2019 has caused a large number of deaths worldwide (1). In December 2020, the United States Food and Drug Administration (FDA) provided emergency approval for the use of the BNT162b2 (Pfizer, New York, USA; BioNTech SE, Mainz, Germany) and mRNA-1273 (Moderna, Cambridge, USA) novel mRNA vaccines against the COVID-19 pandemic. Vaccination started in Japan in February of the following year. These vaccines are now being actively administered worldwide and have shown high efficacy in reducing hospitalization, severe disease, and mortality rates from COVID-19 infection (2). However, reports of adverse reactions due to COVID-19 mRNA vaccination have also increased, and reports of the new onset and exacerbation of various autoimmune diseases are on the rise, in addition to anaphylaxis and injection reactions also seen with conventional recombinant protein vaccines (3).
Immunoglobulin (Ig)G4-related disease (IgG4-RD) is a systemic disease characterized by elevated serum levels of IgG4 and abundant infiltration of IgG4-positive cells into the involved organs (4). The pathogenesis of IgG4-RD is gradually being elucidated, but the origin remains unknown.
We herein report a case of new-onset IgG4-RD that developed after COVID-19 mRNA vaccination.
Case Report
A 78-year-old Japanese woman with no history of autoimmune disease or COVID-19 infection received her second dose of the BNT162b2 COVID-19 mRNA vaccine in February 2022. Two weeks later, she noticed bilateral swelling in the submandibular region. The swelling gradually increased, so she visited an otolaryngologist. Blood testing revealed elevated serum levels of IgG4, and she was referred to our hospital on suspicion of IgG4-RD.
On presentation to our hospital, her general condition was good, and vital signs were normal. A physical examination revealed painless masses bilaterally in the submandibular region (Fig. 1A) but no other abnormalities, including upper eyelid swelling, sicca symptom, or abdominal pain. Blood testing showed elevated levels of serum IgG (2,165 mg/dL; normal, 870-1,700 mg/dL), IgG4 (1,100 mg/dL; normal, 11-121 mg/dL), soluble interleukin-2 receptor (662.0 U/mL; normal, 157-474 U/mL), and C-reactive protein (0.49 mg/dL; normal, <0.14 mg/dL), but no other abnormal blood test data were noted, including autoantibodies and tumor markers (Table 1).
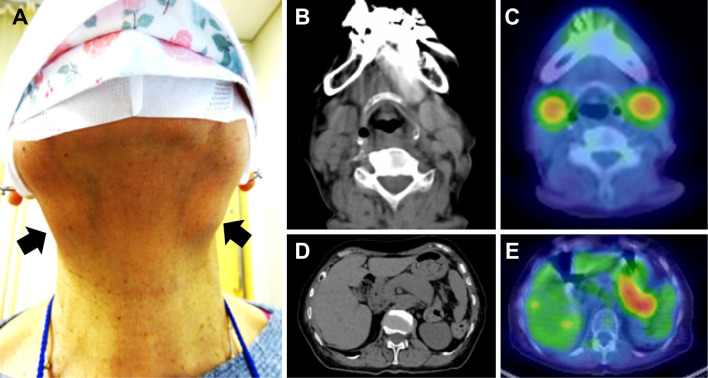
Figure 1.
Facial appearance and CT/PET-CT findings before treatment. A) Facial appearance. Bilateral swelling is evident in the submandibular region (arrows). B) CT of the neck. CT shows swollen submandibular glands. C) PET-CT of the neck. The strong accumulation of FDG is seen in bilateral submandibular glands. D) CT of the abdomen. Diffuse enlargement of the pancreas is apparent. E) PET-CT of the abdomen. The strong accumulation of FDG is seen in the enlarged pancreas.
Table 1.
Laboratory Data on Admission.
| cBC | Chemistry | Serum | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| WBC | 75.3 | ×103/mL | TP | 7.6 | g/dL | CRP | 0.49 | mg/dL | ||
| Neutro | 68.7 | % | Alb | 3.7 | g/dL | RF | 4 | IU/mL | ||
| Lymph | 26.4 | % | T.bil | 0.8 | mg/dL | IgG | 2,165 | mg/dL | ||
| Mono | 4.1 | % | AST | 18 | U/L | IgG4 | 1,100 | mg/dL | ||
| Eosino | 0.4 | % | ALT | 11 | U/L | IgA | 112 | mg/dL | ||
| Baso | 0.4 | % | γ-GTP | 19 | U/L | IgM | 92 | mg/dL | ||
| RBC | 4.01 | ×106/mL | LDH | 198 | U/L | IgE | 1,398 | IU/mL | ||
| Hb | 12.3 | g/dL | ALP | 84 | U/L | C3 | 96 | mg/dL | ||
| Ht | 37.8 | % | AMY | 87 | U/L | C4 | 26 | mg/dL | ||
| Plt | 17.8 | ×104/mL | BUN | 15.1 | mg/dL | CH50 | 57.2 | U/mL | ||
| Cr | 0.65 | mg/dL | sIL-2R | 662 | U/mL | |||||
| Na | 139 | mEq/L | ACE | 7.9 | U/mL | |||||
| K | 4.1 | mEq/L | ANA | (-) | ||||||
| Cl | 102 | mEq/L | aSS-A Ab | (-) | ||||||
| Ca | 8.9 | mg/dL | ||||||||
| CEA | 2.5 | ng/mL | ||||||||
| CA19-9 | <2.1 | U/mL | ||||||||
| Urinalysis | ||||||||||
| Blood | (-) | |||||||||
| Protein | (-) | |||||||||
| Sugar | (-) | |||||||||
Whole-body computed tomography (CT) showed symmetrically enlarged submandibular glands and diffuse pancreatic enlargement with a loss of lobules (Fig. 1B, C). In addition, 18F-fluorodeoxyglucose (FDG)-positron emission tomography (PET) showed an abnormal accumulation in both submandibular glands as well as in the pancreas (Fig. 1D, E). Although the patient declined a biopsy of the submandibular glands, she was considered a possible case of IgG4-RD due to submandibular gland and pancreatic swelling and hyper-IgG4emia, in accordance with the 2020 revision of the comprehensive diagnostic criteria for IgG4-RD (5). The 2018 clinical criteria for autoimmune pancreatitis would have diagnosed a definite case of autoimmune pancreatitis due to the diffuse pancreatic swelling, hyper-IgG4emia and extrapancreatic lesions (sclerosing sialadenitis) (6). Using the American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria (7), the score was 25 points due to the high serum IgG4 level (11 points), single set of submandibular gland swelling (6 points), and pancreatic enlargement (8 points), which would classify the case as IgG4-RD.
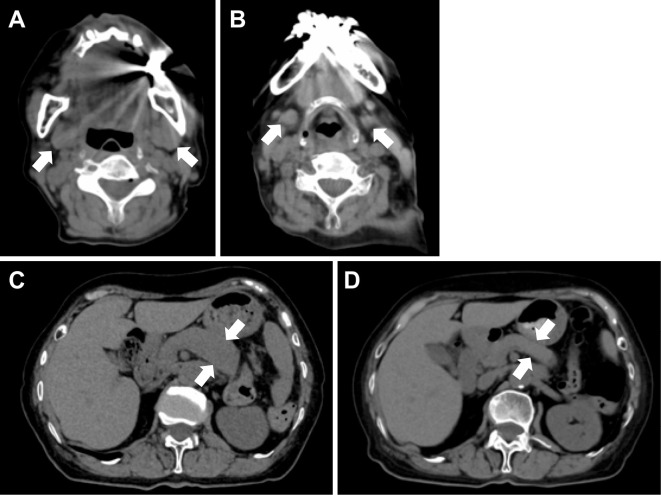
Prednisolone was started at 30 mg/day, and the submandibular mass promptly shrank, while the serum IgG4 levels decreased more gradually. Prednisolone was tapered off, and CT performed again four months after the start of treatment showed improvement in the submandibular gland and pancreatic enlargement (Fig. 2).
Figure 2.
CT of the abdomen after 4 months of treatment. A) CT of the neck before treatment (as in Fig. 1B). B) CT of the neck after four months of treatment. Improvement of pancreatic swelling is observed. C) CT of the abdomen before treatment (as in Fig. 1D). D) CT of the abdomen after four months of treatment. Improvement of pancreatic swelling is observed.
Discussion
In the present case, hyper IgG4emia and a positive inflammatory response suggested multicentric Castleman's disease and eosinophilic polyangiitis granulomatosis as the differential diagnoses. However, we concluded that Castleman's disease was unlikely, as there was no lymph node accumulation on FDG-PET, and eosinophilic polyangiitis granulomatosis was unlikely because there was no eosinophilia and no organ damage, such as that to the lung, kidney, or nerves, as clinical symptoms, although myeloperoxidase anti-neutrophil cytoplasmic antibody (MPO-ANCA) levels were not measured.
This case was one of IgG4-RD after COVID-19 mRNA vaccination. Although the causal relationship between COVID-19 mRNA vaccination and development of IgG4-RD remains controversial, it is certainly interesting that a previously healthy woman began to notice submandibular swelling two weeks after her second vaccination. The obvious suggestion is that vaccination may have triggered the development of IgG4-RD. A small number of cases of new-onset or relapsed autoimmune diseases after COVID-19 mRNA vaccination have been reported (3). An increasing number of new-onset cases of rheumatoid arthritis (8), systemic lupus erythematosus (9), dermatomyositis (10), autoimmune hepatitis (11), and thyroiditis (12) have also been described. To our knowledge, four cases of new-onset or relapsed IgG4-RD have been reported after COVID-19 mRNA vaccination (13-16), with this representing the fifth reported case (Table 2).
Table 2.
Reports of the Onset or Exacerbation of IgG4-related Disease after COVID-19 mRNA Vaccination.
| Reference | Age/Sex (years) | Onset or relapse | Vaccine | Number of vaccinations before onset or relapse | Duration to onset or relapse | Organ involvements | Serum IgG4 (mg/dL) | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|---|
| (13) | 2021 | 66 M | Relapse | mRNA-BNT162b2 | 2 | 2 weeks | Renal dysfunction | NA | PSL 0.5 mg/kg/ day, RTX | Resolved |
| (14) | 2022 | 65 M | Onset | mRNA-BNT162b2 | 1 | 4 weeks | Pancreatitis, liver dysfunction | Normal range | PSL 40 mg/day | Resolved |
| (15) | 2022 | 63 M | Onset | mRNA | 2 | 8 weeks | Pancreatitis, diabetes mellitus, liver dysfunction | 679.9 | PSL 40 mg/day | Resolved |
| (16) | 2022 | 71 M | Onset | mRNA-BNT162b2 | 1 | 1 week | Dyspnea, pleural effusion | 268 | Pleural drainage, partial decortication | Observation |
| Our case | 2022 | 78 F | Onset | mRNA-BNT162b2 | 2 | 2 weeks | Sialadenitis, pancreatitis | 1,100 | PSL 30 mg/day | Resolved |
All patients in these 5 cases were relatively old, being in their 60s and 70s. The vaccine type was not specified in one case, while the remaining four patients received BNT162b2. The number of vaccinations received was one or two, with the onset or relapse occurring at one to eight weeks after vaccination. The affected organs were the pancreas in three cases and the liver in two cases. Acute renal failure, pleural involvement, and sialadenitis occurred in one case each. Serum levels of IgG4 were within the national reference range in one case and elevated in three cases, with the highest levels seen in our case. This was attributed to the fact that even in typical IgG4-RD, the presence of IgG4-related sialadenitis tends to be associated with high IgG4 levels. Treatment comprised the use of glucocorticoids in four cases and rituximab in one case, Masset's patient, whose IgG4-RD had been refractory to treatment prior to vaccination and was given rituximab frequently. The present patient showed a better response to treatment against exacerbation after vaccination. All patients treated with glucocorticoids had a good response to treatment.
The main mechanisms presumed to underlie the induction of autoimmunity by COVID-19 vaccines include molecular mimicry, production of specific autoantibodies, specific vaccine adjuvant effects, and involvement of age-associated B cells (3,17). Vaccines induce adaptive immune responses to exert their protective effects but may induce a state of excessive inflammation. After vaccination, coupled with effective anti-SARS-CoV-2-neutralizing antibody production, healthy individuals experience an acute increase in type I interferon (IFN) expression by peripheral blood mononuclear cells and the accumulation of DNA damage (18). Furthermore, the mRNA in mRNA vaccines is present as antigens and adjuvants and is identified by endosomal Toll-like receptors (TLRs) and cytoplasmic inflammasome components to induce inflammation and immunity (17).
Follicular helper T cells and CD4+ cytotoxic lymphocytes have recently been thought to play major roles in the pathogenesis of IgG4-RD, although details remain unknown (19). Upstream of type II helper T-cell-related inflammation, the marked infiltration of TLR7-expressing M2 macrophages is seen, suggesting a regulatory mechanism (20). Viral infection and endogenous RNA have been considered possible causes of this signaling to TLR7. At the same time, the increased expression of type I IFN has been observed in affected organs (21). The post-vaccination situation, while transient, is considered similar to the situation in the pathogenesis of IgG4-RD, as a condition in which a signal to TLRs results in the overexpression of type I IFNs. Although this alone does not fully explain the pathogenesis, this similarity may be clinically and pathophysiologically important.
The development of immune disorders after vaccination has also been reported for human papillomavirus, influenza virus, and hepatitis B virus vaccines (22). Future studies should examine whether or not the onset and relapse of IgG4-RD is specific to mRNA vaccination, including in terms of the frequency. As one limitation to the interpretation of these cases, the only causal link between COVID-19 mRNA vaccination and the IgG4-RD onset or exacerbation is the short interval between these two events. Obtaining direct proof will require performing studies using animal models predisposed to the development of immunological disorders.
This report describes our experience with a case of IgG4-related sialadenitis and autoimmune pancreatitis following COVID-19 mRNA vaccination. The use of mRNA vaccines requires more studies regarding their effects on the human immune system.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 395: 497-506, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mallapaty S, Callaway E, Kozlov M, Ledford H, Pickrell J, Van Noorden R. How COVID vaccines shaped 2021 in eight powerful charts. Nature 600: 580-583, 2021. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Xu Z, Wang P, et al. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology 165: 386-401, 2022. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto M, Takahashi H, Shinomura Y. Mechanisms and assessment of IgG4-related disease: lessons for the rheumatologist. Nat Rev Rheumatol 10: 148-159, 2014. [DOI] [PubMed] [Google Scholar]
- 5.Umehara H, Okazaki K, Kawa S, et al. The 2020 revised comprehensive diagnostic (RCD) criteria for IgG4-RD. Mod Rheumatol 31: 529-533, 2021. [DOI] [PubMed] [Google Scholar]
- 6.Kawa S, Kamisawa T, Notohara K, et al. Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2018: revision of Japanese clinical diagnostic criteria for autoimmune pancreatitis, 2011. Pancreas 49: e13-e14, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wallace ZS, Naden RP, Chari S, et al. The 2019 American College of Rheumatology/European League Against Rheumatism classification criteria for IgG4-related disease. Ann Rheum Dis 79: 77-87, 2020. [DOI] [PubMed] [Google Scholar]
- 8.Baimukhamedov C, Makhmudov S, Botabekova A. Seropositive rheumatoid arthritis after vaccination against SARS-CoV-2 in- fection. Int J Rheum Dis 24: 1440-1441, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patil S, Patil A. Systemic lupus erythematosus after COVID-19 vaccination: a case report. J Cosmet Dermatol 20: 3103-3104, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Camargo Coronel A, Jiménez Balderas FJ, Quiñones Moya H, et al. Dermatomyositis post vaccine against SARS-CoV2. BMC Rheumatol 6: 20, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bril F, AI Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: causality or casualty? J Hepatol 75: 222-224, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vera-Lastra O, Ordinola Navarro A, Cruz Domiguez MP, Medina G, Sánchez Valadez TI, Jara LJ. Two cases of Graves' disease following SARS-CoV-2 vaccination: an autoimmune/inflammatory syndrome induced by adjuvants. Thyroid 31: 1436-1439, 2021. [DOI] [PubMed] [Google Scholar]
- 13.Masset C, Kervella D, Kandel-Aznar C, Fantou A, Blancho G, Hamidou M. Relapse of IgG4-related nephritis following mRNA COVID-19 vaccine. Kidney Int 100: 465-466, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodrigues T, Komanduri S. Seronegative type I autoimmune pancreatitis with immunoglobulin G4-related disease triggered by the Pfizer-BioNTech COVID-19 vaccine. Gastrointest Endosc 95: AB36, 2022. [Google Scholar]
- 15.Patel AH, Amin R, Lalos AT. Acute liver injury and IgG4-related autoimmune pancreatitis following mRNA-based COVID-19 vaccination. Hepatol Forum 3: 97-99, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tasnim S, Al-Jobory O, Hallak A, Bharadwaj T, Patel M. IgG4 related pleural disease: recurrent pleural effusion after COVID-19 vaccination. Respirol Case Rep 10: e01026, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol 21: 195-197, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ntouros PA, Vlachogiannis NI, Pappa M, et al. Effective DNA damage response after acute but not chronic immune challenge: SARS-CoV-2 vaccine versus systemic lupus erythematosus. Clin Immunol 229: 108765, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maehara T, Moriyama M, Nakamura S. Pathogenesis of IgG4-related disease: a critical review. Odontology 107: 127-132, 2019. [DOI] [PubMed] [Google Scholar]
- 20.Chinju A, Moriyama M, Kakizoe-Ishiguro N, et al. CD163+ M2 macrophages promote fibrosis in IgG4-related disease via Toll-like receptor 7/interleukin-1 receptor-associated kinase 4/NF-κB signaling. Arthritis Rheumatol 74: 892-901, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arai Y, Yamashita K, Kuriyama K, et al. Plasmacytoid dendritic cell activation and IFN-α production are prominent features of murine autoimmune pancreatitis and human IgG4-related autoimmune pancreatitis. J Immunol 195: 3033-3044, 2015. [DOI] [PubMed] [Google Scholar]
- 22.Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol 15: 586-594. [DOI] [PMC free article] [PubMed] [Google Scholar]